Abstract
Background and Objectives
Restless legs syndrome (RLS) is a sensory-motor neurologic disorder. Low-dose opioids are prescribed for patients with refractory or augmented RLS. The long-term safety, dose stability, and efficacy of these medications for RLS treatment is still unclear. In this study, we report the 2-year longitudinal data in a sample of patients treated with opioids for RLS in the community.
Methods
The National RLS Opioid Registry is an observational longitudinal study consisting of individuals taking a prescribed opioid for diagnosed and confirmed RLS, most of whom experienced augmented symptoms from dopamine agonists. Information on opioid dosages, side effects, past and current concomitant RLS treatments, RLS severity, psychiatric symptoms, and opioid abuse risk factors was collected at initial Registry entry and every 6 months thereafter by surveys on REDCap. No feedback or intervention was provided by the study staff to local providers.
Results
Registry participants (n = 448) with 2-year longitudinal data available were mostly White, female, older than 60 years, and, at Registry entry, had been on opioids for a median of 1–3 years at a mean morphine milligram equivalent (MME) of 38.4 (SD = 43.5). No change in RLS severity in the overall cohort was observed over the 2-year follow-up period. The median change in daily opioid dose from baseline to 2 years was 0 MME (interquartile range = 0–10). While 41.1% of participants increased their dose during the follow-up period (median increase = 10 MME), 58.9% decreased their dose or saw no change. Only 8% and 4% saw increases of >25 MME and >50 MME, respectively. Ninety-five percent of those who increased opioid dose >25 or >50 MME had one of the following features: switching opioids, discontinuation of nonopioid RLS treatment medications, at least mild insomnia at baseline, a history of depression, male sex, younger than 45 years, and opioid use for comorbid pain.
Discussion
Low-dose opioid medications continue to adequately control symptoms of refractory RLS over 2 years of follow-up in most of the participants. A minority of patients did see larger dose increases, which were invariably associated with a limited number of factors, most notably changes in opioid and nonopioid RLS medications and opioid use for a non-RLS condition. Continued longitudinal observations will provide insight into the long-term safety and efficacy of opioid treatment of severe, augmented RLS.
Classification of Evidence
This study provides Class IV evidence that opioid doses increase in roughly 40% of patients, in most by small amounts, over a 2-year period when prescribed for adult refractory restless leg syndrome.
Restless legs syndrome (RLS) is a sensory-motor neurologic disorder characterized by an irresistible urge to move the legs often concurrent with leg discomfort. Symptoms are worst at night and are commonly described by patients as unbearable.1 RLS symptoms and their deleterious impact on sleep are associated with high levels of morbidity in affected patients.2
Dopamine agonist medications are commonly used as first-line treatment for RLS and are initially very effective in most patients.3 Unfortunately, only a small subset of patients continues to benefit from these medications beyond 10 years, and many develop a worsening of symptoms known as augmentation.4 First-line options for those with dopaminergic augmentation include iron supplementation (for those with low or low-normal iron indices) or α2δ agents such as gabapentin, pregabalin, and gabapentin enacarbil; however, these are not always efficacious or well tolerated.5 Low-dose opioid medications are also effective in patients who have become refractory to first-line RLS treatments, particularly with augmentation to dopamine agonists.6 Small studies have shown that these medications can control RLS symptoms over 2–10 years with minimal dose escalation.7-9
Rapidly escalating national rates of opioid misuse, abuse and use disorder, and fatal overdose make for an uncertain risk:benefit of this class of medications, even at very low doses, for augmented or refractory RLS. Furthermore, an increasingly strict regulation of their use makes clinicians even more hesitant to prescribe this class of medication.10 Dose escalation of opioids is believed to increase the risk of substance use disorders and adverse outcomes.11 Guidelines suggest various clinical factors to stratify the individual risk of dose escalation and risk of misuse/abuse before initiating opioid therapy in patients with chronic noncancer pain,12 but it is unclear whether such guidelines and clinical tools are relevant for the use of opioids in RLS. Larger-scale multicenter data on the long-term efficacy, complications, and rates of abuse, diversion, and overdose in patients using opioids for RLS are badly needed to establish their safety and efficacy in this population.
The RLS National Opioid Registry was developed to collect longitudinal observational data on RLS treatment efficacy, dosage changes, medication tolerability, RLS severity, and sleep, mood, and anxiety symptoms in a national sample of patients using prescribed opioids for RLS. In this study, we presented data on the first 2 years of participant follow-up. The primary aim of this analysis was to examine the characteristics of long-term opioid medication treatment for RLS, including (1) efficacy for RLS, (2) changes in opioid dose, and (3) risk factors of opioid dose increases.
Methods
Participants
Patients were recruited through Restless Legs Syndrome Foundation (RLSF) advertising or by brochures provided to patients by treating physicians, many of whom practiced in groups certified by the RLSF as RLS Quality Care Centers. Recruitment occurred between December 2017 and September 2019. To meet eligibility criteria, participants had to be taking a prescribed opioid daily for diagnosed RLS and had a previous therapeutic response to a dopamine agonist medication. The requirement for a response to a dopaminergic agent was to better ensure the presence of RLS. Patients were assured that the information they provided for the Registry would not be shared with other healthcare professionals, including their RLS prescribing physician. Similarly, besides survey collection, no clinical oversight, support, or recommendations were provided to any participant or prescriber.
