Abstract
Down syndrome is the main genetic cause of intellectual disability. Many studies describe the clinical characteristics of DS patients; however, few have investigated the clinical profile of mothers who have children with DS. Advanced maternal age (≥ 35 years old) is a risk factor for DS. Although there is an overall increase in pregnancies among women with advanced maternal age, there is still a lack of awareness of the increased risk of aneuploidy. Here, we reported the clinical and epidemiological profile of DS children and their mothers in a public reference hospital in the State of Rio de Janeiro, Brazil. For data collection, we performed a face-to-face interview guided by a structured questionnaire with closed-ended questions. A total of 344 individuals, 172 mothers and their DS children, were included in this study. Our results show that 56% of the mothers sampled were ≥ 35 years of age at childbirth. Although 98% of them received prenatal care, only 4% obtained a prenatal diagnosis of DS. Most mothers reported not drinking alcohol or smoking cigarettes during pregnancy. Furthermore, 91% of women took prenatal vitamins and supplements; however, 47% were not aware of their benefits for a healthy pregnancy. Given the strict correlation between advanced maternal age and DS, prenatal care should include genetic counseling for women over 35 years of age. This study highlights the importance of prenatal care and the urgent need for better DS screening allowing for immediate postnatal care, positively impacting the life expectancy of these patients.
Keywords: Down syndrome, Epidemiological, Maternal age, Maternal habits, Diagnosis
Introduction
Down syndrome (DS) is a genetic condition caused by an extra copy of chromosome 21. The estimated prevalence of DS is ~ 1:700 live births, and it is the most common cause of intellectual disability. In addition to its characteristic neurological and cognitive defects, DS is also associated with congenital heart disease (CHD), an elevated risk of leukemia, respiratory insufficiency, musculoskeletal, and gastrointestinal abnormalities (Bull, 2020).
The genetic mechanisms leading to Down syndrome include Robertsonian translocation, mosaicism, and meiotic nondisjunction. It is estimated that 95% of DS cases have a trisomy of chromosome 21 as a result of meiotic nondisjunction. The remaining cases are observed to be due to Robertsonian translocation (3–4%) and mosaicism (2–3%). Nearly 90% of nondisjunction errors occur during the first maternal meiotic division (Sherman et al 2007; Allen et al. 2009). The meiotic divisions begin during female fetal development and are interrupted with oocytes arrested in prophase I. In the time of puberty, hormones induce maturation, allowing oocytes to progress through the first meiotic division. When ovulation occurs, the oocyte begins the second meiotic division progressing to the metaphase and then having its division arrested. If fertilization of the oocyte occurs, meiosis II continues, and the division process resumes. Thus, advanced maternal age (≥ 35 years old) and meiotic recombination errors have been associated with risk factors for the occurrence of DS (Hassold and Sherman 2000; Chernus et al. 2021).
Several epidemiological studies have investigated possible environmental risk factors for DS occurrence (smoking and alcohol consumption, use of medication during pregnancy, paternal contributions, oral contraceptives, socioeconomic factors, medical conditions, and radiation exposure), but no sufficient evidence was gathered to support their classification as actual risk factors (Ghosh et al. 2011; Coppedè 2016; Cuckle and Benn 2021). Some authors also suggest a complex interaction between genetic and environmental factors, particularly dietary factors such as folic acid consumption (Patterson 2008; Coppedè 2016).
Regarding prenatal, non-invasive screening tests (e.g., triple screen test, ultrasound, and combined test) aim to identify pregnancies at high risk for congenital anomalies. Specific diagnostic tests such as chorionic villus sampling (CVS) or amniocentesis should be recommended for women with positive screening. Nevertheless, definitive diagnostic tests are often invasive (LeFevre and Sundermeyer 2020). Importantly, prenatal diagnosis enables parental enlightening and psychological preparation, genetic counseling, qualified specialist assistance during delivery, and early interventions contributing to better children’s development (Antonarakis et al. 2020).
In respect to the Brazilian population, Bertelli et al. (2009) have previously published on the main clinical characteristics of DS patients. However, few studies have analyzed and described the clinical profile of mothers of children with DS. Analysis of the clinical and epidemiological profile of DS mothers enables the identification of risk factors and gives the opportunity for appropriate counseling, preventing possible adverse effects during pregnancy. This study aimed to investigate the clinical and epidemiological profile of DS children and their mothers in a public reference hospital in the State of Rio de Janeiro, Brazil.
