Abstract
Purpose
Prematurity, prenatal growth restriction, and retinopathy of prematurity (ROP) are associated with altered ocular geometry, such as a steeper corneal shape in childhood, but it is unclear whether perinatal history affects corneal thickness development, so this study investigated whether corneal thickness in adulthood is affected by perinatal history.
Marterials and Methods
The Gutenberg Prematurity Eye Study (GPES) is a retrospective cohort study with a prospective ophthalmologic examination in Germany. The corneal thickness was measured by Scheimpflug imaging (Pentacam HR, Oculus Optikgeräte GmbH, Wetzlar, Germany), and the relationship between perinatal parameters respective birth weight percentile and corneal thickness at different locations was assessed using uni- and multivariable linear regression models. Covariates included age, sex, mean corneal radius, white-to-white distance, gestational age, birth weight percentile, ROP occurrence, and treatment. The main outcome measures were corneal thickness at the apex, the pupil center, and the corneal periphery.
Results
The corneal thickness was measured in 390 participants (754 eyes, mean age 29.7+/-8.7 years, 224 females). In multivariable analyses, a lower birth weight percentile was associated with a lower corneal thickness at the apex (B = 0.20, p = 0.003) and the pupil (B = 0.19, p = 0.007). These effects diminished towards the corneal periphery and were not observed beyond the 4-mm diameter circle around the thinnest corneal position. Neither gestational age, ROP occurrence, or ROP treatment affected the corneal thickness.
Conclusion
A lower birth weight percentile in subjects born preterm as a proxy for restricted fetal growth is associated with corneal thickness thinning in adults aged 18 to 52 years, indicating that corneal thickness development, particularly in the corneal center, may originate in the fetal stage.
Keywords: Corneal thickness, Birth weight, Prematurity, Retinopathy of prematurity, Epidemiology
Introduction
Prematurity, low birth weight (BW), and postnatal occurrence of retinopathy of prematurity (ROP) affect ocular morphologic development.1 Children born preterm with a low BW have increased corneal steepness,1, 2, 3, 4, 5 a smaller anterior chamber depth,2,3 increased lens thickness,2 and a shorter axial length1,5 as well as altered morphology of the posterior pole.6, 7, 8, 9 However, the impact of prematurity and low BW on central corneal thickness (CCT) is controversial with multiple studies linking prematurity to an increased CCT compared to full-term birth in early life,10, 11, 12, 13 while others demonstrated a negative correlation of BW and CCT in older individuals.14,15 Furthermore, other studies reported that ROP contributes to an increase in CCT in newborns independent of prematurity.12
A few studies have evaluated the long-term effects of low BW as a proxy for prematurity on CCT in childhood16 and adolescence17,18 suggesting a decrease in CCT over time. Recently, the Gutenberg Health Study demonstrated in a population-based chohrt that a low BW (<2500 g) is associated with central cornea thinning in adulthood19,20 while less effects were observed on peripheral corneal thickness.20 However, these analyses were limited because gestational age and postnatal ROP status were not surveyed and therefore, these analyses could not assess the effects of the different perinatal parameters on corneal thickness development in adulthood. Furthermore, the authors rather investigated the effects of low birth weight in a population-based setting than the effects of extreme prematurity. Other factors affecting corneal thickness include age, gender, race, environment, and genetic predisposition.21,22 However, it is unclear whether other perinatal parameters influence the peripheral corneal thickness in adulthood and how gestational age and postnatal occurrence of ROP affect this association. Therefore, this study analyzed corneal thickness and its relationship to perinatal history in adults born term and preterm in different corneal regions using Scheimpflug imaging.
Materials and methods
Study population
The Gutenberg Prematurity Eye Study (GPES) is a single-center, retrospective cohort study conducted at the University Medical Center of the Johannes Gutenberg-University Mainz in Germany (UMCM) with prospective data acquisition including an ophthalmic examination in adulthood. The GPES recruited individuals born preterm or at term in the UMCM between 1969 and 2002 aged 18 to 52 years at study examination. Following an invitation algorithm, every former preterm newborn with a gestational age at birth (GA) ≤32 weeks and every second randomly selected preterm newborn with GA 33–36 weeks were approached. For each calendar month (from 1969 to 2002), 6 randomly selected full-term subjects (3 males and 3 females) with a BW between the 10th and 90th percentile were invited to serve as controls as reported earlier.23, 24, 25, 26, 27, 28, 29 The flowchart for eligibility and the effective recruitment efficacy proportion is displayed in Supplementary Figure 1.
Participants were grouped into full-term participants with a GA ≥37 weeks (group 1); preterm participants with a GA between 33 and 36 weeks without ROP (group 2); preterm participants with GA between 29 and 32 weeks without ROP (group 3); preterm participants with GA ≤28 weeks without ROP (group 4); and preterm participants with untreated ROP (group 5) or treated ROP (group 6). In the case of only one eye previously having ROP, the other non-ROP eye was excluded from the analysis. Moreover, participants with a history of corneal surgery and corneal trauma were excluded.
Written informed consent was obtained from all participants before they entered the study. The GPES complies with Good Clinical Practice (GCP), Good Epidemiological Practice (GEP), and the ethical principles of the Declaration of Helsinki. The study protocol and documents were approved by the local ethics committee of the Medical Chamber of Rhineland-Palatinate, Germany (reference no. 2019-14161; original vote: 29.05.2019, latest update: 02.04.2020).
