Significance
Intestinal epithelial cells undergo cell death via pyroptosis in response to invading Gram-negative bacterial pathogens due to the sensing of LPS, a conserved component of their outer envelopes. CASP4, a host cell protease that triggers pyroptosis via the activation of non-noncanonical inflammasomes, is activated upon recognition of LPS. Recent studies have proposed that GBP1, which surrounds invading pathogens, promotes the activation of CASP4 on the surface of intracytosolic bacteria. Here, we show that by targeting the degradation of GBPs, the Shigella type III secreted effector, IpaH9.8, prevents GBP1-mediated LPS release from intracellular Shigella to limit pyroptosis; this suggests that CASP4 is activated in the host cell cytosol rather than on the surface of the invading intracytosolic bacteria.
Keywords: caspase-4, LPS, inflammasome, GBP1
Abstract
Pyroptosis is an inflammatory form of cell death induced upon recognition of invading microbes. During an infection, pyroptosis is enhanced in interferon-gamma-exposed cells via the actions of members of the guanylate-binding protein (GBP) family. GBPs promote caspase-4 (CASP4) activation by enhancing its interactions with lipopolysaccharide (LPS), a component of the outer envelope of Gram-negative bacteria. Once activated, CASP4 promotes the formation of noncanonical inflammasomes, signaling platforms that mediate pyroptosis. To establish an infection, intracellular bacterial pathogens, like Shigella species, inhibit pyroptosis. The pathogenesis of Shigella is dependent on its type III secretion system, which injects ~30 effector proteins into host cells. Upon entry into host cells, Shigella are encapsulated by GBP1, followed by GBP2, GBP3, GBP4, and in some cases, CASP4. It has been proposed that the recruitment of CASP4 to bacteria leads to its activation. Here, we demonstrate that two Shigella effectors, OspC3 and IpaH9.8, cooperate to inhibit CASP4-mediated pyroptosis. We show that in the absence of OspC3, an inhibitor of CASP4, IpaH9.8 inhibits pyroptosis via its known degradation of GBPs. We find that, while some LPS is present within the host cell cytosol of epithelial cells infected with wild-type Shigella, in the absence of IpaH9.8, increased amounts are shed in a GBP1-dependent manner. Furthermore, we find that additional IpaH9.8 targets, likely GBPs, promote CASP4 activation, even in the absence of GBP1. These observations suggest that by boosting LPS release, GBP1 provides CASP4-enhanced access to cytosolic LPS, thus promoting host cell death via pyroptosis.
Host cell death via pyroptosis is a major arm of the human innate immune response and one of the host’s first lines of defense against invading microbial pathogens. Pyroptosis is an inflammatory lytic form of cell death that results in the loss of a niche for intracellular pathogens and the release of proinflammatory cytokines (1). Pyroptosis is triggered by the formation of inflammasomes, multiprotein complexes that assemble upon recognition of cytosolic pathogen-associated molecular patterns, including lipopolysaccharide (LPS) (2–4), the outer component of the cell envelope of Gram-negative bacteria. The assembly of inflammasomes leads to the activation of inflammatory caspases that cleave gasdermin D (GSDMD), a pore-forming protein whose N-terminal domain inserts into and forms pores in the plasma membrane of host cells, eventually leading to their lysis (5–7).
In human epithelial cells, infection with enteric Gram-negative pathogens primarily triggers pyroptosis via caspase-4 (CASP4) (8–11). CASP4 is activated upon binding to the hydrophobic membrane-embedded lipid A portion of LPS (4). As an infection progresses and the host responds, intestinal epithelial cells are exposed to cytokines, including interferon-gamma (IFNγ), which induce the expression of hundreds of genes, including guanylate-binding proteins (GBPs). GBPs play numerous roles in cell-autonomous immunity, including promoting the recognition of cytosolic LPS (cLPS) by CASP4 (12–15).
Upon entering the cytosol of IFNγ-primed epithelial cells, bacteria are encapsulated by GBPs. GBP1 is the first to bind via direct interactions with LPS (16, 17). Thousands of molecules of GBP1 intercalate into the outer bacterial membrane to form a stable coat, referred to as the GBP1 microcapsule or coatomer (16, 18). Subsequently, GBP2, GBP3, and GBP4 (19–21), and in some cases CASP4 (10, 16, 17, 22), are recruited to the bacteria. These observations led to the proposal that GBP1 provides CASP4 access to lipid A, thus enabling the assembly of CASP4 platforms on the surface of Gram-negative bacteria (10, 17).
Shigella species, the causative agents of bacillary dysentery, are a leading contributor to diarrheal mortality worldwide (23, 24). Shigella spp. establish a replicative niche within the cytosol of intestinal epithelial cells and spread from cell to cell using actin-based motility. The virulence of Shigella, like many other Gram-negative bacterial pathogens, is dependent on a type III secretion system (T3SS). T3SSs are complex membrane-embedded nanomachines that serve as a conduit for the direct transfer of bacterial proteins, referred to as effectors, into the cytosol of host cells. Shigella secrete ~30 unique effectors, including OspC3, an effector that directly posttranslationally modifies CASP4 to inhibit pyroptosis (8, 10, 25, 26).
In this study, we demonstrate that Shigella IpaH9.8, an effector that targets the ubiquitination and degradation of GBPs (19–21), cooperates with OspC3 to limit Shigella-triggered pyroptosis. We demonstrate that while intracellular Shigella release LPS into the cytosol of host cells, in the absence of IpaH9.8, these levels are increased threefold to fivefold in a GBP1-dependent manner. Furthermore, we find evidence for GBP1-independent pyroptotic cell death of IFNγ-primed epithelial cells in response to infection with Shigella that lack OspC3 and IpaH9.8 but not with Shigella deficient for only OspC3. These observations demonstrate that GBP1 is not essential for CASP4 activation in response to invading bacteria. Furthermore, they suggest that GBP1 promotes LPS release from bacteria into the host cell cytosol, after which GBP2 and/or GBP4, IFNγ-induced targets of IpaH9.8, accelerate pyroptosis in intestinal epithelial cells due to the activation of noncanonical CASP4 inflammasomes.
Results
Infection with Shigella Strains That Lack OspC3 but Not IpaH9.8 Triggers Pyroptosis of IFNγ-Primed Epithelial Cells.
Shigella species are intracellular pathogens that establish a replicative niche within the cytosol of colonic epithelial cells, spreading from cell to cell using actin-based motility. To do so, it is essential that Shigella, inhibit host cell death. We, and others, previously reported that Shigella OspC3 suppresses cell death via pyroptosis of infected naive untreated epithelial cells via inhibition of CASP4 (8, 10, 25, 27).
Propidium iodide (PI), a membrane-impermeable dye that rapidly enters cells via GSDMD pores, is commonly used as a real-time reporter of pyroptosis. We observed similar evidence of PI uptake of infected cells using a plate reader or automated microscopy–based assays. An advantage of the latter approach was that we could quantify the percentage of pyroptotic cells by analyzing images of cells exposed to both PI and Hoechst, a membrane-permeable dye taken up by all cells, dead or alive. In each case, gentamicin was added to kill any that remained extracellular after providing time for the bacteria to invade host cells.