Methods
A descriptive epidemiological study of mothers with DS children was conducted between 2012 and 2020. A total of 344 individuals were sampled: 172 DS patients and their respective mothers.
For data collection, we performed a face-to-face interview guided by a structured questionnaire with closed-ended questions and analyzed the patients’ medical records. Mothers of DS children were recruited from the Genetic Service of the Instituto de Puericultura e Pediatria Martagão Gesteira (IPPMG) at the Universidade Federal do Rio de Janeiro (UFRJ), Brazil, which is a public pediatric hospital that provides different types of specialized care and receives patients from several cities from Rio de Janeiro state. The studied variables included the maternal history of spontaneous abortions, preexisting chronic diseases, and characteristics of current pregnancy: maternal age, clinical/obstetric intercurrences (e.g., high blood pressure, gestational diabetes, gestational anemia, infections, preeclampsia, preterm labor), medications, smoking, alcohol consumption habits, vitamin and mineral supplementation (folic acid and iron), and prenatal care. The criteria for advanced maternal age was defined as mothers who were 35 years of age or older at the estimated date of delivery.
Data for the respective children with DS was retrieved from the hospital’s medical records. These include time of diagnosis, presence of congenital heart defects, karyotype test results, and postnatal development data.
The Research Ethics Committee of IPPMG /UFRJ approved this survey (CEP/IPPMG 41/10), and all mothers included in this study consented to participation, signing the informed consent form. Statistical analyses were performed using the GraphPad Prism® 3.0 statistical program (GraphPad Software Inc., San Diego, CA, EUA).
Results
Maternal profile
The mean maternal age was 33.2 ± 8.4 years of age at the birth of their offspring. Our results show that 56% of women were aged ≥ 35 years old during the gestational period (Table 1). Prenatal consultation at any point during pregnancy was obtained by 98% of the mothers, and 84.3% of them started prenatal care during the first trimester of pregnancy. Prenatal care included consultations, laboratory diagnostic analyses, and ultrasound screening. However, no specific genetic screening was done, even in mothers of advanced age. Four mothers included in this study did not receive prenatal care.
Table 1.
Characteristics of the study participants
| IPPMG mothers | n | % |
|---|---|---|
| Study cohort | 172 | |
| Maternal age (years) (n = 172) | ||
| 14|–-|19 | 13 | 7.6 |
| 20|–-|24 | 25 | 14.5 |
| 25|–-|29 | 17 | 9.9 |
| 30|–-|34 | 21 | 12.2 |
| 35|–-|39 | 52 | 30.2 |
| 40|–-|44 | 38 | 22.1 |
| 45|–-|49 | 6 | 3.5 |
| Chronic disease (n = 170) | ||
| Yes | 52 | 30.6 |
| No | 118 | 69.4 |
| Miscarriage (n = 169) | ||
| Yes | 50 | 29.6 |
| No | 119 | 70.4 |
| Alcohol (n = 164) | ||
| Yes | 10 | 6.1 |
| No | 154 | 93.9 |
| Cigarette (n = 170) | ||
| Yes | 9 | 5.3 |
| No | 161 | 94.7 |
| Medications during gestation (n = 169) | ||
| Yes | 41 | 24.3 |
| No | 128 | 75.7 |
| Vitamins (n = 169) | ||
| Yes | 153 | 90.5 |
| No | 16 | 9.5 |
| Pregnancy complications (n = 170) | ||
| Yes | 63 | 37.1 |
| No | 107 | 62.9 |
| Prenatal care (n = 172) | ||
| Yes (1º trimester) | 145 | 84.3 |
| Yes (2º trimester) | 15 | 8.7 |
| Yes (3º trimester) | 2 | 1.2 |
| Yes (1º, 2º or 3º trimester) | 6 | 3.5 |
| No | 4 | 2.3 |
Approximately 31% of the women sampled had chronic diseases, mainly hypertension and diabetes. In addition, 37% experienced clinical intercurrences during the DS pregnancy and 30% of the mothers reported at least one miscarriage.
Regarding the gestational period, almost 94% of the mothers denied alcohol or tobacco consumption (Table 1), whereas 24% reported the use of medications such as antihypertensives, antiemetics, anticholinergics, and antispasmodics. Approximately 35% of the mothers were taking medication to manage health conditions acquired before pregnancy.
Concerning the intake of supplements during pregnancy, 91% of mothers reported taking vitamins, mostly folic acid and/or ferrous sulfate. However, it should be noted that 47% of mothers were unaware of the importance of taking supplements during pregnancy.
