Significance
This study reports dual-mode probes that enable ratiometric quantification of hypoxia in a concentration-independent manner based on the magnetic and electronic differences between EuII and EuIII. These results are significant because hypoxia is an important biomarker in a wide range of diseases and there is a critical need to spatially quantify hypoxia to improve our biological understanding of these diseases and identify and evaluate new treatments.
Keywords: hypoxia, MRI, ratiometric, responsive contrast agents
Abstract
Hypoxia is a prognostic biomarker of rapidly growing cancers, where the extent of hypoxia is an indication of tumor progression and prognosis; therefore, hypoxia is also used for staging while performing chemo- and radiotherapeutics for cancer. Contrast-enhanced MRI using EuII-based contrast agents is a noninvasive method that can be used to map hypoxic tumors, but quantification of hypoxia using these agents is challenging due to the dependence of signal on the concentration of both oxygen and EuII. Here, we report a ratiometric method to eliminate concentration dependence of contrast enhancement of hypoxia using fluorinated EuII/III-containing probes. We studied three different EuII/III couples of complexes containing 4, 12, or 24 fluorine atoms to balance fluorine signal-to-noise ratio with aqueous solubility. The ratio between the longitudinal relaxation time (T1) and 19F signal of solutions containing different ratios of EuII- and EuIII-containing complexes was plotted against the percentage of EuII-containing complexes in solution. We denote the slope of the resulting curves as hypoxia indices because they can be used to quantify signal enhancement from Eu, that is related to oxygen concentration, without knowledge of the absolute concentration of Eu. This mapping of hypoxia was demonstrated in vivo in an orthotopic syngeneic tumor model. Our studies significantly contribute toward improving the ability to radiographically map and quantify hypoxia in real time, which is critical to the study of cancer and a wide range of diseases.
Tumor hypoxia develops because of uncontrollable cell proliferation and abnormal vasculature that fails to transport oxygen to the interior cells of tumors (1–3). To dwell in reduced oxygen concentrations, hypoxic cells adjust their cellular environment to possess different chemical and physiological characteristics compared to normoxic cells (4–6). These adaptations lead to poor prognosis, tumor aggressiveness, and therapeutic resistance (7), making hypoxia an important biomarker in the diagnosis of cancer (8). Several imaging modalities have been used to image hypoxia including positron emission tomography, X-ray computed tomography, single-photon emission tomography, optical imaging, and MRI (9–13). The use of MRI is advantageous because it uses nonionizing radiowaves to generate three-dimensional images with near-cellular spatial resolution. Contrast agents are often used in MRI to overcome its inherent poor sensitivity, and responsive contrast agents can be designed to provide molecular information by changing the degree or type of contrast enhancement in response to a molecular event or the presence or absence of an analyte, like O2 (14–20). One of the most promising types of contrast agents for imaging O2 involves EuII (21–26). This ion enhances contrast-like clinically used GdIII, but unlike GdIII, EuII oxidizes to EuIII, an ion that does not enhance contrast in T1-weighted MRI. Thus, the ion provides a clear switch with respect to responsive imaging of O2.
However, the use of contrast-enhanced MRI with responsive contrast agents in tumor imaging is limited because of the dependence of contrast enhancement on the concentration of contrast agents, which is extremely difficult to measure in vivo. Because of this limitation, differentiation between complete response, partial response, and no response is muddled during in vivo MRI (27, 28). Therefore, there is a critical need to employ new strategies to overcome concentration-related limitations to enable responsive contrast-enhanced MRI of hypoxia. In general, several strategies have been used to address concentration dependency of contrast-enhanced MRI (29–33). One of the most promising strategies is the use of ratiometric methods (34–38). Signals arising from individual probes depend on the concentration of a contrast agent; however, the ratio between the concentration-dependent responses causes concentration to become irrelevant if the contrast agent is present above the minimum detectable threshold. Consequently, we hypothesized that dual-mode responsive contrast agents based on 1H- and 19F-MRI coupled with europium in the +2 and +3 oxidation states would enable ratiometric imaging of hypoxia. Here, we report the testing of this hypothesis using fluorinated EuII- and EuIII-containing complexes with varying local concentrations of fluorine (Fig. 1).
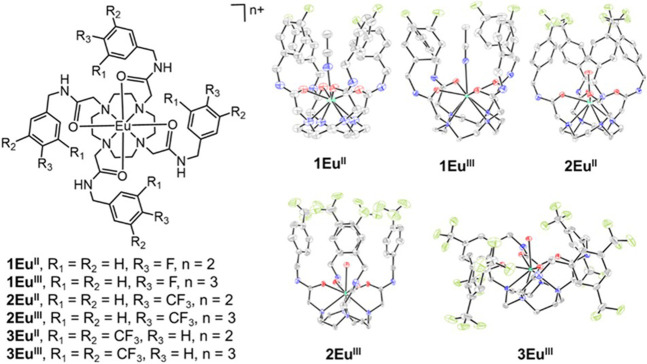
Fig. 1.
(Left) Chemical structures of 1EuII,1EuIII, 2EuII, 2EuIII, 3EuII, and 3EuIII. (Right) Molecular structures in crystals of 1EuII, 1EuIII, 2EuII, 2EuIII, and 3EuIII. Chloride counterions, outer-sphere water molecules, and hydrogen atoms are not shown for clarity. Thermal ellipsoids are drawn at 50% probability. Gray = C; blue = N; red = O; yellow-green = F; sea green = Eu. Crystallographic data for these structures have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers 2203173 (1EuII), 2203172 (1EuIII), 1562330 (2EuII), (22) 1562331 (2EuIII), (22) and 2203174 (3EuIII).