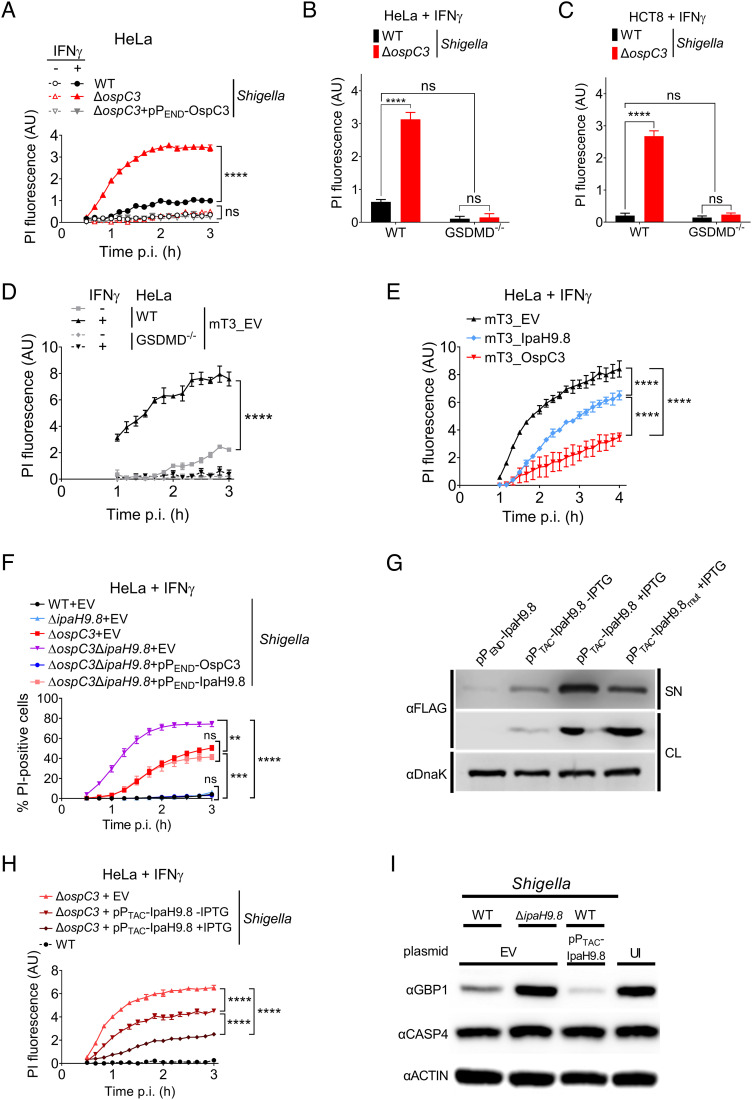
We first compared the fate of IFNγ-primed and untreated epithelial cells infected with ΔospC3 Shigella. Under all conditions tested, regardless of the presence of AfaI, an adhesin that promotes host cell attachment and synchronizes host cell invasion (28), as previously reported (10, 27), infection with ΔospC3 Shigella triggered significant levels of epithelial cell death as assessed by PI uptake of IFNγ-primed HeLa (Fig. 1 A and B and SI Appendix, Fig. S1 A and B) and HCT8 cells (Fig. 1C and SI Appendix, Fig. S1C) or release of lactate dehydrogenase (LDH) (SI Appendix, Fig. S1D). In each case, cell death was dependent on GSDMD (Fig. 1 B and C and SI Appendix, Fig. S1E), establishing that PI uptake reflected activation of pyroptosis. We conducted all the following experiments with AfaI-expressing Shigella.
Fig. 1.
Shigella OspC3 and IpaH9.8 cooperate to suppress bacteria-triggered pyroptosis of IFNγ-primed epithelial cells. (A–F, H, and I) WT or GSDMD−/− HeLa or HCT8 cells unprimed or primed overnight with 10 ng/mL IFNγ were infected with the indicated strains at an MOI of 100 (A), 10 (B–F, and H), or 5 (I). Cells were infected with Shigella strains that carry pBAD33-AfaI and designated plasmids (B and C, H–I). For Shigella, 30 min postinfection (p.i.) (A–C, F, H, and I) and for mT3, 1 h p.i. (D and E), PI (A–E, and H) or PI and Hoechst (F) were added to the medium, and cell death was measured by monitoring PI upt,ake using a plate reader (A–E, and H) or PI and Hoechst uptake using an automated fluorescence microscope (F). A time course of cell death is shown in (A, and D–F) and a 3-h end point in (B). (G) Immunoblots of secretion assays of IpaH9.8-FLAG expressed from designated plasmids by ΔospC3 Shigella. SN (supernatant) and CL (cell lysate) fractions were probed with anti-FLAG to detect secreted and expressed effectors and DnaK, a cytosolic Shigella protein, as a lysis and loading control. (I) Immunoblots of lysates of IFNγ-primed HeLa cells infected with indicated Shigella strains probed with indicated antibodies. EV = empty vector, UI = uninfected, and AU = arbitrary units. Values shown are the mean ± SEM of three experimental replicates. Data were analyzed using two-way ANOVA with Tukey’s post hoc test. **P < 0.01, ***P < 0.001, ****P < 0.0001, ns = nonsignificant.
GBPs are among some of the most highly induced genes upon exposure of epithelial cells to IFNγ and have been shown to promote activation of CASP4. Multiple members of this family, including human GBP1, GBP2, GBP4, and GBP6, are targeted for degradation by Shigella IpaH9.8 (19). However, like others (20, 27), we observed no evidence of cell death of IFNγ-primed HeLa or HCT8 cells infected with ΔipaH9.8 Shigella (SI Appendix, Fig. S1 F and G) under conditions where, as expected, we detected IFNγ-dependent GBP1 expression (SI Appendix, Fig. S1H).
OspC3 and IpaH9.8 Suppress mT3Ec-Triggered Cell Death of IFNγ-Primed Epithelial Cells.
Type III secreted effectors often act in an additive or functionally redundant manner to target host cell processes such that phenotypes associated with their absence from bacteria are not detected using traditional top-down approaches with single deletion strains. Reasoning that IpaH9.8 acts in concert with other effectors to suppress cell death, we investigated whether we could identify a role for IpaH9.8 in regulating cell death using a bottom-up platform.
mT3.1 Escherichia coli, referred to herein as mT3Ec, is a variant of nonpathogenic DH10β E. coli that expresses the Shigella Mxi, Spa, and Inv operons. These operons encode the structural components needed to form the Shigella type III secretion apparatus (T3SA) plus four embedded effectors (29). mT3Ec, like wild-type (WT) Shigella, invades and efficiently escapes into the cytosol of epithelial cells (29). In contrast to WT Shigella, mT3Ec invasion into epithelial cells triggers host cell death via pyroptosis (8). mT3Ec can secrete the full complement of Shigella effectors (8). In a prior study with unprimed epithelial cells, we found that the addition of OspC3, but not IpaH9.8, inhibits mT3Ec-triggered cell death (8).
Given that IFNγ priming resulted in enhanced ΔospC3 Shigella–triggered cell death, we reasoned that IFNγ-primed cells would exhibit a more robust cell death response when infected with mT3Ec. This was the case, as we observed enhanced mT3Ec-triggered cell death of IFNγ-primed as compared to unprimed HeLa and HCT8 epithelial cells, which was dependent on GSDMD (Fig. 1D and SI Appendix, Fig. S1I). This demonstrates that infection with mT3Ec, like ΔospC3 Shigella, triggers pyroptosis of IFNγ-primed epithelial cells.
We next compared the PI uptake of IFNγ-primed cells infected with mT3Ec that contain an empty plasmid (mT3Ec_EV) to variants that express and secrete OspC3 (mT3Ec_OspC3) or IpaH9.8 (mT3Ec_IpaH9.8) (8). The addition of plasmids that express each effector under the control of an isopropyl β-D-1-thiogalactopyranoside (IPTG)-inducible PTAC promoter (pPTAC-OspC3 or pPTAC-IpaH9.8) led to significantly diminished mT3Ec-triggered cell death, OspC3 to a greater extent than IpaH9.8 (Fig. 1E). These observations suggested that IpaH9.8, like OspC3, plays a role in inhibiting Shigella-triggered pyroptosis.
OspC3 and IpaH9.8 Cooperate to Inhibit Shigella-Triggered Cell Death of IFNγ-Primed Epithelial Cells.
