Abstract
Copper, an earth-abundant metal, has reemerged as a viable alternative to the versatile Pd-catalyzed C–N coupling. Coupling sterically hindered reaction partners, however, remains challenging. Herein, we disclose the discovery and development of a pyrrole-ol ligand to facilitate the coupling of ortho-substituted aryl iodides with sterically hindered amines. The ligand was discovered through a library screening approach and highlights the value of mining heteroatom-rich pharmaceutical libraries for useful ligand motifs. Further evaluation revealed that this ligand is uniquely effective in these challenging transformations. The reaction enables the coupling of sterically hindered primary and secondary amines, anilines, and amides with broad functional group tolerance.
Keywords: copper, C–N coupling, aniline, ligand mining, sterically hindered partners
Graphical Abstract
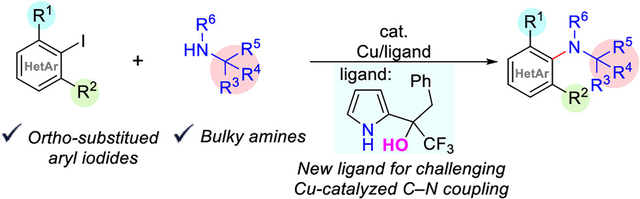
Anilines represent a privileged class of amines in nature, as well as pharmaceuticals, agrochemicals, and other valuable organic materials.1 Consequently, transition metal-catalyzed C–N-bond formation has become critically important to organic synthesis. While a number of d-block metals have been investigated for this reaction, the two most common metals remain palladium (Buchwald–Hartwig amination2) and copper (Ullmann–Goldberg-type3 and Chan−Lam4 amination). Exploration of palladium-catalyzed C–N-coupling reactions started almost 25 years ago concurrently by Buchwald and Hartwig.5 Concentrated effort across a number of groups has positioned the Buchwald–Hartwig reaction as the dominant amination method with broad substrate scope and high selectivities.6 While much progress has been made with the copper-catalyzed variants, the Ullmann-type amination has lagged in terms of both electrophile and amine scope.7 Early advances came independently from Ma,8 Goodbrand,9 Buchwald,10 and others.11,12 Recently, the Ma group introduced oxalamide ligands that enable low catalyst loading and the use of aryl chlorides as electrophiles—addressing key challenges for copper-catalyzed amination.13 Copper-catalyzed C–N-coupling reactions with hindered partners, however, remain an unsolved problem (Figure 1a).12b Currently, there exist only a few examples where an ortho methyl group can be tolerated on the electrophile (Figure 1a).13,14 Furthermore, examples with ortho,ortho′-disubstituted electrophiles with sterically hindered amines have not been reported. Although alternative base metal-catalyzed approaches exist to synthesize sterically encumbered anilines (Figure 1b),15 they rely on fundamentally different building blocks. Here, we describe a novel ligand that enables very challenging and previously unreported direct aminations with sterically hindered coupling partners using copper catalysis (Figure 1c).
Figure 1.

(a) Existing copper-catalyzed C–N-coupling reactions remain limited in sterically demanding environments. (b) Previous approaches for base metal-catalyzed aniline synthesis. (c) A novel pyrrole-ol ligand enables C–N coupling of sterically hindered partners.