Ophthalmologic examination
A detailed ophthalmologic examination including ocular biomicroscopy, Scheimpflug imaging, and a personal interview was conducted between 2019 and 2021. In addition, the perinatal and maternal medical records of each participant were reviewed. Objective refraction and distant-corrected visual acuity (ARK-1s, NIDEK, Oculus, Wetzlar, Germany), and ocular biomicroscopy (LenStar 900, Haag Streit, Köniz, Switzerland) were assessed in both eyes. Corneal and anterior segment tomography was measured using a rotating Scheimpflug camera (Pentacam HR, Oculus Optikgeräte GmbH, Wetzlar, Germany) that allows the three-dimensional examination from the anterior corneal surface to the posterior lens surface. For optimal alignment, corneal tomography was performed while participants had to fixate on a light source. The Scheimpflug camera captures 25 images within 2 s. All corneal thickness measurements were controlled and excluded if a measurement artifact was suspected based on the raw Scheimpflug images. The following corneal parameters were included in the present analysis: corneal thickness at the pupil center (CCT), corneal thickness at the apex (Dapex), minimal corneal thickness (Dmin), and circles around Dmin with 2 mm (D2mm), 4 mm (D4mm), 6 mm (D6mm), 8 mm (D8mm), and 10 mm (D10mm) diameter.
Assessment of pre-, peri- and postnatal history
Medical history data was assessed from medical records in the UMCM including GA (weeks), BW (kg), ROP and stage, ROP treatment with laser or cryotherapy, history of intraventricular hemorrhage, bronchopulmonal dysplasia, necrotizing enterocolitis, maternal placental insufficiency, maternal smoking during pregnancy, maternal preeclampsia and breastfeeding. Perinatal adverse events were defined in congruence with the German query for quality control of the neonatal clinics30 as an occurrence of intraventricular haemorrhage (at least grade 3 or parenchymal haemorrhage), necrotising enterocolitis and moderate or severe broncho-pulmonary dysplasia. BW percentile was calculated according to Voigt et al..31
Covariates
The covariates were selected based on literature review and clinical plausibility21,32, 33, 34 including age,33 gender,32 mean corneal radius,21 and white-to-white distance.21 The following parameters were also considered GA (weeks), BW (kg), BW percentile, ROP (yes), ROP treatment (yes).
Statistical analysis
The main outcome measures were the corneal thickness at the apex, the pupil center, and in circles around the thinnest position. Absolute and relative frequencies were calculated for the dichotomous parameters, mean and standard deviation were calculated for approximately normally distributed data, otherwise median and interquartile range. Linear regression analysis with general estimating equations (GEE) was applied to assess associations and account for correlations between corresponding eyes. First, univariate analyses between the main outcome measure and sex (female), age (years), mean corneal radius (mm), white-to-white distance (mm), GA (weeks), BW (kg), BW percentile, ROP (yes), and ROP treatment (yes), were computed, then, a multivariable model included all these parameters except BW due to the high correlation between gestational age and BW. This is an explorative study so there was no adjustment for multiple testing. All statistical analyses were performed using commercial software (IBM SPSS 20.0; SPSS, Inc., Chicago, IL, USA).
Results
Participant characteristics
In total, 390 participants (754 eyes) with a mean age of 29.7± 8.7 years (224 females) were included in this study. There were 253 eyes of 131 participants with a GA ≥37 weeks (group 1), 241 eyes of 124 participants with a GA between 33 and 36 weeks without ROP (group 2), 159 eyes of 82 participants with a GA between 29 and 32 weeks without ROP (group 3), 27 eyes of 14 participants with GA ≤28 weeks without ROP (group 4), 62 eyes of 31 participants with GA between 24 and 32 weeks with untreated ROP (group 5), and 12 eyes of 8 participants with GA between 24 and 32 and postnatal treatment for ROP (group 6). Of the ROP treated group, 4 participants (6 eyes) had laser coagulation, while 4 participants (6 eyes) had cryocoagulation. The effective recruitment efficacy proportion for each group is presented in Supplementary Figure 1. Overall, 60 participants were excluded because Scheimpflug imaging was not possible, or the scan quality was low (Table 1) or they had a history of corneal surgery.
