Abstract
Ischemic heart disease continues to represent a major health threat for death, disability, and poor quality of life as it also consumes enormous health-related resources. For over a century, the major clinical phenotype was taken to be obstructive atherosclerosis involving the larger coronary arteries (e.g., coronary artery disease [CAD]). However, evolving evidence now indicates that nonobstructive CAD is the predominant phenotype. Patients within this phenotype have been termed to have angina with no obstructive CAD (ANOCA), ischemia with no obstructive CAD (INOCA), or myocardial infarction with no obstructive coronary arteries (MINOCA). But as methods to assess cardiomyocyte injury evolve, these phenotypic distinctions have begun to merge, raising concern about their usefulness.
Also, considerable evidence has suggested several endotypes that link to potential mechanisms. These include coronary microvascular dysfunction, augmented vasoreactivity (failure to relax appropriately, exaggerated constriction [“spasm”], etc.), nonobstructive atherosclerosis, pre-heart failure with preserved ejection fraction, hypercoagulable states, and several others, alone or in combination.
This review summarizes these syndromes and their associated clinical outcomes with an emphasis on potential mechanistic signals. These involve the endothelium, the microvasculature, and cardiomyocyte function. Biomarkers of injury/dysfunction involving these structures are discussed along with a hypothetical construct for management being tested in an ongoing trial.
Keywords: Ischemia, ANOCA, INOCA, MINOCA, Mechanistic signals
1. Introduction
Ischemic cardiovascular disease continues to be the major public health threat in the United States and most of the industrialized world. Ischemic heart disease (IHD), along with ischemic cerebrovascular disease, is responsible for most deaths and much of the disability. Together these ischemic disorders consume an enormous portion of each country's healthcare resources.
Clinically, IHD may present as an acute coronary syndrome (e.g., unstable angina or acute myocardial infarction or as a chronic syndrome with more stable symptoms like angina pectoris. Mechanistically IHD has been traditionally linked with a flow-limiting stenosis in an epicardial coronary artery due to obstruction caused by atherosclerotic plaque.
2. What does IHD look like in 2023?
Emerging evidence indicates that many, perhaps most, chronic IHD occurs among patients with open or non-obstructed epicardial arteries [1], [20]. Hence, the term “open artery ischemia” (OAI) seems appropriate. Limited knowledge is available relative to its clinical phenotypes, other than the fact that they range from ANOCA (ANgina with Open Coronary Arteries), INOCA (Ischemia with NonObstructive Coronary Arteries), to MINOCA (Myocardial Infarction with Open Coronary Arteries). In my opinion studies using high sensitivity and ultra-high sensitivity biomarkers of reversible cardiomyocyte ischemic injury (cardiac troponins [cTn]) and cardiac magnetic resonance imaging (cMRI) suggest that these clinical phenotypes may overlap as HFpEF precursors [2]. For example, prolonged angina maybe associated with cTn efflux and changes in LV strain.
Several different mechanistic endotypes have been confirmed (Table 1). These include:
Table 1.
Potential mechanisms for myocardial ischemia in the absence of obstructive CAD.
|
Coronary microvascular dysfunction (CMD), believed to be present in at least half of the cases, defined as endothelial dysfunction, vascular smooth muscle dysfunction, or both [3]. Heightened coronary vasoreactivity (vasospasm) is estimated to occur in ∼20–25 % at the epicardial level or microvascular level. Current clinical approaches do not permit microvasculature assessment when epicardial vasospasm is present. However, a recent report suggested that adding a second acetylcholine (Ach) challenge after intracoronary nitroglycerin may have the potential to block epicardial spasm exposing only the microvascular spasm [4]. Also, there is some mechanistic overlap between these mechanisms of heightened microvascular coronary vasoreactivity, as well as failure of the vascular smooth muscle to relax. Augmented coronary vascular smooth muscle activation may also contribute to a failure to appropriately relax with vasodilator stimuli. Finally, altered coagulation is observed in ∼5 % of the cases, mostly those with MINOCA, but this estimate is biased by lack of testing when evidence for myocardial injury was not present or not sought.
