Abstract
The conversion of carbonyls to olefins is a transformation of great importance for complex molecule synthesis. Standard methods use stoichiometric reagents that have poor atom economy and require strongly basic conditions, which limit their functional group compatibility. An ideal solution would be to catalytically olefinate carbonyls under nonbasic conditions using simple and widely available alkenes, yet no such broadly applicable reaction is known. Here, we demonstrate a tandem electrochemical/electrophotocatalytic reaction to olefinate aldehydes and ketones with a broad range of unactivated alkenes. This method involves the oxidation-induced denitrogenation of cyclic diazenes to form 1,3-distonic radical cations that rearrange to yield the olefin products. This olefination reaction is enabled by an electrophotocatalyst that inhibits back-electron transfer to the radical cation intermediate, thus allowing for the selective formation of olefin products. The method is compatible with a wide range of aldehydes, ketones, and alkene partners.
Aldehydes and ketones can be olefinated with simple alkenes using electrochemistry and electrophotocatalysis.
INTRODUCTION
Olefins are among the most common structural motifs in organic chemistry and occur widely in compounds of biological, medicinal, and material interest. They are also frequently used as substrates in fine, commodity, and polymer chemical synthesis. As a result, the efficient and selective synthesis of olefins from inexpensive feedstock materials has long been an important challenge in synthetic chemistry. Among the most prevalent strategies for the construction of olefins is via the reaction of carbonyl compounds, including aldehydes and ketones, a process generally termed carbonyl olefination (1). Methods to achieve carbonyl olefination include several well-known “name reactions” such as the Wittig (2, 3), Horner-Wadsworth-Emmons (4, 5), Julia (6, 7), Peterson (8), and Tebbe (9) olefinations (Fig. 1A). While these methods have been of enormous utility to the field of organic chemistry, they rely on the use of stoichiometric and often sensitive reagents (10). These factors can lead to costly and wasteful protocols that present challenges for industrial-scale campaigns. Furthermore, the reliance of these methods on strongly basic conditions renders them incompatible with substrates having base-sensitive functionality. While some modifications have been introduced to allow for the use of weaker bases, the scope of those transformations is generally limited (11, 12). An ideal solution to this problem would be to catalytically olefinate carbonyls under nonbasic conditions using an inexpensive and abundant olefinating reagent, such as olefins (alkenes) themselves. Unfortunately, the standard reactivity patterns of carbonyls and olefins do not readily lend themselves to this type of transformation. Although some success toward this goal has been realized with carbonyl-olefin metathesis (13, 14), these reactions are currently limited to specialized substrates. Here, we demonstrate a general method to olefinate carbonyls with simple alkenes under nonbasic conditions using the combined capabilities of electrochemistry and electrophotocatalysis (Fig. 1B) (15).
Fig. 1. Olefination of carbonyl compounds.
(A) Common reagents for carbonyl olefination. (B) Tandem electrochemical/electrophotocatalytic carbonyl olefination. (C) Single-electron oxidation–induced denitrogenation of cyclic diazenes. UV, ultraviolet. (D) Denitrogenation of diazene 7 under various conditions. Redox potentials shown are versus standard calomel electrode. (E) Mechanistic rationale for the carbonyl olefination described in this work.
The key reactivity sequence of this method involves the controlled generation of a class of reactive intermediates called distonic radical cations—species that incorporate cationic and radical centers on nonadjacent atoms (16). These intermediates are relatively rare because the high reactivity of both radicals and cations makes it difficult to generate both concurrently within a single molecule. Nevertheless, one of the most reliable ways to generate distonic radical cations is via the oxidative denitrogenation of cyclic diazenes 1 (Fig. 1C) (17–20). Because of the high stability of molecular nitrogen, single-electron oxidation of a diazene results in the rapid loss of N2 gas, which leaves behind the organic fragment as a distonic radical cation 2. These intermediates are highly prone to undergo back-electron transfer (BET) to produce a diradical 3, which typically collapses to form a cyclopropane 4. Alternatively, the distonic radical cation can undergo Wagner-Meerwein shift to yield a 1,2-radical cation 5, which then gets reduced to form olefin 6 (21). The selectivity between the two product pathways is determined by the rate of BET between the reduced form of the oxidant and the distonic radical cation intermediate; however, exclusive cyclopropane formation or mixtures of products are the most common outcomes.
