Highlights
-
•
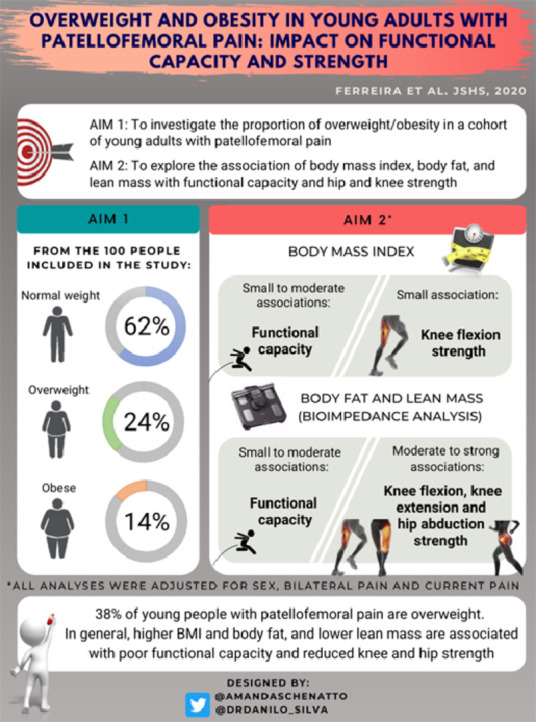
Thirty-eight percent of our cohort of young adults with patellofemoral pain were overweight or obese.
-
•
Higher body mass index was associated with poor functional capacity, but not with knee or hip strength.
-
•
Higher body fat was associated with poor functional capacity and reduced knee and hip strength.
-
•
Lower lean mass was associated with poor functional capacity and reduced knee and hip strength.
-
•
Body mass index, body fat, and lean mass should be considered in the assessment and management of young adults with patellofemoral pain.
Keywords: Body fat distribution, Body mass index, Patellofemoral pain syndrome, Torque
Abstract
Purpose
This study aimed to (a) investigate the proportion of overweight/obesity in a cohort of young adults with patellofemoral pain (PFP) and (b) explore the association of body mass index (BMI), body fat, and lean mass with functional capacity and hip and knee strength in people with PFP.
Methods
We included a mixed-sex sample of young adults (18−35 years old) with PFP (n = 100). Measurements for BMI, percentage of body fat, and lean mass (assessed by bioelectrical impedance) were obtained. Functional capacity was assessed by the Anterior Knee Pain Scale, plank test, and single-leg hop test. Strength of the knee extensors, knee flexors, and hip abductors was evaluated isometrically using an isokinetic dynamometer. The proportion of overweight/obesity was calculated based on BMI. The association between BMI, body fat, and lean mass and functional capacity and strength was investigated using partial correlations, followed by hierarchical regression analysis, adjusted for covariates (sex, bilateral pain, and current pain level).
Results
A total of 38% of our cohort had their BMI categorized as overweight/obese. Higher BMI was associated with poor functional capacity (ΔR2 = 0.06−0.12, p ≤ 0.001) and with knee flexion strength only (ΔR2 = 0.04, p = 0.030). Higher body fat was associated with poor functional capacity (ΔR2 = 0.05−0.15, p ≤ 0.015) and reduced strength (ΔR2 = 0.15−0.23, p < 0.001). Lower lean mass was associated with poor functional capacity (ΔR2 = 0.04−0.13, p ≤ 0.032) and reduced strength (ΔR2 = 0.29− 0.31, p < 0.001).
Conclusion
BMI, body fat, and lean mass should be considered in the assessment and management of young people with PFP because it may be detrimental to function and strength.
Graphical Abstract
1. Introduction
Patellofemoral pain (PFP) is characterized by insidious onset of diffuse anterior knee pain, exacerbated by activities that load the patellofemoral joint (e.g., running, climbing stairs, squatting).1 PFP has an estimated annual prevalence of 23% in the general population and up to 35% prevalence in sporting populations.2 PFP is associated with poor quality of life, poor physical health, and poor psychological health.3, 4, 5 Furthermore, there has been speculation that PFP is a precursor to patellofemoral osteoarthritis (OA).6, 7, 8
Numerous biomechanical, anatomical, and psychological factors have been linked to PFP.1,9, 10, 11 Specifically, impaired knee strength is a risk factor for people with PFP,12 and impaired functional capacity predicts poor outcomes for people with PFP after rehabilitation.13,14 Other potential factors associated with PFP that have received less attention are body composition measures. Findings from a systematic review indicates that young adults with PFP have greater body mass index (BMI)15 than pain-free controls. Greater BMI has also been reported as a clinical predictor of poor long-term outcomes in people with PFP.16 Despite compelling evidence indicating that high BMI is detrimental to people with PFP, its impact on functional capacity and strength in this population has never been explored. Moreover, other measures of body composition (e.g., body fat and lean mass), which seem to provide additional and more accurate implications of overweight and obesity on an individual's health status compared to BMI alone,17,18 have also never been explored in this population.
