Abstract
This study evaluates 21-day risk of myocarditis/pericarditis following COVID-19 vaccination among those aged 12 years and older in Malaysia. We used data from nationwide COVID-19 vaccine registry linked to hospital episode database to identify individuals vaccinated with BNT162b2, CoronaVac, or ChAdOx1 and hospitalised for myocarditis/pericarditis between 1 February 2021 and 28 February 2022. There were 87 myocarditis/pericarditis cases identified within 1–21 days after vaccination. Most cases were reported following BNT16262 vaccination (77.0%) with absolute risk of 0.33 cases/100,000 vaccinated persons or 1.73 per million doses administered. Highest risk was observed following second dose and in younger, male individuals. The risk of myocarditis/pericarditis following CoronaVac and ChAdOx1 were much lower compared to BNT162b2. The findings on higher risk observed among younger following mRNA vaccine were consistent with literature and important for targeted surveillance.
Keywords: COVID-19 vaccine, Myocarditis, Pericarditis, Adverse effects
Introduction
Myocarditis and pericarditis are acute condition associated with inflammation of the heart. The global incidence rate of myocarditis for the year 2017 was estimated to be 39 cases per 100,000 persons [1]. Since the global roll-out of COVID-19 vaccines, post-marketing surveillance data have suggested increased risk of myocarditis/pericarditis after COVID-19 vaccination, particularly mRNA-based COVID-19 vaccine [2], [3]. The Pharmacovigilance Risk Assessment Committee of European’s Medicine Agency evaluated the signals of myocarditis/pericarditis and concluded the events to be “rare” with up to 1 event in 10,000 vaccinated people [4]. Risk of myocarditis/pericarditis following COVID-19 vaccination varies by age, sex, vaccine products, and vaccine dose [5], [6]. Case reports and published studies largely focused on the incidence of myocarditis/pericarditis following administration of mRNA-based COVID-19 vaccination (BNT162b2 and mRNA-1273). A study from Hong Kong compared risk of myocarditis/pericarditis between recipients of BNT162b2 and CoronaVac vaccines [7] while a study conducted in the United Kingdom (UK) describes risk of myocarditis among recipients of ChAdOx1 and BNT162b2 vaccines [8]. Better understanding of the characteristics of the population who acquired myocarditis/pericarditis postvaccination would facilitate prevention measures to mitigate the risk and its complications, as well as facilitate early identification and management.
In Malaysia, the COVID-19 immunisation programme started in February 2021 according to the national priority scheme with the main vaccine products authorised for use includes BNT162b2, CoronaVac, ChAdOx1, and a small proportion of population that received BBIBP-CorV (SinoPharm), Ad5-nCoV (CanSino) and Ad26.COV2.S. Vaccination for adolescent started in September 2021, mainly for BNT162b2 vaccine [9]. Malaysia is one of the few countries with diverse COVID-19 vaccine portfolio administered to the population. This provided an opportunity to examine the risk with direct comparison between different vaccine products. Furthermore, population-based estimates of myocarditis/pericarditis risk following vaccination among Asian population is still lacking. In this study, we aimed to determine the incidence of myocarditis/pericarditis following COVID-19 vaccination in a population vaccinated with either BNT162b2, CoronaVac, or ChAdOx1 vaccines in Malaysia.
Methods
In this population-based cohort, we used data from nationwide vaccine registry and health database from Ministry of Health Malaysia. COVID-19 vaccination registry contains details of all vaccinated individuals in Malaysia including vaccination date, brand, and dose. Hospital admission data was extracted from Malaysia Health Data Warehouse (MyHDW), a health data repository that gathers data from healthcare facilities across Malaysia. Inpatient data includes demographic, hospitalisation details, and diagnoses. For this study, hospitalisation data was restricted to public hospitals due to unavailability of patient identification and low rates of successful data linkage associated with private hospitals data. COVID-19 surveillance data was used to obtain information of SARS-CoV-2 infection. All database was linked deterministically using unique resident identification number. Details of data source have been described previously [10].