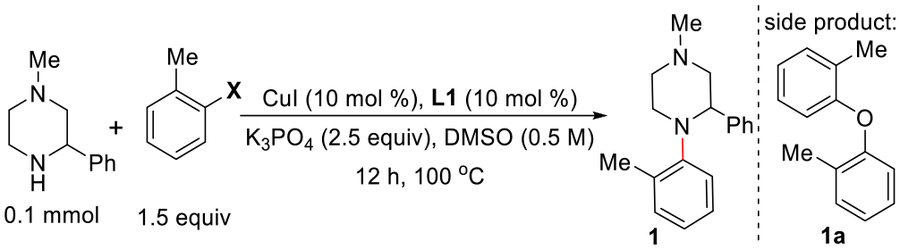
We were interested in identifying a ligand for Cu to couple sterically hindered electrophiles and nucleophiles—a challenge even for Pd-based methods.16 Despite several new ligands for Cu reported in the recent years, coupling of sterically hindered partners remains a formidable challenge. The discovery of a more effective ligand is critical to the success of this transformation. Given the recent success with evaluating pharmaceutical compound libraries as ligands for metal-catalyzed reactions,17 AbbVie’s internal compound library was screened as ligands for copper. The coupling of bromobenzene with 1-methyl-3-phenylpiperazine sought to evaluate 54 nonproprietary compounds that were previously examined for Cu-catalyzed C–O coupling (Scheme 1).17b Interestingly, 2,2,2-trifluoro-1-phenyl-1-(1H-pyrrol-2-yl)ethan-1-ol (L1), a compound that had not been previously applied as a ligand, produced the highest yield of the coupled product (74 HPLC peak area%).14d,18 Surprisingly, the more sterically hindered substrate combination, 2-bromotoluene and 1-methyl-3-phenylpiperazine, coupled to form product only in the presence of L1.
Scheme 1.

Discovery of Trifluoromethylated Pyrole-ol-Based Ligand
aArea% of the coupled products determined by HPLC is reported.
Ligand L1 was chosen for further optimization (Table 1). Formation of an almost equal amount of the C–O coupled product 1a was observed as a side-product in the reaction (entry 1). The addition of molecular sieves to the reaction mixture suppressed the C–O coupling product but failed to improve the yield of the desired product (entry 2). We speculated that base-mediated ligand degradation led to catalyst deactivation through the formation of phenyl(1H-pyrrol-2-yl)methanone L1′.19 Further evaluation of both the target reaction and ligand stability demonstrated rapid, base-mediated decomposition to phenyl(1H-pyrrol-2-yl)methanone L1′, and it functions as an inhibitor for the reaction. Although lowering the reaction temperature suppressed ligand degradation, it also slowed the reaction. In an attempt to increase the lifetime of active catalyst and to block ligand degradation, a variety of mild reductants were evaluated. Interestingly, a stochiometric amount of Hantzsch ester (HE) increased the catalyst lifetime.20 Conversely, ascorbic acid offered no improvement.21 In the presence of one equivalent of Hantzsch ester, the desired product was obtained in 33% yield (entry 3). Next, the more reactive electrophile, 2-iodotoluene, was tested in the reaction. The desired product was obtained in a higher 42% yield along with 50% of 1a (entry 4). As observed earlier, addition of molecular sieves suppressed the ether formation (entry 5). The higher yield of 1 (64%) was observed by conducting the reaction in the presence of 0.5 equiv of HE at 80 °C (entry 6). Fortunately, employing additional base (4 equiv) and amine (1.7 equiv) produced the desired product in 86% yield after 24 h (entry 7).
Table 1.
Initial Reaction Optimization
| |||||
|---|---|---|---|---|---|
| entry | X | additive | 1 (%)a | 1a (%)a | conversion (%)b |
| 1 | Br | - | 17 | 23 | 40 |
| 2 | Br | 100 mg MS (4 Å) | 18 | 1 | 20 |
| 3 | Br | 100 mg MS (4 Å), 0.5 equiv HE | 32 | 2 | 35 |
| 4c | I | - | 42 | 50 | 95 |
| 5c | I | 100 mg MS (4 Å) | 46 | 8 | 59 |
| 6c | I | 100 mg MS (4 Å), 0.5 equiv HE | 64 | 5 | 70 |
| 7 | I | 24 h, 100 mg MS (4 Å), 1 equiv HE 1.7 equiv amine, 4 equiv K3PO3 90 °C |
86 | 7 | 98 |
GC yield.
Based on unreacted aryl halide.
Reaction was conducted at 80 °C.
