Abstract
Study Objectives
This study investigated the role of pubertal status and hormones in the association between sleep satisfaction and self-reported emotion functioning in 256 children and adolescents aged 8–15.
Methods
Self-report data was provided on sleep duration, sleep satisfaction, and emotion reactivity and regulation, and a saliva sample was obtained for hormone measures. A subset of children also wore an Actigraph watch to measure sleep for a week.
Results
Latent-class analysis revealed three classes of sleepers: Satisfied, Moderately Satisfied, and Dissatisfied. Dissatisfied sleepers reported more difficulties with emotion regulation and greater emotion reactivity than Satisfied sleepers. High difficulties with emotion regulation was associated with shorter objective sleep duration, and high emotion reactivity was associated with lower sleep efficiency. For girls, Dissatisfied sleepers reported being further through pubertal development than Satisfied sleepers. There were also significant correlations between pubertal development and shorter sleep duration and longer sleep latency in girls, and shorter and more irregular sleep in boys. Finally, pubertal development in girls was a significant moderator in the relationship between sleep satisfaction and difficulties with emotion regulation in Dissatisfied sleepers, such that being further through puberty and having unsatisfactory sleep resulted in the highest emotion regulation difficulties.
Conclusions
This study expands on previous literature by considering the role of sleep satisfaction and the interaction with puberty development on emotion function. Specifically, a role for pubertal development was identified in the association between unsatisfactory sleep and emotion regulation in girls.
Keywords: sleep quality, sleep satisfaction, puberty status, hormones, emotion reactivity, emotion regulation
Statement of Significance.
This study provides evidence that puberty and poor sleep may have a synergistic impact on emotion function and highlights this period of hormonal change as important for intervention on improved sleep hygiene. Future research should further investigate the role for sex hormone concentration in this association during puberty.
Introduction
Experimental manipulation of sleep shows behavioral and neural reactivity to emotional stimuli after sleep deprivation in adults [1]. As well, there is evidence of self-reported poor sleep quality being associated with difficulty in regulating emotions [2]. Although less research has been performed in school-aged children, similar results to adults have been found for the impact of poor sleep on emotional functioning. McGlinchey and colleagues [3] found that adolescents (ages 11–15) were more susceptible than adults to sleep deprivation as evidenced by reduced vocal expression of positive emotions in adolescents. Studies comparing multiple nights of sleep restriction to sleep extension in children and adolescents found sleep restriction to be associated with worse emotion regulation on tasks and by self-report measures [4, 5] and greater pupil reactivity to negative audio clips [6]. Despite this pattern, an experimental study by Reddy, Palmer, Jackson, Farris, and Alfano [7] did not find an effect of 4-hour sleep restriction compared to an extended sleep period of 9.5 hours on reactivity to emotional images or on an emotion regulation task in adolescents.
Few studies have been conducted on the association between natural variations in sleep quantity and quality and emotion functioning in children and adolescents. The National Sleep Foundation recommends between 9 to 11 hours of sleep for school-aged children (ages 6–13) and 8 to 10 hours for teenagers (14–17 years) [8]. In a large cross-sectional study of adolescents, sleep disturbances, such as difficulty falling asleep and awakenings, were associated with greater mental health complaints, which was partially mediated by poor emotion regulation strategies (greater use of rumination, avoidance, and suppression) [9]. In a sample of children, shorter sleep duration reported by parents was associated with greater reactivity in emotion processing regions, such as the amygdala and insula when viewing negative emotional faces [10]. This study replicates results in adult populations showing increased reactivity of emotion processing regions after insufficient sleep [11]. Together, these studies support similar findings to the impact of poor sleep in adolescents and children as to adults.
During adolescent development, puberty has been associated with changes in sleep. Puberty coincides with the onset of subjective sleep complaints in girls [12, 13] and a shift towards later bedtimes [13]. As well as changes in satisfaction with sleep, one study also reported shortening of sleep duration with progression through the Tanner stages [14]. These changes in sleep satisfaction and duration may be associated with the well-documented shift in sleep timing associated with puberty and adolescence e.g. [15, 16]. Frey and colleagues [17] found that the onset of menarche was associated with a 5-year-long period of shift to preference for a later bedtime before a shift to earlier bedtime in adulthood. Another study found that late bedtimes during childhood were associated with early development of secondary sex characteristics such as pubic hair [18]. This circadian shift towards later bedtimes can result in conflicts between sleep and waking commitments, such as school start times, which can leave adolescents sleep-deprived and with waking deficits [16]. This research supports a role for pubertal timing and sex hormones in sleep quality, duration, and satisfaction that should be investigated for a role in emotion functioning.
Previous research by our group has supported a role for hormones in the relationship between sleep duration and quality and emotion functioning [19, 20]. For instance, greater progesterone concentration in sleep-restricted women in the luteal phase of the menstrual cycle was associated with greater processing of emotional faces reflected by a larger N170 amplitude [20]. This association could be due to the interactive impact of poor sleep and hormone concentration on emotion reactivity when both these factors are often associated with increased reactivity on their own [21, 22]. Increased amygdala reactivity resulting from progesterone has been proposed to occur through activation of GABAA receptors and inhibition of inhibitory interneurons [23]. The effect of poor sleep on amygdala reactivity may be driven by changes to the cholinergic system, potentially due to loss of REM sleep [24, 25]. As well, changes in functional connectivity reported with sleep loss reflect reductions of top–down regulatory control over the amygdala [11, 26]. Thus, we expect the effects of both hormones and sleep loss to produce an amplification on behavior as each of these factors acts via different mechanisms. Specifically, we expect to see a greater impact of sleep loss on emotion for individuals with a specific hormone status, specifically higher cortisol, progesterone and testosterone. For instance, in a sample of university students, we reported a moderating role of cortisol in the association between sleep quality and difficulties with emotion regulation, where poor sleepers with the highest concentration of cortisol had the greatest difficulties with emotion regulation [19].
