Abstract
Background
To apply non-invasive Automatic Tongue Diagnosis System (ATDS) in analyzing tongue features in patients with chronic kidney disease (CKD).
Methods
This was a cross-sectional, case-controlled observational study. Patients with CKD who met the inclusion and exclusion criteria were enrolled and divided into the following groups according to renal function and dialysis status: non-dialysis CKD group; end-stage renal disease (ESRD) group; and control group. Tongue images were captured and eight tongue features—shape, color, fur thickness, saliva, fissure, ecchymosis, teeth marks, and red dots—were imaged and analyzed by ATDS.
Results
117 participants (57 men, 60 women) were enrolled in the study, which included 16 in control group, 38 in non-dialysis CKD group, and 63 in ESRD group. We demonstrated significant differences in the fur thickness (p = 0.045), color (p = 0.005), amounts of ecchymosis (p = 0.010), teeth marks (p = 0.016), and red dot (p < 0.001) among three groups. The areas under receiver operating characteristic curve for the amount of ecchymosis was 0.757 ± 0.055 (95% confidence interval, 0.648–0866; p < 0.001). Additionally, with increase in ecchymosis by one point, the risk of CKD dialysis rose by 1.523 times (95% confidence interval, 1.198–1.936; p = 0.001). After hemodialysis, the amount of saliva (p = 0.038), the area of saliva (p = 0.048) and the number of red dots (p = 0.040) were decreased significantly among patients with ESRD. On the contrary, the percentage of coating (p = 0.002) and area of coating (p = 0.026) were increased significantly after hemodialysis.
Conclusion
Blood deficiency and stasis with qi deficiency or blood heat syndrome (Zheng pattern) is common in patients with CKD. The risk of CKD dialysis increases with increasing ecchymosis. Hemodialysis can affect saliva, tongue coating, and relieve heat syndrome among ESRD patients.
Keywords: Tongue diagnosis, Chronic kidney disease, Automatic Tongue Diagnosis System, Traditional Chinese medicine
At a glance commentary
Scientific background on the subject
This study applied non-invasive automatic tongue diagnosis system in analyzing tongue features in patients with chronic kidney disease.
What this study adds to the field
Blood deficiency and stasis with qi deficiency or blood heat syndrome (Zheng pattern) is common in patients with CKD. The risk of CKD dialysis increases with increasing ecchymosis on the tongue. Hemodialysis can affect saliva, tongue coating and relieve heat syndrome among ESRD patients.
Chronic kidney disease (CKD) is a global financial and medical burden because of its high morbidity and mortality [1]. Furthermore, renal disease is one of the ten leading causes of death in Taiwan. Taiwan has high incidence (27.21/1000 patient-years) and prevalence (15.46%) of CKD. Patients with end-stage renal disease (ESRD) only account for 0.15% of the total patients with CKD; however, over 7% of the annual budget of Taiwan's NHI Program is consumed by dialysis [1]. Additionally, the expenses due to ESRD continue to rise along with the incidence and prevalence of dialysis. The incidence of dialysis in 2000 was 331 per million/year, which rose to 504 per million/year in 2017. Similarly, the prevalence of dialysis was 1448 per million in 2000, which increased to 3480 per million in 2017 [2].
According to 2012 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline, CKD is defined as abnormalities of kidney structures or glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 for more than 3 months [3]. With progressive loss of renal function, many complications, such as volume overload, electrolyte imbalance, anemia, and metabolic acidosis may develop [4]. Patients may present with uremia-related symptoms and signs, such as fatigue, insomnia, pruritus, nausea, and vomiting [5,6]. Traditional Chinese medicine (TCM) has been used to treat CKD for thousands of years in China and was proved to be effective [[7], [8], [9]].
In TCM, tongue diagnosis is an important and unique diagnostic tool. The tongue can reflect the qi-blood, yin-yang status of the internal organs according to the TCM theory [10,11]. Through inspection of the appearance of the tongue, such as tongue color, teeth mark, fur color, and thickness, TCM doctors determine the TCM syndrome, which further guides the treatment.
However, interpretation of tongue diagnosis is often biased due to subjective judgments and environmental factors. In order to obtain an objective diagnosis, in this study, we employed Automatic Tongue Diagnosis System (ATDS) to capture and interpret the appearance of the tongue. In the past, studies have used ATDS to explore the relationship between tongue features and various diseases, such as diabetes mellitus [12], metabolic syndrome [13], breast cancer [14], functional dyspepsia [15], and gastroesophageal reflux [16].
The aims of our study were to evaluate the differences in tongue manifestations between patients with CKD not on dialysis, patients with ESRD, and healthy controls using non-invasive ATDS and to provide valuable information to TCM doctors in accurately differentiating between syndromes and providing treatment.
Materials and methods
Ethics approval
This study was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants before enrollment. Personal data about potential and enrolled participants were collected, shared, and stored in an independent closet for protecting confidentiality before, during, and after the study. The trial was approved by the Institutional Review Board of the Chang Gung Medical Foundation (IRB no. 201801244B0). This trial was registered with the National Institute of Health clinical trial registry (clinicaltrials.gov; NCT04559958).
Participants
Patients were recruited from the outpatient clinic and hemodialysis unit of the Division of Nephrology of Kaohsiung Chang Gung Memorial Hospital in Kaohsiung, Taiwan.