Children’s profile
The mean age of patients with DS was 8.8 ± 5.9 years. Even though over 98% of mothers received prenatal care, in 96% of the cases, the DS baby was diagnosed postnatally (Fig. 1). The diagnosis of DS is usually confirmed by a karyotype test, showing either partial or complete trisomy of chromosome 21. Cytogenetic analysis showed 90% of DS cases in the sample with free chromosome 21 trisomy, 7% with mosaicism, and 3% caused by Robertsonian translocation.
Fig. 1.
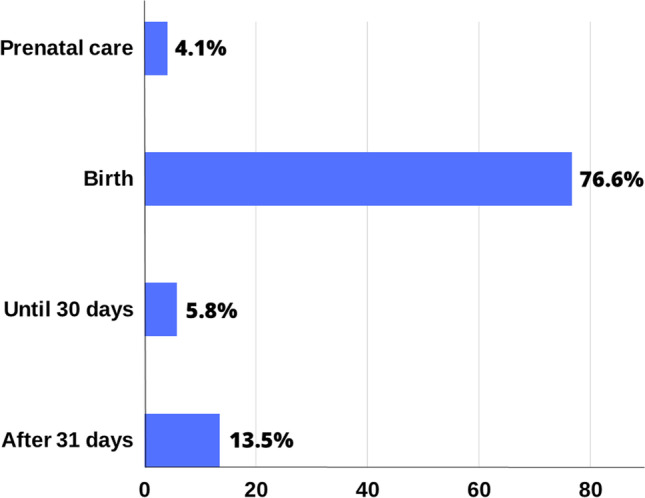
Period of Down syndrome diagnosis (%)
Congenital heart defects were present in 44% of DS patients. The most common cardiac abnormality was interventricular communication and interatrial communication. Other comorbidities have been reported, such as endocrine (especially thyroid disease), gastrointestinal, psychiatric disorders, and lung infections.
Discussion
Childbearing at older ages is becoming common (Barclay and Myrskylã 2016; Howell and Blott 2021) probably because of an increased access of women to education and the prioritization of professional choices (Cusimano et al. 2021). In our study, 56% of the mothers were aged 35 years; supporting the evidence of advanced maternal age and an increased risk for genetic abnormalities.
In Rio de Janeiro, delays in childbearing ranged from 10 (in 2000) to 17.4% (in 2019) in women over 35 years old. For mothers in the 40–44 age group, the percentage of women increased from 1.8% in 2000 to 3.7% in 2019 (MS/SVS/DASIS – Brazilian Live Birth Information System—SINASC). In the USA, the birth rate for women aged 40–44 had risen almost continuously from 1985 to 2019, by an average of 3% per year (Hamilton et al. 2021). Although advanced maternal age in pregnancy has been discussed in the scientific literature for more than 2 decades, a significant part of the population is unaware of or disregards the risks, especially in low-income nations.
Advanced maternal age at conception is a consolidated risk factor for DS (Hassold and Sherman 2000; Chernus et al. 2021), and if this trend continues, it will directly affect prevalence of DS worldwide (Zhang et al. 2020). It is important to note that genetic abnormalities are not the only conditions associated with advanced maternal age during pregnancy. Women aged 35 years or older are more prone to have preexisting clinical conditions such as diabetes, obesity, and hypertension (Lean et al. 2017), contributing to adverse outcomes in pregnancy as pre-eclampsia/eclampsia, maternal hemorrhage, and preterm delivery (Correa-De-Araujo and Yoon 2021).
In addition, spontaneous abortion is frequent in humans and varies between 12 and 15% in each pregnancy (Nagaoka et al. 2012; Yang et al. 2020). Similarly, data from the Brazilian population shows an estimated rate of miscarriage between 11 and 16% (Diniz et al. 2016). In our sample, we did observe a reported rate of previous pregnancy loss of almost 30%, perhaps because of advanced maternal age.
In Brazil’s Public National Health Service, Sistema Único de Saúde (SUS), all pregnant women have access to prenatal care (Leal et al. 2020). In our study, most women received at least one prenatal appointment (98%). A few of the women sampled did not receive or had access to any prenatal care (2%), mostly due to low levels of education, limited knowledge, and/or comprehension of the importance of this measure.
