Significance
Here, we define the molecular pathways responsible for regulating the abundance of Matrin 3 (MATR3), an RNA-binding protein with integral ties to amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). We show that the excitatory neurotransmitter glutamate triggers MATR3 inactivation and degradation in vitro and ex vivo. Subsequent experiments detail a signal transduction pathway controlling not just MATR3 abundance but also its ability to recognize RNA. These observations have significant implications for physiological MATR3 regulation and clarify the mechanism by which pathogenic MATR3 mutations compromise such regulation. Together with our prior work cataloging the sensitivity of neurons to changes in MATR3 abundance, these data detail a pathophysiological cascade contributing to neurodegeneration in familial ALS and FTD due to MATR3 mutations.
Keywords: NMDA, RNA binding protein, ALS, FTD
Abstract
RNA-binding protein (RBP) dysfunction is a fundamental hallmark of amyotrophic lateral sclerosis (ALS) and related neuromuscular disorders. Abnormal neuronal excitability is also a conserved feature in ALS patients and disease models, yet little is known about how activity-dependent processes regulate RBP levels and functions. Mutations in the gene encoding the RBP Matrin 3 (MATR3) cause familial disease, and MATR3 pathology has also been observed in sporadic ALS, suggesting a key role for MATR3 in disease pathogenesis. Here, we show that glutamatergic activity drives MATR3 degradation through an NMDA receptor-, Ca2+-, and calpain-dependent mechanism. The most common pathogenic MATR3 mutation renders it resistant to calpain degradation, suggesting a link between activity-dependent MATR3 regulation and disease. We also demonstrate that Ca2+ regulates MATR3 through a nondegradative process involving the binding of Ca2+/calmodulin to MATR3 and inhibition of its RNA-binding ability. These findings indicate that neuronal activity impacts both the abundance and function of MATR3, underscoring the effect of activity on RBPs and providing a foundation for further study of Ca2+-coupled regulation of RBPs implicated in ALS and related neurological diseases.
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease involving the loss of upper and lower motor neurons, resulting in progressive muscle weakness and paralysis. ALS shares clinical, genetic, and pathological overlap with frontotemporal dementia (FTD), a disorder characterized clinically by altered behavior and speech (1), and pathologically by the degeneration of neurons in frontal and temporal cortices. Accumulating evidence points to the dysfunction of RNA-binding proteins (RBPs) in the pathogenesis of both ALS and FTD, with the vast majority of patients with ALS and approximately half of those with FTD displaying aggregation and/or mislocalization of the RBP TAR DNA-binding protein-43 (TDP43) in affected tissues on autopsy (2, 3). Moreover, while most cases of ALS and FTD are sporadic, rare mutations including in genes encoding RBPs can cause inherited ALS/FTD (4–7), confirming their central contribution to disease pathogenesis.
To date, several disease-linked RBPs have been identified, including but not limited to Matrin 3 (MATR3), TDP43, fused in sarcoma (FUS), and heterogenous nuclear ribonucleoprotein A2/B1. Mutations in the genes encoding these proteins are not only associated with ALS and FTD but also primary myopathy (8, 9). MATR3 is a nuclear protein with two zinc finger (ZF) domains and two RNA recognition motifs (RRMs) that has the capacity to bind both DNA and RNA targets (10–12). Over a dozen point mutations in the MATR3 gene have been linked to familial ALS, combined ALS/FTD, or hereditary distal myopathy (13–21). Furthermore, MATR3 pathology—consisting of MATR3 mislocalization, changes in abundance, and aggregation—has been found in motor neurons of sporadic ALS patients (22, 23), suggesting a fundamental link between MATR3 dyshomeostasis and neuromuscular disease.
Abnormal neuronal activity is frequently observed in ALS and FTD patients (24–27) as well as in disease models (28–33). Both hyper- and hypoexcitability phenotypes can be detected depending on the neuronal population in question, experimental technique, model system, and disease stage. While early changes in neuronal activity are consistently noted prior to symptom onset, it remains unclear whether these processes are directly pathogenic, compensatory, or unrelated, underscoring the need for a better understanding of neuronal excitability and its relationship to other pathological processes (34). In this context, investigations of RBPs such as MATR3 and their response to neuronal activity may reveal crucial disease-related mechanisms contributing to neurodegeneration in ALS and FTD. Indeed, several RBPs are regulated in neurons by receptor activation and/or depolarization (35–40), but the pathways responsible for such regulation, and their association with neuronal dysfunction and death, are incompletely understood.
In previous studies, MATR3 was rapidly cleared from cerebellar granule neurons after treatment with N-methyl-D-aspartate (NMDA) in a protein kinase A (PKA)–dependent manner (41). Separate in vitro studies suggested that MATR3 interacts with Ca2+-bound calmodulin (CaM) (42, 43), a central signal transduction factor that rapidly shuttles to the nucleus upon Ca2+ binding, thereby driving the expression of key plasticity-related transcriptional programs (44–48). Even so, whether or how these processes are related to one another, and whether CaM impacts MATR3 function or levels following NMDA receptor (NMDAR) activation, remains unknown.
Here, we show that MATR3 is degraded after glutamatergic stimulation of cortical neurons through an NMDA-, Ca2+-, and calpain-dependent pathway. MATR3 degradation in response to NMDA takes place over the span of hours; within minutes of NMDAR activation, however, MATR3’s ability to bind RNA is blocked via interaction with Ca2+/CaM, suggesting that neuronal activity exquisitely tunes the function of MATR3 and related RBPs through two complementary mechanisms that operate at distinct timescales.
Results
Glutamatergic Stimulation Reduces MATR3 In Vitro and Ex Vivo.
We first sought to confirm the effect of NMDAergic stimulation on MATR3 levels in neurons. Treatment of mature day in vitro (DIV)14-16 cortical neuron cultures with NMDA resulted in a rapid reduction in MATR3 protein abundance (Fig. 1 A and B). In contrast to previous work suggesting the importance of PKA for MATR3 clearance in cerebellar granule neurons (41), application of the PKA inhibitor H89 failed to significantly affect NMDA-induced MATR3 degradation in cortical neurons (SI Appendix, Fig. S1 A and B). Using a PKA substrate antibody, we also noted an accumulation of phosphorylated MATR3 within minutes of NMDA treatment, but these species were unaffected by H89 (SI Appendix, Fig. S1 C and D), suggesting that in cortical neurons, a kinase other than PKA phosphorylates MATR3 at a residue recognized by this antibody. Together, these observations argue against PKA-mediated clearance of MATR3 in cortical neurons, prompting us to examine the mechanism of NMDA-induced MATR3 degradation in more detail.
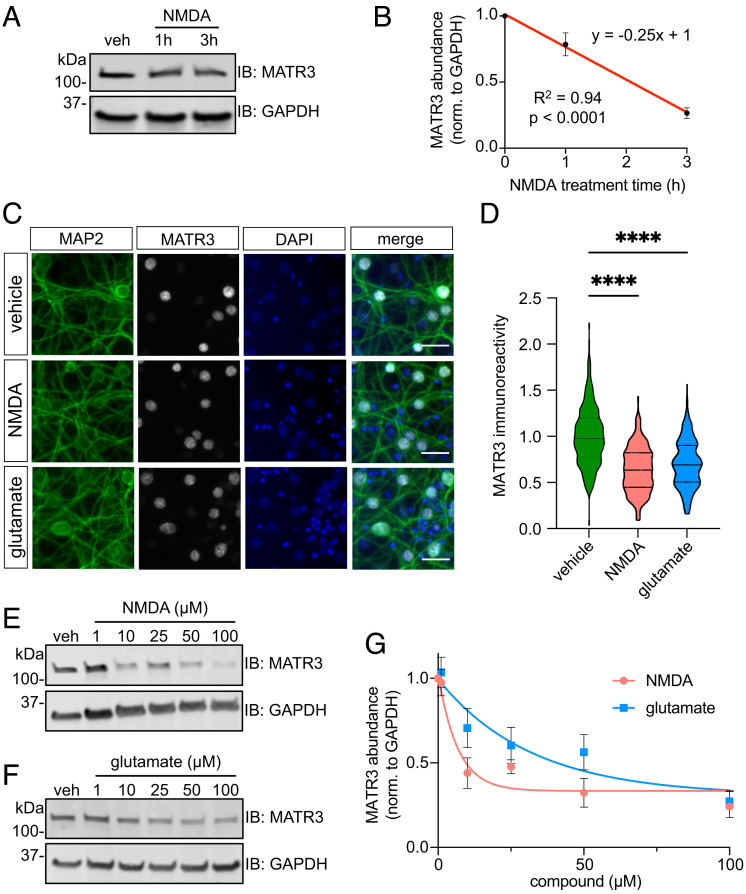
Fig. 1.
Glutamatergic stimulation triggers a rapid MATR3 reduction in primary cortical neurons. (A and B) Application of 100 μM NMDA results in a rapid MATR3 reduction in DIV14-16 cortical neurons. n = 3; significance determined by sum of squares F test. (C and D) MATR3 reduction is recapitulated by immunostaining (vehicle, n = 547; NMDA, n = 419; glutamate, n = 337; ****P < 0.0001; one-way ANOVA with Tukey’s post hoc test). (Scale bars, 20 μm.) (E–G) Both NMDA and glutamate elicit dose-dependent MATR3 clearance (n = 3 per dose; vehicle, y = 0.69−0.15x + 0.33; glutamate, y = 0.69−0.031x + 0.31; nonlinear one phase decay).
Treatment with equivalent doses of NMDA or glutamate for 3 h resulted in comparable reductions of MATR3 immunoreactivity in MAP2-positive neurons without MATR3 aggregation or cytoplasmic mislocalization, indicating that MATR3 clearance occurs primarily in the nuclear compartment (Fig. 1 C and D). Dose–response experiments with NMDA and glutamate confirmed that both compounds were able to effectively decrease MATR3 levels (Fig. 1 E–G).
Previous experiments in wild-type (WT) mice illustrated marked variability in MATR3 protein levels across tissue types and developmental stages, with muscle and spinal cord demonstrating relatively low steady-state MATR3 levels (49). Moreover, MATR3 levels in the CNS are the highest in neonates but begin decreasing in early life. As this decline coincides with the expression of functional NMDARs, we questioned whether NMDAergic maturity may be linked to the observed age-dependent reduction in MATR3 abundance. To test this, we aged primary cortical neurons to DIV6, 8, 10, or 12 and treated each with vehicle or NMDA for 3 h prior to assessment of MATR3 levels by immunoblot (Fig. 2 A and B). We noted a robust reduction in MATR3 levels upon NMDA treatment compared to vehicle control beginning at DIV10, in accordance with previous work demonstrating NMDAergic maturity in rat cortical cultures beginning at this time point (50). Moreover, when comparing vehicle-treated conditions across DIVs, we also observed a decrease in basal MATR3 beginning at the same age (DIV10) (Fig. 2C). These findings suggest that spontaneous, baseline NMDAergic activity may regulate MATR3 levels and account for the in vivo decline of MATR3 during CNS development (49).