As we observed no evidence for IpaH9.8 in suppressing pyroptosis when absent from otherwise WT Shigella, we reasoned that IpaH9.8-mediated GBP degradation acts upstream of OspC3 to limit CASP4 activation. Thus, we compared the fate of IFNγ-primed epithelial cells infected with WT, ΔospC3, or ΔospC3ΔipaH9.8 Shigella. HeLa cells infected with ΔospC3ΔipaH9.8 were ~1.5-fold more likely than those infected with ΔospC3 Shigella to trigger PI uptake (Fig. 1F) or LDH release (SI Appendix, Fig. S1J), thus exhibiting evidence of enhanced cell death via pyroptosis. We found that the introduction of a plasmid that encodes IpaH9.8 under the control of its endogenous promoter (PEND) reduced ΔospC3ΔipaH9.8 Shigella–triggered cell death to levels equivalent to ΔospC3 Shigella. IFNγ-primed HCT8 cells behaved similarly when infected with the same set of strains (SI Appendix, Fig. S1K). ΔospC3ΔipaH9.8 Shigella–triggered cell death was completely GSDMD dependent (SI Appendix, Fig. S1L).
IpaH9.8 is a “second-wave” Shigella effector whose expression is regulated by MxiE, a transcription factor only activated after contact with host cells (30). To gain additional evidence that IpaH9.8 limits Shigella-triggered cell death, we investigated whether we could suppress ΔospC3 Shigella–triggered cell death by increasing the amount and accelerating the timing of translocation of IpaH9.8 into host cells. Thus, we infected IFNγ-primed epithelial cells with ΔospC3 Shigella that carry pPTAC-IpaH9.8. The PTAC promoter is leaky but inducible in Shigella, such that, even in the absence of IPTG, ΔospC3/PTAC-IpaH9.8 Shigella secrete more IpaH9.8 than ΔospC3/ PEND-IpaH9.8, which express IpaH9.8 under the control of its endogenous promoter (Fig. 1G). We observed decreased levels of ΔospC3 Shigella–triggered cell death with increasing levels of IpaH9.8 (Fig. 1H).
In addition to GBPs, IpaH9.8 has also been reported to promote the ubiquitination and degradation of NEMO/IKKγ (31) and U2AF (32). To assess whether IpaH9.8 suppresses cell death via its targeting of GBPs, we tested whether secretion of IpaH9.8_Y86A/Q88A (IpaH9.8mut) (Fig. 1G), a variant impaired in GBP binding (33), suppressed cell death. This was not the case (SI Appendix, Fig. S1M). To confirm that increased IpaH9.8 secretion led to decreased cell death due to increased degradation of GBPs, we compared levels of GBP1 within lysates of cells infected with WT, ΔipaH9.8, and WT/pPTAC -IpaH9.8 Shigella. As expected, we observed decreased levels of GBP1 within lysates of cells infected with WT compared to ΔipaH9.8 Shigella, which were further reduced in cells infected with WT/PTAC-IpaH9.8 Shigella (Fig. 1I). We conducted these studies with cells infected with Shigella that encode OspC3 to ensure that any observed differences in GBP1 levels were not reflective of protein loss due to cell lysis. Together, these data establish a role for IpaH9.8-mediated GBP degradation in inhibition of Shigella-triggered pyroptosis.
OspC3 and IpaH9.8 Cooperate to Promote the Growth of Intracytosolic Shigella.
We next expanded our automated microscopy–based cell death assay to follow the real-time replication of bacteria within infected epithelial cells. To differentiate between intra- and extracellular bacteria, we infected with Shigella that express superfolder green fluorescent protein (sfGFP) under the control of the arabinose-inducible araBAD promoter (PBAD). Following treatment with gentamicin to kill extracellular bacteria, we introduced arabinose to induce sfGFP expression (SI Appendix, Fig. S2A). Under these conditions, sfGFP-expressing bacteria represent live replicating intracellular bacteria.
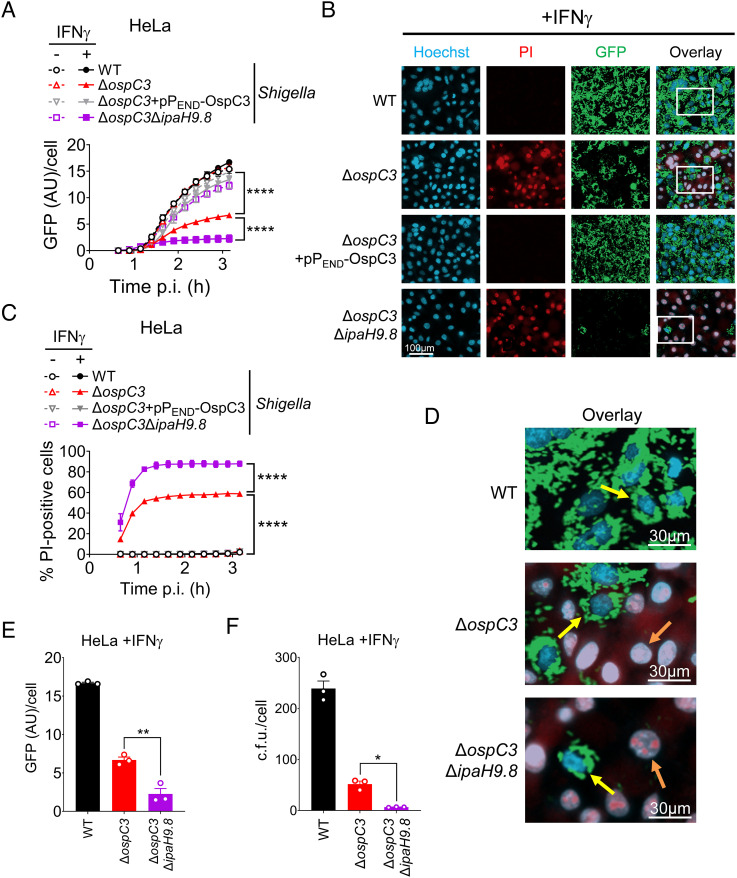
Using this assay, we compared the fate of WT, ΔospC3, ΔospC3/PEND-OspC3, and ΔospC3ΔipaH9.8 Shigella within untreated and IFNγ-primed epithelial cells. We detected evidence of sfGFP-expressing intracellular bacteria starting at ~80 min after the addition of arabinose (SI Appendix, Fig. S2B). We found similar rates of increasing GFP signal/infected cell within untreated epithelial cells infected with all four strains (Fig. 2A), demonstrating that OspC3 and IpaH9.8 are not involved in host cell invasion, as previously reported for OspC3 (8). In contrast, we observed decreased evidence of replication of ΔospC3ΔipaH9.8 and ΔospC3 Shigella, greater with the double deletion strain, within IFNγ-primed cells (Fig. 2A). As expected, only infection of IFNγ-primed epithelial cells with ΔospC3 and ΔospC3ΔipaH9.8 triggered cell death, as assessed by PI uptake (Fig. 2 B–D and SI Appendix, Fig. S2B).
Fig. 2.
Shigella OspC3 and IpaH9.8 cooperate to promote the growth of intracytosolic Shigella. (A–E) WT HeLa cells unprimed or primed overnight with 10 ng/mL IFNγ were infected at an MOI of 3 with designated strains, each of which carries pBAD33-sfGFP, pBR322-AfaI, and pPEND-OspC3 or an EV. Thirty minutes postinfection (p.i.), PI, Hoechst, and arabinose were added to the medium, and cells were imaged using an automated fluorescent microscope. Time course of total GFP divided by the number of Hoechst-positive (Hoechst+) nuclei in (A) and percentage of PI+/Hoechst+ cells in (B). Values shown are the mean ± SEM of three experimental replicates. (C-F) Representative images of cells infected with the designated strains 3 h p.i. (C and E) with enlarged Insets are shown in (D and F), PI (red), Hoechst (blue), and sfGFP (green). Yellow arrows point to live PI−GFP+ cells, while orange arrows point to dead PI+GFP cells. (F) WT HeLa cells primed overnight with 10 mg/mL IFNγ were infected at an MOI of 3 with designated strains that contain pBAD33-sfGFP and pNG162-AfaI. Three hours p.i., the cells were lysed, and intracellular bacteria were enumerated. Three biological replicates were performed, and representative data are shown. Statistical significance when indicated was assessed by one- (E and F) or two-way (A and C) ANOVA with Tukey’s post hoc test. *P < 0.05, **P < 0.01, ****P < 0.0001, ns = nonsignificant.
