Abstract
Alzheimer’s f disease (AD) affects approximately 250,000 Ontarians, a number that is expected to double by 2040. The Ontario Neurodegenerative Disease Research Initiative has developed an in-province genetic test (ONDRISeq), which currently runs in Ontario in an experimental capacity. The aim of this study is to estimate the costs and health outcomes associated with ONDRISeq to diagnose AD relative to out-of-country (OOC) testing (status quo). A cost-utility analysis was developed for a hypothetical cohort of 65-year-olds at risk of AD in Ontario over a 25-year time horizon. Costs and health outcomes (quality-adjusted life years (QALYs)) were assessed from a healthcare payer perspective. Cost-effectiveness was assessed with a $50,000 cost-effectiveness threshold. Probabilistic sensitivity analyses were conducted to evaluate parameter uncertainty. ONDRISeq saved $54 per patient relative to OOC testing and led to a small QALY gain in the base case (0.0014 per patient). Results were most sensitive to testing costs, uptake rates, and treatment efficacy. ONDRISeq represented better value for money relative to OOC testing throughout 75% of 10,000 probabilistic iterations. Using ONDRISeq is expected to provide health system cost savings. Switching to ONDRISeq for AD genetic testing in Ontario would be dependent on the ability to accommodate the expected testing volumes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12687-022-00619-7.
Keywords: Alzheimer’s, Cost-effectiveness, Markov model, Neurogenetics
Introduction
Ontario has a rapidly ageing population with seniors (people aged 65 years and older) representing 16.4% (2.3 million) of Ontarians (Statistics Canada 2017). This figure is expected to reach 25% (4.6 million) by 2041 (Aging with Confidence 2017). Neurodegenerative diseases such as Alzheimer’s disease (AD) are incurable and debilitating conditions characterized by a progressive decline in cognitive, language, motor, or behavioural functions and have strong linkages with age (Nussbaum and Ellis 2003). They have also been recognised as a leading cause of disability worldwide, particularly among senior populations, substantially reducing their quality of life (Chin and Vora 2014) Amongst these disorders, AD is responsible for a sizable proportion of the burden of neurodegenerative diseases and currently affects an estimated 250,000 Ontarians. It is expected that the number affected will surpass 430,000 by 2040, exacerbating the existing impact of the disease on Ontarians and the Ontario health system (Developing Ontario’s dementia strategy 2016).
Though there is currently no cure for AD, there are treatments that alleviate symptoms and may delay the progression of the disease (Weller and Budson 2018). These treatments are most effective when the disease is diagnosed early (Duncan and Siegal 1998; Richards et al. 2015) Genetic testing, using either targeted gene panels, exome, or genome sequencing, is one way to do this. Genetics are acknowledged as an important risk factor in the development of AD with approximately 5–10% of cases having an inheritable genetic component (Garcia and Bustos 2018; Guerreiro et al. 2014; Bertram and Tanzi 2005). With recent advancements in deoxyribonucleic acid (DNA) sequencing technologies, genetic sequencing is now being increasingly applied in many clinical settings, including AD diagnosis (Richards et al. 2015; Jain et al. 2019). Genetic sequencing helps clinicians identify AD cases with a genetic component, facilitating earlier identification and minimizing the probability of misdiagnosis, allowing for timely treatment with the potential to improve the quality of life for patients (Duncan and Siegal 1998; Weimer and Sager 2009). Additionally, genetic sequencing enables clinicians to identify individuals who are at a higher risk of developing the disease, based on genetic variants, but who have not yet developed AD. These patients can be closely followed to ensure timely diagnosis and treatment.
Genetic testing is not yet widely used in Ontario. Physicians typically only recommend genetic testing with specific targeted gene panels to a small subset of patients, namely those who have received a prior clinical diagnosis of AD with some indication of genetic factors of the disease such as a family history of AD. For these individuals, testing may confirm the diagnosis or enable a better understanding of the disease trajectory and future planning (Liu et al. 2015). Currently, AD-related diagnostic targeted gene sequencing is conducted out-of-country (OOC) using LifeLabs’ AD panel, which tests seven genes and is processed in Germany (Farhan et al. 2016; Alzheimer dementia and dementia panel 2019). OOC testing has uncertain clinical benefits and costs up to thousands of dollars. Furthermore, approval from the Ministry of Health (MOH) is required to order the tests. This makes it challenging for physicians to recommend testing to a wider patient base (Expert consultation: Dr. Martin Somerville, October 2019; MOH representative, November 2019). It has been argued that the limited application of genetic testing due to these factors skews the reported incidence rates and may lead to selection bias, resulting in an underreporting of the biologically accurate associations that can be identified through genetic testing (Farhan et al. 2016).