Inclusion criteria
Subjects were deemed eligible upon satisfaction of the following criteria. Patients in both arms of the study were over 20 years old in age. The experimental group had CKD stage 3–5 (estimated glomerular filtration rate < 60 mL/min/1.732). Informed consent was obtained from all patients.
Exclusion criteria
The following were the exclusion criteria: pregnancy; acute infections; cancer with brain metastasis, and presenting with cognitive dysfunction, such as dementia or delirium; inability to protrude the tongue stably; patients with risk of temporomandibular joint dislocation; and those who did not provide informed consent.
Study design
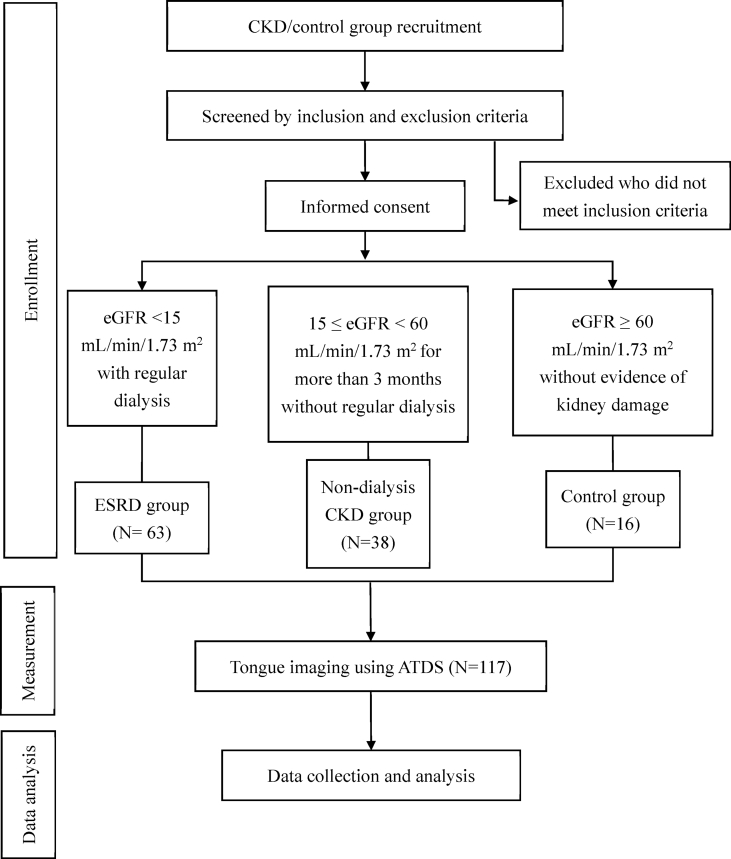
This was a cross-sectional, case-controlled observational study. Participants were divided into the following three groups according to the status of their renal function: non-dialysis CKD group, ESRD group, and control group. Participants with eGFR ≥15 and <60 mL/min/1.73 m2 (calculated using the Modification of Diet in Renal Disease [MDRD] Study equation) for more than 3 months and without regular dialysis were enrolled in non-dialysis CKD group [3]. Participants with eGFR <15 mL/min/1.73 m2 and who underwent regular dialysis were recruited in the ESRD group. Participants with eGFR ≥60 mL/min/1.73 m2 and without evidence of kidney damage, such as albuminuria or abnormal findings on renal imaging, were enrolled in the control group. The lingual information of these three groups was collected and the differences between the characteristics of the tongue images of each group were summed up.
After signing the consent form, the participants underwent tongue image capture with ATDS. The ATDS examination was performed by the same well-trained operator in all patients and in a stable environmental condition. The flow chart of this study is presented in Fig. 1.
Fig. 1.
Flow chart of the study design. Abbreviations: CKD: chronic kidney disease; ESRD: end stage renal disease; eGFR: estimated glomerular filtration rate; ATDS: Automatic Tongue Diagnosis System.
Intervention: automatic tongue diagnosis system
ATDS was used to photograph the tongue and automatically extract the features. It was set at a fixed location and both the operator and patient sat at fixed seats in order to decrease the influence of background surroundings. ATDS can automatically correct any lighting and color deviations using a color calibration bar attached beside the chin support.
First, the operator adjusted the location of the chin support horizontally and vertically according to the patient's height in order to capture the whole tongue. Second, the patient protruded the tongue out and held it relaxed and stable for approximately 5 s, thus, allowing the operator to take a picture. Third, the tongue image was segmented by isolating the tongue region to remove the irrelevant portions within the image, including the teeth, lower facial portion, and background data. Consequently, feature identification and extraction were performed by ATDS.
Outcome measures
Primary outcome measures
The following were the tongue features extracted by ATDS: (1) shape: thin and small, moderate, fat and large; (2) color: slightly white, slightly red, red, dark red, and dark purple; (3) saliva: the total area and amount of saliva (none, little, normal, and excessive); (4) fur: color (white, yellow, and dye), thickness (none, thin, and thick), amount (average covering area, maximum covering area, and minimum covering area); (5) teeth mark: number, average covering area, maximum covering area, and minimum covering area; (6) fissure: amount, average covering area, shortest length, and longest length; (7) ecchymosis: amount, average covering area, maximum covering area, and minimum covering area; and (8) red dots: number, average covering area, maximum covering area, and minimum covering area. The amount of saliva was grouped into three categories: (1) less (<0.05), (2) normal (≥0.05 and <0.15) and (3) more (≥0.15), based on the ratio between the area where saliva was detected to the total area of the tongue [17].