We included individuals aged 12 years and older who received at least one dose of COVID-19 vaccine during the period from 1 February 2021 to 28 February 2022. We restricted the cohort to those who had either BNT162b2, CoronaVac, or ChAdOx1 vaccines and with homologous primary vaccine series (if received more than one vaccine dose). Homologous primary series refers to receipt of the same vaccine brand for the first two vaccination doses. We excluded individuals with a history of hospital admission for myocarditis/pericarditis within 2 years and if they had a previous positive test for SARS-CoV-2 prior to the outcome event.
Exposure was defined as receipt of a dose of BNT162b2, CoronaVac, or ChAdOx1 vaccines. In Malaysia, individuals are eligible to receive booster vaccine dose (dose 3) after at least 90 days of completing the priming dose. For the booster dose, individuals can receive either the same vaccine as the primary series (homologous booster) or a different vaccine (heterologous booster).
Outcome was defined as the first hospital admission for myocarditis/pericarditis within 1 to 21 days of receipt of COVID-19 vaccine (risk period) during the study period. Cases were identified using International Classification of Disease, Tenth Revision (ICD-10) (Appendix 1). Diagnosis of either myocarditis or pericarditis was used as the outcome event due to low prevalence of these events in the population. This combined outcome allowed for a more comprehensive capture of the cases and it was similarly used in other studies [6], [11], [12]. Background risk of myocarditis/pericarditis were extracted from MyHDW for the year 2019.
Cases were summarised descriptively. Cumulative incidence or absolute risk of myocarditis/pericarditis was estimated using number of cases divided by (i) the number of vaccinated persons and (ii) total doses administered with the corresponding 95% confidence interval (CI). Risk associated with the first, second, and booster dose were calculated based on number of cases that occurred within the 21-day risk period by the last vaccine dose administered. Detailed cohort descriptions are provided in Appendix 2. The risk of myocarditis/pericarditis associated with the COVID-19 vaccines was compared with the background risk in 2019, adjusted for 21-day time period to estimate unadjusted risk ratio with 95% CI. The 95% CIs were calculated using the Poisson exact method. Analysis was conducted using STATA SE 15.0.