Fig. 2.
Neuronal activity dynamically regulates MATR3 across in vitro and ex vivo. (A and B) Neurons treated with NMDA show robust NMDA-mediated decrease in MATR3 only at DIV ≥ 10, coinciding with NMDAergic maturity (n = 3; ns, not significant; *P = 0.040, ****P < 0.0001, and ***P = 0.001; two-tailed t tests). (C) Baseline MATR3 levels in vehicle-treated cultures also decline with neuronal maturation (n = 3; ns, not significant; **P < 0.01; one-way ANOVA with Tukey’s post hoc test). (D and E) Neurons treated with NMDA and then subjected to washout fully recover MATR3 within 24 h, but recovery is impaired after stronger stimulus (n = 3; **P < 0.01, ***P < 0.001; ns, not significant; one-way ANOVA with Tukey’s post hoc test). (F and G) Calbindin-positive Purkinje neurons in cerebellar slice cultures displayed significant reductions in MATR3 staining intensity upon glutamate stimulation (vehicle, n = 73; glutamate, n = 76; ****P < 0.0001; two-tailed t test). (Scale bars [F], 20 μm.)
We next asked whether neurons recover MATR3 after NMDA-induced degradation. Following the initial, expected reduction in response to NMDA at 3 h, MATR3 levels returned to pretreatment levels 24 h after mild stimulation with 50 μM NMDA but not with 100 μM NMDA (Fig. 2 D and E). Therefore, while neurons are capable of recovering MATR3, the effectiveness of this process is inversely related to the strength of stimulation.
One potential explanation for these observations is dose-dependent NMDA excitotoxicity and neuron loss. To explore this possibility, we utilized longitudinal fluorescence microscopy, a sensitive method for tracking the survival neurons in culture (SI Appendix, Fig. S2) (51–54). Neurons were transduced with virus expressing a fluorescent marker and were repeatedly imaged after vehicle or NMDA treatment. Individual neurons were identified using custom algorithms, and their time of death determined by a series of validated morphological changes (neurite retraction, soma rounding, and cellular dissolution) (55). In this way, we noted statistically significant but modest toxicity associated with 50 µM and 100 µM NMDA in comparison to vehicle by 24 h. Even so, the effect was minimal at 3 h—at which point we observed >50% reduction in MATR3 levels (Fig. 2 D and E—and comparable among the two NMDA doses, despite the distinct effect of 100 µM NMDA on MATR3 recovery. These findings argue against prominent excitotoxic contributions to NMDA-induced reductions in MATR3.
We next asked whether MATR3 is similarly responsive to glutamatergic stimulation in other contexts. In particular, we focused on ex vivo cerebellar preparations since cerebellar Purkinje neurons display a striking cell-to-cell heterogeneity in MATR3 abundance (49) and susceptibility to mutant MATR3-mediated neurotoxicity (56). We generated acute cerebellar slices from WT C57BL/6J mice and applied vehicle or glutamate before fixation and immunostaining for MATR3 and the Purkinje cell marker calbindin (Fig. 2F). As with isolated cortical neurons (Fig. 1 C and D), Purkinje cells treated with glutamate displayed a significant reduction in MATR3 immunoreactivity compared to vehicle-treated slices (Fig. 2G). These data indicate that activity-dependent MATR3 regulation applies not just to primary cortical neurons but also to Purkinje neurons of the cerebellum; moreover, this pathway may at least in part explain previous observations of MATR3 variance in Purkinje neurons.
MATR3 Is Cleared in an NMDA- and Ca2+-Dependent Process.
Neurons express a variety of receptors activated by glutamate, including the ionotropic kainate, AMPA, and NMDA receptors as well as a host of metabotropic receptors that do not directly pass Na+ or Ca2+ but instead trigger intracellular second messengers (Fig. 3A). Treatment with AP5, a selective antagonist of NMDARs, had no effect on MATR3 abundance on its own. Nevertheless, AP5 blocked MATR3 clearance not only in response to NMDA—as expected from coadministration of the agonist and the antagonist—but also in response to glutamate (Fig. 3 B and C). These results indicate that NMDAR stimulation is both necessary and sufficient for glutamatergic MATR3 reduction in mixed primary cortical neurons.
Fig. 3.
MATR3 reduction upon glutamatergic stimulation is NMDAR and Ca2+ dependent. (A) Schematic of ionotropic and metabotropic receptors activated by glutamate. (B and C) NMDAR activation is both necessary and sufficient for MATR3 clearance (n = 3; **P < 0.01; ns, not significant; one-way ANOVA with Tukey’s post hoc test). (D and E) The Ca2+ chelator BAPTA blocks NMDA-mediated MATR3 reduction (n = 3; ***P < 0.001; ns, not significant; one-way ANOVA with Tukey’s post hoc test). (F and G) Increasing intracellular Ca2+ with ionomycin (INMCN) is sufficient for MATR3 reduction in mature DIV14-16 and immature DIV4-5 neurons (n = 3; *P = 0.034, **P = 0.0080; two-tailed t test).
To determine whether the dependence of MATR3 clearance on NMDARs relies on Ca2+ influx, we treated neurons with the Ca2+ chelator BAPTA before NMDA treatment. This chelation blocked NMDA-induced reduction in MATR3 (Fig. 3 D and E), indicating that MATR3 clearance requires Ca2+ entry into the cell. If an increase in intracellular Ca2+ is the mechanism downstream of NMDAR activation that drives MATR3 reduction, we would expect nonphysiological increase in intracellular Ca2+ independent of receptor engagement to mimic this effect. Indeed, treatment with the ionophore INMCN resulted in MATR3 reduction not only in NMDAergically mature DIV14-16 neurons but also in NMDAergically immature DIV4-5 cells (Fig. 3 F and G). These results suggest that increased intracellular Ca2+ is both necessary and sufficient for MATR3 reduction. Notably, we were unable to detect MATR3 loss in response to INMCN treatment in HEK293T and Neuro2A cell lines (SI Appendix, Fig. S3A), implying that the Ca2+-dependent mechanisms responsible for MATR3 reduction may be cell type specific and limited to neurons.
NMDA-Related Reduction in MATR3 Occurs Posttranscriptionally via Calpain-Mediated Cleavage.
The reduction in MATR3 we observed upon NMDAR stimulation could be due to a decrease in the production of MATR3, degradation of existing MATR3, or both. Using RT-PCR, we observed only a weak trend toward reduced Matr3 production upon NMDA stimulation (Fig. 4A). These data, together with the rapid time frame of reduction (Fig. 1B), point to posttranslational MATR3 degradation as the primary mechanism for its reduction. In a complementary experiment, we transduced cultures with lentivirus expressing exogenous FLAG-tagged MATR3 and noted a rapid reduction in FLAG-MATR3 with NMDA treatment (Fig. 4B), supporting a model in which NMDAR stimulation triggers the MATR3 clearance at the protein level.
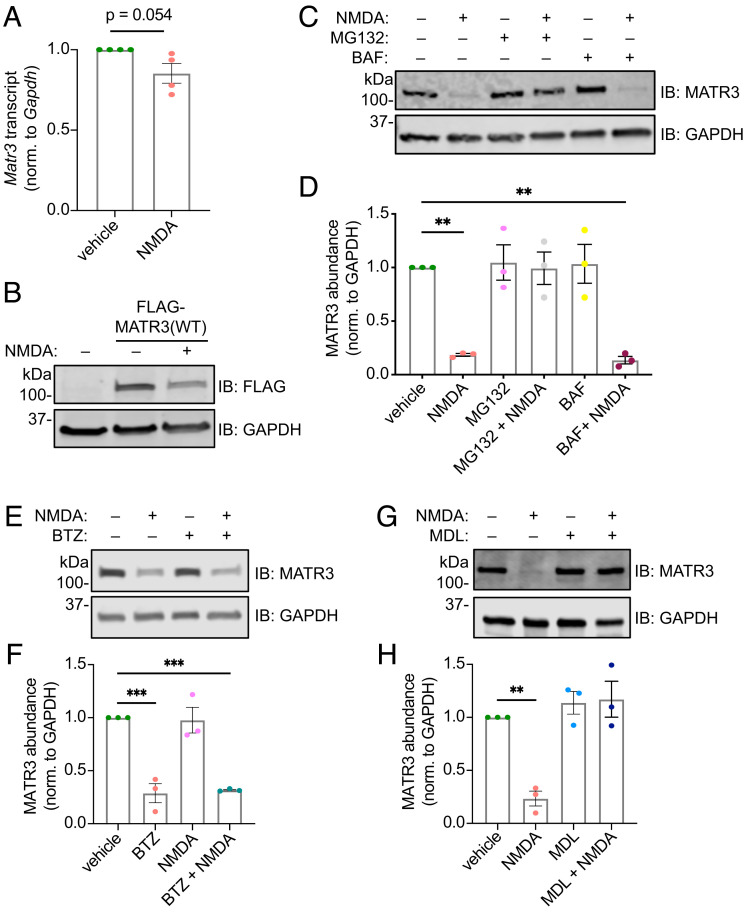
Fig. 4.
NMDA-triggered MATR3 reduction is accomplished posttranslationally via calpains. NMDA application does not significantly alter Matr3 transcript abundance (A; n = 4; P = 0.054; two-tailed t test), but exogenous FLAG-MATR3 (B) is reduced by NMDA application. (C and D) The proteasomal and cysteine protease inhibitor MG132, but not the autophagy inhibitor BAF, blocks MATR3 clearance in response to NMDA (n = 3; **P < 0.01; one-way ANOVA with Tukey’s post hoc test). (E and F) The selective proteasomal inhibitor BTZ fails to block NMDA-triggered MATR3 reduction (n = 3; ***P ≤ 0.001; one-way ANOVA with Tukey’s post hoc test). (G and H) The calpain inhibitor MDL28170 (MDL) effectively impairs MATR3 degradation upon NMDA treatment (n = 3; **P = 0.0040; one-way ANOVA with Tukey’s post hoc test).