Data collection
Information including age, sex, chief complaint, serum blood urea nitrogen, creatinine, eGFR by the MDRD Study equation [18], hemoglobin, hematocrit, albumin, pre- and post-dialysis body weight, and pre- and post-dialysis blood pressure were recorded.
Sample size
We calculated the sample size with power = 0.9, alpha = 0.05, and effect size convention r = 0.6, using G∗Power v3.0.1.0 (http://www.gpower.hhu.de). 120 sample subjects, including 60 subjects undergoing dialysis and 60 subjects not undergoing dialysis were required in this study. However, a total of 117 participants including 63 subjects in ESRD and 54(16 control and 38 non-end-stage CKD) non-dialysis-requiring subjects were enrolled in the study due to the limitations of clinical research. Recalculated power was 0.8045.
Statistical analysis
The data were expressed as means ± standard deviation (SD), percentages, or numbers accordingly. The tongue features among different groups were analyzed and compared using independent t-test for continuous variables and chi-square test (or Fisher's exact) for categorical variables. Furthermore, one-way ANOVA was applied to compare differences between different group means. Logistic regression was used to estimate the odds ratio and the probability of a binary response based on one or more independent variables. We also used a receiver operating characteristic (ROC) curve and area under the ROC curve to evaluate the sensitivity and specificity of the various tongue features in these patients. p-values<0.05 were considered statistically significant. All statistical analyses were performed using SPSS v17.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
Overall, 117 participants (57 men, 60 women) were enrolled in the study, which included 16 in control group, 38 in non-dialysis CKD group, and 63 in ESRD group. The general demographic characteristics of the participants in the three groups are presented in Table 1. The mean age in the control, non-dialysis CKD, and ESRD groups was 63.06 ± 6.90, 63.79 ± 11.60, and 62.38 ± 9.46 years, respectively. There were no significant differences between the three groups in terms of the age, sex distribution, body mass index, diastolic blood pressure, glycated hemoglobin, and low-density lipoprotein.
Table 1.
Demographic characteristics of the study participants.
| Control N = 16 |
CKD N = 38 |
ESRD N = 63 |
p-valuea | |
|---|---|---|---|---|
| Sex (n; male/female) | 7/9 | 20/18 | 30/33 | 0.810 |
| Mean ± standard deviation | ||||
| Age (years) | 63.06 ± 6.90 | 63.79 ± 11.60 | 62.38 ± 9.46 | 0.787 |
| BMI (kg/m2) | 22.88 ± 2.78 | 25.03 ± 4.11 | 23.58 ± 3.83 | 0.090 |
| SBP (mmHg) | 120.06 ± 17.46 | 147.79 ± 11.04 | 143.30 ± 24.05 | <0.001 |
| DBP (mmHg) | 66.19 ± 11.70 | 72.13 ± 9.90 | 72.71 ± 12.25 | 0.124 |
| BUN (mg/dL) | 17.25 ± 2.46 | 57.55 ± 24.39 | 66.48 ± 19.54 | <0.001 |
| Cr (mg/dL) | 0.99 ± 0.19 | 4.41 ± 2.50 | 10.52 ± 2.93 | <0.001 |
| eGFR (mL/min/1.73 m2) | 70.64 ± 8.93 | 18.29 ± 13.96 | 5.14 ± 2.58 | <0.001 |
| Hb (g/dL) | 14.08 ± 0.72 | 10.35 ± 2.22 | 10.48 ± 1.14 | <0.001 |
| Albumin (g/dL) | 4.17 ± 0.21 | 4.13 ± 0.44 | 3.96 ± 0.33 | 0.026 |
| HbA1C (%) | 5.46 ± 0.27 | 6.41 ± 1.64 | 6.40 ± 1.61 | 0.074 |
| LDL Cholesterol (mg/dL) | 96.31 ± 10.96 | 95.87 ± 40.66 | 89.67 ± 36.66 | 0.635 |
Abbreviations: CKD: chronic kidney disease group without dialysis; ESRD: end stage renal disease; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; BUN: blood urea nitrogen; Cr: creatinine; eGFR: estimated glomerular filtration rate; Hb: hemoglobin; HbA1C, glycated hemoglobin; LDL, low-density lipoprotein.
By one-way ANOVA test and chi-square test (or Fisher's exact).