Representative images of IFNγ-primed cells infected with each strain acquired 3 h postinfection (p.i.) revealed the existence of two distinct populations of ΔospC3 and ΔospC3ΔipaH9.8 Shigella infected cells: PI+ cells with no evidence of sfGFP+ Shigella and PI− cells populated with sfGFP bacteria. The number of sfGFP+ ΔospC3 Shigella and ΔospC3ΔipaH9.8 observed within live (PI−) cells was similar to that observed within cells infected with WT Shigella (Fig. 2 B and D). While we were unable to detect bacteria within the pyroptotic PI+ cells, bacteria likely invaded these cells as, under these experimental conditions (MOI 10), we found sfGFP+ WT, ΔospC3, and ΔospC3ΔipaH9.8 Shigella within >95% of unprimed infected cells (SI Appendix, Fig. S2D).
To investigate whether the absence of sfGFP+ bacteria reflected loss of viable bacteria, we conducted gentamicin protection assays with WT, ΔospC3-, and ΔospC3ΔipaH9.8 Shigella–infected IFNγ-primed epithelial cells. We observed similar trends in the intensity of intracellular sfGFP signal associated with each strain and the number of viable intracellular bacteria (Fig. 2 E and F). Together, these observations are consistent with those of others who have reported that the growth of Shigella within the cytosol of pyroptosing cells is inhibited (10).
It has previously been proposed that GSDMD promotes the killing of intracytosolic bacteria directly via disruption of its outer membrane (7). Consistent with this hypothesis, the growth of ΔospC3ΔipaH9.8 Shigella was restored within the cytosol of GSDMD−/− epithelial cells (SI Appendix, Fig. S2 E and F). However, it is also possible that the formation of GSDMD pores enables the uptake of gentamicin into the cytosol of host cells. Future studies are needed to differentiate between these two possibilities. Nevertheless, collectively these studies demonstrate that via the concerted efforts of OspC3 and IpaH9.8, Shigella inhibits host cell death to establish a productive replicative niche within IFNγ-primed epithelial cells.
Intracytosolic Shigella Inhibit Pyroptosis in an IpaH9.8-Dependent Manner.
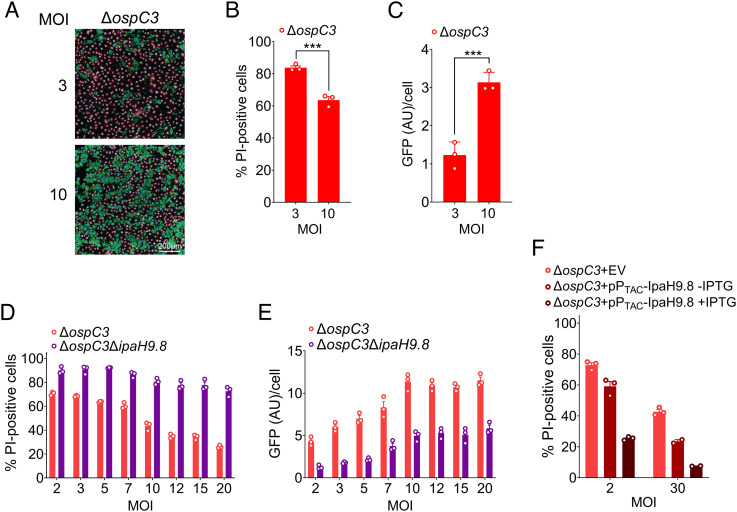
Our finding that a subset of ΔospC3 Shigella–infected epithelial cells evaded cell death was unexpected as it was previously reported that all host cells infected with ΔospC3 Shigella rapidly undergo pyroptosis (10). A significant difference between our experimental design and the earlier study was the multiplicity of infection (MOI) studied. Thus, we compared the fate of primed epithelial cells infected with ΔospC3 Shigella at an MOI of 3 versus 10. Strikingly, with the lower MOI, we observed a higher percentage of PI+ pyroptosing cells (Fig. 3 A and B) and decreased bacterial GFP fluorescence (Fig. 3 A and C).
Fig. 3.
Shigella inhibits pyroptosis in an IpaH9.8-dependent manner. (A–F) WT HeLa cells primed with 10 ng/mL IFNγ overnight were infected with designated strains at the specified MOIs. Strains carry either pBAD33-sfGFP and pNG162-AfaI (A–E) or pBAD33-AfaI and the designated plasmids (F). Thirty minutes p.i., PI, Hoechst, and arabinose were added to the medium, after which cells were imaged using an automated fluorescent microscope.(A-C, E-F) Quantification of % PI-positive cells (PI+/Hoechst+ cells) 1.5 h p.i. (A, C, and F) and GFP fluorescence/Hoechst+ cell at 3 h p.i. (B and E). Representative images of cells infected with ΔospC3 Shigella at designated MOIs at 3 h p.i. (C). Values shown are the mean ± SEM of three experimental replicates. Three biological replicates were performed, and representative data are shown. For (A) and (B), single time points of a time course are shown, and statistical significance was assessed by an ordinary one-way ANOVA. ***P < 0.001.
We expanded these studies to include both ΔospC3 and ΔospC3ΔipaH9.8 Shigella, testing MOIs from 2 to 20. We observed decreasing evidence of pyroptosing cells with increasing MOIs (Fig. 3D and SI Appendix, Fig. S3 A and B). This phenomenon was more striking when infecting with ΔospC3 compared to ΔospC3ΔipaH9.8 Shigella, i.e., 65 to 20% versus 90 to 80% cell death (Fig. 3D and SI Appendix, Fig. S3A). We also observed increased evidence of intracytosolic bacterial replication as the MOI was raised (SI Appendix, Fig. S3 C and D), with a direct correlation between detectable GFP fluorescence and MOI (Fig. 3E).
These observations suggested that a significant driving factor in determining whether infected host cells undergo rapid cell death upon Shigella invasion is the presence of IpaH9.8. To directly test this hypothesis, we infected the cells with ΔospC3/PTAC-IpaH9.8 Shigella, which secretes excess IpaH9.8 (Fig. 1G). Regardless of the MOI, we found that ΔospC3 Shigella–triggered host cell death decreased as levels of IpaH9.8 were increased (Fig. 3F). Remarkably, at an MOI of 30, IPTG-induced expression of IpaH9.8 reduced ΔospC3 Shigella–triggered cell death to almost undetectable levels. These data suggest significant role(s) for GBP1, GBP2, GBP4, and/or GBP6, the targets of IpaH9.8, in promoting rapid host cell death via CASP4-mediated pyroptosis.
GBP1 Enhances LPS Release from Intracytosolic Shigella.
We next investigated the molecular mechanism by which IpaH9.8 limits Shigella-triggered pyroptosis. Upon invasion into host cells, Shigella are encapsulated by GBP1, followed by GBP2, GBP3, and GBP4. GBP1 polymers bind to the exposed outer O-antigen polysaccharide portion of LPS on the surface of the bacteria and then depolymerize into a bacteria-engulfing microcapsule (16, 20), which acts as an LPS surfactant to destabilize the rigidity of the outer bacterial membrane (16). In the case of Shigella, this binding not only impairs IcsA localization and actin tail formation but also increases the in vitro sensitivity of Shigella to polymyxin B, an antimicrobial peptide that targets the outer bacterial envelope (16). As GBP1 promotes destabilization of the outer membrane, we hypothesized that its binding enhances the release of bacterial LPS into the cytosol of host cells.