In the current study, we investigated the role of hormones and pubertal status in the relationship between sleep and emotion during a developmental window in which there is a natural change in sex hormone concentrations due to puberty. This time is an interesting window to investigate these questions due to the changes in both sleep quality and timing, as well as changes in hormone concentration. In this study, we aimed to address several questions. We investigated the effect of different patterns of sleep duration and satisfaction on self-reported emotion functioning. We expected to find more reactivity and difficulties with emotion regulation in children reporting low sleep satisfaction or duration. As we were interested in the role of individual differences and naturally occurring sleep patterns, we used Latent Class Analysis [27]. This analysis allows us to examine different patterns of sleepers naturally present within the sample, and to then compare these groups on emotional outcomes. As such, it allows for a data-driven separation of data into groups for analysis. It provides an opportunity to examine the potential combination of multiple measures of sleep to identify poor sleepers. However, as we were also interested in the role of individual differences in sleep and pubertal status, we also aimed to examine relationships among variables in the continuous data using a correlation/regression strategy. Both data approaches have strengths in investigating the research questions and have connections to past research. Next, we investigated if good and poor sleepers differed on hormone concentrations or on pubertal status. Based on previous findings showing worse sleep with advancing pubertal development [12–14, 18], we expected that groups of good and poor sleepers would differ on pubertal status, and poor sleep would be associated with greater concentration of the sex hormones and more advanced pubertal development. Our final, central aim was to investigate whether there was a role for pubertal status and sex hormone concentration in the relationship between sleep and emotion functioning. Based on our previous research [19, 20], we expected that children with a combination of poor sleep and greater sex hormone or cortisol concentration, or being further through puberty, might have greater emotion complaints due to a synergistic impact of deficits in emotional functioning from both poor sleep and greater hormone concentration. We aimed to investigate the nature of this relationship and whether these variables interact to influence behavior.
Methods
Participants
Participants (N = 1515; Mage = 11.79, SD = 1.81, range 8–15 years), were recruited from 15 elementary schools in a mid-sized city in Canada as part of a larger 3-year longitudinal study on social psychological risk factors for behavioral outcomes. All schools that were approached agreed to participate. Sixty-three percent of the children and adolescents received parental consent to participate in the study. Parent report indicated that 84.20% of the children and adolescents were White, 1.70% were Black, 2.12% were Asian, 2.76% were Hispanic, 0.85% Indigenous, and 7.53% were Mixed ethnicity (a further 0.85% of parents indicated that they preferred not to answer the question). Data on socioeconomic status indicated that mean levels of education for mothers and fathers was, on average, “completed an associate degree and/or technical diploma.” From the total sample, a subset of participants from each grade and sex were randomly chosen to complete a mobile lab session starting in the second year of the study. The current study focused on this subset of students as they completed the measures of interest. The subset consisted of 256 children and early adolescents (127 girls, 129 boys, Mage = 11.25, range 8–15 years). There were no significant differences on the study variables between the full sample and the subset of participants used in the current study (ps > .05 for boys and girls – note that analysis was conducted separately for boys and girls given the different pubertal questions). See Table 1 for sample description.
Table 1.
Number of participants by grade, age, and sex
| Boys | Girls | |||
|---|---|---|---|---|
| Grade | n | M Age | n | M Age |
| 3 | 1 | 8 | 3 | 8 |
| 4 | 27 | 9.11 | 28 | 9.19 |
| 5 | 25 | 10.08 | 24 | 10.25 |
| 6 | 19 | 11.05 | 21 | 11.1 |
| 7 | 25 | 12.16 | 17 | 12.06 |
| 8 | 23 | 13.27 | 20 | 13.25 |
| 9 | 9 | 14 | 14 | 14.14 |
| Total | 129 | 11.25(1.686) | 127 | 11.24(1.785) |
Materials
Subjective sleep
Students were asked to report what time they normally fall asleep and wake up during the week and on weekends, and these variables were used to calculate typical sleep duration during the week and on weekends. To determine sleep satisfaction, the question, “Since the beginning of summer last year, how often has not getting enough sleep bothered you?” was used from a measure of Daily Hassles [28]. Response options included “almost never bothers me,” “sometimes bothers me,” “often bothers me,” and “almost always bothers me.”
Objective sleep
Actigraphy was recorded using an Actiwatch Spectrum Pro (Phillips Respironics, Murrysville, Pennsylvania). Epoch length was set to 15 seconds, and watches were analyzed in Actiware software. Mean sleep duration, sleep efficiency (total sleep time/time between sleep onset and offset) and sleep latency were calculated for the week participants wore the watch (M = 6.58 days of data, SD = 1.24 days). The Sleep Regularity Index (SRI) was also calculated to determine the consistency of sleep/wake states across 24-hour periods [29]. Participants who had fewer than four days of data were removed to avoid artificial inflation of sleep regularity (10 participants). Although diary data was not present to adjust bedtimes, all watch data was screened for obvious artifact and off-wrist data.
Emotion
Emotion regulation was assessed with four items out of the 36 items from the Difficulties in Emotion Regulation Scale (DERS; e.g. “When I’m upset or stressed, I have difficulty concentrating. When I am upset or stressed, I have difficulty thinking about anything else. When I am upset or stressed, I know I can find a way to feel better. When I am upset or stressed, I start to feel bad about myself.” [30]. Responses were given on a 4-point Likert scale ranging from “almost never” to “almost always.” Cronbach’s alpha was .690. Participants also completed three items from the Emotional Reactivity Scale (ERS (e.g. “My feelings get hurt easily. When I am upset/angry, it takes me a long time to calm down. When something bad happens, other people tell me I overreact.”), with responses on a 4-point Likert scale ranging from “not at all like me” to “completely like me” [31]. Cronbach’s alpha was .702. For both measures, the mean score was used for analysis with a higher score reflecting greater difficulties with emotion regulation and greater emotion reactivity.