MS = molecular sieves.
As discussed above, this type of sterically demanding coupling is unprecedented with a Cu catalyst. To better understand the catalyst landscape, we also screened 46 established ligands for Cu in this reaction using a high-throughput approach. To our surprise, none of the established ligands resulted in the formation of 1 in any appreciable yield (Table S1).22 These results suggest that the catalyst derived from L1 and CuI may be unique in its reactivity for sterically demanding C–N couplings.
Next, we evaluated the structure–activity relationships for the pyrrole-ol-based N–O type ligands. Systematically replacing the phenyl ring of L1 resulted in significant variations in yield (L2–L8, Table 2). Pentafluorobenzene-containing ligands (L3, L18, L20), for example, showed no reactivity possibly because of decreased ligand stability under the reaction conditions, ejecting C6F5− as a leaving group. The pyrrole-ol ligand bearing a benzyl substituent (L7), which is presumably less prone to elimination, provided a minor improvement in yield. The unprotected pyrrole moiety proved critical to afford a ligand which promote the overall reaction in reasonable yields (L9–L13, Table 2). Finally, evaluation of the R3- substituent (L14–L18, Table 2) revealed that the trifluoromethyl group is crucial for the ligand reactivity—presumably due to its steric and electronic properties. On the basis of these results, L7 was chosen for further reaction optimization, providing 90% of the desired product under the optimal conditions (described in Table 1, entry 7).
Table 2.
Screening of Pyrole-ol-Based Liganda

|
Reaction conditions: iodide 0.1 mmol, amine 1.7 equiv, CuI 10 mol %, ligand 10 mol %, 100 mg 4 Å MS, HE 1 equiv, K3PO4 4 equiv,DMSO 0.2 M, 90 °C, 24 h.
HE = Hantzsch ester
A diverse range of sterically crowded amines with varied N–H pKa values were explored for the coupling with ortho-substituted aryl iodide. Satisfyingly, the protocol was successful for amines with an N–H pKa window of ~20 to ~ 40.23 Next, we explored the substrate scope with regard to various ortho-substituted aryl iodides and 1-methyl-3-phenylpiperazine. Not surprisingly, larger ortho substituents led to lower yields (1–3, Scheme 2). Next, we tested the scope of ortho substituted aryl iodides with the bioactive and sterically demanding 1-phenyl-1,2,3,4-tetrahydroisoquinoline (4–13).24 The catalyst demonstrated excellent reactivity with electron-neutral (4–6) or electron-donating (7) substituents at the ortho position of the aryl iodide. To our delight, a bulky N,N-di-n-butyl-amino group was well tolerated in the reaction (8). Importantly, 2-bromo-iodobenzene promoted the C–N coupling preferentially at the iodide with >20:1 chemoselectivity (9). Surprisingly, aryl iodides with electron-deficient substituents at the ortho position resulted in low-to-moderate yield (10–12) with significant halide-reduction product. Control reactions revealed that this unwanted side product was not due to hydride donation from the Hantzsch ester (10). Excitingly, hindered 2,6-dimethyl-iodobenzene reacted successfully to form 13, a unprecedented reaction for copper catalysis. The ligand even enabled sterically demanding primary amines to couple with ortho-substituted iodides (16–21). As expected, 2,6-dimethyl-substituted aryl iodides provided satisfactory yield of the desired products (18–20, 26). Additionally, this protocol offered excellent reactivity with 2,6-dimethylaniline (23−26, 28). The reactivity with 2,6-diisopropylaniline further established the versatility of this catalyst system (27). Furthermore, this method is also applicable to cyclic amides (29–30), anilide (31), and an amino acid derivative (22). As an investigation into medicinally relevant heterocycles, a series of heteroaryl iodides and amines were tested. Moreover, pyridine, thiophane, and pyrazole moieties were well tolerated by the catalyst (32–37).
Scheme 2.