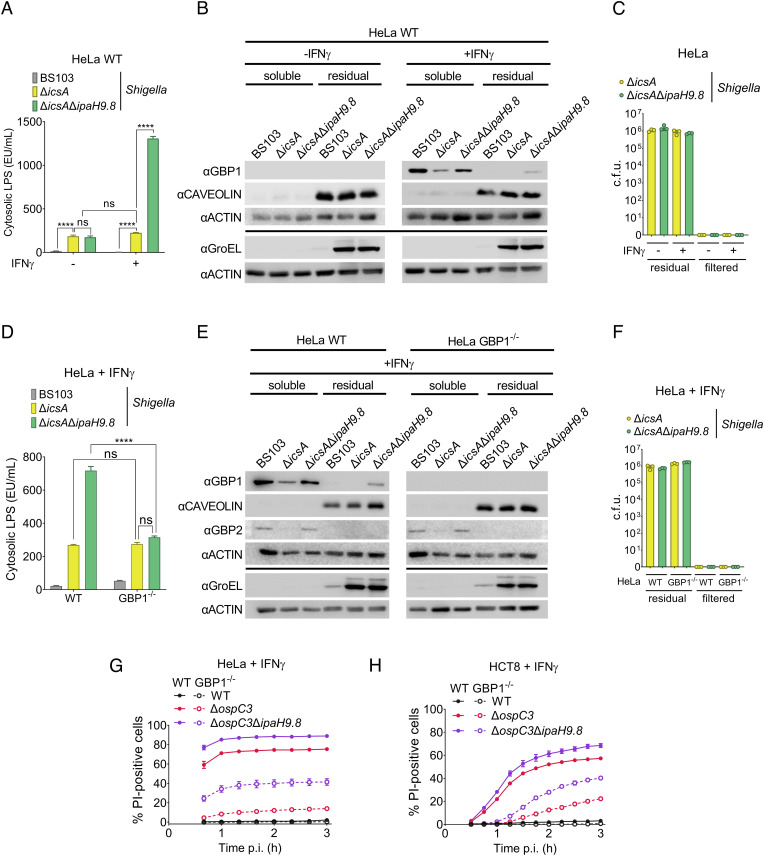
To address this possibility, we developed an assay to monitor cLPS levels adapted from one previously designed to monitor the uptake of extracellular bacterial outer membrane vesicles (OMVs) into the host cell cytosol (34). 2 h p.i. Two hours p.i., we exposed Shigella-infected epithelial cells to digitonin under conditions that selectively lyse the host cell plasma membrane (35). The cytosol and residual fractions were separated via a low-speed spin, after which we filtered the cytosolic fractions to remove any remaining bacteria. Shigella in the residual and filtered supernatant fractions were enumerated, and LPS levels in the cytosolic fractions were quantified using a Limulus amoebocyte lysate (LAL) assay. We assessed fractionation efficiency and GBP1 levels by immunoblotting for GBP1, caveolin, a plasma membrane protein, GroEL, a cytosolic Shigella protein, and actin.
Given that the absence of IpaH9.8 is associated with impaired Shigella cell-to-cell spread within IFNγ-primed epithelial cells due to delayed actin tail formation (20, 21), we conducted these studies with variants of Shigella that lack IcsA, i.e., ΔicsA and ΔicsAΔipaH9.8 Shigella. Infection with neither of these OspC3-containing strains triggered pyroptosis of IFNγ-primed epithelial cells (SI Appendix, Fig. S4A). We also infected cells with noninvasive virulence plasmid–minus BS103 Shigella (20, 21) to assess whether host cell invasion is needed to detect cLPS.
We first compared cLPS levels in lysates of IFNγ-primed and unprimed epithelial cells infected with ΔicsA, ΔicsAΔipaH9.8, and BS103 Shigella (Fig. 4A). We observed similar levels of cLPS within lysates of unprimed cells infected with ΔicsA and ΔicsAΔipaH9.8 Shigella. Strikingly, in contrast we detected ~fivefold higher levels of cLPS within lysates of IFNγ-primed cells infected with ΔicsAΔipaH9.8 compared to ΔicsA Shigella, while the levels of cLPS present in the ΔicsA Shigella–infected cells were equivalent to those found in unprimed cells. As expected, we found diminished levels of GBP1 in the lysates of primed cells infected with ΔicsA as compared to ΔicsAΔipaH9.8 Shigella (Fig. 4B). Equivalent numbers of ΔicsA and ΔicsAΔipaH9.8 Shigella were present within the residual fractions (Fig. 4C). No GBP1 was detected in unprimed cells (Fig. 4B), and no bacteria were found in the filtered cytosolic fractions (Fig. 4C). Complementation of ΔicsAΔipaH9.8 with a plasmid that conditionally expresses WT (pPTAC-IpaH9.8) but not catalytically dead IpaH9.8_C337A (pPTAC-IpaH9.8_C337A) (19) resulted in limited LPS release from intracytosolic bacteria (SI Appendix, Fig. S4 B–D). Together, these data demonstrate that intracellular Shigella shed LPS into the host cell cytosol of unprimed cells, which is substantially enhanced in IFNγ-primed cells due to the action of one or more IFNγ-induced targets of IpaH9.8 expressed in intestinal epithelial cells (GBP1, GBP2, and/or GBP4).
Fig. 4.
GBP1 enhances LPS release from intracellular Shigella but is not essential for the induction of pyroptosis of infected cells. (A–H) WT or GBP1−/− HeLa or HCT8 cells unprimed or primed overnight with 10 ng/mL IFNγ were infected with the indicated Shigella strains that each carry pNG162-AfaI at an MOI of 3. (A–F) Two hours p.i., cells were lysed with 0.005% digitonin and separated into cytosolic and residual fractions. LPS levels in filtered cytosolic extracts were quantified using a LAL assay (A and D); fractionation efficiency and GBP1 levels were assessed by immunoblotting fractions with designated antibodies (B and E), and numbers of bacteria within the residual and filtered supernatant fractions were enumerated (C and F). (G and H) Thirty minutes p.i., gentamicin, PI, and Hoechst were added to the medium, and cells were imaged using an automated fluorescent microscope. Time courses of cell death PI+/Hoechst+ cells are shown. Values shown are the mean ± SEM of three experimental replicates. Where indicated, statistical significance was assessed using two-way ANOVA with Tukey’s post hoc test. ****P < 0.0001, ns = nonsignificant.
To investigate a direct role for GBP1 in mediating LPS release, we generated two independent GBP1−/− HeLa cell lines (GBP1−/− 1-9 and GBP1−/− 1-10) using CRISPR/Cas9 editing. After confirming that each line still expressed its closest homolog, GBP2 (SI Appendix, Fig. S4E), we compared the levels of cLPS present within the parental and modified IFNγ-primed cell lines infected with BS103 and ΔicsAΔipaH9.8 Shigella. For each of the two GBP1−/− cell lines, we observed an ~threefold decrease in detectable cLPS (SI Appendix, Fig. S4F). We extended these studies and compared cLPS levels within lysates of IFNγ-primed WT and GBP1−/− cells infected with BS103, ΔicsA, and ΔicsAΔipaH9.8 Shigella. We again observed substantially lower levels of cLPS within GBP1−/− as compared to WT cells infected with ΔicsAΔipaH9.8 Shigella (Fig. 4D). As expected, we found diminished levels of GBP1 and GBP2 in the lysates of primed cells infected with ΔicsA as compared to ΔicsAΔipaH9.8 Shigella and no GBP1 in GBP1−/− cells (Fig. 4E). Equivalent numbers of ΔicsA and ΔicsAΔipaH9.8 Shigella were present within the residual fractions (Fig. 4F). Strikingly, we detected equivalent levels of cLPS in lysates of IFNγ-primed WT cells infected with ΔicsA Shigella and GBP1−/− cells infected with ΔicsA and ΔicsAΔipaH9.8 Shigella.
As a control, we also investigated whether GSDMD plays a role in promoting LPS from intracellular Shigella. However, in contrast to the absence of GBP1, we observe no differences in cLPS present in the lysates of IFNγ-primed WT and GSDMD−/− cells infected with ΔicsA as compared to ΔicsAΔipaH9.8 (SI Appendix, Fig. S4 G and I). Together, these data suggest that there is a baseline release of LPS from intracytosolic Shigella, which is enhanced in a GBP1-dependent manner.
GBP1 Promotes but Is Not Essential for the Activation of Pyroptosis in Response to Invading Bacteria.