Analysis of tongue features
Differences in the tongue features between the three groups are presented in Fig. 2 and Table 2. The tongue fur in the non-dialysis CKD group (64.66 ± 25.09%) and ESRD group (55.46 ± 24.89%) was significantly thicker than that in the control group (46.63 ± 27.71%, p = 0.045). The color of the tongue was paler in the non-dialysis CKD group and ESRD group than in the control group (42.11% vs. 38.10% vs. 18.75%, respectively, p = 0.005). The amount of ecchymosis in the non-dialysis CKD group (1.66 ± 2.27) and ESRD group (6.05 ± 11.56) was significantly more than that in the control group (0.13 ± 0.34, p = 0.010). The ESRD group had the most ecchymosis on the tongue. The ecchymosis in the ESRD group, non-dialysis CKD group, and control group (1.13 ± 2.50 vs. 0.32 ± 0.84 vs. 0.00 ± 0.00, respectively, p = 0.035) was mostly in the left liver–gall area. The ESRD group had the most teeth marks (1.65 ± 2.16), while the non-dialysis CKD group had the second most teeth marks (1.63 ± 2.38). The number of teeth marks in both the ESRD group and the non-dialysis CKD group was significantly more than that in the control group (0.00 ± 0.00, p = 0.016). The non-dialysis CKD and ESRD groups had the highest (34.82 ± 29.63) and second highest (30.79 ± 30.16) number of red dots, respectively.
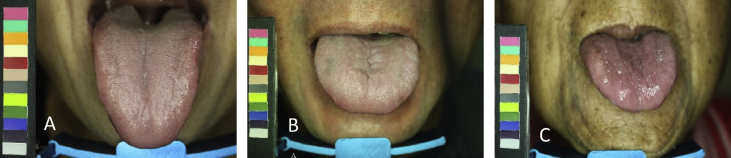
Fig. 2.
Representative tongue images of three groups. (A) Control group. (B) Non-dialysis chronic kidney disease group. (C) End stage renal disease group.
Table 2.
Tongue characteristics of the study participants.
| Control N = 16 |
CKD N = 38 |
ESRD N = 63 |
p-valuea | |
|---|---|---|---|---|
| Tongue coating color (n) | 0.260 | |||
| White | 11 (68.75%) | 12 (31.58%) | 32 (50.79%) | |
| Yellow | 5 (31.25%) | 25 (65.79%) | 26 (41.27%) | |
| Others | 0 (0%) | 1 (2.63%) | 5 (7.94%) | |
| Tongue color (n) | 0.005 | |||
| Pale | 3 (18.75%) | 16 (42.11%) | 24 (38.10%) | |
| Not Pale | 13 (81.25%) | 22 (57.89%) | 39 (61.90%) | |
| Amount of saliva (n) | 0.516 | |||
| Less | 1 (6.25%) | 1 (2.63%) | 3 (4.76%) | |
| Normal | 12 (75%) | 35 (92.11%) | 55 (87.30%) | |
| More | 3 (18.75%) | 2 (5.26%) | 5 (7.94%) | |
| Mean ± standard deviation | ||||
| Area of saliva (cm2) | 1.13 ± 0.40 | 1.26 ± 0.36 | 1.28 ± 0.51 | 0.493 |
| Number of coating | 2.31 ± 1.89 | 1.97 ± 1.91 | 1.75 ± 1.31 | 0.427 |
| Percentage of coating (%) | 46.81 ± 16.16 | 51.68 ± 22.70 | 47.43 ± 23.30 | 0.608 |
| Area of coating (cm2) | 3.92 ± 3.65 | 6.00 ± 4.70 | 4.95 ± 4.50 | 0.261 |
| Thick coating (%) | 46.63 ± 27.71 | 64.66 ± 25.09 | 55.46 ± 24.89 | 0.045 |
| Tongue length and width ratio | 1.03 ± 0.13 | 1.04 ± 0.17 | 1.01 ± 0.15 | 0.548 |
| Number of tongue fissures | 0.06 ± 0.25 | 0.66 ± 1.63 | 1.41 ± 2.72 | 0.055 |
| Amount of ecchymosis | 0.13 ± 0.34 | 1.66 ± 2.27 | 6.05 ± 11.56 | 0.010 |
| Number of teeth marks | 0.00 ± 0.00 | 1.63 ± 2.38 | 1.65 ± 2.16 | 0.016 |
| Number of red dots | 0.19 ± 0.54 | 34.82 ± 29.63 | 30.79 ± 30.16 | <0.001 |
Abbreviations: ANOVA: Analysis of Variance; CKD: chronic kidney disease group without dialysis; ESRD: end stage renal disease.
By one-way ANOVA test and chi-square test (or Fisher's exact).
Ecchymosis and ESRD group
The results of the multiple binary logistic regression analysis are presented in Table 3. After adjusting for the sex and age, for increase in ecchymosis by one point, the risk of ESRD dialysis rose by 1.523 times (95% confidence interval, 1.198–1.936; p = 0.001).
Table 3.
Tongue features analysis in patients with chronic kidney disease.
