Abstract
Biomolecular condensates are nonmembranous structures that are mainly formed through liquid–liquid phase separation. Tensins are focal adhesion (FA) proteins linking the actin cytoskeleton to integrin receptors. Here, we report that GFP-tagged tensin-1 (TNS1) proteins phase-separate to form biomolecular condensates in cells. Live-cell imaging showed that new TNS1 condensates are budding from the disassembling ends of FAs, and the presence of these condensates is cell cycle dependent. TNS1 condensates dissolve immediately prior to mitosis and rapidly reappear while postmitotic daughter cells establish new FAs. TNS1 condensates contain selected FA proteins and signaling molecules such as pT308Akt but not pS473Akt, suggesting previously unknown roles of TNS1 condensates in disassembling FAs, as the storage of core FA components and the signaling intermediates.
Keywords: tensin, TNS1, focal adhesion, biomolecular condensate, cell division
Biomolecular condensates are micron-scale membraneless subcellular organelles that contain biomolecules such as proteins and nucleic acids (1). They form spherical shapes, may merge into one upon contact, and exchange with the surrounding medium within seconds (1–5). They are mainly organized through liquid–liquid phase separation driven by multivalent molecular interactions (1). Although their functions are not fully understood, formation of biomolecular condensates can dramatically enrich relevant molecules, including enzymes and their substrates, transcription factors and coactivators, or elongation factors, within a very small volume of the compartment and significantly accelerate the reactions involved (4–6).
The involvement of phase separation associated with focal adhesion (FA) proteins was recently explored. The small GTPase regulators GIT/PIX proteins form condensates in vitro and in overexpressing cells (5). These condensates serve as modules that can be recruited via specific binding partners to FAs, neuronal synapses, or cell–cell junctions, enabling spatiotemporal regulation of GTPase activities (5). LIMD1 proteins can form opto-genetically induced condensates in cells and be recruited to nascent FAs in a mechanical force–dependent manner to facilitate FA maturation (7). Additionally, p130Cas and FAK undergo phase separation and are sufficient to reconstitute kindlin-dependent integrin clustering in vitro (8). These findings have specified important roles of phase separation of FA proteins. The genesis and dynamics of these condensates in live cells are largely unknown. Therefore, the complete picture of condensate functions at FAs remains elusive.
Results and Discussion
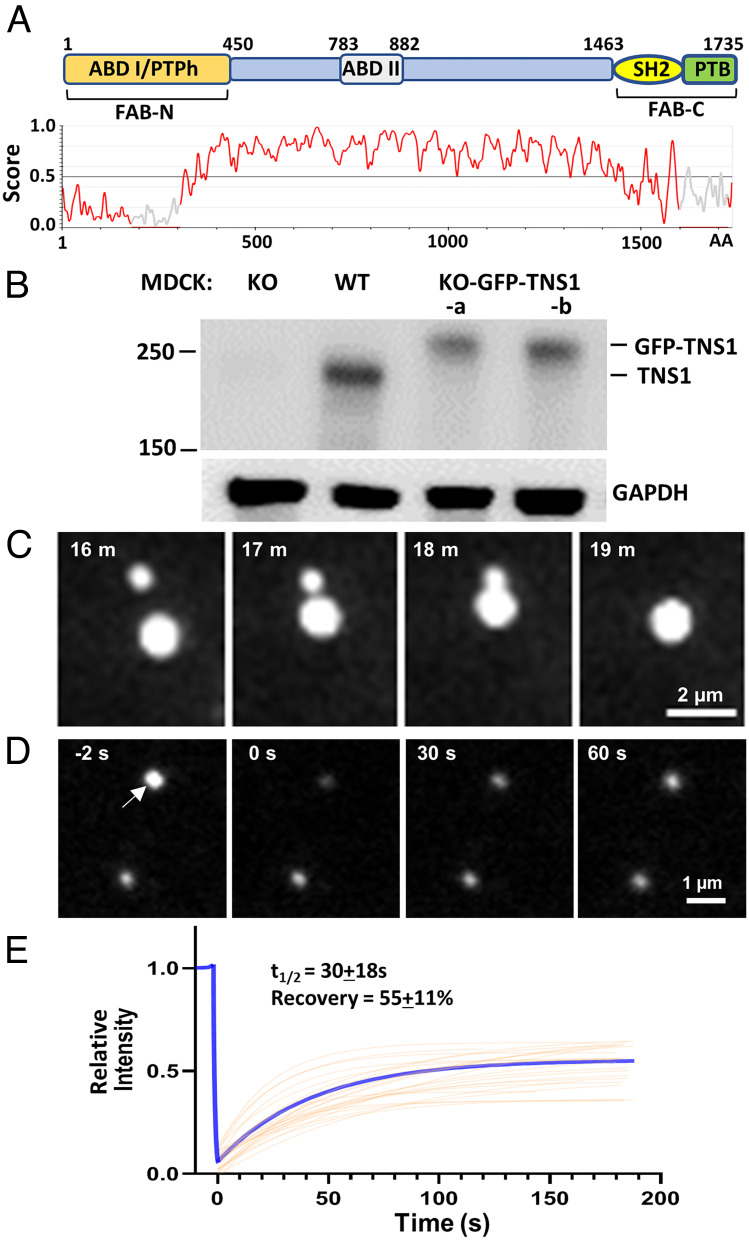
Tensin-1 (TNS1) is a FA protein that links the actin cytoskeleton to integrins and mediates outside-in and inside-out signal transductions that are critical for many biological events (9). Analysis of the human TNS1 protein sequence using the IUPred3 revealed a long stretch intrinsically disordered region that is favorable for forming biomolecular condensates (Fig. 1A). As shown by live-cell imaging of TNS1 knockout MDCK cells (10) expressing GFP-tagged TNS1 proteins (KO-GFP-TNS1)(Fig. 1B), TNS1 formed spherical structures that fused upon contact (Fig. 1C and Movie S1) and exchanged with the surrounding medium demonstrated by fluorescence recovery after photobleaching (FRAP) assays (Fig. 1 D and E and Movie S2). The half-time and percentage of recovery of TNS1 in these condensates (30 ± 18 s and 55 ± 11%) are similar to those of GIT [without PIX cotransfection (5)], suggesting the potential similarity in the interactions that drive phase separation in these condensates. These findings indicate that TNS1 proteins form membraneless biomolecular condensates in cells.