Results and Discussion
Inspired by reported fluorinated europium-containing complexes (22, 39), we synthesized ligands 1–3 that vary in the number of chemically equivalent fluorine atoms. Chemical equivalency of fluorine atoms is important to avoid diluting signal during imaging. Additionally, the presence of more chemically equivalent fluorine atoms is associated with increased sensitivity in imaging; however, the presence of more fluorine atoms is also associated with decreased aqueous solubility. Consequently, ligands 1–3 were selected to provide a range of fluorine atoms to balance these two important parameters. The tetra-amide binding site of the ligand was selected because it forms inert complexes with both EuII and EuIII (23).
Fluorinated ligands were synthesized in two steps from commercially available starting materials. Ligands 1 and 2 were synthesized using reported procedures (22, 39), and ligand 3 was synthesized and characterized using 1H-, 13C-, and 19F-NMR spectroscopy and high-resolution mass spectrometry (see SI Appendix for details). Ligands were metalated with EuCl3·6H2O to produce 1EuIII, 2EuIII, and 3EuIII. The reduced versions of those complexes — 1EuII, 2EuII, and 3EuII— were prepared in quantitative yield by reducing the corresponding EuIII-containing complexes with Zn0 (see the electronic supplementary information for detailed procedures, yields, and characterization).
To study the structures of the six complexes, X-ray crystallography was performed on single crystals of the complexes. In the structures, all the four amide arms of 1EuII, 1EuIII, 2EuII, and 2EuIII are pseudoaxial, resulting in caged structures around monodentate ligands coordinated to the Eu ions. This cage formation can be attributed to the fluorophilic character of the F and CF3 groups and π-stacking of the benzene rings. The amide arms of 3EuII and 3EuIII are pseudoequatorial due to steric hindrance from the multiple CF3 groups on each arm. These structures are expected given the reports of other tetraamide complexes of lanthanides (40–42). Importantly, the crystal structures confirmed the oxidation state of Eu, and each complex has a coordinated solvent molecule, water or acetonitrile, suggesting that they would be appropriate for contrast enhancement in 1H-MRI.
To test the hypoxia-responsive nature of the complexes, we prepared solutions (1 mM) of EuII- and EuIII-containing complexes of 1, 2, and 3 in aqueous 3-morpholinopropane-1-sulfonic acid buffer, pH 7.4, and mixed them to obtain solutions that were 0, 25, 50, and 75% EuII(aq) with respect to the total concentration of Eu in any oxidation state. We measured the T1 relaxation times (influenced by EuII but not EuIII) and the 19F signals (influenced by EuIII but not EuII) of the solutions. The 19F signals were compared to those of an external standard of trifluoroacetic acid to calculate the number of 19F nuclei.
Using the measured T1 times and 19F signals for the series of mixtures of oxidation states, we were able to derive a ratiometric ratio. The observed relaxation rate of the solution (1/T1,obs) is related to the paramagnetic (1/T1,p) and diamagnetic (1/T1,d) relaxation rates (Eq. 1), and rearrangement of Eq. 1 yields Eq. 2. The diamagnetic relaxation rate is a property of the solvent, but in dilute EuII-containing solutions, the paramagnetic term 1/T1,p is proportional to the concentration of EuII (Eq. 3). Combining Eqs. 2 and 3 yields Eq. 4.
| [1] |
| [2] |
| [3] |
| [4] |
Fluorine signal is generated due to the presence of the EuIII-containing complex in solution, and this signal is proportional to the number of moles of 19F nuclei (n19F) in the sample, and hence, to the concentration of the EuIII-containing complex (Eq. 5).
| [5] |
The sum of the percentage of EuII and EuIII is 1 because those are the only two sources of Eu (Eq. 6). Rearrangement of Eq. 6 results in Eq. 7, which when combined with Eq. 5 yields Eq. 8.
| [6] |
| [7] |
| [8] |
Both 1/T1,p and 19F measurements are dependent on the concentration of the probes; however, by taking the ratio of the two measurements (Eq. 3 divided by Eq. 8), the concentration of the complex becomes inconsequential (Eq. 9). We defined the proportionality constant, , as the hypoxia index (units of mol–1 s–1) to equate the two sides of the proportionality in Eq. 9 to yield Eq. 10. Combination of Eq. 2 with Eq. 10 yields Eq. 11 in which T1,obs, T1,d, and 19F signals are obtained using an MRI scanner.
