Abstract
Study Objectives
Insomnia is highly prevalent in patients with coronary heart disease (CHD). However, the potential effect of insomnia on the risk of recurrent major adverse cardiovascular events (MACE) remains uncertain.
Methods
This prospective cohort study included 1082 consecutive patients 2–36 (mean 16) months after myocardial infarction and/or coronary revascularization. Data on clinical insomnia, coronary risk factors, and comorbidity were collected at baseline. Clinical insomnia was assessed using the Bergen Insomnia Scale (BIS). The primary composite endpoint of MACE (cardiovascular death, hospitalization due to myocardial infarction, revascularization, stroke, or heart failure) was assessed with an average follow-up of 4.2 (SD 0.3) years after baseline. Data were analyzed using Cox proportional hazard regression models stratified by prior coronary events before the index event.
Results
At baseline, mean age was 62 years, 21% were females, and 45% reported clinical insomnia. A total of 346 MACE occurred in 225 patients during the follow-up period. For clinical insomnia, the relative risk of recurrent MACE was 1.62 (95% confidence interval [CI]: 1.24–2.11, p < .001) adjusted for age, gender, and previous coronary events. In a multi-adjusted analysis, including coronary risk factors, cardiovascular comorbidity, symptoms of anxiety, and depression, the relative risk was 1.41 (95% CI: 1.05–1.89, p = .023). Clinical insomnia accounted for 16% of the MACE in attributable risk fraction analyses, being third in importance after smoking (27%) and low physical activity (21%).
Conclusions
Clinical insomnia was associated with increased risk of recurrent MACE. These results emphasize the importance of identifying and managing insomnia in CHD outpatients.
Keywords: insomnia, coronary heart disease, recurrent cardiovascular events, secondary prevention, psychosocial factors
Statement of Significance.
Despite insomnia being highly prevalent in patients with coronary heart disease (CHD), the potential effect of insomnia on major adverse cardiovascular events remains uncertain. This study is the first to report a prospective association between a self-report diagnosis of clinical insomnia and either cardiovascular death, hospitalization due to myocardial infarction, revascularization, stroke, or heart failure. Clinical insomnia was the third most important risk factor for major adverse cardiovascular events, after smoking and low physical activity. These results emphasize the importance of assessing insomnia in outpatients with CHD. Future studies should investigate the feasibility and effectiveness of specific insomnia treatments on both insomnia symptoms and prognosis in patients with CHD.
Introduction
Insomnia is characterized by an impairment in sleep initiation, waking up during the night, early morning awakening or non-restorative sleep to the extent that it significantly impacts on daily functioning, cognition and/or emotions [1]. Insomnia is a prevalent condition in the general population (10%) and the most prevalent sleep disorder [2]. International and own data consistently show higher prevalence rates of insomnia (37%–45%) among patients with established coronary heart disease (CHD) [3–5].
Insomnia has been associated with several cardiovascular risk factors such as smoking [5], obesity [5], physical inactivity [5], diabetes [6,7], inflammation [8,9], hypertension [10], and psychosocial factors [3–5, 11–13]. Sleep disturbances, including sleep interruptions and reduced sleep duration and quality, are prevalent and associated with several cardiovascular (CV) risk factors in patients with CHD [14]. However, prospective studies on the association between sleep disturbances and prognosis in patients with CHD are scarce [14].
Poor sleep quality has been identified as an independent risk factor for recurrent cardiac events in women [15] and for a short-term poor clinical outcome in terms of cardiovascular death, recurrent cardiovascular ischemic events, or stroke [16]. A follow-up study of patients with myocardial infarction (MI) found no significant association between insomnia and all-cause mortality at 2-year follow-up [17]. During the period 2 to 6 years post-MI, however, the presence of insomnia was independently associated with increased risk of mortality [17]. Clark et al. investigated the associations between impaired sleep prior to MI, and short- and long-term risk of CV events [18]. Independent associations between impaired sleep and increased risk of CV events were found for short-term (within 28 days post-MI) in men and long-term (10 year follow-up) in women [18].
However, there are several significant methodological limitations in previous studies such as inclusion of only women [15], use of a single screening question for insomnia [17] and not adjusting for all major CV risk factors [15,17], or symptoms of depression and anxiety that commonly co-occur with insomnia and are associated with poor prognosis in patients with CHD [17,18]. To the best of our knowledge, no previous studies have assessed insomnia using diagnostic criteria. Only one previous study has specifically assessed the importance of insomnia for prognosis in patients with CHD by using a single item question in the acute phase of MI [17], whereas the importance of insomnia measured ≥2 months after an acute event is not known. A recent review indicated that future observational studies in patients with CHD investigating the impact of disturbed sleep on mortality and morbidity are warranted before potential intervention trials are tested in this setting [11]. Thus, this is important knowledge with potential impact on clinical management of patients with CHD along with insomnia.
Against this background, we aimed to determine the association between clinical insomnia and recurrent CV events in outpatients with CHD. We hypothesized that the presence of clinical insomnia would be associated with increased risk of recurrent CV event in adjusted analyses even after controlling for coronary risk factors, cardiovascular comorbidity, and symptoms of anxiety and depression.