Evaluation
At enrollment, participants' RLS diagnosis was confirmed by a trained research coordinator using the Hopkins telephone diagnostic interview, an instrument validated to exclude common RLS mimics.13 Baseline information on initial and current opioid dosages, side effects, past and current RLS treatments, augmentation history,14 current RLS severity through the International Restless Legs Syndrome Study Group Severity Scale (IRLS),15 psychiatric history and current symptoms through the Patient Health Questionnaire (PHQ-9),16 and Generalized Anxiety Disorder-7 scale (GAD-7),17 sleep disturbance through the Insomnia Severity Index (ISI),18 and opioid abuse risk factors through the Opioid Risk Tool19 was collected through a phone interview and online (REDCap) survey. Online follow-up surveys were performed every 6 months thereafter. Comprehensive details on recruitment, inclusion and exclusion criteria, and questionnaire items can be found in our previous publication on 1-year longitudinal data from this cohort.20
All opioid dosages were converted to morphine milligram equivalents (MME) using established conversion ratios.21 Conversion ratios for common opioids were as follows: methadone: 4 (for doses ≤20 mg), 8 (21–40 mg), 10 (41–60 mg), and 12 (>60 mg); oxycodone: 1.5; and hydrocodone: 1.
Each questionnaire was assessed on a continuous scale unless noted otherwise. Statistical tests used for this analysis were χ2, Mann-Whitney, Wilcoxon signed-rank, and Spearman correlations. Logistic regression analyses were performed using a forward selection method with a threshold of p < 0.1. In the regression models, multicollinearity of all covariates was found to be at an acceptable level (variance inflation factors were all <1.2). All significance values reported were 2-sided, and analyses were performed using the Prism GraphPad 9 software and Microsoft Excel.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Institutional Review Board of Mass General Brigham, the parent organization of Massachusetts General Hospital. All patients provided verbal informed consent before participating in the registry.
Data Availability
Anonymized data reported in this study will be shared on requests from qualified investigators.
Results
Overview
Five hundred participants were originally enrolled in the registry. Of these original 500 participants, 24 discontinued opioid treatment, 14 were lost to follow-up, 5 were deceased, 4 missed the 2-year survey but remained in the registry, and 3 withdrew before their 2-year follow-up. An additional 2 participants completed the 2-year survey, but their opioid dosing information was uninterpretable and therefore excluded, leaving 448 with opioid dosing data. This constitutes a 94.9% retention rate among those who did not discontinue opioid medications or become deceased.
Table 1 summarizes the baseline characteristics of the 448 analyzed 2-year follow-up sample. Participants were mostly female (57%), predominantly 60 years or older (72%), and almost all White (98%). Participants had been taking opioids for a median of 1–3 years on entry into the registry, and almost all (n = 399; 89%) had a history of dopamine agonist augmentation, assessed by a clinical interview using augmentation diagnostic criteria.14
Table 1.
Baseline Demographics of the 2-Year Study Sample
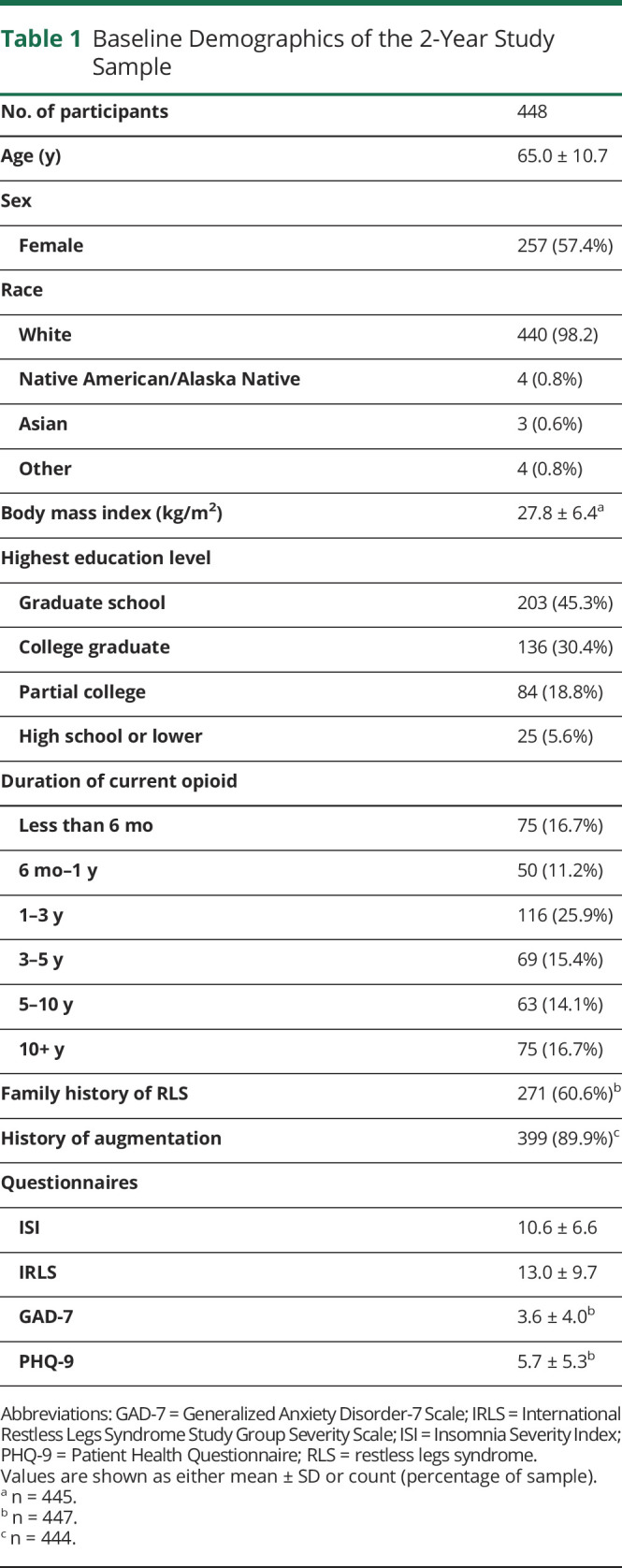
There were no significant differences in age, sex, or baseline IRLS, ISI, GAD-7, or PHQ-9 scores between those who continued participation at 2 years and those for whom 2-year data were unavailable. However, the median baseline daily MME for whom the 2-year follow-up was unavailable was significantly lower than that for participants remaining in the registry (20.0 vs 30.0; p = 0.03).