In this sample, only 4% of DS babies were diagnosed during prenatal care, mainly by ultrasound, and 71% of these mothers were 35 years or older. Similarly, another Brazilian study with a limited sample size of 62 patients did not show any prenatal diagnosis of DS (Bertelli et al. 2009). A recent analysis from Ohio, USA, including 129 patients had 8% of the cases diagnosed with DS during the prenatal period (Seither et al. 2021). These results reveal a low rate of prenatal diagnosis in developed and developing countries, suggesting a need for improvement in DS screening during pregnancy.
In Brazilian public hospitals, ultrasound screening for chromosome anomalies is the primary procedure but cannot be considered a definitive diagnostic method. Although most healthcare professionals recommend at least 3 ultrasound exams during pregnancy, there is substantial heterogeneity in healthcare services in Brazil. The number and quality of ultrasounds depend directly on infrastructure, equipment, and supplies of resources (Leal et al. 2020). Moreover, ultrasound is highly operator-dependent, and many operators might not have adequate training or experience in detecting certain anomalies.
Additional screening tests are not usually available in the national public health system (SUS). In Brazil, there are some reference centers to the Fetal Medicine Services (Kohatsu et al. 2012), where pregnant women identified as high risk, especially after ultrasound exams, are referred to perform invasive tests. In our sample, most expectant mothers were not referred to a genetic service, even with advanced maternal age. Usually, only after birth, when children are suspected to have DS, they are forwarded to our genetic service, where proper counseling and diagnostic tests are provided. These difficulties can also contribute to the low rate of prenatal diagnoses of DS in Brazil.
Alternative prediction models based on DS basic risk factors with cost–benefit analysis, like gestational age, were proposed to improve non-invasive prenatal tests (Khattak et al. 2019; Wanapirak et al. 2019). The first-trimester combined screening (the fetal nuchal translucency added to maternal serum analysis of free-β-human chorionic gonadotropin and pregnancy-associated plasma protein-A) reaches the sensitivity of 80% (Spencer et al. 1999; Nicolaides 2005), according to the Fetal Medicine Foundation guideline.
Nowadays, the application of a new non-invasive prenatal screening technique could improve the detection rate. The cell-free DNA (cfDNA) is the most specific screening test for fetal aneuploidies, with a sensitivity of more than 99% for high-risk pregnancies with DS (Gil et al. 2017). It is based on the presence of small fragments of DNA derived from placental trophoblasts released in the maternal bloodstream. Aneuploidy can be detected with an increased cfDNA from an overrepresented chromosome (Allyse and Wick 2018). Despite this high detection rate for trisomy 21, the diagnosis requires complementary invasive procedures.
In Brazil, abortion is not allowed even after a prenatal diagnosis of DS. Abortion is only possible in cases of rape, fetal anencephaly, or when the mother’s life is in danger. Thus, the detection of fetal anomalies during pregnancy is not a priority. This factor may negatively influence the decision for DS screening in centers where it is available. Nevertheless, many DS newborns need intensive care shortly after birth, and early diagnosis improves delivery planning, especially for those with cardiovascular malformations (Seither et al., 2021). In addition, parents can seek specialized professionals who may teach movement skills, feeding and performing tasks. Moreover, a team of experts can offer encouragement and accurate information about DS. It also provides time for the family to connect with support groups and deal with their own emotions (Skotko 2005).
In this study, we investigated some environmental risk factors for the occurrence of DS: smoking, alcohol consumption, vitamin supplementation, and medications during pregnancy (Cuckle and Benn 2021). However, no significant association could be drawn between these factors and DS risk.
In the last decades, the use of medications during pregnancy has been increasing, especially in developed countries. Women at an advanced age have a higher prevalence of preexisting chronic conditions and are often more exposed to prescribed medications during the organogenesis period (Beŕard and Sheehy 2014; Ayad 2018). Use of medications during pregnancy can increase the risk of spontaneous abortions, prematurity and post-partum depression (Bérard et al. 2019a, b). Pregnant women should be informed since the potential side effects always have to be considered ahead of their benefits (Benevent et al. 2019).
There is a substantial variation found in literature when it comes to the prevalence of medication use amongst pregnant women. The overall drug use of our study was 93%, similar to Ethiopia (87.7%) (Alema et al. 2020), the USA (97.1%) (Haas et al. 2018), France (89.9%) (Bérard et al. 2019a, b), and another Brazilian population study (83.4%) (Melo et al. 2009). When vitamin supplementation is excluded, the present study obtained a prevalence of 24.3% drug use. A cross-sectional multinational survey found a mean frequency of 81.2%; the lowest and the highest prevalence of total medication use during pregnancy was observed in Croatia (62.2%) and the Netherlands (95.1%), respectively (Lupattelli et al. 2014). A Scottish study (McLay et al. 2017) found a lower frequency (45%). All these studies conducted the interviews during pregnancy or within the first 24 h following a live birth. It is important to highlight that our data was collected through retrospective interviews and referred to previous events with potential recall bias, which might explain the lower prevalence reported in our study.