Methods
Design and population
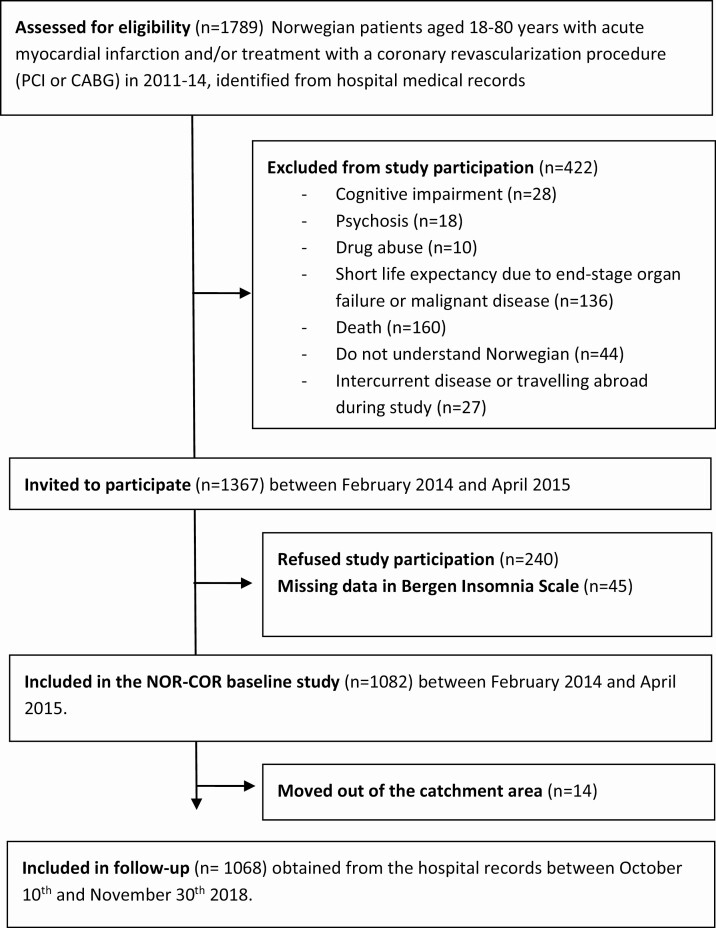
In this pre-planned prospective cohort study [19], 1367 patients were invited and 1082 patients were included between February 2014 and April 2015 (median 16 months, range 2–36 months) after a coronary index event in 2011–2014 (see study flow chart in Figure 1) fulfilling these inclusion criteria: aged 18–80 years, with a coronary index event, which was defined as acute type 1 MI and/or a revascularization procedure (coronary artery bypass grafting or percutaneous coronary intervention). The exclusion criteria were not being able to understand the Norwegian language, cognitive impairment including living in nursing homes, psychosis, drug abuse, short life expectancy due to terminal heart disease (NYHA class 4), lung disease, liver disease, kidney disease (stage 5), or malignant disease. Patients were recruited for a study to evaluate secondary preventive follow-up and to assess medication, lifestyle, and psychosocial factors of importance for the prognosis of CHD. Insomnia was only one of many factors and there was no emphasis on sleep problems. Thirty percent of the participants had one or more coronary events prior to the index event. Baseline data included a clinical examination with collection of blood samples. In addition, participants answered a comprehensive self-reported questionnaire, which included assessment of clinical insomnia using the Bergen Insomnia Scale (BIS) [20]. Forty-five patients had missing data on BIS. They were older, but not significantly different in terms of gender balance [5]. Data on recurrent CV events during follow-up were collected from medical records between October 10 and November 30, 2018. None of the patients had any recurrent events between the index event and the baseline assessments. The two participating Norwegian hospitals were Drammen and Vestfold covering an area of 380,000 inhabitants (corresponding to 7.4% of the Norwegian population). The catchment area is largely representative of Norwegian education, economy, age distribution, morbidity, and mortality and has a representative blend of city and rural districts [19].
Figure 1.
Flow-chart.
Ethics
The Regional Committee of Ethics in Medical Research (2013/1885) approved this study, and all participants gave signed informed consent before study participation.
Variables
Major adverse cardiovascular events (MACE).
Two experienced cardiac researchers registered information on the composite primary outcome MACE obtained from hospital records from October 10 to November 30, 2018. MACE comprised of CV death or readmission for MI, stroke/transitory ischemic attacks or heart failure, or need for a new revascularization.
Insomnia assessment.
Insomnia was assessed using the Bergen Insomnia Scale (BIS) [20], which is a six-item questionnaire based on the clinical diagnosis of insomnia according to the criteria described in the Diagnostic and Statistical manual, 4th version (DSM-IV) [1]. The answers indicate symptoms in the past month and can be used as a diagnostic tool for insomnia vs no insomnia or as a sum-score from 0 to 42. The first four items enquire about difficulties with sleep initiation, maintenance of sleep during the night, awakenings in the morning, and nonrestorative sleep. Items five and six enquire about daytime impairment and satisfaction with sleep. A 30-min cut-off value is used for the first three items. Each item is rated on an 8-point scale from 0 to 7 days per week. A minimum of 3 days on items one, two, three, or four combined with at least 3 days on items five or six indicate a diagnosis of insomnia. Those who met these criteria were categorized with “clinical insomnia,” whereas those who did not meet these criteria were described as “no insomnia.” BIS has normative Norwegian data for comparison and adequate psychometric properties with a 4-week test–retest reliability of 0.92 in the present study [21]. The Cronbach alpha for the BIS sum was 0.88 in the present study [5].
Clinical variables.