Ethical approval
This study was part of the project “Case-based clinical safety monitoring of adverse events following COVID-19 vaccination – SAFECOVAC” which was approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR-21–822-59745). Waiver of informed consent was granted due to the use of secondary data.
Results
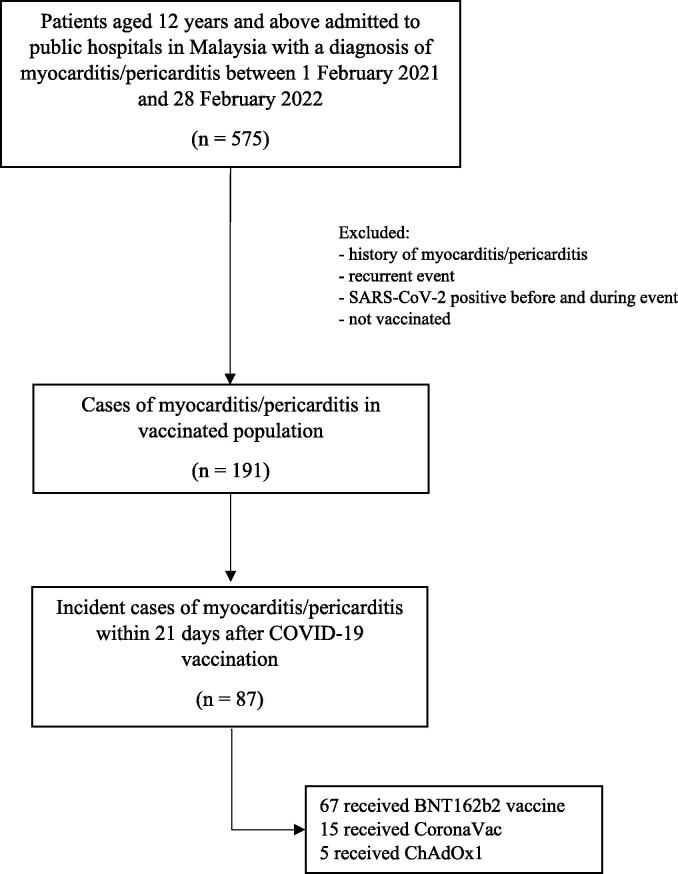
Between 1 February 2021 and 28 February 2022, a total of 191 hospitalisation due to myocarditis/pericarditis that met our inclusion criteria were identified. Of these, 87 cases that occurred within 1–21 days after vaccination were identified (Fig. 1). Sixty-seven (77.0%) cases occurred following BNT162b2 vaccine, 15 (17.2%) in CoronaVac, and 5 (5.7%) in ChAdOx1. Characteristic of myocarditis/pericarditis cases according to the vaccine administered are described in Appendix 3. The patients were mostly male (71.3%) with a median age of 16.7 years. Nearly half of the cases (44.8%) that occurred following vaccination with BNT162b2 were adolescent aged 12–17 years old while all cases from CoronaVac and ChAdOx1 were over 18 years. Most patients were of Malay ethnicity (92.5%). All cases except one were discharged alive and the mean length of stay in hospital was between three to seven days.
Fig. 1.
Flowchart of inclusion and exclusion criteria for analysis.
Table 1 shows the number of myocarditis/pericarditis events by vaccine product and dose number and the cumulative incidence (risk) with 95% CI. The risk of myocarditis/pericarditis following BNT162b2 vaccine was 0.33 per 100,000 vaccinated persons and 1.73 per million doses administered. The risk was higher after the second dose compared to the first dose (0.23 vs 0.19 per 100,000 vaccinated persons). Among individuals vaccinated with CoronaVac and ChAdOx1, the overall risks were 0.15 and 0.18 per 100,000 vaccinated persons, respectively. The incidence of myocarditis/pericarditis following booster dose was highest among those who received BNT162b2 as booster (0.08 per 100,000 vaccinated persons) compared to CoronaVac and ChAdOx1. Subgroup analysis of booster dose recipients for comparison between homologous and heterologous booster showed similar risk estimates for myocarditis/pericarditis.
Table 1.
Cumulative incidence of myocarditis/pericarditis within 1–21 days after SARS-CoV-2 vaccination, by vaccine product and dose.
| Vaccine | Vaccination schedule | Events | Number of persons vaccinateda | Cumulative incidence per 100,000 persons (95% CI) | Number of doses administered |