Atherosclerosis clearly plays a mechanistic role in most cases with OAI, and nonobstructive plaque with positively remodeled epicardial coronary arteries may be the rule rather than the exception. This conclusion is based on intravascular imaging studies using intravascular ultrasound (IVUS) [5], optical coherence tomography, and multi-slice coronary computed tomographic angiography (CTA) [6]. The possibility that the atherosclerosis risk factors (e.g., diabetes/hypertension/hyperlipidemia/smoking) are involved in the mechanisms of OAI also needs to be considered. They may play a role in both atherosclerosis as well microvascular dysfunction and enhanced vasoconstriction. Against this atherosclerosis risk factor possibility, is the observation that aging and male sex are not prominently involved.
More recently, CMD with nonobstructive disease has been suggested to represent a “pre-heart failure with preserved ejection fraction” (HFpEF) endotype [7]. Left-ventricular relaxation abnormalities have been documented in many case series; preserved systolic function is the rule, and hospitalizations for heart failure are a frequent adverse outcome. Studies with long-term follow-up of these patients with no obstructive coronary disease, who have signs and symptoms of ischemia and CMD, observed that admission for HFpEF is prevalent [8].
There are some other less frequently observed endotypes (cardiorenal, cardiopulmonary, retinal, cerebral microvascular disease, etc. These are reviewed in detail in [9] and Fig. 1.
Fig. 1.
Potential therapeutic targets for CMD (left to right): decreased nitric oxide (NO) bioavailability is a commonly used marker of endothelial cell (EC) dysfunction. Reduced NO produced by ECs means less is received by neighboring vascular smooth muscle cells, leading to less vasodilation. Bradykinin, an endothelium-dependent vasodilator, also increases vascular permeability, and prostacyclin (PGI2), inhibits platelet activation and vasodilates the microcirculation. Cardiac macrophages have roles in antigen presentation, phagocytosis and immunoregulation by formation of cytokines and growth factors active in tissue repair after cardiac injury. Since NO can also modify tight junction, this may cause another marker of dysfunction leading to micro bleeding during ischemic injury. Increased EC proliferation has potential to lead to aberrant angiogenesis. Endothelial cell dysfunction may result in changes in factors secreted into the circulation like endothelin-1 (ET-1), a very potent vasoconstrictor.
Reprinted from: Pepine CJ. Microvascular dysfunction treatment options for coronary microvascular dysfunction. Spotlight Series. Cardiology Magazine. 2022 Dec 2.
3. Clinical outcomes over follow-up
The traditional view that OAI was a “benign syndrome” is clearly not correct. Increasingly, these patients are seen by general clinicians or referred to specialists for chest pain. They undergo invasive or noninvasive coronary anatomic imaging, which documents no or nonobstructive disease. However, considerable evidence now documents increased risk for major adverse outcomes in this population. This consists principally of hospitalization for recurrent angina or heart failure, or both. When evaluation is performed some evidence for impaired left-ventricular relaxation is often found either elevated left-ventricular end-diastolic pressure or echo-Doppler abnormalities. Also, all-cause mortality compared with age- and sex-matched reference subjects with “normal” or non-obstructed coronary arteries is somewhat elevated. These patients also have a markedly impaired functional status. Exercise tolerance is decreased in the majority of these patients [10]. Importantly, their quality of life is impaired, further contributing to enormous healthcare resource consumption that rivals that observed among similar patients with obstructive CAD [11].
These OAI syndromes also overlap, and this appreciation is relatively recent. For example, most patients who present with angina and OAI will have some signals for ischemia. These signals for ischemia include angina pectoris, and reversible ECG abnormalities, perfusion defects, and wall motion abnormalities. The problem is that ischemia related to microvascular dysfunction is often “patchy” and none of those prior-mentioned abnormalities have been well validated in patients with coronary microvascular dysfunction. In fact, all of those forementioned testing modalities have been validated by comparison with obstructive CAD, where a relatively well perfused region is compared with a poorly perfused region. The only currently available, clinically useful methods that avoid these pitfalls are P31-cMR spectroscopy [12] and coronary venous blood sampling for metabolites of anaerobic metabolism [13]. Additionally, most of the patients who present with MINOCA also have signals for ischemia or may have angina pre- or post-myocardial infarction. As testing improves, these overlaps will continue to be more prominent. For example, high or ultra-high sensitivity cTn assays now have documented cTn efflux after a prolonged angina episode. This is ischemia-related myocardial injury and those patients would, in many instances, be labeled as MINOCA. Additionally, as cardiac imaging techniques improve (i.e., cMRI), evidence for myocardial injury detected earlier and earlier may be observed in these OAI syndromes.