We identified a high rate of gestational vitamin supplementation (91%) in our sample, especially with folic acid. However, 47% of mothers were not aware of the importance of vitamin supplementation during pregnancy, but the questionnaire did not specify the dose and period of supplementation with folic acid. Perhaps it is still necessary to emphasize the correct time of supplementation, the periconceptional period.
In developing countries, the majority of pregnancies are unplanned. Thus, more effective strategies for the primary prevention of congenital defects could be achieved with national campaigns to increase knowledge and folic acid supplementation, especially among women of childbearing age and with low educational status. Folic acid is effective in the prevention of neural tube defects (Milunsky et al. 1989). Hollis et al. (2013) also suggest a role for the folate pathway in chromosome 21 nondisjunction.
Prenatal alcohol exposure is considered one of the major public health challenges. There is no safe level or timing for drinking for pregnant or lactating women (Oei 2020). Alcohol exposure throughout pregnancy can affect the fetal development of the brain (Sebastiani et al. 2018) and aggravate micronutrient deficits such as folate, vitamin B12, iron, and vitamin A (Young et al. 2014; Sebastiani et al. 2018). In our sample, approximately 94% of women denied alcohol consumption during pregnancy. Our data was similar to other investigations of alcohol use during pregnancy (Oliveira and Simões 2007; Baptista et al. 2017); however, these values are higher in Europe and America (Sebastiani et al. 2018). Although most women would decrease or stop drinking when pregnancy was diagnosed, 12.5% continue to drink in the USA (Oei 2020).
Besides affecting women’s health, smoking during pregnancy has been linked to numerous adverse effects, including fetal morbidity, mortality, and developmental delay. According to Lange et al. (2018), the global prevalence of smoking during pregnancy is 1.7%, while in the Americas region, it is 5.9%. The three countries with the highest estimated prevalence of smoking during pregnancy are Ireland (38.4%), Uruguay (29.7%), and Bulgaria (29.4%) (Lange et al. 2018). Our results (5%) corroborate the findings in Americans and other Brazilian studies (Kataoka et al. 2018; Madeira Domingues et al. 2019).
Conclusion
This study provides an important profile of mothers and DS patients in a reference genetics center in Rio de Janeiro and highlights the need to improve the prenatal diagnosis of DS, not only in Brazil. Moreover, our findings indicate that further investigations are needed. It is important for women to be aware of the effects of folic acid supplementation before pregnancy. The number of women who delay childbearing is increasing, but they are scarcely informed about its risks, especially those related to aneuploidies. In this scenario, the public health policies to warn about adverse risks of delayed pregnancy, the improvement of screening for fetal aneuploidy, and the access to genetic counseling can be helpful to make conscious decisions.
Acknowledgements
The authors would like to thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro- E-26/202.617/2019), UFRJ (Universidade Federal do Rio de Janeiro), and UFF (Universidade Federal Fluminense). This article is part of the doctoral thesis of Aprigio J. in the Postgraduate Program in Genetics, UFRJ, Brazil.
Author contribution
All authors contributed to the study conception and design. JA and CMLC contributed to data collection. All authors read and approved the final manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Federal University of Rio de Janeiro (UFRJ) according to local regulations (approval number CEP/IPPMG 4110). All methods were performed in accordance with the relevant guidelines and regulations. A written informed consent was obtained from each subject participating in the study. All participants were voluntary and understood that they could deny their consent and participation at any time. No incentive was offered.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Authors’ information
J Aprigio, Biologist and Biomedic, PhD student at Universidade Federal do Rio de Janeiro (UFRJ), Pós Graduação em Genética. Rio de Janeiro, RJ, Brazil.
CML Castro, Biologist and Biomedic, Master student at Universidade Federal Fluminense, Programa de Pós Graduação em Medicina (Neurologia/Neurociências), UFF, Niterói, Brazil.
MA Costa Lima, PhD, Biologist, Associate Professor, UERJ. Coordinator of Laboratório de Genética Molecular Humana at Departamento de Genética, UERJ, Rio de Janeiro, RJ, Brazil. http://orcid.org/0000-0002-9629-6138.