Table 1.
Characteristics of the study sample (n = 390) of the Gutenberg prematurity eye study for each group.
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| Gestational age | GA ≥ 37 | GA 33–36 | GA 29–32 | GA ≤ 28 | GA ≤ 32 | GA ≤ 32 |
| no ROP | no ROP | no ROP | ROP without treatment | ROP with treatment | ||
| Participants/eyes (n) | 131 / 253 | 124 / 241 | 82 / 159 | 14 / 27 | 31 / 62 | 8 / 12 |
| Sex (Women) (%) | 76 (58.0%) | 74 (59.7%) | 45 (54.9%) | 8 (57.1%) | 18 (58.1%) | 3 (37.5%) |
| Age (y) | 29.8 ± 9.0 | 29.4 ± 9.2 | 28.1 ± 8.1 | 24.7 ± 7.9 | 25.1 ± 6.1 | 26.3 ± 4.7 |
| Birth weight (g) | 3433 ± 390 | 2076 ± 477 | 1531 ± 328 | 928 ± 202 | 1031 ± 378 | 751 ± 186 |
| Birth weight < 1500 g (yes) | 0 (0%) | 13 (10.5%) | 37 (45.1%) | 14 (100%) | 27 (87.1%) | 8 (100%) |
| Birth weight < 1000 g (yes) | 0 (0%) | 0 (0%) | 5 (6.1%) | 9 (64.3%) | 16 (51.6%) | 7 (87.5%) |
| Birth weight percentile | 49.3 ± 21.4 | 25.4 ± 24.3 | 42.9 ± 24.8 | 46.1 ± 26.0 | 38.4 ± 27.1 | 26.5 ± 27.7 |
| Gestational age (wks) | 39.4 ± 1.3 | 34.3 ± 0.9 | 30.6 ± 1.1 | 26.5 ± 1.6 | 27.7 ± 1.9 | 26.3 ± 1.8 |
| (min–max) | (37–43) | (33–36) | (29–32) | (23–28) | (24–32) | (24–29) |
| ROP stage (1/2/3) | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 21/30/4 | 0/3/9 |
| Perinatal adverse events (yes)a | 1 (0.8%) | 3 (2.4%) | 6 (7.3%) | 2 (14.3%) | 10 (32.3%) | 6 (75.0%) |
| Intraventricular hemorrhage (yes)b | 0 (0%) | 0 (0%) | 1 (1.2%) | 0 (0%) | 2 (6.5%) | 0 (0%) |
| Bronchopulmonary dyplasy (yes)c | 1 (0.8%) | 0 (0%) | 3 (3.7%) | 0 (0%) | 8 (25.8%) | 3 (37.5%) |
| Necrotizing entercolitis (yes)d | 0 (0%) | 3 (2.4%) | 2 (2.4%) | 2 (14.3%) | 2 (6.5%) | 3 (37.5%) |
| Preeclampsia (yes) | 11 (8.4%) | 23 (18.5%) | 9 (11.0%) | 3 (21.4%) | 6 (19.4%) | 2 (25.0%) |
| Placental insufficiency (yes) | 1 (0.8%) | 15 (12.1%) | 2 (2.4%) | 0 (0%) | 1 (3.2%) | 0 (0%) |
| HELLP-syndrome | 0 (0%) | 6 (4.8%) | 1 (1.2%) | 0 (0%) | 3 (9.7%) | 0 (0%) |
| Maternal smoking (yes)e | 6 (4.6%) | 7 (5.6%) | 8 (9.8%) | 1 (7.1%) | 4 (12.9%) | 2 (25.0%) |
| Gestational diabetes (yes) | 1 (0.8%) | 6 (4.8%) | 1 (1.2%) | 0 (0%) | 1 (3.2%) | 0 (0%) |
| Breastfeeding (yes) | 73 (55.7%) | 68 (54.8%) | 44 (53.7%) | 5 (35.7%) | 12 (38.7%) | 3 (37.5%) |
| Ocular parameters | ||||||
| Spherical equivalent (dpt) OD | −0.24 ± 2.58 | −0.02 ± 3.15 | 0.16 ± 1.80 | -0.3 ± 1.9 | 0.50 ± 4.30 | -5.5 ± 7.0 |
| Spherical equivalent (dpt) OS | −0.23 ± 2.60 | −0.01 ± 3.10 | 0.17 ± 1.79 | -0.14 ± 2.2 | 0.49 ± 4.28 | -3.5 ± 7.8 |
| Intraocular pressure (mmHg) OD | 15.2 ± 2.8 | 14.7 ± 2.9 | 15.3 ± 3.3 | 17.0 ± 3.5 | 15.3 ± 4.3 | 17.6 ± 3.4 |
| Intraocular pressure (mmHg) OS | 15.1 ± 2.8 | 14.6 ± 2.8 | 15.2 ± 3.2 | 15.1 ± 3.1 | 15.2 ± 4.2 | 16.0 ± 1.8 |
g - gram; mm-millimeter; dpt – diopter; OD - right eye; OS – left eye.
Perinatal adverse events were defined as:
occurrence of intraventricular hemorrhage (at least grade 3 or parenchymal hemorrhage) and/or
moderate or severe bronchopulmonary dysplasy and/or
occurrence of necrotizing enterocolitis
maternal smoking during pregnancy.
Descriptive ocular geometric parameters
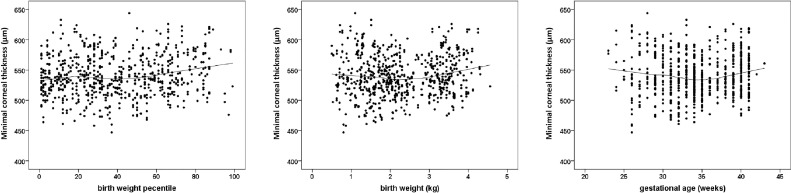
Corneal thickness measurements at different anatomic regions by GA and ROP status are displayed in Table 2. Participants born preterm with a GA between 33 and 36 weeks had a thinner corneal thickness than the control group and like the group with previous treated ROP, had a very low mean BW percentile. Fig. 1 provides the scatterplots of BW percentile, BW, and GA with minimal corneal thickness, showing that a lower BW percentile was associated with a thinner central corneal thickness.
Table 2.
Corneal thickness in different locations of the Gutenberg prematurity eye study study sample (n = 390) for each study group.