| [9] |
| [10] |
| [11] |
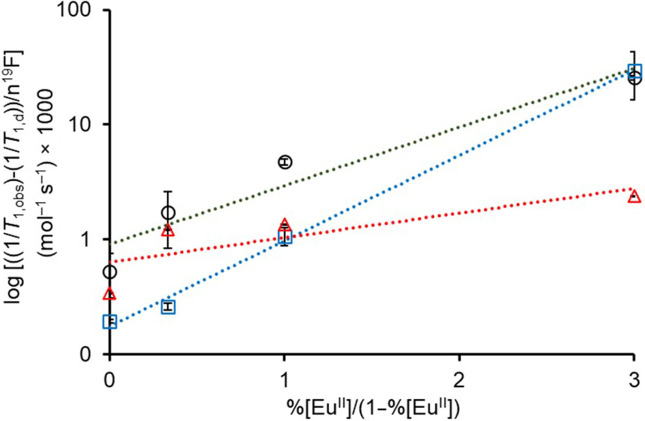
To test Eq. 11 with our data, we plotted the logarithm of versus for each system that contains mixtures of 1EuII, 1EuIII, 2EuII, 2EuIII, 3EuII, and 3EuIII(Fig. 2). All the three plots can be fit with straight lines with hypoxia indices (slopes) of 0.59 ± 0.06, 0.43 ± 0.06, and 0.17 ± 0.06 mol–1 s–1 for the systems containing 1EuII/1EuIII, 2EuII/2EuIII, and 3EuII/3EuIII, respectively. Based on the hypoxia indices, 1EuII/1EuIII possesses the steepest slope with 2EuII/2EuIII and 3EuII/3EuIII having slopes that are 27 and 71%, respectively, less steep. This result can be explained by the denominator of the left side of Eq. 11, which is the number of moles of 19F atoms in each system. A plot of the number of 19F atoms (4, 12, or 24) per molecule versus the hypoxia indices (0.59, 0.43, and 0.17 mol–1 s–1) yields a linear correlation, demonstrating that the number of 19F atoms is the major driver of the slope. This dominance of 19F is not surprising given the similar relaxivities of 1EuII, 2EuII, and 3EuII. Steeper slopes are desirable because they enable more sensitive differentiation between regions with different concentrations of oxygen. Although our systems are concentration independent with respect to the amount of Eu, that only holds true for samples that are detectable. Further, the equation is limited to cases where some EuII is oxidized to EuIII to avoid needing a zero in the denominator; in cases where EuII is not measurably oxidized, as determined by the presence of 19F signal, then the area is extremely hypoxic. Despite 1EuII/1EuIII being the most sensitive with respect to slope of Eq. 11, with only four fluorine atoms, the complex is difficult to detect at low millimolar concentrations. Consequently, for the rest of the studies in this report, we used 2EuII/2EuIII which has much better aqueous solubility than that of 3EuII/3EuIII. The known hypoxia indices, T1,obs, T1,d, and 19F readouts from the scanner, can be used to determine %EuII in a given system as a measure of hypoxia.
Fig. 2.
Plots used to determine hypoxia indices (slopes) using T1 and 19F MR data from mixtures of XEuII and XEuIII [X = 1 (blue, □), 2 (black, ○), and 3 (red, Δ) in aqueous 3-morpholinopropane-1-sulfonic acid buffer (pH 7.4)].
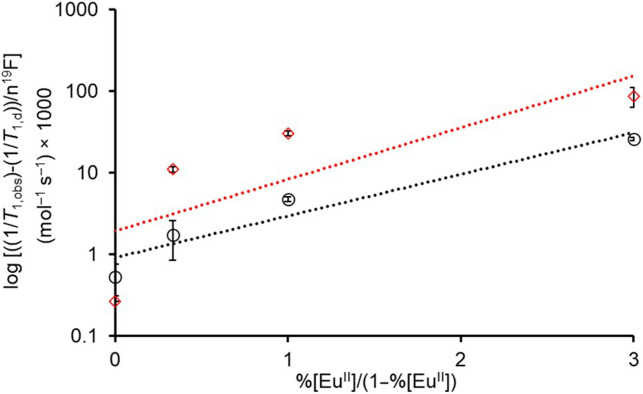
To validate the concentration-independent nature of the hypoxia indices, the experiment was repeated at a concentration of 6 mM of Eu instead of 1 mM using the 2EuII/2EuIIIsystem. The slope acquired at 6 mM was 0.49 ± 0.09 mol–1 s–1 (Fig. 3), which is not different from the slope measured at 1 mM (Student’s t test results are included in the supporting information). These data demonstrate that the hypoxia index is a useful tool for measuring hypoxia in the absence of information about the concentration of Eu.
Fig. 3.
Validation of the concentration-independent nature of the hypoxia indices using 1 and 6 mM solutions of 2EuII/2EuIII system. Based on a t test, there is no significant difference between hypoxia indices of 1 mM (black, ○) solution (0.43 mol–1 s–1, R2 = 0.9917) and 6 mM (red, ◊) solution (0.49 mol–1 s–1, R2 = 0.9183). The solutions were prepared in aqueous 3-morpholinopropane-1-sulfonic acid buffer (pH 7.4).
