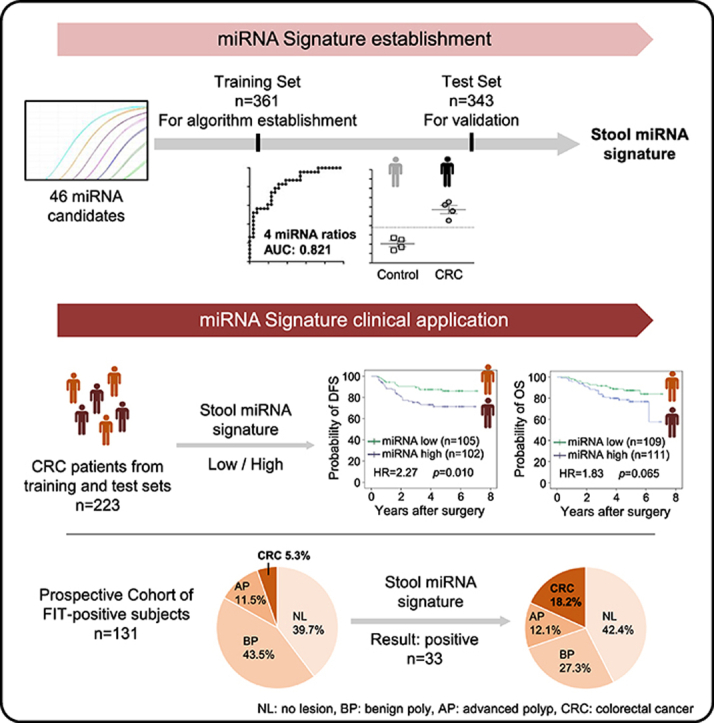
Abstract
Background
Colorectal cancer (CRC) is still among the most lethal and prevalent malignancies in the world. Despite continuous efforts, the diagnosis and prognosis of CRC have never been satisfying, especially the non-invasive assays.
Methods
Our study comprised three independent cohorts of 835 qualified stool samples. From 46 literature-identified miRNA candidates, four miRNA ratios were selected and developed into a miRNA-based signature after applied to the training and test sets. The clinical performances of this signature were further evaluated in the prospective cohorts.
Results
Four miRNA ratios with significant alterations and the highest discriminating power between the CRC and control groups in the training set were successfully validated in the test set. In the training dataset, combining these four miRNA ratios using a logistic regression model improved the area under the curve value to 0.821 and obtained a sensitivity of 73.6% and specificity of 78.9%. This miRNA signature showed consistent performances in the other two sample cohorts, with the highest sensitivity of 85.7% in the prospective cohort. Additionally, the higher miRNA signature was associated with worse disease-free survival (hazard ratio = 2.27) and overall survival (hazard ratio = 1.83) of CRC patients. For fecal immunochemical test (FIT)-positive populations, the positive predictive value for CRC detection in miRNA-positive subjects was 3.43-fold higher in the prospective cohort, compared to FIT alone.
Conclusion
This stool miRNA signature is highly associated with poor outcome of CRC and can be added to FIT tests to help identify the most at-risk group to receive prompt colonoscopy examination.
Keywords: Screening, Colonoscopy, Biomarker, Prioritization
Graphical abstract
At a glance commentary
Scientific background on the subject
Colorectal cancer (CRC) is still the leading cause of cancer death worldwide. Up to date, fecal immunochemical test (FIT)-based screening strategy is considered an effective method for reducing CRC mortality, but the false positive results have caused overloaded demand for colonoscopy.
What this study adds to the field
In this study, we developed a stool miRNA signature that could increase the positive predictive value for CRC detection to 17.9% in FIT-positive populations and was associated with worse disease-free survival and overall survival of CRC patients. It can be used to prioritize the most at-risk population of CRC.
Colorectal cancer (CRC) is the second leading cause of cancer death and the third most commonly diagnosed cancer in the world, with almost 860,000 deaths and 1,800,000 new cases in 2018 [1]. CRC incidence is steadily rising worldwide, especially in developing countries [2]. In Taiwan, it was the most common cancer for 12 consecutive years up to 2017, and it was the third leading cause of cancer mortality with 14.6 per 105 persons in 2018 [3]. The 5-year survival rate of CRC is above 90% for early stage of CRC, but drops to 53% for stage IIIC and 12% for metastatic CRC [2]. Despite ongoing of numerous screening programs, nearly a quarter of CRCs are diagnosed at an advanced stage with metastases [4]. Therefore, developing more efficient strategies for early detection of CRC is still needed.
Population screening and endoscopic surveillance were considered effective strategies to prevent the development of and death from CRC [5,6]. More recently, the fecal immunochemical test (FIT) and the multi-target stool DNA test (Cologuard™) are two non-invasive assays recommended to identify risk groups that should be referred for colonoscopy-based diagnosis [6,7]. Although with superior sensitivity, the Cologuard™ test is high cost and requires sophisticated manipulation in a central laboratory [8,9]. FIT is most commonly used worldwide but has limitations; the positive predictive value (PPV) of FIT is only 5% for CRC and 20% for advanced polyps, and 75% of FIT-positive individuals have positive results due to hemorrhoids or harmless small polyps [10,11]. Since 2004, Taiwanese government launched a nationwide screening program to offer free biannual FIT to individuals aged 50 to 75. After 12 years of FIT screening strategy implementation, a 44% reduction of CRC-related mortality had been observed; however, there are issues with overloaded demand for colonoscopy and reduced patient compliance due to the false positive results of FIT [12]. Therefore, identifying new supplementary tests to improve risk stratification and prioritize FIT-positive individuals for colonoscopy is urgently needed [13].
MicroRNAs (miRNAs) are a group of short non-coding RNA molecules of 18–25 nucleotides in length, that govern various aspects of cellular development and pathological abnormalities [14,15]. Aberrant miRNA expression from colorectal adenoma transformed to carcinoma has been implicated in the pathology of CRC, and miRNAs have received attentions as specific biomarkers for tumor diagnosis, prognosis and treatment response [16,17]. However, there is still no consensus miRNA-based assay for CRC diagnosis or prognosis in clinical practice [18]. Through examination of the paired samples, our previous study has prioritized and verified 46 CRC-associated miRNAs, and determined that miR-223 and miR-92a, which were commonly present in stool and plasma samples, could act as complementary biomarkers to yield the highest sensitivity of 96.8% for CRC [19]. For applying in the clinic, these miRNA biomarkers should be validated in a larger cohort of samples and further in the prospective study. Additionally, their potential roles for predicting outcomes of CRC patients are worthy of further investigation.