Opioid side effects reported at year 2 were very similar to those at baseline. The only difference >5% was appetite change (reported by 6% of participants at year 2 vs 12% at baseline). The most common side effects reported at 2 years were constipation (47%), drowsiness/fatigue (23%), itching (19%), sweating (17%), and stimulation/wakefulness (9%). Only 6% of participants (n = 25) endorsed “feelings of euphoria, warmth, intense relaxation, or giddiness” from their opioid medication(s). No serious side effects were reported by participants who continued in the registry or those who discontinued opioids and were no longer included in this summary.
Self-reported reasons for opioid discontinuation included side effects (n = 6), prescriber's unwillingness to prescribe opioids for RLS (n = 5), symptoms being controlled without opioids (n = 2), “augmentation” (n = 2), tolerance (n = 1), medication ineffectiveness (n = 1), or a combination of reasons (n = 2). An additional 5 participants discontinued their opioid medication for unknown reasons.
RLS Medication Changes
More than four-fifths of participants (81.3%, n = 364) who remained on prescribed opioids and completed 2-year surveys were on the same opioid type(s) as at baseline. An additional 1.8% (n = 8) added a new opioid, 2.7% (n = 12) removed an opioid, and 14.3% (n = 64) switched to a new opioid medication. Nearly half of participants who switched to a new opioid (n = 28; 43.8%) switched to methadone. More than one-tenth of participants (13.6%) added a dopamine agonist and/or an α2δ ligand during the 2-year follow-up period, whereas 10.0% removed such a medication.
At baseline, 23.9% (n = 107) of participants had treated their RLS with iron (either IV or oral supplement). At 2 years, 27.5% (n = 123) of participants reported currently taking iron supplements by mouth or having received an iron infusion in the past 6 months. Moreover, at 2 years, of the 47.5% (n = 211) of participants who had their blood iron levels checked in the past 6 months, 13.3% (n = 28) reported high iron levels, 16.1% (n = 34) reported low iron levels, and 70.6% (n = 149) reported normal iron levels.
Opioid Dose Changes
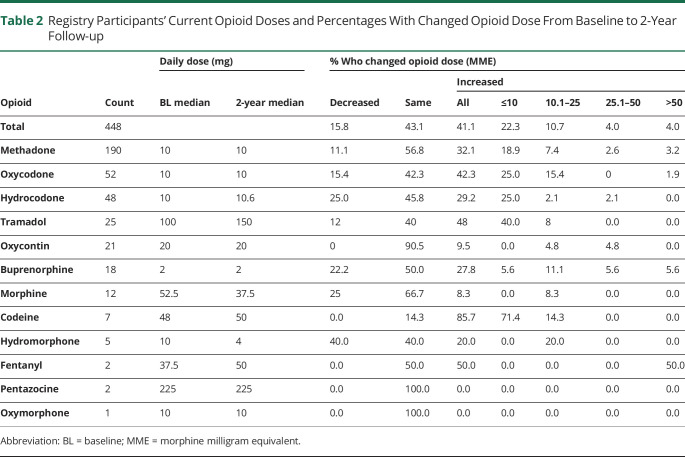
At the 2-year follow-up, the median daily MME was 30.0 (methadone = 7.5 mg, oxycodone = 20 mg) (Figure 1A), which was the same as at baseline. The median change in MME from baseline was 0 (Figure 1B). The mean daily MME at 2 years (excluding 4 outliers) was 43.5 (SD = 51.8), compared with a mean MME of 38.4 (SD = 43.5) at baseline. More than two-fifths (42.0%; n = 188) of patients were on the exact opioid regimen (i.e., opioid type and dosage) as at registry entry. Roughly two-fifths of participants (41.1%, n = 184) increased their opioid dose from baseline to 2 years. Of those who increased opioid dose, more than half (54.9%, n = 101) increased by 10 MME or less (see Table 2 for detailed breakdown).
