Significance
Aerobic glycolysis and abnormal choline phospholipid metabolism are hallmarks of cancer. The inhibition of glycolysis and choline metabolism is a potential strategy for cancer treatment. ENO1 (enolase-1) is a key enzyme of glycolysis and choline kinase α (CHKα) catalyzes the first reaction of phosphatidylcholine production. Herein, we show that ENO1 promotes choline metabolism through the inhibition of ubiquitylation-dependent degradation of CHKα. These results reveal an unprecedented insight into the integrated regulation of cancer metabolism by a crosstalk between glycolytic and phospholipid-synthesizing enzymes. The presented findings uncover that ENO1 is a novel and promising therapeutic target for cancer treatment, which aims to simultaneously curb aerobic glycolysis and phosphatidylcholine production in cancer cells.
Keywords: CHKα, enolase, TRIM25
Abstract
Aberrantly upregulated choline phospholipid metabolism is a novel emerging hallmark of cancer, and choline kinase α (CHKα), a key enzyme for phosphatidylcholine production, is overexpressed in many types of human cancer through undefined mechanisms. Here, we demonstrate that the expression levels of the glycolytic enzyme enolase-1 (ENO1) are positively correlated with CHKα expression levels in human glioblastoma specimens and that ENO1 tightly governs CHKα expression via posttranslational regulation. Mechanistically, we reveal that both ENO1 and the ubiquitin E3 ligase TRIM25 are associated with CHKα. Highly expressed ENO1 in tumor cells binds to I199/F200 of CHKα, thereby abrogating the interaction between CHKα and TRIM25. This abrogation leads to the inhibition of TRIM25-mediated polyubiquitylation of CHKα at K195, increased stability of CHKα, enhanced choline metabolism in glioblastoma cells, and accelerated brain tumor growth. In addition, the expression levels of both ENO1 and CHKα are associated with poor prognosis in glioblastoma patients. These findings highlight a critical moonlighting function of ENO1 in choline phospholipid metabolism and provide unprecedented insight into the integrated regulation of cancer metabolism by crosstalk between glycolytic and lipidic enzymes.
The growth and proliferation of tumor cells require ample production of phosphatidylcholine (PC), a major component of the phospholipid bilayer membrane. In the PC synthesis pathway, choline kinases (CHKs) phosphorylate choline to generate phosphocholine, which is then converted to cytidine diphosphate-choline and subsequently to PC. CHKs have two isoforms: CHKα encoded by CHKA and CHKβ encoded by CHKB, which differ structurally and in their tissue expression patterns (1, 2). In mammalian cells, only CHKα has a central role in maintaining PC biosynthesis, and CHKα, but not CHKβ, has been extensively implicated in human tumorigenesis (3–6). CHKα overexpression, which promotes tumor cell proliferation and survival, has been detected in 40 to 60% of human tumors and is correlated with poor prognosis in patients with early-stage nonsmall-cell lung cancer, hepatocellular carcinoma, and prostate cancer (1, 7–9). In clinical settings, magnetic resonance spectroscopy (MRS) and spectroscopic imaging (MRSI) detection of CHKα-dependent choline metabolites have exhibited great advantages in the diagnosis of cancer and in monitoring the response of tumors to anticancer therapy (10). However, the mechanism underlying CHKα overexpression in cancer, especially the involvement of posttranslational regulation, remains largely unclear.
Enolase, a glycolytic metalloenzyme, catalyzes the conversion of 2-phosphoglycerate (2-PG) to phosphoenolpyruvate (PEP) and therefore plays a pivotal role in glycolysis. Enolase has three isoforms in mammals: α-enolase (enolase1, ENO1), which is ubiquitously expressed in most tissues; β-enolase (ENO3), which is primarily expressed in muscle tissue; and γ-enolase (ENO2), which is mostly expressed in neural tissues (11). Similar to CHKα, ENO1 is overexpressed in various types of cancer, and its overexpression predicts poor survival of patients with gastric cancer and head and neck cancer (12–14). Although ENO1 expression is linked to tumor cell proliferation, migration, and invasion (15, 16), whether ENO1 has any noncanonical functions in tumor growth is unknown.
In this report, we demonstrate that ENO1 interacts with CHKα and tightly governs CHKα expression by abrogating the binding of the ubiquitin E3 ligase tripartite motif-containing protein 25 (TRIM25) to CHKα and TRIM25-dependent CHKα ubiquitylation and degradation. ENO1-stabilized CHKα promoted choline metabolism in brain tumors and subsequent tumor growth.
Results
ENO1 Binds CHKα and Enhances Its Stability.
Many metabolic enzymes are regulated by posttranslational modifications to tightly control their metabolic functions (17–19). To determine whether CHKα expression is regulated at the posttranslational level, thereby contributing to its overexpression in tumor cells, we immunoprecipitated CHKα from U87 human glioblastoma (GBM) cells. Mass spectrometry analyses of the immunoprecipitates showed that ENO1 is a CHKα-associated protein (SI Appendix, Extended Data Fig. S1A). This interaction was validated in both U87 cells and U251 GBM cells by coimmunoprecipitation analyses with an anti-ENO1 antibody (Fig. 1A). An in vitro glutathione-S-transferase (GST) pulldown assay showed that purified and bacterially expressed GST-CHKα bound to ENO1, but not ENO2 or ENO3 (Fig. 1B), indicating a direct interaction between these two proteins. In addition, the depletion of ENO1-associated CHKα in U87 cells with an anti-ENO1 antibody in an immunoprecipitation assay revealed that approximately 25% of CHKα was associated with ENO1 in these cells (SI Appendix, Extended Data Fig. S1B).
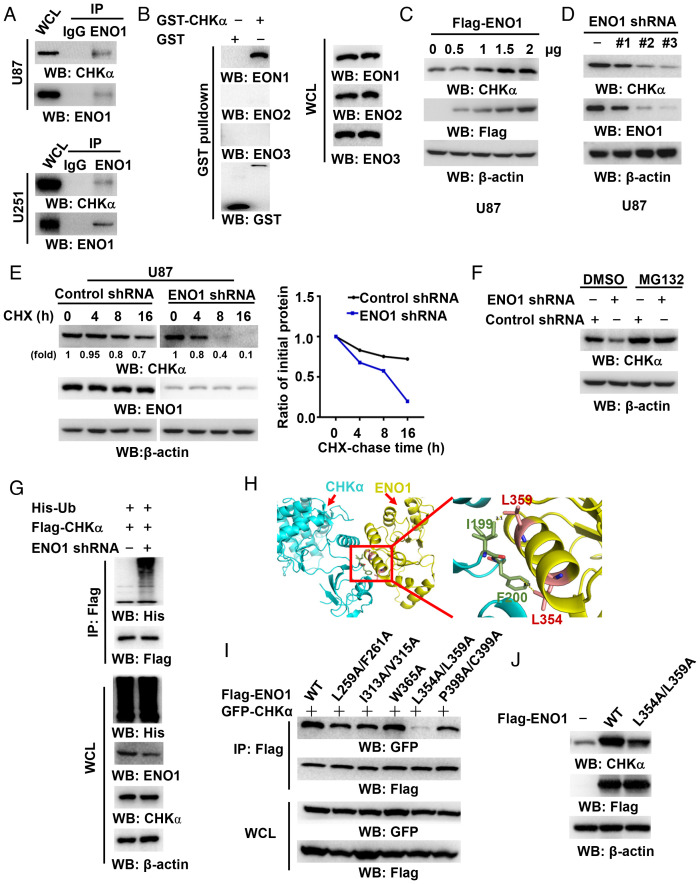
Fig. 1.