MG.Ribeiro, MD, PhD, Geneticist, Associate Professor, UFRJ. Permanent Professor of Pós-Graduação da Clínica Médica, at the Faculty of Medicine, UFRJ, RJ, Brazil. http://orcid.org/0000-0001-8906-0189.
IM Orioli, MD, PhD, Geneticist, Permanent Professor of Pós Graduação em Genética, UFRJ. Coordinator and President of the Technical-Scientific Association of ECLAMC (Estudo Colaborativo Latino Americano de Malformações Congênitas). Coordinator of the Laboratório de Malformações Congênitas, INAGEMP, Rio de Janeiro, RJ, Brazil. http://orcid.org/0000-0003-1863-6229.
MR Amorim, PhD, Biologist, Associate Professor UFF. Coordinator of Laboratório de Genética Humana, UFF. Member of the ECLAMC. Member of Programa de Pós Graduação em Medicina (Neurologia/Neurociências), UFF, Niterói, RJ, Brazil. https://orcid.org/0000-0002-0528-0377.
References
- Alema NM, Semagn G, Melesse S, et al. Patterns and determinants of prescribed drug use among pregnant women in Adigrat general hospital, northern Ethiopia: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):1–9. doi: 10.1186/s12884-020-03327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EG, Freeman SB, Druschel C, et al. Maternal age and risk for trisomy 21 assessed by the origin of chromosome nondisjunction: a report from the Atlanta and National Down Syndrome Projects. Hum Genet. 2009;125(1):41–52. doi: 10.1007/s00439-008-0603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allyse MA, Wick MJ. Noninvasive prenatal genetic screening using cell-free DNA. JAMA - J Am Medical Assoc. 2018;320(6):591–592. doi: 10.1001/jama.2018.9418. [DOI] [PubMed] [Google Scholar]
- Antonarakis SE, Skotko BG, Rafii MS, et al. Down Syndrome. Nat Rev Dis Primers. 2020;6(1):1–20. doi: 10.1038/s41572-019-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad M. Epidemiology of medications use in pregnancy martina. Semin Perinatol. 2018;39(7):508–511. doi: 10.1053/j.semperi.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista FH, Bethania Bispo Rocha K, Lustosa Martinelli J, et al. Prevalência e fatores associados ao consumo de álcool durante a gravidez. Rev Bras Saúde Matern Infant. 2017;17(2):281–289. doi: 10.1590/1806-93042017000200004. [DOI] [Google Scholar]
- Barclay K, Myrskylã M. And Offspring outcomes reproductive aging and counterbalancing. Adv Maternal Age Offspring Outcomes. 2016;42(1):69–94. doi: 10.1111/j.1728-4457.2016.00105.x. [DOI] [Google Scholar]
- Benevent J, Araújo M, Hurault-Delarue C, et al. Pharmacoepidemiology in pregnancy. Pharmacoepidemiol Pregnancy Justine. 2019;74(2):289–300. doi: 10.1016/j.therap.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Bérard A, Sheehy O, Gorgui J, et al. New evidence for concern over the risk of birth defects from medications for nausea and vomitting of pregnancy. J Clin Epidemiol. 2019;116:39–48. doi: 10.1016/j.jclinepi.2019.07.014. [DOI] [PubMed] [Google Scholar]
- Bérard A, Abbas-Chorfa F, Kassai B, et al. The French pregnancy cohort: medication use during pregnancy in the French population. PLoS ONE. 2019;14(7):1–18. doi: 10.1371/journal.pone.0219095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beŕard A and Sheehy O (2014) The Quebec pregnancy cohort - prevalence of medication use during gestation and pregnancy outcomes. PLoS ONE 9(4). 10.1016/j.jclinepi.2019.07.014 [DOI] [PMC free article] [PubMed]
- Bertelli ÉCP, Biselli JM, Bonfim D, et al. Clinical profile of children with down syndrome treated in a genetics outpatient service in the southeast of Brazil. Rev Assoc Med Bras. 2009;55(5):547–552. doi: 10.1590/s0104-42302009000500017. [DOI] [PubMed] [Google Scholar]
- Bull MJ. Down syndrome. N Engl J Med. 2020;382(24):2344–2352. doi: 10.5005/jp/books/12882_18. [DOI] [PubMed] [Google Scholar]
- Chernus JM, Sherman SL, Feingold E. Analyses stratified by maternal age and recombination further characterize genes associated with maternal nondisjunction of chromosome 21. Prenat Diagn. 2021;41(5):591–609. doi: 10.1002/pd.5919. [DOI] [PubMed] [Google Scholar]