The following clinical variables were registered at baseline: Age, gender, coronary history and treatment, diabetes, and CV comorbidity which were all obtained from medical records. A comprehensive self-report questionnaire included level of education (low ≤ 12 years), living alone (yes/no), smoking history (former and current smoking), current smoking (daily smoking at time of inclusion), low physical activity (< 30 minutes moderate activity 3 times a week) [22], high risk (yes/no) of obstructive sleep apnea (OSA) syndrome as assessed by the Berlin Questionnaire [23], use of statins and sleep medication past week (yes/no), and symptoms of anxiety and depression assessed by the Hospital Anxiety and Depression Scale (HADS) [24]. The HADS is a 14-item self-report questionnaire consisting of two seven-item subscales that assess symptoms of anxiety (HADS-A) and depression (HADS-D). A cut-off value of HADS-A or HADS-D equal to or greater than 8 has been reported to represent clinically significant symptoms [25]. In the baseline study, the 4-week test–retest reliabilities were 0.92 for HADS-A and 0.94 for HADS-D [21]. Levels of C-reactive protein (CRP) and low-density lipoprotein (LDL) cholesterol were obtained from non-fasting venous blood samples and analyzed on an Architect ci16200 (Abbott Laboratories, USA) at Drammen hospital to avoid interlaboratory bias. The clinical examination included systolic blood pressure measured with standardized procedure using a validated digital sphygmomanometer (Welch Allyn Connex ProBP 3400) and waist circumference using a non-stretchable tape (Seca 201, Seca, Birmingham, UK).
Statistics.
Descriptive baseline measurements are presented as means with standard deviation (SD) for continuous variables, and as frequencies with percentages for proportions. P-values were calculated with t-test for mean and chi-square for frequencies. We used Cox proportional hazard models to calculate hazard ratio, and 95% confidence interval (CI), estimating the Hazard Ratio (HR) of MACE following the index event. Analysis time in the Cox model was defined as time from the index event, thereby adjusting for baseline variation in the risk by the time since the index event (using left-truncated data with censoring). The participants were followed until the date of death or the end of study (December 1, 2018). Non-modifiable and modifiable covariates were included in the multivariable Cox regression analyses. Model one was adjusted for age and gender. Model two was adjusted for coronary risk factors (CRP, smoking, LDL cholesterol, diabetes, physical activity, waist circumference, and systolic blood pressure), in addition to adjustments in Model one. Model three was adjusted for cardiovascular comorbidity (stroke, peripheral artery disease, and kidney failure) in addition to adjustments in Model two. Model four was adjusted for Hospital Anxiety and Depression Scale (HADS) anxiety ≥ 8 and HADS depression ≥ 8, in addition to adjustments in Model three. OSA risk was not included in the model due to lack of association with MACE together with an overlap with several variables included in the analyses (i.e. age, hypertension, overweight/obesity, and insomnia symptoms). As patients with established CHD prior to inclusion have different risk profile by time, we applied cox regression stratified for CHD prior to the index event. In the stratified analysis, the risk levels can vary differently among patients with and without prior CHD before the index event, while estimating co-variable effects combined to retain statistical power. Event free survival was estimated by the Kaplan–Meier product limit estimator.
Registered variables had few missing values (range: 0%–10%), but in multivariable Cox regression analysis, the combination of missing values for all included variables resulted in 285 excluded cases. We therefore performed sequentially multivariate regression imputation, using chained equations, under a missing at random assumption [26] to utilize all the available data. In addition, we performed supplementary multivariable Cox regression analysis separated on gender and on non-imputed dataset.
We also estimated the population attributable fraction (PAF) for each factor(s), measuring the factor(s) estimated contribution to the overall risk MACE events, compared to a theoretical scenario with the given factor(s) not present [27]. The population attributable fraction was given as one minus the ratio between the expected number of cases in a counterfactual scenario with the given co-variable(s) set to zero, divided by the expected number of cases with the observed covariates. As the effect of each co-variate in the Cox model is multiplicative, the combined PAF will be smaller than the sum of the individual PAF’s, highlighting the lower potential effect of prevention when the overall risk decreases. In practice, our PAF analyses take the prevalence in the given population into account, yielding an estimate for the clinical significance of a risk factor in our outpatient coronary population. To test the sensitivity of our results, we calculated an e-value for the fully adjusted model, adjusted for age, gender, previous coronary events, coronary risk factors, cardiovascular comorbidity, and symptoms of anxiety and depression [28]. Statistical analyses were performed using Stata version 15 (StataCorp LLC, College Station, USA), with the population attributable fraction calculated by the punafcc Stata add-on package.
Results
Mean age was 62 (SD 10) years at baseline, and 21% were females (Table 1). MI was the index event in 79%, whereas the remaining 21% had a stable or unstable angina with stenosis verified with angiography. The mean BIS sum score was 13.9 (SD 10.8), and 488 patients (45%) fulfilled the BIS criteria for clinical insomnia. High risk of OSA was reported by 430 patients (47%), whereas 159 (15%) reported the use of hypnotic medication the past week. Patients were followed for mean 4.2 (range 3.6–4.8, SD 0.4) years after inclusion and 5.7 (range 3.9–7.7, SD 0.9) years from the time of the index event. In total, 346 MACE events were reported in 225 patients, including 39 CVD related deaths. We estimated a risk of 21% (95% CI: 19%, 24%) of MACE events during our observed follow-up, including 3.4% (0.8% per year) CVD-related deaths.
Table 1.