MATR3 turnover in response to NMDA is completely blocked by MG132—a broad inhibitor of cysteine proteases as well as threonine proteases that constitute the proteasome (57)—but not the vacuolar ATPase blocker bafilomycin (BAF) (58) (Fig. 4 C and D), suggesting that MATR3 degradation requires the ubiquitin–proteasome system (UPS) or cysteine proteases. Bortezomib (BTZ), a specific inhibitor of the 26S proteasome subunit (59), failed to prevent MATR3 degradation in response to NMDA, indicating that an MG132-sensitive protease independent of the UPS is responsible for MATR3 cleavage (Fig. 4 E and F). Further supporting this conclusion, we saw no accumulation of higher–molecular weight, ubiquitinated MATR3 species in neurons treated with MG132 and NMDA (SI Appendix, Fig. S3B). Therefore, we asked whether calpains, a group of Ca2+-activated, MG132-sensitive cysteine proteases, may cleave MATR3. Treatment with MDL28170, a specific calpain inhibitor, effectively blocked NMDA-induced MATR3 degradation (Fig. 4 G and H), highlighting calpains as the effector proteases of NMDA-mediated degradation. We were unable to detect lower–molecular weight MATR3 species after NMDA treatment, even in the setting of proteasomal inhibition and using antibodies targeting both N and C termini of MATR3, indicating that these fragments are rapidly cleared in a UPS-independent manner (SI Appendix, Fig. S3C). Accordingly, recent reports have confirmed the in vitro susceptibility of MATR3 to calpains (60) and the apparent difficulty in detecting calpain cleavage products (61, 62).
The two calpains most expressed in the nervous system are calpain 1 (CAPN1) and calpain 2 (CAPN2), with CAPN1 enriched in neurons and CAPN2 expressed predominantly by glia (63, 64). Despite robust expression of CAPN1 and CAPN2 in transfected HEK293T cells (Fig. 5 A and B), only CAPN1 significantly reduced endogenous MATR3 levels in comparison to the negative control (Fig. 5 C and D), suggesting that CAPN1 selectively degrades MATR3. Increasing Ca2+ in HEK293T cells by application of the ionophore INMCN had no effect on MATR3 levels (SI Appendix, Fig. S3A), however, likely due to low expression of the endogenous calpain inhibitor calpastatin, elevated basal Ca2+ concentrations, or a combination of these factors (65, 66).
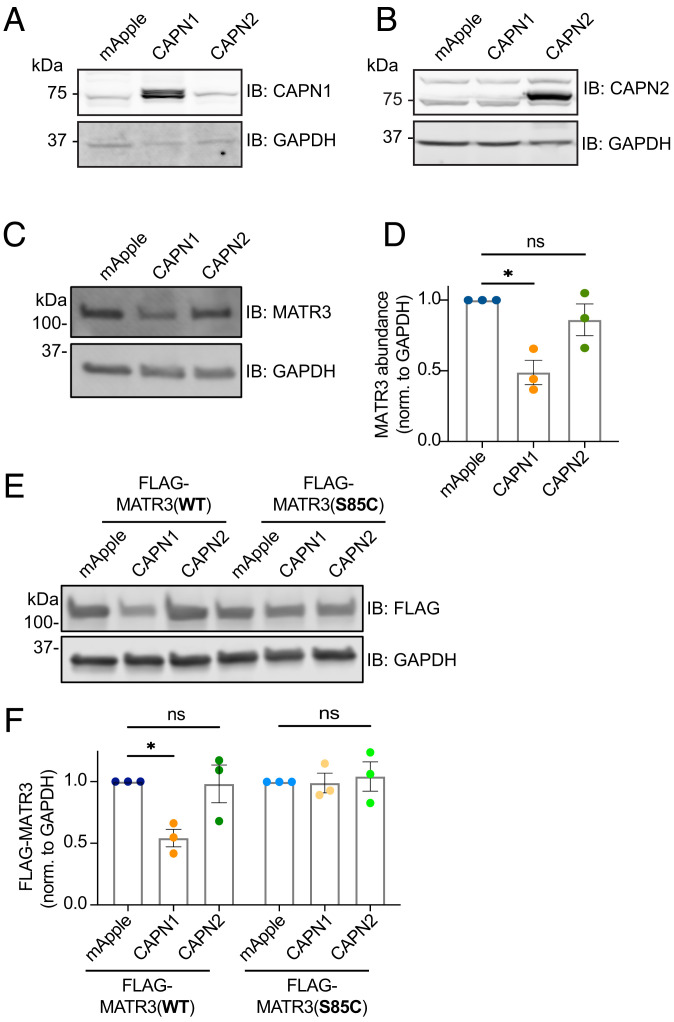
Fig. 5.
MATR3 is a substrate for CAPN1, and the pathogenic S85C mutation renders it resistant to degradation. Transfection of HEK293T cells with calpain expressing constructs resulted in robust expression of CAPN1 (A) and CAPN2 (B) compared to controls. (C and D) CAPN1 but not CAPN2 overexpression resulted in MATR3 degradation (n = 3; *P = 0.010; one-way ANOVA with Tukey’s post hoc test). (E and F) While exogenous FLAG-MATR3(WT) is susceptible to cleavage by CAPN1 (n = 3; *P = 0.036; ns, not significant; one-way ANOVA with Tukey’s post hoc test), the pathogenic S85C mutation is resistant (n = 3; one-way ANOVA with Tukey’s post hoc test).
An online calpain substrate algorithm (67) indicated a probable CAPN1 cleavage site at residues 82 to 85 of MATR3. This stretch of amino acids includes S85, a residue altered by the most common pathogenic MATR3 mutation (S85C). We and others found that MATR3(S85C) is neurotoxic when overexpressed and insoluble in cultured mammalian cells, Drosophila, mice, and patient lymphoblasts (13, 51, 56, 68). Therefore, we wondered whether this mutation drives aberrant accumulation by impairing CAPN1-mediated MATR3 degradation. In HEK293T cells transfected with mApple, CAPN1, or CAPN2 and FLAG-tagged MATR3 variants, exogenous FLAG-MATR3(WT) was effectively degraded by CAPN1. In contrast, FLAG-MATR3(S85C) was completely resistant to CAPN1-mediated cleavage (Fig. 5 E and F). Collectively, these data suggest that NMDAR activation leads to MATR3 degradation through a Ca2+- and calpain-dependent manner, a process that is impaired by the disease-linked S85C mutation.
Ca2+ Promotes a Selective Interaction between Ca2+/CaM and MATR3.
Beyond activation of calpains, Ca2+ may impact several downstream pathways in neurons, many of which are modulated by calmodulin (CaM), a ubiquitous and highly conserved factor. Upon binding Ca2+, CaM undergoes conformational changes that shift its affinity for other proteins, resulting in their activation or inhibition. In prior studies (42, 43), MATR3 bound selectively to Ca2+/CaM via a sequence overlapping the MATR3 RRM2. Using publicly available algorithms (69–71), we confirmed the presence of a predicted CaM-binding site on MATR3 within RRM2 corresponding to the MATR3 fragment used in previous studies (Fig. 6A) (42). We also identified analogous CaM-binding sites buried within the RRM of the related RBP TDP43, suggesting that CaM–RBP interactions may extend beyond MATR3.
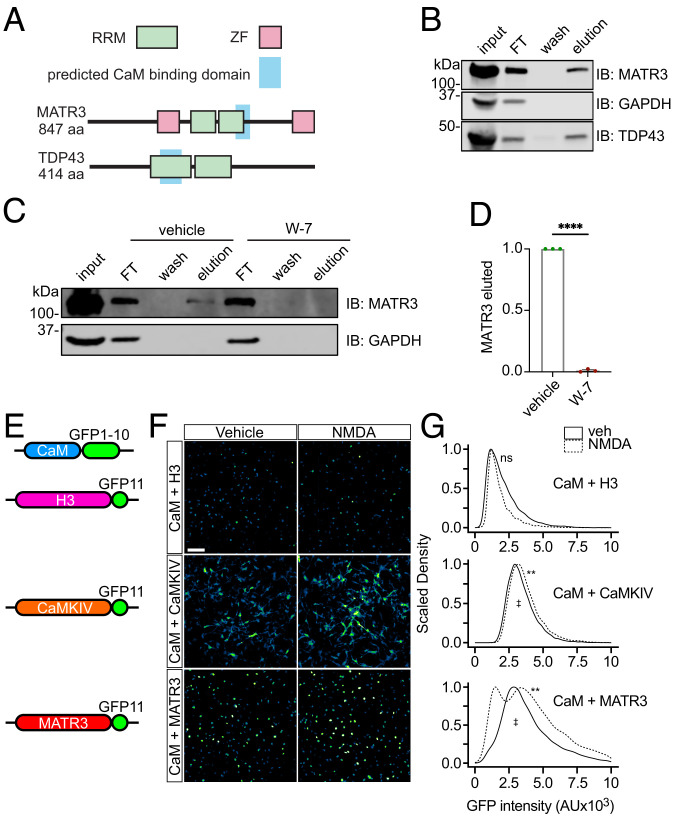
Fig. 6.
CaM binds to MATR3 in a Ca2+-dependent manner. (A) CaM is predicted to interact with the RRMs of both MATR3 and TDP43. (B) Ca2+-bound but not free apo-CaM binds to MATR3 and TDP43. (C and D) The conformational CaM inhibitor W-7 blocks the association between CaM and MATR3 (n = 3; ****P < 0.0001; two-tailed t test). (E) Schematic of split GFP constructs used for detecting in situ interaction of CaM and MATR3. (F and G) Application of NMDA increased the interaction between CaM-GFP1-10 and MATR3-GFP11, as determined by an increase in the reconstituted GFP signal. A similar although more subtle increase was detected in cells expressing CaM-GFP1-10 and CaMKIV-GFP11 but not those transduced with CaM-GFP1-10 and H3-GFP11. n = 200 neurons/condition, 2 reps/condition. (Scale bar, 50 µm.) ns, not significant; **P < 0.01 comparing vehicle to NMDA for each condition, one-sided Kolmogorov–Smirnov test with bootstrapping. ‡P < 0.0001 comparing CaM-GFP1-10 + H3-GFP11 to CaM-GFP1-10 + MATR3-GFP11, one-sided Kolmogorov–Smirnov test with bootstrapping.
To validate these predicted binding events, we incubated lysates from HEK293T cells with CaM-conjugated Sepharose beads in the presence of Ca2+. Following washing, elution was accomplished by incubation with a Ca2+ chelator, selectively liberating proteins bound to Ca2+/CaM. We found that both MATR3 and TDP43 bind CaM in a Ca2+-dependent manner, recapitulating previous findings on MATR3 while also suggesting that CaM may regulate additional disease-associated RBPs such as TDP43 (Fig. 6B). Focusing our studies on MATR3, we sought to further confirm the selectivity of its interaction with CaM. For this, we incubated lysates with CaM-conjugated beads in the absence or presence of W-7, a small-molecule inhibitor that impairs CaM’s ability to adopt its activated confirmation even in the presence of Ca2+. W-7 completely blocked MATR3 binding to CaM (Fig. 6 C and D), indicating that Ca2+ does not promote the CaM–MATR3 interaction through any effects on MATR3 but rather through the Ca2+-triggered conformational changes in CaM.