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| Gestational age | GA ≥ 37 | GA 33–36 | GA 29–32 | GA ≤ 28 | GA ≤ 32 | GA ≤ 32 |
| no ROP | no ROP | no ROP | ROP without treatment | ROP with treatment | ||
| Participants/eyes (n) | 131 / 253 | 124 / 241 | 82 / 159 | 14 / 27 | 31 / 62 | 8 / 12 |
| Corneal thickness (µm) | ||||||
| Apex OD | 550.1 ± 31.7 | 541.8 ± 30.0a | 543.3 ± 37.7 | 564.4 ± 45.5 | 550.0 ± 41.0 | 576b |
| Apex OS | 551.5 ± 33.3 | 540.3 ± 30.2a | 541.1 ± 34.8a | 552.1 ± 43.5 | 553.4 ± 40.3 | 538b |
| Pupil OD | 548.9 ± 31.8 | 541.2 ± 30.0 | 542.4 ± 37.7 | 563.9 ± 46.7 | 549.0 ± 40.4 | 573b |
| Pupil OS | 549.7 ± 33.3 | 538.9 ± 30.3a | 539.3 ± 34.7a | 549.0 ± 40.4 | 551.8 ± 40.4 | 532b |
| Corneal thickness in circles | ||||||
| around corneal thickness minimum (µm) | ||||||
| D 0 mm OD | 545.0 ± 31.8 | 537.4 ± 29.8 | 537.6 ± 36.3 | 559.6 ± 46.3 | 544.9 ± 41.3 | 570b |
| D 0 mm OS | 544.8 ± 33.0 | 533.6 ± 30.6a | 533.8 ± 34.5a | 545.9 ± 44.9 | 547.0 ± 41.2 | 528b |
| D 2 mm OD | 554.7 ± 31.8 | 547.2 ± 30.0 | 547.7 ± 36.6 | 569.9 ± 46.6 | 555.3 ± 40.9 | 579b |
| D 2 mm OS | 554.8 ± 33.0 | 543.6 ± 30.6 | 544.1 ± 34.4 | 555.8 ± 46.6 | 557.2 ± 41.0 | 539b |
| D 4 mm OD | 584.0 ± 32.3 | 576.6 ± 31.1 | 577.6 ± 38.0 | 601.1 ± 49.6 | 586.4 ± 41.0 | 601b |
| D 4 mm OS | 584.2 ± 33.3 | 573.2 ± 31.3a | 573.9 ± 34.9a | 585.6 ± 48.3 | 587.9 ± 41.3 | 572b |
| D 6 mm OD | 633.9 ± 34.7 | 627.0 ± 34.0 | 627.9 ± 40.5 | 655.6 ± 56.0 | 638.6 ± 43.3 | 652b |
| D 6 mm OS | 633.5 ± 35.2 | 623.0 ± 34.0a | 623.9 ± 36.7 | 639.0 ± 55.4 | 640.4 ± 41.6 | 633b |
| D 8 mm OD | 709.6 ± 39.0 | 705.4 ± 40.4 | 706.6 ± 43.1 | 738.3 ± 59.7 | 716.8 ± 46.4 | 746b |
| D 8 mm OS | 709.6 ± 39.1 | 702.2 ± 39.8 | 703.2 ± 39.9 | 722.6 ± 60.5 | 720.9 ± 43.3 | 722b |
| D 10 mm OD | 814.5 ± 49.9 | 816.2 ± 53.5 | 812.3 ± 49.4 | 841.2 ± 60.5 | 821.2 ± 49.9 | 847b |
| D 10 mm OD | 812.6 ± 48.4 | 813.4 ± 50.8 | 810.1 ± 50.7 | 822.5 ± 66.7 | 817.8 ± 42.4 | 827b |
GA – gestational age; ROP – Retinopathy of Prematurity; µm – micrometer, OD – right eye, OS – left eye.
Linear regression analysis was applied to compare the different groups with the full-term control group (reference).
statistical difference (p<0.05) compared to the full-term control group.
For group 6 only median is presented because of the low number of eyes/participants in this group.
Fig. 1.
Relationship of minimal corneal thickness of all participants with a) birth weight percentile, b) birth weight, c) gestational age in the Gutenberg prematurity eye study (n = 390). The line presents the Loess (Locally Weighted Scatterplot Smoothing) curve.
Association analyses
A lower BW percentile was associated with a thinner corneal thickness at the apex (B = 0.20 [95% CI: 0.07; 0.34] µm per percentile increase; p = 0.003), the center of the pupil (B = 0.19 [95% CI: 0.05; 0.32] µm; p = 0.007), minimal corneal thickness (B = 0.20 [95%-CI: 0.06; 0.33] µm; p = 0.005), and in the 2-mm (B = 0.19 [95% CI: 0.05; 0.32] µm; p = 0.007) and 4-mm diameter circles around the corneal minimum (B = 0.16 [95% CI: 0.02; 0.29] µm; p = 0.024) after adjustment for age, sex, corneal radius, and white to white distance (Table 3). These effects diminished towards the corneal periphery and there was no association between the BW percentile and corneal thickness in circles with a diameter of 6 mm, 8 mm, and 10 mm. Gestational age, ROP occurrence, and treatment were not associated with the corneal thickness (Table 3).
Table 3.
Association of perinatal characteristics and corneal thickness for the sample of the Gutenberg prematurity eye study. (n = 390).
| Univariablea |
Multivariableb |
|||
|---|---|---|---|---|
| B [95% CI] | p | B [95% CI] | p | |
| Corneal thickness apex [µm] | ||||
| Gestational age (weeks) | 0.111 (−0.719; 0.941) | 0.79 | 0.380 (−0.507; 1.267) | 0.40 |
| Birth weight (kg) | 2.380 (−1.309; 6.069) | 0.21 | – | – |
| Birth weight percentile | 0.187 (0.055; 0.319) | 0.006 | 0.204 (0.069; 0.338) | 0.003 |
| ROP (yes) | 5.204 (−2.822; 13.230) | 0.20 | 4.407 (−3.742; 10.56) | 0.35 |