Given that GBP1 promotes LPS release from Shigella and LPS promotes CASP4 activation, we next compared the fate of IFNγ-primed WT and GBP1−/− HeLa cells when infected with WT, ΔospC3, and ΔospC3ΔipaH9.8 Shigella. Consistent with prior studies with IFNγ-primed HeLa cells (10), we observed almost complete suppression of ΔospC3 Shigella–triggered cell death in the absence of GBP1 (Fig. 4G). However, in contrast, we observed only ~50% suppression of ΔospC3ΔipaH9.8 Shigella–triggered cell death, which was suppressed to almost undetectable levels in the presence of disulfiram, a small-molecule inhibitor of GSDMD and pyroptosis (36) (SI Appendix, Fig. S5A).
We also compared the fate of WT and GBP1−/− HCT8 cells (SI Appendix, Fig. S5B) infected with WT, ΔospC3, and ΔospC3ΔipaH9.8 Shigella. In this case, we again observed significant suppression of ΔospC3 and moderate suppression of ΔospC3ΔipaH9.8 Shigella–triggered cell death in the absence of GBP1 (Fig. 4H), albeit in this case, the onset of cell death was delayed by at least 60 min. Cell death was further suppressed in the presence of disulfiram (SI Appendix, Fig. S5C), demonstrating that during this period, all PI uptake reflected death via pyroptosis.
To confirm that IpaH9.8 targeting of other GBPs, and not one of its other targets, limits cell death of GBP1−/− cells, we next compared the fate of WT and GBP1−/− HeLa and HCT8 cells infected with ΔospC3ΔipaH9.8 that carry an empty vector (EV) or plasmids that encode WT (pPTAC-IpaH9.8) or IpaH9.8_Y86A/Q88A (pPTAC-IpaH9.8_Y86A/Q88A), the mutant impaired in GBP binding. As expected, with each of the four cell lines, we found that the introduction of WT, but not IpaH9.8_Y86A/Q88A, resulted in significantly decreased ΔospC3ΔipaH9.8-triggered cell death (SI Appendix, Fig. S5 D-G).
The major difference between GBP1−/− cells infected with ΔospC3 versus ΔospC3ΔipaH9.8 Shigella is that those infected with bacteria that secrete Ipa9.8 have significantly lower levels of GBP2, GBP4, and GBP6. Thus, together, these studies demonstrate that GBP1 is not essential for the activation of noncanonical CASP4 inflammasomes and suggest that other IFNγ-induced targets of IpaH9.8 that are expressed in intestinal epithelial cells, e.g., GBP2 and/or GBP4, promote CASP4 activation, even in the absence of GBP1.
Discussion
To establish a replicative niche within the cytosol of human intestinal epithelial cells, it is essential for invading pathogens to evade host cell death via pyroptosis. In the case of Gram-negative bacterial pathogens, host cell death is induced upon CASP4 recognition of LPS, a response that is enhanced in IFNγ-primed cells. Upon entry into the cytosol of IFNγ-primed cells, bacteria are rapidly engulfed in a GBP1 microcapsule (16), followed by GBP2, GBP3, and GBP4, and in a small subset CASP4 (10, 16). These observations have led to the proposal that GBPs promote the assembly of CASP4 inflammasomes on the surface of bacteria (10, 17).
Shigella IpaH9.8, one of its type III secreted effectors, targets the ubiquitination and degradation of multiple human GBPs, e.g., GBP1, GBP2, GBP4, and GBP6. Previous studies have shown that in the absence of IpaH9.8, CASP4 is recruited to <10% of intracytosolic Shigella (10, 16). In this study, using a variety of complementary approaches, we have established that IpaH9.8 acts upstream of OspC3 to suppress Shigella-triggered cell death. Using a quantitative LAL assay, we found that Shigella releases LPS into the cytosol of unprimed host cells. In the absence of IpaH9.8, within IFNγ-primed cells, these levels are increased threefold to fivefold in a GBP1-dependent manner. Consistent with this hypothesis, Zhu et al. found that GBP1 enhances the release of labeled LPS from intracellular S. Typhimurium using a fluorescent microscopy–based assay (18). These observations suggest that GBP1 mediates LPS release from intracellular bacteria into the host cell cytosol.
It has been proposed that GBP1 is essential for the activation of CASP4 inflammasomes. Yet, there is extensive evidence of CASP4 activation in response to the invasion of Gram-negative bacteria into unprimed (3, 8, 11) and GBP-deficient IFNγ-primed epithelial cells (27). Based on our observation that Shigella shed LPS into the cytosol of unprimed epithelial cells, we propose that these relatively low levels of LPS are sufficient to bind to and activate CASP4, even in the absence of GBPs, to trigger, albeit delayed, pyroptosis.
As previously reported, we also found that infection with ΔospC3 Shigella triggers substantially lower levels of cell death of IFNγ-primed GBP1−/− HeLa and HCT8 cells as compared to their WT counterparts. However, the absence of GBP1 only resulted in moderate suppression of ΔospC3ΔipaH9.8 Shigella–triggered cell death. As ΔospC3ΔipaH9.8 Shigella does not translocate IpaH9.8 into host cells, IFNγ-primed epithelial cells infected with this strain, as compared to those infected with ΔospC3 Shigella, have detectable GBP2 and GBP4 (19). Thus, we propose that in the absence of GBP1, the relatively low levels of LPS that are spontaneously shed into the host cell cytosol from bacteria interact with GBP2 and/or GBP4, perhaps in concert with GBP3, which was previously demonstrated to promote CASP4 activation (17), to promote pyroptosis in a GBP1-independent manner. Consistent with this hypothesis, Dickinson et al. recently found that GBP2, like GBP1, upon direct binding to LPS, polymerizes with LPS into large complexes (37), perhaps forming cytosolic platforms for the assembly of noncanonical CASP4 inflammasomes.
Interestingly, we found that IpaH9.8-mediated GBP degradation played an increasing role in preventing cell death as numbers of intracytosolic Shigella were increased. Furthermore, we found distinct populations of infected cells, i.e., dead cells with no evidence of bacteria and live ones full of bacteria. These observations suggest that the recognition of invading Shigella by infected cells is an “all or none” phenomenon. It is difficult to determine what is a physiologically relevant MOI at the time of infection, particularly after the establishment of an infection. Presumably, higher numbers of Shigella are present within the cytosol of intestinal epithelial cells at later points in an infection when the epithelial cells are exposed to IFNγ, thus ensuring host cell survival and bacterial replication under these conditions due to increased secretion of IpaH9.8 and perhaps other effectors.
In summary, here we present complementary experimental approaches that demonstrate that Shigella IpaH9.8 modulates cell death via its targeted degradation of GBPs. We show that IpaH9.8 limits LPS release from intracellular Shigella in a GBP1-dependent manner and that it is not essential for Shigella-triggered cell death via pyroptosis. These observations suggest that the recruitment of CASP4 to the surface of bacteria via GBPs is not necessary for its activation but rather that GBP1 promotes the release of LPS into the host cell cytosol, whereby interactions with additional GBPs promote CASP4 activation. Given that GBP1 promotes pyroptosis in response to transfected LPS and cytosolic OMVs, it is likely that GBP1 also enhances the recognition of LPS released from intracellular bacteria (12, 15), a topic for future investigation.
Materials and Methods
Bacterial Strains and Plasmids.
All strains used are listed in SI Appendix, Table S1 and plasmids in SI Appendix, Table S2.
Generation of IpaH9.8 Expression Plasmids.
pPEND-IpaH9.8, pPTAC-IpaH9.8_Y86A/Q88A, and pPTAC-IpaH9.8_C337A were each generated using Gateway™ cloning. For pPEND-IpaH9.8mut, a pENTR221 entry clone was developed using a PCR-amplified fragment of the Shigella virulence plasmid that contains IpaH9.8 plus 295 upstream nucleotides generated using oligos END98-5 and END98-3 (SI Appendix, Table S3). For PTAC-IpaH9.8mut, a pENTR221 entry clone was developed using a synthetic DNA fragment containing the open reading frame encoding IpaH9.8_Y86A/Q88A plus an upstream consensus ribosome binding sequence flanked by attB sites (Twist Bioscience). For pPTAC-IpaH9.8_C337A, the fragment used to generate a pENTR223 entry clone was generated via overlap PCR. Once sequence-verified, the introduced regions were transferred into pCMD136-ccdB-FLAG and pDSW206-ccdB-FLAG.