| B ± SE | Wald | p-valuea | Exp (B) | 95% Exp (B) | |
|---|---|---|---|---|---|
| Constant | 30.119 ± 19.729 | 2.331 | 0.127 | ||
| Sex | −5.463 ± 2.951 | 3.427 | 0.064 | 0.004 | 0.001–1.379 |
| Age | −0.250 ± 0.131 | 3.665 | 0.056 | 0.779 | 0.603–1.006 |
| SBP | 0.002 ± 0.036 | 0.004 | 0.952 | 1.002 | 0.934–1.076 |
| BUN | −0.219 ± 0.103 | 4.541 | 0.033 | 0.803 | 0.657–0.983 |
| Cr | 1.368 ± 0.702 | 3.793 | 0.051 | 3.928 | 0.991–15.563 |
| eGFR | −0.534 ± 0.303 | 3.116 | 0.078 | 0.586 | 0.324–1.061 |
| Hb | 0.384 ± 0.609 | 0.397 | 0.528 | 1.468 | 0.445–4.838 |
| Albumin | −0.734 ± 2.194 | 0.112 | 0.738 | 0.480 | 0.007–35.365 |
| Thick coating | −0.044 ± 0.039 | 1.311 | 0.252 | 0.957 | 0.887–1.032 |
| Ecchymosis | 0.834 ± 0.408 | 4.182 | 0.041 | 2.302 | 1.035–5.119 |
| Tooth marks | −0.271 ± 0.332 | 0.664 | 0.415 | 0.763 | 0.398–1.463 |
| Red dots | −0.033 ± 0.041 | 0.656 | 0.418 | 0.968 | 0.894–1.048 |
| Tongue color | −0.436 ± 1.764 | 0.061 | 0.805 | 0.647 | 0.020–20.498 |
By logistic regression analysis.
Diagnostic accuracy of tongue features
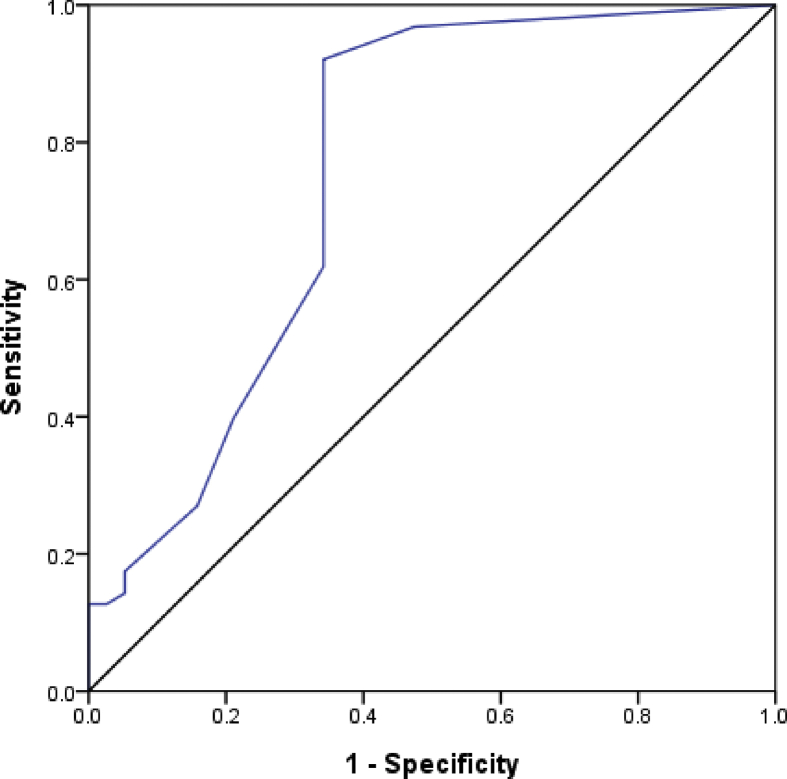
Among the tongue features, there was significant difference in the amount of ecchymosis between the groups. The diagnostic accuracy was analyzed using an ROC curve and presented in Fig. 3. The area for the amount of ecchymosis was 0.757 ± 0.055 (95% confidence interval, 0.648–0.866; p < 0.001).
Fig. 3.
Area on ROC curve for ecchymosis of tongue in chronic kidney disease. Area: 0.757 ± 0.055; p < 0.001; 95% confidence interval = 0.648–0.866.
Characteristics of ESRD patients before and after hemodialysis
According to Table 4, the results showed that the amount of saliva in patients with ESRD decreased significantly (p = 0.038). The area of saliva (p = 0.048) and the number of red dots (p = 0.040) also decreased significantly after hemodialysis. Other results showed that the percentage of coating (p = 0.002) and area of coating (p = 0.026) increased significantly after hemodialysis.
Table 4.
Characteristics of patients with end-stage renal disease before and after hemodialysis.