Cumulative incidence per million doses (95% CI) |
|---|---|---|---|---|---|---|
| BNT162b2 | Primary series | |||||
| Dose 1 | 26 | 13,573,466 | 0.19 (0.13, 0.28) | 13,573,466 | 1.92 (1.25, 2.81) | |
| Dose 2 | 31 | 13,388,261 | 0.23 (0.16, 0.33) | 13,388,261 | 2.32 (1.57, 3.29) | |
| Booster dose | ||||||
| Dose 3 | 10 | 11,861,042 | 0.08 (0.04, 0.16) | 11,861,042 | 0.84 (0.40, 1.55) | |
| Homologous b | 4 | 5,257,500 | 0.07 (0.02, 0.19) | 5,257,500 | 0.76 (0.21, 1.95) | |
| Heterologous c | 6 | 6,603,542 | 0.09 (0.03, 0.19) | 6,603,542 | 0.91 (0.33, 1.98) | |
| Total | 67 | 20,177,088 | 0.33 (0.26, 0.42) | 38,822,769 | 1.73 (1.34, 2.19) | |
| CoronaVac | Primary series | |||||
| Dose 1 | 10 | 9,881,811 | 0.10 (0.05, 0.19) | 9,881,811 | 1.01 (0.49, 1.86) | |
| Dose 2 | 4 | 9,798,795 | 0.04 (0.01, 0.11) | 9,798,795 | 0.41 (0.11 (1.05) | |
| Booster dose | ||||||
| Dose 3 | 1 | 1,107,802 | 0.09 (0.00, 0.50) | 1,107,802 | 0.90 (0.02, 5.03) | |
| Homologous b | 1 | 1,089,606 | 0.09 (0.00, 0.51) | 1,089,606 | 0.92 (0.02, 5.11) | |
| Heterologous c | – | 18,196 | NA | 18,196 | NA | |
| Total | 15 | 9,900,007 | 0.15 (0.09, 0.25) | 20,788,408 | 0.72 (0.40, 1.19) | |
| ChAdOx1 | Primary series | |||||
| Dose 1 | 1 | 2,040,770 | 0.05 (0.00, 0.27) | 2,040,770 | 0.49 (0.01, 2.73) | |
| Dose 2 | 2 | 2,023,822 | 0.09 (0.01, 0.36) | 2,023,822 | 0.99 (0.12, 3.57) | |
| Booster dose | ||||||
| Dose 3 | 2 | 1,517,906 | 0.13 (0.02, 0.48) | 1,517,906 | 1.32 (0.16, 4.76) | |
| Homologous b | 1 | 728,337 | 0.14 (0.00, 0.77) | 728,337 | 1.37 (0.03, 7.65) | |
| Heterologous c | 1 | 789,569 | 0.13 (0.00, 0.71) | 789,569 | 1.27 (0.03, 7.06) | |
| Total | 5 | 2,830,339 | 0.18 (0.06, 0.41) | 5,582,498 | 0.90 (0.29, 2.09) |
Defined as total persons who received the vaccine as 1st dose, 2nd dose, or 3rd doses. Person who received 3 vaccine doses contributed to dose 1, dose 2, and dose 3 categories. Total number of persons vaccinated was the sum of individuals who received at least one dose of the vaccine for the primary series and/or booster dose.
Refers to vaccine administered as the booster dose is the same type as the first and second doses (e.g., BNT162b2/BNT162b2/BNT162b2).
Refers to vaccine administered as the booster dose is different as the first and second doses (e.g., CoronaVac/CoronaVac/BNT162b2).
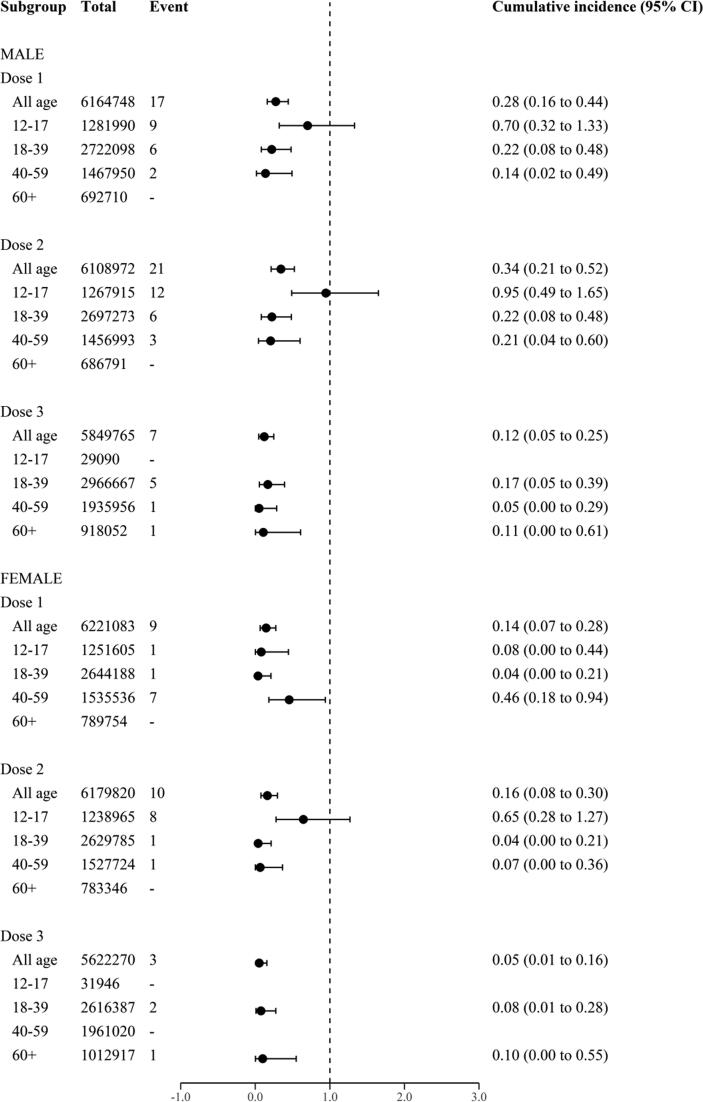