A well-known example of this type of reaction is the denitrogenation of the bicyclic diazene 7, which yields either housane (8) or cyclopentene (9) depending on the reaction pathway (Fig. 1D) (21). Conditions that do not involve single-electron oxidation—such as pyrolysis, direct photolysis, and photosensitized photolysis—result in almost exclusive formation of housane (8) (22). On the other hand, the strongly oxidizing photocatalyst 2,4,6-triphenylpyrylium tetrafluoroborate [TPT+; E1/2(PC−/PC) = −0.32 V versus standard calomel electrode (SCE)] (23) was reported to produce some amount of the cyclopentene product (9), though only in an 81:19 ratio in favor of the housane (21). A catalytic method for diazene denitrogenation to allow exclusive access to the olefin product remains an open challenge. [Note: While a single example of exclusive cyclopentene generation was reported using a triarylamine photocatalyst (24), a subsequent report (25)—confirmed in our laboratory—showed that this result could not be reproduced].
The product distribution noted above arises from TPT radical-mediated competitive BET to the intermediate distonic radical cation, leading primarily to cyclopropane formation via the diradical pathway. We speculated that a photooxidant that was less competent at BET in its reduced form would facilitate progression to the olefin product. However, the single-electron reduction products of most photocatalysts are too reducing to be effective for this purpose (26, 27). In addition, we found that the commonly used photocatalyst Ir(dFCF3ppy)2(dtbbpy)+, which is too poorly oxidizing to achieve SET of diazene 7 and instead effects denitrogenation by energy transfer, results in exclusive formation of housane (8) when used to denitrogenate 7 under irradiation (Fig. 1D).
We reasoned that to shift the reaction pathway in favor of the desired olefin product, it would be necessary to use a photocatalyst that had a much higher oxidation potential in its reduced form, thereby slowing the rate of BET and allowing the Wagner-Meerwein shift to better compete. We speculated that the trisaminocyclopropenium ion radical dication (TAC•2+, 10), which is a powerful photooxidant, might provide high selectivity for the olefin-forming pathway since its single-electron reduction product (TAC+) has a relatively high oxidation potential of E1/2(PC+/PC•2+) = +1.3 V versus SCE (28). We found that 10 effected the exclusive conversion of 7 to cyclopentene 9 when irradiated with white light for 16 hours; under these conditions, no housane was observed.
We recognized that this highly selective reactivity could enable a powerful synthesis of olefins if it could be paired with the facile formation of diazenes from simple precursors. In general, diazenes are challenging to prepare because of their high reactivity and propensity to undergo tautomerization to hydrazones (29). While cyclic diazenes can be readily synthesized by [3 + 2]-cycloaddition of diazo reagents with alkenes (30), the generation of the requisite diazo reagents, particularly those that are not stabilized, is also notoriously difficult (31, 32). In addition to their low stability, diazo compounds are often toxic, highly reactive, and even explosive. State-of-the-art procedures for the generation of unstabilized diazo compounds rely on the use of flow chemistry (33) and stoichiometric metal-based oxidants (31–33). We hypothesized that it might be possible to generate diazo reagents in situ via the electrochemical oxidation of hydrazones. Limited examples of this type of transformation have been reported, mostly in the context of stabilized diazo products (34, 35). Our strategy would maintain a low steady-state concentration of these intermediates by allowing them to react in situ with the alkene substrate to generate the cyclic diazenes. Notably, the requisite hydrazone precursors are easily prepared by condensation of hydrazine with aldehydes or ketones. Thus, we envisioned an integrated oxidative approach to the olefination of carbonyl compounds entailing (i) in situ hydrazone formation, (ii) electrochemical oxidation to the diazo compound and spontaneous [3 + 2]-cycloaddition with an alkene, and (iii) electrophotocatalytic oxidative denitrogenation to form the product alkene (Fig. 1E). The realization of this sequence would offer an attractive method to olefinate aldehydes and ketones with cheap and abundant alkenes without the need for strong base or onerous stoichiometric by-products.
