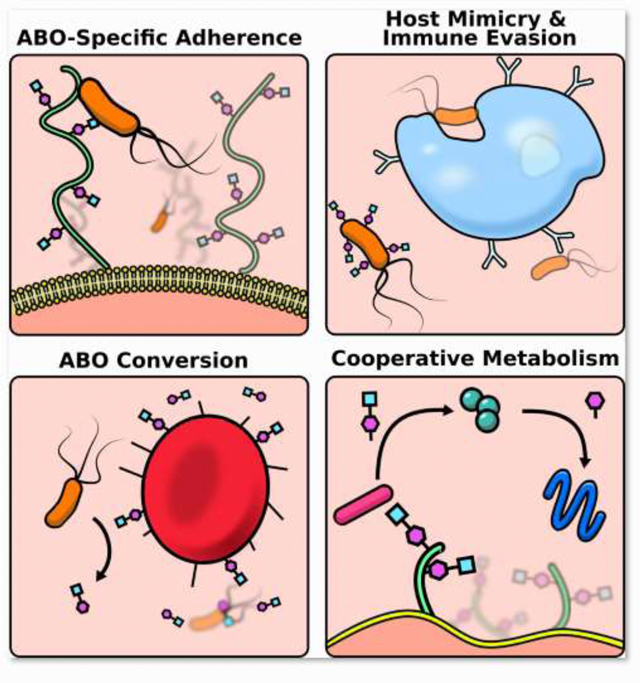
Graphical abstract
Keywords: Microbiome, blood type, pathogenic bacteria
Abstract
The complex communities of microbes that constitute the human microbiome are influenced by host and environmental factors. Here we address how a fundamental aspect of human biology, blood type, contributes to shaping this microscopic ecosystem. Although this question remains largely unexplored, we glean insights from decades of work describing relationships between pathogens and blood type. The bacterial strategies, molecular mechanisms, and host responses that shaped those relationships may parallel those that characterize how blood type and commensals interact. Understanding these nuanced interactions will expand our capacity to analyze and manipulate the human microbiome.
ABCs of ABO
At the beginning of the 20th century Dr. Karl Landsteiner discovered the ABO blood group, which not only informed safe blood transfusions but profoundly contributed to our understanding of the human immune system[1]. Over a century later there are 36 recognized blood groups but the ABO/H, secretor and Lewis systems remain the most clinically relevant and are known together as the histo-blood group antigens (HBGA)[2] [3]. An individual’s blood type can be defined by two factors: 1) specific antigen expression on red blood cells (RBC) (Fig. 1) and 2) presence of serum antibodies reactive to blood antigens not found in that individual (Fig. 2) [4]. Although these carbohydrate antigens are canonically associated with RBC, they are also widely expressed on tissues throughout the body [5], and in nearly 80% of people they are abundant in the mucous and other secretions (Fig. 3)[6]. Thus, HBGAs represent critical players at human-microbe interfaces. Here, we explore the current understanding of interactions between blood glycans and microbes -both commensal and pathogenic- and how those relationships might impact the microbiome.
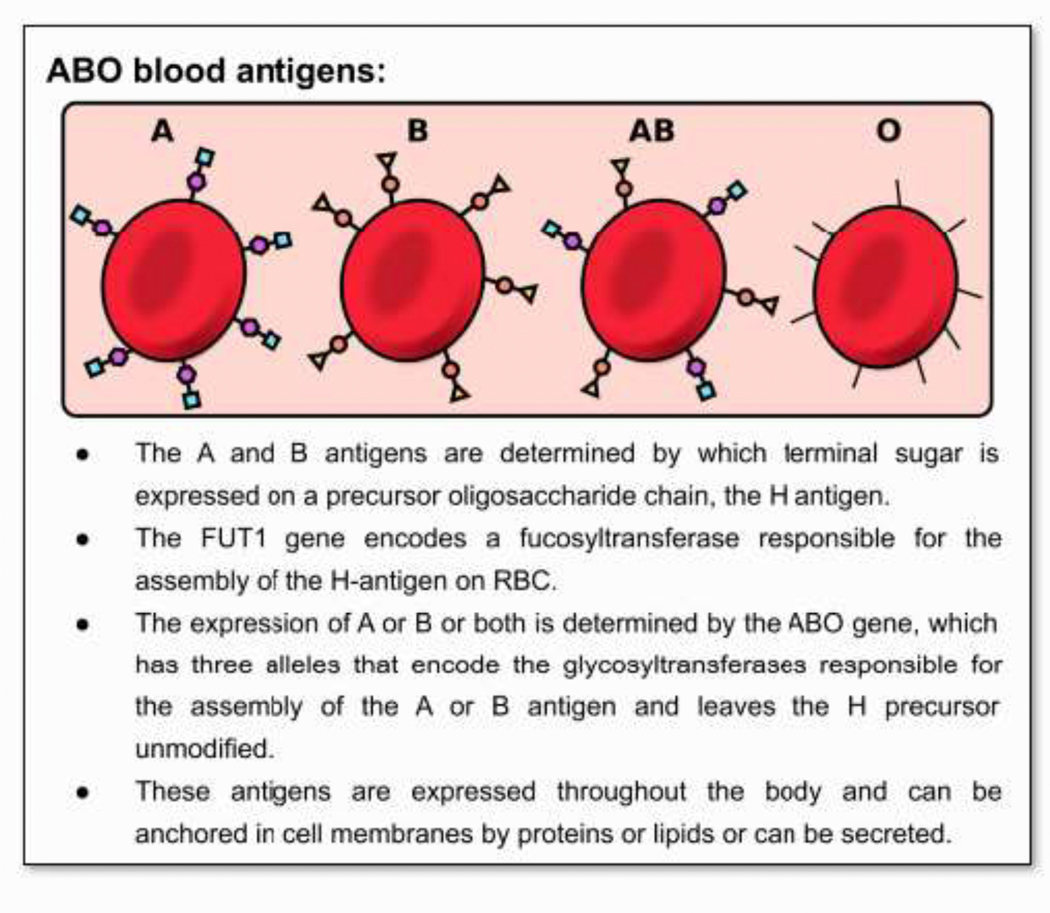
Figure 1. ABO blood antigens:
• The A and B antigens are determined by which terminal sugar is expressed on a precursor oligosaccharide chain, the H antigen.