| ROP treatment (yes) | 22.39 (−6.659; 51.46) | 0.13 | 18.38 (−8.032; 44.78) | 0.17 |
| Corneal thickness pupil [µm] | ||||
| Gestational age (weeks) | 0.094 (−0.738; 0.927) | 0.82 | 0.361 (−0.538; 1.260) | 0.43 |
| Birth weight (kg) | 2.154 (−1.539; 5.847) | 0.25 | – | – |
| Birth weight percentile | 0.172 (0.039; 0.304) | 0.011 | 0.188 (0.052; 0.323) | 0.007 |
| ROP (yes) | 5.087 (−3.102; 13.276) | 0.22 | 3.339 (−4.231; 10.90) | 0.39 |
| ROP treatment (yes) | 21.27 (−7.835; 50.37) | 0.15 | 17.08 (−9.482; 43.65) | 0.21 |
| D 0 Corneal thickness [µm] | ||||
| Gestational age (weeks) | 0.130 (−0.709; 0.968) | 0.76 | 0.371 (−0.530; 1.272) | 0.42 |
| Birth weight (kg) | 2.408 (−1.293; 6.110) | 0.20 | – | – |
| Birth weight percentile | 0.184 (0.051; 0.370) | 0.007 | 0.195 (0.060; 0.331) | 0.005 |
| ROP (yes) | 5.139 (−3.537; 13.82) | 0.27 | 3.337 (−4.767; 11.44) | 0.42 |
| ROP treatment (yes) | 22.36 (−6.999; 51.72) | 0.14 | 18.87 (−8.095; 45.84) | 0.17 |
| D 2 Corneal thickness [µm] | ||||
| Gestational age (weeks) | 0.087 (−0.752; 0.926) | 0.84 | 0.357 (−0.547; 1.262) | 0.44 |
| Birth weight (kg) | 2.114 (−1.589; 5.817) | 0.26 | – | – |
| Birth weight percentile | 0.171 (0.037; 0.304) | 0.012 | 0.185 (0.050; 0.319) | 0.007 |
| ROP (yes) | 5.528 (−3.063; 14.12) | 0.21 | 3.635 (−4.441; 11.68) | 0.38 |
| ROP treatment (yes) | 22.72 (−6.79; 52.15) | 0.13 | 18.55 (−8.544; 45.65) | 0.18 |
| D 4 Corneal thickness [µm] | ||||
| Gestational age (weeks) | −0.031 (−0.894; 0.832) | 0.94 | 0.334 (−0.596; 1.265) | 0.48 |
| Birth weight (kg) | 1.293 (−2.494; 5.081) | 0.50 | – | – |
| Birth weight percentile | 0.136 (−0.001; 0.272) | 0.05 | 0.157 (0.020; 0.293) | 0.024 |
| ROP (yes) | 6.600 (−2.237; 15.44) | 0.14 | 3.910 (−4.256; 12.08) | 0.35 |
| ROP treatment (yes) | 25.47 (−5.641; 56.58) | 0.11 | 19.06 (−9.112; 47.23) | 0.19 |
| D 6 Corneal thickness [µm] | ||||
| Gestational age (weeks) | −0.259 (−1.195; 0.677) | 0.59 | 0.272 (−0.732; 1.277) | 0.60 |
| Birth weight (kg) | −0.236 (−4.294; 3.823) | 0.91 | – | – |
| Birth weight percentile | 0.073 (−0.074; 0.220) | 0.33 | 0.112 (−0.031; 0.256) | 0.13 |
| ROP (yes) | 7.707 (−2.010; 17.42) | 0.12 | 2.957 (−5.645; 11.56) | 0.50 |
| ROP treatment (yes) | 30.82 (−5.538; 67.17) | 0.10 | 20.95 (−10.95; 52.85) | 0.19 |
| D 8 Corneal thickness [µm] | ||||
| Gestational age (weeks) | −0.666 (−1.694; 0.363) | 0.21 | 0.252 (−0.837; 1.341) | 0.65 |
| Birth weight (kg) | −2.758 (−7.275; 1.760) | 0.23 | – | – |
| Birth weight percentile | −0.015 (−0.181; 0.150) | 0.86 | 0.068 (−0.090; 0.226) | 0.40 |
| ROP (yes) | 8.731 (−1.334; 18.795) | 0.09 | 2.016 (−6.541; 10.57) | 0.64 |
| ROP treatment (yes) | 36.22 (−6.629; 79.06) | 0.10 | 22.51 (−15.30; 60.32) | 0.24 |
| D 10 Corneal thickness [µm] | ||||
| Gestational age (weeks) | −0.774 (−2.024; 0.476) | 0.23 | 0.926 (−0.342; 2.194) | 0.15 |
| Birth weight (kg) | −4.134 (−9.850; 1.582) | 0.16 | – | – |
| Birth weight percentile | −0.094 (−0.311; 0.122) | 0.39 | 0.038 (−0.159; 0.235) | 0.71 |
| ROP (yes) | 12.75 (1.494; 24.01) | 0.03 | 8.351 (−2.131; 18.83) | 0.11 |
| ROP treatment (yes) | 43.66 (−17.27; 104.6) | 0.16 | 33.96 (−21.98; 89.89) | 0.23 |
B – Beta; CI – Confidence interval; µm – micrometer; Linear regression analysis using generalized estimating equations to control for correlations between right and left eyes.
Crude model without adjustment.
Model with inclusion of age (years), sex (female), corneal radius (mm), white-to-white distance (mm), gestational age (weeks), birth weight percentile, ROP (yes), ROP treatment (yes). Birth weight (kg) was not included into this multivariable model due to the high correlation with gestational age.
Discussion
In 754 eyes of 390 adults of the Gutenberg Prematurity Eye Study, a low BW percentile was associated with a thinner cornea at the apex and the pupil, while these differences diminished towards the periphery. In contrast, low gestational age, postnatal ROP occurrence, and treatment were not associated with corneal thickness, suggesting that fetal growth restriction rather than preterm birth and ROP influence corneal thickness in adulthood.