Overweight and obesity are associated with impaired functional capacity and knee strength in people with knee OA,18, 19, 20, 21, 22 with body fat and lean mass presenting a higher association (∆R² = 0.162 and ∆R² = 0.093, respectively) with impaired functional capacity than BMI (∆R² = 0.005).18 Although these findings are mostly related to the tibiofemoral joint (i.e., tibiofemoral OA), previous studies23 have reported that overweight and obesity also have deleterious effects on patellofemoral OA, likely via metabolic factors (i.e., increased leptin due to obesity has been associated with reduced patellar cartilage volume)24 and/or mechanical factors (i.e., increased loading by obesity may also affect patellar cartilage and its biomechanical properties).25,26 Additionally, studies have reported that a weight loss of 10% or higher led to clinically important improvements in pain, functional capacity, and knee strength in people with knee OA (both tibiofemoral and tibiofemoral plus patellofemoral OA).27, 28, 29, 30 However, as highlighted in a recent editorial, interventions targeting weight loss do not exist in the PFP literature.31 The detrimental effects of overweight and obesity have been extensively explored in several other musculoskeletal conditions.18,32, 33, 34 However, it remains underexplored in PFP. More research is needed to understand the impact of overweight and obesity on clinically important PFP outcomes, such as functional capacity and strength. Considering that overweight and obesity are modifiable factors, our findings could provide novel insights toward changing traditional rehabilitation of PFP.35 Therefore, the aims of our study were twofold: (a) to investigate the proportion of overweight and obesity in a cohort of young adults with PFP and (b) to explore the association of BMI, body fat, and lean mass with functional capacity and knee strength in young adults with PFP.
2. Methods
This cross-sectional study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology guideline recommendations.36 The study was approved by the University Ethics Committee (No. 1.484.129), and each participant provided informed written consent prior to data collection.
2.1. Recruitment
Participants were recruited from the community between October 2018 and November 2019 via advertisements at universities and fitness centers and via posts on social media. Participants were between 18 and 35 years of age. Eligibility criteria were assessed by a physiotherapist (with >7 years of experience in assessing people with PFP). Eligibility criteria included (a) anterior knee pain in at least one limb provoked by at least two of the following activities: running, walking, hopping, landing, squatting, negotiating stairs, kneeling, or prolonged sitting,1 (b) insidious onset of symptoms with a duration of at least 3 months, and (c) anterior knee pain in the previous month of at least 30 mm on a 100-mm visual analogue scale.37 Exclusion criteria included the following: a history of patellar subluxation, a history of surgery on any lower limb joint, the presence of meniscal injury38 or ligament instability,39 and self-reported back, hip, ankle, or foot pain. To control for potential carry-over effects from previous treatments that might influence the outcomes assessed, those who had received acupuncture, physiotherapy, or any other treatment for PFP during the preceding 6 months were also excluded from our study. Participants were asked to refrain from taking, in the 7 days prior to data collection, any medications and to avoid participating in any type of physical activity they were unaccustomed to.
2.2. Sample size calculation
An a priori sample size calculation was performed using G*Power Statistical Power Analysis Software V3.1 (Universität Düsseldorf, Düsseldorf, Germany). In our pilot study, with data drawn from 30 participants with PFP, BMI uniquely explained 4.8% of the variance in the single-leg hop test while the covariates explained 39.0%. We chose the single-leg hop test to power our study because it presented the smallest ΔR2 among all variables. We estimated that we would need at least 94 participants to detect a ∆R² of 0.048 with 80% of power and an α level of 0.05, using 4 predictor variables.
2.3. Overview of the experimental approach
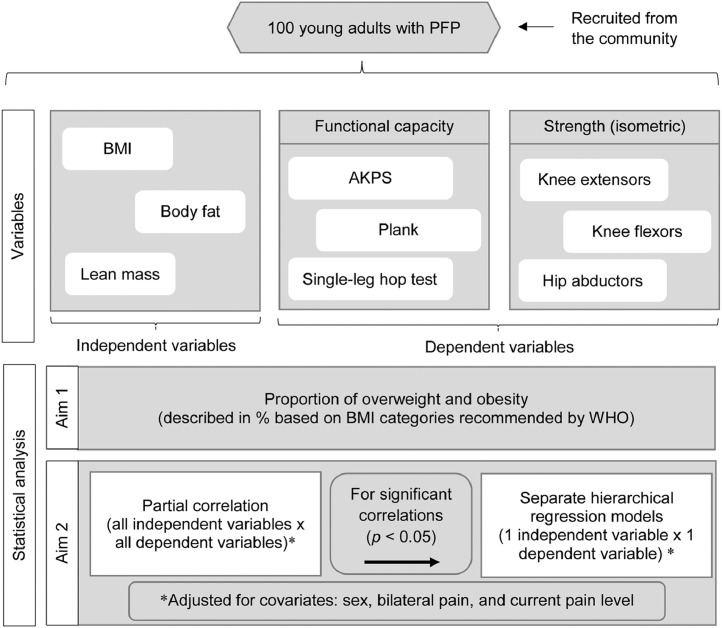
A total of 100 young adults with PFP were invited to attend a one-day assessment at the Sao Paulo State University. BMI, percentages of body fat and lean mass, functional capacity (measured by the Anterior Knee Pain Scale (AKPS), plank test, and single-leg hop test), and strength measures (peak isometric knee extension, knee flexion, and hip abduction torque) were obtained. After data collection completion, the proportion of overweight and obesity in our sample (based on BMI) was calculated and described as a percentage of the entire cohort (Aim 1). Then, we used partial correlation and hierarchical regression analysis to investigate the relationship of BMI, body fat, and lean mass with functional capacity and strength in young adults with PFP (Aim 2). All analyses were adjusted for covariates (sex, bilateral pain, and current pain level) on the basis of previously reported associations40, 41, 42 or plausible hypotheses. Fig. 1 shows in detail the experimental design of our study.