• The FUT1 gene encodes a fucosyltransferase responsible for the assembly of the H-antigen on RBC.
• The expression of A or B or both is determined by the ABO gene, which has three alleles that encode the glycosyltransferases responsible for the assembly of the A or B antigen and leaves the H precursor unmodified.
• These antigens are expressed throughout the body and can be anchored in cell membranes by proteins or lipids or can be secreted.
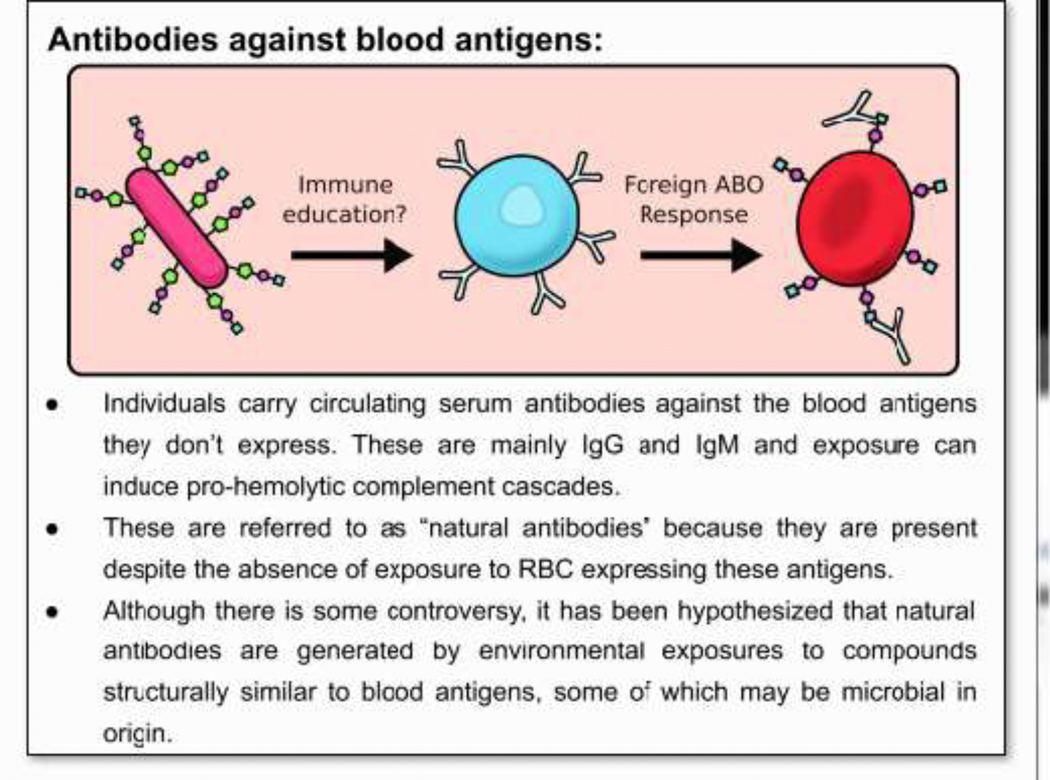
Figure 2. Antibodies against foreign blood antigens.
• Individuals carry circulating serum antibodies against the blood antigens they don’t express. These are mainly IgG and IgM and exposure can induce pro-hemolytic complement cascades.
• These are referred to as “natural antibodies” because they are present despite the absence of exposure to RBC expressing these antigens.
• Although there is some controversy, it has been hypothesized that natural antibodies are generated by environmental exposures to compounds structurally similar to blood antigens, some of which may be microbial in origin.