In the present study, we collected a total of 1183 stool samples to further validate those 46 miRNAs [19]. In addition to evaluating the discriminating power for CRC, the association between the miRNA signature and patient survival was also demonstrated. Further verification with prospectively collected samples, the miRNA signature was proved that it can be used to identify those who are the most at-risk for CRC from FIT-positive individuals.
Materials and methods
Study design
The study followed a three-cohort design: (1) In the training set, 46 miRNA candidates were assessed in 361 stool samples collected between January 2012 and August 2013. (2) In the independent test set, these miRNAs were validated and four relevant miRNA ratios were identified in 343 stool samples collected between August 2013 and July 2017. In the retrospective study, a total of 223 CRC patients from the training and test set were followed up to 21 March 2019, and the primary outcomes of recurrence or death were recorded and further used to evaluate the prognostic power of the miRNA ratio-based signature. (3) Finally, in the prospective cohort, the clinical performance of this miRNA signature to discriminate CRC patients was further assessed using 131 stool samples from iFOBT-positive individuals, prospectively collected between February 2018 and May 2018. A stepwise outline of this study is shown in Supplementary Fig. 1.
In total, this study enrolled 1183 subjects from Chang Gung Memorial Hospital in Taiwan. For the retrospective study, pre-treated CRC patients were recruited from the colorectal surgery department and control groups containing normal and benign polyp groups were recruited as volunteers from the Health-Check Center. In the control groups, subjects who underwent only sigmoidoscopy or had no pathological examination were excluded from further analyses. For the prospective study, only subjects with positive results of FIT assays and definitive colonoscopy results were recruited. FIT-positive subjects who were aged between 50 and 75 and participating in the national screening program were recruited as the prospective cohort to clarify the clinical use of our miRNA signature. Among the 230 CRC patients, no patient had Lynch syndrome or familial adenomatous polyposis (FAP), and 10 patients had first-degree relatives with CRC. Tumors were staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging manual [20]. According to the colonoscopy results, hyperplastic polyp, adenomatous polyp, and tubular adenoma with the largest diameter <1 cm were classified as benign polyps, while tubular adenoma with the largest diameter ≧ 1 cm, tubulovillous adenoma, and villous adenoma were classified as advanced polyps. Participants with no lesion were defined as the normal group, and no individual with inflammatory bowel disease was included. The clinicopathologic features of all individuals included in the study are shown in Table 1. This study was approved by the Institutional Review Board of Chang Gung Medical Foundation, Taipei, Taiwan (100–4602B, 102–5224B, and 201503735B0D001), and written informed consents were obtained from all participants.
Table 1.
Clinicopathological characteristics of the study subjects.
| Training Set (n = 361) |
Test Set (n = 343) |
Prospective Cohort (n = 131) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CRC (n = 129) | BP (n = 95) | NL (n = 137) | CRC (n = 94) | BP (n = 113) | NL (n = 136) | CRC (n = 7) | AP (n = 15) | BP (n = 57) | NL (n = 52) | |
| Age, mean (SD) | 62.7 (11.4) | 51.2 (10.5) | 45.9 (10.3) | 62.0 (10.3) | 58.8 (8.8) | 57.1 (9.0) | 59.6 (5.5) | 64.3 (7.6) | 64.1 (6.6) | 64.0 (6.6) |
| Gender, no. (%) | ||||||||||
| Female | 54 (41.9) | 21 (22.1) | 60 (43.8) | 45 (47.9) | 37 (32.7) | 72 (52.9) | 2 (28.6) | 3 (20.0) | 13 (22.8) | 19 (36.5) |
| Male | 75 (58.1) | 74 (77.9) | 77 (56.2) | 49 (52.1) | 76 (67.3) | 64 (47.1) | 5 (71.4) | 12 (80.0) | 44 (77.2) | 33 (63.5) |
| Stage, no. (%) | ||||||||||
| Stage 0 | 2 (1.6%) | – | – | 1 (1.1%) | – | – | 0 (0.0%) | – | – | – |
| Stage I | 31 (24.0%) | – | – | 27 (28.7%) | – | – | 1 (14.3%) | – | – | – |
| Stage II | 40 (31.0%) | – | – | 23 (24.5%) | – | – | 3 (42.9%) | – | – | – |
| Stage III | 40 (31.0%) | – | – | 38 (40.4%) | – | – | 1 (14.3%) | – | – | – |
| Stage IV | 13 (10.1%) | – | – | 5 (5.3%) | – | – | 0 (0.0%) | – | – | – |
| Missing | 3 (2.3%) | – | – | 0 (0%) | – | – | 2 (28.6%) | – | – | – |
| Location, no. (%) | ||||||||||
| Rectum | 47 (36.4%) | – | – | 29 (30.9%) | – | – | 0 (0.0%) | – | – | – |
| Left | 38 (29.5%) | – | – | 31 (33.0%) | – | – | 3 (42.9%) | – | – | – |
| Right | 38 (29.5%) | – | – | 30 (31.9%) | – | – | 1 (14.3%) | – | – | – |
| Missing or multiple | 6 (4.7%) | – | – | 4 (4.3%) | – | – | 3 (42.9%) | – | – | – |
| Size, no. (%) | ||||||||||
| <4 cm | 65 (50.4%) | – | – | 54 (57.4%) | – | – | 3 (42.9%) | – | – | – |
| ≥4 cm | 61 (47.3%) | – | – | 39 (41.5%) | – | – | 2 (28.6%) | – | – | – |
| Missing | 3 (2.3%) | – | – | 1 (1.1%) | – | – | 2 (28.6%) | – | – | – |
Abbreviations: CRC, colorectal cancer; AP, advanced polyp; BP, benign polyp; NL, no lesion.
Sample collection and miRNA detection
In the retrospective study, the stool samples of participants were collected before colonoscopy or surgery performance; each sample was dipped with a designated FIT swab and inserted into a preservation buffer. After being thoroughly mixed, the stool samples were aliquoted and stored. In the prospective study, the residual samples of FIT tests from participants who engaged with the national screening program were directly collected prior to colonoscopic examination. All stool samples were stored at −80 °C until use. The stool samples from participants who didn't finish colonoscopy or surgery within 3 months were excluded from further data analyses. The FIT test was performed using the OC-Sensor Diana Latex Reagent (Eiken Chemical, Tokyo, Japan); the detection range was 10–1000 ng/mL with a cutoff of 100 ng/mL. Detailed descriptions of the procedures for miRNA detection can be found in Supplementary information.