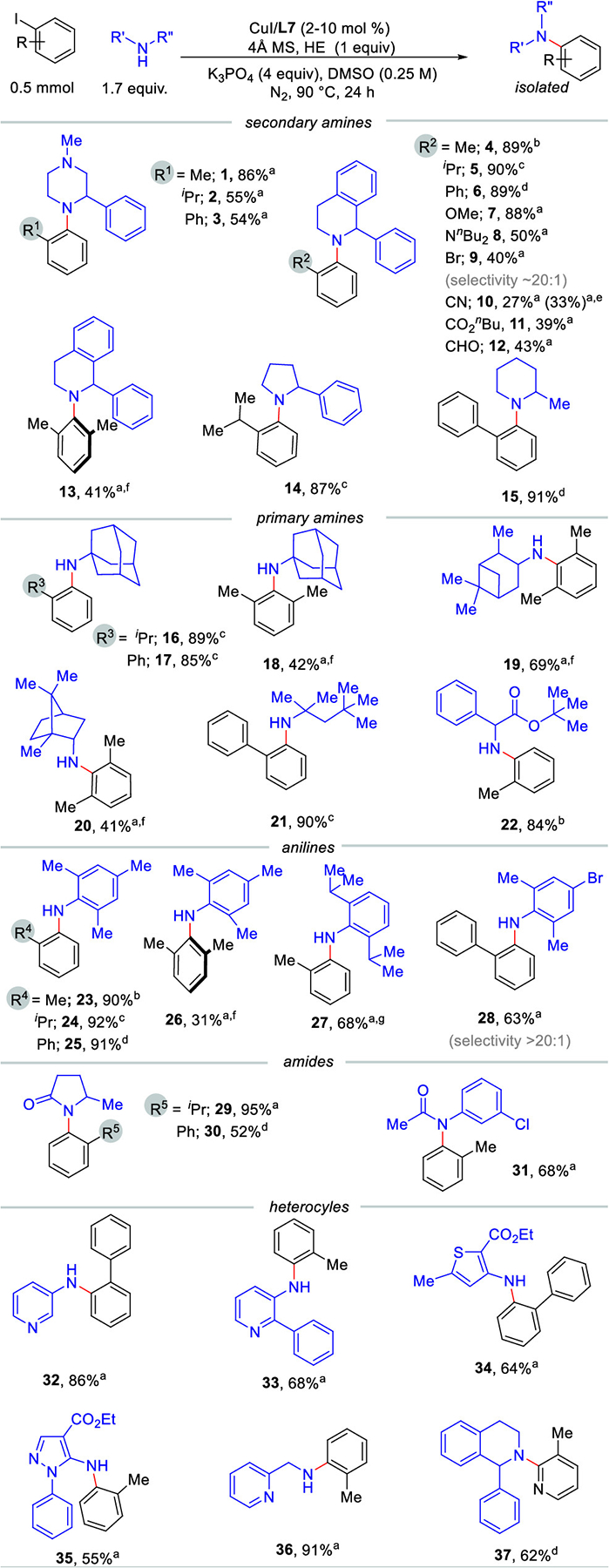
Substrate Scope for C−N Coupling Reaction
a10 mol % Cu/L7; b2 mol % Cu/L7; c5 mol % Cu/L7; d8 mol % Cu/L7; ewithout HE; f48 h; g60 h. HE = Hantzsch ester
The origin for the effectiveness of pyrrole-ol-based ligands for enabling the Cu-catalyzed coupling of sterically hindered coupling partners remains worthy of further mechanistic consideration. We expect L7 to be deprotonated under the reaction conditions and act as either mono- or bis-anionic ligand for Cu.25 Likely, L7 forms a highly electron-rich Cu(I) or anionic Cu(I)-complex that promotes oxidative addition even with sterically hindered electrophiles.26
In summary, we have disclosed a new ligand that enables an unprecedented Cu-catalyzed method for coupling ortho- and ortho,ortho′-substituted aryl iodides with sterically hindered primary aliphatic amines, anilines, and amides. The unique reactivity and novel pyrrole-ol structural motif of ligand L7 was discovered by mining AbbVie’s internal compound library—demonstrating the value of screening pharmaceutical compound libraries for ligand discovery in challenging metal-catalyzed reactions. Complementary to the rational ligand design and optimization, this approach has the potential to identify previously unexplored and nonobvious compounds as ligands. Our future studies will focus on detailed mechanistic studies to elucidate the role of pyrrole-ol ligands in Cu-catalyzed reactions.