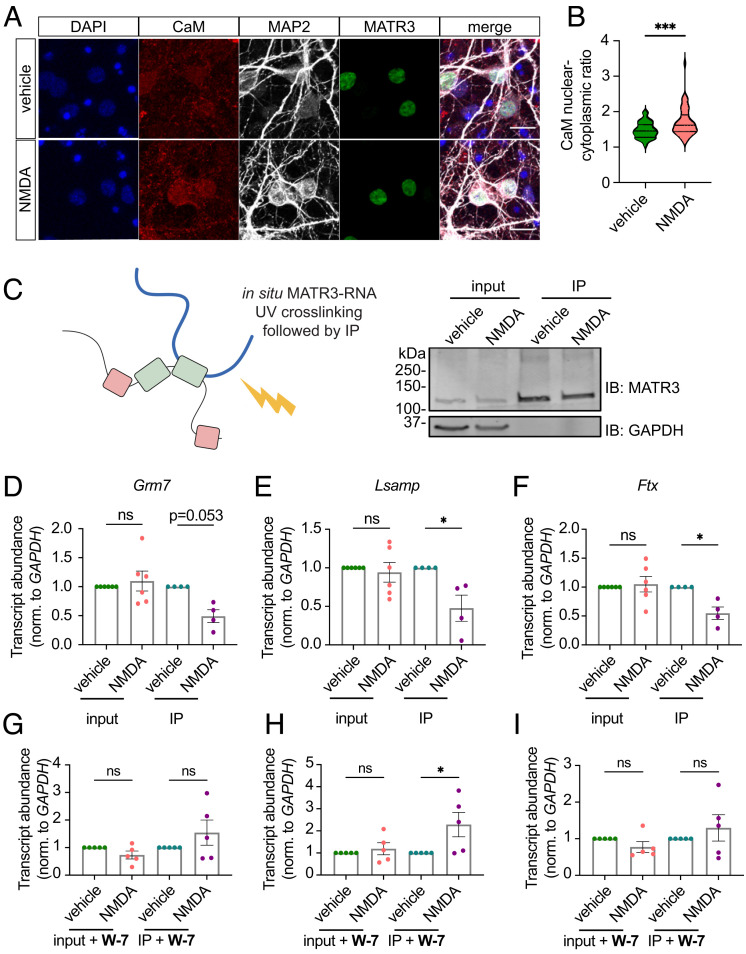
We next sought to confirm these findings in situ by transducing mature (DIV15) neurons with lentiviral vectors encoding MATR3 labeled at the C terminus with green fluorescent protein (GFP) blade 11 (MATR3-GFP11) and CaM fused to GFP blades 1 to 10 (CaM-GFP1-10). Separate cultures of neurons were transduced with CaM-GFP1-10 and histone H3 fused to GFP blade 11 (H3-GFP11) as a negative control, or CaM-GFP1-10 and CaM kinase IV labeled with GFP blade 11 (CaMKIV-GFP11) as a positive control (Fig. 6E). In this experiment, reconstitution of GFP fluorescence should be proportional to the interaction among fused proteins. As expected, we detected moderate GFP fluorescence in cells expressing the positive control (CaM-GFP1-10 + CaMKIV-GFP11) but little in the negative control (CaM-GFP1-10 + H3-GFP11) (Fig. 6 F and G). We also observed reconstituted fluorescence in neurons transduced with CaM-GFP1-10 and MATR3-GFP11, suggesting baseline interaction among CaM and MATR3. Notably, NMDA application resulted in a significant increase in GFP intensity within the nuclei of neurons expressing CaM-GFP1-10 and MATR3-GFP11, but not CaM-GFP1-10 and H3-GFP11, consistent with an NMDA-induced interaction between nuclear CaM and MATR3 (Fig. 6 F and G). Density plots of GFP intensity after NMDA application also highlighted the presence of a separate neuronal population displaying reduced GFP intensity; we suspect that this peak reflects MATR3 degradation in response to NMDA (Figs. 1 and 2) and the ensuing decrease in GFP signal.
Activity Rapidly Impairs MATR3 Binding to Substrate RNAs.
Given the previously validated location of the CaM-binding site within the MATR3 RRM2 domain (42), we wondered whether CaM binding could interfere with the ability of MATR3 to recognize its RNA substrates. Upon neuronal depolarization, CaM rapidly translocates to the nucleus, where it drives the expression of activity-dependent genes (44–48). Using immunofluorescence, we confirmed that NMDA treatment of mature primary neurons results in a modest increase in nuclear CaM within a matter of minutes (Fig. 7 A and B). This effect, along with its adoption of the activated Ca2+-bound conformation, would be expected to enhance its association with resident nuclear proteins such as MATR3. In keeping with these results, we observed primarily nuclear GFP signal in NMDA-treated neurons expressing CaM-GFP1-10 and MATR3-GFP11 (Fig. 6).
Fig. 7.
Ca2+/CaM enriches in the nucleus of stimulated neurons and inhibits MATR3 RNA binding. (A and B) Ca2+/CaM is enriched within the nucleus of neurons treated with NMDA (vehicle, n = 67; NMDA, n = 48; ***P = 0.0001; two-tailed t test). (Scale bars [A], 20 μm.) (C) Schematic of ultraviolet (UV) cross-linking followed by IP (UV-CLIP). (D–F) While NMDA treatment did not alter total levels of MATR3 target RNAs (n = 6 for each candidate), less RNA was cross-linked to MATR3 in stimulated conditions, indicating impaired RNA binding (n = 4 for each candidate; *P < 0.05; two-tailed t test). (G–I) Pretreatment with CaM inhibitor W-7 prior to NMDA stimulation did not alter total levels of MATR3 target RNAs (n = 5 for each candidate). Although W-7 pretreatment increased Lsamp RNA cross-linked to MATR3 (*P = 0.045, two-tailed t test), it did not alter the amount of Grm7 or Ftx cross-linked to MATR3 (n = 5 per condition; ns, not significant; two-tailed t test).
To determine whether CaM binding displaces RNA from MATR3, we irradiated primary neurons after 5 m of NMDA treatment, thereby cross-linking RNA–MATR3 complexes. We then immunoprecipitated MATR3, isolating bound high-confidence neuronal RNA targets identified in previous studies (72), and measured their abundances by quantitative RT-PCR (UV-CLIP; Fig. 7C). MATR3 pulled down less cross-linked RNA in the context of NMDA stimulation compared to vehicle control, despite no change in the overall abundance of each target (Fig. 7 D–F). Furthermore, pretreatment with the CaM inhibitor W-7 prevented the NMDA-induced drop in MATR3 binding for these targets (Fig. 7 G–I), and in the case of one MATR3 substrate (Lsamp), W-7 pretreatment led to an increase in MATR3 binding. These results suggest that NMDA stimulation impairs RNA recognition by MATR3 through a Ca2+/CaM-dependent manner, affecting the function of MATR3 without directly reducing its abundance.
Based on our previous data suggesting destabilization of TDP43 upon interruption of RNA binding (52), and the observation that W-7 completely blocked NMDA-induced MATR3 degradation (SI Appendix, Fig. S4 A and B), we asked whether CaM activation might likewise promote MATR3 clearance by inhibiting its RNA binding. To answer this, we employed optical pulse labeling, a technique in which proteins of interest are fused to the photoconvertible protein Dendra2. Brief illumination with UV light triggers the irreversible conversion of Dendra2 from a green fluorescent protein to a red fluorophore (SI Appendix, Fig. S4C). The time-dependent loss of red fluorescence for each cell can then be used to determine a half-life for Dendra2-tagged proteins. Using this method, we observed no differences in the turnover of MATR3(WT)-Dendra2 and a variant of MATR3 with its key RNA-binding domain deleted (ΔRRM2) (SI Appendix, Fig. S4D). These data suggest that, in contrast to TDP43, impaired RNA binding per se does not destabilize MATR3.
Discussion
In this work, we focused on the physiological regulation of MATR3 abundance and function in neurons. Application of glutamate resulted in the rapid clearance of MATR3 in an NMDAR-dependent manner. In contrast to previous observations, however, PKA inhibition only produced a trend toward blunted MATR3 clearance upon NMDA treatment. Both Ca2+ and calpains were required for neuronal MATR3 reduction in response to NMDA, with CAPN1 being sufficient for MATR3 clearance. Based on the predicted location of the CAPN1 cleavage site, we found that the most common pathogenic MATR3 mutant, S85C, renders the protein resistant to CAPN1 cleavage. Together with our prior data showing that MATR3 levels are critical determinants of neuronal survival (51), these findings suggest that interference with physiological regulation of MATR3 function and abundance may represent a key pathogenic mechanism underlying S85C-induced neurodegeneration and myopathy.
Our results in cortical neurons conflict with those observed in cerebellar granule neurons, in which PKA inhibition by H89 completely blocks MATR3 degradation in response to NMDA (41). We found that H89 had little effect on MATR3 phosphorylation, implying the presence of an alternative MATR3 kinase acting on the consensus PKA motif (RRXpS/T) recognized by the PKA phospho-substrate antibody (73, 74). The identity of the kinase(s) acting on MATR3 after NMDAR activation remains unknown, as does the functional significance of this modification. As PKA does not appear to directly phosphorylate MATR3 in cortical neurons, we propose that the effect of H89 on MATR3 turnover may be due to additional PKA targets that affect Ca2+ dynamics and, indirectly, MATR3 clearance. PKA is itself activated by Ca2+/CaM through adenylyl cyclase (75–77) and in turn phosphorylates key residues on the intracellular portions of NMDARs, thereby increasing Ca2+ current (78, 79). This feedforward mechanism for increasing Ca2+ influx in response to NMDAR stimulation depends on both CaM and PKA, and its attenuation upon inhibition of CaM (SI Appendix, Fig. S4 A and B) and PKA (SI Appendix, Fig. S1 A and B) would be expected to blunt Ca2+-dependent MATR3 clearance indirectly by influencing the complex cross talk between Ca2+ and Ca2+-dependent signaling pathways in neurons.