Sociodemographic, clinical, and psychosocial characteristics at baseline
| Clinical insomnia (N = 488) | No insomnia (N = 594) | P-value* | Total (n = 1082) | |
|---|---|---|---|---|
| Sociodemographic factors | ||||
| Age, mean (SD) | 60.2 (10) | 62.5 (9) | <0.001 | 61.5 (10) |
| Female gender, n (%) | 130 (27) | 96 (16) | <0.001 | 226 (21) |
| Low education (≤ 12 years), n (%) | 354 (74) | 396 (67) | 0.015 | 750 (70) |
| Living alone, n (%) | 104 (23) | 90 (16) | 0.005 | 194 (19) |
| Clinical factors | ||||
| Coronary index diagnosis | ||||
| Acute myocardial infarction, n (%) | 390 (80) | 468 (79) | 0.648 | 858 (79) |
| Stable or unstable angina, n (%) | 98 (20) | 126 (21) | 224 (21) | |
| More than 1 coronary event prior to the index event, n (%) | 150 (31) | 175 (30) | 0.649 | 234 (30) |
| Participation in cardiac rehabilitation, n (%) | 222 (46) | 287 (48) | 0.055 | 509 (47) |
| Coronary risk factors at interview | ||||
| CRP mg/L, mean (SD)† | 2.8 (3) | 2.3 (3) | 0.001 | 2.5 (3) |
| LDL cholesterol mmol/L, mean (SD) | 2.1 (1) | 2.1 (1) | 0.087 | 2.1 (1) |
| Current smoking, n (%) | 114 (24) | 105 (19) | 0.033 | 219 (21) |
| Diabetes, n (%) | 102 (21) | 81 (14) | 0.002 | 183 (17) |
| Low physical activity‡, n (%) | 310 (64) | 330 (56) | 0.011 | 640 (60) |
| Systolicblood pressure, mmHg mean (SD) | 137 (19) | 140 (19) | 0.017 | 138 (19) |
| Waistcircumference in 1 cm, mean (SD) | 104 (13) | 101 (12) | 0.007 | 102 (12) |
| Not taking statins (past week) n (%) | 42 (9) | 39 (7) | 0.204 | 81 (8) |
| OSA risk (yes/no), n (%) | 243 (58) | 187 (37) | <0.001 | 430(47) |
| Cardiovascular comorbidity | ||||
| Stroke/TIA, n (%) | 24 (5) | 49 (8) | 0.030 | 73 (7) |
| Peripheral artery disease, n (%) | 41 (8) | 49 (8) | 0.928 | 90 (8) |
| Kidney failure, n (%) | 62 (14) | 71 (13) | 0.645 | 133 (13) |
| Heart failure, n (%) | 52 (11) | 84 (14) | 0.085 | 136 (13) |
| Psychosocial factors | ||||
| Bergen Insomnia Scale sum, mean (SD) | 23.3 (9) | 6.4 (5) | <0.001 | 13.9 (11) |
| Taking sleep medication past week, n (%) | 115 (24) | 44 (8) | <0.001 | 159 (25) |
| HADS-A ≥ 8, n (%) | 170 (37) | 51 (9) | <0.001 | 231 (21) |
| HADS-D ≥ 8, n (%) | 113 (24) | 43 (7) | <0.001 | 156 (14) |
*p-values calculated with t-test for mean and chi-square for n.
†Excluded cases > 15 mg/L.
‡Low physical activity is defined as < physical activity for 30 min 2–3 times a week.
SD, standard deviation; CRP, C-reactive protein; LDL, low density lipoprotein; TIA, transient ischemic attack; HADS-A, hospital anxiety and depression rating scale-anxiety subscale; HADS-D, hospital anxiety and depression rating scale-depression subscale.
Kaplan–Meier event free survival estimates show statistically significant differences (p-value < .001) between the non-insomnia and clinical insomnia groups, in analyses stratified by prior coronary events (combined estimates; Figure 2). The figure shows combined estimates for those with and without prior coronary events.
Figure 2.
Kaplan–Meier plot with event free survival in patients with coronary heart disease with and without insomnia.
Clinical insomnia and the BIS sum-score were significantly associated with MACE with an estimated hazard ratio (HR) for clinical insomnia of 1.62 (95% CI: 1.24, 2.11) in age- and gender-adjusted analyses stratified by prior coronary events (combined estimates; Table 2). For clinical insomnia, the HR of MACE was moderately reduced to 1.49 (95% CI: 1.14, 1.97) when the estimate also was adjusted for coronary risk factors, whereas additional adjustments for cardiovascular comorbidities hardly changed the estimates (HR 1.48, 95% CI: 1.12, 1.96). The findings for BIS were similar. The associations between clinical insomnia and MACE were further attenuated, but still significant, with additional adjustments for symptoms of anxiety and depression. The estimated HR of MACE for clinical insomnia was now 1.41 (95% CI: 1.05, 1.89), and for the BIS sum-score 1.01 (1.00, 1.03). Separate analyses on gender yielded a crude HR of 1.94 (95% CI: 1.06, 3.53) for women and 1.46 (95% CI: 1.08, 1.97) for men. Supplementary analyses on non-imputed dataset yielded slightly attenuated estimates and wider 95% CIs, due to missing data and reduced statistical power (Supplementary Table S1). High OSA risk was not associated with MACE in unadjusted analyses (HR 1.18; 95% CI: 0.89, 1.56, p = .246) and is not included in the further models (Supplementary Table S2). The association between clinical insomnia and MACE remained unchanged when also adjusted for time between the index event and inclusion (Supplementary Table S3).
Table 2.