ENO1 binds CHKα and enhances its stability. (A) Endogenous ENO1 in U87 cells (Top) and U251 cells (Bottom) was immunoprecipitated. Immunoblotting was performed with the indicated antibodies. (B) Bacterially purified GST-CHKα were incubated with U87 cell lysate. A GST pulldown assay was performed. Immunoblotting was performed with the indicated antibodies. (C) A vector expressing Flag-ENO1 was transfected into U87 cells at the indicated amount. Immunoblotting analyses were performed with the indicated antibodies. (D) U87 cells stably expressing with different ENO1 shRNAs were analyzed by immunoblotting with the indicated antibodies. (E) U87 cells with or without ENO1 shRNA expression were treated with cycloheximide (CHX) (100 μg/mL) and harvested at the indicated periods of time. Immunoblotting analyses were performed with the indicated antibodies (Left). The expression levels of CHKα were quantified (Right). (F) U87 cells with or without ENO1 shRNA expression were treated with MG132 (10 μM) for 10 h. Immunoblotting analyses were performed with the indicated antibodies. (G) U87 cells were transfected with vectors expressing His-Ub and Flag-CHKα with or without ENO1 shRNA and treated with MG132 (10 μM) for 10 h. Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies. Ubiquitin, Ub; WCL, whole-cell lysate. (H) Protein–protein docking analyses of CHKα and ENO1 show the potential interacting residues. (I) HEK293T cells expressing GFP-CHKα and indicated Flag-ENO1 proteins were treated with MG132 (10 μM) for 10 h, followed by immunoprecipitation and immunoblotting analyses with the indicated antibodies. (J) U87 cells expressing indicated Flag-ENO1 proteins were analyzed by immunoblotting analyses with the indicated antibodies.
ENO1 and CHKα are overexpressed in multiple types of cancer (1, 12–14). Compared to high-grade GBM cells, HS683 grade II glioma cells exhibited reduced expression of ENO1 and CHKα and interaction between ENO1 and CHKα (SI Appendix, Extended Data Fig. S1C), as determined by immunoprecipitation analyses with limited ENO1 antibody, which was saturated in binding to ENO1 in the cells. Notable, the association ENO1 with CHKα was also detected in HCT116 human colon cancer cells and Huh7 human hepatocellular carcinoma cells (SI Appendix, Extended Data Fig. S1D). These results suggested that the ENO1–CHKα interaction is not cell line specific and enhanced in malignant tumors.
To examine the relationship between these two interacting proteins, we ectopically expressed Flag-ENO1 in GBM cells and found that Flag-ENO1 expression, which did not alter the mRNA levels of CHKA (SI Appendix, Extended Data Fig. S1E), substantially increased CHKα protein levels (SI Appendix, Extended Data Fig. S1F) in a Flag-ENO1 expression-dependent manner (Fig. 1C). In addition, the expression of catalytically inactive Flag-ENO1 S40A also induced CHKα expression, indicating that ENO1 increases CHKα expression independent of its catalytical activity (SI Appendix, Extended Data Fig. S1F). Conversely, the expression of different shRNAs against ENO1, which did not affect the CHKA mRNA levels (SI Appendix, Extended Data Fig. S1G), reduced CHKα expression in both U87 (Fig. 1D) and U251 (SI Appendix, Extended Data Fig. S1H) cells. These results indicated that ENO1 upregulates CHKα expression in a CHKA transcription-independent manner.
To determine whether ENO1 upregulates CHKα expression by regulating CHKα stability, we treated U87 cells with cycloheximide, an inhibitor of mRNA translation, to eliminate the effect of transcription and translation on CHKα. We found that ENO1 shRNA expression substantially shortened the half-life of CHKα (Fig. 1E) but not CHKβ (SI Appendix, Extended Data Fig. S2A). To determine whether ENO1 regulates CHKα stability through the ubiquitylation proteasome pathway, we treated U87 cells with MG132, a proteasome inhibitor, which abrogated the ENO1 shRNA-reduced CHKα expression (Fig. 1F). In addition, ENO1 shRNA largely enhanced the ubiquitylation of CHKα in both U87 (Fig. 1G) and U251 cells (SI Appendix, Extended Data Fig. S2B). Similarly, the depletion (SI Appendix, Extended Data Fig. S2C) and overexpression (SI Appendix, Extended Data Fig. S2D) of ENO1 in LN229 cells decreased and increased CHKα expression, respectively. In contrast, ectopic expression (SI Appendix, Extended Data Fig. S2E) or depletion (SI Appendix, Extended Data Fig. S2F) of ENO2 in GBM cells did not alter CHKα expression. These results indicated that ENO1 directly interacts with CHKα and enhances CHKα stability by inhibiting the ubiquitylation and proteasomal degradation of CHKα.
To identify the binding residues of ENO1 to CHKα, we performed molecular docking analyses and revealed that L359 and L354, which is not shared with ENO2 (SI Appendix, Extended Data Fig. S2G), in the binding region of ENO1 that potentially interacts with CHKα (Fig. 1H). The mutations of these residues, but not other surrounding amino acids (L259, F261, I313, V315, W365, P398, and C399), showed that only L354A/L359A mutation, which did not alter ENO1 activity (SI Appendix, Extended Data Fig. S2H), remarkably reduced the binding of ENO1 to CHKα (Fig. 1I) with corresponding decrease of CHKα expression (Fig. 1J). These results suggested that ENO1 L354/L359 are involved in binding to CHKα and subsequently promote CHKα expression.
TRIM25 Binds CHKα and Polyubiquitylates CHKα at K195 for Its Degradation.
To determine the mechanism underlying ENO1-regulated CHKα ubiquitylation, we examined CHKα-associated proteins using mass spectrometry analyses and found that TRIM25, a RING domain-containing E3 ubiquitin ligase, was a CHKα-interacting protein (SI Appendix, Extended Data Fig. S1A). The interaction between endogenous CHKα and TRIM25 was validated by a coimmunoprecipitation assay (Fig. 2A). In addition, purified and bacterially expressed GST-CHKα bound to purified and bacterially expressed His-TRIM25 (Fig. 2B). The expression of different TRIM25 truncation mutants showed that TRIM25 with PRY-SPRY domain deletion was unable to bind to CHKα (Fig. 2C), suggesting a critical role of the PRY-SPRY domain in the binding of TRIM25 to CHKα. These results indicated that TRIM25, similar to ENO1, directly interacts with CHKα.