Data processing and statistical analysis
Descriptive statistics are summarized and presented as the percentage, mean or median, and standard deviation (SD). Intergroup comparisons were conducted using the Mann–Whitney U (MWU) test. A P-value less than 0.05 (two-tailed) was considered statistically significant. Disease-free survival (DFS) and overall survival (OS) of CRC patients were measured from date of surgery to documented first recurrence or death, and censored at last follow-up. All statistical analyses, including the area under the receiver operating characteristic (ROC) curve (AUC) of a specific miRNA, the logistic regression, and the univariable and multivariable analyses of Cox proportional hazards models, were conducted using IBM SPSS Statistics 20 (IBM Corp., Armonk, NY, USA). Survival curves were plotted using the Kaplan–Meier method and compared using the log-rank test. Plots were graphed using the Prism 7 software (GraphPad Software, La Jolla, California, USA).
Results
Identification of four miRNA ratios exhibiting the best performances for discriminating CRC patients from control subjects
To clearly demonstrate performance of the 46 most reported miRNAs selected in our previous study [19], a total of 704 stool samples (after we excluded those with low sample quality and without a complete clinical examination) were divided into the training and test sets according to the collection date (Supplementary Fig. 1). Multiplex reverse transcription-quantitative polymerase chain reaction (RT-qPCR)-based profiling of the 46 miRNAs revealed that 23 of the miRNAs were undetectable in more than 50% of the normal-group samples (Supplementary Fig. 2A), and the detection of miR-106a was significantly interfered by the cDNA template of miR-17 (Supplementary Fig. 2B in our previous study [19]). These 24 miRNAs were therefore excluded to increase the data reliability. The expression levels of the 22 detectable miRNAs were compared between the benign polyp and normal groups in the training and test set. As shown in Supplementary Fig. 2B and C, there was no between-group difference for these miRNAs in the training set, and similar trends were verified in the test set. Based on their similar expression patterns, the benign polyp and normal groups were combined into the control group for further data processing.
To adjust for intrinsic variations between samples and improve the experimental accuracy, we selected an endogenous normalizer miR-222, which showed relatively little alteration between the CRC and control groups (Supplementary Fig. 3A), low undetected rate, and the lowest SD in total control samples from both the training and test sets (Supplementary Fig. 3B). Then, the step of endogenous normalization was conducted by calculating miR-222-based miRNA ratios. The mean levels, fold-changes, and discriminating the area under the curve (AUC) values of the 21 miRNA ratios between the CRC and control groups were evaluated in the training set first. The top six miRNA ratios with the highest AUC values (>0.6) and significant alterations in the CRC group are listed in Supplementary Table 1A. These miRNA ratios were then validated using the test set. The top four miRNA ratios (miR-223/miR-222, miR-92a/miR-222, miR-16/miR-222, and miR-20a/miR-222) were still significantly different between the CRC and control groups, although the AUC value of miR-20a/miR-222 was a little lower than 0.6 in the test set (Supplementary Table 1B). As shown in Supplementary Fig. 4A and 4B, the expression levels of these four miRNA ratios in the training and test sets were higher in the CRC group than in the control group, registering increases ranging from 1.5- to 10.9-folds.
Developing and validating a miRNA ratio-based signature for CRC detection
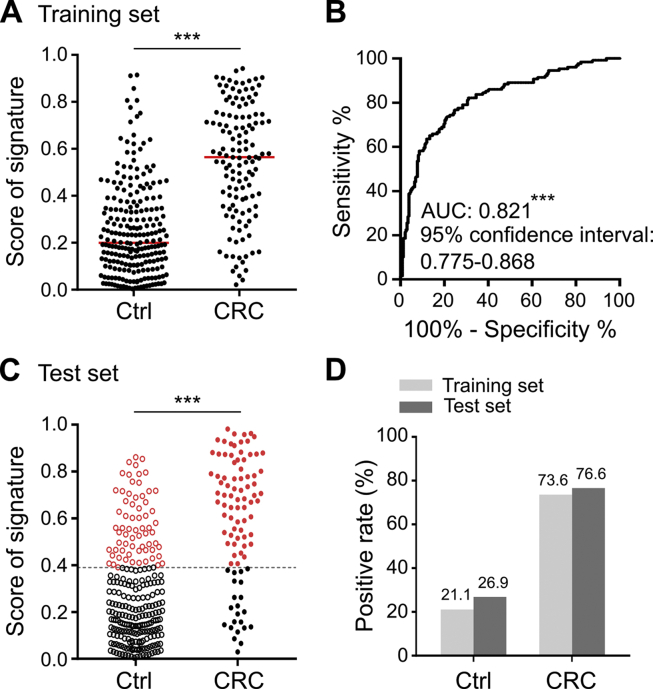
To assess whether combining the four validated miRNA ratios into a miRNA signature could increase the detection rate of CRC, we first conducted a logistic regression model using the training set data. As shown in Fig. 1B, combining the four miRNA ratios had the highest discriminating power for detecting CRC (AUC = 0.821) than that of the individual ratio (Supplementary Table 1A). The logistic regression algorithm was used to summarize different weighted miRNA ratios into a risk score for each sample. The risk scores of CRC samples (median = 0.56) were significantly higher than those of control samples (median = 0.20) in the training set (Fig. 1A). Based on the optimal cut-off value from the ROC curve, the sensitivity and specificity of the miRNA signature were 73.6% and 78.9%, respectively (Fig. 1D). To further validate discriminating performance of the miRNA signature, the miRNA data of the test set were fitted into the same regression algorithm using the same cut-off value developed from the training set. Compared with the control samples, the risk scores of CRC samples in the test set were also significantly increased (median from 0.21 to 0.66) (Fig. 1C), and the miRNA signature had a similar sensitivity (76.6%) and specificity (73.1%) for CRC detection in the test set (Fig. 1D). The risk scores of the miRNA signature were further assessed in stool samples collected average 6 months after surgery from six CRC patients from the test set. As shown in Supplementary Fig. 5, the stool miRNA signature showed reduced risk scores and negative results in these post-operated samples. CRC could be detected in five of these six patients by the miRNA signature in pre-operated stool samples. Collectively, these data support the idea that the risk scores of the developed miRNA signature were highly associated with the existence of CRC tumors.