The Ontario Neurodegenerative Disease Research Initiative (ONDRI) is a multi-platform, longitudinal, observational cohort study characterizing multiple neurodegenerative diseases that developed an alternative to OOC testing (Farhan et al. 2016). This alternative, called ONDRISeq, is a next-generation sequencing-based panel that targets 80 genes associated with multiple neurodegenerative diseases, including 21 AD-specific genes (Farhan et al. 2016). ONDRISeq can be conducted in Ontario with existing apparatus and personnel trained in genetic sequencing (Farhan et al. 2016). ONDRISeq is more comprehensive and costs less per test than OOC testing; however, this local, more comprehensive, and less expensive alternative, by reducing a barrier to access together with increased awareness of availability, might increase referrals for targeted gene sequencing. The increased referrals, despite the reduced cost of each test, could pose a higher cost burden to the healthcare system due to a higher volume of earlier and prolongated treatment (Expert consultation: Dr. Martin Somerville, October 2019; ONDRI Taskforce, October 2019). Therefore, it is imperative to determine whether additional investment in the early detection of AD through genetic testing is cost-effective from the MOH’s perspective. The primary objective of this economic evaluation is to estimate the costs and health benefits associated with implementing ONDRISeq in Ontario to identify genetically indicated AD, relative to the status quo (i.e., OOC testing) using a cost-utility analysis.
Methods
A cost-utility analysis was conducted using a Markov model, built in TreeAge Pro®, that allowed accounting for disease progression over time across different health states (Zhang et al. 2019).
Target population, perspective, time horizon, and discounting
The target population was a hypothetical cohort of 65-year-old patients at risk of AD in Ontario, as modelled in previous economic evaluations of AD (Lee et al. 2017). At the start of the model, a proportion of patients were expected to have undiagnosed AD while the rest were at risk of developing the disease. Individuals with pathogenic variants were considered to have a higher risk of developing AD. The evaluation was conducted from the Ontario publicly funded healthcare payer’s perspective (MOH). The time horizon was 25 years, with annual Markov cycles. A within-cycle correction was employed to estimate cumulative outcomes across discrete-time steps. All costs and outcomes were discounted at a rate of 1.5% per annum as per the Canadian Agency for Drugs and Technologies in Health (CADTH) Guidelines for Economic Evaluations (Guidelines for the Economic Evaluation of Health Technologies 2017).
Intervention and comparators
This evaluation compared ONDRISeq to the status quo in Ontario, OOC testing. Though ONDRISeq assesses 80 genes clinically associated with a range of neurodegenerative disorders including AD, PD, amyotrophic lateral sclerosis, frontotemporal dementia, and cerebrovascular disease, this evaluation focused on ONDRISeq’s ability to identify AD through 21 AD-related genes (Farhan et al. 2016). Currently, AD-related genetic sequencing for Ontarians is conducted using LifeLabs’ AD panel, which tests seven genes and is processed in Germany (Alzheimer dementia and dementia panel 2019).
Model structure
The model started with a decision tree that determined whether or not patients enrolled in genetic sequencing testing. For simplicity, the model assumed that patients who participated in the first cycle were not given a chance to enroll again. Patients then entered a Markov model. After the first cycle, the Markov model followed patients based on whether they were diagnosed and started treatment. The Markov model (Fig. 1) accounts for the disease progression of the average 65-year-old patient either with existing AD (left branch) or at risk of developing it (right branch).
Fig. 1.
Markov model following a cohort of 65-year-old Ontarians with (AD) or without (No AD) clinically diagnosed AD who undergo genetic sequencing via ONDRISeq or OOC
Patients entered the model with or without the disease as determined by the prevalence rate of AD in Ontario. A proportion of these patients (10%) (Guerreiro et al. 2014) were expected to have a genetic mutation associated with AD that could be identified by OOC testing or ONDRISeq. The uptake rate was modelled to account for the proportion of patients who were invited and decided to undergo testing. This rate determines the extent to which screening programs are effective at enrolling patients. Some inputs were based on expert opinion. This was gathered by consulting with a team of experts including fifteen ONDRI partner clinicians and a Senior Program Consultant at the Ministry of Health Laboratories and Genetics branch. Based on this expert elicitation, uptake was expected to be higher for ONDRISeq (20%), compared to OOC testing (10%), due to an overall increased capacity, faster test processing, and reduced barriers to physicians in requesting the tests. The following health states were modelled: mild AD (diagnosed or undiagnosed), moderate AD (diagnosed or undiagnosed), severe AD (always diagnosed), and death. Patients with undiagnosed AD at the beginning of the model and a genetic mutation had a higher probability of being diagnosed by genetic sequencing at early disease stages compared to those without a genetic mutation. Patients who were diagnosed at any stage were assumed to start treatment, which slowed their disease progression by half, as modelled by Lee et al. (Lee et al. 2017). A risk of dying was modelled at all stages of the Markov process, with the degree of risk depending on whether or not they had AD and increasing with disease severity.
Patients without the disease also had a chance to participate in genetic sequencing; those with an AD-related genetic mutation were expected to be at a higher risk of developing AD. Patients without AD had a probability of developing the disease based on their genetic risk and the base case annual AD incidence rate.