Figure 1. (A) Total Daily MME Distribution at 2 Years and (B) Changes in Daily MME From Baseline to 2 Years.
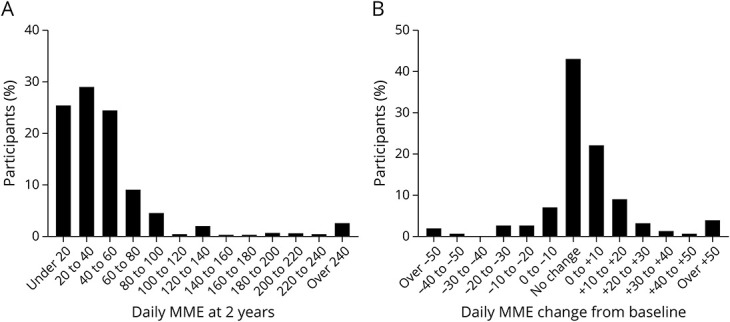
MME = morphine milligram equivalent
Table 2.
Registry Participants' Current Opioid Doses and Percentages With Changed Opioid Dose From Baseline to 2-Year Follow-up
A portion of participants saw dose increases that resulted in them crossing risk thresholds established by the Centers for Disease Control and Prevention22: a total of 36 participants (8.0%) increased their daily MME dose from below 50 MME to ≥ 50 MME, and 15 participants (3.3%) increased their daily MME dose from below 90 MME to ≥ 90 MME.
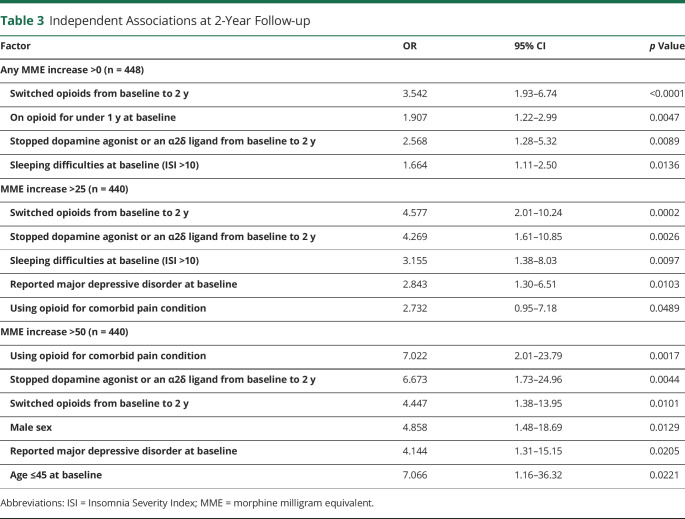
Opioid dose increases of any size from baseline to 2 years were independently associated with the following factors: switching specific opioid medication (OR 3.54, 1.93–6.74), being on prescribed opioid(s) for <1 year at baseline (OR 1.91, 1.22–2.99), stopping a dopamine agonist or α2δ ligand (OR 2.57, 1.28–5.32), and having a baseline ISI score of more than 10 (OR 1.66, 1.11–2.50) (Table 3).
Table 3.
Independent Associations at 2-Year Follow-up
Just 8.0% (n = 36) of all participants increased their opioid dose by > 25 MME (translating to a methadone dose change of 6.25 mg if dose was ≤20 mg or 2–3 mg if dose was >20 mg, or an oxycodone dose of 16.67 mg) over the 2-year follow-up period. Such larger dose increases (>25 MME) were independently associated with the following factors: switching specific opioid medication from baseline to 2 years (OR 4.58, 2.01–10.24), stopping a dopamine agonist or α2δ ligand (OR 4.27, 1.61–10.85), having a baseline ISI score of more than 10 (OR 3.16, 1.38–8.03), reporting a history of major depressive disorder (MDD) at baseline (OR 2.84, 1.30–6.51), and the use of opioid(s) for non-RLS comorbid pain (OR 2.73, 0.95–7.18) (Table 3). Nearly all participants (35/36 = 97.2%) who increased by > 25 MME satisfied at least one of these 5 conditions. By contrast, among participants who did not satisfy any of these conditions (n = 111), less than 1% saw dose increases >25 MME during the follow-up period (0.9%; n = 1). Male sex had a trend association (OR 2.15, 0.99–4.71). Notably, baseline RLS severity (as assessed by IRLS) and Opioid Risk Tool scores were not associated with >25 MME dose increases from baseline to 2 years.
Opioid dose increases of >50 MME (n = 18) during the 2-year follow-up period were independently associated with male sex (OR 4.86, 1.48–18.69), use of opioid(s) for non-RLS comorbid pain (OR 7.02, 2.01–23.79), stopping a dopamine agonist or α2δ ligand (OR 6.67, 1.73–24.96), switching specific opioid medication from baseline to 2 years (OR 4.45, 1.38–13.95), a reported history of MDD at baseline (OR 4.14, 1.31–15.15), and an age of 45 years or younger at baseline (OR 7.07, 1.16–36.32) (Table 3). Nearly all participants who increased dose by > 50 MME satisfied at least one of these 6 criteria (17/18 = 94.4%). On the contrary, of the 89 who did not satisfy any of these conditions, only 1 increased dose by > 50 MME (1.1%). Again, baseline RLS severity and Opioid Risk Tool scores were not associated with such opioid dose increases.