Fig. 1.
Detection rate of the miRNA ratio-based signature in the control and CRC groups. (A) With the data of the training set, logistic regression was used to integrate the summed effects of the four miRNA ratios into a miRNA signature for discriminating the CRC group from the control group. Scores ranging from 0 to 1 were generated for each sample; the dot plot denotes the distribution of scores in the control and CRC groups. The red bar shows the median of the respective groups. (B) The discriminating power for CRC was assessed using the ROC curve of the scores of the miRNA signature. The optimal cut-off value was calculated from the Youden's index on this ROC curve. (C) Scores obtained from the same regression algorithm based on the four miRNA ratios for each sample in the test set are shown. The cut-off value mentioned above is indicated by the dotted line. (D) The samples with scores higher than the cut-off value are marked as positive. The positive rates of the control and CRC groups in the two sample sets are further represented in a bar chart.
Correlation between the miRNA signature scoring and the tumor stage, location or size of CRC patients
The overall 223 CRC patients recruited in the retrospective study were divided into subgroups based on pathological characteristics, including tumor stage, location, and size (Table 1). The four miRNA ratios were compared between these subgroups first. As shown in Supplementary Fig. 6, miR-223/miR-222 exhibited the largest differences based on these groupings, showing significant increases in CRC from the early to late stages, right to left sides, and small to large tumors. The other miRNA ratios showed similar trends between these CRC subgroups, although not all the P-values of intergroup comparisons were significant. As shown in Table 2, the risk scores of our miRNA signature showed significant increases in CRC samples from the early to late stages, right to left sides, and small to large tumors. Notably, we also observed that these risk scores were significantly associated with tumor recurrence, but not correlated with age and gender. These results suggest that the risk score of our miRNA signature could be used as a predictor for tumor malignance.
Table 2.
Relationships between the miRNA signature scores and clinical characteristics in CRC patients.
| Variable | Number | miRNA Score | p-valuea |
|---|---|---|---|
| Age | |||
| <60 | 89 | 0.60 ± 0.27 | 0.070 |
| ≧60 | 134 | 0.55 ± 0.24 | |
| Gender | |||
| Female | 99 | 0.58 ± 0.27 | 0.436 |
| Male | 124 | 0.56 ± 0.24 | |
| Tumor stage | |||
| Early (0, I & II) | 124 | 0.53 ± 0.26 | 0.016 |
| Late (III & IV) | 96 | 0.62 ± 0.24 | |
| Tumor location | |||
| Right | 68 | 0.44 ± 0.23 | <0.001 |
| Left | 146 | 0.63 ± 0.24 | |
| Tumor size | |||
| <4 cm | 119 | 0.53 ± 0.25 | 0.005 |
| ≧4 cm | 100 | 0.62 ± 0.25 | |
| Recurrence | |||
| No | 165 | 0.54 ± 0.26 | 0.024 |
| Yes | 42 | 0.65 ± 0.23 | |
Mann-Whitney U test.
Association of the miRNA signature with worse DFS and OS of CRC patients
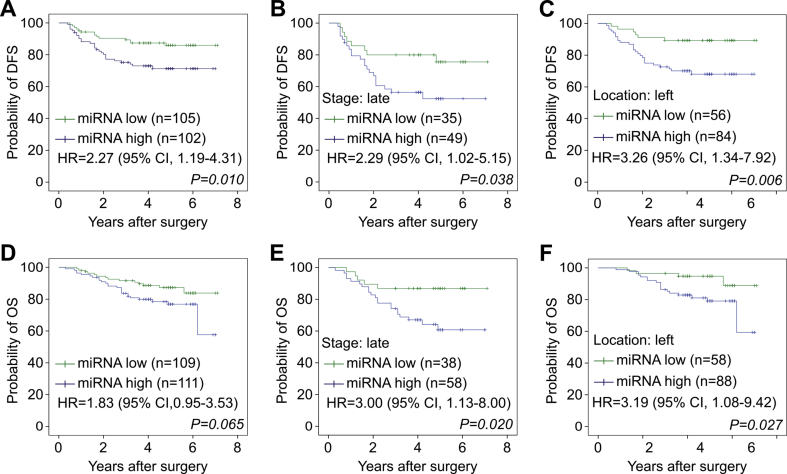
These CRC patients retrospectively enrolled in this study were followed for at least 20 months, and the recurrence and survival statuses were retrieved for prognostic analysis. To analyze the association of the miRNA signature and the prognosis of CRC, we used the median of the miRNA signature scores from all patients to dichotomize CRC cases as exhibiting low miRNA signature (the lower 50th percentile) or high miRNA signature (the upper 50th percentile). As shown in Fig. 2A, high miRNA signature among CRC patients was associated with reduced DFS (hazard ratio (HR) = 2.27, p = 0.010). This association was stronger in the CRC subgroups with the late stage (HR = 2.29, P = 0.038, Fig. 2B) and left colon location (HR = 3.26, P = 0.006, Fig. 2C). The high miRNA signature among CRC patients was also associated with reduced OS (HR = 1.83, P = 0.065, Fig. 2D), especially for those CRC patients with the late stage (HR = 3.0, P = 0.020, Fig. 2E) and left location (HR = 3.19, P = 0.027, Fig. 2F). As shown in Table 3, the univariable analyses of Cox regression among the clinicopathological features indicated that CRC patients at late stage showed worse DFS and OS compared with those at early stage (HR = 4.41, P < 0.001 and HR = 2.95, P = 0.001, respectively). Furthermore, our multivariable analysis demonstrated that the miRNA signature was an independent prognostic predictor for worse DFS (HR = 1.95, P = 0.043) (Table 3). These results show that, in addition to correlating tumor malignance, the developed stool miRNA signature could be used to predict poor prognosis among CRC patients.
Fig. 2.
Kaplan–Meier analyses of disease-free survival (DFS) and overall survival (OS) in CRC patients according to their stool miRNA signature scores. The samples were divided into two groups, miRNA-high and miRNA-low, based on the median of scores from all CRC patients. (A–C) DFS of the miRNA-high and miRNA-low groups in total CRC patients (A) or CRC subgroups (B and C) are shown. (D–F) OS of the miRNA-high and miRNA-low groups in total CRC patients (D) or CRC subgroups (E and F) are shown.
Table 3.