RESULTS
In practice, the optimized procedure begins with condensation of the carbonyl substrate 11 with N-Boc (N-tert-butoxycarbonyl) hydrazine in the presence of molecular sieves at room temperature, followed by addition of trifluoroacetic acid (TFA) and mild heating to remove the Boc group and reveal the unsubstituted hydrazone 13. (Hydrazine itself can be used to directly prepare 13, which obviates the need for the deprotection procedure, but we found the handling of N-Boc hydrazine to be more convenient.) Fortunately, TFA is a component of the electrochemical setup, so this reaction mixture can be directly subjected to the next phase of the procedure, which involves white light irradiation of the hydrazone along with the olefin reactant 15 (10 equiv.), 10–mol % TAC catalyst 10, lithium perchlorate (LiClO4) electrolyte, and TFA in a divided electrochemical cell with a carbon felt anode and platinum wire anode at a controlled potential (Ecell = 1.5 V versus SCE). Depending on the substrate, this step is conducted at either room temperature or 50°C for 20 hours. Under these conditions, the hydrazone 13 undergoes electrochemical oxidation to form diazo compound 14, which reacts with olefin 15 to form diazene 16. Meanwhile, anodic oxidation of TAC+ generates the photocatalytic species TAC•2+, which in its photoexcited state (TAC•2+*) oxidizes diazene 16 to selectively form the distonic radical cation 17 by denitrogenation, and H2 generation at the cathode balances the redox reaction. Wagner-Meerwein shift of 17 to form radical cation 18 followed by reduction then furnishes the olefin product 19. The distonic radical cation intermediate can be produced in two regioisomeric forms, leading to potential regioisomeric olefin products, but this ratio is usually electronically biased to selectively furnish one isomer.
We first investigated this olefination procedure in the context of unactivated, aliphatic aldehydes. With nonanal as the substrate, the reaction proved to be compatible with a variety of readily available alkene coupling partners (Fig. 2). For example, we found that the use of allyl chloride led to the production of homoallylic chloride 20 in 68% yield. Notably, it would be difficult to generate this compound via many standard olefination procedures, including the Wittig reaction, because of the sensitivity of the chloride group to strong base. We also found that products 21 to 24, derived from inexpensive and commercially available N, N-dimethylallylamine, allyl acetate, methallylamine, and 3-butenoic acid, respectively, could be generated in good to high yields (52 to 82%). We next investigated the reaction of more complex aldehydes. Derivatives of isonicotinaldehyde and piperonal were readily olefinated with allyl chloride to furnish 25 and 26, respectively. A highly sensitive α-amino-aldehyde was also suitable, giving rise to 27 in 62% yield. Furthermore, the isoxazole-containing product 28 was generated in good yield from the corresponding precursor aldehyde and allyl chloride.
Fig. 2. Substrate scope studies for tandem electrochemical/electrophotocatalytic carbonyl olefination with common allylic olefins.
Electrocatalytic/electrophotocatalytic (EC/EPC) reaction conditions: Anodic chamber: TAC (10 mol %, 0.05 mmol), LiClO4 [10 equiv (eq), 5 mmol], hydrazone reaction mixture [tert-butyl carbazate (1.0 equiv, 0.5), aldehyde/ketone substrate (1 equiv, 0.5 mmol), and degassed MeCN (2 ml) followed by deprotection with TFA (6 equiv, 3 mmol)], alkene substrate (10 equiv, 5 mmol), and a carbon felt anode. Cathodic chamber: LiClO4 (10 equiv, 5 mmol), TFA (6 equiv, 3 mmol), and a platinum cathode. Aldehyde substrates were conducted at room temperature (rt), while ketone substrates were conducted at 50°C. In all cases, a single regioisomer is formed. E/Z ratio > 10:1 except 29 (1:1), 30 (1:1), 33 (3:1), 34 (4:1), 35 (1:1), 36 (5:1), and 37 (3:1). Product 36 exists as a mixture of tautomers and so was characterized as its O-acylated derivative. Me, methyl; Ac, acetyl; CFL, compact fluorescent light.