Fig. 1.
GFP-tagged TNS1 proteins form condensates in cells. (A) Schematic diagram and IUPred3 identification of the disordered region of the human TNS1 protein. A score >0.5 indicates disordered. ABD, actin-binding domain; PTPh, protein tyrosine phosphatase homology; SH2, Src homology 2; PTB, phosphotyrosine-binding; FAB, focal adhesion binding. (B) Equal amounts of protein lysates from MDCK wild-type, TNS1-KO, and two KO-GFP-TNS1 cell lines were immunoblotted against TNS1 and GAPDH antibodies. (C) Live-cell imaging showing two TNS1 condensates were fused into one. (D) FRAP studies. The top TNS1 condensate (arrow) larger than the laser beam was bleached at 0’’, and its fluorescence recovery was recorded; the bottom punctum was an unbleached control. (E) The average fitting curve (blue) of the FRAP experiments suggested the half-time (t1/2) and percentage of recovery were 30 ± 18 s and 55 ± 11% (n = 25), respectively.
Interestingly, new TNS1 condensates were budding from rear ends of nascent FA sites. As the nascent FA extended toward the leading edge of the plasma membrane, the TNS1 signal intensified and assembled into a punctum at the rear end of FAs and segregated away toward the cell center (Fig. 2 A–C and Movie S3). The formation of TNS1 condensates is most obvious in spreading cells without any TNS1 condensates integrating into FAs. These data demonstrate that TNS1 condensates are derived from disassembling FAs. While FA condensates are thought to be precursors to nascent FAs (5, 7, 8), our results support that phase separation of FA proteins may drive FA disassembly.
Fig. 2.
Dynamics of cellular TNS1 condensates. (A) TNS1 condensates (arrows) were derived from and displaced the rear end of GFP-TNS1-positive focal adhesions. (B) Intensity quantification of developing condensates [as framed in the yellow square in (A)] revealed four phases: precondensate formation (phase 1), condensate developing (phase 2), departing (phase 3), and departed (phase 4). The intensity values were measured and normalized based on the highest intensity during each time course as 100. The averages (connected dots) and SDs (shaded area) were plotted (n = 20 condensates from five cells). The intensities at phase 4 were significantly lower than phase 1 (two-tailed Student’s t test, P < 0.0001), suggesting that condensate formation/departure has displaced a portion of FA. (C) A kymograph showed the condensate development in the tracking area (yellow line). (D) Live-cell imaging of GFP-TNS1 condensates during mitosis (h:min): prophase (00:00, defined by the nuclear envelope breakdown), metaphase [00:15, the GFP-excluded area (arrow) indicated chromosome alignment at the metaphase plane], cytokinesis (00:25, the formation of two daughter cells). No condensates were detected from 00:00 to 00:25. (E) Condensate counts in each cell (left vertical axis) and the relative fluorescence intensity in cytoplasm (right vertical axis, the highest intensity was set as 100). Averages were connected by lines, and shaded regions denote SDs (n = 6 dividing cells); (F) Area/size of condensates (n = 15 to 100 each time point).