Univariable and multivariable analyses of the association between clinicopathological variables and miRNA signature status with DFS and OS of patients.
| Clinicopathological feature | DFS |
OS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis |
Multivariable analysis |
Univariable analysis |
Multivariable analysis |
|||||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age | ||||||||||||
| >60 | 1.099 | 0.590–2.049 | 0.766 | 1.179 | 0.612–2.272 | 0.623 | ||||||
| ≤60 | 1 | Reference | 1 | Reference | ||||||||
| Gender | ||||||||||||
| Male | 1.370 | 0.735–2.555 | 0.322 | 0.826 | 0.441–1.549 | 0.552 | ||||||
| Female | 1 | Reference | 1 | Reference | ||||||||
| Tumor stage | ||||||||||||
| Late (III & IV) | 4.412 | 2.257–8.624 | <0.001 | 4.101 | 2.091–8.045 | <0.001 | 2.952 | 1.515–5.752 | 0.001 | 2.773 | 1.415–5.433 | 0.003 |
| Early (0, I & II) | 1 | Reference | 1 | Reference | 1 | Reference | 1 | Reference | ||||
| Tumor location | ||||||||||||
| Left & Rectum | 1.598 | 0.763–3.349 | 0.214 | 0.621 | 0.316–1.222 | 0.168 | ||||||
| Right | 1 | Reference | 1 | Reference | ||||||||
| Tumor size | ||||||||||||
| ≥4 cm | 1.517 | 0.826–2.785 | 0.179 | 1.617 | 0.858–3.048 | 0.137 | ||||||
| <4 cm | 1 | Reference | 1 | Reference | ||||||||
| miRNA signature | ||||||||||||
| high | 2.269 | 1.194–4.313 | 0.012 | 1.949 | 1.021–3.718 | 0.043 | 1.833 | 0.952–3.531 | 0.070 | 1.599 | 0.825–3.097 | 0.164 |
| low | 1 | Reference | 1 | Reference | 1 | Reference | 1 | Reference | ||||
Clinical performances of the stool miRNA signature in FIT-positive populations
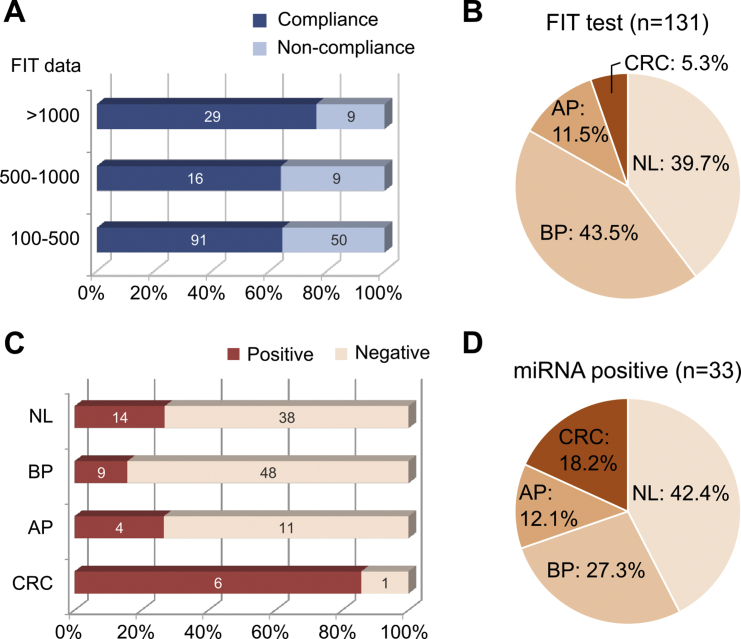
To further evaluate the clinical applicability of our miRNA signature, we prospectively collected 204 FIT-positive residua from the national screening program as a prospective cohort. After a follow-up of 3 months, only 131 samples from the subjects compliant with the FIT results and completely finished colonoscopy examination could be used for further evaluation (Fig. 3A). As shown in Supplementary Fig. 8A, a significantly increased trend of the risk scores in the CRC group relative to the control group could be validated in the prospective cohort, maintaining a high sensitivity (85.7%) and specificity (78.9%) (Supplementary Fig. 8B). As shown in Fig. 3C, the stool miRNA signature was relatively sensitive for the CRC group (85.7%), but not for the normal, benign- and advanced-polyp groups (26.9%, 15.8%, and 26.7%, respectively). These data were consistent with that the four miRNA ratios were upregulated only in CRC samples, not in advanced polyp samples (Supplementary Fig. 4C). The high correlation with CRC promotes us to evaluate the PPV for CRC of the stool miRNA signature. According to the proportion of CRC group in the miRNA-positive samples (Fig. 3D) and FIT-positive samples (Fig. 3B), we demonstrated that the PPV of the stool miRNA signature for CRC was 3.43-fold higher than that of FIT (18.2% and 5.3%, respectively). These data collectively indicated that the developed miRNA signature was specifically sensitive to CRC discrimination and further to its malignance and could be used for early stratifying patients with the most risk from those FIT-positive subjects.
Fig. 3.
Clinical performances of the miRNA signature in FIT-positive samples. (A) Distribution of the FIT results in 204 samples prospectively collected from the participants of the national screening program. Only 136 patients were compliant with the FIT results and took colonoscopy examination within 3 months. Among these compliance, 5 patients with no pathological examination of polypectomy were excluded for further analyses. (B) Proportions of the normal (NL), benign polyp (BP), advanced polyp (AP), and colorectal cancer (CRC) groups in 131 FIT-positive samples are shown. (C) A stool sample with a miRNA signature score higher than the cut-off value is interpreted as positive. The numbers of positive and negative samples from the NL, BP, AP, and CRC groups in the 131 FIT-positive samples are shown. (D) The proportions of N, BP, AP, and CRC groups in 33 samples with both positive results of the FIT test and the miRNA signature are further calculated.