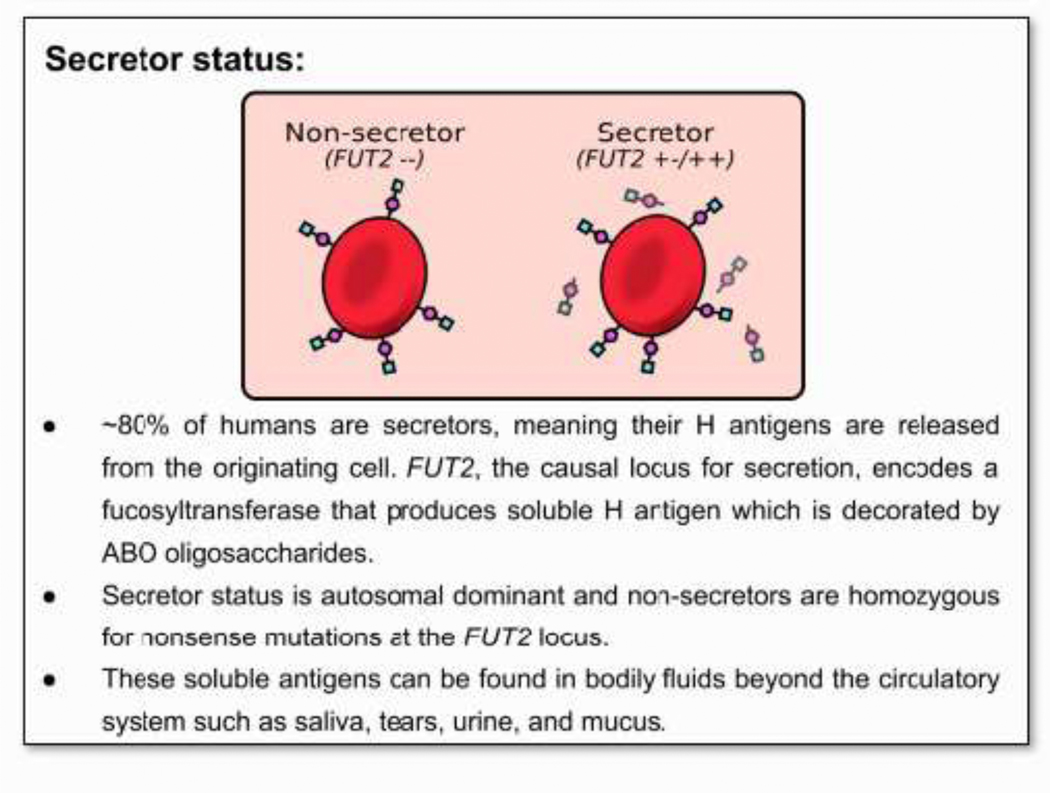
Figure 3. Secretor status.
• ~80% of humans are secretors, meaning their H antigens are released from the originating cell. FUT2, the causal locus for secretion, encodes a fucosyltransferase that produces soluble H antigen which is decorated by ABO oligosaccharides.
• Secretor status is autosomal dominant and non-secretors are homozygous for nonsense mutations at the FUT2 locus.
• These soluble antigens can be found in bodily fluids beyond the circulatory system such as saliva, tears, urine, and mucus.
ABO polymorphisms are conserved in many primate lineages, indicating that the evolution of this system far predates modern humans[7] [8]. The high level of conservation of these polymorphisms are suggestive of balancing selection. The patchwork geographic distribution of blood group frequencies cannot be explained by migration and genetic drift alone, and implicates endemic disease as a strong selecting force for blood group diversity[9][10] [11]. Indeed, many pathogens are associated with host blood type and secretor status, including malaria, noroviruses, and cholera [12] . Infection likely drove the balancing selection that has maintained these polymorphisms. Intriguingly, a number of chronic diseases are associated with blood type and have also been linked with the microbiome, such as Type 2 diabetes, gastric cancers, and asthma[13][14] [15][16][17]. What remains to be thoroughly explored is how commensal microbes interact with host blood groups and the influence of those interactions on the microbiome and on host health.
Barely a decade after Landsteiner described the ABO blood system, Dr. Alfred Nissle suggested people might have protective microbes in their guts. This concept was not fully pursued until mid-century with the development of anaerobic culture techniques, and has exploded in the last two decades, due to enormous advances in sequencing technologies [18]. This frenzy of research has provided unprecedented appreciation and insight into the complex microbial communities that colonize and support the human body [19]. While this work has uncovered associations between our microbes and nearly every aspect of human physiology, the mechanisms driving many of these links remain to be described. Although we enjoy relative immunologic harmony with our commensal microbes, we need not start from scratch as we try to uncover the ways in which they might associate with blood type, as decades of research on pathogens has illuminated some of the mechanisms and implications of microbes interacting with HBGAs[20].
Attachment and Adherence
For many microbes, success in the host begins with the ability to adhere, and host glycans are ideal receptors. The diversity of blood glycan structures likely arose as a means to inhibit the binding of pathogens to host cells, but these molecules also present a scaffold for commensals [21] [22] [6]. It has been proposed that the secretion of these glycans also evolved as a strategy to bind up pathogens before they encounter infectable cells [23]. These evolutionary skirmishes have resulted in differential disease risk based on host blood type. For example, enterotoxigenic Escherichia coli (H10407) expresses an adhesion molecule that specifically targets the A blood glycan which results in children with type A or AB blood being disproportionately affected by the diarrheal disease caused by this pathogen[24]. Helicobacter pylori binds to fucosylated blood antigens on the gastric epithelium and while H. pylori can be found in healthy individuals of all blood types, it can better access this receptor in type O individuals, putting them at greater risk for the overgrowth and pathogenesis of H. pylori [25] [26]. The commensal Lactobacilli have diversified and target A, B, and H antigens in a strain specific manner; with L. gasseri OLL2827 targeting the H-antigen, L. gasseri OLL2755, OLL2877 the B-antigen, and L. brevis OLL2772 the A antigen[27] [28]. An indirect benefit of our commensal bacteria adhering to these receptors is that they prevent pathogens from getting a foothold via adhesion exclusion[29].