Pubertal status
Puberty status was measured by administering the Pubertal Development Scale [32]. Participants were asked to rate their pubertal development on various symptoms such as growth in height, presence of body hair, and skin changes. Boys were asked about growth of facial hair and a deeper voice, while girls were asked about breast development and whether they had reached menarche. Each item was rated on a 4-point Likert scale from “not yet started” to “seems complete.” The Cronbach’s alpha was .495 for girls, and .763 for boys.
Saliva sampling
Saliva was collected in Salivettes (Sarstedt Inc). Participants were instructed to chew the cotton swab for 60 seconds and then place back in the tube. Saliva samples were then frozen at −20 degrees until analysis.
Procedure
Students were invited to participate in the study during visits by research assistants to schools. For each of the 3 years of the larger longitudinal study, students completed a survey that was completed in two separate halves, occurring approximately 1 month apart within a 4-month period (January–April). The survey included the subjective sleep, emotion regulation, and pubertal status measures. Trained researchers administered the surveys to participants in their classrooms during school hours. Surveys were completed at the time of day that was convenient for the school; therefore, all participants did not complete the survey measures at the same time during the day. Participants were compensated with small gifts (e.g. backpacks, pencils). Parents reported on demographic variables, such as child age, grade, and sex, initially in a survey that was completed at home and submitted with the parents’ consent form. All procedures were cleared by a university research ethics board.
In the second year of the larger study, a subset of participants from each grade and sex were randomly chosen to participate in a mobile lab session which was an RV parked at school in either a morning (09:00–10:30 am) or afternoon session (01–02:30 pm). Students came to the mobile lab individually and completed a performance task battery while EEG was recorded (not included here). During their time in the mobile lab, they provided a saliva sample in order to assess hormonal concentrations (thus, some participants provided the sample in the morning session and the others in the afternoon session). Actigraph watches for these students also were handed out in waves of 10 to 20 students as the watches became available, and so were not directly connected to the dates of mobile lab participation. Participants were instructed to wear the watch on their non-dominant hand for one week. The mobile lab sessions were conducted between October and June in the second year of the study. The mobile lab sessions took a longer time to complete than the surveys as the lab sessions were conducted individually and only two participants could complete the session each school day. The survey data and actigraphy data from this second-year time point were analyzed for this report.
Data analysis
Hormones
Progesterone, cortisol, and testosterone were quantified using liquid chromatography coupled with tandem mass spectrometry following methodology outlined in Gao, Stalder, and Kirschbaum’s recent article [33]. However, the hormone concentrations for progesterone appeared artificially high, perhaps due to interference from the cotton swab for measuring sex hormones [34]. The mean progesterone in girls was 165 pg/ml (range = 60.8–362), while other studies have shown anovulatory levels before menarche to be 41 pg/ml, with 100 pg/ml used as a sign of ovulation [35], and means of 14.5 to 43.4 pg/ml in children under the age of 12 (BioVendor, Czech Republic). The progesterone data from girls was therefore excluded.
Missing data
Missing data occurred because some students did not complete all the questions in surveys (average missing data 7.9% on emotion questionnaires and 10.5% on sleep questions), and because some students did not complete each part of the survey (the survey was split into two parts completed at different time periods; missing data was due to absenteeism but also occasionally to time conflicts, students declining to participate in one part of the survey, and students moving to another school district). Thus, missing data were estimated using the full information maximum likelihood (FIML) estimation method in the Latent Class Analysis [36]. Additionally, only a subset of participants wore Actigraphy watches (see Objective Measures in Results section) due to manufacturer delay.
Statistical analysis
Latent class analysis (LCA) was conducted using Mplus Version 8.0 (Muthen and Muthen). Latent class indicators included subjective sleep satisfaction, and subjective sleep duration during the week and weekend, while controlling for both age and sex (see Supplementary Material for more detail on LCA models). To address the first research question, classes based on sleep indicators were compared on emotion outcomes using one-way ANOVAs. Additionally, we conducted correlations between sleep and emotion variables not considering class for both subjective and objective measures of sleep (as no clear latent class model was produced for the smaller sample of objective measures). To address the relationship between sleep and pubertal status or hormone concentration, we compared classes on pubertal status/concentration with one-way ANOVAs and conducted correlations between pubertal status and sleep outcomes. Finally, to address the central question of whether there is a role for pubertal status in the relationship between sleep and emotion functioning, we conducted correlations separately in each class and performed a moderation regression analysis using sleep class as a predictor for difficulties with emotion regulation and pubertal development in girls as a moderator. We used the Bejamini and Hochberg [37] method for false discovery rate for the correlational analyses, and we conducted this method separately based on research question and dependent variable.
Results
Relationships between sleep quality and emotion functioning
Subjective measures of sleep
Latent Class Analysis revealed three distinct classes using subjective measures of sleep (sleep duration during the week, sleep duration during the weekend, and the extent “bothered” by sleep pattern). Class 1 had 99 participants (46 girls), Class 2 had 72 participants (34 girls), and Class 3 had 70 participants (37 girls). Class 1 reported “almost never” being bothered by their sleep (M = 1.0), Class 2 reported being “sometimes” bothered (M = 2.0), and finally, Class 3 reported being between “often” and “almost always” bothered by sleep (M = 3.4, SD =.5, (F(2,229) = 1606.15, p < .001). As the groups did not differ on self-reports of sleep duration during the week, (range = 9.10 to 9.44 hours, F(2,226) = 1.103, p =.334), or on weekends, (range = 9.04 to 9.62, F(2,206 = 1.140, p =.322), the classes were labeled as Satisfied, Moderately Satisfied and Dissatisfied respectively. To determine that the 3-class solution was the best model fit, we considered entropy, class size, BLRT (bootstrap loglikelihood ratio test) and LMR-LRT (Lo-Mendell-Rubin-adjusted likelihood ratio). This model fit had an entropy greater than .7, class sizes larger than 5% of the sample, and a significant BLRT and LMR-LRT. The 4- class solution had a BIC that increased, a nonsignificant LMR-LRT, and a class with less than 5% of the sample.