Fig. 2.
TRIM25 binds CHKα and polyubiquitylates CHKα at K195 for its degradation. (A) Endogenous TRIM25 in U87 cells was immunoprecipitated. Immunoblotting analyses were performed with the indicated antibodies. (B) Bacterially purified His-TRIM25 and GST-CHKα were mixed. A GST pulldown assay was performed. Immunoblotting analyses were performed with the indicated antibodies. (C) A schematic shows WT and truncated TRIM25 (Top). Flag-CHKα was coexpressed with or without the indicated HA-TRIM25 proteins in HEK293T cells in the presence of MG132 (10 μM) for 10 h. Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies (Bottom). RING, RING-finger domain; B1, the 1st B-box domain; B2, the 2nd B-box domain; middle, middle region; PRY, PRY domain; SPRY, SPRY domain. (D) U87 cells with or without expression of the indicated HA-TRIM25 proteins were analyzed by immunoblotting analyses with the indicated antibodies. Arrow indicates TRIM25-∆PRY-SPRY. (E) U87 (Left) and U251 (Right) cells were stably transfected with vectors expressing the indicated shRNA. Immunoblotting analyses were performed with the indicated antibodies. (F) An in vitro ubiquitylation assay was performed by incubating purified GST-CHKα with the indicated purified HA-TRIM25 proteins. Immunoblotting analyses were performed with the indicated antibodies. (G) U87 cells were transfected the indicated plasmids. The cells were treated with MG132 (10 μM) for 10 h. Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies. (H) HEK293T cells expressing His-ubiquitin, HA-TRIM25, and the indicated Flag-CHKα proteins were treated with MG132 (10 μM) for 10 h. Immunoprecipitation and immunoblotting were performed with the indicated antibodies. (I) U87 cells expressing Flag-CHKα or Flag-CHKα K195R were treated with CHX (100 μg/mL) for indicated periods of time. Immunoblotting analyses were performed with the indicated antibodies (Top). The expression levels of CHKα were quantified (Bottom).
To determine the impact of TRIM25 binding on CHKα, we overexpressed TRIM25 in U87 cells and observed a decrease in CHKα expression in wild-type (WT) TRIM25—but not TRIM25 with RING domain deletion (ΔRING)—or TRIM25 ΔPRY-SPRY-expressing cells (Fig. 2D), and this decrease was abrogated by MG132 treatment (SI Appendix, Extended Data Fig. S3A). In contrast, depletion of TRIM25 by expressing its different shRNAs in U87 and U251 cells, which did not change CHKA mRNA expression (SI Appendix, Extended Data Fig. S3B), greatly enhanced CHKα expression (Fig. 2E) with a correspondingly increased half-life of CHKα (SI Appendix, Extended Data Fig. S3C). Similarly, depletion (SI Appendix, Extended Data Fig. S3D) and overexpression (SI Appendix, Extended Data Fig. S3E) of TRIM25 in LN229 cells increased and decreased CHKα expression, respectively. These results indicate that TRIM25 induces proteasome-dependent degradation of CHKα.
To determine whether TRIM25 is an E3 ligase for CHKα, we performed an in vitro ubiquitylation assay and showed that purified WT TRIM25, but not the RING domain-or the PRY-SPRY domain-deleted mutant, polyubiquitylated bacterially purified CHKα (Fig. 2F). Consistently, TRIM25 overexpression enhanced the polyubiquitylation of CHKα in U87 cells (Fig. 2G). These results indicate that TRIM25 polyubiquitylates CHKα in a RING domain-dependent manner.
The mutation of several Lys (K) residues in CHKα, which were potential ubiquitylation residues predicted by using the PLMD 3.0 database (http://plmd.biocuckoo.org/http://plmd.biocuckoo.org/), showed that only CHKα K195R was highly resistant to polyubiquitylation by TRIM25 in HEK293T cells (Fig. 2H), suggesting that K195 is a primary polyubiquitylation residue. In addition, an in vitro ubiquitylation assay showed that bacterially purified CHKα K195R had reduced ubiquitylation by TRIM25 (SI Appendix, Extended Data Fig. S3F). Notably, CHKα K195R expressed in U87 (Fig. 2I) and LN229 (SI Appendix, Extended Data Fig. S3G) cells had a much longer half-life than its WT counterpart. These results indicate that TRIM25 binds CHKα and polyubiquitylates CHKα at K195 for its degradation.
The Binding of ENO1 to CHKα Reduces the TRIM25–CHKα Interaction, Thereby Inhibiting TRIM25-Mediated CHKα Polyubiquitylation and Degradation.
Given that the binding of ENO1 and TRIM25 to CHKα upregulates and downregulates expression of CHKα, respectively, we next examined whether the expression and binding of ENO1 to CHKα regulates the association between TRIM25 and CHKα. Coimmunoprecipitation analyses showed that ectopically expressed Flag-ENO1 reduced the interaction between endogenous TRIM25 and endogenous CHKα in U87 and LN229 cells (Fig. 3A). Conversely, this interaction was enhanced by ENO1 depletion (Fig. 3B). An in vitro GST pulldown assay showed that bacterially purified ENO1, but not the ENO1 substrate 2-PG or the product PEP, reduced the binding of purified CHKα to TRIM25 (SI Appendix, Extended Data Fig. S4A), indicating that ENO1, rather than its metabolic substrate or product, impairs the interaction between CHKα and TRIM25. Consistent with this finding, purified ENO1 reduced TRIM25-mediated CHKα polyubiquitylation in vitro (Fig. 3C). In addition, the decrease in CHKα expression induced by ENO1 depletion was rescued by TRIM25 depletion in U87 cells (Fig. 3D). As a control, we did not observe obvious alteration in expression of ATBF1, a known substrate of TRIM25 (20), in U87 cells overexpressing ENO1 (SI Appendix, Extended Data Fig. S4B). These results indicate that the binding of ENO1 to CHKα reduces the interaction between TRIM25 and CHKα, thereby inhibiting TRIM25-mediated CHKα polyubiquitylation and degradation.
Fig. 3.