Fig. 1.
Flowchart describing the experimental approach. AKPS = Anterior Knee Pain Scale; BMI = body mass index; PFP = patellofemoral pain; WHO = World Health Organization.
2.4. Participant characteristics
Demographics (age, sex, height, and body mass) were obtained, along with duration of symptoms (months) and presence of bilateral pain. Height and body mass were assessed with participants wearing light clothing and no shoes. Body mass was measured to the nearest 0.1 kg, and height was measured to the nearest 0.1 cm using a calibrated scale with a stadiometer (Welmy 110 CH; Welmy®, Sao Paulo, Brazil). Body mass and height were used to calculate BMI (kg/m2).
Before data collection, all participants rated their current knee pain intensity on a 0−100 visual analogue scale, with 0 indicating no pain and 100 indicating the worst pain possible.37
2.5. Bioelectrical impedance analysis
Body fat and lean mass were measured using a bioelectrical impedance analyzer (Omron HBF 514C; Omron Healthcare Co., Kyoto, Japan). The device uses 8 electrodes in a tetrapolar arrangement and requires participants to stand on metal footpads in bare feet and hold a pair of electrodes fixed on the display unit, with arms extended in front of their chest. The manufacturers’ valid and reliable equations43 were used to predict body fat and lean mass (expressed as a percentage of total body mass). Due to the inclusion of a mixed-sex sample with wide ranges of body mass, body fat, and lean mass were reported as a percentage to facilitate the interpretation of results. Participants were instructed to avoid alcohol and caffeine consumption for 24 h prior to measurement, to avoid vigorous exercise for 12 h prior to measurement, and to avoid food and beverages for 2 h prior to measurement.
2.6. Functional capacity
Self-reported functional capacity was assessed by the AKPS. Objective functional capacity was assessed by the plank test and single-leg hop test in a randomized order. Data collection for all lower limb assessments was performed in the symptomatic leg or most symptomatic leg (in case of bilateral symptoms).5
The AKPS is a valid and reliable 13-item questionnaire used to assess functional capacity of people with PFP; the overall score ranges from 0 (maximum functional limitation) to 100 (no functional limitation).37
To perform the plank test, participants assumed a prone position supported by their forearms and feet, with the shoulders and elbows flexed at 90°. They were instructed to raise their pelvis from the floor, maintaining a straight line from head to ankles, and to hold this static position as long as possible. If necessary, verbal instructions were given to correct participants’ positioning. The evaluator ended the test when participants were no longer able to maintain the proper position after 2 warnings or when the participant ended it due to fatigue. Participants performed a single trial, and the test duration was recorded with a stopwatch.44
To perform the single-leg hop test, the participant stood on the tested leg with the heel positioned on a marked line and with the other leg lifted from the floor by flexing the contralateral knee. The participants were told to hop as far as possible, keeping their arms behind their back, taking off and landing on the same foot, and maintaining their balance for about 2 s after landing. The hop distance (cm) was measured, with a measuring tape that was affixed to the floor, from the heel in the starting position to the heel in the landing position. A hop was considered successful when the participant was able to maintain balance for at least 2 s after landing. A hop was considered unsuccessful when the participant touched the floor with the contralateral lower extremity, lost balance, pushed with the contralateral foot, or did not keep the hands behind the back. Each participant was given 3 practice trials before the test. Three successive trials were then recorded. One minute of rest was provided between each trial. The average value of the 3 trials was used for statistical analysis.5
Before the strength tests were administered, participants were given a minimum of a 10-min rest after performing the functional tests (plank test and single-leg hop test) in order to avoid pain summation and limit neuromuscular fatigue. Additionally, participants were asked to perform the next test only when they felt completely recovered from the previous one.