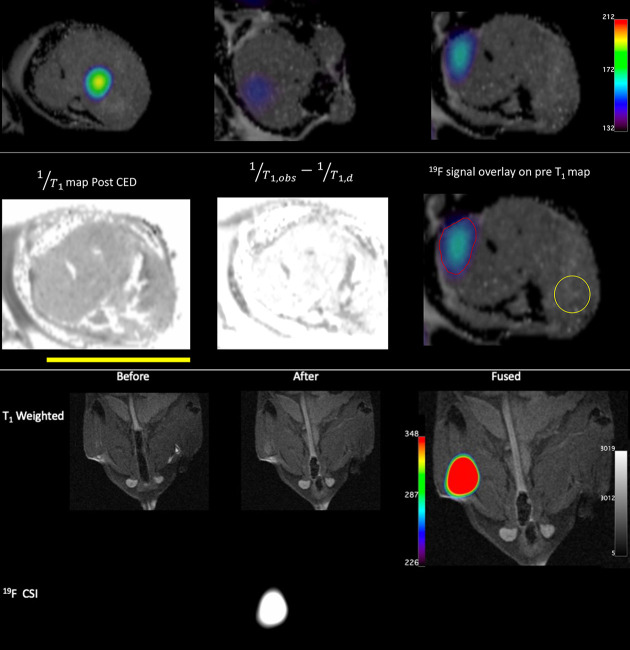
Inspired by our in vitro results, we wanted to learn the potential for application of our method in vivo. We chose 2EuII for in vivo experiments because it has the best combination of 19F signal, hypoxia index slope, and solubility among 1EuII, 2EuII, and 3EuII. We performed intratumoral injections of 2EuII (~150 µL, 6 mM in 3-morpholinopropane-1-sulfonic acid buffer, pH 7.4) to osteosarcoma in immunocompetent wild-type C57BL/6 mice using convection-enhanced delivery. Osteosarcoma was chosen because as a highly aggressive solid tumor, it develops areas of hypoxia secondary to outgrowing its blood supply. Further, there are strong links between hypoxia and chemotherapeutic resistance, promoting metastatic potential and alterations in immune microenvironment for osteosarcoma (43, 44). These hypoxia-mediated features are key facets that drive poor outcomes for patients and, thus, the immunocompetent osteosarcoma model provides an extremely valuable system to image and understand the role of hypoxia in tumor development progression (45). We measured T1,obs and 19F signals. T1-weighted MRI (Fig. 4) of the tumor showed the hypoxic region of the tumor. 19F chemical shift imaging (CSI) map (Fig. 4) showed the normoxic region of the tumor, where 2EuII is oxidized into 2EuIII. Overlaying of the T1-weighted image with the 19F CSI provided a complete picture of the tumor (Fig. 4). As a control for normoxic tissue, 2EuII (100 µL, 6 mM) was injected into the thigh muscle of a healthy mouse, and 19F signal was observed as expected (Fig. 4). Compared to limitations of biopsy, including invasiveness and ambiguous diagnosis of tumors, the method described here provides a platform for the quantification of relative levels of hypoxia in tumor microenvironments.
Fig. 4.
In vivo imaging experiments of 2EuII. (Top Row) Overlay of T1-weighted 1H-MRI and 19F-MR images of osteosarcoma after convection-enhanced delivery of 2EuII. The three images are from three slices across the tumor and show pockets of 19F signal at different locations across the tumor. (Middle Row) Images of the rightmost slice from the Top row. The leftmost image is a 1/T1 map after convection-enhanced delivery of 2EuII. The middle image is a difference map of T1,obs–1 – T1,d–1 used to calculate the percent of EuII from Eq. 11. The rightmost image is the overlay from the top image with two areas of interest denoted by red and yellow boundaries. 19F atoms are observable inside the red boundary, and there is no detectable 19F in the yellow circle. The combination of 19F content with a hypoxia index of 0.43 mol–1 s–1 corresponds essentially to all Eu in the +2 oxidation state in the yellow circle and all Eu in the +3 oxidation state in the red circle. (Bottom) T1-weighted MRI and 19F CSI of thigh muscle of a healthy mouse injected with 2EuII as a control for normoxic tissue. The yellow bar is 1 cm.
In conclusion, we report a method that enables quantify relative levels of hypoxia in tumor-bearing mice using fluorinated Eu-containing complexes without knowledge of the concentration of the contrast agent. We present hypoxia index curves for systems containing 1EuII/1EuIII, 2EuII/2EuIII, and 3EuII/3EuIII that serve as calibration plots to quantify hypoxia in vivo. Our in vivo studies demonstrated that combined T1-weighted MRI signal and 19F signal can be used to characterize the extent of hypoxia in tumors by overcoming the concentration dependence of contrast agent. Coupled with studies involving the partial pressure of oxygen, which we are pursuing, we expect that these studies will provide the foundation for measuring hypoxia in a wide range of disease models that involve hypoxia, including studies involving tumor growth over time and models of ischemia. Further, the combination of these results with strategies that enable systemic delivery (21) would provide a path beyond studies requiring intratumoral injections.
Materials and Methods
Commercially available chemicals were of reagent-grade purity or better and were used without further purification unless otherwise noted. DOWEX-Na+ was prepared as previously reported (23). Water was purified using a PURELAB Ultra Mk2 water purification system. Samples containing europium were prepared in a wet (water allowed but no O2) glovebox under an atmosphere of N2.
1H-, 13C-, and 19F-NMR spectra were acquired using an Agilent A-400 (399.72 MHz for 1H, 100.49 MHz for 13C, and 376 MHz for 19F) spectrometer or a Bruker BioSpec 9.4 T horizontal bore MRI scanner. Chemical shifts are reported relative to residual solvent signals or internal standard [CH3OD: 1H δ 3.31, 13C δ 49.01, 19F δ –78.20 (NaOTf internal standard in CH3OD); CDCl3: 1H δ 7.27, 13C δ 77.23, 19F δ 0.00 (CFCl3 internal standard in CDCl3); D2O: 1H δ 4.79, 19F: δ –78.20 (NaOTf internal standard in D2O)]. NMR data are assumed to be first order and the multiplicity is reported as “s” = singlet, “d” = doublet, “q” = quartet, and “brs” = broad singlet. Italicized elements are those that are responsible for the shifts. Correlation spectroscopy, distortionless enhancement by polarization transfer, and heteronuclear multiple quantum coherence spectra were used to assign the spectral peaks.