The Binding of ENO1 to CHKα inhibits TRIM25-mediated CHKα polyubiquitylation and degradation. (A) A control vector or a vector expressing Flag-ENO1 was transfected into U87 (Left) and LN229 (Right) cells. Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies. WCL, whole-cell lysate. (B) U87 cells stably expressing a control shRNA or ENO1 shRNA were treated with MG132 (10 μM) for 10 h. Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies. WCL, whole-cell lysate. (C) An in vitro ubiquitylation assay was performed by incubating purified GST-CHKα with or without purified His-TRIM25 in the presence or absence of purified ENO1. Immunoblotting analyses were performed with the indicated antibodies. (D) TRIM25 shRNA was coexpressed with or without ENO1 shRNA in U87 cells. Immunoblotting analyses were performed with the indicated antibodies. (E) U87 cells were transfected with a vector expressing the indicated Flag-CHKα proteins. Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies. WCL, whole-cell lysate. (F) U87 cells were transfected with a vector expressing the indicated Flag-CHKα proteins. Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies. WCL, whole-cell lysate. (G) Knock-in expression of CHKα I199N/F200N in LN229 cells was constructed. Immunoblotting analyses were performed with the indicated antibodies. KI, knock-in; C1, clone 1; C2, clone 2. (H) LN229 and LN229 CHKα I199N/F200N knock-in cells were treated with CHX (100 μg/mL) for the indicated periods of time. Immunoblotting analyses were performed with the indicated antibodies (Left). The expression levels of CHKα were quantified (Right). KI, knock-in. (I) LN229 and LN229 CHKα I199N/F200N knock-in cells with or without TRIM25 shRNA expression were analyzed by immunoblotting with the indicated antibodies. KI, knock-in; C1, clone 1; C2, clone 2.
The expression of different truncation mutants of CHKα in HEK293T cells showed that CHKα 210 to 457, but not CHKα 160 to 457 or CHKα 81 to 457, lost its binding to both ENO1 and TRIM25 (SI Appendix, Extended Data Fig. S4C), suggesting that amino acids 161 to 209 are the region for both proteins to bind CHKα. This finding was validated by deletion of amino acids 161 to 209 of CHKα, which disrupted the association of CHKα with endogenous ENO1 or TRIM25 (Fig. 3E). Mutations of hydrophobic residues in the 161 to 209 region of CHKα and immunoprecipitation of the mutants showed that CHKα I199N/F200N displayed strongly reduced binding to ENO1 compared to WT CHKα (Fig. 3F and SI Appendix, Extended Data Fig. S4D). In contrast, this mutant had an increased association with endogenous TRIM25 with a corresponding decrease in CHKα expression (Fig. 3F). Consistently, molecular docking analyses revealed that CHKα I199 and F200 potentially interacts with ENO1 L354 and L359, respectively (Fig. 1H). These results suggest that ENO1 and TRIM25 bind to the same region of CHKα and that the binding of ENO1 inhibits the binding of TRIM25 to CHKα.
Knock-in expression of CHKα I199N/F200N in LN229 GBM cells using Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 genome-editing technology (SI Appendix, Extended Data Fig. S4 E and F) showed that CHKα I199N/F200N had a reduced expression level compared to its WT counterpart (Fig. 3G). Consistently, CHKα I199N/F200N had a much shorter half-life than its WT counterpart in LN229 cells (Fig. 3H). Notably, the reduction in CHKα I199N/F200N expression was largely rescued by expression of TRIM25 shRNA, which greatly depleted endogenous TRIM25 expression (Fig. 3I). In addition, we detected a moderate decrease in enolase activity in CHKα I199N/F200N-expressing LN229 cells, suggesting a mutual regulation between ENO1 and CHKα (SI Appendix, Extended Data Fig. S4G). These results further indicate that the binding of ENO1 to CHKα stabilizes CHKα.
ENO1-Upregulated CHKα Expression Promotes PC Production and Enhances GBM Cell Proliferation.
We next examined the effect of ENO1-regulated CHKα expression on choline metabolism and showed that overexpression (Fig. 4A and SI Appendix, Extended Data Fig. S5A) and depletion (Fig. 4B and SI Appendix, Extended Data Fig. S5B) of ENO1 in U87 and U251 cells resulted in an increase and decrease in PC levels, respectively. Notably, the ENO1 depletion-reduced PC levels were rescued by TRIM25 depletion (Fig. 4B and SI Appendix, Extended Data Fig. S5B). In addition, although CHKα I199N/F200N and CHKα K195R did not alter their activities in phosphorylating choline in vitro compared to their WT counterparts (SI Appendix, Extended Data Fig. S5C), knock-in expression of CHKα I199N/F200N in LN229 cells resulted in a decrease in PC levels (Fig. 4C and SI Appendix, Extended Data Fig. S5D), which corresponded to the expression levels of CHKα (Fig. 3G). As expected, the CHKα I199N/F200N-reduced PC production was rescued by TRIM25 depletion (Fig. 4C and SI Appendix, Extended Data Fig. S5D). Consistent with the effect of ENO1-regulated CHKα expression on PC production, we showed that knock-in expression of CHKα I199N/F200N in LN229 cells (Fig. 4D and SI Appendix, Extended Data Fig. S5E) or reconstituted expression of Flag-CHKα I199N/F200N in endogenous CHKα-depleted U87 cells (SI Appendix, Extended Data Fig. S5F) inhibited the cell proliferation. This inhibition was rescued by WT CHKα overexpression, which elicited substantially increased cell proliferation (SI Appendix, Extended Data Fig. S5G). Similar to the effect induced by Flag-CHKα I199N/F200N expression, the expression of Flag-ENO1 L354A/L359A reduced U87 cell proliferation compared to WT Flag-ENO1 expression (Fig. 4E).
Fig. 4.
ENO1-upregulated CHKα expression promotes phosphatidylcholine production and enhances GBM cell proliferation. (A) Flag-ENO1 was stably expressed in U87 cells. The amounts of PC in the indicated cells were measured. Data are the mean ± SD (n = 3). ***P < 0.001. (B) The amounts of PC in U87 cells stably expressing the indicated shRNA were measured. Data are the mean ± SD (n = 3). ***P < 0.001, ****P < 0.0001. (C) The amounts of PC in LN229 and LN229 I199N/F200N knock-in cells with or without expressing TRIM25 shRNA were measured. Data are the mean ± SD (n = 3). ***P < 0.001, ****P < 0.0001. KI, knock-in; C1, clone 1. (D) The proliferation of LN229 cells with or without knock-in expression of CHKα I199N/F200N was measured. Data are the mean ± SD (n = 6). ****P < 0.0001. KI, knock-in; C1, clone 1. (E) U87 cells with expression of WT Flag-ENO1 or Flag-ENO1 L354A/L359A were analyzed by immunoblotting analyses with the indicated antibodies (Left). The proliferation of the cells was measured at day 4 (Right). Data are the mean ± SD (n = 6). ****P < 0.0001.
As expected, ENO1 depletion inhibited GBM cell proliferation, and this inhibition was partially rescued by overexpression of CHKα (SI Appendix, Extended Data Fig. S5H), suggesting that ENO1 depletion-reduced glycolysis could not be compensated by CHKα expression. In line with the role of CHKα in cell growth, overexpression of CHKα in HEB normal human astrocytes increased PC production (SI Appendix, Extended Data Fig. S5I) and cell proliferation (SI Appendix, Extended Data Fig. S5J).
As expected, the ectopic expression of Flag-ENO1 in HEB cells, which increased the interaction between CHKα and ENO1 and CHKα expression (SI Appendix, Extended Data Fig. S5K), also enhanced PC production (SI Appendix, Extended Data Fig. S5L) and cell proliferation (SI Appendix, Extended Data Fig. S5M). These results indicate that ENO1-upregulated CHKα expression promotes PC production and enhances GBM cell proliferation.