Hazard ratio for major adverse cardiovascular events in patients with CHD (N = 1068)
| Model 1* | Model 2† | Modell 3‡ | Modell 4§ | |||||
|---|---|---|---|---|---|---|---|---|
| HR | P-value | HR | P-value | HR | P-value | HR | P-value | |
| Clinical insomnia(yes/no) | 1.62 (1.24, 2,11) | <0.001 | 1.49 (1.14, 1.97) | 0.004 | 1.48 (1.12, 1.96) | 0.006 | 1.41 (1.05, 1.89) | 0.023 |
| Bergen insomnia scale sum | 1.02 (1.01, 1.03) | <0.001 | 1.02 (1.01, 1.03) | 0.003 | 1.02 (1.00, 1.03) | 0.020 | 1.01 (1.00, 1.03) | 0.099 |
| OSA risk (yes/no) | 1.22 (0.92, 1.62) | 0.170 | – | – | – | |||
| Taking sleep medication past week (yes/no) | 0.87 (0.64, 1.19) | 0.443 | – | – | – |
All analyses based on imputed dataset. Insomnia and BIS are not imputed.
*Adjusted for age and gender, stratified for prior coronary events with combined estimates presented.
†Adjusted for coronary risk factors (CRP, smoking, LDL cholesterol, diabetes, physical activity, waist circumference, and systolic blood pressure), in addition to adjustments in Model 1.
‡Adjusted for cardiovascular comorbidity (stroke, peripheral artery disease, and kidney failure) in addition to adjustments in Model 2.
§Adjusted for Hospital Anxiety and Depression Scale (HADS) anxiety ≥ 8 and HADS depression ≥ 8 in addition to adjustments in Model 3. RR, relative risk.
Clinical insomnia accounted for 16% (95% CI: 4%–26%) of the MACE in attributable risk fraction (PAF) analysis (Table 3). Only history of smoking (27%; 95% CI: 5%, 44%) and low physical activity (21%; 95% CI: 5%, 35%) accounted for a higher percentage of MACE. In total, all the preventable and potentially modifiable risk factors included in the model accounted for 69% (95% CI: 53%–80%) of the MACE. Both crude HR and age and gender adjusted HR for the coronary risk factors and psychosocial factors included in the attributable risk fraction analyses are shown in Supplementary Table S4. A similar table from the NORCOR cohort has been published elsewhere [29].
Table 3.
Attributional risk fraction (ARF)
| ARF | 95% CI | P-value | |
|---|---|---|---|
| History of smoking | 27% | 5.44% | 0.018 |
| Low physical activity* | 21% | 5.35% | 0.012 |
| Clinical insomnia | 16% | 4.26% | 0.009 |
| Not participating in cardiac rehabilitation | 16% | 1.28% | 0.035 |
| Central obesity† | 12% | −6.27% | 0.181 |
| Not taking statin | 7% | 4.9% | <0.001 |
| Hypertension | 7% | −7.19% | 0.311 |
| Diabetes mellitus | 6% | 0.12% | 0.064 |
| HADS‡ Anxiety or Depression score ≥8 | 1% | −3.5% | 0.531 |
| LDL§ cholesterol ≥1.8 mmol/L | −5% | −24.11% | 0.557 |
| All risk factors combined | 69% | 53.80% | <0.001 |
All analyses based on imputed dataset.
*Less than 30 min moderate activity 3 times a week.
†Waist circumference ≥102 cm in males and ≥88 cm in females.
‡HADS, hospital anxiety and depression rating scale.
§LDL, low density lipoprotein.
HADS, Hospital anxiety and depression scale.
Discussion
In this follow-up study of outpatients with CHD, we found clinical insomnia measured 2–36 months after a cardiac event to be independently associated with increased risk of MACE during the follow-up period of mean 4.2 years. The results remained significant and considerable even after adjustments for traditional CV risk factors, CV comorbidity, and symptoms of anxiety and depression. Clinical insomnia was among the three factors that most strongly predicted MACE (16%), being third after smoking (27%), and physical inactivity (21%), highlighting the need to assess and address insomnia in patients with CHD.
The risk of MACE was 41 percentage points increased in patients with a BIS diagnosis of clinical insomnia. This is slightly lower than the 60% increase (95% CI: 9%–134%) in risk for all-cause mortality shown in the previous study assessing insomnia with a single item question [17]. Given the uncertainty in the estimates with overlapping 95% CI and deviating outcome variables, our results are in line with those of Conden et al. [17]. However, in the latter study, the risk for mortality was not significantly increased at 2-year follow-up, but for the period of 2 to 6 years after the event. Furthermore, the adjustments in our study included all major coronary risk factors (smoking, hypertension, obesity, physical inactivity, diabetes, LDL cholesterol, and inflammation), whereas Conden et al. [17] only included diabetes and physical activity. In addition, we included adjustments for anxiety and depression symptoms in the fully adjusted model. These differences may explain the slight differences in results.
The reported risk for recurrent MACE in our study was lower than found in a study of sleep quality in women where poor sleep quality was associated with 2.5 times increased risk of recurrent cardiac events (cardiovascular death, recurrent acute myocardial infarction, or revascularization procedure) compared good sleep quality. In the same study, those who reported “not feeling well rested” after sleep had a similar increased risk [15]. That study included only women in the age range of 30–65 and sleep quality was assessed with the short version of Karolinska Sleep Questionnaire [15]. Another study also found sleep disturbance associated with increased long-term risk of incidence and death of acute MI, stroke, and heart failure in women at 10-year follow-up [18]. These studies suggest an elevated risk in women and the results are supported by an elevated relative risk for women compared to men in gender separated analyses in our study, although the 95% CI for men and women largely overlapped. The lower risk reported in our study may be explained by the lower (21%) female participation rate, which is a limitation in our study. Future studies may consider gender differences in CHD prevalence to provide sufficient power to conduct gender-specific analyses.