Elemental analyses (C, H, and N) were performed by Midwest Microlab (Indianapolis). Thermal gravimetric analysis (TGA) was performed at 10 °C/min under flowing nitrogen using an SDT-2960 TGA and differential thermal analysis analyzer. Concentrations of Eu were determined using energy-dispersive X-ray fluorescence (EDXF) spectroscopy at the Lumigen Instrument Center in the Department of Chemistry at Wayne State University. All dilutions were performed with 2% HNO3, which was also used for blank samples during calibration. Calibration curves were created using the 153Eu isotope ion count for 1 to 200 ppb concentration range (diluted from Fluka ICP standard solution). High-resolution electrospray ionization mass spectrometry (HRMS) was recorded using a waters LCT Premier Xe time-of-flight high-resolution mass spectrometer.
UV–visible absorbance spectra were measured using a Shimadzu UVmini-1240 spectrophotometer, and samples were loaded in quartz cuvettes under an atmosphere of N2. Emission and excitation spectra were recorded using a HORIBA Jobin Yvon Fluoromax-4 spectrofluorometer. MRI studies were performed at Baylor College of Medicine. T1-weighted and 19F scans were performed with a Bruker BioSpec 9.4 T horizontal bore MRI scanner equipped with 20 cm bore. Images were acquired with a body coil while using a heater set to 37 °C.
Synthetic Procedures and Characterization.
EuII- and EuIII-containing complexes of 2,2,2,2-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetrakis(N-(4-(trifluoromethyl)benzyl)acetamide) (2EuII and 2EuIII, respectively) and EuIII-containing complex of 2,2,2,2-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetrakis(N-(4-fluorobenzyl)acetamide) (1EuIII) were synthesized following reported procedures (22, 39). Fluorinated tetraamide ligands and their corresponding EuII- and EuIII-containing complexes were synthesized as shown in SI Appendix, Fig. S1. Characterization is reported in this manuscript for ligands and metal complexes that has not been reported elsewhere.
2-chloro-N-(3,5-bis(trifluoromethyl)benzyl)acetamide (6).
To a stirring solution of (3,5-bis(trifluoromethyl)phenyl)methanamine (5.2500 g, 21.592 mmol) and K2CO3 (3.133 g, 22.672 mmol) in CH2Cl2 (100 mL) at 0 °C, a solution of chloroacetyl chloride (2.60 mL, 23.32 mmol) in CH2Cl2 (20 mL) was added dropwise. The resultant reaction mixture was stirred for 30 min at 0 °C followed by 30 min at ambient temperature. The mixture was filtered through a fine glass frit, and the solid was washed with CH2Cl2 (5 × 5 mL). The organic layer was washed with HCl (2 M, aqueous, 2 × 10 mL) followed by brine (3 × 10 mL), and the organic layer was dried over sodium sulfate. The solvent was removed under reduced pressure to yield a tan solid that was crystallized from CH2Cl2 to yield a white solid (2.967 g, 43%). 1H NMR (399.72 MHz, CDCl3): δ = 4.13 (s, 2H; ClCH2), 4.62 (d, J = 4.0 Hz, 2H; NCH2), 7.17 (brs, 1H; NH), 7.75 (s, 2H; CH), 7.81 (s, 1H; CH) ppm; 13C NMR (100.49 MHz, CDCl3): δ = 42.7 (ClCH2), 43.1 (NCH2), 121.9 (CH), 123.3 (q, 1J = 272 Hz, CF3), 127.8 (CH), 132.3 (q, 2J = 33 Hz, CCF3), 140.1, 166.7 ppm; 19F NMR (376 MHz, CDCl3): δ = –63.4 (s, CF3) ppm; HRMS (m/z): [M + H]+ calcd for C11H8ClF6NO, 320.0277; found, 320.0274.
2,2′,2″,2‴-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetrakis(N-(4-(trifluoromethyl)benzyl)acetamide) (3).
A stirring mixture of 6 (1.0000 g, 3.1286 mmol), K2CO3 (3.4539 g, 25.0288 mmol), cyclen (0.2520 g, 1.463 mmol), potassium iodide (0.010 mg, 0.060 mmol), and acetonitrile (150 mL) was heated to 120 °C. After 10 h, the mixture was filtered while hot through a fine glass frit, and the filtrate was concentrated under reduced pressure to yield a pale-yellow solid that was crystallized from acetonitrile to yield a white solid (2.082 g, 51%). 1H NMR (399.72 MHz, CH3OD): δ = 2.72 (brs, 20H*, CH2), 4.50 (s, 8H; NCH2CO), 4.80 (s, 8H; CH2), 7.80 (s, 4H, CH), 7.86 (s, 8H, CH) ppm, *peak overlaps with residual methanol peak; 13C NMR (100.49 MHz, CH3OD): δ = 43.5 (CH2), 52.3 (CH2), 59.5 (CH2), 122.2 (CH), 125.2 (q, 1J = 271 Hz, CF3), 129.5 (CH), 133.3 (q, 2J = 33 Hz, CCF3), 144.3, 173.8 ppm; 19F NMR (376 MHz, CH3OD): δ = –62.5 (s, CF3) ppm. HRMS (m/z): [M + H]+ calcd for C52H48O4N8F24, 1305.3494; found, 1305.3444.
Europium(III) 2,2′,2″,2‴-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetrakis-(N-(4- (trifluoromethyl)benzyl)acetamide) trichloride (3EuIII).