We then compared the classes on emotion variables. There was a significant effect of class on both emotion reactivity (F(2,221) = 9.542, p< .001) and difficulties with emotion regulation (F(2,220) = 11.97, p<.001). The Dissatisfied sleepers (M = 2.21, SD = .734) reported more reactivity (t(160) = −4.554, p < .001) and regulation difficulties (M = 2.57, SD = .785) (t(160) = −4.707, p< .001) than the Satisfied sleepers (M = 1.72, SD = .610; M = 2.05, SD = .602). The Dissatisfied sleepers also reported more emotion reactivity than the Moderately Satisfied sleepers (M = 1.92, SD = .760) (t(126) = 2.138, p = .034), and the Moderately Satisfied sleepers (M = 2.35, SD = .628) reported more Difficulties with Emotion Regulation than the Satisfied sleepers (t(155) = −2.995, p = .003).
To further investigate the association between sleep and emotion, we conducted correlations between subjective and objective sleep quality and self-reported emotion without separating by classes. See Table 2 for correlations. Difficulties with emotion regulation was correlated with the extent participants felt bothered by their sleep schedule, such that feeling more bothered by their sleep was associated with greater difficulties. Additionally, emotion reactivity was correlated with the extent participants felt bothered, such that feeling more bothered by their sleep was associated with greater emotion reactivity.
Table 2.
Correlations between objective and subjective sleep variables and emotion functioning
| Difficulties with emotion regulation | P-value | Emotion reactivity | P-value | |
|---|---|---|---|---|
| Extent bothered | 0.323 | <.001* | 0.310 | <.001* |
| Sleep duration during the week (Subj) | −0.129 | .061 | −0.100 | .148 |
| Sleep duration during the weekend (Subj) | −0.061 | .400 | 0.015 | .833 |
| Sleep regularity index (Obj) | 0.037 | .740 | −0.142 | .194 |
| Sleep duration (Obj) | −0.323 | .002* | −0.198 | .056 |
| Sleep efficiency | −0.180 | .082 | −0.211 | .041† |
| Sleep latency | 0.196 | .059 | 0.029 | .783 |
*Indicates statistical significance.
†This significant association did not survive use of the Bejamini & Hochberg method.
Objective measures of sleep
As there was a smaller sample size of participants who provided at least one night of actigraphy data, we computed the LCA separately for the subset of participants who wore the watch. This analysis included 99 participants, due to a delay in receiving the Actigraph watches from the manufacturer at the onset of the study, or to participants not wearing the watch. These students were relatively evenly spread across grades (19 Grade 4, 17 Grade 5, 14 Grade 6, 14 Grade 7, 19 Grade 8 and 16 Grade 9). Class indicators included mean duration, mean latency, and mean sleep efficiency for the week period, and the sleep regularity index. Within this sample, the Latent Class Analysis did not come to a good model fit, producing non-significant LRT and BLRT values. As such, the LCA results for the objective data was not used any further. Additionally, as the LCA did not come to a good fit with these data, we moved forward with a correlational approach to address relationships between our research questions on the objective sleep data with puberty and emotion measures.
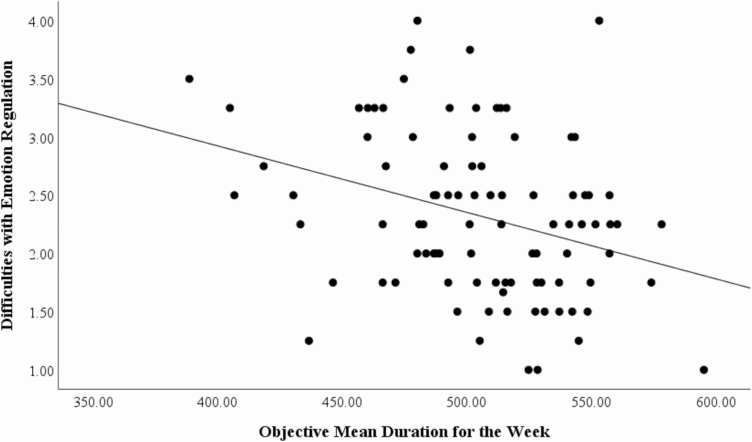
To further investigate the association between sleep and emotion, we conducted correlations without separating by classes. See Table 2 for correlations. Difficulties with emotion regulation was correlated with mean sleep duration from the watches, such that a shorter sleep duration was associated with greater difficulties with emotion regulation (See Figure 1; one multivariate outlier removed).
Figure 1.
Correlation between mean duration on the watches and difficulties with emotion regulation in the sample of children who wore the Figure 1. Correlation between mean duration on the watches and difficulties with emotion regulation in the sample of children who wore the watches (r = −0.323, p = .002).
Relationships between sleep and pubertal status/hormone concentration
Pubertal status
Sleep classes were then compared on their pubertal status separately for boys and girls. In girls only, there was a significant effect of class membership on pubertal status (F(2,103) = 4.284, p = .016). Scores indicated that Dissatisfied sleepers (M = 2.55, SD = .787) were further through pubertal development than the Satisfied sleepers (M = 2.05, SD = .722) (t(74) = 2.924, p = .005).