What may be the underlying mechanisms for the association between clinical insomnia and increased risk of MACE? To date, most studies on mechanisms have focused on lifestyle factors including smoking, alcohol use, physical inactivity, poor nutrition and non-adherence to treatments, associations with other CV risk factors (hypertension, diabetes, and obesity), and biological mechanisms such as low-grade inflammation. In a previous study, we found clinical insomnia to be associated with smoking, physical inactivity, diabetes, and CRP levels [5]. However, when we adjusted for CV risk factors, including CRP in the present study, the relative risk decreased from 1.62 to 1.49. Thus, CV risk factors are to some extent involved in the relationship between clinical insomnia and future MACE. However, it is uncertain at this stage whether the CV risk factors act as confounders, mediators, or both because we do not fully understand the causal directions between insomnia and CV risk factors.
Our finding that high OSA risk was not associated with MACE was unexpected, as OSA has been associated with CVD [30]. We assessed OSA risk with the Berlin Questionnaire. The screening properties of the Berlin Questionnaire in a CHD population are not known and even though its properties for the accuracy of identifying OSA have been questioned [31,32], it has still been regarded as a valuable tool in epidemiological studies [33]. However, OSA was not assessed by polysomnography and we cannot exclude OSA as a potential confounding factor for the association between clinical insomnia and MACE. Therefore, the relationship between OSA pathology and MACE cannot be ruled out as a confound within the analyses. There is a need to further investigate the causal relationship between objectively assessed OSA, insomnia, and CVD.
The co-occurrence of symptoms of clinical insomnia, anxiety, and depression symptoms is well known [5], but the causal directions and/or underlying mechanisms between these symptoms remain to be fully understood. A bidirectional relationship between insomnia and both anxiety and depression has been proposed [11] and depression is also associated with poor prognosis in patients with CHD [34]. The underlying mechanisms suggested to link both depression and insomnia to poor cardiac prognosis include low grade inflammation, cardiac autonomic dysfunction, platelet hyperactivity, and impaired endothelial function [35,36]. Interestingly, we found that clinical insomnia was correlated with recurrent cardiovascular events even after controlling for anxiety and depression symptoms, thus indicating a risk over and above that of anxiety and depression for recurrent MACE. Our result is in line with that of other studies with adjustment for depression [15]. However, our study is the first to also adjust for anxiety symptoms that has been closely connected to insomnia [36].
Altogether, our results from multi-adjusted analyses may be interpreted as follows: in patients with CHD, the presence of clinical insomnia increases the risk of prospective MACE at a given time point with 41 percentage points. One might argue that an unknown confounding variable can explain the observed association. However, to find such an association, the confounder needs to be strongly associated with both clinical insomnia and MACE. Using E-value calculations, we find that the potential confounder will first produce such an association if it has risk ratios of about 1.8 with both clinical insomnia and MACE [28]. Noteworthy, BIS sum score was not associated with increased risk of MACE when we added adjustments for anxiety and depression symptoms. This emphasizes the importance of clinical insomnia symptoms, in terms of diagnostic criteria for insomnia, for the prognosis in patients with CHD.
The diagnostic criteria of DSM-IV insomnia predicted 16% of MACE in the present study and stand out as the third most important potentially modifiable risk factor for recurrent MACE in the NOR-COR population. Only smoking (27%) and low physical activity (21%) contributed more to MACE risk. This result was in line with that reported for not participating in cardiac rehabilitation. This is indeed an interesting finding as large efforts are made to change risk behaviors such as smoking and low physical activity, whereas insomnia is hardly attended to or screened for among patients with CHD in clinical practice. Our results therefore emphasize the importance of screening for and managing insomnia in patients with CHD. Effective psychological treatment is known and cognitive behavior therapy for insomnia (CBT-I) is the first line treatment for chronic insomnia [37]. The effectiveness of CBT-I is well documented in the population with heart failure [37–39]. However, heart failure and CHD are two different conditions and there is still sparse evidence of the effectiveness of CBT-I in patients with CHD in general [38,40] and its effectiveness in older patients with CHD has yet to be established. Recently, a web-based CBT-I intervention was found feasible in older patients with prevalent CHD [41]. However, to the best of our knowledge, the effect of these psychological therapies on insomnia symptoms and CV prognosis in patients with CHD is not yet investigated. Therefore, there is a need for future studies with sound methods investigating the feasibility and effectiveness of psychological insomnia treatment on both insomnia symptoms and prognosis in patients with CHD. If CBT-I is not effective or available, short-term pharmacological treatment (<4 weeks) with, e.g. hypnotics may be used for insomnia [42]. However, hypnotics have been associated with incident CVD and mortality in post-menopausal women [43].
The strengths of this study include the catchment area being representative for the Norwegian population, when it comes to sociodemographic and clinically relevant factors [19]; all MACE events were extracted from the hospital records by experienced cardiologists, a high participation rate (83%), few missing data and only 14 patients lost to follow-up together with consecutive recruitment of patients from routine practice from two general hospitals. These strengths ensure a clinically representative CHD outpatient study group albeit a survival bias may be present in the study population, because of inclusion mean 16 (range 2–36) months after the index event. One hundred sixty patients died between the cardiac event and inclusion, and they may have been in a poorer clinical and psychosocial condition compared to the included patients.