Also interesting is that women represent up to 65 % of these OAI syndromes, but reasons for this sex disparity are unclear. This proportion will likely increase with increased adoption of coronary CTA and cMRI, as women are traditionally reluctant to undergo invasive coronary angiography.
Data from several meta-analyses show that the rates for serious adverse outcomes in OAI patients approximate ∼3 % per year. For MINOCA, mortality can approach 5 % at 1 year with 1-year MACE rates like those of patients with acute myocardial infarction due to an obstructive coronary artery [14].
Considerable data on this OAI syndrome originated from the Women's Ischemia Syndrome Evaluation (WISE) program, which we started in 1996 and has continued to today through multiple, separate NHLBI funded cohorts. The surprise was that among a consecutive case cohort of women referred for coronary angiography to evaluate suspected IHD in the WISE original cohort, about two-thirds had no obstructive CAD. About 500 of these women have had invasive functional coronary testing from the original WISE and WISE-CVD cohorts, and CMD is highly prevalent and predicted adverse outcomes. Neither symptom characteristics nor stress-testing characteristics predicted the presence or absence of obstructive CAD nor the presence or absence of CMD. In the original WISE cohort, the only predictor of adverse outcome that we observed was angina persisting at 1-year follow-up [11]. In that cohort, we also observed that at 5 years, the rate of death or myocardial infarction exceeded 13 % or about 2.6 % per year. By 10 years, 20 % had died and 62 % of these deaths were adjudicated cardiovascular [15], and 25 % of all deaths occurred among women with no obstructive disease.
Also interesting is the WISE IVUS sub-study, designed to evaluate 100 women who had coronary angiograms locally read as “normal”, showed evidence for plaque in >80 % who were tested in only one 10-millimeter proximal left coronary artery segment (Fig. 2) [5]. In this example, it is apparent that a large plaque is totally concealed by positive remodeling in cross sections near the arrow. Thus, an open artery on a coronary angiogram does not assure absence of coronary atherosclerosis.
Fig. 2.
Left coronary angiogram and left anterior descending IVUS images from a 65-year-old woman with diabetes, hypertension, dyslipidemia, and hormone replacement therapy. Note plaque in proximal left anterior descending with maintenance of lumen area.
Reprinted with permission: M.A. Khuddus, C.J. Pepine, E.M. Handberg, C.N. Bairey Merz, G. Sopko, A.A. Bavry, S.J. Denardo, S.P. McGorray, K.M. Smith, B.L. Sharaf, S.J. Nicholls, S.E. Nissen, R.D. Anderson, An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE), J. Interv. Cardiol. 23 (2010) 511–519.
These findings have evolved into new endotypes for chronic ischemic syndromes, and support the need for a new taxonomy, as we had suggested (Fig. 3) [16]: the open artery ischemia syndromes, ANOCA, INOCA, and MINOCA. Their clinical presentations are not that different from those of patients with obstructive coronary disease. The notion that they had more atypical symptoms versus patients with obstructive coronary disease has not held in large cohort studies but “the 2021 ACC/AHA Chest Pain Guidelines recommended not using the term ‘atypical chest pain’”. Among patients with documented nonobstructive CAD, persistent stable symptoms, and documented myocardial ischemia, the patients are often younger, with high comorbidity burden”, and the overwhelming majority are women. The Guideline also recommended that it is reasonable to assess for microvascular dysfunction and enhance risk stratification using invasive coronary function testing, stress positron emission tomography with assessment of myocardial blood flow reserve (MBFR), or stress CMR with assessment of MBFR” [17].
Fig. 3.
A plea for new stable ischemic heart disease taxonomy. CAD, coronary artery disease; LVH, left-ventricular hypertrophy.