2.7. Knee and hip strength
After functional capacity assessments, maximal voluntary isometric contractions (MVICs) of the knee extensors, knee flexors, and hip abductors were measured using an isokinetic dynamometer (Biodex System 4 Pro; Biodex Medical Systems Inc., New York, NY, USA). Knee extensor and knee flexor strength was assessed (Biodex System 4 Pro; Biodex Medical Systems Inc.) in the same seating position, with the hips and non-tested knee flexed at 90°. Four straps were used to stabilize the trunk, pelvis, and the tested limb. The dynamometer's rotational axis was aligned with the lateral epicondyle of the femur, and the resistance pad was placed 5 cm above the lateral malleolus. The MVICs of the knee extensors and flexors were assessed with the tested limb at 60° of knee flexion.45
Hip abductor strength was assessed (Biodex System 4 Pro; Biodex Medical Systems Inc.) in a side-lying position, with the tested limb on top of the non-tested limb, in neutral hip flexion/extension and medial/lateral rotation alignment and with an extended knee. Four straps were used to stabilize the trunk and non-tested limb. The dynamometer's rotational axis was aligned with the hip joint center in the frontal plane of the tested limb, and the resistance pad was placed on the lateral aspect of the distal thigh, 5 cm above the patella. MVICs for the hip abductors were assessed with the tested limb at 30° of hip abduction.45
The testing order for the muscle groups was randomized. The assessor provided standardized verbal encouragement during contractions to elicit maximal effort. Participants performed 2 submaximal contractions of 6 s, with an interval of 30 s between trials for familiarization with each test position. Then, 3 maximal isometric contractions of 6 s, with an interval of 1 min between each trial, were performed in order to determine the MVIC for each muscle group. The highest peak torque (N·m) achieved in one of the 3 trials was used in the statistical analysis.45,46
2.8. Statistical analysis
Normality and variance homogeneity of data were tested using the Shapiro-Wilk and Levene tests, respectively. Duration of symptoms and results of the plank test were non-normally distributed; therefore, results of the plank test were log transformed before being included in the partial correlation and hierarchical regression analyses. Descriptive statistics were used to describe participant characteristics, body composition, functional capacity, and strength measures for the total sample. Descriptive statistics were also described according to BMI categories suggested by the World Health Organization (WHO). Normally distributed variables were reported as mean ± SD, and non-normally distributed variables were reported as median (interquartile range).
The first aim of our study was to determine the proportion of overweight and obesity in our sample of young adults with PFP. Overweight and obesity were defined based on the following BMI categories recommended by the WHO:47 participants with a BMI of <18.5 kg/m2 were categorized as underweight, 18.5 kg/m2 ≤ BMI < 25 kg/m2 were categorized as normal weight, 25 kg/m2 ≤ BMI < 30 kg/m2 were categorized as overweight, and a BMI of ≥30 kg/m2 were categorized as obese. The proportion for each category was presented as a percentage of the entire cohort. No participants were underweight.
We also compared demographics and participant characteristics, body composition, functional capacity, and knee and hip strength measurements across all 3 BMI categories by running a one-way analysis of variance or a Kruskal–Wallis test. Bonferroni's post hoc test for multiple pairwise comparisons was performed when overall differences were statistically significant (p < 0.05). Comparisons between BMI categories for bilateral pain and sex were made using χ2 tests, with pairwise multiple comparisons using Bonferroni correction of p values when overall differences were statistically significant (p < 0.05). These analyses are reported in Supplementary Table 1.
Our second aim was to explore the association of BMI, body fat, and lean mass with functional capacity and strength in young adults with PFP. We used partial correlation coefficients48,49 to determine the relationship between independent variables (BMI, body fat, and lean mass) and dependent variables (AKPS, plank test, single-leg hop test, and peak isometric strength of the knee extensors, knee flexors, and hip abductors). All partial correlation analyses were adjusted for the following covariates: (a) sex (previous studies have found sex differences in the clinical presentation of people with PFP40,41), (b) bilateral pain (our sample included participants with unilateral and bilateral symptoms; since it may influence the outcomes, we included bilateral pain as a covariate), and (c) current pain level (PFP is characterized by intermittent pain42 that may highly influence the outcomes for each participant individually). The classification of correlation was defined as small: r = 0.10−0.30, moderate: r = 0.31−0.50, and strong: r = 0.51−1.00.49 The variables that presented significant correlations (p < 0.05) were included in hierarchical regression models.
Separate hierarchical regression models were used to determine the unique association of each measure of BMI, body fat, and lean mass with each measure of functional capacity and strength that presented significant correlations. All regression models were also adjusted for covariates by first entering the covariates (sex, bilateral symptoms, and current pain level) into the hierarchical regression model (Model 1). Then, either BMI, body fat, or lean mass was added into the model (Model 2), which means that all changes in the results of the regression models, from the first step to the second step, were due to the insertion of the independent variable (either BMI, body fat, or lean mass).
An α level of 0.05 was set for all statistical tests. All analyses were performed using the PASW Statistics software (Version 18.0; SPSS Inc., Chicago, IL, USA).
3. Results
From October 2018 to November 2019, 100 young adults with PFP (60 females and 40 males) were enrolled in our study. Descriptive statistics for all variables are presented in Table 1.
Table 1.