ENO1-Upregulated CHKα Expression Promotes Choline Metabolism in Brain Tumors and Brain Tumor Growth.
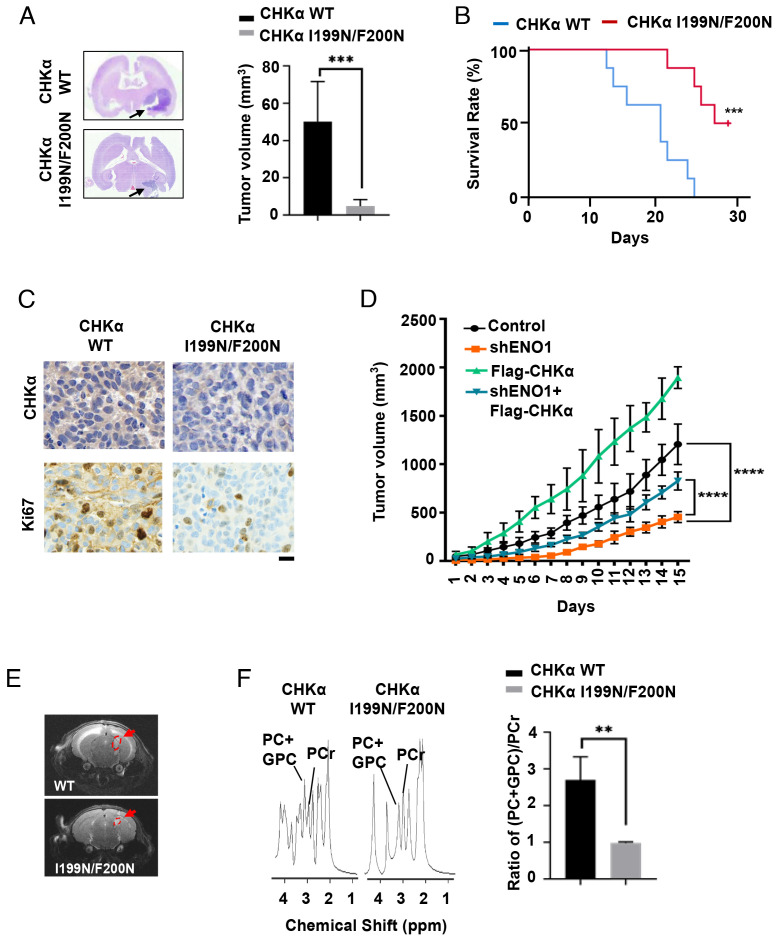
To determine the effect of ENO1-regulated CHKα expression on tumor growth, we intracranially injected LN229 CHKα I199N/F200N knock-in cells into athymic nude mice and found that CHKα I199N/F200N expression reduced tumor growth (Fig. 5A) and prolonged mouse survival time (Fig. 5B). Consistently, the expression of Flag-CHKα I199N/F200N in CHKα-depleted U87 cells inhibited tumor growth when these cells were subcutaneously injected into athymic nude mice (SI Appendix, Extended Data Fig. S6). The immunohistochemical (IHC) analysis showed that CHKα I199N/F200N in tumor cells was expressed at a reduced level compared to its WT counterpart, and the expression levels of CHKα were correlated with those of Ki-67, a marker of cell proliferation (Fig. 5C). As expected, ENO1 depletion blunted tumor growth, and the ENO1 depletion-inhibited tumor growth was partially rescued by overexpression of CHKα (Fig. 5D).
Fig. 5.
ENO1-upregulated CHKα expression promotes choline metabolism in brain tumors and brain tumor growth. (A) LN229 cells with or without knock-in expression of CHKα I199N/F200N were intracranially injected into the brains of athymic nude mice (n = 6 per group). Tumor growth was examined 21 d after injection. Representative hematoxylin-and-eosin-stained coronal brain sections are shown. The tumor regions are indicated by arrows (Left). Tumor volumes were measured (Right). ***P < 0.001. (B) LN229 cells with or without knock-in expression of CHKα I199N/F200N were intracranially injected into the brains of athymic nude mice (n = 8 per group). Mouse survival time was recorded and visualized using Kaplan–Meier survival curves. Data represent the mean ± SD. ***P < 0.001. (C) IHC analyses of the indicated tumors expressing the indicated CHKα proteins were performed with the indicated antibodies. Representative staining images are shown. (Scale bar, 15 μm.) (D) U87 cells with or without stably expressing ENO1 shRNA in the presence or absence of Flag-CHKα expression were injected subcutaneously into nude mice (n = 5 per group). The tumor volume was measured every other day. The data are means ± SD. ****P < 0.0001. (E and F) LN229 cells with or without knock-in expression of CHKα I199N/F200N were intracranially injected into the brains of athymic nude mice (n = 6 per group). MRSI was acquired from the whole brain showing a high signal intensity tumor lesion (indicated by red arrows) (E). PC levels in tumor tissues were determined by MRS-1H spectroscopy. The tumor metabolic profiles, which were normalized by N-acetyl, were estimated, including PC, GPC and PCr. Data represent the mean ± SD. **P < 0.01 (F). PC, phosphatidylcholine; GPC, glycerophosphorylcholine; PCr, phosphocreatine.
To determine the role of CHKα-regulated choline metabolism in the context of tumorigenesis, we performed analyses of metabolic flux in mouse brains by NMR 1H spectrum. The MRS fitting spectrum shows that CHKα I199N/F200N expression reduced tumor growth (Fig. 5E) and PC levels in tumor tissues (Fig. 5F). These results suggest that ENO1-upregulated CHKα expression promotes choline metabolism in brain tumors and brain tumor growth.
ENO1 Levels Are Positively Correlated with CHKα Levels and the Clinical Aggressiveness of GBM.
To determine the clinical importance of ENO1-upregulated CHKα expression, we analyzed 101 primary human GBM specimens. The results showed that levels of ENO1 were positively correlated with CHKα expression levels (Fig. 6 A and B), and the expression levels of ENO1 and CHKα were negatively correlated with survival durations of GBM patients (Fig. 6C). These results suggest that ENO1-upregulated CHKα expression and subsequent choline metabolism are physiologically relevant in brain tumor development and play a role in the clinical aggressiveness of GBM.
Fig. 6.
ENO1 levels are positively correlated with CHKα levels and the clinical aggressiveness of GBM. (A and B), IHC analyses of 101 human GBM specimens with indicated antibodies. Representative images of two cases are shown (A). (Scale bars, 20 mm.) IHC staining was scored, and correlation analysis was performed by means of Pearson correlation test. Note that the scores of some samples overlap (B). (C) Kaplan–Meier Plots of the overall survival durations of GBM patients (n = 101) with expression levels of CHKα and ENO1 (P < 0.001) in GBM specimens. The P values were calculated using the log-rank test. The table shows the multivariate analysis results after adjustment for patients.