Across all vaccine products, the risk of myocarditis/pericarditis was consistently higher among males compared to females. Fig. 2 shows the risk of myocarditis/pericarditis stratified by dose number, sex, and age among individuals who received BNT162b2. Highest risk was observed in male aged 12–17 years following second dose (0.95 per 100,000 vaccinated persons). Subgroup analysis was not conducted for the CoronaVac and ChAdOx1 due to small number of events.
Fig. 2.
Cumulative incidence (risk) of myocarditis/pericarditis within 1–21 days after BNT162b2 vaccine, stratified by vaccine dose, sex, and age. Risks are described as risk per 100,000 vaccinated persons and the corresponding 95% confidence interval (CI).
Table 2 summarised the risk of myocarditis/pericarditis following SARS-CoV-2 infection and background incidence for the year 2019 for comparison with the risk following vaccination. Notably, the risk of myocarditis/pericarditis diagnosed within 21-day following SARS-CoV-2 infection was 5.62 per 100,000 persons. Compared to the background incidence of myocarditis/pericarditis in 2019, there was a higher risk differences among the vaccinated. The risk of developing myocarditis/pericarditis following SARS-CoV-2 infection, however, was much higher when compared to the vaccinated and the background rate (risk ratio 133.8; 95% CI 76.1 to 286.2).
Table 2.
Myocarditis/pericarditis following SARS-CoV-2 vaccination, SARS-CoV-2 infection, and compared to background risk.
|
SARS-CoV-2 vaccination |
SARS-CoV-2 infectiona | Background (2019)b | |||
|---|---|---|---|---|---|
| BNT162b2 | CoronaVac | ChAdOx1 | |||
| Total events | 67 | 15 | 5 | 157 | 200 |
| Persons | 20,177,008 | 9,900,007 | 2,830,339 | 2,793,430 | 27,397,700 |
| Risk per 100,000 persons (95% CI) | 0.33 (0.26 to 0.42) |
0.15 (0.09 to 0.25) |
0.18 (0.06 to 0.41) |
5.62 (4.78 to 6.57) |
0.04 (0.02 to 0.07) |
| Risk ratioc (95% CI) |
7.91 (4.08 to 15.5) |
3.61 (1.51 to 8.09) |
4.21 (1.20 to 13.7) |
133.8 (76.1 to 286.2) |
Reference |
Abbreviation: CI, confidence interval.
Events post SARS-CoV-2 infection were defined as diagnosis within 1–21 days of positive test.
Background risk were calculated from the hospital episode database for the year 2019 and Malaysian population as the denominator and standardised to 21 days.
Risk ratio was calculated as risk per 100,000 persons for the respective cohort (BNT162b2, CoronaVac, ChAdOx1, and SARS-CoV-2 infection) divided by risk in the population for the year 2019 (background 2019).