Model inputs
Prevalence, incidence, and mortality rates of AD were estimated from dementia rates in the Ontario population (Aging with Confidence 2017; Developing Ontario’s dementia strategy 2016) Additional inputs related to disease management, utility estimates, transition probabilities, and treatment efficacy rates were retrieved from a cost-effectiveness analysis conducted by Lee et al. in 2017 (Lee et al. 2017). ONDRISeq was expected to be more sensitive (sensitivity = 1) than OOC testing (sensitivity = 0.86) due to a more comprehensive gene panel (Expert consultation: Dr. Martin Somerville, October 2019; ONDRI Taskforce, October 2019). All model inputs are summarized in Table 1. Incremental net monetary benefits (INMBs) were estimated to evaluate cost-effectiveness considering a base case threshold (λ) of $50,000 per QALY (Ochalek et al. 2018; Woods et al. 2016). The alternative with the highest net monetary benefit represented the cost-effective option at that specific threshold.
Table 1.
Base case model inputs, ranges for sensitivity analyses (with distribution shape), and sources
| Parameter | Value | Minimum value | Maximum value | Distribution shape | Source |
|---|---|---|---|---|---|
| Cost of clinical diagnosis | $678 | $366 | $879 | Gamma | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Annual cost of patients with no disease (follow-up) | $35,352 | $25,481 | $44,551 | Gamma | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Annual cost of mild disease (includes homecare) | $35,352 | $25,481 | $44,551 | Gamma | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Annual cost of moderate disease | $49,617 | $36,677 | $58,583 | Gamma | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Annual cost of severe disease | $88,195 | $73,000 | $104,000 | Gamma | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Annual cost of treatment | $4679 | $2000 | $6000 | Gamma | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Cost of ONDRISeq | $340 | $0 | $1500 | Gamma | Farhan et al. (Liu et al. 2015) |
| Cost of out-of-country genetic testing | $1,500 | $0 | $2000 | Gamma | LifeLabs Web site (Alzheimer dementia and dementia panel 2019) |
| Annual incidence among high-risk patients | 10% | 30% | 60% | Beta | Estimated based on Government of Ontario data (Aging with Confidence 2017; Developing Ontario’s dementia strategy 2016) |
| Annual incidence among low-risk patients | 5% | 0% | 29% | Beta | Estimated based on Government of Ontario data (Aging with Confidence 2017; Developing Ontario’s dementia strategy 2016) |
| Transition probability from mild to moderate AD without treatment (annual) | 27% | 15% | 35% | Beta | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Transition probability from moderate to severe AD without treatment (annual) | 32% | 20% | 40% | Beta | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Treatment efficacy (reduction of disease progression) | 0.5 | 0.253 | 1 | Beta | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Utility of no disease | 0.71 | 0.6 | 0.8 | Beta | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Utility of mild AD | 0.68 | 0.5 | 0.8 | Beta | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Utility of moderate AD | 0.54 | 0.4 | 0.6 | Beta | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Utility of severe AD | 0.37 | 0.25 | 0.45 | Beta | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Uptake rate of out-of-country testing | 10% | 0% | 40% | Triangular | Expert elicitation from ONDRISeq task force and Dr. Somerville |
| Uptake rate of ONDRISeq | 20% | 0% | 40% | Triangular | Expert elicitation from ONDRISeq task force and Dr. Somerville |
| Prevalence of mild AD | 0.70 | 0 | 0.7 | Beta | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: Canada. 2017) |
| Prevalence of moderate AD | 0.28 | 0 | 0.28 | Beta | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Prevalence of severe AD | 0.02 | 0 | 0.02 | Beta | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Prevalence of AD among 65-year-olds | 0.05 | 0 | 0.5 | Beta | ICES (Djalalov et al. 2012) |
| Sensitivity of ONDRISeq | 1 | 0 | 1 | NA | Expert elicitation from ONDRISeq task force |
| Specificity of ONDRISeq | 1 | 0 | 1 | NA | Expert elicitation from ONDRISeq task force |
| Sensitivity of out of country testing | 0.84 | 0 | 1 | NA | Expert elicitation from ONDRISeq task force |
| Specificity of out of country testing | 1 | 0 | 1 | NA | Expert elicitation from ONDRISeq task force |
| Sensitivity of clinical diagnosis for AD among patients with mild disease | 0.54 | 0.46 | 0.62 | NA | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Sensitivity of clinical diagnosis for AD among patients with moderate disease | 0.86 | 0.80 | 0.92 | NA | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Specificity of clinical diagnosis | 0.84 | 0.80 | 0.92 | NA | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: Canada. CADTH Methods and Guidelines. 2017) |
| Baseline probability of death | 0.01 | 0 | 0.03 | Beta | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Multiplier of mortality for mild AD | 2.92 | 1.50 | 5.00 | NA | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Multiplier of mortality for moderate AD | 3.85 | 2.00 | 8.00 | NA | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Multiplier of mortality for severe AD | 9.52 | 7.00 | 12.00 | NA | Lee et al. (Guidelines for the Economic Evaluation of Health Technologies: 2017) |
| Proportion of patients with genetic mutation (at high risk) | 10% | 0% | 40% | Beta | Expert elicitation from ONDRISeq task force |
All values presented in this table are adjusted for inflation and purchasing power parity
Costs
This economic evaluation included direct health care costs. Each health state had an associated cost related to genetic screening, clinical diagnosis, and treatment. Data on the cost of the ONDRISeq panel was retrieved from a publication on ONDRISeq and next-generation sequencing (Farhan et al. 2016). The cost of OOC genetic testing was retrieved through the LifeLabs Web site (Alzheimer dementia and dementia panel 2019). Average costs for OOC testing were corroborated by a representative from the MOH (Personal communication, November 2019). Treatment costs were specific to disease severity and were bundled to include follow-up, homecare, long-term care, medical visits, and laboratory/imaging fees (Lee et al. 2017). All costs were converted to 2019 Canadian dollars using the inflation adjustment factor for the consumer price index from the Bank of Canada (Armstrong 2018) and purchasing power parity from the Organization for Economic Co-operation and Development (Purchasing power parities (PPP)Paris, France: 2018).