Discussion
Aberrantly enhanced choline metabolism has been proposed as a novel hallmark of cancer, and imaging-based monitoring of high production of choline phospholipid, the major membrane phospholipid, appears to be a potential diagnostic approach for cancer and the therapeutic response (10). CHKα is a key metabolic enzyme governing choline metabolism, and its high expression promotes tumor progression and predicts poor prognosis of cancer patients (1, 7–9). We revealed for the first time that CHKα expression can be regulated at the posttranslational level. We demonstrated that CHKα interacts with ENO1. In addition, ENO1 expression levels control CHKα expression in a manner independent of the transcriptional regulation of the CHKA gene. In the CHKα protein complex, TRIM25 is another CHKα-associated protein that polyubiquitylates CHKα at K195, leading to the proteasomal degradation of CHKα. ENO1 bound to I199/F200 of CHKα, which is in the same binding region as TRIM25, thereby inhibiting the interaction between CHKα and TRIM25 and abrogating TRIM25-dependent CHKα polyubiquitylation and degradation. ENO1-stabilized CHKα promoted choline metabolism, tumor cell proliferation, and brain tumor growth. The MRS-1H spectroscopy analyses revealed that disruption of the binding of ENO1 to CHKα by knock-in expression of CHKα I199N/F200N in brain tumors in mice reduced choline phospholipid production and tumor growth.
ENO1 is primarily known for its metabolic role in glucose metabolism. In models of autoimmune diseases, impaired glycolysis in regulatory T cells (Treg cells) demonstrated the critical role of ENO1 in the regulation of gene expression and subsequent suppression of the differentiation of Treg cells (21). We revealed for the first time that ENO1 possesses a glycolysis-independent function in cancer cells by directly interacting with CHKα and subsequently stabilizing CHKα by inhibiting the binding of TRIM25 to CHKα. The clinical significance of ENO1-mediated CHKα expression is evidenced by the positive correlation of ENO1 expression with CHKα expression in human GBM specimens and the positive association of this relationship with poor prognosis in GBM patients. The discovery of the noncanonical function of ENO1 in controlling CHKα expression and choline phospholipid metabolism provides unprecedented insight into the regulation of cancer metabolism by crosstalk between glycolytic and lipidic enzymes in tumor cells and identifies the moonlighting function of ENO1 as a novel and promising therapeutic target for cancer treatment.
Materials and Methods
Materials.
Normal rabbit immunoglobulin (sc-2027), normal mouse immunoglobulin (sc-2025), and mouse antibody against ENO1 (sc-100812) and CHKα (sc-376489) were purchased from Santa Cruz Biotechnology (Shanghai, China). Rabbit antibodies that recognize human CHKα (13520-1-AP), HA (51064-2-AP), FLAG (20543-1-AP) and mouse antibodies that recognize HA tag (66006-2-Ig), FLAG (66008-4-Ig), GST (66001-2-Ig), and 6×His-tag (66005-1-Ig) were purchased from Proteintech (Wuhan, China). Rabbit antibody against ENO1 (ab227978) was obtained from Abcam (Shanghai, China). Rabbit antibodies against TRIM25 (A12938), 6×His-tag (AE003) and mouse antibody against GFP (AE012) were purchased from Abcolonal (Wuhan, China). 2-PG (19,710) and PEP (P7002) were purchased from Sigma (Shanghai, China). Puromycin (HY-B1743S), hygromycin (HY-BO490), MG132 (HY-13259) and CHX (HY-12320) were purchased from MedChemExpress (Shanghai, China). Lipofectamine 2000 (L3000015) transfection reagents were obtained from Thermo Fisher Scientific (Waltham, MA).
Cell Culture and Transfection.
U251, U87, LN229, HS683, HEB, and HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% bovine calf serum. These cells were routinely tested for mycoplasma. All cells were authenticated using short-tandem repeat profiling. Transfection was performed using Lipofectamine 2000 according to the manufacturer’s instructions (22).
DNA Constructs and Mutagenesis.
PCR-amplified human CHKα, CHKα (a.a. 81 to 457), CHKα (a.a. 160 to 457), CHKα (a.a. 210 to 457), CHKα I199N/200N, CHKα K195R, TRIM25, TRIM25 (ΔRING), TRIM25 (ΔRING-BBOX), TRIM25 (ΔPYR-SPRY), ENO1, and ENO1 L354A/L359A were cloned into the PCDNA3.1 (+)-Flag, PCDNA3.1 (+)-HA, PCDNA3.1 (+)-GFP, PCDH/puro-flag, PCDH/puro-HA, pColdI, or pGEX-4T-1 vector. The mutations were generated using a QuikChange site-directed mutagenesis kit (Stratagene, CA).
The following pLKO.1 shRNAs were used:
Control shRNA oligonucleotide: 5′-TTCTCCGAACGTGTCACGT-3′;
Human ENO1 shRNA #1 oligonucleotide: 5′-ATGATCGAGATGGATGGAACA-3′;
Human ENO1 shRNA #2 oligonucleotide:5′-GACTTCAAGTCTCCCGATGAC-3′;
Human ENO1 shRNA #3 oligonucleotide: 5′-GAACTTCAGAAACCCCTTGGC-3′;
Human TRIM25 shRNA #1 oligonucleotide;5′-AACTGAACCACAAGCTGATA-3′;
Human TRIM25 shRNA #2 oligonucleotide;5′-CAGCTACAACAAGAATACACGGAAA-3′;
Human TRIM25 shRNA #3 oligonucleotide;5′-CTGGTGCCACACTCTCCATCT-3′;
CRISPR/Cas9-Mediated Genome Editing.
Genomic mutations were introduced into cells using the CRISPR/Cas9 system, as described previously (23, 24). Single-guide RNAs were designed to target the genomic area adjacent to the indicated mutation sites using the CRISPR design tool (www.benchling.com). The annealed-guide RNA oligonucleotides were inserted into the PX458 vector and digested with the BbsI restriction enzyme. Cells were seeded at 50% confluence, followed by cotransfection of single-guide RNAs (3 μg) and 10 nmol single-stranded donor oligonucleotide (3 μL) as a template to introduce mutations with human CHKα I199N/F200N single-stranded oligo donor sequence: tgccatctgttttagggggctgaggccatggttctggagagcgttatgtttgccattctcgcagagaggtcacttgggccaaaattatacggtaacaatccacagggtcgactggagcagttcatcccggtaagatttgttcataaacgcttagttgacatatgccacagacagaagatggcttctttgtctcattgctg. Two days post-transfection, cells were treated with puromycin (1 μg/mL) for 3 d, trypsinized, and diluted to obtain single cells and seeded into 96-well plates. Genomic DNA was extracted from puromycin-positive cells, followed by sequencing of the PCR products spanning the mutation sites.
Mass Spectrometry Analysis.