Masquerading and Mimicry
Once anchored in the host, a microbe needs to avoid detection and clearance. An effective tactic to evade host immune responses is directly co-opting or mimicking host antigens[30]. A particularly nefarious strategy employed by Group A Streptococcus is to lyse host red blood cells and then cloak themselves in the erythrocyte membrane to evade detection by circulating immune cells[31]. A more refined strategy is to express molecules that mimic host glycans[32]. For instance, E. coli O86 expresses a glycan with high similarity to the blood group B antigen[33], and consequently, individuals with type A or O blood can more effectively clear this bacteria due to the presence of natural antibodies against the B blood antigen which cross react with E. coli’s mimic[34]. This strategy is shared by some viruses as well, and notably may be relevant to the observations that both SARS-CoV-1 and SARS-CoV-2 impact type-A individuals to a greater extent[35] [36].
The existence of natural antibodies against blood glycans is somewhat enigmatic- arising in the absence of canonical immunization. Some evidence suggests that high titers of blood antigen antibodies may be driven by widespread mimicry of blood group antigens by commensal microbes. Antibodies to blood group antigens appear in the first few months of life and increase to adult levels between the ages of 510 [37]. Although the expression of these antibodies is ubiquitous in healthy adults, titer levels can vary significantly between individuals, as well as within an individual over time [37] [38]. For example, people recovering from bowel surgeries have increased titers of anti-blood group antibodies[39].
These observations strongly support a role for environmental exposure, specifically via the gastrointestinal tract, for the presence of antibodies to blood group antigens. Indeed, germ free animals have virtually no antibodies against HBGAs[40]. Furthermore, individuals living in more industrialized regions have decreased ABO antibody titers which is proposed to be driven by reduced exposure to bacterial antigens due to changes in lifestyle and diet[41]. An ambitious three-year study performed in the 1950’s found that thirty days after hatching, germ free chickens had no anti-B antibodies while conventional chicks did, lending early experimental support to the theory that gut microbiota may have molecules antigenically similar to blood glycans [42]. Since then, it has been shown that many strains of gram-negative bacteria have structures with sufficient homology to HBGAs to induce the production of antibodies to HGBAs in both animal and human studies[43] [44] [45]. While it is clear that exposure to a complex microbiome results in the production of anti-ABO antibodies, inoculation with just a few strains of bacteria can induce high levels of these antibodies as well. Two individuals receiving plasma donations suffered hemolytic reactions despite previously receiving successful transplants from the same donor. It was later discovered that the donor had begun taking a probiotic which contained strains of Lactobacilli, Bifidobacteria, and Bacillus subtilis that resulted in massive anti-B titers in his plasma[46]. A number of other specific strains with this capacity have been identified, among them are E. coli O86, Citrobacter freundii, Bifidobacterium longum, and Lactobacillus reuteri [43] [40]. This diverse cohort of bacteria suggests that molecular homology or mimicry of HBGAs among bacteria is a widespread phenomenon. Even some viruses and plants exhibit these glycan moieties which can result in antibody production[45] [43].
Farmers and Foragers
Bacteria not only target HBGAs as receptors but can utilize them as a nutrient source. Some bacteria even have the capacity to induce host expression of these glycans. The infant gut is dominated by sialylated glycans and the mature gut is defined by fucosylated glycans including the ABO blood antigens. This shift to fucosylated glycans, like the induction of anti-ABO antibodies, is dependent on colonization of the gut by microbes[47]. The induction of glycan expression is of direct benefit to those bacteria that utilize them as food, but the benefits aren’t one sided; germ-free mice with immature fucosylation patterns were unable to recover in models of inflammatory intestinal diseases[48]. While the maturation of the glycan profile in the gut coincides with the maturation of the microbiome, it has been shown that even colonization with a single strain, Bacteroides fragilis or Bacteroides thetaiotaomicron, can result in a mature glycan expression[48]. To reap the rewards of promoting fucosylation, a number of Bacteroides, Ruminococcus, and Bifidobacterium strains encode either α -N-acetylgalactosaminidases and/or alpha-galactosidases, enzymes that can cleave A and B glycan moieties resulting in the harvest of these nutrients[49] [50] [51] [52]. Intriguingly, the abundance and diversity of Bifidobacterium was found to be higher in people who are type A and secretors, perhaps indicating that the host glycan profile can be particularly beneficial to blood glycan-consuming bacteria [53]. Another study identified some members of the genus Ruminococcous as well as B. fragilis as features predictive of ABO status although they did not report which blood type they were predictive of [54].