| Before (n = 50) | After (n = 50) | p-valuea | |
|---|---|---|---|
| SBP (mmHg) | 146.02 ± 24.21 | 144.90 ± 27.99 | 0.831 |
| DBP (mmHg) | 71.00 ± 11.57 | 71.58 ± 12.96 | 0.814 |
| Body weight (kg) | 61.92 ± 10.55 | 59.61 ± 10.20 | 0.268 |
| BUN (mg/dL) | 69.62 ± 19.56 | 64.86 ± 17.72 | 0.205 |
| Cr (mg/dL) | 10.43 ± 2.36 | 10.12 ± 2.21 | 0.508 |
| eGFR (mL/min/1.73 m2) | 4.83 ± 1.29 | 4.99 ± 1.25 | 0.471 |
| Hb (g/dL) | 10.48 ± 1.13 | 10.48 ± 1.03 | 0.993 |
| Albumin (g/dL) | 4.01 ± 0.27 | 4.04 ± 0.24 | 0.604 |
| Tongue coating color (n) | 0.492 | ||
| White | 25 (50%) | 23 (46%) | |
| Yellow | 17 (34%) | 22 (44%) | |
| Others | 8 (16%) | 5 (10%) | |
| Tongue color (n) | 0.673 | ||
| Pale | 16 (32%) | 18 (36%) | |
| Not Pale | 34 (68%) | 32 (64%) | |
| Amount of saliva (n) | 0.038 | ||
| Less | 3 (6%) | 10 (20%) | |
| Normal | 42 (84%) | 39 (78%) | |
| More | 5 (10%) | 1 (2%) | |
| Mean ± standard deviation | |||
| Area of saliva (cm2) | 1.29 ± 0.49 | 1.10 ± 0.43 | 0.048 |
| Number of coating | 1.82 ± 1.42 | 1.64 ± 1.26 | 0.505 |
| Percentage of coating (%) | 43.14 ± 22.21 | 57.54 ± 23.32 | 0.002 |
| Area of coating (cm2) | 4.32 ± 4.38 | 6.36 ± 4.63 | 0.026 |
| Thick coating (%) | 53.16 ± 25.43 | 57.94 ± 23.25 | 0.329 |
| Tongue length and width ratio | 1.00 ± 0.15 | 1.03 ± 0.13 | 0.295 |
| Number of tongue fissures | 2.02 ± 3.63 | 2.18 ± 5.03 | 0.856 |
| Amount of ecchymosis | 5.48 ± 13.35 | 4.14 ± 6.36 | 0.523 |
| Number of teeth marks | 1.56 ± 2.06 | 1.68 ± 2.21 | 0.779 |
| Number of red dots | 25.32 ± 32.08 | 23.06 ± 26.51 | 0.040 |
Abbreviations: SBP: systolic blood pressure; DBP: diastolic blood pressure; BUN: blood urea nitrogen; Cr: creatinine; eGFR: estimated glomerular filtration rate; Hb: hemoglobin.
By paired t test and chi-square test (or Fisher's exact).
Discussion
According to our results, patients with CKD have thicker tongue fur, paler tongue, more ecchymosis, teeth marks, and red dots than those in the control group. In the TCM theory, thick tongue fur represents phlegm-dampness, pale tongue corresponds to blood deficiency, ecchymosis mirrors blood stasis, teeth marks represent qi deficiency, and red dot correlates to heat [10,11]. Therefore, “blood deficiency and stasis with qi deficiency or blood heat syndrome (Zheng pattern)” may be common in patients with CKD. The hemoglobin levels in our results also demonstrated that patients in the non-dialysis CKD group and ESRD group had significantly lower Hb than those in the control group. This finding is consistent with the TCM theory, which states that a pale tongue represents blood deficiency [10,11]. Zhao et al. [19] launched a nationwide expert questionnaire and demonstrated that “qi deficiency of the spleen and kidney,” “yang deficiency of the spleen and kidney,” “blood stasis,” and “water dampness” syndromes are common in patients with CKD. Our findings using scientific instruments agree with Zhao's results. Increased ecchymosis and increased pallor of the tongue among CKD patients could indicate anemia and ischemic vascular diseases.
The ROC curve is an effective assessment of sensitivity and specificity of using the tongue's features to evaluate CKD. An area value larger than 0.5 indicates diagnostic accuracy. In our study, the amount of ecchymosis corresponded to an area larger more than 0.5. Hence, ecchymosis of the tongue may be diagnostic and may provide some insight on CKD status.
Hemodialysis could significantly decrease the amount of saliva, the coverage area of saliva and the number of red dots on tongues of CKD patients undergoing dialysis. On the other hand, it significantly increases the percentage of coating and area of coating among patients with ESRD. The decline in saliva may be related to fluid removal (2.04 ± 0.84 L/time) after hemodialysis. The tongue coating represents the physiological and pathological status of the stomach “Qi” based on traditional Chinese medicine theories. The percentage of coating and area of coating increased significantly after hemodialysis may represent the better status of the stomach “Qi” and the essence “Qi” of food or nutrition. Hemodialysis can affect the saliva, tongue coating and relieve heat syndrome among ESRD patients.
The tongue is a muscle which can reflect the physiological and pathological changes of health and disease. Tongue diagnosis is one of the non-invasive diagnostic methods that allow TCM practitioners to differentiate between TCM syndromes in Chinese communities. It is commonly recognized that the tongue is connected with the internal organ by meridians. Therefore, tongue features can reflect the body's yin, yang, qi, and blood and body fluid statuses. Based on the TCM theory, the tongue is subdivided into five areas that correspond to different internal organs [10,11,[20], [21], [22]].
The normal tongue should have a light red body with a thin white coating. The abnormal changes in the color, shape, fissures, red dots, ecchymosis, and moisture in the tongue coating and body color can reflect diseases of internal organs. For example, a tongue with teeth marks on the sides can be a sign of qi deficiency. A tongue with dark purple color and ecchymosis is often caused by blood stasis. A tongue with pale color can be a sign of blood deficiency [10,11,[20], [21], [22]]. In the past, traditional tongue diagnosis depended on the subjective and qualitative observation by a TCM physician. Tongue inspection and diagnosis with a computerized automatic tongue diagnosis system can provide objective and quantitative observations. TCM physicians can capture the quantitative features to improve the reliability and consistency of tongue diagnosis [19,[23], [24], [25], [26]].