Discussion
Using large-linked database, this study describes the incidence of myocarditis/pericarditis following SARS-CoV-2 vaccination in Malaysia. We compared the risk estimates between mRNA vaccine (BNT162b2), inactivated vaccine (CoronaVac), and adenovector vaccine (ChAdOx1) for primary series and booster dose, including both homologous and heterologous booster groups. Our study shows that the risk of myocarditis/pericarditis in 1–21 days following vaccination with BNT162b2 was two times higher than CoronaVac and ChAdOx1. Stratifying by age and sex, greater risk was observed among male aged 12–17 years after the second dose of BNT162b2 vaccine.
Our findings are consistent with previous studies that observed association of carditis risk with BNT161b2, but not CoronaVac [7], [13] or ChAdOx1 [14]. This further supports the evidence that the potential mechanisms of postvaccination myocarditis/pericarditis is specific to mRNA platform. Our study also confirmed that men were at higher risk of postvaccination myocarditis/pericarditis than men, particularly the younger age as reported in earlier studies [3], [12], [15]. There were concerns about potential risk of developing myocarditis/pericarditis following booster dose of mRNA vaccine. Previous studies showed that the risk of myocarditis/pericarditis following booster dose of BNT162b2 to be either similar or lower than after primary vaccination [15], [16]. Our findings add to the evidence by showing that the risk after BNT162b2 booster dose in our population is lower than after the first two doses, while the estimates between BNT162b2 administered as homologous and heterologous booster dose were within similar range. The small number of events following booster dose precludes further analysis for meaningful comparison.
We found an above expected incidence of myocarditis/pericarditis in the vaccinated population compared to the background estimate in 2019. This was similarly observed in prior studies [11], [17] and other possible attributes include increased awareness of potential risk of acute myocarditis/pericarditis following COVID-19 vaccination. Nevertheless, the absolute incidence in the vaccinated population remained low with 3 cases for every one million persons vaccinated. It is also important to note that the incidence of myocarditis/pericarditis following SARS-CoV-2 infection was substantially higher than the vaccinated people and background risk in our population. This indicates that by reducing the risk of SARS-CoV-2 infection, vaccination provides protection against complications associated with infection, including carditis. Hence, the risk of carditis and other complications associated with vaccination needs be weighed against the benefits. Further research shall be conducted especially among population with potentially higher risk to provide real-world evidence on both short- and long-term outcomes.
Among the Asian countries, the incidence of myocarditis/pericarditis following BNT162b2 vaccination have been reported from Singapore (0.35 per 100,000 doses), and Taiwan (1.35 per 100,000 vaccinees), and Hong Kong (0.57 per 100,00 doses) [7], [17], [18]. Hong Kong also reported the incidence of 5.57 and 37.32 per 100,000 persons vaccinated after the first and second dose, respectively, for the male adolescents [11]. Although our result showed a slightly lower incidence in our population, direct comparisons were not possible due to various case definition, risk period, study design used, as well as different dosing interval applied in different countries [5], [19]. For instance, the study from Taiwan did not restrict the time since vaccination for their case definition and this will allow more cases to be included in the analysis [18]. The background incidence of myocarditis/pericarditis in our population was low and the proportion of people given BNT162b2 vaccine was less compared to countries like Hong Kong and Singapore given that other vaccines were rolled out at the similar scale. Collectively, some of our results are confirmatory and we also demonstrate that the risk of myocarditis/pericarditis was observed after BNT162b2 booster dose given as either homologous or heterologous.
Nonetheless, our study describes population-based estimates on risk of myocarditis/pericarditis post-vaccination among the Asian with multi-ethnic composition in a setting where 3 types of COVID-19 vaccine were administered in the same population. Despite the strength of population-based data and nationwide registry, there are limitations to consider. First, we did not include cases treated in outpatient or those that did not require medical attention. Secondly, we only used diagnoses codes to identify cases and did not include laboratory parameters to confirm events since such data are not available. Hence, we may have underestimated the true incidence of myocarditis/pericarditis after vaccination. Thirdly, due to observational nature of the study, we are unable to make causal inference on the effect of the vaccines. Lastly, we cannot rule out misclassification bias and unmeasured confounding from the use of secondary database for this study.