We also found that cerebellar Purkinje neurons—a cell type previously reported to display altered MATR3 metabolism as baseline and in disease models—clear MATR3 in response to glutamatergic stimulation. These observations suggest that differences in Purkinje cell activity may partially explain disparities in MATR3 abundance noted in previous studies (49). Future experiments may examine the behavioral threshold required to drive MATR3 clearance in Purkinje neurons, perhaps through motor tasks (80). Intriguingly, a knock-in mouse homozygous for the S85C mutation exhibited markedly reduced MATR3 staining in Purkinje cells from an early time point followed by age-dependent Purkinje cell loss (56). The mechanism through which this mutation affects MATR3 metabolism in Purkinje neurons remains unclear, however, since no MATR3 mutation identified to date results in cerebellar degeneration or Purkinje cell loss.
We additionally found that MATR3 reduction in response to NMDA depends on Ca2+, and furthermore, that elevated Ca2+ is sufficient to drive MATR3 degradation not only in NMDAergically mature DIV14-16 but also in immature DIV4-5 (Fig. 3 F and G) neurons, indicating a key role for Ca2+ downstream of NMDAR recruitment. Although increases in intracellular Ca2+ can activate several proteolytic pathways, including autophagy (81, 82) and the UPS (83, 84), neither of these processes were responsible for MATR3 clearance. Rather, calpain inhibition fully blocked NMDA-triggered degradation, suggesting that calpains are the final effectors of MATR3 clearance. A growing body of evidence implicates calpain cleavage of RBPs in the pathogenesis of ALS/FTD and related disorders. Activated calpains degrade survival motor neuron (SMN) (85) and are capable of cleaving MATR3, FUS, and TDP43 in vitro and in vivo (60, 86). Calpain-mediated TDP43 cleavage can be modulated through phosphorylation (87), but whether the many reported (88) posttranslational modifications of MATR3 determine its susceptibility to clearance is unknown. Furthermore, overexpression of calpastatin, an endogenous calpain inhibitor, prevented cleavage of calpain targets while increasing motor function and survival in a transgenic mutant SOD1 mouse model of ALS (89). Although the connection with human disease is still unclear, these observations highlight the importance of calpain-mediated protein turnover mechanisms for ALS pathogenesis and perhaps therapeutic purposes.
In accordance with previous in vivo data showing an age-dependent decrease in MATR3 levels in the developing mouse nervous system, we observed a reduction in steady-state MATR3 levels that coincided with NMDAergic maturity in isolated primary cortical neurons. Supporting the concept of developmental regulation of RBPs by a similar mechanism, the RBP muscleblind-like 2 (MBNL2) is degraded by calpains upon NMDAR stimulation in mature neurons, enabling dynamic regulation of MBLN2 substrate RNAs (35). Notably, MATR3 haploinsufficiency causes microcephaly and refractory epilepsy (90), suggesting that developmental deficiencies in MATR3 may lead to conditions that are distinct from ALS/FTD, though the contribution of developmental abnormalities to adult-onset degenerative disease remains unclear.
Ca2+ and calpains regulate RBPs beyond simply degrading them, although these nondegradative mechanisms are less defined. Increases in cellular Ca2+ result in the cytoplasmic redistribution of TDP43 and the ALS-linked RBP FUS (91), while this same stimulus drives the nuclear enrichment of CPEB4 (92), indicating that Ca2+ can bidirectionally regulate localization in a unique and selective manner. An RNAi screen of calcium signaling proteins in Drosophila demonstrated that calpain A is critical for TDP43 cytoplasmic localization after Ca2+ increase (93), and degradation of nuclear pore complex components by Ca2+-activated calpains resulted in aberrant nucleocytoplasmic transport in mouse CNS (94). These results suggest that calpains may modulate RBP localization as well as abundance. Even so, we failed to detect any changes in MATR3 distribution after NMDA treatment (Fig. 1C), suggesting that Ca2+- and calpain-mediated MATR3 regulation occurs primarily within the nucleus, without a change in MATR3 localization.
Conversely, we confirmed that Ca2+/CaM translocates to the nucleus after stimulation and further showed that CaM interacts directly with nuclear MATR3. This interaction interferes with MATR3’s ability to recognize its RNA substrates, potentially by displacing RNA from the RRM2 domain. A similar phenomenon of inhibited nucleic acid recognition through activated CaM binding has been reported for CPSF30 in Arabidopsis (95), as well as the CNS-specific mammalian PCP4 (96). Our preliminary results for MATR3 and TDP43 suggest that this may be a widely applicable mechanism for rapidly tuning RBP function in response to neuronal activity. It is also possible that a similar phenomenon regulates local mRNA sequestration and translation in axonal terminals and dendrites. Indeed, Ca2+/CaM-mediated modulation of RBP–mRNA interactions may underlie rapid derepression of local translation for many RBPs, in a functionally analogous manner to what has been shown in select cases of neurotransmitter or trophic factor signaling (38, 97, 98).
While a recent study identified MATR3 as a substrate of both CAPN1 and CAPN2, these experiments were performed in vitro (60), limiting the effects of factors such as subcellular localization and substrate accessibility that are critical for calpain function (35). In testing MATR3 susceptibility to the two main calpains present in the CNS, we found that WT MATR3—but not the pathogenic S85C mutant—is a substrate of CAPN1 (Fig. 5 D and E). The S85C mutation is the most common disease-linked variant in MATR3, with a phenotypic spectrum that encompasses both ALS and distal myopathy with vocal cord and pharyngeal weakness (14, 99–101). In patient tissue (13) as well as cellular (51) and animal (56, 68, 102) models, this mutation reduces MATR3 solubility through unknown mechanisms. Resistance to CAPN1 may promote aggregation of MATR3(S85C); alternatively, insolubility may itself interfere with CAPN1-mediated degradation. Like neurons, skeletal muscle cells are excitable and rely not only on Ca2+ levels for their function but also on CAPN1 activation as a proteolytic mechanism to control myofiber size and number (103). Therefore, our findings may offer insight into the unique disease spectrum of the S85C mutation that encompasses motor neuron and skeletal muscle pathology.
In summary, we demonstrated that glutamatergic stimulation and the resulting increase in Ca2+ result in suppression of MATR3 function through two complementary mechanisms (Fig. 8). Within minutes of Ca2+ influx, Ca2+/CaM binding to MATR3 rapidly impairs its ability to bind RNAs. Over extended periods of time, activation of calpains results in MATR3 cleavage. What consequences these regulatory processes have for MATR3 gene targets, particularly those that encode activity-related proteins, are currently unclear, as are the implications of this Ca2+-mediated control for neuronal physiology and for RBP-linked neuromuscular disease. Intriguingly, motor neurons lack key Ca2+-buffering proteins such as parvalbumin and calbindin (104, 105), rendering them more susceptible to aberrant Ca2+ signaling and offering a possible explanation for selective activity-dependent pathology in disease. We expect that continued investigations into this phenomenon will offer insights into the basic biology of activity-dependent RBP regulation, which can in turn be leveraged for therapies targeting neurological disorders such as ALS and FTD characterized by both aberrant neuronal activity and RBP dysfunction.
Fig. 8.
A model depicting regulation of MATR3 abundance and RNA binding in a Ca2+/CaM-dependent manner. MATR3 possesses two ZF domains and two RRMs, with RRM2 being the dominant RNA-binding domain. Ca2+ influx through NMDARs activates calmodulin (CaM), which displaces bound RNA from MATR3 via overlapping interactions with RRM2. Simultaneously, Ca2+ activates calpain-1 (CAPN1), which is capable of degrading RNA-deficient MATR3. By virtue of its location within the predicted CAPN1 cleavage site, the pathogenic S85C mutation interferes with CAPN1-mediated MATR3 degradation.
Materials and Methods
Plasmids and Expression Vector Delivery.
For lentiviral expression plasmids, the FLAG-MATR3 open reading frame (ORF) was PCR amplified from pGW1 FLAG-MATR3-Dendra2 using primers that attached HpaI sites to the 5′ and 3′ ends of the ORF. The resulting amplicon was inserted into the HpaI site of pLV-EF1a. CAPN1 (#60941) and CAPN2 (#60942) overexpression constructs were purchased from Addgene. Expression plasmids for pGW1 FLAG-MATR3(WT) and FLAG-MATR3(S85C) were generated by amplifying MATR3(WT) and MATR3(S85C) from pGW1 FLAG-MATR3-EGFP constructs (51) using Q5 Hot Start High-Fidelity DNA Polymerase (New England Biolabs) and flanking primers that eliminated linker sequences and EGFP. A similar strategy was used for creating MATR3(ΔRRM2)-Dendra2. FUGW-H3-GFP1-10, FUGW-CaM-GFP1-10, FUGW-MATR3-GFP11, and FUGW-CaMKIV-GFP11 were synthesized using GeneArt (ThermoFisher).
HEK293T cells (ATCC CRL-3216) were transfected with Lipofectamine 2000 (ThermoFisher) in accordance with the manufacturer’s instructions. For lentivirus experiments, viral particles were generated by the University of Michigan Vector Core and neurons transduced at DIV5-6 overnight. The next day, media were removed from cells, after which they were washed once in phosphate buffered saline (PBS) before being placed in virus-free conditioned media until experimentation on DIV14-16.
Primary Cortical Neuron Culture and Pharmacological Treatments.
Primary cortical neurons were isolated from E19-20 rats and plated as previously described (106). Unless otherwise stated, neurons were treated at DIV14-16 with 100 μM NMDA (Sigma) or L-glutamate (Sigma) for 3 h or at the doses and times detailed in Figs. 1 and 2. For blocking experiments, cells were cotreated with 100 μM D-AP5 (Tocris), 10 μM MG132 (Sigma), 20 nM BAF (Sigma), 100 nM BTZ (Sigma), or 20 μM MDL28170 (ThermoFisher). For Ca2+ chelation and PKA inhibition experiments, neurons were pretreated for 30 m with 2 mM BAPTA (Cayman Chemical) or 20 μM H89 (Tocris), respectively, prior to NMDA application. To increase intracellular Ca2+, INMCN (ThermoFisher) was applied at 10 μg/mL for 3 h. Cerebellar slices were incubated with 500 μM glutamate or vehicle for 3 h prior to immunostaining. All pharmacological treatments were performed at 37 °C.
Immunoblot.