- Coppedè F (2016) Risk factors for Down syndrome. Archives of Toxicology 90(12). Springer Berlin Heidelberg: 2917–2929. 10.1007/s00204-016-1843-3 [DOI] [PubMed]
- Correa-De-Araujo R, Yoon SS. Clinical outcomes in high-risk pregnancies due to advanced maternal age. J Women’s Health. 2021;30(2):160–167. doi: 10.1089/jwh.2020.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuckle H, Benn P. Review of epidemiological factors (other than maternal age) that determine the prevalence of common autosomal trisomies. Prenat Diagn. 2021;41(5):536–544. doi: 10.1002/pd.5822. [DOI] [PubMed] [Google Scholar]
- Cusimano MC, Baxter NN, Sutradhar R, et al. Delay of pregnancy among physicians vs nonphysicians. JAMA Intern Med. 2021;181(7):905–912. doi: 10.1001/jamainternmed.2021.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo SCCS, Pelloso SM, de Carvalho MD, B, , et al. Uso de medicamentos por gestantes usuárias do Sistema Único de Saúde. Acta Paulista De Enfermagem. 2009;22(1):66–70. doi: 10.1590/s0103-21002009000100011. [DOI] [Google Scholar]
- Diniz D, Medeiros M, Madeiro A. National abortion survey 2016. Ciência e Saúde Coletiva. 2016;22(2):653–660. doi: 10.1590/1413-81232017222.23812016. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Hong CS, Feingold E, et al. Epidemiology of Down syndrome: new insight into the multidimensional interactions among genetic and environmental risk factors in the oocyte. Am J Epidemiol. 2011;174(9):1009–1016. doi: 10.1093/aje/kwr240. [DOI] [PubMed] [Google Scholar]
- Gil MM, Accurti V, Santacruz B, et al. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2017;50(3):302–314. doi: 10.1002/uog.17484. [DOI] [PubMed] [Google Scholar]
- Haas DM, Marsh DJ, Dang DT, et al. Prescription and other medication use in pregnancy. Obstet Gynecol. 2018;131(5):789–798. doi: 10.1097/AOG.0000000000002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton BE, Martin JA and Osterman MJK (2021) Births: Provisional data for 2020. NVSS Vital Statistics Rapid Release (012): 1–21. 10.15620/cdc:104993
- Hassold T, Sherman S. Down syndrome : genetic recombination and the origin of the extra chromosome 21. Clin Genet. 2000;57(2):95–100. doi: 10.1034/j.1399-0004.2000.570201.x. [DOI] [PubMed] [Google Scholar]
- Hollis ND, Allen EG, Oliver TR, et al. Preconception folic acid supplementation and risk for chromosome 21 nondisjunction: a report from the National Down Syndrome Project. Am J Med Genet A. 2013;161A(3):438–444. doi: 10.1002/ajmg.a.35796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A, Blott M. Very advanced maternal age. Obstet Gynaecol. 2021;23(1):38–47. doi: 10.1111/tog.12710. [DOI] [Google Scholar]
- Kataoka MC, Paula A, Carvalheira P, et al. Smoking during pregnancy and harm reduction in birth weight : a cross-sectional study. BMC Pregnancy Childbirth. 2018;18(1):67. doi: 10.1186/s12884-018-1694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak MT, Supriyanto E, Aman MN, et al. Predicting Down syndrome and neural tube defects using basic risk factors. Med Biol Eng Compu. 2019;57:1417–1424. doi: 10.1007/s11517-019-01969-0. [DOI] [PubMed] [Google Scholar]
- Kohatsu M, Carvalho MH, Vieira Francisco RP, et al. Analysis of fetal and maternal results from fetal genetic invasive procedures: an exploratory study at a University Hospital. Rev Assoc Med Bras. 2012;58(6):703–708. doi: 10.1590/S0104-42302012000600016. [DOI] [PubMed] [Google Scholar]
- Lange S, Probst C, Rehm J, et al. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Health. 2018;6(7):e769–e776. doi: 10.1016/S2214-109X(18)30223-7. [DOI] [PubMed] [Google Scholar]
- Leal do MC, Esteves-Pereira AP, Viellas EF, et al. (2020) Prenatal care in the Brazilian public health services. Revista de Saude Publica 54: 08 10.11606/s1518-8787.2020054001458 [DOI] [PMC free article] [PubMed]
- Lean SC, Derricott H, Jones RL, et al. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS ONE. 2017;12(10):1–15. doi: 10.1371/journal.pone.0186287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFevre NM, Sundermeyer RL. Fetal aneuploidy: screening and diagnostic testing. Am Fam Physician. 2020;101(8):481–488. [PubMed] [Google Scholar]