Protein samples were digested in-gel in 50 mM ammonium bicarbonate buffer containing Rapigest (Waters Corp., Milford, MA) overnight at 37 °C with 200 ng of sequencing-grade modified trypsin (Promega, Madison, WI). The digest was analyzed by LC-MS/MS on an Obitrap-Elite mass spectrometer (Thermo Fisher Scientific, Waltham, MA). Proteins were identified by searching for the fragment spectra in the Swiss-Prot protein database using the Mascot search engine (version 2.3; Matrix Science, London, UK) and SEQUEST v.1.27 (University of Washington, Seattle, WA) via the Proteome Discoverer software program (version1.4; Thermo Fisher Scientific).
Lentiviral Generation and Infection.
Lentiviral constructs expressing CHKα, ENO1 and TRIM25 constructs were cotransfected into HEK293T-packaging cells along with packaging and envelope using (Invitrogen) (25). The cells were seeded at 50 to 60% confluence. Lentiviruses were harvested 72 h after transfection, centrifuged to remove cell debris, and filtered through a 0.45-µm filter (Millipore). Cells were infected with lentivirus in the presence of 10 μg/mL polybrene. The selection of stable clones was carried out using puromycin or hygromycin.
RT-PCR Analysis.
Total cellular RNA was isolated with a TRIzol reagent kit (Invitrogen) following the manufacturer’s protocol. First-strand cDNA was synthesized using the Super Script II First-Strand Synthesis System. Real-time PCR assays were performed by the SYBR Premix Ex Taq system (Bimake), and the reaction included 95 °C for 30 s, 95 °C for 10 s, 60 °C for 10 s, 72 °C for 20 s (45 cycles), and 72 °C extension for 1 min (26, 27). The following primer pairs were used: human CHKα, 5′-TGGTTCTGGAGAGCGTTATGT-3′ (forward) and 5′-CATTTTCTCGGCGATTTCTGC-3′ (reverse); human β-actin, 5′-TGGCACCCAGCACAATGAA-3′′ (forward) and 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ (reverse).
Immunoprecipitation and Immunoblot Analysis.
The extraction of proteins using a modified buffer from cultured cells was followed by immunoprecipitation and immunoblot using antibodies as described previously (28).
Purification of Recombinant Proteins.
WT and mutant GST-CHKα, GST-ENO1, and His-TRIM25 were expressed in bacteria and purified as described previously (29).
Cell Proliferation Assay.
2 × 103 cells suspended in 200 µL of medium were seeded in each well of a 96-well plate. Cell proliferation was measured using a CCK-8 kit.
Animal Experiments.
As described previously (30), we intracranially injected 1 × 106 LN229 cells (in 6 μL of DMEM per mouse) with or without knock-in expression of CHKα I199N/F200N into 4-wk-old female BALB/c Nude mice. Six mice per group were used in each experiment. Twenty-1 d after injection, the mice were sacrificed and brains of the mice were harvested, fixed in 4% formaldehyde, and embedded in paraffin. Tumor formation and phenotype were determined by histological analysis of hematoxylin and eosin-stained sections. For the subcutaneous tumor formation assay, 2 × 106 cells were injected subcutaneously into the nude mice. Tumor volume was measured with a caliper and calculated using the following formula: V = ab2/2, where a is the longer dimension and b is the shorter one. The animals were treated in accordance with relevant institutional and national guidelines and regulations. The use of the animals was approved by the institutional research ethics committee of Qingdao University.
Immunohistochemical Analysis.
Sections of paraffin-embedded xenograft tissue were stained with an antibody against Ki-67 or with nonspecific IgG as a negative control. Immunohistochemical staining was performed using a VECTASTAIN ABC kit (Vector Laboratories) according to the manufacturer’s instructions. Human GBM tissues were stained with antibodies against CHKα and ENO1. The IHC scores were assessed by two independent authors blinded to the patients’ clinicopathological data. We quantitatively scored the tissue sections according to the staining intensity (0, no signal; 1, weak; 2, moderate; and 3, strong) and percentage of positive cells (1, 0 to 25%; 2, 26 to 50%; 3, 51 to 75%; and 4, >75%). We then combined the intensity and proportion scores to obtain a total score as described previously. The specimens with scores R 4 were classified as high expression, while those with scores <4 were classified as low expression. Scores were compared with the overall survival, which was defined as the time from the date of surgery to death or the last known date of follow-up (25).
The deidentified human GBM specimens and clinical information were purchased from Qingdao Biomedical Technology Co., Ltd. (RIXCN®, Article number: ZL-BraG180sur01). This study was approved by the Ethics Committee of Qingdao University.
GST Pulldown Assay.
GST-tagged protein pulldown assays were performed as described previously (31).
CHKα Activity Assay.
Recombinant purified CHKα protein (1 μg) was incubated for 10 min at 37 °C in a buffer containing 100 mM Tris (pH 8.0), 10 mM MgCl2, 10 mM adenosine triphosphate (ATP), and 200 μM choline chloride (5). CHKα activity was calculated by measuring ADP generation with a Transcreener ADP2 FP Assay kit (BellBrook Labs) following the manufacturer’s instructions.
Measurement of Cellular Phosphatidylcholine Levels.
Cellular phosphatidylcholine levels were measured by using a Phosphatidylcholine Assay Kit (Abcam) following the manufacturer’s instructions.
Analysis of Metabolic Flux by NMR In Vivo 1H Spectrum.
A 7.0T MRI Bruker® system (7.0T MRI Bruker ®, Germany) was used to obtain the original data. The set scan sequences and parameters were as follows: 1) 3D section RM FSE T2WI: repetition time (TR) 4,311 ms, echo time (TE) 33 ms, field of view (FOV) 0.1 × 0.1 mm, average 4, slice thickness 0.3 mm, interslice distance 0.6 mm, imaging matrix 256 × 256, scan time 5:23; 2) T1WI/SAG: TR 100 ms, TE 3.1 ms, FOV = 0.1 × 0.1 mm, average 1, slice thickness 0.1 mm, spacing 0.3 mm, imaging matrix 256 × 256, scan time 4:03 3). Three-channel body coil MRS - 1H spectrum PRESS.ppg13 sequence set parameters: number of scans 10, receiver gain 64.0 ppm, relaxation delay 0.0028 s, acquisition time 0.6204 min, magnetic field frequency 300.33 MHz, spectral width 3,301.1 Hz, frequency lower limit −239 Hz, spectral size 2,048 pixels, digital resolution 1.61 pixels, TR 100 ms, TE 3.1 ms, number of accumulations 45 times.
The original data were preprocessed with jMRUI_v6.0 beta (jMRUI software, European Union) to obtain the MRS fitting spectrum. The peak calibration was performed on the final curve to obtain relevant compound information. The analysis of the 1H spectrum was mainly the H atom signal distribution of characteristic groups. The resulting 1H spectra were Fourier transformed, phase-corrected, and baseline corrected to give a chemical shift of 4.7 ppm for the scaled water peak and 2.02 ppm for N-acetyl as the second reference, and the aligned lines were back labeled and integrated one by one to determine the N-acetyl compound resonance peak 2.02 ppm, phosphocreatine (PCr) 3.02 to 3.08 ppm, (PC + GPC) 3.22 to 3.5 ppm, and other signals, determined from the respective integral values: (PC + GPC)/PCr.