Conclusion
Myocarditis/pericarditis are rare following COVID-19 vaccination, with absolute event risk of less than five for every 1 million persons vaccinated in Malaysia within 21 days of vaccination. The risk was higher among recipients of BNT162b2 compared to CoronaVac and ChAdOx1. There was preponderance of cases in young male individuals following second dose of BNT162b2 which suggests enhanced surveillance in this group to prevent further complications. Nevertheless, the finding should be interpreted within the context of the benefits of COVID-19 vaccination since it still outweighs the possible harm of SARS-CoV-2 infection for the population.
Funding
This research was supported by a grant from the Ministry of Health Malaysia – Sukuk Prihatin (NMRR-21–822-59745). The funding body had no role in study design, data analysis, or the presentation of data and writing of the manuscript.
The SAFECOVAC study group
Members of the SAFECOVAC study group are listed in Appendix 4.
Data availability
The data that supports the findings of this study are available within the article and its supplementary materials. Data will not be available for others and any request will be subjected to approval from the data custodians. Malaysia COVID-19 vaccine administration data are available at https://github.com/MoH-Malaysia/covid19-public, redacted of personal identifying information.
CRediT authorship contribution statement
Norazida Ab Rahman: Conceptualization, Methodology, Formal analysis, Writing – original draft, Visualization, Funding acquisition. Ming Tsuey Lim: Investigation, Writing – review & editing, Project administration. Fei Yee Lee: Investigation, Writing – review & editing, Project administration. Emelyne Bani Anak Jam: Investigation, Writing – review & editing, Project administration. Kalaiarasu M Peariasamy: Supervision, Funding acquisition, Writing – review & editing. Sheamini Sivasampu: Supervision, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the Director General of Health Malaysia for his permission to publish this study. We thank all investigators and collaborators for their immense contribution and support. We would also like to acknowledge all data providers who make data available for research and personnels who were involved in data collection process.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2023.100303.
Contributor Information
Norazida Ab Rahman, Email: norazida@crc.gov.my.
Ming Tsuey Lim, Email: mtlim2000@yahoo.com.
Fei Yee Lee, Email: feiyee7890@gmail.com.
Emelyne Bani Anak Jam, Email: emyroberto@gmail.com.
Kalaiarasu M Peariasamy, Email: drkalai@moh.gov.my.
Sheamini Sivasampu, Email: sheamini@crc.gov.my.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- 1.Wang X., Bu X., Wei L., Liu J., Yang D., Mann D.L., et al. Global, Regional, and National Burden of Myocarditis From 1990 to 2017: A Systematic Analysis Based on the Global Burden of Disease Study 2017. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.692990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozkurt B., Kamat I., Hotez P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlstad Ø., Hovi P., Husby A., Härkänen T., Selmer R.M., Pihlström N., et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. 2022;7(6):600–612. doi: 10.1001/jamacardio.2022.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EMA/PRAC/683817/2021. Signal assessment report on Myocarditis, pericarditis with Tozinameran (COVID-19 mRNA vaccine (nucleoside modified) – COMIRNATY) 2021 [Available from: https://www.ema.europa.eu/en/documents/prac-recommendation/signal-assessment-report-myocarditis-pericarditis-tozinameran-covid-19-mrna-vaccine_en.pdf.