Key model assumptions
The model assumed that everyone was eligible for genetic sequencing but that clinicians did not recommend this testing to all patients suspected of having AD. It further assumed that clinicians would refer more patients for genetic testing with ONDRISeq than the OOC alternative and that patients are only given one opportunity to undergo genetic testing (decision node). ONDRISeq was assumed to be more sensitive than OOC testing at identifying AD with a genetic component, with genetic testing improving clinical diagnosis. Finally, the model assumed AD progresses from mild to moderate to severe disease: patients cannot transition from mild to severe disease in the same cycle nor can they revert to a less severe disease state; however, they can remain in any health state for multiple study cycles.
Sensitivity analyses
We ran sensitivity analyses to assess the robustness of our model and to examine the effect of specific parameters on the main outcomes. Probabilistic distributions were assigned to each model input based on recommendations by Briggs et al. (Briggs et al. 2006) (Table 1) to conduct a probabilistic sensitivity analysis (PSA). Inputs with expected correlation (sensitivity and specificity estimates) were not varied in the PSA. Convergence was assessed after 10,000 Monte Carlo iterations. Cost-effectiveness acceptability curves were estimated to determine the robustness of the results across different cost-effectiveness thresholds. Furthermore, we conducted a univariate sensitivity analysis, changing one parameter at a time while keeping all the other parameters constant at their base case (deterministic) value to examine their effect on incremental costs, incremental effectiveness, as measured in QALYs, and cost-effectiveness (as measured by INMBs). A threshold analysis was conducted to understand how the cost-effectiveness estimates changed when varying the cost per test for ONDRISeq and the ONDRISeq uptake rate simultaneously. These factors were hypothesized to have the largest impact on the cost-effectiveness of ONDRISeq over the alternative.
Scenario analysis
A scenario analysis was conducted to evaluate the cost-effectiveness of ONDRISeq relative to a new alternative with no genetic screening for AD (i.e., no screening). This alternative was tested as a scenario analysis, as OOC testing better represents the status quo in Ontario. Deterministic and probabilistic results were computed, and cost-effectiveness acceptability curves were estimated.
Validation
The model was validated iteratively throughout its development according to CADTH Guidelines (Guidelines for the Economic Evaluation of Health Technologies, 2017). Face validity was ensured by the active participation and judgment of content experts throughout the process. Furthermore, the modelling exercise was validated by testing extreme and zero values, as well as with univariate sensitivity analyses. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist was employed to follow the recommended structure of economic evaluations (Supplemental File 1) (Husereau et al. 2013).
Results
Base case scenario
Results for the deterministic base case are presented per patient and summarized in Table 2. Screening for AD with local ONDRISeq was cost-saving compared to OOC testing in Ontario. ONDRISeq yielded a lower total cost than the OOC alternative, though there was a minimal difference in QALYs between alternatives and is expected to save $53 per patient. Considering a base case cost-effectiveness threshold of $50,000 per additional QALY, the mean INMB of ONDRISeq compared to OOC testing was estimated at $127.49.
Table 2.
Deterministic results for ONDRISeq versus out-of-country genetic testing
| Total cost (CAD) per patient | Total QALYs per patient | Incremental cost (ONDRISeq—OOC) per patient | Incremental QALYs (ONDRISeq—OOC) per patient | Incremental cost- effectiveness ratio per patient | Incremental net monetary benefit (at $50,000 per QALY; CAD) per patient | |
|---|---|---|---|---|---|---|
| OOC | $324,058 | 10.801 | ||||
| ONDRISeq | $324,005 | 10.802 | − $53.56 | 0.0014 | Dominates OOC | $127.49 |
Sensitivity analyses
-
Probabilistic sensitivity analysis
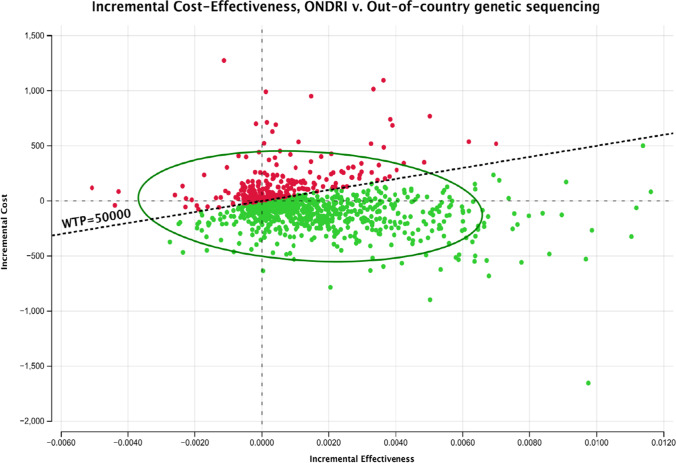
Results of a multivariate PSA suggested that ONDRISeq was expected to represent a mean of $232 in cost savings and a mean increase of 0.002 QALYs compared to OOC testing. The 10,000 iterations are summarized in the incremental cost-effectiveness scatterplot (Fig. 2). The cost-effectiveness acceptability curves suggested that, at a threshold of $50,000 per QALY, ONDRISeq dominated the OOC alternative in 75.5% of 10,000 iterations. Furthermore, ONDRISeq had a higher probability than OOC testing of being cost-effective at any threshold.