The ability to liberate host blood glycans as a nutrient source may facilitate the success of certain bacteria in hosts of different blood types, particularly in the absence of standard dietary polysaccharides. Deprivation of dietary polysaccharides will cause B. thetaiotamicron to switch to host mucins [55], suggesting that the impact of host blood type on these nutrient pools may only be evident when dietary polysaccharides become limited. In addition to providing the direct benefit of nutrient acquisition, the cleavage of HBGAs could benefit the broader microbial community via cross-feeding or syntrophy [56]. Microbial grazing on host products does not generally seem to be problematic for the host, however if there is a sustained dearth of dietary polysaccharides, as can be the case in a western diet, extensive consumption of host mucins can result in damage to gut barrier integrity and inflammation[57] [58]. Furthermore, if these bacteria escape the gut environment, the ability to harvest host glycans may shift from an adaptive feeding strategy to a virulence factor [59]. Outside of the intestinal milieu, bacterial scavenging of host blood glycans can lead to dire consequences. Septic Clostridium tertium can enzymatically cleave A-blood glycans into a “b-like” glycan resulting in a temporary functional conversion of blood type[60]. This feat is apparently shared with some environmental bacteria, as a body recovered from the River Thames was found to have different blood types in different tissues[61].
Quandaries and Questions
The relationships between bacteria and host blood glycans are abundant and complex. The very existence of different blood types is likely the result of millennia of evolutionary arms races and peace treaties between microbes and mammals. While these relationships and the mechanisms driving them have been well explored in culturable strains of bacteria, they remain relatively unexamined with our commensal bacteria.
Although multiple studies have found that secretor status is significantly associated with the overall composition of the microbiome and the relative abundance of specific taxa [53][62] [63], there is a dearth of studies examining the role of HBGA expression on the microbiome, and the few that have directly asked this question report contradicting results. Some indicate host ABO blood type does influence the composition of the microbiome [64] [65], while others have not found any associations [54] [66]. What might underlie these conflicting reports? The studies that didn’t identify associations were performed on large cohorts, which is important for robust analysis but can also introduce more hidden variables than the analysis of a smaller more homogenous cohort, such as that of reference 64 which was composed entirely of healthy, non-vegetarian, Finnish individuals. A major confounder is likely diet, the profound influence of diet on the microbiome has been well established and dietary differences could easily obscure the more subtle changes driven by host blood type[67] [68]. While existing studies have produced mixed results on the apparent effect size of blood type on the microbiome, evidence suggests that this variability is driven by underlying heterogeneity that we do not completely understand, supporting the consideration of blood type as a potential confounder in human microbiome studies. The ability of diet to obscure an influence of blood type on gut microbiome composition, is supported by experiments in mice which have shown that in a diet devoid of plant polysaccharides, the host carbohydrate repertoire significantly influences the microbiome. However, the effects of host carbohydrates are lost in a diet rich in plant polysaccharides[62]. Additionally, the few studies on blood type and microbiome focus primarily on healthy adult cohorts, but the influence of blood type may be more relevant in the maturing gut and more evident in states of dysbiosis or disease[69]. Taken together age, diet, and health status may dilute the effect of blood type on the microbiome which could explain conflicting reports. Future studies should address and explore these potential confounders to shed insight on the nuanced relationship of host blood type and the microbiome. This knowledge would have important implications for analyzing the composition of individual microbiomes, for informing microbial prevention or treatment strategies, and would provide insight on the mechanisms driving host and microbe interactions.
A mechanistic understanding of how microbes interact with host blood glycans can facilitate interventions targeting the microbiome, such as pre or probiotics and fecal microbiota transplants[71]. Insight on how host blood type, diet, and the microbiota interact could help researchers understand which bacteria would be likely to engraft, which strains would be most effective, and which might run the risk of being inflammatory instead of beneficial. Furthermore, this mechanistic understanding could also support the implementation of some of the techniques microbes have evolved. Indeed researchers are already exploring the capacity of bacteria to cleave blood glycans to effectively turn donations of any blood type to type O, which can be received by any recipient [50] [52]. Others are exploring the use of the B-like antigen from E. coli O86 to adsorb anti-B antibodies in donor fluids, tissues, and organs so they can be given safely to individuals with type B blood[70].
Understanding interactions at the host: microbe interface may also clarify if blood type could represent a risk factor that turns a commensal into pathobiont. Furthermore, microbial mimicry of host has been implicated in autoimmune disease, as have changes in the microbiome- it would be compelling to investigate of the importance of HBGA mimics in autoimmune disease [72] [73]. Another avenue ripe for exploration is the chronic diseases that have been both associated with blood type and the microbiome[74][75]. While our understanding of the importance of blood type on the microbiome is nascent, it would be useful to include host blood type and diet data in microbiome studies to allow researchers to stratify their analyses so that these relationships can be untangled and explored.
Highlights.
A historical perspective on associations between ABO blood type and bacteria.
Mechanisms that drive relationships between host blood type and commensal and pathogenic bacteria.
Review of conflicting evidence for an influence of host blood type on the intestinal microbiome.
How this knowledge may inform the development of microbiome-targeted intervention strategies.