We next examined the reaction of ketones to generate trisubstituted olefin products. In these cases, the reactions were conducted at 50°C because of the slower rate of the [3 + 2]-cycloaddition step with disubstituted diazo compounds. Using this modified procedure, olefin 29 was obtained in 77% yield, albeit as a 1:1 mixture of E/Z isomers. We also found this procedure to be compatible with substantial molecular complexity, including the nitrogen-containing heterocycles pyridine 30, piperidine 31, and pyrazole 32. Steroid derivative 33, bearing a free alcohol moiety, was generated in high yield. In addition, products 34 to 36, derived from the drug molecules pentoxifylline, zomepirac, and warfarin, respectively, were produced in modest to good yields, despite the presence of potentially sensitive functionality. This reaction also worked with a β-ketoamide, a substrate that is base sensitive because of its relatively high acidity (37). In addition, the imide product 38 was formed in high yield with this procedure. Many of these products would be very difficult to form using standard olefination methods because of the presence of base- or nucleophile-sensitive functionality.
Next, we investigated the use of more highly substituted alkenes in this reaction (Fig. 3). Inexpensive alkenes such as cyclohexene, 1-methylcyclohexene, pinene, and carene reacted with nonanal to yield 39 to 42, respectively. Notably, the reaction with limonene occurred exclusively on the sterically less hindered 1,1-disubstitued alkene to furnish 43, leaving the trisubstituted alkene untouched. We also investigated olefination with a 1,1-disubstituted alkene, 2-ethyl-1-butene. In these cases, the olefin position was generally retained from starting material to product. Thus, reaction of nonanal, 2-cyclopentylacetaldehyde, 4-benzyloxybutanal, and 4-phthalimidobutanal under the standard conditions furnished 44 to 47 in good yields. The steroidal product 48 was also generated with high efficiency. Meanwhile, products 49 to 51 bearing free amines could also be accessed. Reaction of ethyl pyruvate led to adduct 52 in high yield; however, in this case, the olefin was found to have isomerized into conjugation with the carboxyl group.
Fig. 3. Substrate scope studies for tandem electrochemical/electrophotocatalytic carbonyl olefination with common allylic olefins.
EC/EPC reaction conditions: Anodic chamber: TAC (10 mol %, 0.05 mmol), LiClO4 [10 equiv (eq), 5 mmol], hydrazone reaction mixture [tert-butyl carbazate (1.0 equiv, 0.5), aldehyde/ketone substrate (1 equiv, 0.5 mmol), and degassed MeCN (2 ml) followed by deprotection with TFA (6 equiv, 3 mmol)], alkene substrate (10 equiv, 5 mmol), and a carbon felt anode. Cathodic chamber: LiClO4 (10 equiv, 5 mmol), TFA (6 equiv, 3 mmol), and a platinum cathode. Reactions of unsymmetrical and 1,1-disubstituted substrates were conducted at 50°C, while intramolecular substrates were conducted at room temperature (rt). In all cases, a single regioisomer was formed. Yield of 56 was determined by 1H nuclear magnetic resonance because of the volatility of the product. Et, ethyl; Bn, benzyl.
In addition to the intermolecular transformation, the reaction was amenable to intramolecular olefination reactions to generate substituted cyclic olefins. Cyclohexene and cyclopentene ring structures 53 to 57 were synthesized in good yields from the corresponding acyclic aldehydes. Adduct 55 was formed in 63% yield despite the presence of an α-chloro aldehyde on the substrate. In addition, cyclopentene 57 was generated despite the presence of a hindered quaternary carbon center. A ketone substrate could also be used to generate tetrasubstituted alkene 58. Although we used substrates bearing isopropylidene groups as the alkene for the formation of 53 to 58 out of convenience, it was also possible to use a 1,2-disubstituted alkene (see the Supplementary Materials). Terminal olefin substrates were not effective for the intramolecular reactions.