Discussion
Relative to Cologuard™ test, FIT is still the most commonly used CRC screening test worldwide; it is considered to be an effective method that can reduce CRC mortality by 10%–52% [21]. However, increasing participation in screening programs based on FIT, which has an average positive rate of 8%, has significantly increased the demand for timely colonoscopy and the burden on public health workers [12]. More than 50% of positive results on FIT are false positives [11], potentially explaining the insufficient awareness of FIT results and low compliance in completing the recommended colonoscopy [12]. Data from our prospective cohort indicated that 68 of the total 204 recruited participants (33.3%) had not undertaken colonoscopy within 3 months of obtaining the positive FIT result. Among that reporting non-compliance, the FIT results of 18 subjects were over 500 ng/mL (Fig. 3A). In an effort to improve this screening strategy, we herein assessed 46 selected miRNA candidates using residual samples of FIT and developed a miRNA signature composed of four miRNA ratios that can be used for CRC detection. Assessment of the performance of this signature in our prospective cohort revealed that the PPV of the miRNA signature was 18.2% for CRC, and thus was greatly improved over the 5.3% PPV of FIT. Hence, positive-FIT subjects with further positive results on this miRNA signature should be prioritized for colonoscopy examination.
The other noninvasive fecal test, Cologuard™, has been approved by the United States Food and Drug Administration for screening in general-risk adults in 2014. Different from our microRNA signature, the Cologuard™ test is designed to measure multiple types of molecular markers (7 KRAS gene mutations, NDRG4 and BMP3 gene methylation, β-actin, and hemoglobin). In a large cohort study of 9989 subjects, the Cologuard™ test has shown high sensitivity of 92.3% for CRC and 42.4% for advanced precancerous lesions, compared to 73.8% for CRC and 23.8% for advanced precancerous lesions of FIT alone [22]. However, the Cologuard™ test is not cost-effective and at present is only introduced into clinical practice in the United States triennially [8]. In addition, the sophisticated Cologuard™ test requires large amounts of stool samples to be analyzed in a central lab, which will reduce the feasibility as a first-line routine test worldwide. In contrast to the Cologuard™ test, our miRNA-based signature incorporates one unique molecular type and directly uses the residuum of FIT tests. The reagents for RNA extraction, RT reaction, and qPCR are easier to access, while the detection procedures can be easily operated in the clinical laboratory setting without the requirement of special ventilation equipment for dealing with large amounts of stool samples. Together, our stool miRNA assay should be relatively inexpensive and feasible for clinical translation.
While FIT and CologuardTM tests, which are only used as screening tools, our stool miRNA signature can be additionally used as a prognostic predictor for worse DFS and OS, especially for CRC with the late-stage and at the left location. Once diagnosed as CRC, the higher score of the miRNA signature could be used as another warning sign for both patients and physicians. Our approach also has advantages over other reported stool microRNA studies by directly using a fixed volume of stool sample from FIT buffer for extraction, a fixed volume of purified RNA for RT, and an established multiplex RT-qPCR method for miRNA detection [19]. This makes these miRNA results can be easily implemented into the regular FIT tests to increase the effectiveness for both diagnosis and prognosis of CRC.
Because the detection accuracies of CRC remain unsatisfactory, many other screening markers are still under development [8]. Stool-based tests are noninvasive and more acceptable to patients for CRC detection, which detect biomarkers produced directly from colonic epithelium or surrounding microenvironment in the intestine [23]. Compared to stool protein and microbiota, stool miRNAs as disease biomarkers are well studied in the past two decades [24]. Through being important modulators in the initiation and progression of CRC, miRNAs have emerged as a class of cellular molecules with potential in diagnostic, prognostic, and therapeutic implications [25]. In the current study, we demonstrated that our miRNA signature can not only be used for screening, but for disease monitoring.
Although accumulated studies have searched for miRNA biomarkers for CRC detection, few miRNAs have moved into clinical practice [18]. We believe that these reported candidates hold great potential and just need to be confirmed in adequate samples from independent cohorts. Hence, rather than performing a new genome-wide miRNA expression profiling, we sought to enroll more subjects and validate miRNAs selected from literature reviews. Using our well-characterized case and control samples, we identified miRNA ratios with the highest discriminating power for CRC (miR-223, miR-92a, miR-16 and miR-20a with miR-222 as the endogenous normalizer). Among the miRNAs included in the selected ratios, miR-20a and miR-92a were found in comprehensive meta-analyses to be promising miRNAs for CRC diagnosis, exhibiting upregulation in both feces and blood [26,27]. Both of these miRNAs are encoded from the miR-17–92 cluster; this miRNA cluster has been implicated in a wide variety of malignancies, and its encoded members are referred as the OncomiRs [28]. miR-223 is less commonly reported in this context, but elevated miR-223 was identified as a fecal biomarker for CRC diagnosis in several Asian studies [29]. The expressional alteration and regulation of miR-16 in CRC is controversial [30,31], but upregulated expression of miR-16 was recently reported in CRC tissue versus adjacent normal tissues, and was associated with worse survival rate of patients [30,32]. Notably, elevated levels of miR-16 and miR-223 were detected in EpCAM+ extracellular vesicles (EVs) in plasma samples from CRC patients prior to surgery compared with healthy individuals [33]. EVs, which can be secreted by cancer cells into their microenvironment, can protect encapsulated miRNAs from degradation by RNase in feces [34]. This may be a mechanism that stabilizes cancer-specific miRNAs, potentially helping to explain why CRC-related miRNAs can be detected in feces. Among these detectable miRNAs, the abundant miR-222 showed relatively little variation between sample groups (Supplementary Fig. 3) and was used to adjust various RNA amounts between samples. Consistently, we had previously reported that the expression of miR-222 was no change in 62 paired CRC and adjacent normal tissues [19].
In addition to diagnosis, their value for CRC prognosis has been explored. The higher expression levels of miR-223, miR-92a, miR-16, and miR-20a in tissue samples have been mentioned to correlate with poor DFS or OS, respectively, but their value as strong prognostic predictors has not been supported by comprehensive investigations [26,27,30,35]. Two groups previously reported that higher expression levels of miR-92a in serum and miR-20a in plasma predict poorer OS for CRC patients [36,37]. However, no stool miRNA has been reported to have prognostic values for CRC. In the present study, we demonstrate that the combined assessment of these four well-investigated miRNAs into one miRNA signature in feces could be used as a prognostic predictor for worse DFS and OS of CRC patients (Table 3). The tumor stage is the other clinical feature significantly associated with worse DFS and OS in our study (Table 3). Noticeably, our stool miRNA signature could further differentiate worse DFS and OS in the late-stage CRC (Fig. 2B and E), predicting those of the worse outcomes.