-
Univariate analyses
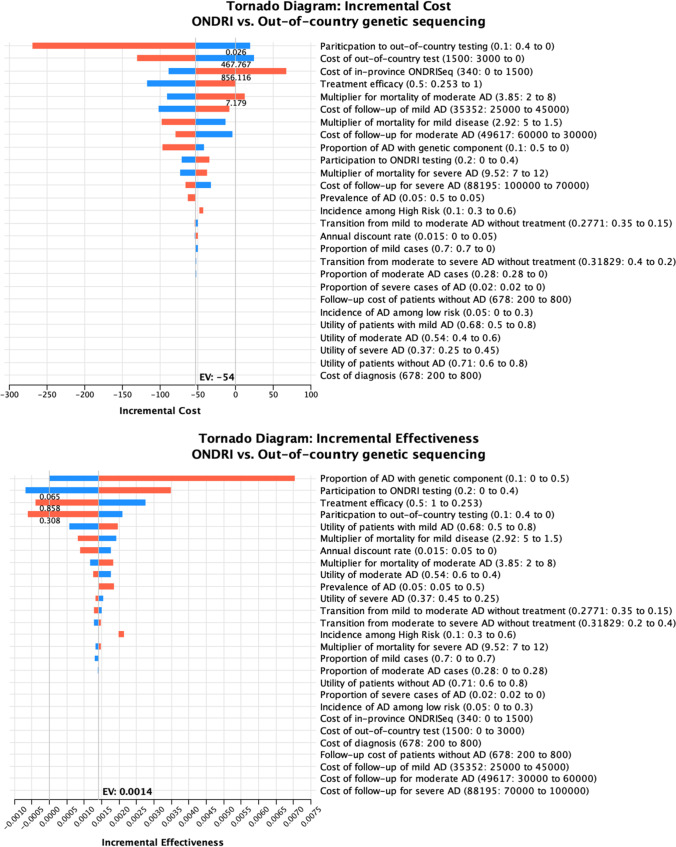
The first univariate analysis evaluated the effect of individual model inputs on the incremental cost of OOC testing versus ONDRISeq. The incremental cost was most sensitive to the ONDRISeq and OOC uptake rates and the unit cost of genetic tests (Fig. 3).
The second sensitivity analysis assessed the effect of individual model parameters on the effectiveness outcomes. The incremental QALYs were most sensitive to the genetic sequencing (OOC and ONDRISeq) uptake rates, treatment efficacy, and the proportion of high-risk patients (i.e., patients with a genetic mutation associated with AD) (Fig. 3).
A final sensitivity analysis determined overall value for money (i.e., cost-effectiveness) was most significantly impacted by the risk of AD in the population, and the per-test cost of ONDRISeq. The higher the risk of AD in the population, the higher the net monetary benefit of ONDRISeq relative to OOC testing. Furthermore, a sufficiently high cost per test (i.e., $1600 or greater) would result in a higher net monetary benefit for OOC testing relative to ONDRISeq making OOC testing the cost-effective alternative at a $50,000 per QALY threshold (Fig. 3).
-
Threshold analysis
ONDRISeq was expected to remain cost-effective relative to OOC testing, regardless of cost, given ONDRISEq uptake rates of 17% or less. At higher uptake rates (> 80% ONDRISeq uptake rate), ONDRISeq is only expected to be cost-effective if the cost per test remains below $900, given everything else remains constant.
Fig. 2.
Incremental cost-effectiveness (ONDRISeq vs. out of country). The scatterplot summarizes the incremental costs and QALYs of ONDRISeq relative to OOC (total of 10,000 probabilistic iterations). Observations below the $50,000 threshold (i.e., green plots) represent iterations of the model in which ONDRISeq represented the cost-effective alternative, relative to out-of-country testing. The oval represents the 95% confidence ellipse, which encloses 95% of the iterations of the probabilistic sensitivity analyses
Fig. 3.
Tornado diagram—incremental costs and QALYs. Tornado diagrams depicting the effect of varying the values of inputs on the incremental cost, QALYs, and net monetary benefits of ONDRISeq vs. OOC genetic sequencing. Blue and red bars represent low and high values for individual parameters, respectively. EV expected value, NMB net monetary benefit, WTP willingness to pay
Scenario analysis
The deterministic results suggest that ONDRISeq costs an additional $19 per patient, relative to “no screening” (total costs = $324,005 and $323,986, respectively) and represents an additional 0.0021 QALYs per patient (ICER = $8920). Therefore, at a cost-effectiveness threshold of $50,000 per additional QALY, ONDRISeq is expected to be cost-effective relative to “no screening.” The probabilistic sensitivity analysis estimated that ONDRISeq had a higher probability of being cost-effective relative to “no screening,” throughout 10,000 iterations, at cost-effectiveness thresholds above $22,000 per additional QALY.