Cells were washed in PBS and lysed in radioimmunoprecipitation assay (RIPA) buffer (Pierce) supplemented with complete protease inhibitor cocktail (Roche). For experiments investigating MATR3 phosphorylation, the lysis buffer was also supplemented with PhosSTOP phosphatase inhibitor (Roche). Resuspended cells were sonicated using a Fisherbrand Model 505 Sonic Dismembrator (ThermoFisher) at 80% amplitude, 5-s on/5-s off for 2 m. Lysates were cleared by centrifugation at 21,000× g at 4 °C for 15 m. Then, 5 to 20 μg proteins were loaded onto 4 to 20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels (Bio-Rad) and run at 100 V, after which proteins were transferred onto 0.2-μm polyvinylidene difluoride (PVDF) membranes (Bio-Rad) at 100 V for 2 h at 4 °C. Membranes were incubated in blocking buffer [3% BSA and 0.2% Tween-20 in Tris-buffered saline (TBST)] and then blotted with the following primary antibodies diluted in blocking buffer: rabbit anti-MATR3 N terminus [1:1,000, Abcam EPR10634(B)] for all blots except for SI Appendix, Fig. S3 as noted, mouse anti-GAPDH (1:1,000, MilliporeSigma MAB374), rabbit anti-MATR3 C terminus [1:1,000, Abcam EPR10635(B)] for SI Appendix, Fig. S3B, mouse anti-ubiquitin (1:100, Santa Cruz sc-8017), rabbit anti-TDP43 (1:5,000, Proteintech 10782-2-AP), rabbit anti-phospho-PKA substrate (1:1,000; Cell Signaling #9624), rabbit anti-CAPN1 (1:1,000; Abcam #28258), and mouse anti-calpain 2 (1:1,000; Santa Cruz sc-373955). The following day, they were washed 3 × 5 m in TBST and incubated with donkey anti-mouse 800 (1:10,000, LI-COR 925-32213) and donkey anti-rabbit 680 (1:10,000 LICOR 926-68073) in blocking buffer for 1 h at RT. Membranes were then washed again 3 × 5 m in TBST and imaged on an Odyssey CLx Imaging System (LI-COR).
Immunocytochemistry.
Primary cortical neurons were fixed in 4% paraformaldehyde (PFA) in PBS supplemented with 2 mM CaCl2 at room temperature (RT) for 10 m, permeabilized with 0.1% Triton X-100 in PBS, and treated with 10 mM glycine in PBS. They were then placed in blocking solution (0.1% Triton X-100, 2% fetal calf serum, and 3% bovine serum albumin (BSA) in PBS) for 1 h at RT, after which they were probed overnight at 4 °C with the following antibodies diluted in blocking solution: rabbit anti-MATR3 [1:1,000, Abcam EPR10635(B)], chicken anti-MAP2 (1:1,000, Novus Biologicals NB300-213), mouse anti-calmodulin (1:1,000, ThermoFisher MA3-917), rabbit anti-CAPN1 (1:250; Abcam #28258), and mouse anti-calpain 2 (1:250; Santa Cruz sc-373955). The next day, samples were washed 3 × 5 m in PBS and then incubated 1 h at RT with the following secondary antibodies all diluted 1:1,000 in blocking solution: donkey anti-rabbit Alexa Fluor 647 (ThermoFisher A-31573), goat anti-mouse Alexa Fluor 568 (ThermoFisher A-11031), and goat anti-chicken Alexa Fluor 488 (ThermoFisher A-11039). After 3 × 5-m PBS washes, neurons were imaged using an Eclipse Ti inverted microscope (Nikon) with PerfectFocus, Semrock filters, Lambda 421 lamp (Sutter Instrument), and an Andor Zyla 4.2(+) sCMOS camera (Oxford Instruments), with custom BeanShell scripts controlling image acquisition and stage movements via μManager (107). For confocal microscopy, neurons were mounted in ProLong Gold Antifade Mountant with DAPI (ThermoFisher P36935) and imaged with a Nikon A1 inverted point-scanning confocal microscope (Nikon) controlled by NIS-Elements software. For CaM N/C ratio determination, custom scripts were used to create nuclear and cytoplasmic regions of interest using DAPI and MAP2 signals, respectively.
Cerebellar Slice Culture and Immunohistochemistry.
WT C57BL/6J mice were anesthetized by isoflurane inhalation. Slices were prepared using a VT1200 vibratome (Leica) at a thickness of 300 μM as before (108, 109) and placed in prewarmed aCSF with 5% CO2 and 95% O2 for 30 m before transferring to Neurobasal Medium (ThermoFisher). They were treated with vehicle or 500 μM glutamate for 3 h at 37 °C, after which they were processed as before (110). Briefly, slices were fixed in 4% PFA at 4 °C overnight. The following day, samples were permeabilized with 1% Triton X-100 in PBS for 1.5 h, treated with 20 mM glycine in PBS for 1 h, blocked in 5% normal goat serum in PBS, and probed overnight at 4 °C with rabbit anti-MATR3 [1:1,000, Abcam EPR10635(B)] and mouse anti-calbindin (1:1,000, MilliporeSigma C9848). After washing 3 × 5 m in PBS, slices were incubated overnight at 4 °C with goat anti-mouse Alexa Fluor 568 and goat anti-rabbit Alexa Fluor 488 (ThermoFisher A-11008), both diluted 1:500 in blocking solution. Stained slices were washed 3 × 5 m in PBS and then mounted in ProLong Gold Antifade Mountant with DAPI. Slices were imaged using a 40× objective Nikon A1 inverted point-scanning confocal microscope, as above.
Immunoprecipitation.
DIV14-16 primary neurons were treated with vehicle (dimethyl sulfoxide, DMSO), 100 μM NMDA, 10 μM MG132, or both NMDA and MG132, then collected in PBS, lysed in RIPA buffer supplemented with protease and phosphatase inhibitors and 2 mM of the deubiquitinating enzyme inhibitor N-ethylmaleimide (MilliporeSigma), and sonicated at 80% amplitude, 5-s on/5-s off for 2 m in a Fisherbrand Model 505 Sonic Dismembrator. Lysates were cleared by centrifugation at 21,000× g at 4 °C for 15 m and resuspended in dilution buffer (50 mM Tris, 10 mM ethylenediaminetetraacetic acid (EDTA), 1.5 M NaCl, 1% Na deoxycholate, and 10% Triton X-100, pH 8.0) before incubation overnight at 4 °C with 1 μg rabbit anti-MATR3 antibody per sample (ThermoFisher A300-591A) conjugated to Dynabeads Protein G (ThermoFisher). The following day, beads were washed 3 × 5 m in wash buffer (20 mM Tris, 1% Triton X-100, 2 mM EDTA, 150 mM NaCl, and 0.1% SDS, pH 8.1) before elution by heating in SDS-PAGE sample buffer at 95 °C for 10 m.
CaM-Sepharose Pulldown.
HEK293T cells were collected in PBS, resuspended in buffer (10 mM Tris and 5 mM MgCl2, pH 7.4) supplemented with cOmplete EDTA-free protease inhibitor (Roche), and lysed by passing through an 18-G × 1½’’ needle and sonication at 80% amplitude, 5-s on/5-s off for 2 m in a Fisherbrand Model 505 Sonic Dismembrator. Cleared lysates were rotated overnight at 4 °C with 100 μL per sample of Calmodulin Sepharose 4B resin (Cytiva Life Science) in CaM-binding buffer (50 mM Tris-HCl, 150 mM NaCl, 2 mM CaCl2, and 1 μM dithiothreitol (DTT), pH 7.5). The next day, beads were washed 3 × 5 m in CaM-binding buffer and eluted in CaM elution buffer with EGTA (50 mM Tris-HCl, 150 mM NaCl, 2 mM EGTA, and 1 μM DTT, pH 7.5) or BAPTA (50 mM Tris-HCl, 150 mM NaCl, 2 mM BAPTA, and 1 μM DTT, pH 7.5). For inactivation of CaM, CaM-binding buffer was supplemented with 100 μM W-7 (Tocris).
Split GFP.
DIV15 primary neurons were transduced with lentivirus expressing FUGW-CaM-GFP1-10 and FUGW-MATR3-GFP11, FUGW-CaMKIV-GFP11, or FUGW-H3-GFP1-10. At DIV19, transduced neurons were treated with vehicle or 100 μM NMDA for 3 h. Neurons were then photobleached (100 × 200 ms, 488 nM at 100% power, Sutter 421 multi-LED) using a Nikon Eclipse Ti inverted microscope before automated imaging using customized scripts written in the BeanShell environment of µManager (51). Separate Python scripts were used for image processing, neuron segmentation, establishment of regions of interest surrounding each cell, and measurement of mean GFP intensity (integrated density/area) for each neuron.
UV-CLIP and RT-PCR.
DIV14-16 primary neurons were pretreated with vehicle (DMSO) or 100 μM W-7 for 30 m prior to application of vehicle or 100 μM NMDA for 3 to 5 m before cross-linking at 254 nm in a Stratalinker 2400 (Stratagene) at 1,500 mJ/cm2. The cells were then lysed in RIPA buffer supplemented with cOmplete protease inhibitor (Roche) and RNaisin ribonuclease inhibitor (Promega) and sonicated at 80% amplitude, 5-s on/5-s off for 2 m using a Fisherbrand Model 505 Sonic Dismembrator. Cleared lysates were incubated overnight at 4 °C with 2 μg rabbit anti-MATR3 antibody/sample (ThermoFisher A300-591A) conjugated to Dynabeads Protein G (ThermoFisher). The following day, beads were washed 1× in NaCl wash buffer (20 mM Tris, 1% Triton X-100, 2 mM EDTA, 150 mM NaCl, and 0.1% SDS, pH 8.1), 1x in LiCl wash buffer (10 mM Tris, 1% NP-40, 1 mM EDTA, 0.25% Na-deoxycholate, and 250 mM LiCl, pH 8.0), and 2× in TE buffer (10 mM Tris and 1 mM EDTA, pH 8.0).
Samples were then split for either protein analysis, for which MATR3 complexes were removed from beads by heating at 95 °C for 10 m in SDS-PAGE sample buffer, or for RNA analysis. For the latter, beads were resuspended in elution buffer (50 mM Tris, 1 mM EDTA, 150 mM NaCl, 1% SDS, and 50 mM NaHCO3, pH 8.1) supplemented with RNasin (Promega) and incubated with proteinase K (New England Biolabs) for 2 h at 50 °C. RNA was extracted with TRIzol reagent (ThermoFisher) and chloroform, the suspension centrifuged at 21,000× g at 4 °C for 15 m, the aqueous phase removed, and RNA precipitated overnight at −20 °C and resuspended in water by heating at 65 °C for 10 m. cDNA was generated using iScript Reverse Transcriptase Supermix (Bio-Rad), and qPCR was performed with 200 nM each of forward and reverse primers and PowerUp SYBR Green Master Mix (ThermoFisher) according to manufacturer’s instructions.
Statistical Analysis.