Adapted from: C.J. Pepine, P.S. Douglas, Rethinking stable ischemic heart disease: is this the beginning of a new era? J. Am. Coll. Cardiol. 60 (2012) 951–956; C.J. Pepine, K.C. Ferdinand, L.J. Shaw, K.A. Light-McGroary, R.U. Shaw, M. Gulati, C. Duvernoy, M.N. Walsh, C.N. Bairey Merz; ACC CVD in Women Committee, Emergence of nonobstructive coronary artery disease: a woman's problem and need for change in definition on angiography. J. Am. Coll. Cardiol. 27 (2015) 1918–1933; C. N. Bairey Merz, C.J. Pepine, M.N. Walsh, J.L. Fleg, Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation, 135 (2017) 1075–1092.
Also interesting is that their ischemia testing imaging results are highly variable; they can be abnormal and then, several months later, normal without any specific therapy. This is best illustrated by CIAO-ISCHEMIA [18] among patients screened for the ISCHEMIA trial who had three to four abnormal wall motion segments by stress echocardiography but no obstructive disease on CTAs. When retested at 1 year, half of the patients no longer had an ischemic response on echocardiography. Similar results were found with angina: at 1 year, angina disappeared in at least half of the subjects with no structured treatment. Of mechanistic interest, these wall motion and angina findings were not correlated.
The notion of a pre-HFpEF syndrome [7] is growing as more studies report findings in patients studied with positron emission tomography (to measure coronary flow reserve) and echo-Doppler (to measure left-ventricular relation indices) [8]. Limitations in microvascular flow with limitations in left-ventricular relaxation are prevalent and closely associated [8]. This area is one phase of the current NHLBI-funded WISE pre-HFpEF project (NCT03876223).
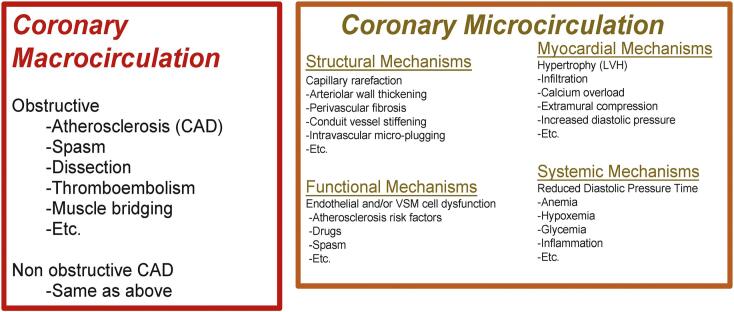
Obstructive CAD typically involves the macrocirculation, but it is also likely that many CAD patients have coexisting microcirculation disorders as CMD is a well-recognized pathophysiologic mechanism in many associated disorders (e.g., hypertension, diabetes, aortic stenosis, viral infections, transplant vasculopathy, etc.). However, current methods limit evaluation of the microcirculation with an upstream narrowing of hemodynamic significance. Patients without an obstructive lesion in the macrocirculation can have multiple reasons for microcirculation failure and result in ischemia (Fig. 3) [16].
How big is the problem and what are the outcomes of patients with OAI? Data from the NCDR registry and the WISE program indicate that at least 4 million Americans, with signs and symptoms suggesting ischemia, have nonobstructive coronary disease. The WISE observed a ∼3 % 10-year mortality and ∼30 % adverse outcome rate in a nationally representative cohort [11]. Adverse outcome risks in these patients are substantial and their predictors are just beginning to evolve. One clear piece of evidence is that the relationship between atherosclerosis presence and severity does not predict ischemia testing results. As noted in CIAO-ISCHEMIA [18] nor does the ischemia testing abnormality persist on retesting after 1 year.
HeartFlow, Inc., has pioneered application of computational fluid dynamics to coronary CTA for risk assessment. A very complete review summarizes the wealth of information that can be obtained from coronary CTA [6] (Table 2).
Table 2.
Coronary CTAa for risk assessment.
| • Angiography (stenosis detection and severity) |
| • Fraction flow reserve (epicardial conductance) |
| • Perfusion with hyperemia (microcirculation) |
| • Plaque anatomy (tissue composition, morphology, high-risk lesion) |
| • Inflammation |
| • Ventricular size and function (ejection fraction) |
| • Ventricular wall motion analysis |
| • Quantify atherosclerosis burden (Leaman score, anatomic or functional SYNTAX score) |
| • Calcification activity |
CTA, computed tomographic angiography.