Supplementary Material
ACKNOWLEDGMENTS
AbbVie contributed to the design, study conduct, and financial support for this research. AbbVie participated in the interpretation of data, writing, reviewing, and approving the publication. We acknowledge funds from Indiana University in partial support of this work. We also acknowledge the NIH (R01GM121840) for partial support of this work.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acscatal.0c02965
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.0c02965.
Experimental details and spectroscopic data (PDF)
The authors declare the following competing financial interest(s): E.C.S., M.C.H., T.S.F., and S.S. are AbbVie employees and may own AbbVie stocks. A.J.N. and V.S.C. were employees of AbbVie and may own AbbVie stocks. A.J.N. is currently an employee of Dow Chemicals. V.S.C. is currently an employee of Seattle Genetics. A.M. is a postdoctoral associate, and S.P.C. is an Associate Professor at Indiana University and have no conflicts of interest to disclose. The authors wish to acknowledge Dr. Seble Wagaw and Dr. DavidM. Barnes for helpful discussions. S.W. and D.M.B. are AbbVie employees and may own AbbVie stocks.
Contributor Information
Atanu Modak, Department of Chemistry, Indiana University, Bloomington, Indiana 47405-7102, United States.
Alex J. Nett, Process Research and Development, AbbVie Inc., North Chicago, Illinois 60064, United States
Elizabeth C. Swift, Process Research and Development, AbbVie Inc., North Chicago, Illinois 60064, United States.
Michael C. Haibach, Process Research and Development, AbbVie Inc., North Chicago, Illinois 60064, United States
Vincent S. Chan, Process Research and Development, AbbVie Inc., North Chicago, Illinois 60064, United States
Thaddeus S. Franczyk, Process Research and Development, AbbVie Inc., North Chicago, Illinois 60064, United States
Shashank Shekhar, Process Research and Development, AbbVie Inc., North Chicago, Illinois 60064, United States.
Silas P. Cook, Department of Chemistry, Indiana University, Bloomington, Indiana 47405–7102, United States.
REFERENCES
- (1).Kahl T; Schröder K-W; Lawrence FR; Marshall WJ; Höke H; Jäckh R Aniline. Ullmann’s Encyclopedia of Industrial Chemistry 2011, 465–478. [Google Scholar]
- (2).Forero-Cortés PA; Haydl AM The 25th Anniversary of the Buchwald–Hartwig Amination: Development, Applications, and Outlook. Org. Process Res. Dev 2019, 23, 1478–1483. [Google Scholar]
- (3).(a) Ullmann F; Bielecki J Ueber Synthesen in der Biphenylreihe. Ber. Dtsch. Chem. Ges 1901, 34, 2174–2185. [Google Scholar]; (b) Ullmann F; Sponagel P Ueber die Phenylirung von Phenolen. Ber. Dtsch. Chem. Ges 1905, 38, 2211–2212. [Google Scholar]
- (4).(a) Chan DMT; Monaco KL; Wang R-P; Winters MP New N- and O-arylations with phenylboronic acids and cupric acetate. Tetrahedron Lett. 1998, 39, 2933–2936. [Google Scholar]; (b) Lam PYS; Clark CG; Saubern S; Adams J; Winters MP; Chan DMT; Combs A New aryl/heteroaryl C–N bond cross-coupling reactions via arylboronic acid/cupric acetate arylation. Tetrahedron Lett. 1998, 39, 2941–2944. [Google Scholar]