Syndrome differentiation is an important concept in TCM practice and it includes a comprehensive analysis of a patient's clinical information from four diagnostic procedures—inspection, auscultation, questioning, and pulse analysis. Syndrome differentiation guides the treatment [27]. For example, patients with qi deficiency may have fatigue, weakness, and decreased appetite; those with blood deficiency may have anemia, dizziness, and insomnia; those with blood stasis may have stabbing pain; and those with blood heat may have thirst, pruritus, and irritability [11]. Patients with CKD often present with fatigue, decreased appetite, pruritus, anemia, and sleep disturbances [4,5]. These symptoms are consistent with “blood deficiency and stasis with qi deficiency or blood heat syndrome (Zheng pattern).”
To the best of our knowledge, there is only one English article that reported the relationship between patients with CKD and tongue manifestations. Pieralisi et al. [28] demonstrated high tongue coating frequency in these patients, which was frequently colonized by yeasts. Our results also demonstrated that patients with CKD had thicker tongue fur than those in the control group.
TCM physicians perform tongue diagnosis by observing a patient's tongue directly. This practice relies on the physician's subjective and qualitative inspection, which would be biased by environmental lighting, color perception, and subjective judgment. ATDS was developed to obtain tongue pictures and automatically analyze the tongue features, thus, producing objective and quantitative results [[19], [20], [21]]. Nowadays, many researchers employ artificial intelligence (AI) for medical purposes, such as image interpretation and disease detection. An unified AI model to segment the tongue images from both stand-alone ATDS and mobile phone may be developed in the future. The AI approach may reach higher accuracy in tongue segmentation than its traditional counterpart [29]. We believe that our results will help AI-driven tongue diagnosis to benefit clinical physicians in establishing such observational diagnoses.
There were several limitations to our study. First, the sublingual region was not analyzed due to instrumental limitations. Second, some participants might have underlying systemic diseases, such as hypertension and diabetes mellitus, which might have affected the tongue presentation. Third, this study was a single-center, cross-sectional study. Larger, multi-center, prospective studies are warranted to confirm our results. Fourth, tongue inspection is easily biased by food or a patient's habits. For example, the tongue becomes redder after eating spicy food. The tongue coating would be stained by the food's color. Similarly, the thickness of tongue fur can diminish because of the habit of tongue brushing. Fifth, tongue inspection in this study could evaluate the differences in blood and qi deficiencies between patients with CKD and the controls. However, the results of this study had difficulty in evaluating the differences in the yin and yang deficiencies between these groups. Sixth, most of the control group had less teeth marks; however, the absence of teeth marks could be due to selection bias.
Conclusions
Blood deficiency and stasis with qi deficiency or blood heat syndrome (Zheng pattern) is common in patients with CKD. Increased ecchymosis on the tongue may correspond to increasing risk of CKD. Hemodialysis can affect saliva, tongue coating and relieve heat syndrome among ESRD patients. TCM tongue inspection can be expected to serve as preliminary clinical indices in patients with CKD. Further evidence clinical trials are required to confirm these findings.
Conflicts of interest
The authors declare they have no conflict of interest.
Acknowledgments
This study was supported by the Chung Gung Medical Research Fund (CMPRG8H0691). We thank the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital. The authors would like to express our thanks to Professor John Y. Chiang from National Sun Yat-sen University and Mr. Chen-Ting Dai for their assistance with this study.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Tsai M.H., Hsu C.Y., Lin M.Y., Yen M.F., Chen H.H., Chiu Y.H., et al. Incidence, prevalence, and duration of chronic kidney disease in Taiwan: results from a community-based screening program of 106,094 individuals. Nephron. 2018;140(3):175–184. doi: 10.1159/000491708. [DOI] [PubMed] [Google Scholar]
- 2.Xu Z.C., Wu M.S., Huang S.Z., Lin Y.F., Xu Y.H., Qiu Y.W. 1 ed. National Health Research Institutes; Miaoli: 2020. 2019 Annual Report on Kidney Disease in Taiwan. [Google Scholar]
- 3.Levin A., Stevens P.E. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 4.Drawz P., Rahman M. Chronic kidney disease. Ann Intern Med. 2015;162(11):Itc1–Itc16. doi: 10.7326/AITC201506020. [DOI] [PubMed] [Google Scholar]
- 5.Meyer T.W., Hostetter T.H. Uremia. N Engl J Med. 2007;357(13):1316–1325. doi: 10.1056/NEJMra071313. [DOI] [PubMed] [Google Scholar]