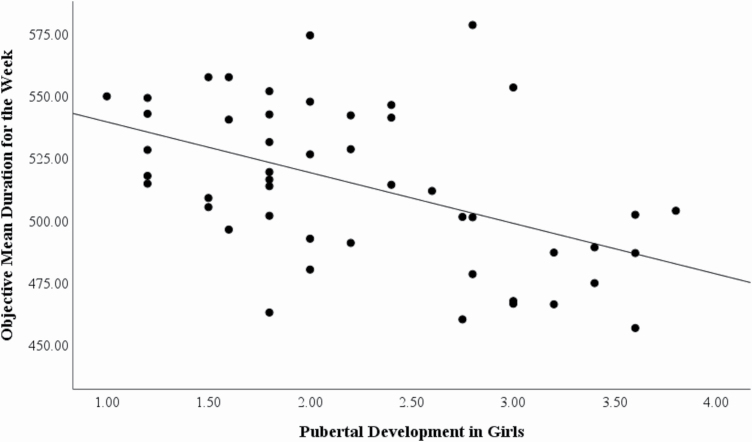
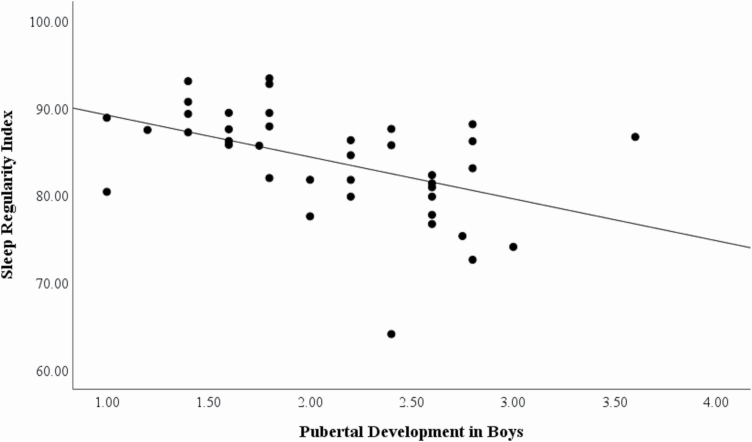
We conducted correlations of the puberty scale score with sleep quality. In girls, puberty score was correlated with objective mean duration of sleep (r = −.490, p < .001; 2 multivariate outliers removed; see Figure 2), objective sleep latency (r = .459, p = .001), and the extent to which they felt bothered by their sleep (r = .243, p = .014), such that pubertal development was associated with shorter sleep duration, longer sleep latency, and more dissatisfaction. In boys, puberty score was correlated with the sleep regularity index (r = −.476, p = .002; see Figure 3), such that being further through puberty was associated with more irregular sleep.
Figure 2.
Correlation between pubertal development in girls and the mean sleep duration measured by actigraphy (r = −0.490, p < .001).
Figure 3.
Correlation between pubertal development in boys and the Sleep Regularity Index (r = .476, p = .002).
Hormone concentration
There were no significant differences between classes based on hormone concentration, or any correlations with hormone concentration and emotion functioning. Outliers greater than 3 SD over their group mean were removed (9 for cortisol and 2 for testosterone) (see Table 3).
Table 3.
Hormone concentrations by class
| Satisfied (n) | Satisfied (M) | Moderately satisfied (n) | Moderately satisfied (M) | Dissatisfied (n) | Dissatisfied (M) | F(df) | P-value | |
|---|---|---|---|---|---|---|---|---|
| Cortisol | 87 | 1.95 (1.04) | 69 | 1.88 (0.79) | 63 | 2.00 (0.88) | 0.310 (2,216) | .734 |
| Testosterone | 49 | 70.70 (35.62) | 38 | 81.75 (48.58) | 28 | 72.78 (37.78) | 0.832 (2,112) | .438 |
Investigation of the role of pubertal status/hormone concentration in the relationship between sleep and emotion functioning
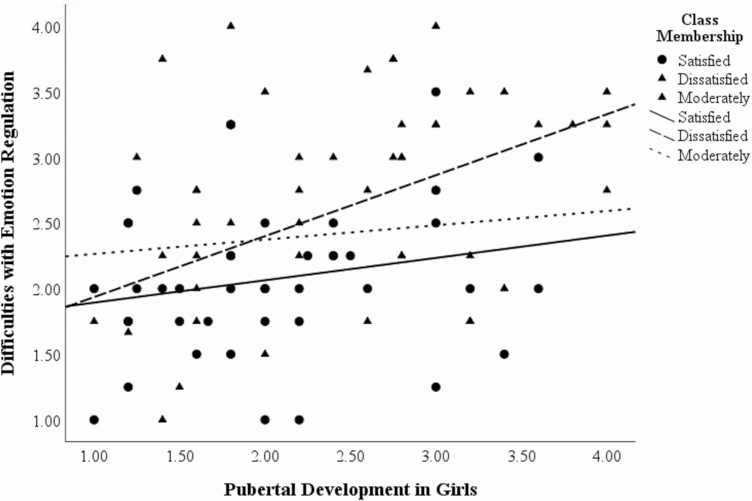
To investigate pubertal development for a role in the relationship between sleep and emotion functioning, we examined correlations between hormone concentration and pubertal development score with emotion functioning within each class. In the Dissatisfied sleeper group only, there was a significant correlation between difficulties with emotion regulation and pubertal development in girls (r = .422, p = .013), such that being further along in development was associated with greater emotion regulation difficulties (Figure 4). Although this association would not survive correction due to multiple comparisons, we present the more robust moderation model as a test of our hypotheses. Since class is a categorical variable with more than two groups, we used dummy coding with the Satisfied sleepers as the reference group. The Satisfied sleepers were selected as a reference group to be able to compare each of the groups to the most satisfied sleepers, so we could understand the contributions of poorer sleep compared to satisfied sleep. On the first step, we included the two dummy coded variables, and step one accounted for a significant portion of difficulties with emotion regulation, R2 = .115, F(2,103), = 6.68, p = .002. An interaction term was created for each dummy coded variable with mean-centered pubertal development in girls. These interaction terms were entered in the second step. The second step led to a significant change in R2 (R2 = .081, F(2,101) = 5.075, p = .008). When comparing the interaction terms, there was a significant interaction between pubertal development in girls and the dummy coded variables for the Dissatisfied compared to Satisfied sleeper variable (b = .292, t = 3.12, p = .002). In a separate analysis with the Dissatisfied and Satisfied sleepers alone, simple slopes analyses revealed a significant effect of class at high pubertal development in girls (+1SD, b = .409, t(72) = 2.816, p = .006).