Previous studies evaluating the effect of prematurity, low BW, and postnatal ROP occurrence or treatment on CCT development have mostly focused on infancy and childhood.35,14,15 At birth, preterm newborns with a low BW have thicker corneas,10, 11, 12, 13 which decreases until full-term age.13,35,36 This decrease may be modulated by ROP in preterm newborns, with a lower CCT decrease in preterm infants with ROP (10.93 µm/week) than in preterm infants without ROP (16.11 µm/week) at 30–38 weeks.12 Regarding full-term newborns, both the central and peripheral corneal thickness was thicker in newborns with a lower BW compared to newborns with a higher BW.37
This decrease of CCT in the newborn period seems to cause a shift in childhood as previous studies suggest a correlation between low BW and reduced corneal thickness in children aged 3 years and older.16,18 Likewise, children born preterm versus children born at term aged 7–14 years also had a descriptively thinner corneal thickness (535 ± 103 µm vs. 561 ± 33 µm; p = 0.09).38 Furthermore, Marques et al. reported a significantly thinner cornea in eyes with a history of ROP,39 whereas the Wiesbaden Prematurity Study observed no difference in corneal thickness between children born preterm with and without ROP compared to children born at term at the age of 4 to 10 years.1
The association of low BW and central corneal thickness was also reported in a cohort of Chinese adolescents,17 and the Gutenberg Health Study also reported that adults of a low BW (<2500 g) had a 5-µm thinner central corneal thickness compared to normal BW individuals.19 In another analysis of the participants of the Gutenberg Health Study the authors could show that low birth weight is associated particularly with lower central corneal thickness, while less effects are present in the corneal periphery.20 However, this data was limited because birth-weight was self-reported and GA as well as postnatal ROP status were not considered. The present study extends these findings, presenting the first data regarding the effects of extreme prematurity, fetal growth restriction, and ROP occurrence and treatment on corneal thickness. In the oldest and largest cohort of adults born preterm with and without ROP, we demonstrated that a low BW percentile as a proxy for fetal growth restriction was associated with a thinner cornea in the apex and these differences diminished towards the periphery, suggesting fetal adverse growth as a causal mechanism. Growth restriction during decisive periods of intrauterine organ development may cause morphologic and functional long-term sequelae in these organs,40,41 as supported by the present findings, suggestting long-term effects of fetal growth restriction on corneal shape. We speculate that this may be caused by structural changes due to an alteration in the corneal remodeling processes or ultrastructural changes in collagen fiber layers potentially predisposing to ectatic diseases in later life.
Other factors reducing the CCT include African-American race,42 female gender,43,44 age,45,46 large body height,47 and low BMI.48 Some of these parameters are partially influenced by low BW percentile49 and may to some extent explain those associations. Alterations of the corneal thickness and structure are of clinical importance in the assessment of intraocular pressure, and in the pathophysiology of corneal diseases, i.e. ectatic diseases with corneal thinning such as keratoconus.
Strengths and limitations
The findings of the current study are derived from a single study center in a hospital-based cohort leading to limitations in terms of generalizability. Furthermore, there may be bias in UM contact data availability and consent to participate in our study. However, the effective recruitment efficacy proportion was relatively high (Supplementary Figure 1). In addition, individuals born extremely preterm are at increased risk of reduced visual acuity and nystagmus, thus may not be able to adequately fixate during Scheimpflug imaging. Another limitation is that the number of participants with treated ROP was low. Nonetheless, a strength of the present analysis is the ocular examination including Scheimpflug imaging of the oldest and largest cohort of adults born preterm with and without ROP in the medical literature. The review of the medical charts of each participant and their mothers allowed a detailed view of the perinatal history. The approach used also made it possible to build statistical models including several perinatal parameters potentially affecting corneal thickness in the long term.
Conclusion
In summary, our study highlights that a low BW percentile as a surrogate marker of fetal growth restriction has life-long effects on corneal thickness, particularly in the corneal center.
Conflict of interest
The author reports no conflict of interest.
Acknowledgments
The study team thanks all study participants who took part in the GPES, which includes an enthusiastic team to explore perinatal factors on long-term eye development. The GPES was supported by the Ernst- und Berta-Grimmke Stiftung and Stufe 1 support of the UM and by the Else Kröner Fresenius-Stiftung. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Schuster AK holds the professorship for ophthalmic healthcare research endowed by “Stiftung Auge” and financed by “Deutsche Ophthalmologische Gesellschaft” and “Berufsverband der Augenärzte Deutschlands e.V.” Pfeiffer N receives financial support and grants from Novartis, Ivantis, Santen, Thea, Boehringer Ingelheim Deutschland GmbH & Co. KG, Alcon, and Sanoculis. Schuster AK receives research support from Allergan, Bayer, Heidelberg Engineering, PlusOptix and Norvartis.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.optom.2022.07.001.
Appendix. Supplementary materials
References
- 1.Fieß A., Kolb-Keerl R., Knuf M., et al. Axial length and anterior segment alterations in former preterm infants and full-term neonates analyzed with scheimpflug imaging. Cornea. 2017;36:821–827. doi: 10.1097/ICO.0000000000001186. [DOI] [PubMed] [Google Scholar]
- 2.Wu W.C., Lin R.I., Shih C.P., et al. Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology. 2012;119:1907–1916. doi: 10.1016/j.ophtha.2012.02.040. [DOI] [PubMed] [Google Scholar]
- 3.Cook A., White S., Batterbury M., Clark D. Ocular growth and refractive error development in premature infants with or without retinopathy of prematurity. Investig Ophthalmol Vis Sci. 2008;49:5199–5207. doi: 10.1167/iovs.06-0114. [DOI] [PubMed] [Google Scholar]
- 4.Fieß A., Schuster A.K., Pfeiffer N., Nickels S. Association of birth weight with corneal power in early adolescence: results from the National Health and Nutrition Examination Survey (NHANES) 1999–2008. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ojaimi E., Robaei D., Rochtchina E., Rose K.A., Morgan I.G., Mitchell P. Impact of birth parameters on eye size in a population-based study of 6-year-old Australian children. Am J Ophthalmol. 2005;140:535–537. doi: 10.1016/j.ajo.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 6.Fieß A., Christian L., Kolb-Keerl R., et al. Peripapillary choroidal thickness in former preterm and full-term infants aged from 4 to 10 years. Investig Ophthalmol Vis Sci. 2016;57:6548–6553. doi: 10.1167/iovs.16-20128. [DOI] [PubMed] [Google Scholar]