Intriguingly, TNS1 condensates dissolved when cells undergo mitosis (Fig. 2D and Movie S4). Condensates started to disappear minutes prior to prophase, and no condensate was detected from prophase to cytokinesis (Fig. 2 D and E). Parallel to the disappearance, the fluorescence intensities in cytoplasm were markedly increased (Fig. 2 D and E), suggesting that TNS1 condensates were dissolved, instead of being degraded, into the cytoplasm. Although trypsin induced cell detachment and spherical cell morphology similar to mitotic cells, the TNS1 condensates remained in the cytoplasm (Movie S5), indicating that the dissolution process is not dependent on the attachment status or cell shape, but rather cell cycle regulated. The current literature has focused on the assembly of biomolecular condensates, and little is known about the disassembly process. Our results indicate that TNS1 condensate disassembly is rapid and tightly regulated by a currently unknown mechanism.
Upon the completion of cytokinesis, many smaller TNS1 condensates rapidly appeared (Fig. 2 D–F). The reappearance of TNS1 condensates corresponded with reattaching/flattening of daughter cells after cell division, a process requiring rapid formation and turnover of nascent FAs. Since the number of TNS1 condensates increases in postmitotic cells (Fig. 2F), our observation supports the notion that at least some of the TNS1 condensates are spawn from dynamic FAs as a product of FA disassembly rather than consumed as a precursor of FA assembly. After cell division, the average condensate sizes gradually increased, while the condensate numbers reduced, and both values reached premitosis values in 80 to 90 min (Fig. 2 E and F). These findings are consistent with condensates merged by contact-induced fusion (Fig. 1C).
Since condensates were derived from FAs, we tested and found that FA proteins including ILK, paxillin, ACTN4, DLC1, vinculin, and p130Cas were colocalized to TNS1 condensates. Meanwhile, FAK, zyxin, talin-1, talin-2, ACTN1, VASP, filamin A, Src, and integrin b1 were not (Fig. 3A). Surprisingly, Akt, a protein ser/thr kinase that mediates many signaling events (11), was detected at TNS1 condensates. T308 and S473 are two major phosphorylation sites on Akt. Phosphorylation of T308 activates AKT kinase activity, and further S473 phosphorylation maximizes Akt’s kinase activity (11, 12). Interestingly, only pT308 of Akt was found at TNS1 condensates (Fig. 3B). These findings indicate that TNS1 condensates selectively recruit partners to form biomolecular condensates that are not identical to GIT/PIX, LIMD1, p130Cas, or FAK condensates (5, 7, 8). While we do not know all proteins that associate with FA condensates, the selective protein recruitment likely defines the specific functions of these condensates.
Fig. 3.
Phase separation of GFP-TNS1 recruits selected proteins to condensates in KO-GFP-TNS1 cells, and endogenously tagged TNS1 proteins also form condensates in TNS1-GFP-KI cells. Cells were immunofluorescence-stained with indicated antibodies. Representative results show TNS1 condensates colocalized with (A) ILK, but not FAK, and (B) pT308Akt, not pS473Akt. (C) Immunoblots of wild-type and TNS1-GFP-KI cell lysates against indicated antibodies. (D) TNS1 condensates were derived from disassembling focal adhesions in TNS1-GFP-KI cells. (E) pT308Akt localized to condensates in TNS1-GFP-KI cells.
To unequivocally demonstrate that endogenous TNS1 proteins may form condensates, we generated MDCK cells carrying GFP cDNA knock-in at the end of the TNS1 coding sequence (TNS1-GFP-KI) using CRISPR/Cas9 homology-directed repair approaches. Immunoblot analysis confirmed that the TNS1-GFP band shows similar intensity as that of the wild type and is larger in size, consistent with the presence of GFP in knock-in cells (Fig. 3C). The absence of an untagged TNS1 band in TNS1-GFP-KI suggested that these cells are homozygous (Fig. 3C). In agreement with our earlier findings using KO-GFP-TNS1 cells, endogenous TNS1-GFP-tagged proteins were detected at focal adhesions and formed condensates that contain pT308Akt (Fig. 3 D and E).
Based on our findings on TNS1 condensates, we propose that phase separation at FAs promotes FA disassembly and releases biomolecular condensates driven by various FA scaffold proteins, such as TNS1, GIT/PIX, and LIMD1. These condensates store their own sets of FA proteins and/or signaling molecules as future building materials and signaling intermediates in responding to immediate needs of forming new FAs and transmitting signaling cues, respectively. Why TNS1 condensates dissolve prior to cell division is currently unknown, but it is likely that the dissolution allows equal separation of molecules within condensates to daughter cells for reassembling nascent FAs, and/or the dissolution may release key regulators that are critical for processing mitosis. Our current studies open areas of research on the dynamics, regulations, and function of biomolecular condensates.