Discussion
Summary of results
Our evaluation showed ONDRISeq genetic testing for AD was cost-saving compared to OOC testing. In univariate sensitivity analyses, the ONDRISeq and OOC uptake rates had the largest overall impact on costs. On the other hand, the proportion of high-risk patients had the largest impact on the incremental QALYs. ONDRISeq was cost-effective relative to “no screening” for cost-effectiveness thresholds over $22,000 per additional QALY.
Increasing uptake rates in genetic sequencing are associated with earlier diagnosis of AD. However, the total cost of screening is expected to increase substantially as more patients are referred. As such, ONDRIseq could become more expensive than OOC testing with an uptake rate of over 80% (compared to a base case rate of 10% for OOC). Additionally, the per-test cost of ONDRISeq had a considerable effect on the expected total cost. Although the base case assumed a cost of $340 per test (Farhan et al. 2016), a threefold increase (i.e., $1000 per test) would represent a higher total cost for ONDRISeq compared to OOC.
The overall health differences between the two alternatives were relatively small as, with the current treatment options, early identification of the disease does not avoid the progression of and eventual mortality due to AD. Treatment options are limited and most likely to be effective at the initial stages of the disease; therefore, the benefits of genetic testing lie predominantly in early diagnosis of disease, enabling timely treatment, and research. Further, the implementation of ONDRISeq will result in an overall reduction in testing costs, based on the available costing information, making it relatively more affordable compared to the OOC status quo.
This study is the first, to our knowledge, to assess the cost-effectiveness of two genetic testing alternatives for AD in Canada. A 2012 study by Djalalov and colleagues explored the cost-effectiveness of genetic testing for the apolipoprotein e4 allele (one of the genes associated with AD) and preventative treatment in Ontarians with amnestic mild cognitive impairment (who are at increased risk for developing AD) compared to the standard of care (Djalalov et al. 2012). Testing and preventative treatment among this population were determined to be cost-effective in a little more than half of their model scenarios with a willingness to pay threshold of $50,000 (Djalalov et al. 2012) Similar to our findings, the cost-effectiveness was influenced most strongly by the effectiveness of AD treatments. This suggests that the cost-effectiveness of genetic testing in individuals with or at risk of AD is heavily dependent on the effectiveness of treatment. Previous economic evaluations have estimated the value for money of early AD treatment options (Green et al. 2019; Ito et al. 2021) However, these studies have usually failed to incorporate risk factors based on genetic and biomarker profiles. Therefore, work on developing and refining treatments, particularly those tailored based on genetic risk factors, is warranted.
What this study adds
Genetic testing is one of the fastest growing segments in laboratory diagnostics and is a significant contributor to healthcare costs in Ontario. From April 1, 2013, to March 31, 2014, approximately $55.1 million was spent on Ontario-based genetic testing and $24 million on OOC genetic testing (Sullivan et al. 2015). There has been a movement on the part of the Ontario government to repatriate certain types of genetic testing to increase the provincial capacity; however, they have been less clear about creating a strategic plan to this effect (). The Genetic Testing Advisory Committee, which investigated genetic testing in Ontario from 2014 to 2016, suggested that “a full study of test costs across the sector would inform a rationalization of the test menu, and would also support ongoing updates to the Schedule of Benefits” (Sullivan et al. 2015). This evaluation is a first step towards informing the discussion of the costs and health benefits of neurodegenerative disease testing supported by the MOH and the potential savings of repatriating AD testing to Ontario.
Policy implications
This research is positioned to inform the MOH’s decisions regarding the repatriation of genetic testing for AD and other dementias. As OOC genetic testing can be quite costly, repatriating genetic testing can be a cost-effective decision, provided the expertise and capacity exist or can be created within Ontario’s laboratories.
Limitations
Our study is not without its limitations. First, ONDRISeq is designed to screen for multiple neurodegenerative diseases which can be examined together. However, we limited the scope of this evaluation to AD, for simplicity. Many of the neurodegenerative diseases for which ONDRISeq tests co-occur in the general population. Testing for multiple neurodegenerative diseases with a single test could lead to rectifying misdiagnoses, meaning the benefits identified by this evaluation may be underestimated. Conversely, testing for multiple neurodegenerative diseases at once could lead to more false negatives, leading to undue distress for the patient. Second, the genetic testing was modeled as a one-time decision at 65 years for the at-risk Ontario population. In practice, it is possible that genetic testing could be repeated throughout an individual’s life to account for the potential impact of epigenetics; however, with limited and highly variable data available from clinicians, there was insufficient information to model this alternative. We relied on existing comparable literature to inform our model input parameters. Most transition probabilities and clinical inputs were retrieved from one of the few robust and comprehensive economic evaluations of AD (Lee et al. 2017) that could be generalizable to our study. Even though there was parameter uncertainty, our base case findings were confirmed to a large extent by the comprehensive sensitivity analyses conducted. Univariate and probabilistic sensitivity analyses were employed to evaluate the uncertainty around key model parameters, such as the accuracy of genetic testing, and the cost of OOC testing, that were based on expert elicitation. Additionally, these results are specific to a health system perspective. Conducting the evaluation from a societal perspective would allow accounting for costs borne by patients and their families (i.e., productivity losses, out-of-pocket and caregiver costs). Nonetheless, we expect the results to further favour in-province testing when considering a societal perspective, due to its capacity to delay disease progression and reduce the high indirect costs associated with severe AD. Finally, this is a static model that followed the study population until their death. There are both age- and sex-linked differential risks of AD, which may alter the findings presented here; however, there was insufficient data available to model this differential risk.