Figure 4.
Correlation between difficulties with emotion regulation and pubertal development in girls by class (Dissatisfied sleepers: r = .422, p = .013).
Discussion
The current study investigated the association between sleep satisfaction and quality and emotion reactivity and regulation in a sample of children and adolescents. The central aim was to investigate a role for puberty and hormones in the relationship.
Relationships between sleep quality and emotion functioning
Three classes emerged that differed on the extent they felt satisfied with their sleep pattern. Dissatisfied sleepers reported greater emotion reactivity than Moderately satisfied and Satisfied sleepers. Dissatisfied sleepers reported greater difficulties with emotion regulation than the Satisfied sleepers, and Moderately satisfied sleepers reported greater difficulties than Satisfied sleepers. These results support previous findings that have shown that poor sleep is associated with greater emotion reactivity and regulation complaints [3–7]. These findings also suggest a potential role for sleep satisfaction, as the classes had differences in their emotion functioning despite not being different in their sleep durations. In fact, all groups had average durations within the acceptable ranges outlined by the National Sleep Foundation [8]. Despite this, previous research has shown that even with no differences in sleep duration, some individuals may be satisfied with their sleep, while others feel sleep restricted due to variability in sleep need [38]. Asking participants to rate their sleep satisfaction may be a way to gage the discrepancy between actual sleep duration and ideal sleep duration to highlight sleep debt, as low satisfaction despite a sleep duration within recommended range for one’s age group may be due to greater sleep need for an individual. A study in adolescents found that sleep debt after a stressful life event was associated with more negative affect and less positive affect the following morning or greater “affective spillover” [39]. The current study indicates that despite comparable sleep durations, the students could be experiencing sleep debt that is influencing their emotion functioning.
In the sample of participants not separated by class, associations between sleep and emotion functioning emerged. For emotion reactivity, there was a significant relationship with sleep satisfaction. As expected, better quality was associated with lower emotion reactivity. For emotion regulation, more emotion regulation difficulties were associated with lower sleep satisfaction and shorter sleep duration. These results support the previous literature that finds associations between sleep quality and duration and emotion functioning in children, adolescents and adults [1, 3–5, 9, 10]. Having associations with subjective emotion functioning and objective sleep measures lends some support to the role of sleep in emotion, instead of the association being fully explained by another variable such as low life satisfaction or greater life complaints.
Relationships between sleep and pubertal status/hormone concentration
When comparing the classes on pubertal status, for girls, Dissatisfied sleepers reported being further through puberty than Satisfied sleepers. Thus, it is possible that girls further through puberty may not be meeting their sleep need. An association between pubertal development and sleep complaints is supported by Knutson [12], who found the relationship in girls and not boys for more insomnia complaints and waking feeling tired, although not for reporting insufficient sleep on a yes or no response. It is possible that with a four-point scale in the current study, we were better able to measure the more subtle differences in sleep satisfaction. Another study found a link between sleep changes and pubertal timing in girls only [18]. In their longitudinal study, they found that girls with later bedtimes and shorter sleep durations in childhood experienced earlier onset of secondary sex characteristics. The timing of puberty has been linked to the developmental decrease in delta power during slow-wave sleep [40]. These studies highlight changes in sleep and sleep quality that occur during puberty that could have an impact on sleep satisfaction as puberty progresses. This interpretation is also supported by the correlations with puberty score that we found in the full sample, where in girls, being further through puberty was associated with shorter sleep duration and longer sleep latency, both of which may contribute to worse sleep satisfaction. In boys, puberty was associated with less consistency in sleep periods. This inconsistency could be due to the circadian shift that occurs with puberty, particularly in boys, and staying up late on the weekends resulting in an inconsistent bedtime [41]. As boys were less developed than girls in this age group, it is possible that with more pubertal development, we may see greater effects emerge in boys.
Investigation of the role of pubertal status/hormone concentration in the relationship between sleep and emotion functioning
Next, we conducted correlations within each class between puberty score and emotion outcomes to determine if a combination of sleep and puberty status were associated with emotion outcomes. There was a significant correlation in the Dissatisfied sleeper group only, indicating that being further through puberty was associated with greater emotion regulation difficulties in the poor sleepers. When tested as a moderation model, only the interaction between Dissatisfied sleepers and puberty score in girls was a significant predictor of difficulties with emotion regulation. Simple slopes revealed that there was an effect of class at higher pubertal development scores. Due to issues with the measurement of progesterone in the current sample, we were unable to test the role of progesterone concentration in this relationship. This result is similar to our previous work in which sleep-restricted women in the luteal phase of the menstrual cycle with greater progesterone concentrations showed more neural reactivity to emotional images [20]. Since poor sleep on its own is associated with poor emotion regulation, and higher levels of progesterone are also associated with greater emotion reactivity, together these conditions may have a synergistic impact on emotion regulation. Progesterone administration and being in the luteal phase has been associated with greater amygdala activation [22, 42]. This greater emotion reactivity may thus be exacerbated by poor sleep, which would also bias an individual towards greater reactivity and emotion regulation difficulties. As adolescence is a time that is associated with the development of emotion regulation [43], the combination of poor sleep and hormonal fluctuations could have an important impact on the development of emotion regulation and reactivity. In a study by Forbes, Williamson, Ryan and Dahl [44], girls with depression who were in mid-to-late puberty experienced greater negative affect than boys, girls without depression, or girls who had not yet undergone puberty, showing a potentially summative impact on affect. Adolescence has been shown to be a time for the onset of emotional disorders, especially in girls [45, 46], which may be associated with white matter structure development [47]. Due to the changes in sleep and emotion functioning during this time, it is likely that adolescence is an important window for setting up good sleep behaviors to assist with emotional development and protect against negative emotional outcomes.