Individuals who started their opioid medication less than 1 year before entering into the registry saw markedly lower rates of increase from 1-year follow-up to 2-year follow-up (26.3%, n = 20) than during the first year (48.7%; n = 37; p = 0.004). These participants also saw lower rates of large increases in the second year of follow-up: being on a prescribed opioid medication for RLS for <1 year at registry entry was associated with >25 MME increases during the first year of follow-up (OR 4.28, 1.74–11.00) but not during the second year of follow-up.
Less than one-tenth of participants increased opioid dose during each of the first 2 years of follow-up (n = 42). These participants had lower baseline daily MME values than other participants (17.3 vs 30.0; p = 0.007). These individuals also had higher baseline IRLS scores, although this difference was not significant (16.5 vs 12.0; p = 0.27). On the 2-year surveys, the participants who increased doses both years had higher daily MME than others (40.0 vs 30.0; p = 0.009), and higher IRLS scores (15.0 vs 12.0; p = 0.08).
RLS Symptoms
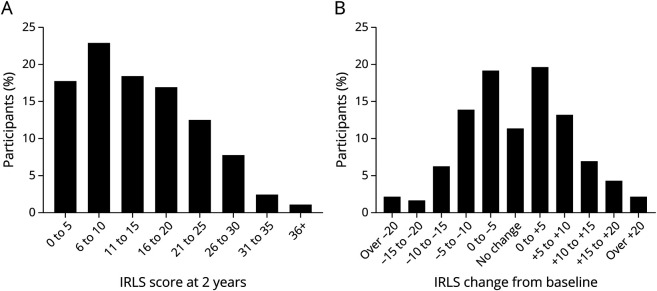
Of the 448 participants who remained on prescribed opioids and completed 2-year surveys, IRLS, ISI, and PHQ-9 scores did not change from baseline to 2 years. RLS symptom severity remained in the low-to-moderate range (2-year IRLS mean = 13.6) (Figure 2A), consistent with severity at baseline (IRLS mean = 13.0) and at 1 year (IRLS mean = 13.9). Sleep disturbance remained consistently common in this population from baseline (ISI mean = 10.6), to 1 year (ISI mean = 10.4) and to 2 years (ISI mean = 10.2). There was a slight increase in GAD-7 scores (3.6 vs 4.1; p = 0.003).
Figure 2. (A) Total IRLS Distribution at 2 Years and (B) Changes in IRLS From Baseline to 2 Years.
IRLS = International Restless Legs Syndrome Study Group Severity Scale
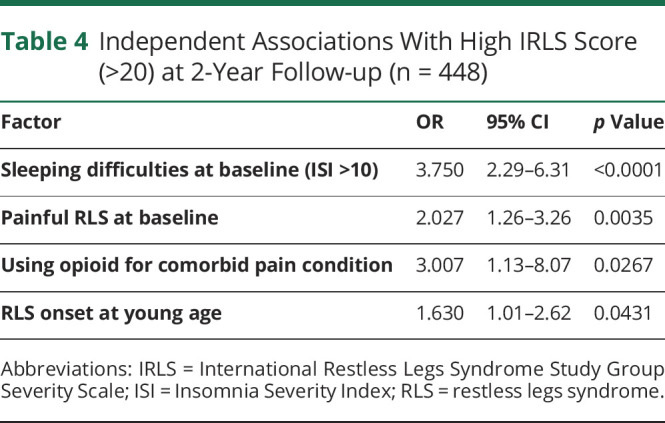
Large and roughly equivalent fractions of the cohort had increases (45.9%) or decreases (42.8%) in the IRLS score during follow-up (Figure 2B). The only significant association with change in IRLS from baseline to the 2-year follow up was the baseline IRLS score (r = −0.51, p < 0.001). Almost a quarter of participants (23.8%) continue to experience severe RLS (indicated by IRLS score >20). Severe RLS at year 2 was independently associated with the following factors: having a baseline ISI score of more than 10 (OR 3.75, 2.29–6.31), reporting painful RLS at baseline (OR 2.03, 1.26–3.26), use of opioid(s) for non-RLS comorbid pain (OR 3.01, 1.13–8.07), and an age of 45 years or younger at baseline (OR 1.63, 1.01–2.62) (Table 4). Individuals taking non-opioid RLS medications in addition to prescribed opioid(s) (dopamine agonist and/or α2δ ligand, 48.4% of participants) had higher 2-year IRLS scores than those on opioid monotherapy (15.9 vs 11.5; p < 0.001).
Table 4.
Independent Associations With High IRLS Score (>20) at 2-Year Follow-up (n = 448)
Comparison of Most Commonly Used Opioids
The most commonly used opioid in this sample was methadone (used by 50.9% of participants at 2 years; n = 228), followed by oxycodone (15.0%; n = 67), hydrocodone (11.6%; n = 52), oxycontin (7.1%; n = 32), and tramadol (6.7%; n = 30). IRLS scores at 2 years were significantly lower for participants using methadone (median = 10.0) than for participants taking oxycodone (19.0; p < 0.0001) or hydrocodone (18.0; p < 0.0001). 2-year ISI scores were also lower for participants taking methadone (median = 9.0) compared with participants using oxycodone (median = 13.0, p < 0.0001) or hydrocodone (12.0, p = 0.02). PHQ-9 and GAD-7 scores were significantly lower for participants using methadone when compared with participants taking oxycodone, but not compared with participants prescribed hydrocodone.