- (5).(a) Louie J; Hartwig JF Palladium-catalyzed synthesis of arylamines from aryl halides. Mechanistic studies lead to coupling in the absence of tin reagents. Tetrahedron Lett. 1995, 36, 3609–3612. [Google Scholar]; (b) Guram AS; Rennels RA; Buchwald SL A Simple Catalytic Method for the Conversion of Aryl Bromides to Arylamines. Angew. Chem., Int. Ed. Engl 1995, 34, 1348–1350. [Google Scholar]
- (6).Dorel R; Grugel CP; Haydl AM The Buchwald–Hartwig Amination After 25 Years. Angew. Chem., Int. Ed 2019, 58, 17118–17129. [DOI] [PubMed] [Google Scholar]
- (7).(a) Shaughnessy KH; Ciganek E; DeVasher RB Copper-Catalyzed Amination of Aryl and Alkenyl Electrophiles. Org. React 2014, 1–668. [Google Scholar]; (b) Evano G; Blanchard N; Toumi M Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis. Chem. Rev 2008, 108, 3054–3131. [DOI] [PubMed] [Google Scholar]
- (8).Ma D; Zhang Y; Yao J; Wu S; Tao F Accelerating Effect Induced by the Structure of α-Amino Acid in the Copper-Catalyzed Coupling Reaction of Aryl Halides with α-Amino Acids. Synthesis of Benzolactam-V8. J. Am. Chem. Soc 1998, 120, 12459–12467. [Google Scholar]
- (9).Goodbrand HB; Hu N-X Ligand-Accelerated Catalysis of the Ullmann Condensation: Application to Hole Conducting Triarylamines. J. Org. Chem 1999, 64, 670–674. [Google Scholar]
- (10).Klapars A; Antilla JC; Huang X; Buchwald SL A General and Efficient Copper Catalyst for the Amidation of Aryl Halides and the N-Arylation of Nitrogen Heterocycles. J. Am. Chem. Soc 2001, 123, 7727–7729. [DOI] [PubMed] [Google Scholar]
- (11).Bernhardson DJ; Widlicka DW; Singer RA Cu-Catalyzed Couplings of Heteroaryl Primary Amines and (Hetero)aryl Bromides with 6-Hydroxypicolinamide Ligands. Org. Process Res. Dev 2019, 23, 1538–1551. [Google Scholar]
- (12).(a) Wang D; Ding K 2-Pyridinyl β-ketones as new ligands for room-temperature CuI-catalysed C−N coupling reactions. Chem. Commun 2009, 1891–1893. [DOI] [PubMed] [Google Scholar]; (b) Bhunia S; Pawar GG; Kumar SV; Jiang Y; Ma D Selected Copper-Based Reactions for C-N, C-O, C-S, and C-C Bond Formation. Angew. Chem., Int. Ed 2017, 56, 16136–16179. [DOI] [PubMed] [Google Scholar]
- (13).(a) Gao J; Bhunia S; Wang K; Gan L; Xia S; Ma D Discovery of N-(Naphthalen-1-yl)-N′-alkyl Oxalamide Ligands Enables Cu-Catalyzed Aryl Amination with High Turnovers. Org. Lett 2017, 19, 2809–2812. [DOI] [PubMed] [Google Scholar]; (b) De S; Yin J; Ma D Copper-Catalyzed Coupling Reaction of (Hetero)Aryl Chlorides and Amides. Org. Lett 2017, 19, 4864–4867. [DOI] [PubMed] [Google Scholar]