Adapted from P.W. Serruys, H. Hara, S. Garg, H. Kawashima, B.L. Norgaard, M.R. Dweck, J.J. Bax, J. Knuuti, K. Nieman, J.A. Leipsic, S. Mushtaq, D. Andreini, Y. Onuma, Coronary computed tomographic angiography for complete assessment of coronary artery disease: JACC State-of-the-Art Review, J. Am. Coll. Cardiol. 78 (2021) 713–736.
Where is the evidence for management? (Table 3) Unfortunately, no randomized trial evidence base exists to guide management of these patients. We are conducting a randomized trial sufficient to gather clinical outcome data; WARRIOR (Women's IschemiA TReatment Reduces Events In NonObstructive CoRonary Disease, NCT #03417388) sponsored by the US Department of Defense [19]. Clinically stable women with INOCA, by either an invasive coronary angiogram or a CTA, are randomized to either (1) an intensive medical treatment strategy that includes aspirin, a high-potency statin, and a high dose of a renin-angiotensin system blocker (angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker), plus usual preventative treatment delivered in an intensive fashion, or (2) usual care. Average follow-anticipated ∼1.5 years and outcomes include clinical events, angina severity, and quality of life.
Table 3.
Potential therapies for CMD (an incomplete list).
| Potential clues to mechanisms | |
|---|---|
| Pharmacologic | Nonpharmacologic |
| Nitrates | Exercise |
| Statins | Physical therapy |
| ACEIs or ARBs | Cognitive behavioral therapy |
| Calcium antagonists | Transcendental meditation |
| P2Y12 inhibitors | Transcutaneous electrical |
| Tricyclic antidepressants | Nerve stimulation |
| Estrogens | Ultrasound-guided shock wave therapy |
| PDE-5 inhibitors | Gene therapy |
| L-arginine | Cell-based therapy (autoCD34+) |
| Ranolazine | Coronary sinus reducer |
| Vasodilating beta-blockers | Etc. |
| Ivabradine | |
| Rho kinase inhibitors | |
| Endothelin receptor blockers | |
| Metformin | |
| Etc. | |
4. Multi-organ microvascular dysfunction
A unifying hypothesis suggests that microvascular dysfunction of specific organs is an expression of a systemic problem that worsens with age and is accelerated by vascular risk factors (Fig. 4) [9]. Investigating relationships across a spectrum of microvascular disorders/diseases affecting the brain, retina, kidney, lung, and myocardium has the potential to reveal shared pathologic mechanisms that could inform novel management strategies.
Fig. 4.
Multi-organ microvascular disease.
Microvascular dysfunction affecting the heart, brain, retina, lung, and kidney, representing different manifestations of small vessel disease. They share pathophysiologic mechanisms and can occur concomitantly.
ANOCA = angina with no obstructive coronary artery disease; HFpEF = heart failure with preserved ejection fraction; INOCA = ischemia with no obstructive coronary artery disease; MINOCA = myocardial infarction with no obstructive coronary arteries.
Reprinted with permission: D.S. Feuer, E.M. Handberg, B. Mehrad, J. Wei, C.N. Bairey Merz, C.J. Pepine, and E.C. Keeley, Microvascular Dysfunction as a Systemic Disease: A Review of the Evidence, Am J Med. 135 (2022) 1059–1068. https://doi.org/10.1016/j.amjmed.2022.04.006.
5. Summary and conclusions
In summary, OAI is prevalent, and much more likely among women than men. It occurs with all degrees of ischemia on stress testing, regardless of the stress testing modality. It is highly variable and does not persist over time in about half of the patients. The severity of ischemia in these patients does not correlate with the severity of their nonobstructive atherosclerotic lesions in large vessels nor does it correlate with the severity of angina. Clearly there are many knowledge gaps to be investigated in future studies. Nevertheless, it is very important to explain to these patients that a “normal” coronary angiogram or angiogram showing nonobstructive disease does not equate to “benign” or low risk.
Funding
Dr. Pepine receives/has received grants and research support related to this topic from the NIH/NHLBI grants R01HL146158 (PI: Bairey Merz), U54AG065141 (PI: Cheng), and R01HL090957 (MPI: Pepine), and NIH/NCATS grant UL1TR001427 (PI: Mitchell).