Mammalian Cell Culture.
HeLa and HCT8 cells obtained from the American Type Culture Collection were cultured in a 5% CO2 incubator at 37°C. HeLa cells were maintained in high-glucose Dulbecco's modified eagle medium (DMEM) (Thermo Fisher Scientific #11965) and HCT8 cells in GlutaMAX-supplemented Roswell Park Memorial Institute (RPMI) 1600 (Thermo Fisher Scientific #61870127). In both cases, media were supplemented with 10% heat-inactivated fetal bovine serum (FBS) (R&D systems #S11150), 100 IU/mL penicillin, and 100 µg/mL streptomycin) (Life Technologies #15140). One day prior to infection, cells were seeded in antibiotic-free media at 2 × 104 (for automated fluorescent microscopy assay) or 4 × 104 cells/well (for real-time cytotoxicity assay) into 96-well tissue culture–treated plates (Corning 3603 for a plate reader, Greiner μClear #655096 for automated microscopy assays) and when indicated treated with 10 ng/mL IFNγ overnight (0.5% BSA, PeproTech) or disulfiram (30 µM) in dimethyl sulfoxide (Cayman Chemical) 1 h prior to and throughout the infection.
Knockout Cell Lines.
All mammalian knockout cells were generated via CRISPR/Cas9 editing. To target GSDMD, a single sgRNA (sgRNA-GSDMD1)–Cas9 RNP (ribonucleoprotein) complex was used (Synthego), while to target GBP1, a mixture of three sgRNAs (sgRNA-GBP1-1, sgRNA-GBP1-2, and sgRNA-GBP1-3) in complex with Cas9 was used (Gene Knockout Kit v2) (Synthego). Guide sequences are defined in SI Appendix, Table S3. In each case, the RNP complexes were electroporated into 1 × 106 cells (HeLa or HCT8) using a Neon system (Thermo Fisher Scientific). Pools were monitored for knockout efficiency after a 2- to 3-d recovery period. Cells were diluted to isolate single cells, which were expanded to generate clonal cell lines. Knockouts were verified by lysing 1 × 106 cells seeded in wells of 6-well plates for 15 min with 250 μL ice-cold radioimmunoprecipitation assay (RIPA) buffer (25 mM Tris, pH 8, 150 mM NaCl, 0.1% SDS, and 1% NP-40 plus complete cocktail protease inhibitors; Millipore Sigma #11836170001). A cell scraper was used to remove lysed cells from the plate, which was transferred to a 1.5-mL tube and centrifuged at 13,000 g for 15 min. The supernatant was resuspended in protein-loading dye, and samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, and immunoblotted with antibodies using conditions described in SI Appendix, Table S4.
Bacterial Infections.
For Shigella infections, single Congo red colonies were picked and grown overnight in 2 mL TCS (trypticase soy) broth at 30°C with aeration. For mT3Ec infections, single colonies were picked and grown overnight in 2 mL TCS broth at 37°C with aeration. The next day, each culture was diluted 1:100 into TCS broth and grown at 37°C with aeration for 2 to 3 h until the cultures reached an OD(optical density)600 of 0.8 to 1.0. For mT3Ec and in cases where IpaH9.8 expression was induced in Shigella, IPTG at a final concentration of 1 mM was added to the media 1 h after back-dilution. After a total of 2 to 3 h after back-dilution, when the cultures reached an OD600 of 0.8 to 1.0, bacteria were pelleted and resuspended in prewarmed low-glucose DMEM (no phenol red, Invitrogen #11054-020) plus 1% FBS then diluted to the indicated MOI for infections. In parallel, cell lines were washed 3 times with the same DMEM. Bacteria were added to each well at the designated MOI, and the plates were centrifuged at 2,000 rpm for 10 min to synchronize infection. The plates were incubated at 37°C in a 5% CO2 incubator for 30 min, after which each well was washed three times with HBSS (Hanks' Buffered Salt Solution)/10% FBS (Fetal Bovine Serum)/50 mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid) containing gentamicin (GENT) (50 µg/mL) to kill and remove extracellular bacteria.
Plate Reader–Based Cell Death Assay.
Infections were carried out as described above in 96-well clear bottom tissue culture–treated plates (see above). During the washing step, PI was added to a final concentration of 3 µM to identify cells undergoing death via pyroptosis. After the washes, 200 µL of HBSS/FBS/HEPES/GENT was added to each well. PI uptake of infected cells was monitored using a SpectraMax i3x plate reader (Molecular Devices). A 3 × 3 grid of fluorescent output reads covering each well were taken and averaged every 10 min over a defined time course.
Automated Fluorescent Microscopy Assay.
Infections were carried out as described above in 96-well clear bottom tissue culture–treated plates (see above). During the washing step, PI was added to a final concentration of 3 µM to identify cells undergoing death via pyroptosis, 16.2 µM Hoechst 33342 to identify all cells, and when indicated, 0.2% arabinose to monitor the growth of intracellular Shigella via sfGFP expression. The plate was imaged using an ImageXpress PICO automated microscope (Molecular Devices) using a 20×/0.4 lens with DAPI, Texas Red, and, when indicated, FITC filters. Images were obtained every 10 to 15 min. Images of each well were analyzed using CellReporterXpress software (Molecular Devices). The percentage of dead cells was calculated by dividing the total number of PI-positive cells by the total number of Hoechst-stained cells imaged in each well. Similarly, the percentage of invaded cells was calculated by dividing the total number of GFP-positive cells by the total number of Hoechst-stained cells imaged in each well. GFP fluorescence was calculated by dividing the total GFP area by the number of Hoechst-stained cells imaged in each well. In each case, the results from three replicate wells were averaged.
LDH Assay.
HeLa cells seeded in 96-well plates (2 × 104 cells/well) were treated overnight with 10 ng/mL IFNγ after which infections were carried out as described above. Three hours p.i., LDH was measured using a CyQUANT™ LDH Cytotoxicity Kit (Invitrogen #C20300) per the manufacturer’s instructions. Absorbance measurements at 490 nm and 680 nm were taken immediately after addition of Stop Solution using a SpectraMax i3x plate reader (Molecular Devices). To determine LDH activity, the 680-nm absorbance value was subtracted from the 490-nm absorbance value, a media control value was subtracted from all other values, and cytotoxicity was calculated for each sample as LDH activity as a percentage of the total LDH activity for cells treated with the 10X lysis buffer provided by the manufacturer.
Secretion Assay.
Overnight cultures grown in TCS broth were diluted 1:100 into 2 mL of TCS broth and incubated at 37°C with aeration. IPTG (1 mM) was added when cultures reached OD600 of ~0.4. After 2 h, when mid-log growth was observed, equivalent numbers of bacteria from each culture were pelleted, resuspended in 2 mL of phosphate-buffered saline (PBS) supplemented with 10 µM Congo red (Sigma), and incubated for 30 min. Bacterial cultures were centrifuged, and the cell pellets were resuspended in protein-loading dye. Proteins in the supernatant fractions were TCA (trichloroacetic acid)-precipitated [10% (vol/vol)] and resuspended in loading dye. Proteins from supernatant fractions and cell lysates were separated via SDS-PAGE and immunoblotted with designated antibodies.
Gentamicin Protection Assay.
HeLa cells were seeded in 96-well plates (4 × 104 cells/well) and primed overnight with 10 ng/mL IFNγ overnight. Infections were carried out as described above. Three hours p.i., cells were washed three times with PBS before being lysed with 0.1% Triton X-100 in PBS. Bacteria were plated and enumerated.
Cell Lysates of Infected Cells.