Statistical analyses were performed in Prism 7 (GraphPad) or R. Data were plotted using Prism 7, and significance determined via the two-tailed t test. One-way ANOVA with Tukey’s posttest for more than two comparisons or unpaired t test for comparing two groups was used to assess for significant differences among protein abundances, RNA levels, nuclear/cytoplasmic ratios, and half-lives. Data are shown as mean ± SEM unless otherwise stated.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This work was funded by the National Institute for Neurological Disorders and Stroke (NINDS) R01NS097542 and R01NS113943 (S.B.), National Institute on Aging P30AG072931 (S.B.), the family of Angela Dobson and Lyndon Welch, the Robert Packard Center for Amyotrophic Lateral Sclerosis (ALS) Research (S.B.), National Institute of General Medical Sciences (NIGMS) T32 GM007863 (A.M.M.), NINDS F31 NS110119 (A.M.M.), and NIGMS T32 GM007315 (J.J.W). Portions of Figs. 2, 3, 7, and 8 were made with BioRender.
Author contributions
A.M.M., J.J.W., and S.B. designed research; A.M.M., J.J.W., C.A.G., Q.A.D., X.L., and H.H. performed research; E.H.M.T. and V.G.S. contributed new reagents/analytic tools; A.M.M., J.J.W., and S.B. analyzed data; and A.M.M., J.J.W., and S.B. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. U.P. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Purice M. D., Taylor J. P., Linking hnRNP function to ALS and FTD pathology. Front. Neurosci.-switz 12, 326 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai T., et al. , TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602–611 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Neumann M., et al. , Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Kabashi E., et al. , TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet. 40, 572–574 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Vance C., et al. , Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwiatkowski T. J., et al. , Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Ticozzi N., et al. , Mutational analysis reveals the FUS homolog TAF15 as a candidate gene for familial amyotrophic lateral sclerosis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 156B, 285–290 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Mackenzie I. R., et al. , TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron 95, 808–816.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H. J., et al. , Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 495, 467–473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belgrader P., Dey R., Berezney R., Molecular cloning of Matrin 3. A 125-kilodalton protein of the nuclear matrix contains an extensive acidic domain. J. Biol. Chem. 266, 9893–9899 (1991). [PubMed] [Google Scholar]
- 11.Hibino Y., Nakamura K., Tsukada S., Sugano N., Purification and characterization of nuclear scaffold proteins which bind to a highly repetitive bent DNA from rat liver. Biochim. Biophys. Acta (BBA) - Gene Struct. Expression 1174, 162–170 (1993). [DOI] [PubMed] [Google Scholar]
- 12.Hibino Y., et al. , Molecular properties and intracellular localization of rat liver nuclear scaffold protein P130. Biochim. Biophys. Acta (BBA) - Gene Struct. Expression, 1759, 195–207 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Senderek J., et al. , Autosomal-dominant distal myopathy associated with a recurrent missense mutation in the gene encoding the nuclear matrix protein, Matrin 3. Am. J. Hum. Genet. 84, 511–518 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson J. O., et al. , Mutations in the Matrin 3 gene cause familial amyotrophic lateral sclerosis. Nat. Neurosci. 17, 664–666 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millecamps S., et al. , Genetic analysis of matrin 3 gene in French amyotrophic lateral sclerosis patients and frontotemporal lobar degeneration with amyotrophic lateral sclerosis patients. Neurobiol. Aging 35, e13–2882.e15 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Lin K.-P., et al. , Mutational analysis of MATR3 in Taiwanese patients with amyotrophic lateral sclerosis. Neurobiol. Aging 36, e1–2005.e4 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Origone P., et al. , A novel Arg147Trp MATR3 missense mutation in a slowly progressive ALS Italian patient. Amyotroph. Lateral Scler. Frontotemporal Degener. 16, 530–531 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Leblond C. S., et al. , Replication study of MATR3 in familial and sporadic amyotrophic lateral sclerosis. Neurobiol. Aging 37, e17–209.e21 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Xu L., Li J., Tang L., Zhang N., Fan D., MATR3 mutation analysis in a Chinese cohort with sporadic amyotrophic lateral sclerosis. Neurobiol. Aging 38, e3–218.e4 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Marangi G., et al. , Matrin 3 variants are frequent in Italian ALS patients. Neurobiol. Aging 49, e1–218.e7 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Narain P., et al. , Identification and characterization of novel and rare susceptible variants in Indian amyotrophic lateral sclerosis patients. Neurogenetics 20, 197–208 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Dreser A., et al. , The ALS-linked E102Q mutation in Sigma receptor-1 leads to ER stress-mediated defects in protein homeostasis and dysregulation of RNA-binding proteins. Cell Death Differ. 24, 1655–1671 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tada M., et al. , Matrin 3 is a component of neuronal cytoplasmic inclusions of motor neurons in sporadic amyotrophic lateral sclerosis. Am. J. Pathol. 188, 507–514 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Kanai K., et al. , Altered axonal excitability properties in amyotrophic lateral sclerosis: Impaired potassium channel function related to disease stage. Brain 129, 953–962 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Vucic S., Nicholson G. A., Kiernan M. C., Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain 131, 1540–1550 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Marchand-Pauvert V., et al. , Absence of hyperexcitability of spinal motoneurons in patients with amyotrophic lateral sclerosis. J. Physiol. 597, 5445–5467 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa Y., et al. , Detecting motor unit abnormalities in amyotrophic lateral sclerosis using high-density surface EMG. Clin. Neurophysiol. 142, 262–272 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Pieri M., et al. , Altered excitability of motor neurons in a transgenic mouse model of familial amyotrophic lateral sclerosis. Neurosci. Lett. 351, 153–156 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Wainger B. J., et al. , Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 7, 1–11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devlin A.-C., et al. , Human iPSC-derived motoneurons harbouring TARDBP or C9ORF72 ALS mutations are dysfunctional despite maintaining viability. Nat. Commun. 6, 339 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremblay E., Martineau É., Robitaille R., Opposite Synaptic alterations at the neuromuscular junction in an ALS mouse model: When motor units matter. J. Neurosci. 37, 8901–8918 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-Silva M., et al. , Hypoexcitability precedes denervation in the large fast-contracting motor units in two unrelated mouse models of ALS. Elife 7, e30955 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amalyan S., et al. , Enhanced motor cortex output and disinhibition in asymptomatic female mice with C9orf72 genetic expansion. Cell Rep. 40, 111043 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Elbasiouny S. M., Motoneuron excitability dysfunction in ALS: Pseudo-mystery or authentic conundrum? J. Physiol. 600, 4815–4825 (2022), 10.1113/jp283630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L.-H., et al. , Calpain-2 mediates MBNL2 degradation and a developmental RNA processing program in neurodegeneration. J. Neurosci. Off. J. Soc. Neurosci. 42, 5102–5114 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fredj N. B., et al. , Depolarization-induced translocation of the RNA-binding protein Sam68 to the dendrites of hippocampal neurons. J. Cell Sci. 117, 1079–1090 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Tiruchinapalli D. M., et al. , Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and β-Actin mRNA in dendrites and spines of hippocampal neurons. J. Neurosci. 23, 3251–3261 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antar L. N., Afroz R., Dictenberg J. B., Carroll R. C., Bassell G. J., Metabotropic glutamate receptor activation regulates Fragile X mental retardation protein and Fmr1 mRNA localization differentially in dendrites and at synapses. J. Neurosci. 24, 2648–2655 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nalavadi V. C., et al. , Regulation of zipcode binding protein 1 transport dynamics in axons by myosin Va. J. Neurosci. Off. J. Soc. Neurosci. 32, 15133–15141 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weskamp K., et al. , Shortened TDP43 isoforms upregulated by neuronal hyperactivity drive TDP43 pathology in ALS. J. Clin. Invest. 130, 1139–1155 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giordano G., et al. , Activation of NMDA receptors induces protein kinase A-mediated phosphorylation and degradation of matrin 3. Blocking these effects prevents NMDA-induced neuronal death. J. Neurochem. 94, 808–818 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Valencia C. A., Ju W., Liu R., Matrin 3 is a Ca2+/calmodulin-binding protein cleaved by caspases. Biochem. Biophys. Res. Commun. 361, 281–286 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Shen X., et al. , Scanning the human proteome for calmodulin-binding proteins. Proc. Natl. Acad. Sci. U.S.A. 102, 5969–5974 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deisseroth K., Heist E. K., Tsien R. W., Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature 392, 198–202 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Mermelstein P. G., Deisseroth K., Dasgupta N., Isaksen A. L., Tsien R. W., Calmodulin priming: Nuclear translocation of a calmodulin complex and the memory of prior neuronal activity. Proc. Natl. Acad. Sci. U.S.A. 98, 15342–15347 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma H., et al. , γCaMKII shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell 159, 281–294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen S. M., et al. , Excitation-transcription coupling in parvalbumin-positive interneurons employs a novel CaM kinase-dependent pathway distinct from excitatory neurons. Neuron 90, 292–307 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen S. M., et al. , Calmodulin shuttling mediates cytonuclear signaling to trigger experience-dependent transcription and memory. Nat. Commun. 9, 2451 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rayaprolu S., et al. , Heterogeneity of Matrin 3 in the developing and aging murine central nervous system. J. Comp. Neurol. 524, 2740–2752 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng C., Fass D. M., Reynolds I. J., Emergence of excitotoxicity in cultured forebrain neurons coincides with larger glutamate-stimulated [Ca2+]i increases and NMDA receptor mRNA levels. Brain Res. 849, 97–108 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Malik A. M., et al. , Matrin 3-dependent neurotoxicity is modified by nucleic acid binding and nucleocytoplasmic localization. Elife 7, e35977 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores B. N., et al. , An intramolecular salt bridge linking TDP43 RNA binding, protein stability, and TDP43-dependent neurodegeneration. Cell Rep. 27, 1133–1150.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Archbold H. C., et al. , TDP43 nuclear export and neurodegeneration in models of amyotrophic lateral sclerosis and frontotemporal dementia. Sci Rep-uk 8, 4606 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weskamp K., Safren N., Miguez R., Barmada S., Monitoring neuronal survival via longitudinal fluorescence microscopy. J. Vis. Exp. 143 (2019), 10.3791/59036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arrasate M., Finkbeiner S., Automated microscope system for determining factors that predict neuronal fate. Proc. Natl. Acad. Sci. U.S.A. 102, 3840–3845 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kao C. S., et al. , Selective neuronal degeneration in MATR3 S85C knock-in mouse model of early-stage ALS. Nat. Commun. 11, 5304 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsubuki S., Saito Y., Tomioka M., Ito H., Kawashima S., Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine. J. Biochem. 119, 572–576 (1996). [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto A., et al. , Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in Rat Hepatoma cell line, H-4-II-E cells. Cell Struct. Funct. 23, 33–42 (1998). [DOI] [PubMed] [Google Scholar]