- 7.Fieß A., Christian L., Janz J., et al. Functional analysis and associated factors of the peripapillary retinal nerve fibre layer in former preterm and full-term infants. Br J Ophthalmol. 2017;101:1405–1411. doi: 10.1136/bjophthalmol-2016-309622. [DOI] [PubMed] [Google Scholar]
- 8.Fieß A., Janz J., Schuster A.K., et al. Macular morphology in former preterm and full-term infants aged 4 to 10 years. Graefes Arch Clin Exp. Ophthalmol. 2017;255:1433–1442. doi: 10.1007/s00417-017-3662-5. [DOI] [PubMed] [Google Scholar]
- 9.Fieß A., Kolb-Keerl R., Schuster A.K., et al. Correlation of morphological parameters and visual acuity with neurological development in former preterm children aged 4–10 years. Acta Ophthalmol (Copenh) 2018;96:e1010–e1017. doi: 10.1111/aos.13755. [DOI] [PubMed] [Google Scholar]
- 10.Kirwan C., O'Keefe M., Fitzsimon S. Central corneal thickness and corneal diameter in premature infants. Acta Ophthalmol Scand. 2005;83:751–753. doi: 10.1111/j.1600-0420.2005.00559.x. [DOI] [PubMed] [Google Scholar]
- 11.Autzen T., Bjornstrom L. Central corneal thickness in premature babies. Acta Ophthalmol (Copenh) 1991;69:251–252. doi: 10.1111/j.1755-3768.1991.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 12.Kardaras D., Papageorgiou E., Gaitana K., et al. The association between retinopathy of prematurity and ocular growth. Investig Ophthalmol Vis Sci. 2019;60:98–106. doi: 10.1167/iovs.18-24776. [DOI] [PubMed] [Google Scholar]
- 13.Jethani J., Shah K., Jethani M. Evaluating the change in central corneal thickness in neonates (term and preterm) in Indian population and the factors affecting it. Indian J Ophthalmol. 2015;63:501–503. doi: 10.4103/0301-4738.162601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunay M., Celik G., Gunay B.O., Dogru M., Gursoy T., Ovali H.F. Central corneal thickness measurements in premature infants. Int J Ophthalmol. 2014;7:496–500. doi: 10.3980/j.issn.2222-3959.2014.03.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kıvanç S.A., Akova Budak B., Olcaysü O.O. Association of birth parameters with corneal thickness in prematurely born and full-term school-aged children. Cornea. 2016;35:634–637. doi: 10.1097/ICO.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 16.Yeter V., Aritürk N., Bİrİncİ H., Süllü Y., Güngör İ. Effects of birth weight on anterior segment measurements in full-term children without low birth weight by dual-scheimpflug analyzer. Am J Ophthalmol. 2015;160:832–840.e831. doi: 10.1016/j.ajo.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Pan C.-.W., Qian Y.-.X., Zhong H., Li J., Liu H., Chen Q. Central corneal thickness and its association with birth parameters in chinese adolescents. Ophthalmic Epidemiol. 2019;26:360–366. doi: 10.1080/09286586.2019.1632903. [DOI] [PubMed] [Google Scholar]
- 18.Fledelius H.C. Ophthalmic changes from age of 10 to 18 years. A longitudinal study of sequels to low birth weight. III. Ultrasound oculometry and keratometry of anterior eye segment. Acta Ophthalmol (Copenh) 1982;60:393–402. doi: 10.1111/j.1755-3768.1982.tb03030.x. [DOI] [PubMed] [Google Scholar]
- 19.Fieß A., Schuster A.K., Nickels S., et al. Association of low birth weight with altered corneal geometry and axial length in adulthood in the German Gutenberg health study. JAMA Ophthalmol. 2019;137:507–514. doi: 10.1001/jamaophthalmol.2018.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fieß A., Urschitz M.S., Marx-Groß S., et al. Association of birth weight with central and peripheral corneal thickness in adulthood-results from the population-based german gutenberg health study. Children (Basel) 2021;8:1006. doi: 10.3390/children8111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann E.M., Lamparter J., Mirshahi A., et al. Distribution of central corneal thickness and its association with ocular parameters in a large central European cohort: the Gutenberg health study. PLoS ONE. 2013;8:e66158. doi: 10.1371/journal.pone.0066158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y., Vitart V., Burdon K.P., et al. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat Genet. 2013;45:155–163. doi: 10.1038/ng.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fieß A., Fauer A., Mildenberger E., et al. Refractive error, accommodation and lens opacification in adults born preterm and full-term: results from the Gutenberg Prematurity Eye Study (GPES) Acta Ophthalmol (Copenh) 2022 doi: 10.1111/aos.15132. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Fieß A., Gißler S., Fauer A., et al. Short report on retinal vessel metrics and arterial blood pressure in adult individuals born preterm with and without retinopathy of prematurity: results from the Gutenberg prematurity eye study. Acta Ophthalmol (Copenh) 2022 doi: 10.1111/aos.15132. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Fieß A., Gißler S., Mildenberger E., et al. Anterior chamber angle in adults born extremely, very, and moderately preterm with and without retinopathy of prematurity-results of the gutenberg prematurity eye study. Children (Basel) 2022;9:281. doi: 10.3390/children9020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fieß A., Gißler S., Mildenberger E., et al. Optic nerve head morphology in adults born extreme, very and moderate preterm with and without retinopathy of prematurity: results from the Gutenberg prematurity eye study. Am J Ophthalmol. 2022;239:212–222. doi: 10.1016/j.ajo.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Fieß A., Hufschmidt-Merizian C., Gißler S., et al. Dry eye parameters and lid geometry in adults born extremely, very, and moderately preterm with and without ROP: results from the Gutenberg prematurity eye study. J Clin Med. 2022;11:2702. doi: 10.3390/jcm11102702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fieß A., Nauen H., Mildenberger E., et al. Ocular geometry in adults born extremely, very and moderately preterm with and without retinopathy of prematurity: results from the Gutenberg prematurity eye study. Br J Ophthalmol. 2022 doi: 10.1136/bjophthalmol-2021-320907. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fieß A., Pfisterer A., Gißler S., et al. Retinal thickness and foveal hypoplasia in adults born preterm with and without retinopathy of prematurity – the Gutenberg prematurity eye study. Retina. 2022 doi: 10.1097/IAE.0000000000003501. [DOI] [PubMed] [Google Scholar]
- 30.IQTIG – Institut für Qualitätssicherung und Transparenz im Gesundheitswesen. Beschreibung der Qualitätsindikatoren und Kennzahlen nach QSKH-RL. Neonatologie. Erfassungsjahr. 2018. Vol 2021. online2019.https://iqtig.org/downloads/auswertung/auswertung/2018/neo/QSKH_NEO_2018_QIDB_V2003_2019-2004-2025.pdf.