HeLa cells seeded in 6-well plates (1 × 106 cells/well) were infected as described above. Two hours p.i., each well was washed three times with prewarmed PBS, after which the cells were incubated for 15 min on ice in 250 μL RIPA buffer, which does not lyse bacterial cells. A cell scraper was used to remove lysed cells from the plate, and the mixture was centrifuged at 13,000rpm for 15 min. The supernatant was resuspended in protein-loading dye, and samples were separated by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with antibodies using conditions described in SI Appendix, Table S4.
Intracytosolic LPS Assay.
HeLa cells were seeded in 6-well plates at a density of 1 × 106 cells/well and, when indicated, treated with 10 ng/mL of IFNγ overnight. In the morning, cells were infected with Shigella (MOI of 3), as described above. Two hours p.i., cells were washed three times with prewarmed PBS before being treated with 500 µL prewarmed TrypLE Trypsin (Life Technologies #12605). After a 5- to 10-min incubation at 37°C, the cells were resuspended in 500 µL prewarmed DMEM with 10% FBS and transferred to 1.5-mL Eppendorf tubes which were spun at 100 g for 10 min. The supernatants were discarded, and pellets were resuspended in 500 µL ice-cold 0.005% digitonin in PBS and incubated at 4°C for 10 min on a rotator. The lysed cells were centrifuged at 400 g for 10 min to separate soluble and residual fractions. The supernatant (cytosolic) fractions (200 µL) were applied to AcroPrep Advance 96-well Filter Plates (0.2 µm, polyethersulfone membrane, Pall Corp, #8019) to remove intact bacteria. Filtered cytosolic fractions were diluted 1:100 in endotoxin-free water, after which LPS was quantified using a LAL chromogenic assay according to the manufacturer’s instructions (Associates of Cape Cod #E0005 and #C0031). The pellet (residual) fractions were each resuspended in 500 μL of 0.1% CHAPS (3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate) in HBSS and incubated at RT for 10 min. Samples were immunoblotted with designated antibodies to confirm fractionation and assess GBP1 degradation. When comparing specific variables, we quantified all samples in parallel as significant differences in absolute, but not relative, cLPS levels were detected on different days with the same samples, likely due to the sensitivity of the assay in detecting minor alterations in LPS standard levels. To quantify the relative numbers of infecting bacteria per condition, the residual fraction for each sample was serially diluted and plated, and colony-forming units were quantified using ImageJ.
Statistical Analyses.
Prism 9.4.1 (GraphPad Software) was used to graph data and conduct all statistical analyses. Differences were considered statistically significant if the P value was <0.05.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Cerina Karr and Elizabeth Turcotte for assistance in generating knockout cell lines and Drs. Ann Hochschild, Thomas Bernhardt, and Marcia Goldberg for supplying plasmids. Drs. Russell Vance and Miriam Kutsch for critically reading the manuscript. This work was supported by the NIH grants AI064285 (C.F.L.), AI28360 (C.F.L.), AI007061 (K.K.), and AI139425 (J.C.).
Author contributions
L.G., K.K., X.M., and C.F.L. designed research; L.G., K.K., T.C.S., P.J.C., X.M., and N.H.E. performed research; L.G., K.K., T.C.S., P.J.C., X.M., J.C., and C.F.L. analyzed data; and L.G., K.K., and C.F.L. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Liu X., Lieberman J., A mechanistic understanding of pyroptosis: The fiery death triggered by invasive infection. Adv. Immunol. 135, 81–117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagar J. A., Powell D. A., Aachoui Y., Ernst R. K., Miao E. A., Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kayagaki N., et al. , Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Shi J., et al. , Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Kayagaki N., et al. , Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Shi J., et al. , Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Liu X., et al. , Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mou X., Souter S., Du J., Reeves A. Z., Lesser C. F., Synthetic bottom-up approach reveals the complex interplay of Shigella effectors in regulation of epithelial cell death. Proc. Natl. Acad. Sci. U.S.A. 115, 6452–6457 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naseer N., et al. , Salmonella enterica Serovar Typhimurium induces NAIP/NLRC4- and NLRP3/ASC-independent, Caspase-4-dependent inflammasome activation in human intestinal epithelial cells. Infect Immun. 90, e0066321 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wandel M. P., et al. , Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat. Immunol. 21, 880–891 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knodler L. A., et al. , Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16, 249–256 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finethy R., et al. , Inflammasome activation by bacterial outer membrane vesicles requires guanylate binding proteins. mBio 8, e01188-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagrange B., et al. , Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11. Nat. Commun. 9, 242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilla D. M., et al. , Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc. Natl. Acad. Sci. U.S.A. 111, 6046–6051 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos J. C., et al. , LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J. 37, e98089 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutsch M., et al. , Direct binding of polymeric GBP1 to LPS disrupts bacterial cell envelope functions. EMBO J. 39, e104926 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos J. C., et al. , Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat. Commun. 11, 3276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu S., Cryo-ET of a human GBP coatomer governing cell-autonomous innate immunity to infection. bioRxiv (2021), 10.1101/2021.08.26.457804 (Accessed 27 August 2021). [DOI]
- 19.Li P., et al. , Ubiquitination and degradation of GBPs by a Shigella effector to suppress host defence. Nature 551, 378–383 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Piro A. S., et al. , Detection of cytosolic Shigella flexneri via a C-terminal triple-arginine motif of GBP1 inhibits actin-based motility. mBio 8, e01979-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wandel M. P., et al. , GBPs Inhibit motility of Shigella flexneri but are targeted for degradation by the bacterial ubiquitin ligase IpaH9.8. Cell Host Microbe. 22, 507–518.e505 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisch D., et al. , Human GBP1 differentially targets salmonella and toxoplasma to license recognition of microbial ligands and caspase-mediated death. Cell Rep. 32, 108008 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalil I. A., et al. , Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect Dis. 18, 1229–1240 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotloff K. L., Riddle M. S., Platts-Mills J. A., Pavlinac P., Zaidi A. K. M., Shigellosis. Lancet 391, 801–812 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T., et al. , The Shigella OspC3 effector inhibits caspase-4, antagonizes inflammatory cell death, and promotes epithelial infection. Cell Host Microbe. 13, 570–583 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Oh C., Verma A., Hafeez M., Hogland B., Aachoui Y., Shigella OspC3 suppresses murine cytosolic LPS sensing. iScience 24, 102910 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z., et al. , Shigella evades pyroptosis by arginine ADP-riboxanation of caspase-11. Nature 599, 290–295 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Clerc P., Sansonetti P. J., Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 55, 2681–2688 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du J., et al. , The type III secretion system apparatus determines the intracellular niche of bacterial pathogens. Proc. Natl. Acad. Sci. U.S.A. 113, 4794–4799 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mavris M., Sansonetti P. J., Parsot C., Identification of the cis-acting site involved in activation of promoters regulated by activity of the type III secretion apparatus in Shigella flexneri. J. Bacteriol. 184, 6751–6759 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashida H., et al. , A bacterial E3 ubiquitin ligase IpaH9.8 targets NEMO/IKKgamma to dampen the host NF-kappaB-mediated inflammatory response. Nat. Cell Biol. 12, 66–73; sup pp 61–69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuda J., et al. , Shigella effector IpaH9.8 binds to a splicing factor U2AF(35) to modulate host immune responses. Biochem. Biophys. Res. Commun. 333, 531–539 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Ji C., et al. , Structural mechanism for guanylate-binding proteins (GBPs) targeting by the Shigella E3 ligase IpaH9.8. PLoS Pathog. 15, e1007876 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanaja S. K., et al. , Bacterial outer membrane vesicles mediate cytosolic localization of LPS and Caspase-11 activation. Cell 165, 1106–1119 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramsby M., Makowski G., Differential detergent fractionation of eukaryotic cells. Cold Spring Harb. Protoc. 2011, prot5592 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Hu J. J., et al. , FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 21, 736–745 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickinson M. S., et al. , LPS-aggregating proteins GBP1 and GBP2 are each sufficient to enhance Caspase-4 activation both in cellulo and in vitro. Proc. Nat. Acad. of Sci. U. S. A., in press. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.