- 59.Bonvini P., Zorzi E., Basso G., Rosolen A., Bortezomib-mediated 26S proteasome inhibition causes cell-cycle arrest and induces apoptosis in CD-30+ anaplastic large cell lymphoma. Leukemia 21, 838–842 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Marco G. D., et al. , Effects of intracellular calcium accumulation on proteins encoded by the major genes underlying amyotrophic lateral sclerosis. Sci. Rep.-uk 12, 395 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi S., et al. , Calpain-mediated X-linked Inhibitor of apoptosis degradation in neutrophil apoptosis and its impairment in chronic neutrophilic leukemia*. J. Biol. Chem. 277, 33968–33977 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Chen Z., Boor P. J., Finnerty C. C., Herndon D. N., Albrecht T., Calpain-mediated cleavage of p53 in human cytomegalovirus-infected lung fibroblasts. Faseb Bioadv. 1, 151–166 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamakubo T., Kannagi R., Murachi T., Matus A., Distribution of calpains I and II in rat brain. J. Neurosci. 6, 3103–3111 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akashiba H., Matsuki N., Nishiyama N., Calpain activation is required for glutamate-induced p27 down-regulation in cultured cortical neurons. J. Neurochem. 99, 733–744 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Wang Y., Zhang Y., Regulation of TET Protein Stability by Calpains. Cell Rep. 6, 278–284 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y., Li Q., Youn J. Y., Cai H., Protein phosphotyrosine phosphatase 1B (PTP1B) in calpain-dependent feedback regulation of vascular endothelial growth factor receptor (VEGFR2) in Endothelial Cells IMPLICATIONS IN VEGF-DEPENDENT ANGIOGENESIS AND DIABETIC WOUND HEALING*. J. Biol. Chem. 292, 407–416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Z.-X., et al. , Precise prediction of calpain cleavage sites and their aberrance caused by mutations in cancer. Front. Genet. 10, 715 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramesh N., Kour S., Anderson E. N., Rajasundaram D., Pandey U. B., RNA-recognition motif in Matrin-3 mediates neurodegeneration through interaction with hnRNPM. Acta Neuropathol. Commun. 8, 138 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yap K. L., et al. , Calmodulin target database. J. Struct. Funct. Genom. 1, 8–14 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Mruk K., Farley B. M., Ritacco A. W., Kobertz W. R., Calmodulation meta-analysis: Predicting calmodulin binding via canonical motif clustering. J. Gen. Physiol. 144, 105–114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abbasi W. A., Asif A., Andleeb S., Ul Amir Afsar Minhas F., CaMELS: In silico prediction of calmodulin binding proteins and their binding sites. Proteins Struct. Funct. Bioinform. 85, 1724–1740 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Uemura Y., et al. , Matrin3 binds directly to intronic pyrimidine-rich sequences and controls alternative splicing. Genes Cells 22, 785–798 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Pearson R. B., Kemp B. E., Protein kinase phosphorylation site sequences and consensus specificity motifs: Tabulations. Methods Enzymol. 200, 62–81 (1991). [DOI] [PubMed] [Google Scholar]
- 74.Douglass J., et al. , Identifying protein kinase target preferences using mass spectrometry. Am. J. Physiol. Cell Physiol. 303, C715–27 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vorherr T., et al. , The calmodulin binding domain of nitric oxide synthase and adenylyl cyclase. Biochemistry-us 32, 6081–6088 (1993). [DOI] [PubMed] [Google Scholar]
- 76.Gu C., Cooper D. M. F., Calmodulin-binding sites on adenylyl cyclase Type VIII*. J. Biol. Chem. 274, 8012–8021 (1999). [DOI] [PubMed] [Google Scholar]
- 77.Masada N., Ciruela A., MacDougall D. A., Cooper D. M. F., Distinct mechanisms of regulation by Ca2+/calmodulin of Type 1 and 8 Adenylyl Cyclases support their different physiological roles*. J. Biol. Chem. 284, 4451–4463 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skeberdis V. A., et al. , Protein kinase A regulates calcium permeability of NMDA receptors. Nat. Neurosci. 9, 501–510 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Murphy J. A., et al. , Phosphorylation of Ser1166 on GluN2B by PKA is critical to synaptic NMDA receptor function and Ca2+ signaling in spines. J. Neurosci. 34, 869–879 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakamura T., Sato A., Kitsukawa T., Sasaoka T., Yamamori T., Expression pattern of immediate early genes in the cerebellum of D1R KO, D2R KO, and wild type mice under vestibular-controlled activity. Front. Cell Dev. Biol. 3, 38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knöferle J., et al. , Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc. Natl. Acad. Sci. U.S.A. 107, 6064–6069 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vingtdeux V., et al. , AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 285, 9100–9113 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Djakovic S. N., Schwarz L. A., Barylko B., DeMartino G. N., Patrick G. N., Regulation of the proteasome by neuronal activity and calcium/calmodulin-dependent protein kinase ii*. J. Biol. Chem. 284, 26655–26665 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park J. Y., Jang S. Y., Shin Y. K., Suh D. J., Park H. T., Calcium-dependent proteasome activation is required for axonal neurofilament degradation. Neural. Regen. Res. 8, 3401–3409 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fuentes J. L., Strayer M. S., Matera A. G., Molecular determinants of survival motor neuron (SMN) protein cleavage by the calcium-activated protease, calpain. Plos One 5, e15769 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamashita T., et al. , A role for calpain-dependent cleavage of TDP-43 in amyotrophic lateral sclerosis pathology. Nat. Commun. 3, 1307 (2012). [DOI] [PubMed] [Google Scholar]
- 87.Yamashita T., Teramoto S., Kwak S., Phosphorylated TDP-43 becomes resistant to cleavage by calpain: A regulatory role for phosphorylation in TDP-43 pathology of ALS/FTLD. Neurosci. Res. 107, 63–69 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Hornbeck P. V., et al. , PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 43, D512–D520 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rao M. V., Campbell J., Palaniappan A., Kumar A., Nixon R. A., Calpastatin inhibits motor neuron death and increases survival of hSOD1(G93A) mice. J. Neurochem. 137, 253–265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zech M., et al. , MATR3 haploinsufficiency and early-onset neurodegeneration. Brain 144, e72–e72 (2021). [DOI] [PubMed] [Google Scholar]
- 91.Tischbein M., et al. , The RNA-binding protein FUS/TLS undergoes calcium-mediated nuclear egress during excitotoxic stress and is required for GRIA2 mRNA processing. J. Biol. Chem. 294, 10194–10210 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kan M.-C., et al. , CPEB4 is a cell survival protein retained in the nucleus upon ischemia or endoplasmic reticulum calcium depletion. Mol. Cell Biol. 30, 5658–5671 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park J. H., et al. , Cytosolic calcium regulates cytoplasmic accumulation of TDP-43 through Calpain-A and Importin α3. Elife 9, e60132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamashita T., Aizawa H., Teramoto S., Akamatsu M., Kwak S., Calpain-dependent disruption of nucleo-cytoplasmic transport in ALS motor neurons. Sci. Rep.uk 7, 39994 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delane K. J., et al. , Calmodulin interacts with and regulates the RNA-binding activity of an Arabidopsis polyadenylation factor subunit. Plant Physiol. 140, 1507–1521 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saladino P., et al. , RNA-binding activity of the rat calmodulin-binding PEP-19 protein and of the long PEP-19 isoform. Int. J. Mol. Med. 29, 141–145 (2011). [DOI] [PubMed] [Google Scholar]
- 97.Darnell J. C., et al. , FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sasaki Y., et al. , Phosphorylation of zipcode binding protein 1 is required for brain-derived neurotrophic factor signaling of local -actin synthesis and growth cone turning. J. Neurosci. 30, 9349–9358 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feit H., et al. , Vocal cord and pharyngeal weakness with autosomal dominant distal myopathy: Clinical description and gene localization to 5q31. Am. J. Hum. Genet. 63, 1732–1742 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kraya T., Schmidt B., MüLLER T., Hanisch F., Impairment of respiratory function in late-onset distal myopathy due to MATR3 Mutation. Muscle Nerve 51, 916–918 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Palmio J., et al. , Re-evaluation of the phenotype caused by the common MATR3 p.Ser85Cys mutation in a new family. J. Neurol. Neurosurg. Psychiatry 87, 448 (2016). [DOI] [PubMed] [Google Scholar]
- 102.Zhao M., et al. , Knockdown of genes involved in axonal transport enhances the toxicity of human neuromuscular disease-linked MATR3 mutations in Drosophila. Febs Lett. 594, 2800–2818 (2020). [DOI] [PubMed] [Google Scholar]
- 103.Kemp C. M., Oliver W. T., Wheeler T. L., Chishti A. H., Koohmaraie M., The effects of Capn1 gene inactivation on skeletal muscle growth, development, and atrophy, and the compensatory role of other proteolytic systems. J. Anim. Sci. 91, 3155–3167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ince P., et al. , Parvalbumin and calbindin D-28k in the human motor system and in motor neuron disease. Neuropath. Appl. Neuro. 19, 291–299 (1993). [DOI] [PubMed] [Google Scholar]
- 105.Alexianu M. E., et al. , The role of calcium-binding proteins in selective motoneuron vulnerability in amyotrophic lateral sclerosis. Ann. Neurol. 36, 846–858 (1994). [DOI] [PubMed] [Google Scholar]
- 106.Saudou F., Finkbeiner S., Devys D., Greenberg M. E., Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95, 55–66 (1998). [DOI] [PubMed] [Google Scholar]
- 107.Edelstein A., Amodaj N., Hoover K., Vale R., Stuurman N., Computer control of microscopes using µManager. Curr. Protoc. Mol. Biol. 92, 14.20.1–14.20.17 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mercer A. A., Palarz K. J., Tabatadze N., Woolley C. S., Raman I. M., Sex differences in cerebellar synaptic transmission and sex-specific responses to autism-linked Gabrb3 mutations in mice. Elife 5, e07596 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ankri L., Yarom Y., Uusisaari M. Y., Slice it hot: Acute adult brain slicing in physiological temperature. J. Vis. Exp. Jove. 92, e52068 (2014), 10.3791/52068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schultz M. L., et al. , Synthetic high-density lipoprotein nanoparticles for the treatment of Niemann-Pick diseases. Bmc Med. 17, 200 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.