- 31.Voigt M., Fusch C., Olbertz D. Vol. 66. Geburtsh Frauenheilk; 2006. pp. 956–970. (Analyse Des Neugeborenenkollektivs der Bundesrepublik Deutschland 12. Mitteilung: Vorstellung Engmaschiger Perzentilwerte (-kurven) Für Die Körpermaße Neugeborener). [Google Scholar]
- 32.Elflein H.M., Pfeiffer N., Hoffmann E.M., et al. Correlations between central corneal thickness and general anthropometric characteristics and cardiovascular parameters in a large European cohort from the Gutenberg health study. Cornea. 2014;33:359–365. doi: 10.1097/ICO.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 33.Wong T.T., Wong T.Y., Foster P.J., Crowston J.G., Fong C.W., Aung T. The relationship of intraocular pressure with age, systolic blood pressure, and central corneal thickness in an asian population. Investig Ophthalmol Vis Sci. 2009;50:4097–4102. doi: 10.1167/iovs.08-2822. [DOI] [PubMed] [Google Scholar]
- 34.Fieß A., Marx-Groß S., Wasielica-Poslednik J., et al. Peripheral corneal thickness and associated factors - results from the population-based German Gutenberg health study. Acta Ophthalmol (Copenh) 2022 doi: 10.1111/aos.15057. ; 100:e1298–e1305. [DOI] [PubMed] [Google Scholar]
- 35.Remón L., Cristóbal J.A., Castillo J., Palomar T., Palomar A., Pérez J. Central and peripheral corneal thickness in full-term newborns by ultrasonic pachymetry. Investig Ophthalmol Vis Sci. 1992;33:3080–3083. [PubMed] [Google Scholar]
- 36.Ehlers N., Sorensen T., Bramsen T., Poulsen E.H. Central corneal thickness in newborns and children. Acta Ophthalmol (Copenh) 1976;54:285–290. doi: 10.1111/j.1755-3768.1976.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 37.Portellinha W., Belfort R., Jr. Central and peripheral corneal thickness in newborns. Acta Ophthalmol (Copenh) 1991;69:247–250. doi: 10.1111/j.1755-3768.1991.tb02719.x. [DOI] [PubMed] [Google Scholar]
- 38.Ecsedy M., Kovacs I., Mihaltz K., et al. Scheimpflug imaging for long-term evaluation of optical components in Hungarian children with a history of preterm birth. J Pediatr Ophthalmol Strabismus. 2014;51:235–241. doi: 10.3928/01913913-20140521-04. [DOI] [PubMed] [Google Scholar]
- 39.Marques S.N., Barros R.S., Miranda F.A., et al. Scheimpflug imaging for evaluation of optical components in Portuguese children with history of preterm birth. Oftalmologia. 2016;40:139–144. [Google Scholar]
- 40.Barker D.J. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807–813. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 41.Barker D.J. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588s–595s. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 42.Dai E., Gunderson C.A. Pediatric central corneal thickness variation among major ethnic populations. J AAPOS: Off Publ Am Assoc Pediatr Ophthalmol Strabismus. 2006;10:22–25. doi: 10.1016/j.jaapos.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Pfeiffer N., Torri V., Miglior S., Zeyen T., Adamsons I., Cunha-Vaz J. Central corneal thickness in the European glaucoma prevention study. Ophthalmology. 2007;114:454–459. doi: 10.1016/j.ophtha.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 44.Vijaya L., George R., Arvind H., et al. Central corneal thickness in adult South Indians: the Chennai glaucoma study. Ophthalmology. 2010;117:700–704. doi: 10.1016/j.ophtha.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 45.Kawase K., Tomidokoro A., Araie M., Iwase A., Yamamoto T. Ocular and systemic factors related to intraocular pressure in Japanese adults: the Tajimi study. Br J Ophthalmol. 2008;92:1175–1179. doi: 10.1136/bjo.2007.128819. [DOI] [PubMed] [Google Scholar]
- 46.Rochtchina E., Mitchell P., Wang J.J. Relationship between age and intraocular pressure: the blue mountains eye study. Clin Exp Ophthalmol. 2002;30:173–175. doi: 10.1046/j.1442-9071.2002.00519.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H., Xu L., Chen C., Jonas J.B. Central corneal thickness in adult Chinese. Association with ocular and general parameters. The Beijing eye study. Graefes Arch Clin Exp Ophthalmol. 2008;246:587–592. doi: 10.1007/s00417-007-0760-9. [DOI] [PubMed] [Google Scholar]
- 48.Tomidokoro A., Araie M., Iwase A. Corneal thickness and relating factors in a population-based study in Japan: the Tajimi study. Am J Ophthalmol. 2007;144:152–154. doi: 10.1016/j.ajo.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 49.Xia Q., Cai H., Xiang Y.B., et al. Prospective cohort studies of birth weight and risk of obesity, diabetes, and hypertension in adulthood among the Chinese population. J Diabetes. 2019;11:55–64. doi: 10.1111/1753-0407.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.