- 6.Almeras C., Argilés A. The general picture of uremia. Semin Dial. 2009;22(4):329–333. doi: 10.1111/j.1525-139X.2009.00575.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhong Y., Deng Y., Chen Y., Chuang P.Y., Cijiang He J. Therapeutic use of traditional Chinese herbal medications for chronic kidney diseases. Kidney Int. 2013;84(6):1108–1118. doi: 10.1038/ki.2013.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong Y., Menon M.C., Deng Y., Chen Y., He J.C. Recent advances in traditional Chinese medicine for kidney disease. Am J Kidney Dis. 2015;66(3):513–522. doi: 10.1053/j.ajkd.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Xiong W., He F.F., You R.Y., Xiong J., Wang Y.M., Zhang C., et al. Acupuncture application in chronic kidney disease and its potential mechanisms. Am J Chin Med. 2018;46(6):1169–1185. doi: 10.1142/S0192415X18500611. [DOI] [PubMed] [Google Scholar]
- 10.Kirschbaum B. 1st ed. Eastland Press; 2000. Atlas of Chinese tongue diagnosis. [Google Scholar]
- 11.Sun G., Eisenstark D.D., Zhang Q.R. 1st ed. People's Medical Publishing House; Beijing: 2014. Fundamentals of Chinese medicine. [Google Scholar]
- 12.Hsu P.C., Wu H.K., Huang Y.C., Chang H.H., Lee T.C., Chen Y.P., et al. The tongue features associated with type 2 diabetes mellitus. Medicine (Baltimore) 2019;98(19):e15567. doi: 10.1097/MD.0000000000015567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee T.C., Lo L.C., Wu F.C. Traditional Chinese medicine for metabolic syndrome via TCM pattern differentiation: tongue diagnosis for predictor. Evid Based Complement Alternat Med. 2016;2016:1971295. doi: 10.1155/2016/1971295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo L.C., Cheng T.L., Chen Y.J., Natsagdorj S., Chiang J.Y. TCM tongue diagnosis index of early-stage breast cancer. Complement Ther Med. 2015;23(5):705–713. doi: 10.1016/j.ctim.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Kim J., Han G., Ko S.J., Nam D.H., Park J.W., Ryu B., et al. Tongue diagnosis system for quantitative assessment of tongue coating in patients with functional dyspepsia: a clinical trial. J Ethnopharmacol. 2014;155(1):709–713. doi: 10.1016/j.jep.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Wu T.C., Lu C.N., Hu W.L., Wu K.L., Chiang J.Y., Sheen J.M., et al. Tongue diagnosis indices for gastroesophageal reflux disease: a cross-sectional, case-controlled observational study. Medicine(Baltimore) 2020;99(29) doi: 10.1097/MD.0000000000020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo L.C., Chen Y.F., Chen W.J., Cheng T.L., Chiang J.Y. The study on the agreement between automatic tongue diagnosis system and traditional Chinese medicine practitioners. Evid Based Complement Alternat Med. 2012;2012:505063. doi: 10.1155/2012/505063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J., Chen J.H., Yan J., Zhu H., Shu Y., Wang X.F., et al. Analysis of the results of questionnaire survey on TCM syndromes types of chronic kidney disease and treatment principles. Shi jie ke xue ji shu: Zhong yi yao xian dai hua. 2019;21:1068–1073. [Google Scholar]
- 20.Tania M.H., Lwin K., Hossain M.A. Advances in automated tongue diagnosis techniques. Integr Med Res. 2019;8(1):42–56. doi: 10.1016/j.imr.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q., Yue X.Q., Ling C.Q. [Researches into the modernization of tongue diagnosis: in retrospect and prospect] Zhong Xi Yi Jie He Xue Bao. 2003;1(1):66–70. doi: 10.3736/jcim20030128. Chinese. [DOI] [PubMed] [Google Scholar]
- 22.Anastasi J.K., Currie L.M., Kim G.H. Understanding diagnostic reasoning in TCM practice: tongue diagnosis. Altern Ther Health Med. 2009;15(3):18–28. [PubMed] [Google Scholar]
- 23.Jung C.J., Jeon Y.J., Kim J.Y., Kim K.H. Review on the current trends in tongue diagnosis systems. Integr Med Res. 2012;1(1):13–20. doi: 10.1016/j.imr.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu C.C. A novel approach based on computerized image analysis for traditional Chinese medical diagnosis of the tongue. Comput Methods Programs Biomed. 2000;61(2):77–89. doi: 10.1016/s0169-2607(99)00031-0. [DOI] [PubMed] [Google Scholar]
- 25.Kim M., Cobbin D., Zaslawski C. Traditional Chinese medicine tongue inspection: an examination of the inter-and intrapractitioner reliability for specific tongue characteristics. J Altern Complement Med. 2008;14(5):527–536. doi: 10.1089/acm.2007.0079. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien K.A., Abbas E., Zhang J., Guo Z.X., Luo R., Bensoussan A., et al. Understanding the reliability of diagnostic variables in a Chinese medicine examination. J Altern Complement Med. 2009;15(7):727–734. doi: 10.1089/acm.2008.0554. [DOI] [PubMed] [Google Scholar]
- 27.Jiang M., Lu C., Zhang C., Yang J., Tan Y., Lu A., et al. Syndrome differentiation in modern research of traditional Chinese medicine. J Ethnopharmacol. 2012;140(3):634–642. doi: 10.1016/j.jep.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 28.Pieralisi N., de Souza Bonfim-Mendonça P., Negri M., Jarros I.C., Svidzinski T. Tongue coating frequency and its colonization by yeasts in chronic kidney disease patients. Eur J Clin Microbiol Infect Dis. 2016;35(9):1455–1462. doi: 10.1007/s10096-016-2684-y. [DOI] [PubMed] [Google Scholar]
- 29.Hung Y.C., Chiang J.Y., Hu W.L. Tongue inspection and diagnosis: past, present, and future. Jpn J Med. 2020;3:452–455. [Google Scholar]