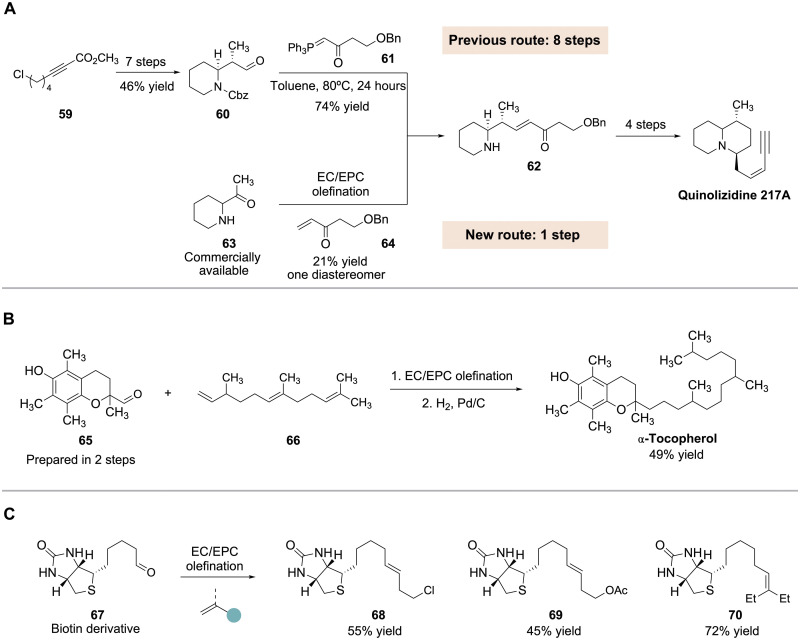
To explore the synthetic utility of this procedure, we sought to apply it to the construction of more complex structures of biological relevance. To demonstrate that this method can serve as a replacement for Wittig olefination in certain circumstances, we set out to synthesize a known intermediate toward the natural product quinolizidine 217A (Fig. 4A). This alkaloid had previously been prepared by a sequence involving Wittig olefination of aldehyde 60 with phosphorus ylide 61 to form advanced intermediate 62; however, the precursor aldehyde 60 required seven steps to prepare (36). By contrast, we were able to prepare piperidine 62 from the commercially available ketone 63 and unsaturated ketone 64 (prepared in two steps) using our olefination procedure. As an additional demonstration, we applied this chemistry to the synthesis of α-tocopherol. As shown in Fig. 4B, aldehyde 65, which is available in two steps, was reacted with triene 66 under our standard conditions. Although our protocol resulted in a mixture of isomers in this case, global hydrogenation of the resulting triene then furnished α-tocopherol in 49% overall yield. Last, we investigated the preparation of biotin derivatives via the olefination of aldehyde 67. Use of allyl chloride led to the formation of the homoallylic chloride 68 in 55% yield. Meanwhile, allyl acetate produced a 45% yield of adduct 69, while reaction of 2-ethyl-1-butene furnished trisubstituted alkene 70 in 72% yield.
Fig. 4. Application of tandem electrochemical/electrophotocatalytic carbonyl olefination to complex molecule synthesis.
(A) Alternative route to quinolizidine 217A intermediate. (B) Two-step conversion of 65 to α-tocopherol. (C) Preparation of biotin derivatives. Cbz, benzyloxycarbonyl; Ph, phenyl.
DISCUSSION
The conversion of aldehydes and ketones to olefins is a foundational transformation in organic chemistry, yet the strongly basic conditions of common olefination methods often necessitate wasteful and time-consuming protection group strategies due to incompatibilities with sensitive functionality. The method described here provides a strategy for carbonyl olefination with a broad functional group tolerance due to its lack of strongly basic reagents. By using olefins themselves as the olefinating agents, this method allows the use of inexpensive and readily available materials. Moreover, this method generates an arguably less offensive waste stream compared to traditional chemistries, as the main by-products are N2 and H2 gases. Most notably, the tandem utilization of electrochemical and electrophotocatalytic oxidations has enabled the controlled generation and destruction of otherwise highly reactive intermediates to realize a useful method of general applicability.
MATERIALS AND METHODS
All reactions were conducted under a nitrogen atmosphere using anhydrous and deoxygenated solvents with oven-dried glassware and standard Schlenk-line techniques. A representative olefination reaction was conducted as follows:
To a dry 10-ml round-bottom flask equipped with a stir bar, tert-butyl carbazate (1.0 equiv, 0.5 mmol, 66 mg) was added. The flask was purged with nitrogen and charged with nonanal (1 equiv, 71 mg, 0.5 mmol) and degassed MeCN (2 ml). The resulting reaction mixture was stirred for 3 hours, after which time TFA (6 equiv, 3 mmol, 0.383 ml) was added to the flask. The reaction mixture was heated to 50°C for 2 hours. This mixture is referred to below as the hydrazone solution.