Acknowledgments
Funding Sources: This work was supported by the National Institutes of Health NIAID training grant (Training Program in Immunology; T32-AI07405) award to Kathleen L Arnolds.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bayne-Jones S: Dr. Karl Landsteiner Nobel Prize Laureate in Medicine, 1930. Science 1931, 73:599–604. [DOI] [PubMed] [Google Scholar]
- 2.Storry JR, Castilho L, Chen Q, Daniels G, Denomme G, Flegel WA, Gassner C, de Haas M, Hyland C, Keller M, et al. : International society of blood transfusion working party on red cell immunogenetics and terminology: report of the Seoul and London meetings. ISBT Sci Ser 2016, 11:118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ABO H, and Lewis Systems. In Human Blood Groups. . John Wiley & Sons, Ltd; 2013:11–95. [Google Scholar]
- 4.Dean L: The ABO blood group. National Center for Biotechnology Information (US); 2005. [PubMed] [Google Scholar]
- 5.Ravn V, Dabelsteen E: Tissue distribution of histo-blood group antigens. APMIS 2000, 108:1–28. [DOI] [PubMed] [Google Scholar]
- 6.Henry S, Oriol R, Samuelsson B: Lewis Histo-Blood Group System and Associated Secretory Phenotypes. Vox Sang 1995, 69:166–182. [DOI] [PubMed] [Google Scholar]
- 7.Ségurel L, Thompson EE, Flutre T, Lovstad J, Venkat A, Margulis SW, Moyse J, Ross S, Gamble K, Sella G, et al. : The ABO blood group is a trans-species polymorphism in primates. Proc Natl Acad Sci 2012, 109:18493–18498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ségurel L, Gao Z, Przeworski M: Ancestry runs deeper than blood: The evolutionary history of ABO points to cryptic variation of functional importance. Bioessays 2013, 35:862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FARHUD DD, ZARIF YEGANEH M: A Brief History of Human Blood Groups. Iran J Public Health 2013, 42:1–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Glass RI, Holmgren J, Haley CE, Khan MR, Svennerholm A, Stoll BJ, Hossain KMB, Black RE, Yunus M, Barua D: PREDISPOSITION FOR CHOLERA OF INDIVIDUALS WITH O BLOOD GROUP POSSIBLE EVOLUTIONARY SIGNIFICANCE. Am J Epidemiol 1985, 121:791–796. [DOI] [PubMed] [Google Scholar]
- 11.Miller LH: Impact of malaria on genetic polymorphism and genetic diseases in Africans and African Americans. Proc Natl Acad Sci U S A 1994, 91:2415–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewald DR, Sumner SC: Blood Type Biochemistry and Human Disease. Wiley Interdiscip Rev Syst Biol Med 2016, 8:517–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meo SA, Rouq FA, Suraya F, Zaidi SZ: Association of ABO and Rh blood groups with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci 2016, 20:237–242. [PubMed] [Google Scholar]
- 14.Fagherazzi G, Gusto G, Clavel-Chapelon F, Balkau B, Bonnet F: ABO and Rhesus blood groups and risk of type 2 diabetes: evidence from the large E3N cohort study. Diabetologia 2015, 58:519–522. [DOI] [PubMed] [Google Scholar]
- 15.Cao X, Wen Z-S, Sun Y-J, Li Y, Zhang L, Han Y-J: Prognostic value of ABO blood group in patients with surgically resected colon cancer. Br J Cancer 2014, 111:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho I, Blaser MJ: The Human Microbiome: at the interface of health and disease. Nat Rev Genet 2012, 13:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mroczek B, Sitko Z, Sujewicz A, Wolińska W, Karpeta-Pawlak I, Kurpas D: Blood Group and Incidence of Asthma and Chronic Obstructive Pulmonary Disease. Adv Exp Med Biol 2018, 1114:31–39. [DOI] [PubMed] [Google Scholar]
- 18.Pariente N: A field is born. Nat Res 2019, doi: 10.1038/d42859-019-00006-2. [DOI] [Google Scholar]
- 19.Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R: Current understanding of the human microbiome. Nat Med 2018, 24:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooling L: Blood Groups in Infection and Host Susceptibility. Clin Microbiol Rev 2015, 28:801–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varki A: Nothing in Glycobiology Makes Sense, except in the Light of Evolution. Cell 2006, 126:841–845. [DOI] [PubMed] [Google Scholar]
- 22.Sharon N: Carbohydrate—Lectin Interactions in Infectious Disease. In Toward Anti-Adhesion Therapy for Microbial Diseases. Edited by Kahane I, Ofek I. Springer US; 1996:1–8. [DOI] [PubMed] [Google Scholar]
- 23.Varki A: Biological roles of glycans. Glycobiology 2017, 27:3–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar P, Kuhlmann FM, Chakraborty S, Bourgeois AL, Foulke-Abel J, Tumala B, Vickers TJ, Sack DA, DeNearing B, Harro CD, et al. : Enterotoxigenic Escherichia coli–blood group A interactions intensify diarrheal severity. J Clin Invest [date unknown], 128:3298–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boren T, Falk P, Roth KA, Larson G, Normark S: Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 1993, 262:1892–1895. [DOI] [PubMed] [Google Scholar]
- 26.Chakrani Z, Robinson K, Taye B: Association Between ABO Blood Groups and Helicobacter pylori Infection: A Meta-Analysis. Sci Rep 2018, 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UCHIDA H, KAWAI Y, KINOSHITA H, KITAZAWA H, MIURA K, SHIIBA K, HORII A, KIMURA K, TAKETOMO N, ODA M, et al. : Lactic Acid Bacteria (LAB) Bind to Human B- or H-Antigens Expressed on Intestinal Mucosa. Biosci Biotechnol Biochem 2006, 70:3073–3076. [DOI] [PubMed] [Google Scholar]