- Lupattelli A, Spigset O, Twigg MJ, et al. Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open. 2014;4(2):e004365. doi: 10.1136/bmjopen-2013-004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira Domingues RMS, Figueiredo VC, do Leal MC. Prevalence of pre-gestational and gestational smoking and factors associated with smoking cessation during pregnancy, Brazil, 2011–2012. PLoS ONE. 2019;14(5):2011–2012. doi: 10.1371/journal.pone.0217397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay JS, Izzati N, Pallivalapila AR, et al. Pregnancy, prescription medicines and the potential risk of herb-drug interactions: a cross-sectional survey. BMC Complement Altern Med. 2017;17(1):1–7. doi: 10.1186/s12906-017-2052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milunsky A, Jick H, Jick SS, et al. Multivitamin folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA J Am Med Assoc. 1989;262(20):2847–2852. doi: 10.1001/jama.1989.03430200091032. [DOI] [PubMed] [Google Scholar]
- Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13(7):493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides KH. First-trimester screening for chromosomal abnormalities. Semin Perinatol. 2005;29(4):190–194. doi: 10.1053/j.semperi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Oei JL. Alcohol use in pregnancy and its impact on the mother and child. Addiction. 2020;115(11):2148–2163. doi: 10.1111/add.15036. [DOI] [PubMed] [Google Scholar]
- Oliveira TR, Simões SMF. O consumo de bebida alcóolica pelas gestantes: um estudo exploratório. Escola Anna Nery. 2007;11(4):632–638. doi: 10.1590/s1414-81452007000400012. [DOI] [Google Scholar]
- Patterson D. Folate metabolism and the risk of Down syndrome. Downs Syndr Res Pract. 2008;12(2):93–97. doi: 10.3104/updates.2051. [DOI] [PubMed] [Google Scholar]
- Sebastiani G, Borrás-Novell C, Casanova MA, et al. The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Nutrients. 2018;10(8):1–17. doi: 10.3390/nu10081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seither K, Tabbah S, Tadesse DG, et al. Neonatal complications of Down syndrome and factors necessitating intensive care. Am J Med Genet A. 2021;185(2):336–343. doi: 10.1002/ajmg.a.61948. [DOI] [PubMed] [Google Scholar]
- Sherman SL, Allen EG, Bean LH, Freeman SB. Epidemiology of Down syndrome. Ment Retard Dev Disabil Res Rev. 2007;13(3):221–227. doi: 10.1002/mrdd.20157. [DOI] [PubMed] [Google Scholar]
- Skotko B. Mothers of children with Down syndrome reflect on their postnatal support. Pediatrics. 2005;115(1):64–77. doi: 10.1542/peds.2004-0928. [DOI] [PubMed] [Google Scholar]
- Spencer K, Souter V, Tul N, et al. A screening program for trisomy 21 at 10–14 weeks using fetal nuchal translucency, maternal serum free β-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 1999;13(4):231–237. doi: 10.1046/j.1469-0705.1999.13040231.x. [DOI] [PubMed] [Google Scholar]
- Wanapirak C, Buddhawongsa P, Himakalasa W, et al. Fetal Down syndrome screening models for developing countries; Part II: Cost-benefit analysis. BMC Health Serv Res. 2019;19(1):1–9. doi: 10.1186/s12913-019-4699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Tao T, Zhao X, et al. Association between fetal chromosomal abnormalities and the frequency of spontaneous abortions. Exp Ther Med. 2020;19(4):2505–2510. doi: 10.3892/etm.2020.8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JK, Giesbrecht HE, Eskin MN, et al. Nutrition implications for fetal alcohol spectrum disorder. Adv Nutr. 2014;5(6):675–692. doi: 10.3945/an.113.004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen L, Xuemiao W, et al. Changes in maternal age and prevalence of congenital anomalies during the enactment of China’s universal two-child policy (2013–2017) in Zhejiang Province, China: an observational study. PLoS Med. 2020;17(2):1–19. doi: 10.1371/JOURNAL.PMED.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]