To a flask containing 2,2′,2″,2‴-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetrakis(N-(3,5-bis(trifluoromethyl)benzyl)acetamide) (0.5000 g, 0.3833 mmol) and EuCl3·6H2O (0.1275 g, 0.3484 mmol), CH3OH (4 mL) and CH3CN (125 mL) were added. The resulting mixture was heated at 80 °C. After 24 h, the mixture was filtered while hot through a fine glass frit, and the solvent was evaporated under reduced pressure. The crude product was crystallized from acetonitrile to yield a white solid (230 mg, 45%). 1H NMR (399.72 MHz, D2O): δ = –12.27 (brs), –9.86 (brs), –8.79 (brs), –4.61 (brs), –3.46 (brs), 2.03 (brs), 3.11 (brs), 3.76 (brs), 7.01 (brs), 7.17 (brs), 21.58 (brs) ppm. 19F NMR (376 MHz, D2O): δ = –62.54 (s, CF3) ppm. HRMS (m/z): [M – 3Cl]3+ calcd for C52H48EuF24N8O4Cl3, 485.7543; found, 485.7537 with expected isotope pattern. Elemental analysis (calculated, found for C52H48F24N8O4EuCl3·6H2O): C (37.37, 37.02), H (3.62, 2.96), N (6.70, 6.66); TGA (SI Appendix, Fig. S3) shows a mass loss of 6.4%, corresponding to six molecules of H2O, which is consistent with the elemental analysis data.
Europium(II) 2,2′,2″,2‴-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetrakis-(N-4-(trifluoromethyl)benzyl)acetamide) dichloride (3EuII).
Under an atmosphere of N2, a solution of 3EuIII (131.15 mg, 0.09 mmol) was prepared in water (10 mL, degassed), and the pH of the resulting solution was adjusted to 6.5 using HCl (1 M, degassed, aqueous). To the resulting solution, Zn dust (150 mg, 2.3 mmol) was added, and the mixture was stirred vigorously for 30 min. The mixture was filtered through a 0.2-µm filter, and the filtrate was swirled with DOWEX-Na+ (0.1 g) for 1 min and filtered with a 0.2-µm filter. The DOWEX step was repeated a total of three times to yield a pale-yellow solution of 3EuII (quantitative). Solutions of 3EuII were characterized with luminescence spectroscopy to confirm loss of EuIII (SI Appendix, Fig. S2), and the concentration of 3EuII was determined with EDXF spectroscopy.
Crystallographic Data.
A solution of either 1EuIIICl3 or 3EuIIICl3 (20 mg) in acetonitrile (15 drops) was filtered through a 0.2-µm hydrophilic filter into a 5-mm NMR tube. After evaporation, colorless crystals of 1EuIIICl3 and yellow color crystals of 3EuIIICl3 formed. A solution of 1EuIICl2 in methanol was filtered through a 0.2-µm hydrophilic filter into a 5-mm NMR tube. Yellow-colored crystals formed in a desiccator placed in a wet glove box under N2 atmosphere.
1EuIIICl3 and 1EuIICl2 were mounted on a MicroMount (MiTeGen) with paratone oil (Parabar 10312, Hampton Research) on a Bruker X8 APEX-II diffractometer with Mo radiation and a graphite monochromator. The X-ray diffraction intensities were measured using a Bruker APEX-II CCD detector. 3EuIIICl3 was mounted on a MicroMount (MiTeGen) with paratone oil (Parabar 10312, Hampton Research) on a Bruker D8 Venture diffractometer with kappa geometry, an Incoatec IµS microfocus source X-ray tube (Mo Kα radiation), and a multilayer mirror for monochromatization. All data were acquired with an Oxford 800 Cryostream low-temperature apparatus. The intensities were integrated using SAINT, and a multiscan absorption correction was applied with SADABS using Apex 3. The structures were solved by Intrinsic Phasing using ShelXT (46) and refined with ShelXL (47) using Olex2 (48).
All nonhydrogen atoms were refined anisotropically. The hydrogen atoms were positioned with idealized geometry and refined isotropically using a riding model. 1EuIIICl3 was modeled with whole molecule disorder over 4 sites (0.247:0.240:0.250:262). 1EuIICl2 was a superstructure and was a pseudo-merohedral twin, as determined by Platon’s TwinRotMat with a twin law of (1 0 0, 0 −1 0, 1 0 −1), and was refined with a batch scale factor of 0.742(5). 3EuIIICl3 had a disordered acetonitrile that could not be modeled, so a solvent mask in Olex2 was used and half of an acetonitrile molecule per formula unit was removed. The –CF3 groups were disordered over 2 or 3 sites and the crystal was treated as an inversion twin with a 0.039(12) batch scale factor.
Convection-enhanced delivery of 1EuII.
For convection-enhanced delivery, we used a Harvard Apparatus PHD 2000 infusion system to infuse 120 µL at a rate of 1.5 µL/min. Infusion catheters were placed such that the tip of the catheter was in the center of the tumor.
Hypoxia MRI.