Next steps—further research
The focus of this evaluation was on AD; however, AD is only one of the many neurodegenerative diseases prevalent in the aging population that can be tested for with genetic screening. Future research should explore if ONDRISeq as a complete 80-gene panel is more effective, both clinically and economically than the array of single disease OOC genetic tests that would be necessary to gain the same results. Additionally, a better understanding of the set-up and scaling costs of ONDRISeq is of particular interest, particularly as it relates to the capacity for this kind of testing facility within Ontario and the related impact on the MOH allocated budget. Further, considering that the results were highly sensitive to the cost of OOC testing, developing more comprehensive study designs could allow identifying more accurate estimates (e.g., activity-based costing studies).
Finally, future research can be conducted to explore further benefits associated with genomic testing for AD, which are not considered in this evaluation. These include non-health benefits, such as the ability of the patients and their relatives to make informed decisions (e.g., reproductive, career, or lifestyle-related) (Using cost-effectiveness analysis to quantify the value of genomic-based diagnostic tests: recommendations for practice and research. 2017). Value of information analyses and economic evaluations conducted from a broader societal perspective can be employed to better understand the trade-offs between costs, health and non-health benefits (Using cost-effectiveness analysis to quantify the value of genomic-based diagnostic tests: recommendations for practice and research. 2017).
Conclusion
Currently, there are limited treatment options available for individuals with neurodegenerative diseases. These treatment options provide temporary relief of symptoms, may delay disease progression, and are most effective in the early stages of the disease. Genetic testing to confirm a diagnosis may provide an opportunity for earlier diagnosis and more timely treatment, slowing the progression of the disease. Furthermore, these tests may allow for a better understanding of the projected trajectory of the disease and allow patients and families to better prepare for the future. When identified early, informed individuals can make decisions about their lifestyle that may delay the onset of symptoms and address symptoms that do occur with treatment options early.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dr. Martin Somerville, the ONDRI scientists and clinicians (ondri.ca), and representatives from the MOH for contributing their expertise to this project. This research was conducted with the support of the Ontario Brain Institute, an independent nonprofit corporation, funded partially by the Ontario government. The opinions, results and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred.
Author contribution
NI, DT, and SM designed the study, analyzed the results, and drafted this manuscript. RHH and BC consulted on study design, provided feedback and guidance on analyses, and critically reviewed the manuscript. AAD, JFR, and RAH were consulted on the application of ONDRISeq and provided background on genetic concepts. All authors read and approved the final manuscript.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nicolas Iragorri, Email: nicolas.iragorri@mail.utoronto.ca.
Danielle Toccalino, Email: danielle.toccalino@mail.utoronto.ca.
Sujata Mishra, Email: sujata.mishra@mail.utoronto.ca.
Brian CF. Chan, Email: brian.chan@uhn.ca
Allison A. Dilliott, Email: allison.dilliott@mcgill.ca
John F. Robinson, Email: robinson@robarts.ca
Robert A. Hegele, Email: hegele@robarts.ca
Rebecca Hancock-Howard, Email: rebecca.hancock@utoronto.ca.
References
- Aging with Confidence (2017) Ontario’s Action Plan for Seniors. Toronto, Ontario: Government of Ontario 1–39
- Alzheimer dementia and dementia panel (2019) LifeLabs Genetics [Available from: https://www.lifelabsgenetics.com/search-details/?id=12828&type=CN&prov=on accessed November 14 2019
- Armstrong RA. Visual problems associated with traumatic brain injury. Clin Exp Optom. 2018;101(6):716–726. doi: 10.1111/cxo.12670. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. The genetic epidemiology of neurodegenerative disease. J Clin Invest. 2005;115(6):1449–1457. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs A, Sculpher M, Claxton K. (2006) Decision modelling for health economic evaluation: OUP Oxford
- Chin JH, Vora N. The global burden of neurologic diseases. Neurology. 2014;83(4):349–351. doi: 10.1212/wnl.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Developing Ontario’s dementia strategy (2016) a discussion paper. Toronto, Ontario: Ministry of Health and Long Term Care 1–54
- Diagnostic testing: molecular diagnostics Ottawa, Ontario: Children’s Hospital of Eastern Ontario; [Available from: https://www.newbornscreening.on.ca/en/diagnostic-testing/molecular-diagnostics2020.