- (14).(a) Job GE; Buchwald SL Copper-Catalyzed Arylation of β-Amino Alcohols. Org. Lett 2002, 4, 3703–3706. [DOI] [PubMed] [Google Scholar]; (b) Antilla JC; Klapars A; Buchwald SL The Copper-Catalyzed N-Arylation of Indoles. J. Am. Chem. Soc 2002, 124, 11684–11688. [DOI] [PubMed] [Google Scholar]; (c) Antilla JC; Baskin JM; Barder TE; Buchwald SL Copper-Diamine-Catalyzed N-Arylation of Pyrroles, Pyrazoles, Indazoles, Imidazoles, and Triazoles. J. Org. Chem 2004, 69, 5578–5587. [DOI] [PubMed] [Google Scholar]; (d) Altman RA; Anderson KW; Buchwald SL Pyrrole-2-carboxylic Acid as a Ligand for the Cu-Catalyzed Reactions of Primary Anilines with Aryl Halides. J. Org. Chem 2008, 73, 5167–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Altman RA; Buchwald SL 4,7-Dimethoxy-1,10-phenanthroline: An Excellent Ligand for the Cu-Catalyzed N-Arylation of Imidazoles. Org. Lett 2006, 8, 2779–2782. [DOI] [PubMed] [Google Scholar]
- (15).(a) Rucker RP; Whittaker AM; Dang H; Lalic G Synthesis of Hindered Anilines: Copper-Catalyzed Electrophilic Amination of Aryl Boronic Esters. Angew. Chem., Int. Ed 2012, 51, 3953–3956. [DOI] [PubMed] [Google Scholar]; (b) Gui J; Pan C-M; Jin Y; Qin T; Lo JC; Lee BJ; Spergel SH; Mertzman ME; Pitts WJ; La Cruz TE; Schmidt MA; Darvatkar N; Natarajan SR; Baran PS Practical olefin hydroamination with nitroarenes. Science 2015, 348, 886. [DOI] [PubMed] [Google Scholar]
- (16).(a) Ruiz-Castillo P; Blackmond DG; Buchwald SL Rational Ligand Design for the Arylation of Hindered Primary Amines Guided by Reaction Progress Kinetic Analysis. J. Am. Chem. Soc 2015, 137, 3085–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Park NH; Vinogradova EV; Surry DS; Buchwald SL Design of New Ligands for the Palladium-Catalyzed Arylation of α-Branched Secondary Amines. Angew. Chem., Int. Ed 2015, 54, 8259–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Khadra A; Mayer S; Mitchell D; Rodriguez MJ; Organ MG A General Protocol for the Broad-Spectrum Cross-Coupling of Nonactivated Sterically Hindered 1° and 2° Amines. Organometallics 2017, 36, 3573–3577. [Google Scholar]; (d) Hill LL; Moore LR; Huang R; Craciun R; Vincent AJ; Dixon DA; Chou J; Woltermann CJ; Shaughnessy KH Bulky Alkylphos-phines with Neopentyl Substituents as Ligands in the Amination of Aryl Bromides and Chlorides. J. Org. Chem 2006, 71, 5117–5125. [DOI] [PubMed] [Google Scholar]
- (17).(a) Hansen EC; Pedro DJ; Wotal AC; Gower NJ; Nelson JD; Caron S; Weix DJ New ligands for nickel catalysis from diverse pharmaceutical heterocycle libraries. Nat. Chem 2016, 8, 1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chan VS; Krabbe SW; Li C; Sun L; Liu Y; Nett AJ Identification of an Oxalamide Ligand for Copper-Catalyzed C-O Couplings from a Pharmaceutical Compound Library. ChemCatChem 2019, 11, 5748–5753. [Google Scholar]
- (18).(a) Rao H; Fu H; Jiang Y; Zhao Y Copper-Catalyzed Arylation of Amines Using Diphenyl Pyrrolidine-2-phosphonate as the New Ligand. J. Org. Chem 2005, 70, 8107–8109. [DOI] [PubMed] [Google Scholar]; (b) Zhang H; Cai Q; Ma D Amino Acid Promoted CuI-Catalyzed C-N Bond Formation between Aryl Halides and Amines or N-Containing Heterocycles. J. Org. Chem 2005, 70, 5164–5173. [DOI] [PubMed] [Google Scholar]
-
(19).