Characteristics of study participants.
| Variable | All sample | BMI groups |
||
|---|---|---|---|---|
| Normal weight (BMI = 18.5 to 25 kg/m²) |
Overweight (BMI = 25 to 30 kg/m²) |
Obese (BMI ≥ 30 kg/m²) |
||
| Demographics | ||||
| n | 100 | 62 | 24 | 14 |
| Age (year) | 24.11 ± 4.83 | 23.30 ± 4.60 | 24.16 ± 3.77 | 27.57 ± 6.13 |
| Body mass (kg) | 72.10 ± 15.48 | 64.25 ± 8.14 | 76.15 ± 9.93 | 99.90 ± 13.53 |
| Height (cm) | 168.88 ± 8.42 | 168.95 ± 8.03 | 169.14 ± 9.97 | 168.10 ± 7.82 |
| BMI (kg/m²) | 25.36 ± 4.78 | 22.55 ± 1.65 | 26.94 ± 1.31 | 35.10 ± 3.77 |
| Bilateral pain (%) | 57.0 | 54.8 | 70.8 | 42.8 |
| Sex (female, %) | 60.0 | 59.7 | 50.0 | 78.6 |
| Worst pain level in the previous month (VAS) | 50.70 ± 20.79 | 48.62 ± 21.59 | 50.83 ± 17.29 | 59.64 ± 21.61 |
| Current pain level (VAS) | 17.06 ± 21.45 | 12.19 ± 19.08 | 17.50 ± 17.87 | 37.89 ± 25.54 |
| Duration of symptoms (month)a | 36 (12−96) | 36 (11−94) | 36 (12−96) | 54 (5−123) |
| Body composition measures | ||||
| Body fat−bioimpedance (%) | 31.13 ± 10.74 | 27.05 ± 8.91 | 32.54 ± 8.60 | 46.75 ± 5.44 |
| Lean mass–bioimpedance (%) | 31.36 ± 7.31 | 32.96 ± 7.45 | 31.51 ± 6.06 | 23.97 ± 3.30 |
| Functional capacity measures | ||||
| Functional capacity (AKPS) | 78.44 ± 10.23 | 80.37 ± 9.47 | 78.91 ± 9.57 | 69.07 ± 10.07 |
| Plank (s)a | 55 (34−90) | 60 (39−100) | 58 (46−89) | 22 (15−32) |
| Single-leg hop test (cm) | 90.11 ± 25.25 | 94.80 ± 23.61 | 93.89 ± 24.82 | 62.88 ± 14.78 |
| Strength measures | ||||
| Peak isometric knee extension (N·m) | 181.98 ± 62.87 | 178.70 ± 64.63 | 196.64 ± 63.65 | 171.42 ± 52.54 |
| Peak isometric knee flexion (N·m) | 99.80 ± 33.02 | 96.06 ± 34.03 | 112.50 ± 32.36 | 94.59 ± 25.02 |
| Peak isometric hip abduction (N·m) | 68.59 ± 23.74 | 69.38 ± 24.75 | 74.45 ± 22.74 | 55.08 ± 15.48 |
Note: Data are presented as mean ± SD unless otherwise stated.
Data are presented as median (interquartile ranges).
Abbreviations: AKPS = Anterior Knee Pain Scale; BMI = body mass index; VAS = visual analogue scale.
3.1. Proportion of overweight and obesity in young adults with PFP
Of the 100 participants included in our study, 62% were categorized as normal weight, 24% as overweight, and 14% as obese.
3.2. Correlation coefficient findings
The partial correlation coefficients between variables of interest are reported in Table 2. Significant negative correlations were found for BMI with AKPS, plank, and single-leg hop test (r = −0.28 to −0.41, small to moderate). And a significant positive correlation was found for BMI with knee flexion strength (r = 0.22, small). No significant correlations were found between BMI and knee extension and hip abductor strength. Significant negative correlations were found for body fat with AKPS, plank, single-leg hop test, and strength measures (r = −0.25 to −0.49, small to moderate). Significant positive correlations were found for lean mass with AKPS, plank, single-leg hop test, and strength measures (r = 0.22 to 0.57, small to strong).
Table 2.
Pearson correlation coefficients (r) among BMI, body fat percentage, lean mass percentage, functional capacity, and strength measures.
| Variables | BMI (kg/m²) | Body fat percentage | Lean mass percentage |
|---|---|---|---|
| AKPS | −0.28 (0.005)* | −0.25 (0.015)* | 0.22 (0.032)* |
| Planka | −0.34 (0.001)* | −0.37 (<0.001)* | 0.38 (<0.001)* |
| Single-leg hop test (cm) | −0.41 (<0.001)* | −0.46 (<0.001)* | 0.44 (<0.001)* |
| Peak isometric knee extension | 0.17 (0.087) | −0.43 (<0.001)* | 0.57 (<0.001)* |
| Peak isometric knee flexion | 0.22 (0.031)* | −0.40 (<0.001)* | 0.56 (<0.001)* |
| Peak isometric hip abduction | −0.07 (0.510) | −0.49 (<0.001)* | 0.57 (<0.001)* |
Note: Data were adjusted for sex, presence of bilateral pain, and current pain level (VAS), and presented as r (p).
Log transformed.
p < 0.05.
Abbreviations: AKPS = Anterior Knee Pain Scale; BMI = body mass index; VAS = visual analogue scale.