Declaration of competing interest
Dr. Pepine receives grants and research support from NIH/NHLBI — R01HL146158 (WISE-HFpEF), U54AG065141 (MAE-WEST), R01HL090957 (WISE-CVD), and HL064829 (WISE), HL132448 (Brain-Gut-Microbiome), HL033610 (Neuroimmune Activation in HTN), HL087366 (CCTRN2); PCORI/PCORnet — ADAPTABLE, INVESTED; NIH/National Center for Advancing Translational Sciences UL1TR001427; US Department of Defense W811XWH-17-2-0300 (WARRIOR); Industry — Amarin, AstraZeneca, BioCardia (CardiAmp-HF, CardioAmp-CMI), Caladrius (FREEDOM), DCRI, Mesoblast (DREAM-HF), Pfizer, Sanofi, XyloCor Therapeutics (EXACT); the Gatorade Foundation through the University of Florida Department of Medicine; and the McJunkin Family Foundation Trust.
If published, please submit to NIHMS for inclusion in PubMed Central, citing NIH/NHLBI grants R01HL146158 (PI: Bairey Merz), U54AG065141 (PI: Cheng), and R01HL090957 (MPI: Pepine), and NIH/NCATS grant UL1TR001427 (PI: Mitchell).
References
- 1.Kunadian V., Chieffo A., Camici P.G., Berry C., Escaned J., Maas A., Prescott E., Karam N., Appelman Y., Fraccaro C., Buchanan G.Louise, Manzo-Silberman S., Al-Lamee R., Regar E., Lansky A., Abbott J.D., Badimon L., Duncker D.J., Mehran R., Capodanno D., Baumbach A. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504–3520. doi: 10.1093/eurheartj/ehaa503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quesada O., Elboudwarej O., Nelson M.D., Al-Badri A., Mastali M., Wei J., Zarrabi B., Suppogu N., Aldiwani H., Mehta P., Shufelt C., Cook-Wiens G., Berman D.S., Thomson L.E.J., Handberg E., Pepine C.J., Van Eyk J.E., Merz C.N.B. Ultra-high sensitivity cardiac troponin-I concentration and left ventricular structure and function in women with ischemia and no obstructive coronary artery disease. Am. Heart J. Plus. 2022;13 doi: 10.1016/j.ahjo.2022.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camici P.G., Crea F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 4.Seitz A., Feenstra R., Konst R.E., Martinez Pereyra V., Beck S., Beijk M., van de Hoef T., van Royen N., Bekeredjian R., Sechtem U., Damman P., Piek J.J., Ong P. Acetylcholine rechallenge: a first step toward tailored treatment in patients with coronary artery spasm. JACC Cardiovasc. Interv. 2022;15:65–75. doi: 10.1016/j.jcin.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Khuddus M.A., Pepine C.J., Handberg E.M., Bairey Merz C.N., Sopko G., Bavry A.A., Denardo S.J., McGorray S.P., Smith K.M., Sharaf B.L., Nicholls S.J., Nissen S.E., Anderson R.D. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) J. Interv. Cardiol. 2010;23:511–519. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serruys P.W., Hara H., Garg S., Kawashima H., Norgaard B.L., Dweck M.R., Bax J.J., Knuuti J., Nieman K., Leipsic J.A., Mushtaq S., Andreini D., Onuma Y. Coronary computed tomographic angiography for complete assessment of coronary artery disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021;78:713–736. doi: 10.1016/j.jacc.2021.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Pepine C.J., Petersen J.W., Bairey Merz C.N. A microvascular-myocardial diastolic dysfunctional state and risk for mental stress ischemia: a revised concept of ischemia during daily life. JACC Cardiovasc. Imaging. 2014;7:362–365. doi: 10.1016/j.jcmg.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Taqueti V.R., Solomon S.D., Shah A.M., Desai A.S., Groarke J.D., Osborne M.T., Hainer J., Bibbo C.F., Dorbala S., Blankstein R., Di Carli M.F. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur. Heart J. 2018;39:840–849. doi: 10.1093/eurheartj/ehx721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feuer D.S., Handberg E.M., Mehrad B., Wei J., Bairey Merz C.N., Pepine C.J., Keeley E.C. Microvascular dysfunction as a systemic disease: a review of the evidence. Am. J. Med. 2022;135:1059–1068. doi: 10.1016/j.amjmed.2022.