Probable path for ligand degradation and catalyst poisonings:

- (20).(a) Andersen J; Madsen U; Björkling F; Liang X Rapid Synthesis of Aryl Azides from Aryl Halides under Mild Conditions. Synlett 2005, 2005, 2209–2213. [Google Scholar]; (b) Jin Z; Xue P; Fu E Application of Hantzsch Dihydropyridine in Copper-Catalyzed [3 + 2] Cycloaddition of Terminal Alkynes with Azides. Synth. Commun 2014, 44, 68–75. [Google Scholar]
- (21).Knöpfel TF; Carreira EM The First Conjugate Addition Reaction of Terminal Alkynes Catalytic in Copper: Conjugate Addition of Alkynes in Water. J. Am. Chem. Soc 2003, 125, 6054–6055. [DOI] [PubMed] [Google Scholar]
- (22). See Supporting Information for details.
- (23).Bordwell FG Equilibrium acidities in dimethyl sulfoxide solution. Acc. Chem. Res 1988, 21, 456–463. [Google Scholar]
- (24).(a) Minor DL; Wyrick SD; Charifson PS; Watts VJ; Nichols DE; Mailman RB Synthesis and Molecular Modeling of 1-Phenyl-1,2,3,4-tetrahydroisoquinolines and Related 5,6,8,9-Tetrahydro-13bH-dibenzo[a, h]quinolizines as D1 Dopamine Antagonists. J. Med. Chem 1994, 37, 4317–4328. [DOI] [PubMed] [Google Scholar]; (b) Bolchi C; Pallavicini M; Fumagalli L; Straniero V; Valoti E One-Pot Racemization Process of 1-Phenyl-1,2,3,4-tetrahydroisoquinoline: A Key Intermediate for the Antimuscarinic Agent Solifenacin. Org. Process Res. Dev 2013, 17, 432–437. [Google Scholar]; (c) Chen K-X; Xie H-Y; Li Z-G; Gao J-R Quantitative structure−activity relationship studies on 1-aryl-tetrahydroisoquinoline analogs as active anti-HIV agents. Bioorg. Med. Chem. Lett 2008, 18, 5381–5386. [DOI] [PubMed] [Google Scholar]
- (25).(a) Royappa AT; Royappa AD; Moral RF; Rheingold AL; Papoular RJ; Blum DM; Duong TQ; Stepherson JR; Vu OD; Chen B; Suchomel MR; Golen JA; André G;Kourkoumelis N; Mercer AD; Pekarek AM; Kelly DC Copper(I) oxalate complexes: Synthesis, structures and surprises. Polyhedron 2016, 119, 563–574. [Google Scholar]; (b) Hsu SCN; Chang Y-L; Chuang W-J; Chen H-Y; Lin IJ; Chiang MY; Kao C-L; Chen H-Y Copper(I) Nitro Complex with an Anionic [HB(3,5-Me2Pz)3]- Ligand: A Synthetic Model for the Copper Nitrite Reductase Active Site. Inorg. Chem 2012, 51, 9297–9308. [DOI] [PubMed] [Google Scholar]; (c) Ahmad K; Khan BA; Akram B; Khan J; Mahmood R; Roy SK Theoretical investigations on copper catalyzed CN cross-coupling reaction between aryl chlorides and amines. Comput. Theor. Chem 2018, 1134, 1–7. [Google Scholar]
- (26).Giri R; Brusoe A; Troshin K; Wang JY; Font M; Hartwig JF Mechanism of the Ullmann Biaryl Ether Synthesis Catalyzed by Complexes of Anionic Ligands: Evidence for the Reaction of Iodoarenes with Ligated Anionic CuI Intermediates. J. Am. Chem. Soc 2018, 140, 793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