Limitations and Future Directions
Due to the nature of correlational data in the current study, there are open questions about the direction of effects in the relationship between sleep and emotion. Additionally, as the questionnaire and actigraphy data were collected at different times, there may be an effect of timing and separation of data collection on relationships between subjective and objective sleep data, and objective sleep data and pubertal status. As such, the relationships identified in this sample may therefore reflect one’s typical sleep behavior, as opposed to the prior night’s sleep in particular. Since some measures were delivered based on convenience for the participating schools, there was some variability in time of day of measures taken between individual participants. Saliva measures also were taken from either the morning or afternoon session based on scheduling of the various schools. There is the possibility for a response bias in the current study in that the simple measures of subjective sleep and emotion were administered at the same time, possibly inflating their association. We think the findings cannot be completely attributed to any such inflation because the results were supported by associations with objective measures of sleep from actigraphy in the current sample. Future studies however could address this issue by implementing other objective measures. As this study had a correlational strategy, there is a potential for confounding variables, such as school setting, etc, to have impacted the results. Although Grade 9 was the only grade that was faced with a change in school setting, there may have been variability in school start times between different schools and this variation could be a potential confounding factor. As such, there is a need for further experimental research in this population.
This study did not have a direct measure of sleep need, so although differences in sleep satisfaction may be reflective of differences in sleep need but not sleep duration, future research is needed to directly measure the role of sleep need and verify the role of sleep satisfaction. Additionally, as the question of sleep satisfaction from this study has not been previously validated, future research should investigate these questions with other measures of sleep satisfaction and subjective sleep assessment. Due to the length of the DERS scale for this population, only 4 of 36 questions were used from the scale; this combination of questions has not been previously externally validated. However, this simpler measure did relate to constructs as expected.
To address some of the questions of causality, in-laboratory studies could be conducted to examine the role of sleep the night before on emotion functioning, and how daily fluctuations in sleep quality may contribute to next day functioning. This study did not find the expected relationships with hormone concentration and emotional functioning; however, issues with sampling time and methods may have masked effects. The question of the role of sex and stress hormone concentration in this association for children and adolescents should thus be followed up in future research. More research could additionally be done in boys during puberty, as greater effects with puberty may emerge at a greater age than the current sample. As well, as this report focused on cross-sectional data, future research should focus on longitudinal data to determine cause and effect relationships between sleep and puberty. As actigraphy data was not collected with diary data, there could be some measurement error in timing of sleep and wake onset, and these associations with pubertal status should be investigated in future studies with concurrent diary collection. However, based on the high quality of individual watch data, we feel the actigraphy data is quite reliable.
Conclusions
The current study contributes to the literature by exploring both subjective and objective measures of sleep in a large sample of children to investigate the association with emotion functioning. It also allows for the consideration of the role of puberty and development on this relationship. These results indicate that puberty may be a time period in which there can be a synergistic impact with poor sleep on emotion complaints, at least in girls, and may be an important intervention period for better sleep hygiene practices as these children also transition to later bedtimes and develop sleep habits in adolescence and adulthood.
Supplementary Material
Funding
This research was funded by the Social Science and Humanities Research Council (SSHRC) of Canada.
Disclosure Statement
None declared.
Data Availability
Data cannot be shared for ethical/privacy reasons.
References
- 1. Cote KA, et al. The role of sleep in processing emotional information. In: Dringenberg HC, ed. Handbook of Behavioral Neuroscience. Vol 30. Amsterdam: Academic Press; 2019:505–518. [Google Scholar]
- 2. Palmer CA, et al. Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev. 2017;31:6–16. [DOI] [PubMed] [Google Scholar]
- 3. McGlinchey EL, et al. The effect of sleep deprivation on vocal expression of emotion in adolescents and adults. Sleep. 2011;34(9):1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vriend JL, et al. Manipulating sleep duration alters emotional functioning and cognitive performance in children. J Pediatr Psychol. 2013;38(10):1058–1069. [DOI] [PubMed] [Google Scholar]
- 5. Baum KT, et al. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry. 2014;55(2):180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McMakin DL, et al. The impact of experimental sleep restriction on affective functioning in social and nonsocial contexts among adolescents. J Child Psychol Psychiatry. 2016;57(9):1027–1037. [DOI] [PubMed] [Google Scholar]
- 7. Reddy R, et al. Impact of sleep restriction versus idealized sleep on emotional experience, reactivity and regulation in healthy adolescents. J Sleep Res. 2017;26(4):516–525. [DOI] [PubMed] [Google Scholar]
- 8. Hirshkowitz M, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. [DOI] [PubMed] [Google Scholar]
- 9. Palmer CA, et al. Associations among adolescent sleep problems, emotion regulation, and affective disorders: findings from a nationally representative sample. J Psychiatr Res. 2018;96:1–8. [DOI] [PubMed] [Google Scholar]