Among those who switched from another opioid to methadone (n = 28) during the follow-up period, 2-year IRLS scores were significantly lower than at baseline (11.1 vs 18.8; p < 0.001). However, most participants of this group (85.7%, n = 24) did see increases in daily MME from baseline to 2-years (median increase = 20 MME). Registry participants who switched to opioids other than methadone had slightly worse RLS severity than at baseline (16.3 vs 15.4; p = NS).
Table 2 summarizes a comparison of daily opioid doses and percentages who changed dose among participants who remained on specific opioids during the 2-year follow-up period. The percentage of participants who increased dose did not significantly differ between participants on methadone, oxycodone, and hydrocodone, and the regression analysis did not reveal any of these 3 medications to be significantly associated with dose increases at 2 years.
Reported side effects differed slightly between participants prescribed the 3 most common opioids. The most commonly reported side effects among participants taking methadone were constipation (59%), drowsiness (25%), sweating (19%), and itching (17%). Commonly reported side effects on oxycodone were constipation (40%), itching (28%), drowsiness (27%), and sweating (19%). Last, the most common side effects among participants using hydrocodone were constipation (31%), itching and drowsiness (both 21%), and sweating (10%).
Classification of Evidence
This study provides Class IV evidence that opioid doses increase in roughly 40% of patients, in the majority by small amounts, over a 2-year period when prescribed for adult refractory restless leg syndrome.
Discussion
Opioids are prescribed for patients with RLS when they are refractory to, or intolerant of, first-line treatments (α2δ agents, dopamine agonists) or have severe augmentation to dopamine agonists. Controlled trials6 demonstrate that efficacious opioid doses for such patients are low compared (mean MME = 33) with the doses of those with chronic opioid use for noncancer pain in which MME are roughly 60 MME.23,24 The aim of this analysis was to assess the longitudinal efficacy and stability of opioid dosing for patients with refractory RLS.
Methadone was the most commonly prescribed opioid in our participants. Although the only large clinical trial with opioids was with oxycodone ER,6 methadone has become the standard treatment in refractory, augmented patients due to its long half-life (which corresponds to the prolonged daily duration of most augmented patients' symptoms) and relatively lower risk of misuse due to once daily dosing and lack of euphoric effects.25 For this reason, the few small long-term studies with opioids for refractory RLS have also all primarily used methadone.7-9
The median opioid dose change was 0 MME, and RLS symptom severity was unchanged from baseline to 2 years in this large cohort of the National RLS Opioid Registry. These findings suggest that prescribed opioid medications can control RLS symptoms over time with minimal dose escalation, consistent with previous small, long-term studies of opioid therapy in RLS that demonstrated relatively stable dosing over 2–10 years of follow-up.7,8
RLS severity in the overall cohort was unchanged from baseline, with overall low-to-moderate symptoms. Roughly equivalent fractions of the Registry participants had increases as decreases in IRLS over the 2-year follow-up period. There were no consistent associations with change in RLS severity over the follow-up period (other than baseline severity), and thus, these changes on an individual level may be explained by a multitude of factors such as medication switches, dose alterations, augmentation, and natural progression of disease.
Although most of the participants either decreased or did not change their opioid dose during the follow-up period, there was a notable minority of participants who increased their dose. While most increases were small, 4% of participants increased the dose by 25–50 MME and another 4% increased the dose by > 50 MME. These individuals deserve particular attention because larger dose escalations and higher doses raise the risk for dangerous side effects and overdose.
A small number of discrete factors accounted for nearly all individuals with opioid dose increases >25 or >50 MME. Discontinuation of a nonopioid RLS therapeutic medication or the use of the opioid to additionally treat a non-RLS comorbid pain condition might be considered predictable associations with larger MME increases during the 2-year follow-up period. Eliminating dopaminergic agents, as is recommended in the context of augmentation of RLS symptoms, usually necessitates a greater therapeutic burden carried by the opioid. Similarly, in patients using prescribed opioids for a non-RLS comorbid pain condition, increases in opioid doses may be related to exacerbation of the other non-RLS pain conditions, in which opioids are often prescribed at higher doses than in the treatment of RLS. In fact, opioid dose escalation does occur in those with chronic noncancer pain conditions, although its frequency and causes are uncertain.23,24,26
Switching from one opioid to another during the follow-up period was also associated with larger opioid dose increases. Because registry participants switching to methadone made up a large portion of the individuals who switched opioids during the follow-up period (43.8%), this finding may largely be an artifact of the high MME conversion ratio used for methadone in this analysis. As such, nearly all participants who switched to methadone saw a substantial increase in daily calculated MME (median increase = 20 MME). Of note, there is some controversy and uncertainty regarding the optimal equivalence determination for methadone.27,28 Furthermore, the nonlinear relationship of methadone dose to MME may contribute to the elevated number of participants with large MME increases as, for instance, an increase in methadone dose from 20 to 25 mg is calculated as a 40-MME increase.