- Djalalov S, Yong J, Beca J, et al. Genetic testing in combination with preventive donepezil treatment for patients with amnestic mild cognitive impairment: an exploratory economic evaluation of personalized medicine. Mol Diagn Ther. 2012;16(6):389–399. doi: 10.1007/s40291-012-0010-7[publishedOnlineFirst:2012/11/29]. [DOI] [PubMed] [Google Scholar]
- Duncan BA, Siegal AP. Early diagnosis and management of Alzheimer’s disease. J Clin Psychiatry. 1998;59(Suppl 9):15–21. [PubMed] [Google Scholar]
- Dynacare selected to perform non-invasive prenatal testing in Ontario Brampton, Ontario: Dynacare; 2015 [Available from: https://www.dynacare.ca/news/dynacare-selected-to-perform-non-invasive-prenatal.aspx2020.
- Farhan SMK, Dilliott AA, Ghani M, et al. The ONDRISeq panel: custom-designed next-generation sequencing of genes related to neurodegeneration. NPJ Genom Med. 2016;1:16032. doi: 10.1038/npjgenmed.2016.32[publishedOnlineFirst:2016/09/21]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JC, Bustos RH. (2018) The Genetic Diagnosis of Neurodegenerative Diseases and Therapeutic Perspectives. Brain Sci 8(12) 10.3390/brainsci8120222 [published Online First: 2018/12/16] [DOI] [PMC free article] [PubMed]
- Green C, Handels R, Gustavsson A, et al. Assessing cost-effectiveness of early intervention in Alzheimer’s disease: an open-source modeling framework. Alzheimers Dement. 2019;15(10):1309–1321. doi: 10.1016/j.jalz.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Bras J, Hardy J, et al. Next generation sequencing techniques in neurological diseases: redefining clinical and molecular associations. Hum Mol Genet. 2014;23(R1):R47–53. doi: 10.1093/hmg/ddu203[publishedOnlineFirst:2014/05/06]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidelines for the Economic Evaluation of Health Technologies (2017) Canada. CADTH Methods and Guidelines. 4 ed. Ottawa, Ontario: Canadian 76
- Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049. doi: 10.1136/bmj.f1049. [DOI] [PubMed] [Google Scholar]
- Ito K, Chapman R, Pearson SD, et al. Evaluation of the cost-effectiveness of drug treatment for Alzheimer disease in a simulation model that includes caregiver and societal factors. JAMA Netw Open. 2021;4(10):e2129392. doi: 10.1001/jamanetworkopen.2021.29392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Andrade D, Donner E, et al. Development of criteria for epilepsy genetic testing in Ontario Canada. Can J Neurol Sci. 2019;46(1):7–13. doi: 10.1017/cjn.2018.341[publishedOnlineFirst:2018/11/14]. [DOI] [PubMed] [Google Scholar]
- Lee SAW, Sposato LA, Hachinski V, et al. Cost-effectiveness of cerebrospinal biomarkers for the diagnosis of Alzheimer’s disease. Alzheimers Res Ther. 2017;9(1):18. doi: 10.1186/s13195-017-0243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YT, Lee YC, Soong BW. What we have learned from the next-generation sequencing: Contributions to the genetic diagnoses and understanding of pathomechanisms of neurodegenerative diseases. J Neurogenet. 2015;29(2–3):103–112. doi: 10.3109/01677063.2015.1060972[publishedOnlineFirst:2015/06/11]. [DOI] [PubMed] [Google Scholar]
- Nussbaum RL, Ellis CE. Alzheimer's Disease and Parkinson's Disease. NEJM. 2003;348(14):1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- Ochalek J, Lomas J, Claxton K. (2018) Assessing health opportunity costs for the Canadian health care systems 2018
- Purchasing power parities (PPP) Paris, France: Organisation for Economic Co-operation and Development; 2018 [Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm#indicator-chart accessed November 29 2019
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30[publishedOnlineFirst:2015/03/06]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Canada (2017) Ontario [Province] and Canada [Country] (table). Census profile. 2016 Census. Statistics Canada Catalogue no. 98-316-X2016001. Ottawa. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E. Accessed 23 Nov2022
- Sullivan T, Gordon P, Minto S. (2015) Laboratory Services Expert Panel Review. Toronto, Ontario: Laboratory Services Expert Panel 91
- Using cost-effectiveness analysis to quantify the value of genomic-based diagnostic tests: recommendations for practice and research (2017). Genet Test Mol Biomarkers 12(21):705–716. 10.1089/gtmb.2017.0105 [DOI] [PubMed]
- Weimer DL, Sager MA. Early identification and treatment of Alzheimer’s disease: social and fiscal outcomes. Alzheimers Dement. 2009;5(3):215–226. doi: 10.1016/j.jalz.2009.01.028[publishedOnlineFirst:2009/04/11]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller J, Budson A (2018) Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Res 2018;7:F1000 Faculty Rev-161. 10.12688/f1000research.14506.1
- Woods B, Revill P, Sculpher M, et al. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–935. doi: 10.1016/j.jval.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lim CY, Maiti T, et al. Analysis of conversion of Alzheimer’s disease using a multi-state Markov model. Stat Methods Med Res. 2019;28(9):2801–2819. doi: 10.1177/0962280218786525[publishedOnlineFirst:2018/07/25]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.