3.3. Regression models
The results of the hierarchical regression analyses are reported in Tables 3, 4, and 5. After adjusting for covariates, BMI significantly explained 6% of the variance in AKPS (p < 0.001), 9% of the variance in plank (p < 0.001), 12% of the variance in single-leg hop test (p < 0.001), and 4% of the variance in knee flexion strength (p = 0.030) (Table 3).
Table 3.
Hierarchical linear regression between BMI and functional capacity, and knee flexion strength.
| Dependent variable | Model | Independent variable | R² | ∆R² | ∆F | β (95%CI) |
|---|---|---|---|---|---|---|
| AKPS | 1 | Covariatesa | 0.20 | |||
| 2 | BMI | 0.26 | 0.06 | 8.29⁎⁎ | −0.57 (−0.96 to −0.17) | |
| Plankb | 1 | Covariatesa | 0.26 | |||
| 2 | BMI | 0.35 | 0.09 | 12.64⁎⁎ | −0.02 (−0.03 to −0.01) | |
| Single-leg hop test | 1 | Covariatesa | 0.30 | |||
| 2 | BMI | 0.42 | 0.12 | 18.94⁎⁎ | −1.90 (−2.76 to −1.03) | |
| Peak isometric knee flexion strength | 1 | Covariatesa | 0.07 | |||
| 2 | Body fat | 0.11 | 0.04 | 4.81* | 1.53 (0.14 to 2.92) |
Adjusted for sex, presence of bilateral pain, and current pain level.
Log transformed.
p < 0.05;
p < 0.01.
Abbreviations: 95%CI = 95% confidence interval; AKPS = Anterior Knee Pain Scale; BMI = body mass index.
Table 4.
Hierarchical linear regression between body fat and functional capacity, and strength measures.
| Dependent variable | Model | Independent variable | R² | ∆R² | ∆F | β (95%CI) |
|---|---|---|---|---|---|---|
| AKPS | 1 | Covariatesa | 0.20 | |||
| 2 | Body fat | 0.25 | 0.05 | 6.19* | −0.33 (−0.59 to −0.06) | |
| Plankb | 1 | Covariatesa | 0.26 | |||
| 2 | Body fat | 0.36 | 0.10 | 15.10⁎⁎ | −0.01 (−0.02 to −0.01) | |
| Single leg hop test | 1 | Covariatesa | 0.30 | |||
| 2 | Body fat | 0.45 | 0.15 | 25.03⁎⁎ | −1.41 (−1.97 to −0.85) | |
| Peak isometric knee extension strength | 1 | Covariatesa | 0.05 | |||
| 2 | Body fat | 0.23 | 0.18 | 21.81⁎⁎ | −2.60 (−3.71 to −1.49) | |
| Peak isometric knee flexion strength | 1 | Covariatesa | 0.07 | |||
| 2 | Body fat | 0.22 | 0.15 | 18.50⁎⁎ | −1.26 (−1.85 to −0.68) | |
| Peak isometric hip abductor strength | 1 | Covariatesa | 0.04 | |||
| 2 | Body fat | 0.27 | 0.23 | 30.43⁎⁎ | −1.12 (−1.53 to −0.72) |
Adjusted for sex, presence of bilateral pain, and current pain level.
Log transformed.
p < 0.05;
p < 0.01.
Abbreviations: 95%CI = 95% confidence interval; AKPS = Anterior Knee Pain Scale.
Table 5.
Hierarchical linear regression between lean mass and functional capacity, and strength measures.
| Dependent variable | Model | Independent variable | R² | ∆R² | ∆F | β (95%CI) |
|---|---|---|---|---|---|---|
| AKPS | 1 | Covariatesa | 0.20 | |||
| 2 | Lean mass | 0.24 | 0.04 | 4.73* | 0.58 (0.05 to 1.11) | |
| Plankb | 1 | Covariatesa | 0.26 | |||
| 2 | Lean mass | 0.37 | 0.11 | 16.24⁎⁎ | 0.03 (0.01 to 0.04) | |
| Single-leg hop test (cm) | 1 | Covariatesa | 0.30 | |||
| 2 | Lean mass | 0.43 | 0.13 | 22.14⁎⁎ | 2.69 (1.55 to 3.82) | |
| Peak isometric knee extension strength | 1 | Covariatesa | 0.05 | |||
| 2 | Lean mass | 0.36 | 0.31 | 46.43⁎⁎ | 5.02 (3.55 to 6.48) | |
| Peak isometric knee flexion strength | 1 | Covariatesa | 0.07 | |||
| 2 | Lean mass | 0.36 | 0.29 | 43.53⁎⁎ | 2.55 (1.78 to 3.32) | |
| Peak isometric hip abductor strength | 1 | Covariatesa | 0.04 | |||
| 2 | Lean mass | 0.35 | 0.31 | 45.02⁎⁎ | 1.88 (1.32 to 2.44) |
Adjusted for sex, presence of bilateral pain, and current pain level.
Log transformed.
p < 0.05;
p < 0.01.
Abbreviations: 95%CI = 95% confidence interval; AKPS = Anterior Knee Pain Scale.