- 5.Pillay J., Gaudet L., Wingert A., Bialy L., Mackie A.S., Paterson D.I., et al. Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review. BMJ (Clinical research ed) 2022;378:e069445. doi: 10.1136/bmj-2021-069445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong H.L., Hu M., Zhou C.K., Lloyd P.C., Amend K.L., Beachler D.C., et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet (London, England) 2022;399(10342):2191–2199. doi: 10.1016/S0140-6736(22)00791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai F.T.T., Li X., Peng K., Huang L., Ip P., Tong X., et al. Carditis After COVID-19 Vaccination With a Messenger RNA Vaccine and an Inactivated Virus Vaccine : A Case-Control Study. Ann Intern Med. 2022;175(3):362–370. doi: 10.7326/M21-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patone M., Mei X.W., Handunnetthi L., Dixon S., Zaccardi F., Shankar-Hari M., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husin M., Tok P.S.K., Suah J.L., Thevananthan T., Tng B.H., Peariasamy K.M., et al. Real-world effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection among adolescents (12 to 17-year-olds) in Malaysia. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2022;121:55–57. doi: 10.1016/j.ijid.2022.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ab Rahman N., Lim M.T., Lee F.Y., Lee S.C., Ong S.M., Sivasampu S., et al. A case-based monitoring approach to evaluate safety of COVID-19 vaccines in a partially integrated health information system: a study protocol. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.834940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chua G.T., Kwan M.Y.W., Chui C.S.L., Smith R.D., Cheung E.C.L., Ma T., et al. Epidemiology of Acute Myocarditis/Pericarditis in Hong Kong Adolescents Following Comirnaty Vaccination. Clin Infect Dis. 2022;75(4):673–681. doi: 10.1093/cid/ciab989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchan S.A., Seo C.Y., Johnson C., Alley S., Kwong J.C., Nasreen S., et al. Epidemiology of Myocarditis and Pericarditis Following mRNA Vaccination by Vaccine Product, Schedule, and Interdose Interval Among Adolescents and Adults in Ontario, Canada. JAMA Netw Open. 2022;5(6):e2218505. doi: 10.1001/jamanetworkopen.2022.18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou O.H.I., Zhou J., Lee T.T.L., Kot T., Lee S., Wai A.K.C., et al. Comparisons of the risk of myopericarditis between COVID-19 patients and individuals receiving COVID-19 vaccines: a population-based study. Clinical research in cardiology : official journal of the German Cardiac Society. 2022;111(10):1098–1103. doi: 10.1007/s00392-022-02007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrao G., Franchi M., Cereda D., Bortolan F., Leoni O., Vignati E., et al. Increased risk of myocarditis and pericarditis and reduced likelihood of severe clinical outcomes associated with COVID-19 vaccination: a cohort study in Lombardy, Italy. BMC Infect Dis. 2022;22(1):844. doi: 10.1186/s12879-022-07823-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patone M., Mei X.W., Handunnetthi L., Dixon S., Zaccardi F., Shankar-Hari M., et al. Risk of Myocarditis After Sequential Doses of COVID-19 Vaccine and SARS-CoV-2 Infection by Age and Sex. Circulation. 2022;146(10):743–754. doi: 10.1161/CIRCULATIONAHA.122.059970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C., Fu F., Ding L., Fang J., Xiao J. Booster dose of COVID-19 mRNA vaccine does not increase risks of myocarditis and pericarditis compared with primary vaccination: New insights from the vaccine adverse event reporting system. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.938322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yap J., Tham M.Y., Poh J., Toh D., Chan C.L., Lim T.W., et al. Pericarditis and myocarditis after COVID-19 mRNA vaccination in a nationwide setting. Ann Acad Med Singap. 2022;51(2):96–100. [PubMed] [Google Scholar]
- 18.Su W-J, Liu Y-L, Chang C-H, Lin Y-C, Huang W-I, Wu L-C, et al. Risk of Myocarditis and Pericarditis Following Coronavirus Disease 2019 Messenger RNA Vaccination—A Nationwide Study. medRxiv. 2022:2022.10.11.22280860. [DOI] [PMC free article] [PubMed]
- 19.Ling R.R., Ramanathan K., Tan F.L., Tai B.C., Somani J., Fisher D., et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med. 2022;10(7):679–688. doi: 10.1016/S2213-2600(22)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available within the article and its supplementary materials. Data will not be available for others and any request will be subjected to approval from the data custodians. Malaysia COVID-19 vaccine administration data are available at https://github.com/MoH-Malaysia/covid19-public, redacted of personal identifying information.
The authors do not have permission to share data.