To both chambers of an oven-dried electrochemical H cell, stir bars were added. To the anodic chamber, TAC+ClO4− (10 mol %, 0.05 mmol, 24 mg), LiClO4 (10 equiv, 5 mmol, 532 mg), and a carbon felt anode were added. To the cathodic chamber, LiClO4 (10 equiv, 5 mmol, 532 mg) and a platinum cathode were added. The cell was sealed using a rubber septum and parafilm and then flushed with nitrogen gas for 10 min. To the anodic chamber, allyl chloride (10 equiv, 382 mg, 5 mmol) and MeCN were added. To the cathodic chamber, TFA (6 equiv, 3 mmol, 0.383 ml) and MeCN were added. The solution was stirred at room temperature with the cell set at a constant potential of 1.5 V and irradiated with a 23-W compact fluorescent light. The reaction vessel was cooled with an oscillating fan for the duration of the procedure. The hydrazone reaction mixture was added to the anodic chamber of the H cell via syringe pump set at 0.5 ml/hour. After 20 hours, the solutions from both reaction chambers were combined and washed with saturated sodium bicarbonate solution. The carbon felt anode was sonicated in a dichloromethane solution and combined with the reaction mixture. The combined fractions were extracted using dichloromethane. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated. The crude reaction mixture was purified by column chromatography on silica gel using 90:10 hexanes:ethyl acetate to afford 1-chlorododec-3-ene 20 in 68% yield (69 mg, 0.34 mmol, E/Z = >10:1).
Acknowledgments
Funding: Funding for this work was provided by the National Institutes of Health (R35GM127135).
Author contributions: T.H.L. conceived and directed the project and helped prepared the manuscript. K.A.S. designed and performed the experiments and helped prepare the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Sections S1 to S7
Table S1
References
REFERENCES AND NOTES
- 1.T. Takeda, Modern Carbonyl Olefination: Methods and Applications (John Wiley & Sons, 2006). [Google Scholar]
- 2.Nicolaou K. C., Härter M. W., Gunzner J. L., Nadin A., The wittig and related reactions in natural product synthesis. Liebigs Ann. 1997, 1997, 1283–1301 [Google Scholar]
- 3.Maryanoff B. E., Reitz A. B., The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem. Rev. 89, 863–927 (1989). [Google Scholar]
- 4.Wadsworth W. S. Jr., Synthetic applications of phosphoryl-stabilized anions. Org. React. 25, 73–253 (1977). [Google Scholar]
- 5.Bisceglia J. A., Orelli L. R., Recent progress in the Horner-Wadsworth-Emmons reaction. Curr. Org. Chem. 19, 744–775 (2015). [Google Scholar]
- 6.Blakemore P. R., The modified Julia olefination: Alkene synthesis via the condensation of metalated heteroarylalkylsulfones with carbonyl compounds. J. Chem. Soc. Perkin Trans. 1, 2563–2585 (2002). [Google Scholar]
- 7.Rinu P. X. T., Radhika S., Anilkumar G., Recent applications and trends in the Julia-Kocienski olefination. Org. Supramol. Chem. 7, e202200760 (2022). [Google Scholar]
- 8.van Staden L. F., Gravestock D., Ager D. J., New developments in the Peterson olefination reaction. Chem. Soc. Rev. 31, 195–200 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Pine S. H., Carbonyl methylenation and alkylidenation using titanium-based reagents. Org. React. 43, 1–91 (1993). [Google Scholar]
- 10.Coyle E. E., Doonan B. J., Holohan A. J., Walsh K. A., Lavigne F., Krenske E. H., O’Brien C. J., Catalytic Wittig reactions of semi- and nonstabilized ylides enabled by ylide tuning. Angew. Chem. Int. Ed. 53, 12907–12911 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Blanchette M. A., Choy W., Davis J. T., Essenfeld A. P., Masamune S., Roush W. R., Sakai T., Horner-Wadsworth-Emmons reaction: Use of lithium chloride and an amine for base-sensitive compounds. Tetrahedron Lett. 25, 2183–2186 (1984). [Google Scholar]