- 10. Reidy BL, et al. Decreased sleep duration is associated with increased fMRI responses to emotional faces in children. Neuropsychologia. 2016;84:54–62. [DOI] [PubMed] [Google Scholar]
- 11. Yoo SS, et al. The human emotional brain without sleep - a prefrontal amygdala disconnect. Curr Biol. 2007;17(20):R877–R878. [DOI] [PubMed] [Google Scholar]
- 12. Knutson KL. The association between pubertal status and sleep duration and quality among a nationally representative sample of U.S. adolescents. Am J Hum Biol. 2005;17(4):418–424. [DOI] [PubMed] [Google Scholar]
- 13. Pieters S, et al. Puberty-dependent sleep regulation and alcohol use in early adolescents. Alcohol Clin Exp Res. 2010;34(9):1512–1518. [DOI] [PubMed] [Google Scholar]
- 14. Rutters F, et al. Sleep duration and body-weight development during puberty in a Dutch children cohort. Int J Obes. 2010;34(10):1508–1514. [DOI] [PubMed] [Google Scholar]
- 15. Crowley SJ, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014;9(11):e112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crowley SJ, et al. An update on adolescent sleep: new evidence informing the perfect storm model. J Adolesc. 2018;67:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frey S, et al. Consequences of the timing of menarche on female adolescent sleep phase preference. PLoS One. 2009;4(4):e5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foley JE, et al. Changes to sleep-wake behaviors are associated with trajectories of pubertal timing and tempo of secondary sex characteristics. J Adolesc. 2018;68:171–186. [DOI] [PubMed] [Google Scholar]
- 19. Lustig KA. The interaction of sleep and hormones on emotion functioning (Unpublished doctoral dissertation). Brock University Digital Repository; 2021. [Google Scholar]
- 20. Lustig KA, et al. Sex hormones play a role in vulnerability to sleep loss on emotion processing tasks. Neurobiol Sleep Circadian Rhythms. 2018;5:94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Derntl B, et al. Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. Psychoneuroendocrinology. 2008;33(8):1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Wingen GA, et al. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience. 2011;191:38–45. [DOI] [PubMed] [Google Scholar]
- 23. Andréen L, et al. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinology. 2009;34(8):1121–1132. [DOI] [PubMed] [Google Scholar]
- 24. Vazquez J, et al. Basal forebrain acetylcholine release during REM sleep is significantly greater than during waking. Am J Physiol Regul Integr Comp Physiol. 2001;280(2):R598–R601. [DOI] [PubMed] [Google Scholar]
- 25. Mu P, et al. Cholinergic system in sleep regulation of emotion and motivation. Pharmacol Res. 2019;143:113–118. [DOI] [PubMed] [Google Scholar]
- 26. Motomura Y, et al. Sleep debt elicits negative emotional reaction through diminished amygdala-anterior cingulate functional connectivity. PLoS One. 2013;8(2):e56578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bergman LR, et al. A person-oriented approach in research on developmental psychopathology. Dev Psychopathol. 1997;9(2):291–319. [DOI] [PubMed] [Google Scholar]
- 28. Willoughby T. A short-term longitudinal study of Internet and computer game use by adolescent boys and girls: prevalence, frequency of use, and psychosocial predictors. Dev Psychol. 2008;44(1):195–204. [DOI] [PubMed] [Google Scholar]
- 29. Phillips AJK, et al. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep. 2017;7(1):3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gratz KL, et al. Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopathol Behav Assess. 2004;26(1):41–54. [Google Scholar]
- 31. Nock MK, et al. The emotion reactivity scale: development, evaluation, and relation to self-injurious thoughts and behaviors. Behav Ther. 2008;39(2):107–116. [DOI] [PubMed] [Google Scholar]
- 32. Carskadon MA, et al. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14(3):190–195. [DOI] [PubMed] [Google Scholar]
- 33. Gao W, et al. Quantitative analysis of estradiol and six other steroid hormones in human saliva using a high throughput liquid chromatography-tandem mass spectrometry assay. Talanta. 2015;143:353–358. [DOI] [PubMed] [Google Scholar]
- 34. Shirtcliff EA, et al. Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26(2):165–173. [DOI] [PubMed] [Google Scholar]
- 35. Gray SH, et al. Salivary progesterone levels before menarche: a prospective study of adolescent girls. J Clin Endocrinol Metab. 2010;95(7):3507–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schafer JL, et al. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- 37. Benjamini Y, et al. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57(1):289–300. [Google Scholar]
- 38. Mercer PW, et al. Differences in reported sleep need among adolescents. J Adolesc Health. 1998;23(5):259–263. [DOI] [PubMed] [Google Scholar]
- 39. Chue AE, et al. The role of sleep in adolescents’ daily stress recovery: negative affect spillover and positive affect bounce-back effects. J Adolesc. 2018;66:101–111. [DOI] [PubMed] [Google Scholar]
- 40. Campbell IG, et al. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. Proc Natl Acad Sci U S A. 2012;109(15):5740–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Randler C. Age and gender differences in morningness-eveningness during adolescence. J Genet Psychol. 2011;172(3):302–308. [DOI] [PubMed] [Google Scholar]
- 42. van Wingen GA, et al. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2008;13(3):325–333. [DOI] [PubMed] [Google Scholar]
- 43. Ahmed SP, et al. Neurocognitive bases of emotion regulation development in adolescence. Dev Cogn Neurosci. 2015;15:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forbes EE, et al. Positive and negative affect in depression: influence of sex and puberty. Ann N Y Acad Sci. 2004;1021:341–347. [DOI] [PubMed] [Google Scholar]
- 45. Angold A, et al. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29(5):1043–1053. [DOI] [PubMed] [Google Scholar]
- 46. Dahl RE, et al. (Eds.). Annals of the New York Academy of Sciences: Vol. 1021. Adolescent Brain Development: Vulnerabilities and Opportunities. New York: New York Academy of Sciences; 2004. [DOI] [PubMed] [Google Scholar]
- 47. Ladouceur CD, et al. White matter development in adolescence: the influence of puberty and implications for affective disorders. Dev Cogn Neurosci. 2012;2(1):36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared for ethical/privacy reasons.