A recent report by Duran-Sanchon et al. indicated that 2 stool miRNAs (miR-421 and miR-27a-3p) plus fecal hemoglobulin concentration and age/sex can be applied to detect advanced polyps and CRC and to avoid 34% of colonoscopy examination [38,39]. These two miRNAs are different from the ones we have selected and validated in our current study. This discrepancy could be due to our miRNA targets being highly detectable at late stages and having potential for outcome monitoring in CRC, while the other two miRNAs (miR-421 and miR-27a-3p) were screened from early-stage CRC and advanced polyps. However, both of the two studies used almost the same reagents of RNA extraction and TaqMan miRNA assays to detect stool miRNAs in the original FIT buffer. It is worthy of future investigation to include miR-421 and miR-27a-3p for further validation study in our patient cohort. According the data shown in Fig. 3 and Supplementary Fig. 4C, our stool miRNA signature could well differentiate CRC from control groups, but was not sensitive to advanced polyps. This reflects that the miRNA candidates were selected from previous studies that focused on dysregulated miRNAs between CRC and normal groups. Due to the sequential transformation from polyps to malignant tissues in CRC development, early detection and removal of precancerous lesions are considered a crucial part of efforts to reduce CRC incidence [6]. Future work aimed at identifying and exploring miRNAs that are sensitive for benign and advanced polyps is warranted and could help improve the miRNA signature for the purpose of CRC screening [39]. It is a pity that there are no complete FIT results in our training and test sets of the present study, and combining FIT values in the regression algorithm of our stool miRNA signature may be one strategy to increase the detection rate and is worthy of further investigation. Although we validated our stool miRNA signature in a small cohort of prospectively collected samples, these findings should be further verified in well-designed clinical trials and other independent research centers. However, we are optimistic that the miRNA signature identified herein could help improve the diagnosis and prognosis prediction of CRC.
Conclusions
In this study, we assembled four miRNA ratios into a stool miRNA signature that can detect CRC patients and predict patients with worse prognosis. If the FIT result is positive, this miRNA signature can be assessed in the residual sample and the results can be used to prioritize the most at-risk persons to receive prompt colonoscopic confirmation and to suggest more frequent follow-up for those at risk for a poor prognosis of CRC.
Grant support
This work was supported by Chang Gung Memorial Hospital (CIRPG3B0023 and CLRPD1J0012), Ministry of Health and Welfare of Taiwan (MOHW107-TDU-B-212-114023), and Ministry of Science and Technology of Taiwan (107-2320-B-182-024).
Availability of data
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of interest
Each author that there is no conflict to disclose. The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Acknowledgements
We thank professors Hua-Chien Chen and Shu-Jen Chen for inspiration of using stool miRNA for CRC detection. This work was financially supported by the “Molecular Medicine Research Center, Chang Gung University” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2022.01.011.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Rawla P., Sunkara T., Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onyoh E.F., Hsu W.F., Chang L.C., Lee Y.C., Wu M.S., Chiu H.M. The rise of colorectal cancer in asia: epidemiology, screening, and management. Curr Gastroenterol Rep. 2019;21(8):36. doi: 10.1007/s11894-019-0703-8. [DOI] [PubMed] [Google Scholar]
- 4.Xie Y.H., Chen Y.X., Fang J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5(1):22. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladabaum U., Dominitz J.A., Kahi C., Schoen R.E. Strategies for colorectal cancer screening. Gastroenterology. 2020;158(2):418–432. doi: 10.1053/j.gastro.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 6.Strum W.B. Colorectal adenomas. N Engl J Med. 2016;375(4):389–390. doi: 10.1056/NEJMc1604867. [DOI] [PubMed] [Google Scholar]
- 7.Meklin J., Syrjanen K., Eskelinen M. Colorectal cancer screening with traditional and new-generation fecal immunochemical tests: a critical review of fecal occult blood tests. Anticancer Res. 2020;40(2):575–581. doi: 10.21873/anticanres.13987. [DOI] [PubMed] [Google Scholar]
- 8.Tepus M., Yau T.O. Non-invasive colorectal cancer screening: an overview. Gastrointest Tumors. 2020;7(3):62–73. doi: 10.1159/000507701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebner D.W., Kisiel J.B. Stool-based tests for colorectal cancer screening: performance benchmarks lead to high expected efficacy. Curr Gastroenterol Rep. 2020;22(7):32. doi: 10.1007/s11894-020-00770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang P.Y., Chen C.C., Chiang J.M., Chang S.C., Wang M.C., Chen J.S., et al. A simple and highly specific MassARRAY-based stool DNA assay to prioritize follow-up decisions in fecal immunochemical test-positive individuals. Cancers (Basel) 2019;11(3):423. doi: 10.3390/cancers11030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y.Y., Chen T.H., Su M.Y., Ning H.C., Kuo C.J., Lin W.P., et al. Accuracy of immunochemical fecal occult blood test for detecting colorectal neoplasms in individuals undergoing health check-ups. Adv Digestive Med. 2014;1:74–79. [Google Scholar]