For assessment of hypoxia after convection-enhanced delivery, we generated T1 maps of tumors. We also measured the 19F signal using CSI. We conducted these measurements 1-h post convection-enhanced delivery. T1 measurements. Standard rapid acquisition with relaxation pulse sequences were used, and T1 maps were acquired and processed using Paravision 360 software. 1/T1 maps and 1/T1 difference maps were calculated using Image J.19F imaging (CSI). 19F-magnetic resonance spectroscopic imaging was performed using a fast spin echo imaging sequence with the following imaging parameters: field of view = 32 mm × 32 mm; image size = 16 × 16 voxels, slice thickness = 15 mm; repetition time = 250 ms; echo time = 0.647 ms; number of averages = 8; 1,024 free induction decay data points, spectral width = 25 kHz. 19F overlays and signal intensities were generated and measured using Osirix.
Validation of imaging with pimonidazole staining (hypoxyprobe).
Pimonidazole staining was performed using the Hypoxyprobe kit (HP1-100Kit) per manufacturer’s protocol. After MRI, tumors were extracted and sliced to align with MR images. To validate the hypoxia imaging, we performed pimonidazole staining for in-situ detection of hypoxic regions within the tumors. Specifically, pimonidazole hydrochloride (60 mg/kg per manufacturer’s recommendation) was injected via tail vein 60 to 90 min prior to killing the mouse. Subsequently, immunohistochemistry staining using Mab1 clone 4.3.11.3 was used to stain and confirm areas of hypoxia with MRI data (SI Appendix, Fig. S4).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We acknowledge the NIH (R01EB026453) for financial support. The Lumigen Instrument Center X-ray crystallography lab is partially supported by the NIH (3R01EB027103-02S1), and NMR lab is partially supported by the NIH (S10OD028488). A.G.S. was supported by the NIH (T32GM142519). We thank the Small Animal Imaging facility at Texas Children’s Hospital as well as the Baylor College of Medicine Small Animal MRI ATC Core for access to imaging equipment and image-processing resources.
Author contributions
S.A.A.S.S., J.T.Y., R.G.P., and M.J.A. designed research; S.A.A.S.S., C.J.O., J.R., C.L.W., A.G.S., L.K., and R.G.P. performed research; S.A.A.S.S., J.T.Y., R.G.P., and M.J.A. analyzed data; and S.A.A.S.S., C.J.O., J.R., C.L.W., A.G.S., L.K., J.T.Y., R.G.P., and M.J.A. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Jason T. Yustein, Email: jason.yustein@emory.edu.
Robia G. Pautler, Email: rpautler@bcm.edu.
Matthew J. Allen, Email: mallen@chem.wayne.edu.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Liu J.-N., Bu W., Shi J., Chemical design and synthesis of functionalized probes for imaging and treating tumor hypoxia. Chem. Rev. 117, 6160–6224 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Eales K. L., Hollinshead K. E. R., Tennant D. A., Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 5, e190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nabavizadeh S. A., et al. , Arterial spin labeling and dynamic susceptibility contrast-enhanced MR imaging for evaluation of arteriovenous shunting and tumor hypoxia in glioblastoma. Sci. Rep. 9, 1–8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor C. T., Scholz C. C., The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 18, 573–587 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paredes F., Williams H. C., Martin A. S., Metabolic adaptation in hypoxia and cancer. Cancer Lett. 502, 133–142 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng Z., et al. , Hypoxic exosomal HIF-1α-stabilizing circZNF91 promotes chemoresistance of normoxic pancreatic cancer cells via enhancing glycolysis. Oncogene 40, 5505–5517 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Bristow R. G., Hill R. P., Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 8, 180–192 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Bhandari V., et al. , Molecular landmarks of tumor hypoxia across cancer types. Nat. Genet. 51, 308–318 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Subasinghe S. A. A. S., Pautler R. G., Samee M. A. H., Yustein J. T., Allen M. J., Dual-mode tumor imaging using probes that are responsive to hypoxia-induced pathological conditions. Biosensors 12, 478 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu L., et al. , Tumor microenvironment responsive “head-to-foot” self-assembly nanoplatform for positron emission tomography imaging in living subjects. ACS Nano 15, 18250–18259 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Liu J., et al. , Tumor microenvironment modulation platform based on composite biodegradable bismuth–manganese radiosensitizer for inhibiting radioresistant hypoxic tumors. Small 17, 2101015 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Yang X., et al. , An oxygen-enriched thermosensitive hydrogel for the relief of a hypoxic tumor microenvironment and enhancement of radiotherapy. Biomater. Sci. 9, 7471–7482 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y., et al. , Rational construction of a reversible arylazo-based NIR probe for cycling hypoxia imaging in vivo. Nat. Commun. 12, 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahsner J., Gale E. M., Rodríguez-Rodríguez A., Caravan P., Chemistry of MRI contrast agents: Current challenges and new frontiers. Chem. Rev. 119, 957–1057 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barandov A., et al. , Sensing intracellular calcium ions using a manganese-based MRI contrast agent. Nat. Commun. 10, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierre V. C., Harris S. M., Pailloux S. L., Comparing strategies in the design of responsive contrast agents for magnetic resonance imaging: A case study with copper and zinc. Acc. Chem. Res. 51, 342–351 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Xie D., Yu M., Kadakia R. T., Que E. L., 19F Magnetic resonance activity-based sensing using paramagnetic metals. Acc. Chem. Res. 53, 2–10 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Botár R., et al. , Stable and inert Mn(II)-based and pH-responsive contrast agents. J. Am. Chem. Soc. 142, 1662–1666 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Guo S., et al. , Reductive microenvironment responsive gadolinium-based polymers as potential safe MRI contrast agents. Biomater. Sci. 7, 1919–1932 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Wang H., et al. , Ultrasensitive magnetic resonance imaging of systemic reactive oxygen species in vivo for early diagnosis of sepsis using activatable nanoprobes. Chem. Sci. 10, 3770–3778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rashid M. M., et al. , Systemic delivery of divalent europium from ligand screening with implications to direct imaging of hypoxia. J. Am. Chem. Soc. 144, 23053–23060 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basal L. A., et al. , Fluorinated EuII-based multimodal contrast agent for temperature- and redox-responsive magnetic resonance imaging. Chem. Sci. 8, 8345–8350 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekanger L. A., et al. , Spectroscopic characterization of the +3 and +2 oxidation states of europium in a macrocyclic tetraglycinate complex. Inorg. Chem. 55, 9981–9988 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekanger L. A., Polin L. A., Shen Y., Haacke E. M., Allen M. J., Evaluation of Eu(II)-based positive contrast enhancement after intravenous, intraperitoneal, and subcutaneous injections. Contrast Media Mol. Imaging 11, 299–303 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekanger L. A., et al. , A Eu(II)-containing cryptate as a redox sensor in magnetic resonance imaging of living tissue. Angew. Chem. Int. Ed. 54, 14398–14401 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenora C. U., Structural features of Europium(II)-containing cryptates that influence relaxivity. Chem. Eur. J. 23, 15404–15414 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekanger L. A., Allen M. J., Overcoming the concentration-dependence of responsive probes for magnetic resonance imaging. Metallomics 7, 405–421 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts T. P. L., Physiologic measurements by contrast-enhanced MR imaging: Expectations and limitations. J. Magn. Res. Imaging 7, 82–90 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Raghunand N., Howison C., Sherry A. D., Zhang S., Gillies R. J., Renal and systemic pH imaging by contrast-enhanced MRI. Magn. Reason. Med. 49, 249–257 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Gianolio E., et al. , Dual MRI-SPECT agent for pH-mapping. Chem. Commun. 47, 1539–1541 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Frullano L., Catana C., Benner T., Sherry A. D., Caravan P., Bimodal MR–PET agent for quantitative pH imaging. Angew. Chem. Int. Ed. 49, 2382–2384 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gianolio E., Napolitano R., Fedeli F., Arena F., Aime S., Poly-β-cyclodextrin based platform for pH mapping via a ratiometric 19F/1H MRI method. Chem. Commun. 40, 6044–6046 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Ekanger L. A., Ali M. M., Allen M. J., Oxidation-responsive Eu2+/3+-liposomal contrast agent for dual-mode magnetic resonance imaging. Chem. Commun. 50, 14835–14838 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorarinsdottir A. E., Du K., Collins J. H., Harris T. D., Ratiometric pH imaging with a CoII2 MRI probe via CEST effects of opposing pH dependences. J. Am. Chem. Soc. 139, 15836–15847 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Janasik D., et al. , Ratiometric pH-responsive 19F magnetic resonance imaging contrast agents based on hydrazone switches. Anal. Chem. 94, 3427–3431 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genicio N., Bañobre-López M., Gröhn O., Gallo J., Ratiometric magnetic resonance imaging: Contrast agent design towards better specificity and quantification. Coord. Chem. Rev. 447, 214150 (2021). [Google Scholar]
- 37.Toljić Đ, Angelovski G., A low-molecular-weight ditopic MRI probe for ratiometric sensing of zwitterionic amino acid neurotransmitters. Chem. Commun. 55, 11924–11927 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Connah L., Angelovski G., Synergy of key properties promotes dendrimer conjugates as prospective ratiometric bioresponsive magnetic resonance imaging probes. Biomacromolecules 19, 4668–4676 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Blackburn O. A., et al. , Substituent effects on fluoride binding by lanthanide complexes of DOTA-tetraamides. Dalton Trans. 45, 3070–3077 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Srivastava K., Weitz E. A., Peterson K. L., Marjańska M., Pierre V. C., Fe-and Ln-DOTAm-F12 are effective paramagnetic fluorine contrast agents for MRI in water and blood. Inorg. Chem. 56, 1546–1557 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Thompson A. L., et al. , On the role of the counter-ion in defining water structure and dynamics: Order, structure and dynamics in hydrophilic and hydrophobic gadolinium salt complexes. Dalton Trans. 47, 5605–5616 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Dickins R. S., et al. , Structural rigidity and luminescence of chiral lanthanide tetraamide complexes based on 1,4,7,10-tetraazacyclododecane. Angew. Chem. Int. Ed. 36, 521–523 (1997). [Google Scholar]
- 43.Yang C., et al. , Bone microenvironment and osteosarcoma metastasis. Int. J. Mol. Sci. 21, 6985 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu L., Zhang J., Li Y., Effects of microenvironment in osteosarcoma on chemoresistance and the promise of immunotherapy as an osteosarcoma therapeutic modality. Front. Immunol. 13, 871076 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W., et al. , Hypoxia-immune-related microenvironment prognostic signature for osteosarcoma. Front. Cell Dev. Biol. 10, 974851 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheldrick G. M., SHELXT- Integrated space-group and crystal structure determination. Acta Cryst. 71, 3–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheldrick G. M., Crystal structure refinement with SHELXL. Acta Cryst. 71, 3–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dolomanov O. V., Bourhis L. J., Gildea R. J., Howard J. A. K., Puschmann H., OLEX2: A complete structure solution refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.