Materials and Methods
MDCKTns1KO-GFP-TNS1 cells were generated by transfection of pEGFP-TNS1 plasmids into MDCKTns1KO cells (10). Live-cell images and FRAP were acquired as described (13). Additional details are provided in Supporting information.
Supplementary Material
Appendix 01 (PDF)
MDCKTns1KO-GFP-TNS1 cells were recorded in time lapse (hr:min). Two condensates (arrows) were fused into one. Scale bar, 10 μm.
FRAP of TNS1 condensates in MDCKTns1KO-GFP-TNS1 cells. The top GFP-TNS1 condensate (yellow box) was bleached at 00:00 (min:sec) and its fluorescence recovery were recorded in a time course; the bottom condensate was an unbleached control. Scale bar, 1 μm.
Development of TNS1 condensates at focal adhesion sites. MDCKTns1KO-GFP-TNS1 cells were recorded in time lapse (hr:min). GFP-TNS1 condensates (arrow) were budding from GFP-TNS1 positive focal adhesion sites. Scale bar, 10 μm.
Presence of TNS1 condensates is cell cycle dependent. MDCKTns1KO-GFP-TNS1 cells were recorded in time lapse (hr:min). Scale bar, 20 μm.
TNS1 condensates were present after the cell was rounded up by trypsin treatment. Time stamp in hr:min; scale bar, 10 μm.
Acknowledgments
We thank Po-Yuan Tung and Jordan Lang for their efforts on the early stage of this work. This work was supported in part by the Team Research Award (S.H.L.) from UC Davis and R01GM148706 (S.Y.) from the NIH.
Author contributions
Y.-R.J.L., S.Y., and S.H.L. designed research; Y.-R.J.L., S.Y., and S.H.L. performed research; Y.-R.J.L., S.Y., and S.H.L. contributed new reagents/analytic tools; Y.-R.J.L., S.Y., and S.H.L. analyzed data; and Y.-R.J.L., S.Y., and S.H.L. wrote the paper.
Competing interests
The authors declare no competing interest.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Banani S. F., Lee H. O., Hyman A. A., Rosen M. K., Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dundr M., et al. , In vivo kinetics of Cajal body components. J. Cell Biol. 164, 831–842 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phair R. D., Misteli T., High mobility of proteins in the mammalian cell nucleus. Nature 404, 604–609 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Lu Y., et al. , Phase separation of TAZ compartmentalizes the transcription machinery to promote gene expression. Nat. Cell Biol. 22, 453–464 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu J., et al. , GIT/PIX condensates are modular and ideal for distinct compartmentalized cell signaling. Mol. Cell 79, 782–796.e6 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Ramella M., Ribolla L. M., de Curtis I., Liquid-liquid phase separation at the plasma membrane-cytosol interface: Common players in adhesion, motility, and synaptic function. J. Mol. Biol. 434, 167228 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Wang Y., et al. , LIMD1 phase separation contributes to cellular mechanics and durotaxis by regulating focal adhesion dynamics in response to force. Dev. Cell 56, 1313–1325.e7 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Case L. B., De Pasquale M., Henry L., Rosen M. K., Synergistic phase separation of two pathways promotes integrin clustering and nascent adhesion formation. Elife 11, e72588 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao Y. C., Lo S. H., Tensins - emerging insights into their domain functions, biological roles and disease relevance. J. Cell Sci. 134, jcs254029 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z. Y., et al. , Hyperactivity of Mek in TNS1 knockouts leads to potential treatments for cystic kidney diseases. Cell Death Dis. 10, 871 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manning B. D., Cantley L. C., AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alessi D. R., et al. , Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 (1996). [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X., et al. , Condensation of pericentrin proteins in human cells illuminates phase separation in centrosome assembly. J. Cell Sci. 134, jcs258897 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
MDCKTns1KO-GFP-TNS1 cells were recorded in time lapse (hr:min). Two condensates (arrows) were fused into one. Scale bar, 10 μm.
FRAP of TNS1 condensates in MDCKTns1KO-GFP-TNS1 cells. The top GFP-TNS1 condensate (yellow box) was bleached at 00:00 (min:sec) and its fluorescence recovery were recorded in a time course; the bottom condensate was an unbleached control. Scale bar, 1 μm.
Development of TNS1 condensates at focal adhesion sites. MDCKTns1KO-GFP-TNS1 cells were recorded in time lapse (hr:min). GFP-TNS1 condensates (arrow) were budding from GFP-TNS1 positive focal adhesion sites. Scale bar, 10 μm.
Presence of TNS1 condensates is cell cycle dependent. MDCKTns1KO-GFP-TNS1 cells were recorded in time lapse (hr:min). Scale bar, 20 μm.
TNS1 condensates were present after the cell was rounded up by trypsin treatment. Time stamp in hr:min; scale bar, 10 μm.
Data Availability Statement
All study data are included in the article and/or SI Appendix.