- 12.Wang Y.W., Chen H.H., Wu M.S., Chiu H.M. Taiwanese Nationwide Colorectal Cancer Screening Program. Current status and future challenge of population-based organized colorectal cancer screening: lesson from the first decade of Taiwanese program. J Formos Med Assoc. 2018;117(5):358–364. doi: 10.1016/j.jfma.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Hull M.A., Rees C.J., Sharp L., Koo S. A risk-stratified approach to colorectal cancer prevention and diagnosis. Nat Rev Gastroenterol Hepatol. 2020;17(12):773–780. doi: 10.1038/s41575-020-00368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebert L.F.R., MacRae I.J. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou K., Liu M., Cao Y. New insight into microRNA functions in cancer: oncogene-microRNA-tumor suppressor gene network. Front Mol Biosci. 2017;4:46. doi: 10.3389/fmolb.2017.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadaka A.O., Pretorius A., Klein A. Biomarkers for stratification in colorectal cancer: MicroRNAs. Cancer Control. 2019;26(1) doi: 10.1177/1073274819862784. :1073274819862784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saberinia A., Alinezhad A., Jafari F., Soltany S., Akhavan Sigari R. Oncogenic miRNAs and target therapies in colorectal cancer. Clin Chim Acta. 2020;508:77–91. doi: 10.1016/j.cca.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Chen B., Xia Z., Deng Y.N., Yang Y., Zhang P., Zhu H., et al. Emerging microRNA biomarkers for colorectal cancer diagnosis and prognosis. Open Biol. 2019;9(1):180212. doi: 10.1098/rsob.180212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang P.Y., Chen C.C., Chang Y.S., Tsai W.S., You J.F., Lin G.P., et al. MicroRNA-223 and microRNA-92a in stool and plasma samples act as complementary biomarkers to increase colorectal cancer detection. Oncotarget. 2016;7(9):10663–10675. doi: 10.18632/oncotarget.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiser M.R. AJCC 8th Edition: Colorectal cancer. Ann Surg Oncol. 2018;25(6):1454–1455. doi: 10.1245/s10434-018-6462-1. [DOI] [PubMed] [Google Scholar]
- 21.Robertson D.J., Selby K. Fecal immunochemical test: the world's colorectal cancer screening test. Gastrointest Endosc Clin N Am. 2020;30(3):511–526. doi: 10.1016/j.giec.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Imperiale T.F., Ransohoff D.F., Itzkowitz S.H., Levin T.R., Lavin P., Lidgard G.P., et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287–1297. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 23.Zou J., Xiao Z., Wu Y., Yang J., Cui N. Noninvasive fecal testing for colorectal cancer. Clin Chim Acta. 2022;524:123–131. doi: 10.1016/j.cca.2021.10.030. [DOI] [PubMed] [Google Scholar]
- 24.Rashid H., Hossain B., Siddiqua T., Kabir M., Noor Z., Ahmed M., et al. Fecal MicroRNAs as potential biomarkers for screening and diagnosis of intestinal diseases. Front Mol Biosci. 2020;7:181. doi: 10.3389/fmolb.2020.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampath S.S., Venkatabalasubramanian S., Ramalingam S. Role of MicroRNAs in the progression and metastasis of colon cancer. Endocr Metab Immune Disord Drug Targets. 2021;21(1):35–46. doi: 10.2174/1871530320666200825184924. [DOI] [PubMed] [Google Scholar]
- 26.Peng Q., Shen Y., Lin K., Zou L., Shen Y., Zhu Y. Identification of microRNA-92a and the related combination biomarkers as promising substrates in predicting risk, recurrence and poor survival of colorectal cancer. J Cancer. 2019;10(14):3154–3171. doi: 10.7150/jca.30306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moody L., Dvoretskiy S., An R., Mantha S., Pan Y.X. The efficacy of miR-20a as a diagnostic and prognostic biomarker for colorectal cancer: a systematic review and meta-analysis. Cancers (Basel) 2019;11(8):1111. doi: 10.3390/cancers11081111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang L.L., Wang X.H., Sun B.F., Zhang X.D., Zhu X.H., Yu Z.J., et al. Expression, regulation and mechanism of action of the miR-17-92 cluster in tumor cells (Review) Int J Mol Med. 2017;40(6):1624–1630. doi: 10.3892/ijmm.2017.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yau T.O., Tang C.M., Harriss E.K., Dickins B., Polytarchou C. Faecal microRNAs as a non-invasive tool in the diagnosis of colonic adenomas and colorectal cancer: a meta-analysis. Sci Rep. 2019;9(1):9491. doi: 10.1038/s41598-019-45570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diamantopoulos M.A., Kontos C.K., Kerimis D., Papadopoulos I.N., Scorilas A. Upregulated miR-16 expression is an independent indicator of relapse and poor overall survival of colorectal adenocarcinoma patients. Clin Chem Lab Med. 2017;55(5):737–747. doi: 10.1515/cclm-2016-0756. [DOI] [PubMed] [Google Scholar]
- 31.Qian J., Jiang B., Li M., Chen J., Fang M. Prognostic significance of microRNA-16 expression in human colorectal cancer. World J Surg. 2013;37(12):2944–2949. doi: 10.1007/s00268-013-2205-4. [DOI] [PubMed] [Google Scholar]
- 32.Hasakova K., Bezakova J., Vician M., Reis R., Zeman M., Herichova I. Gender-dependent expression of leading and passenger strand of miR-21 and miR-16 in human colorectal cancer and adjacent colonic tissues. Physiol Res. 2017;66(Suppl 4):S575–S582. doi: 10.33549/physiolres.933808. [DOI] [PubMed] [Google Scholar]
- 33.Ostenfeld M.S., Jensen S.G., Jeppesen D.K., Christensen L.L., Thorsen S.B., Stenvang J., et al. miRNA profiling of circulating EpCAM(+) extracellular vesicles: promising biomarkers of colorectal cancer. J Extracell Vesicles. 2016;5:31488. doi: 10.3402/jev.v5.31488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koga Y., Yasunaga M., Moriya Y., Akasu T., Fujita S., Yamamoto S., et al. Exosome can prevent RNase from degrading microRNA in feces. J Gastrointest Oncol. 2011;2(4):215–222. doi: 10.3978/j.issn.2078-6891.2011.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z.W., Yang Y.M., Du L.T., Dong Z., Wang L.L., Zhang X., et al. Overexpression of miR-223 correlates with tumor metastasis and poor prognosis in patients with colorectal cancer. Med Oncol. 2014;31(11):256. doi: 10.1007/s12032-014-0256-5. [DOI] [PubMed] [Google Scholar]
- 36.Pesta M., Kucera R., Topolcan O., Karlikova M., Houfkova K., Polivka J., et al. Plasma microRNA levels combined with CEA and CA19-9 in the follow-up of colorectal cancer patients. Cancers(Basel) 2019;11(6):864. doi: 10.3390/cancers11060864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu G.H., Zhou Z.G., Chen R., Wang M.J., Zhou B., Li Y., et al. Serum miR-21 and miR-92a as biomarkers in the diagnosis and prognosis of colorectal cancer. Tumour Biol. 2013;34(4):2175–2181. doi: 10.1007/s13277-013-0753-8. [DOI] [PubMed] [Google Scholar]
- 38.Duran-Sanchon S., Moreno L., Gomez-Matas J., Auge J.M., Serra-Burriel M., Cuatrecasas M., et al. Fecal MicroRNA-based algorithm increases effectiveness of fecal immunochemical test-based screening for colorectal cancer. Clin Gastroenterol Hepatol. 2021;19(2):323–330. doi: 10.1016/j.cgh.2020.02.043. e1. [DOI] [PubMed] [Google Scholar]
- 39.Duran-Sanchon S., Moreno L., Auge J.M., Serra-Burriel M., Cuatrecasas M., Moreira L., et al. Identification and validation of MicroRNA profiles in fecal samples for detection of colorectal cancer. Gastroenterology. 2020;158(4):947–957. doi: 10.1053/j.gastro.2019.10.005. e4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.