Male sex and younger age were both found to be independently associated with especially large dose increases of >50 MME. Both these factors have previously been associated with opioid dose escalation and/or high dose prescribing in chronic noncancer pain.29-32 A history of depressive disorder was also associated with opioid dose increases of both >25 and >50 MME. Similar to younger age and male sex, depression has been associated with high-dose opioid prescribing in chronic noncancer pain.31 Given the high comorbidity of RLS and depression,33,34 this association with larger dose increases is worth noting.
Of the original 500 RLS Registry participants, 2-year data were unavailable for 10% of participants. Of note, the median baseline MME for these participants was significantly lower than that for remaining participants who continued using the prescribed opioids. However, there were no significant differences in age, sex, or baseline IRLS between the 2 groups. Considering that the 2 most common reasons provided for discontinuation were side effects and unwillingness of the prescriber to continue opioid treatment, it seems possible that both side effects and prescriber reluctance contributed to the lower baseline doses observed in this group.
Participants who were on opioids for less than a year at entry into the registry were significantly less likely to see dose increases during year 2 of follow-up when compared with that during year 1. This suggests that individuals just starting opioid medications for RLS may require dose increases early on, but that further dose increases are less frequent because an optimal dose is achieved approximately 1 year into treatment.
Patients with RLS, particularly those with daytime symptoms from dopamine agonist–related augmentation, are often prescribed methadone due to its long duration of action. Participants switching to methadone from another opioid during the 2-year follow-up had significant reductions in RLS symptom severity scores after the switch. By contrast, participants switching to other opioids did not see similar decreases in RLS severity. This suggests that methadone may be particularly effective for controlling RLS symptoms, although future comparative trials are needed to test this. As previously mentioned, most individuals switching to methadone did see MME increases, although this may be an artifact of the high conversion ratio used in this analysis.
This study is limited in its generalizability due to Registry participants being predominantly White, elderly, and highly educated. Thus, an extrapolation of these results to more diverse and younger populations should be performed with caution. Some baseline data are also limited in their reliance on participants' recollection of events, occurring sometimes decades in the past (e.g., age of symptom onset, mental health history, etc).
It is important to note that Registry participants' care is managed by participants' own providers without any intervention from the Registry research team at Massachusetts General Hospital, which provides no clinical feedback or guidance to either participants or to providers. In fact, most RLS providers are likely unaware of their patients' involvement in the registry.
In conclusion, this analysis provides preliminary evidence from a large national registry that low-dose opioids can control RLS symptoms over time with minimal dose escalation in most patients with refractory, augmented RLS. Continued data collection may help in identifying those patients with RLS for whom opioids continue to be effective and well tolerated at low doses.
Acknowledgment
The authors thank the participants of the RLS Registry, the MDs who referred patients for Registry participation, and the RLS Foundation, Baszucki Brain Research Fund, Florence Petrlik Family Foundation, Diane and Richard Brainerd, Steven Silin, and Jerry Blakeley for research support.
Glossary
- GAD-7
generalized anxiety disorder-7 scale
- IRLS
International Restless Legs Syndrome Study Group Severity Scale
- ISI
insomnia severity index
- MDD
major depressive disorder
- MME
morphine milligram equivalent
- PHQ-9
Patient Health Questionnaire
- RLS
restless legs syndrome
- RLSF
Restless Legs Syndrome Foundation
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
CME Course: NPub.org/cmelist
Study Funding
The National RLS Opioid Registry has received research support from the RLS Foundation, Baszucki Brain Research Fund, Florence Petrlik Family Foundation, Diane and Richard Brainerd, Steven Silin, and Jerry Blakeley.
Disclosure
J. Winkelman receives receives royalties from UpToDate; consultation fees from Emalex, Noctrix, and Disc Medicine; and research support from NIDA, the RLS Foundation, and the Baszucki Brain Research Fund. All other authors report no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 2014;15:860-873. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165(11):1286-1292. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 3.Scholz H, Trenkwalder C, Kohnen R, Kriston L, Riemann D, Hornyak M. Dopamine agonists for the treatment of restless legs syndrome. Cochrane Database Syst Rev. 2011;2011(3):CD006009. Accessed February 1, 2022. cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006009.pub2/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis–Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med. 2016;21:1-11. doi: 10.1016/j.sleep.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Silber MH, Buchfuhrer MJ, Earley CJ, et al. The management of restless legs syndrome: an updated algorithm. Mayo Clin Proc. 2021;96(7):1921-1937. doi: 10.1016/j.mayocp.2020.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Trenkwalder C, Beneš H, Grote L, et al. Prolonged release oxycodone–naloxone for treatment of severe restless legs syndrome after failure of previous treatment: a double-blind, randomised, placebo-controlled trial with an open-label extension. Lancet Neurol. 2013;12:1141-1150. doi: 10.1016/s1474-4422(13)70239-4. [DOI] [PubMed] [Google Scholar]