- 28.Uchida H, Kinoshita H, Kawai Y, Kitazawa H, Miura K, Shiiba K, Horii A, Kimura K,Taketomo N, Oda M, et al. : Lactobacilli binding human A-antigen expressed in intestinal mucosa. Res Microbiol 2006, 157:659–665. [DOI] [PubMed] [Google Scholar]
- 29.Sassone-Corsi M, Raffatellu M: No Vacancy: How Beneficial Microbes Cooperate with Immunity To Provide Colonization Resistance to Pathogens. J Immunol 2015, 194:4081–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cress BF, Englaender JA, He W, Kasper D, Linhardt RJ, Koffas MAG: Masquerading microbial pathogens: capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol Rev 2014, 38:660–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wierzbicki IH, Campeau A, Dehaini D, Holay M, Wei X, Greene T, Ying M, Sands JS, Lamsa A, Zuniga E, et al. : Group A Streptococcal S Protein Utilizes Red Blood Cells as Immune Camouflage and Is a Critical Determinant for Immune Evasion. Cell Rep 2019, 29:2979–2989.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szymanski CM, Schnaar RL, Aebi M: Bacterial and Viral Infections. In Essentials of Glycobiology. Edited by Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, et al. Cold Spring Harbor Laboratory Press; 2015. [PubMed] [Google Scholar]
- 33.Guo H, Yi W, Shao J, Lu Y, Zhang W, Song J, Wang PG: Molecular Analysis of the O-Antigen Gene Cluster of Escherichia coli O86:B7 and Characterization of the Chain Length Determinant Gene (wzz). Appl Environ Microbiol 2005, 71:7995–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Check JH, O’Neill EA, O’Neill KE, Fuscaldo KE: Effect of Anti-B Antiserum on the Phagocytosis of Escherichia coli. Infect Immun 1972, 6:95–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guillon P, Clément M, Sébille V, Rivain J-G, Chou C-F, Ruvoën-Clouet N, Le Pendu J: Inhibition of the interaction between the SARS-CoV Spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology 2008, 18:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J, Yang Y, Huang H, Li D, Gu D, Lu X, Zhang Z, Liu L, Liu T, Liu Y, et al. : Relationship between the ABO Blood Group and the COVID-19 Susceptibility. Epidemiology; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philar M: Human Blood Groups 2nd ed. by Geoff Daniels. [date unknown]. [Google Scholar]
- 38.de França NDG, Poli MCC, Ramos PG de A, Borsoi CS da R, Colella R: Titers of ABO antibodies in group O blood donors. Rev Bras Hematol E Hemoter 2011, 33:259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soltis RD, Wilson ID: Serum immunoglobulin M concentrations following bowel resection in chronic inflammatory bowel disease. Gastroenterology 1975, 69:885–892. [PubMed] [Google Scholar]
- 40.Khasbiullina NR, Shilova NV, Navakouski ME, Nokel AY, Knirel YA, Blixt O, Bovin NV: Repertoire of Abs primed by bacteria in gnotobiotic mice. Innate Immun 2018, 24:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazda T, Yabe R, NaThalang O, Thammavong T, Tadokoro K: Differences in ABO antibody levels among blood donors: a comparison between past and present Japanese, Laotian, and Thai populations. Immunohematology 2007, 23:38–41. [PubMed] [Google Scholar]
- 42.Springer GF, Horton RE, Forbes M: ORIGIN OF ANTI-HUMAN BLOOD GROUP B AGGLUTININS IN WHITE LEGHORN CHICKS. J Exp Med 1959, 110:221–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Springer GF, Williamson P, Readler BL: Blood Group Active Gram-Negative Bacteria and Higher Plants*. Ann N Y Acad Sci 1962, 97:104–110. [DOI] [PubMed] [Google Scholar]
- 44.Springer GF, Williamson P, Brandes WC: BLOOD GROUP ACTIVITY OF GRAM-NEGATIVE BACTERIA. J Exp Med 1961, 113:1077–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Springer GF: Blood-group and Forssman antigenic determinants shared between microbes and mammalian cells. Prog Allergy 1971, 15:9–77. [PubMed] [Google Scholar]
- 46.Daniel-Johnson J, Leitman S, Klein H, Alter H, Lee-Stroka A, Scheinberg P, Pantin J,Quillen K: Probiotic-associated high-titer anti-B in a group A platelet donor as a cause of severe hemolytic transfusion reactions. Transfusion (Paris) 2009, 49:1845–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nanthakumar NN, Dai D, Newburg DS, Walker WA: The role of indigenous microflora in the development of murine intestinal fucosyl- and sialyltransferases. FASEB J Off Publ Fed Am Soc Exp Biol 2003, 17:44–46. [DOI] [PubMed] [Google Scholar]
- 48.Nanthakumar NN, Meng D, Newburg DS: Glucocorticoids and microbiota regulate ontogeny of intestinal fucosyltransferase 2 requisite for gut homeostasis. Glycobiology 2013, 23:1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tailford LE, Crost EH, Kavanaugh D, Juge N: Mucin glycan foraging in the human gut microbiome. Front Genet 2015, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu QP, Sulzenbacher G, Yuan H, Bennett EP, Pietz G, Saunders K, Spence J, Nudelman E, Levery SB, White T, et al. : Bacterial glycosidases for the production of universal red blood cells. Nat Biotechnol 2007, 25:454–464. [DOI] [PubMed] [Google Scholar]