Limitations
This study has certain limitations. First, self-reported data from questionnaires may not yield similar diagnostic results as that of a clinical interview for the disorder of insomnia. Second, this is a non-randomized observational study, and the presence of unmeasured confounders can never be excluded. Some potential confounders in this study may be OSA, sleep duration, intake of alcohol and caffeine containing beverages, metabolic stress (i.e. cortisol/cytokines/interleukins), and psychological concepts like hyperarousal. However, the results showed that OSA risk or higher alcohol consumptions were not associated with MACE in the present study. OSA risk was assessed by the Berlin Questionnaire and we do not have objective data such as polysomnography in the assessment of OSA. Third, assessing clinical insomnia, anxiety symptoms, and depression symptoms at the same time (at baseline) hamper causal/temporal interpretations of these closely related symptoms, leading to uncertainty if they should be interpreted as confounder, mediators, or both. Fourth, we assessed clinical insomnia at one time point (baseline) with a large time-range (2–36 months) after the index event. Therefore, there may be different reasons for clinical insomnia within a short time period (e.g. response to the acute cardiac event and rehabilitation efforts) compared to longer time period after the index event but we do not have any data to assess these reasons. However, we did not find a significant difference in clinical insomnia prevalence rates between time groups after the index event [5] and the estimates of the association between MACE and clinical insomnia did not change after adjusting for time between the index event and baseline. In addition, we do not have data on insomnia prior to the index event Whether insomnia occurred prior to a CV event or as a consequence of it may have clinical implications. Therefore, assessment of insomnia onset is recommended in future studies. Furthermore, BIS is based on the DSM-IV insomnia criteria which cannot distinguish between acute and chronic insomnia. Finally, missing data of MACE which occurred outside the catchment area of Drammen and Vestfold hospitals may have occurred, but the risk is low because hospital discharge reports are sent routinely to local hospitals.
Conclusion
This is the first study to detect a prospective association between a self-report diagnosis of clinical insomnia and MACE in outpatients with CHD. Those who fulfilled the BIS criteria for clinical insomnia in the period following a cardiac event had a 41% to 62% elevated risk of a recurrent MACE. The 41% elevated risk represents a conservative estimate, adjusted for most possible confounders, including symptoms of anxiety and depression. Clinical insomnia was the third most important predictor of MACE and efforts should be made to assess and effectively manage insomnia in patients with CHD.
Supplementary Material
Work Performed: Department of Behavioural Medicine, University of Oslo, Oslo, Norway
The NORwegian CORonary (NOR-COR) Prevention project. https://clinicaltrials.gov/ct2/results?term=ID+NCT02309255, Registered at December 5, 2014.
Data Availability
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
Funding
The study was funded by the Univeristy of Oslo and the NORCOR corornary prevention research group at Vestre Viken trust. Lars Aastebøl Frøjd has received financial support from the Research Council of Norway (project 271555/20). All authors declare no conflict of interest.
Disclosure Statement
None declared.
References
- 1. Association AP. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC.: American Psychiatric Association, 2000. [Google Scholar]
- 2. Morin CM, et al. Insomnia disorder. Nat Rev Dis Primers. 2015;1:15026. doi: 10.1038/nrdp.2015.26. [DOI] [PubMed] [Google Scholar]
- 3. Coryell VT, et al. Clinical correlates of insomnia in patients with acute coronary syndrome. Int Heart J. 2013;54(5):258–265. doi: 10.1536/ihj.54.258. [DOI] [PubMed] [Google Scholar]
- 4. Da Costa D, et al. Prevalence and determinants of insomnia after a myocardial infarction. Psychosomatics. 2017;58(2):132–140. doi: 10.1016/j.psym.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 5. Frøjd LA, et al. Insomnia in patients with coronary heart disease: prevalence and correlates. J Clin Sleep Med. 2021;17(5):931–938. doi: 10.5664/jcsm.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garg H. Role of optimum diagnosis and treatment of insomnia in patients with hypertension and diabetes: a review. J Family Med Prim Care. 2018;7(5):876–883. doi: 10.4103/jfmpc.jfmpc_337_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cappuccio FP, et al. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laugsand LE, et al. Insomnia and high-sensitivity C-reactive protein: the HUNT study, Norway. Psychosom Med. 2012;74(5):543–553. doi: 10.1097/PSY.0b013e31825904eb. [DOI] [PubMed] [Google Scholar]
- 10. Meng L, et al. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res. 2013;36(11):985–995. doi: 10.1038/hr.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Madsen MT, et al. Sleep disturbances in patients with coronary heart disease: a systematic review. J Clin Sleep Med. 2019;15(3):489–504. doi: 10.5664/jcsm.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsuda R, et al. The prevalence of poor sleep quality and its association with depression and anxiety scores in patients admitted for cardiovascular disease: a cross-sectional designed study. Int J Cardiol. 2017;228:977–982. doi: 10.1016/j.ijcard.2016.11.091. [DOI] [PubMed] [Google Scholar]
- 13. Banack HR, et al. The association between sleep disturbance, depressive symptoms, and health-related quality of life among cardiac rehabilitation participants. J Cardiopulm Rehabil Prev. 2014;34(3):188–194. doi: 10.1097/HCR.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 14. Spiesshoefer J, et al. Sleep—the yet underappreciated player in cardiovascular diseases: a clinical review from the German Cardiac Society Working Group on sleep disordered breathing. Eur J Prev Cardiol. 2019;28(2):189–200. [DOI] [PubMed] [Google Scholar]