- 7.Silver N, Allen RP, Senerth J, Earley CJ. A 10-year, longitudinal assessment of dopamine agonists and methadone in the treatment of restless legs syndrome. Sleep Med. 2011;12(5):440-444. doi: 10.1016/j.sleep.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Ondo WG. Methadone for refractory restless legs syndrome. Mov Disord. 2005;20(3):345-348. doi: 10.1002/mds.20359. [DOI] [PubMed] [Google Scholar]
- 9.Walters AS, Winkelmann J, Trenkwalder C, et al. Long-Term follow-up on restless legs syndrome patients treated with opioids. Mov Disord. 2001;16(6):1105-1109. doi: 10.1002/mds.1214. [DOI] [PubMed] [Google Scholar]
- 10.Zhu W, Chernew ME, Sherry TB, Maestas N. Initial opioid prescriptions among U.S. Commercially insured patients, 2012-2017. N Engl J Med. 2019;380(11):1043-1052. doi: 10.1056/nejmsa1807069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes CJ, Krebs EE, Hudson T, Brown J, Li C, Martin BC. Impact of opioid dose escalation on the development of substance use disorders, accidents, self-inflicted injuries, opioid overdoses and alcohol and non-opioid drug-related overdoses: a retrospective cohort study. Addiction. 2020;115(6):1098-1112. doi: 10.1111/add.14940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.e22. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hening WA, Allen RP, Washburn M, Lesage S, Earley CJ. Validation of the Hopkins telephone diagnostic interview for restless legs syndrome. Sleep Med. 2008;9(3):283-289. doi: 10.1016/j.sleep.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 14.García-Borreguero D, Allen RP, Kohnen R, et al. Diagnostic standards for dopaminergic augmentation of restless legs syndrome: report from a world association of sleep medicine–international restless legs syndrome study group consensus conference at the max planck institute. Sleep Med. 2007;8(5):520-530. doi: 10.1016/j.sleep.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Walters AS, LeBrocq C, Dhar A, et al. Validation of the international restless legs syndrome study group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121-132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 16.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 18.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297-307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 19.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med. 2005;6:432-442. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 20.Winkelman JW, Purks J, Wipper B. Baseline and 1-year longitudinal data from the national restless legs syndrome opioid registry. Sleep. 2021;44(2):zsaa183. doi: 10.1093/sleep/zsaa183. [DOI] [PubMed] [Google Scholar]
- 21.Opioid oral morphine milligram equivalent (MME) conversion factors|guidance portal [online]. Accessed June 14, 2022. hhs.gov/guidance/document/opioid-oral-morphine-milligram-equivalent-mme-conversion-factors-0.
- 22.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman CR, Bradshaw DH. Only modest long-term opioid dose escalation occurs over time in chronic nonmalignant pain management. J Pain Palliat Care Pharmacother. 2013;27:370-377. [DOI] [PubMed] [Google Scholar]
- 24.Chang H-Y, Murimi IB, Jones CM, Alexander GC. Relationship between high-risk patients receiving prescription opioids and high-volume opioid prescribers. Addiction. 2018;113(4):677-686. doi: 10.1111/add.14068. [DOI] [PubMed] [Google Scholar]
- 25.Cai NS, Quiroz C, Bonaventura J, et al. Opioid-galanin receptor heteromers mediate the dopaminergic effects of opioids. J Clin Invest. 2019;129(7):2730-2744. doi: 10.1172/jci126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portenoy RK. Opioid therapy for chronic nonmalignant pain: a review of the critical issues. J Pain Symptom Manage. 1996;11(4):203-217. doi: 10.1016/0885-3924(95)00187-5. [DOI] [PubMed] [Google Scholar]
- 27.Rennick A, Atkinson T, Cimino NM, Strassels SA, McPherson ML, Fudin J. Variability in opioid equivalence calculations. Pain Med. 2016;17(5):892-898. doi: 10.1111/pme.12920. [DOI] [PubMed] [Google Scholar]
- 28.Fudin J, Raouf M, Wegrzyn EL, Schatman ME. Safety concerns with the centers for disease control opioid calculator. J Pain Res. 2017;11:1-4. doi: 10.2147/jpr.s155444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplovitch E, Gomes T, Camacho X, Dhalla IA, Mamdani MM, Juurlink DN. Sex differences in dose escalation and overdose death during chronic opioid therapy: a population-based cohort study. PLoS One. 2015;10:e0134550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buntin-Mushock C, Phillip L, Moriyama K, Palmer PP. Age-dependent opioid escalation in chronic pain patients. Anesth Analgesia. 2005;100(6):1740-1745. doi: 10.1213/01.ane.0000152191.29311.9b. [DOI] [PubMed] [Google Scholar]
- 31.Richards GC, Mahtani KR, Muthee TB, et al. Factors associated with the prescribing of high-dose opioids in primary care: a systematic review and meta-analysis. BMC Med. 2020;18(1):68. doi: 10.1186/s12916-020-01528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobus AM, Smith DH, Morasco BJ, et al. Correlates of higher-dose opioid medication use for low back pain in primary care. J Pain. 2012;13(11):1131-1138. doi: 10.1016/j.jpain.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hornyak M. Depressive disorders in restless legs syndrome: epidemiology, pathophysiology and management. CNS Drugs. 2010;24(2):89-98. doi: 10.2165/11317500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Picchietti D, Winkelman JW. Restless legs syndrome, periodic limb movements in sleep, and depression. Sleep. 2005;28(7):891-898. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data reported in this study will be shared on requests from qualified investigators.