- 51.Hoskins LC, Agustines M, McKee WB, Boulding ET, Kriaris M, Niedermeyer G: Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J Clin Invest 1985, 75:944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahfeld P, Sim L, Moon H, Constantinescu I, Morgan-Lang C, Hallam SJ, Kizhakkedathu JN, Withers SG: An enzymatic pathway in the human gut microbiome that converts A to universal O type blood. Nat Microbiol 2019, doi: 10.1038/s41564-019-0469-7. [DOI] [PubMed] [Google Scholar]
- 53.Wacklin P, Mäkivuokko H, Alakulppi N, Nikkilä J, Tenkanen H, Räbinä J, Partanen J, Aranko K, Mättö J: Secretor Genotype (FUT2 gene) Is Strongly Associated with the Composition of Bifidobacteria in the Human Intestine. PLoS ONE 2011, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davenport ER, Goodrich JK, Bell JT, Spector TD, Ley RE, Clark AG: ABO antigen and secretor statuses are not associated with gut microbiota composition in 1,500 twins. BMC Genomics 2016, 17:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martens EC, Chiang HC, Gordon JI: Mucosal Glycan Foraging Enhances Fitness and Transmission of a Saccharolytic Human Gut Bacterial Symbiont. Cell Host Microbe 2008, 4:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koropatkin NM, Cameron EA, Martens EC: How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 2012, 10:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahowald MA, Rey FE, Seedorf H, Turnbaugh PJ, Fulton RS, Wollam A, Shah N, Wang C, Magrini V, Wilson RK, et al. : Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci U S A 2009, 106:5859–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johansson MEV, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, et al. : Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63:281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao Y, Rocha ER, Smith CJ: Efficient utilization of complex N-linked glycans is a selective advantage for Bacteroides fragilis in extraintestinal infections. Proc Natl Acad Sci U S A 2014, 111:12901–12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerbal A, Maslet C, Salmon C: Immunological Aspects of the Acquired B Antigen. Vox Sang 1975, 28:398–403. [DOI] [PubMed] [Google Scholar]
- 61.Rahfeld P, Withers SG: Toward universal donor blood: Enzymatic conversion of A and B to O type. J Biol Chem 2020, 295:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kashyap PC, Marcobal A, Ursell LK, Smits SA, Sonnenburg ED, Costello EK, Higginbottom SK, Domino SE, Holmes SP, Relman DA, et al. : Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc Natl Acad Sci 2013, 110:17059–17064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar H, Wacklin P, Nakphaichit M, Loyttyniemi E, Chowdhury S, Shouche Y, Mättö J, Isolauri E, Salminen S: Secretor Status Is Strongly Associated with Microbial Alterations Observed during Pregnancy. PLOS ONE 2015, 10:e0134623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mäkivuokko H, Lahtinen SJ, Wacklin P, Tuovinen E, Tenkanen H, Nikkilä J, Björklund M, Aranko K, Ouwehand AC, Mättö J: Association between the ABO blood group and the human intestinal microbiota composition. BMC Microbiol 2012, 12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gampa A, Engen PA, Shobar R, Mutlu EA: Relationships between gastrointestinal microbiota and blood group antigens. Physiol Genomics 2017, 49:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turpin W, Bedrani L, Espin-Garcia O, Xu W, Silverberg MS, Smith MI, Guttman DS, Griffiths A, Moayyedi P, Panaccione R, et al. : FUT2 genotype and secretory status are not associated with fecal microbial composition and inferred function in healthy subjects. Gut Microbes 2018, 9:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. : Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. : Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rausch P, Rehman A, Künzel S, Häsler R, Ott SJ, Schreiber S, Rosenstiel P, Franke A, Baines JF: Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci 2011, 108:19030–19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shang W, Zhai Y, Ma Z, Yang G, Ding Y, Han D, Li J, Zhang H, Liu J, Wang PG, et al. : Production of human blood group B antigen epitope conjugated protein in Escherichia coli and utilization of the adsorption blood group B antibody. Microb Cell Factories 2016, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duvallet C, Zellmer C, Panchal P, Budree S, Osman M, Alm EJ: Framework for rational donor selection in fecal microbiota transplant clinical trials. PLOS ONE 2019, 14:e0222881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou S, Xu R, He F, Zhou J, Wang Y, Zhou J, Wang M, Zhou W: Diversity of Gut Microbiota Metabolic Pathways in 10 Pairs of Chinese Infant Twins. PloS One 2016, 11:e0161627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie H, Guo R, Zhong H, Feng Q, Lan Z, Qin B, Ward KJ, Jackson MA, Xia Y, Chen X, et al. : Shotgun Metagenomics of 250 Adult Twins Reveals Genetic and Environmental Impacts on the Gut Microbiome. Cell Syst 2016, 3:572–584.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ewald DR, Sumner SCJ: Human microbiota, blood group antigens, and disease. Wiley Interdiscip Rev Syst Biol Med 2018, 10:e1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Durack J, Lynch SV: The gut microbiome: Relationships with disease and opportunities for therapy. J Exp Med 2019, 216:20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]