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis J.F., McGorray S., Lin L., Pepine C.J., Chaitman B., Doyle M., Edmundowicz D., Sharaf B.L., Merz C.N., National Heart L., Blood I. Exercise treadmill testing using a modified exercise protocol in women with suspected myocardial ischemia: findings from the National Heart, Lung and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) Am. Heart J. 2005;149:527–533. doi: 10.1016/j.ahj.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 11.Shaw L.J., Merz C.N., Pepine C.J., Reis S.E., Bittner V., Kip K.E., Kelsey S.F., Olson M., Johnson B.D., Mankad S., Sharaf B.L., Rogers W.J., Pohost G.M., Sopko G., I. Women's Ischemia Syndrome Evaluation The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women's Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 12.Buchthal S.D., den Hollander J.A., Merz C.N., Rogers W.J., Pepine C.J., Reichek N., Sharaf B.L., Reis S., Kelsey S.F., Pohost G.M. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N. Engl. J. Med. 2000;342:829–835. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 13.Wargovich T.J., MacDonald R.G., Hill J.A., Feldman R.L., Stacpoole P.W., Pepine C.J. Myocardial metabolic and hemodynamic effects of dichloroacetate in coronary artery disease. Am. J. Cardiol. 1988;61:65–70. doi: 10.1016/0002-9149(88)91306-9. [DOI] [PubMed] [Google Scholar]
- 14.Lindahl B., Baron T., Erlinge D., Hadziosmanovic N., Nordenskjold A., Gard A., Jernberg T. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135:1481–1489. doi: 10.1161/CIRCULATIONAHA.116.026336. [DOI] [PubMed] [Google Scholar]
- 15.Sharaf B., Wood T., Shaw L., Johnson B.D., Kelsey S., Anderson R.D., Pepine C.J., Bairey Merz C.N. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am. Heart J. 2013;166:134–141. doi: 10.1016/j.ahj.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepine C.J., Douglas P.S. Rethinking stable ischemic heart disease: is this the beginning of a new era? J. Am. Coll. Cardiol. 2012;60:957–959. doi: 10.1016/j.jacc.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 17.Writing Committee M., Gulati P.D., Levy D., Mukherjee E., Amsterdam D.L., Bhatt K.K., Birtcher R., Blankstein J., Boyd R.P., Bullock-Palmer T., Conejo D.B., Diercks F., Gentile J.P., Greenwood E.P., Hess S.M., Hollenberg W.A., Jaber H., Jneid J.A., Joglar D.A., Morrow R.E., O'Connor M.A.Ross, Shaw L.J. AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021;78(2021):e187–e285. doi: 10.1016/j.jacc.2021.07.053. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds H.R., Picard M.H., Spertus J.A., Peteiro J., Lopez Sendon J.L., Senior R., El-Hajjar M.C., Celutkiene J., Shapiro M.D., Pellikka P.A., Kunichoff D.F., Anthopolos R., Alfakih K., Abdul-Nour K., Khouri M., Bershtein L., De Belder M., Poh K.K., Beltrame J.F., Min J.K., Fleg J.L., Li Y., Maron D.J., Hochman J.S. Natural history of patients with ischemia and no obstructive coronary artery disease: the CIAO-ISCHEMIA Study. Circulation. 2021;144:1008–1023. doi: 10.1161/CIRCULATIONAHA.120.046791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handberg E.M., Merz C.N.B., Cooper-Dehoff R.M., Wei J., Conlon M., Lo M.C., Boden W., Frayne S.M., Villines T., Spertus J.A., Weintraub W., O'Malley P., Chaitman B., Shaw L.J., Budoff M., Rogatko A., Pepine C.J. Rationale and design of the women's ischemia trial to reduce events in nonobstructive CAD (WARRIOR) trial. Am. Heart J. 2021;237:90–103. doi: 10.1016/j.ahj.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Boden W.E., Marzilli M., Crea F., Mancini G.B.J., Weintraub W.S., Taqueti V.R., Pepine C.J., Escaned J., Al-Lamee R., Gowdak L.H.W., Berry C., Kaski J.C. and on behalf of the Chronic Myocardial Ischemic Syndromes Task Force, Evolving management paradigm for stable ischemic heart disease patients: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023;81:505–514. doi: 10.1016/j.jacc.2022.08.814. [DOI] [PMC free article] [PubMed] [Google Scholar]