- 12.Jedinak L., Rush L., Lee M., Hesek D., Fisher J. F., Boggess B., Noll B. C., Mobashery S., Use of silver carbonate in the Wittig reaction. J. Org. Chem. 78, 12224–12228 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert T. H., Development of a hydrazine-catalyzed carbonyl-olefin metathesis reaction. Synlett 30, 1954–1965 (2019). [Google Scholar]
- 14.Albright H., Davis A. J., Gomez-Lopez J. L., Vonesh H. L., Quach P. K., Lambert T. H., Schindler C. S., Carbonyl-olefin metathesis. Chem. Rev. 121, 9359–9406 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H., Steiniger K. A., Lambert T. H., Electrophotocatalysis: Combining light and electricity to catalyze reactions. J. Am. Chem. Soc. 144, 12567–12583 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Yates B. F., Bouma W. J., Radom L., Distonic radical cations: Guidelines for the assessment of their stability. Tetrahedron 42, 6225–6234 (1986). [Google Scholar]
- 17.Karatsu T., Hotta H., Kitamura A., Photochemistry of Azoalkane: Formation of the rearrangement product by photoinduced electron transfer of the pyrazoline derivative. J. Chem. Soc. Chem. Commun. 20, 1451–1452 (1991). [Google Scholar]
- 18.Adam W., Sendelbach J., Photosensitized electron transfer from azoalkanes: Generation, fragmentation, and rearrangement of radical cations structurally related to dicyclopentadiene. J. Org. Chem. 58, 5310–5315 (1993). [Google Scholar]
- 19.Adam W., Sendelbach J., Denitrogenation of bicyclic azoalkanes through photosensitized electron transfer: Generation and intramolecular trapping of radical cations. J. Org. Chem. 58, 5316–5322 (1993). [Google Scholar]
- 20.Engel P. S., Robertson D. M., Scholz J. N., Shine H. J., Reaction of azoalkanes with isolable cation radical salts. J. Org. Chem. 57, 6178–6187 (1992). [Google Scholar]
- 21.Adam W., Wagner-Meerwein M., Wagner-Meerwein rearrangements of radical cations generated by triphenylpyrylium tetrafluoroborate photosensitized electron transfer of azoalkanes. J. Am. Chem. Soc. 109, 1570–1572 (1987). [Google Scholar]
- 22.Adam W., Denninger U., Finzel R., Kita F., Platsch H., Walter H., Zang G., Comparative study of the pyrolysis, photoinduced electron transfer (PET), and laser-jet and 185-Nm photochemistry of alkyl-substituted bicyclic azoalkanes. J. Am. Chem. Soc. 114, 5027–5035 (1992). [Google Scholar]
- 23.Hola E., Ortyl J., Pyrylium salt as a visible-light-induced photoredox catalyst for polymer and organic synthesis—Perspectives on catalyst design and performance. Eur. Polym. J. 150, 110365 (2021). [Google Scholar]
- 24.Adam W., Sahin C., Tris(aryl)aminium hexachloroantimonates: Convenient one-electron oxidants for chemical electron transfer with bicyclic azoalkanes and bicyclo[2.1.0]pentanes. Tetrahedron Lett. 35, 9027–9030 (1994). [Google Scholar]
- 25.Park Y. S., Wang S. C., Tantillo D. J., Little R. D., A highly selective rearrangement of a housane-derived cation radical: An electrochemically mediated transformation. J. Org. Chem. 72, 4351–4357 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Prier C. K., Rankic D. A., MacMillan D. W. C., Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero N. A., Nicewicz D. A., Organic photoredox catalysis. Chem. Rev. 116, 10075–10166 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Huang H., Strater Z. M., Rauch M., Shee J., Sisto T. J., Nuckolls C., Lambert T. H., Electrophotocatalysis with a trisaminocyclopropenium radical dication. Angew. Chem. Int. Ed. 58, 13318–13322 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.M. C. Elliott, Imines and their N-substituted derivatives: Hydrazones and other =NN derivatives including diazo compounds, in Comprehensive Organic Functional Group Transformations II.A. R. Katritzky and R. J. K. Taylor, Eds. (Amsterdam: Elsevier, 2005), vol. 3, pp. 469–523. [Google Scholar]
- 30.1,3-Dipolar Cycloaddition Chemistry, A. Padwa, Ed. (John Wiley & Sons, 1984), vol. 1 and 2. [Google Scholar]
- 31.M. Regitz, G. Maas, Diazo Compounds (Academic Press, 1986). [Google Scholar]