In Vitro Ubiquitylation Assay.
In vitro ubiquitination assays were performed as described previously (32). In brief, purified GST-CHKα or GST-CHKα K195R with purified His-TRIM25 were incubated with 50 to 500 nM E1, 0.5 to 5 μM His-E2 (Ubc4), 10 μM GST-Ub, and 2 mM ATP in a reaction buffer (50 mM Tris-HCl, pH 7.5, 2.5 mM MgCl2, and 0.5 mM dithiothreitol) for 90 min at room temperature.
Statistical Analysis.
All quantitative data are presented as the mean ± SD of at least three independent experiments. A two-group comparison was conducted using a two-sided, two-sample Student’s t test. A simultaneous comparison of more than two groups was conducted using one-way ANOVA. Values of P < 0.05 were considered statistically significant.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This study was supported by grants from the Ministry of Science and Technology of the People's Republic of China (2020YFA0803300, J.F. and Z.L.), the National Natural Science Foundation of China (82188102, 82030074, Z.L. and 82073061, J.F), the Postdoctoral Science Foundation of China (2022TQ0164, Q.M.), the Zhejiang Natural Science Foundation Key Project (LD21H160003, Z.L.), the Zhejiang University Research Fund (188020*194221901/029, Z.L.), and the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (2019R01001, Z.L.). Z.L. is the Kuancheng Wang Distinguished Chair.
Author contributions
J.F. and Z.L. designed research; Q.M., H.J., L.M., G.Z., Q.X., D.G., N.H., H.L., Z.M., J.L., L.Z., Q.L., X.W., M.L., and S.L. performed research; Q.M. analyzed data; and Z.L. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Jing Fang, Email: jfang@qdu.edu.cn.
Zhimin Lu, Email: zhiminlu@zju.edu.cn.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Chen X., et al. , Molecular structure and differential function of choline kinases CHKalpha and CHKbeta in musculoskeletal system and cancer. Cytokine Growth Factor Rev. 33, 65–72 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Malito E., Sekulic N., Too W. C., Konrad M., Lavie A., Elucidation of human choline kinase crystal structures in complex with the products ADP or phosphocholine. J. Mol. Biol. 364, 136–151 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arlauckas S. P., Popov A. V., Delikatny E. J., Choline kinase alpha-putting the chok-hold on tumor metabolism. Prog. Lipid Res. 63, 28–40 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu G., Aoyama C., Young S. G., Vance D. E., Early embryonic lethality caused by disruption of the gene for choline kinase alpha, the first enzyme in phosphatidylcholine biosynthesis. J. Biol. Chem. 283, 1456–1462 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu R., et al. , Choline kinase alpha 2 acts as a protein kinase to promote lipolysis of lipid droplets. Mol. Cell 81, 2722–2735.e9 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Ma Q., Meng Z., Meng Y., Liu R., Lu Z., A moonlighting function of choline kinase alpha 2 in the initiation of lipid droplet lipolysis in cancer cells. Cancer Commun. (Lond). 41, 933–936 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez de Molina A., et al. , Expression of choline kinase alpha to predict outcome in patients with early-stage non-small-cell lung cancer: A retrospective study. Lancet oncol. 8, 889–897 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Kwee S. A., Hernandez B., Chan O., Wong L., Choline kinase alpha and hexokinase-2 protein expression in hepatocellular carcinoma: Association with survival. PLoS One 7, e46591 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Challapalli A., et al. , Exploiting altered patterns of choline kinase-alpha expression on human prostate tissue to prognosticate prostate cancer. J. Clin. Pathol. 68, 703–709 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Glunde K., Jiang L., Moestue S. A., Gribbestad I. S., MRS and MRSI guidance in molecular medicine: Targeting and monitoring of choline and glucose metabolism in cancer. NMR Biomed. 24, 673–690 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Didiasova M., Schaefer L., Wygrecka M., When place matters: Shuttling of enolase-1 across cellular compartments. Front. Cell Dev. Biol. 7, 61 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao H., et al. , Enolase1 overexpression regulates the growth of gastric cancer cells and predicts poor survival. J. Cell Biochem. 120, 18714–18723 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Altenberg B., Greulich K. O., Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics 84, 1014–1020 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Tsai S. T., et al. , ENO1, a potential prognostic head and neck cancer marker, promotes transformation partly via chemokine CCL20 induction. Eur. J. Cancer 46, 1712–1723 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Fu Q. F., et al. , Alpha-enolase promotes cell glycolysis, growth, migration, and invasion in non-small cell lung cancer through FAK-mediated PI3K/AKT pathway. J. Hematol. Oncol. 8, 22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Principe M., et al. , Alpha-enolase (ENO1) controls alpha v/beta 3 integrin expression and regulates pancreatic cancer adhesion, invasion, and metastasis. J. Hematol. Oncol. 10, 16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu D., et al. , The evolving landscape of noncanonical functions of metabolic enzymes in cancer and other pathologies. Cell Metab. 33, 33–50 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Lu Z., Hunter T., Metabolic kinases moonlighting as protein kinases. Trends Biochem. Sci. 43, 301–310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Egervari G., Wang Y., Berger S. L., Lu Z., Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 19, 563–578 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong X. Y., et al. , Oestrogen causes ATBF1 protein degradation through the oestrogen-responsive E3 ubiquitin ligase EFP. Biochem. J. 444, 581–590 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Rosa V., et al. , Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 16, 1174–1184 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H., Xia Y., Fang D., Hawke D., Lu Z., Caspase-10-mediated heat shock protein 90 beta cleavage promotes UVB irradiation-induced cell apoptosis. Mol. Cell. Biol. 29, 3657–3664 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X., et al. , Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol. Cell 61, 705–719 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X., et al. , Nucleus-translocated ACSS2 promotes gene transcription for lysosomal biogenesis and autophagy. Mol. Cell 66, 684–697.e689 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang W., et al. , Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature 480, 118–122 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X., et al. , A splicing switch from ketohexokinase-C to ketohexokinase-A drives hepatocellular carcinoma formation. Nat. Cell Biol. 18, 561–571 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu D., et al. , The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature 580, 530–535 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Lu Z., et al. , Activation of protein kinase C triggers its ubiquitination and degradation. Mol. Cell. Biol. 18, 839–845 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia Y., et al. , MEKK1 mediates the ubiquitination and degradation of c-Jun in response to osmotic stress. Mol. Cell. Biol. 27, 510–517 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J. H., et al. , EGFR-phosphorylated platelet isoform of phosphofructokinase 1 promotes PI3K activation. Mol. Cell 70, 197–210.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian X., et al. , PTEN suppresses glycolysis by dephosphorylating and inhibiting autophosphorylated PGK1. Mol. Cell 76, 516–527.e7 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Lee J. H., et al. , Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat. Commun. 8, 949 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.