After adjusting for covariates, body fat significantly explained 5% of the variance in AKPS (p = 0.015), 10% of the variance in plank (p < 0.001), 15% of the variance in single-leg hop test (p < 0.001), 18% of the variance knee extension strength (p < 0.001), 15% of the variance in knee flexion strength (p < 0.001), and 23% of the variance in hip abductor strength (p < 0.001) (Table 4).
After adjusting for covariates, lean mass significantly explained 4% of the variance in AKPS (p = 0.032), 11% of the variance in plank (p < 0.001), 13% of the variance in single-leg hop test (p < 0.001), 31% of the variance in knee extension strength (p < 0.001), 29% of the variance in knee flexion strength (p < 0.001), and 31% of the variance in hip abductor strength (p < 0.001) (Table 5).
4. Discussion
A total of 38% of our cohort had their BMI categorized as overweight or obese. Higher BMI and body fat, and lower lean mass, were associated with poor functional capacity, even after adjusting for confounders such as sex, presence of bilateral pain, and current pain level. Additionally, higher body fat and lower lean mass, but not BMI, were associated with reduced knee and hip strength, even after adjusting for confounders. Our study provides novel data related to body fat and lean mass measures in young adults with PFP that can be used to further advance assessment and treatment strategies.
As noted, a BMI of ≥25 kg/m² was observed in 38% of our cohort, indicating that overweight and obesity are health problems coexisting with physical impairments5,45 in a large proportion of young adults with PFP. This finding is consistent with a previous systematic review of 33 cross-sectional studies, which reported that young adults with PFP have greater BMI compared with pain-free controls.15 Interestingly, evidence from 2 systematic reviews of prospective studies suggests that BMI might not be a risk factor for PFP.12,15 This indicates that overweight may be a consequence of living with PFP (rather than overweight or obesity predisposing PFP), with PFP known to lead to reduced physical activity levels and psychological impairments.4,10,50 However, many of the studies included in these systematic reviews12,15 (six of 12 studies) investigated BMI as a risk factor for PFP in military populations. Future prospective studies are needed to confirm if higher BMI values are a risk factor for (or consequence of) PFP in other populations such as young adults, adolescents, and recreational sports population.
Our findings suggest that higher BMI, higher body fat, and lower lean mass are associated with poor subjective and objective functional capacity. Previous research in other musculoskeletal conditions assessing the relationship of BMI, body fat, and lean mass with functional capacity has yielded conflicting findings.18,19,51 Davis et al.18 reported an association between body fat and lean mass and objective functional capacity in older adults with knee OA. However, BMI was not associated with either subjective or objective functional capacity. Nonetheless, when people with different levels of overweight are compared, those with higher BMI reported poor subjective functional capacity in knee OA19 and chronic low back pain.51 These findings provide important clinical implications, given that patients with PFP reporting poor functional capacity are most likely to have poor long-term outcomes.13,14 Exercise therapy targeting the hip and knee is a key recommendation of international consensus and clinical practice guidelines to improving pain and function in people with PFP.35,52,53 However, a prognostic study indicated that more than half of people with PFP report unfavorable outcomes 5−8 years after being enrolled in exercise-based rehabilitation.14 This suggests a need for alternative approaches that are more tailored to patient needs.31 Our findings suggest that greater BMI, higher body fat, and lower lean mass are predictive of poor functional capacity and reduced knee and hip strength. Therefore, considering management approaches beyond exercise therapy that incorporate weight management may be a potential alternative to enhance long-term outcomes for patients with PFP. This approach has been reported to be successful in people with knee OA, with weight management interventions leading to improvements in pain, functional capacity, and knee strength.27, 28, 29, 30
According to our findings, BMI was associated with knee flexion strength but not with knee extension and hip strength. A potential explanation for this finding is that BMI does not take into account specific components of body composition (i.e., body fat and lean mass); consequently, the relative contribution of these components to muscle strength cannot be determined. Additionally, although we included sex as a covariate during statistical analysis and did not find a significant difference between BMI groups for proportion of males and females (Supplementary Table 1), the inclusion of a non-balanced mixed-sex sample across BMI categories is a limitation of our study. A previous systematic review has indicated larger hip strength deficits for female populations compared to male and mixed-sex populations, which supports the notion that males and females present different deficits and may represent distinct subgroups within PFP.40
Our novel findings indicate that higher body fat and lower lean mass are associated with reduced knee and hip strength. Overweight has been associated with alterations in skeletal muscle, such as increased lipid accumulation between (intermuscular fat) and within skeletal muscle fibers,54,55 which in turn seems to affect muscular quality (muscle strength relative to unit of muscle mass) in people with knee OA.54,56,57 These physiological changes in the skeletal muscle as a consequence of overweight may negatively influence knee extensor strength and physical function in people with knee OA.56,58 Fat infiltration in muscle fibers could not be detected by bioelectrical impedance and therefore is a limitation of our study. Further research is warranted to explore this hypothesis.