- 32.Allouche E. M. D., Charette A. B., Non-Stabilized diazoalkane synthesis via the oxidation of free hydrazones by iodosylbenzene and application in in situ MIRC cyclopropanation. Chem. Sci. 10, 3802–3806 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rullière P., Benoit G., Allouche E. M. D., Charette A. B., Safe and facile access to nonstabilized diazoalkanes using continuous flow technology. Angew. Chem. Int. Ed. 57, 5777–5782 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Tanbouza N., Petti A., Leech M. C., Caron L., Walsh J. M., Lam K., Ollevier T., Electrosynthesis of stabilized diazo compounds from hydrazones. Org. Lett. 24, 4665–4669 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Okimoto M., Numata K., Tomozawa K., Shigemoto T., Hoshi A. M., Takahashi Y., Electrooxidative conversion of dibenzoylbenzene dihydrazones into the corresponding bis-diazo compounds and bis-dimethyl acetals. Aust. J. Chem. 58, 560–563 (2005). [Google Scholar]
- 36.Fellah M., Santarem M., Lhommet G., Mouriès-Mansuy V., Total synthesis of Quinolizidine (−)-217A. J. Org. Chem. 75, 7803–7808 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Sahu S., Das B., Maji M., Stereodivergent total synthesis of hapalindoles, fischerindoles, hapalonamide H, and ambiguine H alkaloids by developing a biomimetic, redox-neutral, cascade prins-type cyclization. Org. Lett. 20, 6485–6489 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Gelat F., Poisson T., Biju A., Pannecoucke A. X., Besset T., Trifluoromethylthiolation of α-chloroaldehydes: Access to quaternary SCF3-containing centers. Eur. J. Org. Chem. 27, 3693–3696 (2018). [Google Scholar]
- 39.Albright H., Riehl P., McAtee C., Reid J., Ludwig J., Karp L., Zimmerman P., Sigman M., Schindler C., Catalytic carbonyl-olefin metathesis of aliphatic ketones: Iron(III) homo-dimers as Lewis acidic superelectrophiles. J. Am. Chem. Soc. 141, 1690–1700 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukherjee J., Sil S., Pahari A., Chattopadhyay S., A modular synthesis of some biologically relevant cyclic peptides through late-stage functionalization. Synthesis 48, 1181–1190 (2016). [Google Scholar]
- 41.Diéguez H., López A., Domingo V., Arteaga J., Dobado J., Herrador M., Quílez del Moral J., Barrero A., Weakening C−O bonds: Ti(III), a new reagent for alcohol deoxygenation and carbonyl coupling olefination. J. Am. Chem. Soc. 132, 254–259 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Bordessa A., Colin-Cassin C., Grillier-Vuissoz I., Kuntz S., Mazerbourg S., Husson G., Vo M., Flament S., Martin H., Chapleur Y., Boisbrun M., Optimization of troglitazone derivatives as potent anti-proliferative agents: Towards more active and less toxic compounds. Eur. J. Med. Chem. 83, 129–140 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Corona C., Bryant B., Arterburn J., Synthesis of a biotin-derived alkyne for Pd-catalyzed coupling reactions. Org. Lett. 8, 1883–1886 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braddock D., Pouwer R., Burton J., Broadwith P., Clarification of the stereochemical course of nucleophilic substitution of arylsulfonate-based nucleophile assisting leaving groups. J. Org. Chem. 74, 6042–6049 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Gajewski J., Warner J., Stereochemistry and kinetic deuterium isotope effects in the thermal 1,3-sigmatropic rearrangement of (−)-(R,R)-trans-2-methyl-1-(1-tert-butylvinyl)cyclopropane: Evidence for a biradical intermediate. J. Am. Chem. Soc. 106, 802–803 (1984). [Google Scholar]
- 46.Ishmuratov G., Yakovleva M., Zaripova G., Botsman L., Muslukhov R., Tolstikov G., Novel synthesis of (4R)-4-methylpentanolide from (L)-(−)-menthol. Chem. Nat. Compd 40, 548–551 (2004). [Google Scholar]
- 47.Baba T., Avasthi K., Suzuki A., The reaction of organoboranes with lithium salts of trisylhydrazones of cycloalkanones followed by treatment with iodine. BCSJ 56, 1571–1572 (1983). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sections S1 to S7
Table S1
References