- 15. Leineweber C, et al. Poor sleep increases the prospective risk for recurrent events in middle-aged women with coronary disease. The Stockholm Female Coronary Risk Study. J Psychosom Res. 2003;54(2):121–127. doi: 10.1016/s0022-3999(02)00475-0. [DOI] [PubMed] [Google Scholar]
- 16. Andrechuk CR, et al. Sleep quality and adverse outcomes for patients with acute myocardial infarction. J Clin Nurs. 2016;25(1-2):223–230. doi: 10.1111/jocn.13051. [DOI] [PubMed] [Google Scholar]
- 17. Condén E, et al. Insomnia predicts long-term all-cause mortality after acute myocardial infarction: a prospective cohort study. Int J Cardiol. 2016;215:217–222. doi: 10.1016/j.ijcard.2016.04.080. [DOI] [PubMed] [Google Scholar]
- 18. Clark A, et al. Sleep impairment and prognosis of acute myocardial infarction: a prospective cohort study. Sleep. 2014;37(5):851–858. doi: 10.5665/sleep.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Munkhaugen J, et al. The role of medical and psychosocial factors for unfavourable coronary risk factor control. Scand Cardiovasc J. 2016;50(1):1–8. doi: 10.3109/14017431.2015.1111408. [DOI] [PubMed] [Google Scholar]
- 20. Pallesen S, et al. A new scale for measuring insomnia: the Bergen Insomnia Scale. Percept Mot Skills. 2008;107(3):691–706. doi: 10.2466/pms.107.3.691-706. [DOI] [PubMed] [Google Scholar]
- 21. Peersen K, et al. Reproducibility of an extensive self-report questionnaire used in secondary coronary prevention. Scand J Public Health. 2017;45(3):269–276. doi: 10.1177/1403494816688375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurtze N, et al. Reliability and validity of self-reported physical activity in the Nord-Trøndelag Health Study: HUNT 1. Scand J Public Health. 2008;36(1):52–61. doi: 10.1177/1403494807085373. [DOI] [PubMed] [Google Scholar]
- 23. Netzer NC, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 24. Zigmond AS, et al. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25. Bjelland I, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 26. Donders AR, et al. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59(10):1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 27. Newson RB. Attributable and unattributable risks and fractions and other scenario comparisons. The Stata Journal: Promoting communications on statistics and Stata 2013;13(4):672–98 doi: 10.1177/1536867x1301300402 [published Online First: Epub Date]. [DOI] [Google Scholar]
- 28. VanderWeele TJ, et al. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 29. Sverre E, et al. Preventable clinical and psychosocial factors predicted two out of three recurrent cardiovascular events in a coronary population. BMC Cardiovasc Disord. 2020;20(1):61. doi: 10.1186/s12872-020-01368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Badran M, et al. Epidemiology of sleep disturbances and cardiovascular consequences. Can J Cardiol. 2015;31(7):873–879. doi: 10.1016/j.cjca.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 31. Amado-Garzónl S, et al. Sensitivity and specificity of four screening tests sleep-disordered breathing in patients with and without cardiovascular disease. Sleep Sci. 2021;14(4):311–318. doi: 10.5935/1984-0063.20200104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chiu HY, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. doi: 10.1016/j.smrv.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 33. Senaratna CV, et al. Validity of the Berlin questionnaire in detecting obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2017;36:116–124. doi: 10.1016/j.smrv.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 34. Hare DL, et al. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35(21):1365–1372. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- 35. Davidson KW, et al. Selected psychological comorbidities in coronary heart disease: challenges and grand opportunities. Am Psychol. 2018;73(8):1019–1030. doi: 10.1037/amp0000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pedersen SS, et al. Psychosocial perspectives in cardiovascular disease. Eur J Prev Cardiol. 2017;24(3_suppl):108–115. doi: 10.1177/2047487317703827. [DOI] [PubMed] [Google Scholar]
- 37. Redeker NS, Yaggi HK, Jacoby D, et al. Cognitive behavioral therapy for insomnia has sustained effects on insomnia, fatigue, and function among people with chronic heart failure and insomnia: the HeartSleep Study. Sleep 2022;45(1) doi: 10.1093/sleep/zsab252 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Conley S, et al. Cognitive behavioral therapy for insomnia in the context of cardiovascular conditions. Curr Sleep Med Rep. 2015;1(3):157–165. doi: 10.1007/s40675-015-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harris KM, et al. Pilot randomized trial of brief behavioral treatment for insomnia in patients with heart failure. Heart Lung. 2019;48(5):373–380. doi: 10.1016/j.hrtlng.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 40. Rybarczyk B, et al. Testing two types of self-help CBT-I for insomnia in older adults with arthritis or coronary artery disease. Rehabil Psychol. 2011;56(4):257–266. doi: 10.1037/a0025577. [DOI] [PubMed] [Google Scholar]
- 41. Javaheri S, et al. Reducing coronary heart disease risk through treatment of insomnia using web-based cognitive behavioral therapy for insomnia: a methodological approach. Behav Sleep Med. 2020;18(3):334–344. doi: 10.1080/15402002.2019.1584896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Riemann D, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. doi: 10.1111/jsr.12594. [DOI] [PubMed] [Google Scholar]
- 43. Haines A, et al. The association of hypnotics with incident cardiovascular disease and mortality in older women with sleep disturbances. Sleep Med. 2021;83:304–310. doi: 10.1016/j.sleep.2021.04.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.