4.1. Clinical implications
Our findings suggest that BMI, body fat, and lean mass should be considered in the assessment and management of PFP because, depending on their levels, they may be detrimental to functional capacity and particularly strength. Weight management interventions (diet only or a combination of diet plus exercise) have been reported to be effective to improving pain, functional capacity, and knee strength in people with knee OA.27, 28, 29, 30,59 Considering that 38% of our cohort were overweight or obese, weight management and advice on a healthier lifestyle60 should be considered for young adults with PFP. Additionally, we found that lean mass was the best predictor of strength, explaining 31%, 29%, and 31% of the variance in knee extensor, knee flexor, and hip abductor strength, respectively, while body fat explained 18%, 15%, and 23%, respectively. These findings may suggest that developers of interventions targeting weight loss as a treatment modality for PFP need to consider the impact of the weight loss intervention on lean mass specifically. Therefore, interventions focusing on combined diet and strength training rather than diet only may be the best option for people with PFP, particularly for those categorized as obese,61,62 because they seem to have the lowest knee and hip strength (Supplementary Table 1). This hypothesis should be a research priority because there has been no randomized controlled trial in PFP targeting weight loss.31
4.2. Limitations
Due to the first aim of the study (to determine the proportion of overweight/obesity in our sample of young adults with PFP), we did not create equal samples within BMI categories, which could have influenced our results regarding the second aim (to examine associations between body composition and functional capacity and strength). Due to our sample size, we chose to account for sex as a covariate within the regression model rather than stratify the cohort into subgroups. Future studies stratifying the sample based on sex are needed in order to better understand its contribution in the relationship of BMI, body fat, and lean mass with functional capacity and strength in PFP. Although we have attempted to control for some important covariates in our analyses (sex, bilateral pain, and current pain level), caution should be taken when interpreting our findings because the participants' physical activity level was not assessed in our cohort; further inquiry into the influence of physical activity level is warranted to determine whether it may serve as a potential covariate for the observed relationships. Our findings are also limited to individuals 18−35 years old. Adolescents and older populations with PFP may present different distributions of BMI, body fat, and lean mass,15,63 and this requires consideration in future research. Last, we only investigated the impact of BMI, body fat, and lean mass on functional capacity and strength measures; future research should explore the impact of overweight and obesity in psychological, biomechanical, and other clinical outcomes in people with PFP.
5. Conclusion
Higher BMI, higher body fat, and lower lean mass are associated with poor functional capacity in young adults with PFP. Higher body fat and lean mass, but not BMI, were also associated with reduced knee and hip strength. These findings indicate that when managing people with PFP, assessment of BMI, body fat, and lean mass is warranted because it has an impact on clinical outcomes.
Acknowledgment
Amanda Schenatto Ferreira is supported by a PhD scholarship from Sao Paulo Research Foundation - FAPESP (scholarship No. 2018/17106-0).
Authors’ contributions
ASF and DdOS conceived the study's design and carried out data collection, data analysis and interpretation, and drafting of the manuscript; BFM contributed to statistical analysis and data interpretation and helped revise the manuscript; BT participated in the design of the study and contributed to data collection; MFP participated in the design of the study and helped revise the manuscript; FMdA provided a critical review of the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found, in the online version, at doi:10.1016/j.jshs.2020.12.002.
Supplementary materials
References
- 1.Crossley KM, Stefanik JJ, Selfe J, et al. 2016 Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 1: Terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br J Sports Med. 2016;50:839–843. doi: 10.1136/bjsports-2016-096384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith BE, Selfe J, Thacker D, et al. Incidence and prevalence of patellofemoral pain: A systematic review and meta-analysis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coburn SL, Barton CJ, Filbay SR, Hart HF, Rathleff MS, Crossley KM. Quality of life in individuals with patellofemoral pain: A systematic review including meta-analysis. Phys Ther Sport. 2018;33:96–108. doi: 10.1016/j.ptsp.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Priore LB, Azevedo FM, Pazzinatto MF, et al. Influence of kinesiophobia and pain catastrophism on objective function in women with patellofemoral pain. Phys Ther Sport. 2019;35:116–121. doi: 10.1016/j.ptsp.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Nunes GS, de Oliveira Silva D, Crossley KM, Serrão FV, Pizzari T, Barton CJ. People with patellofemoral pain have impaired functional performance, that is correlated to hip muscle capacity. Phys Ther Sport. 2019;40:85–90. doi: 10.1016/j.ptsp.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Eijkenboom JFA, Waarsing JH, Oei EHG, Bierma-Zeinstra SMA, van Middelkoop M. Is patellofemoral pain a precursor to osteoarthritis? Patellofemoral osteoarthritis and patellofemoral pain patients share aberrant patellar shape compared with healthy controls. Bone Jt Res. 2018;7:541–547. doi: 10.1302/2046-3758.79.BJR-2018-0112.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crossley KM. Is patellofemoral osteoarthritis a common sequela of patellofemoral pain? Br J Sports Med. 2014;48:409–410. doi: 10.1136/bjsports-2014-093445. [DOI] [PubMed] [Google Scholar]
- 8.Wyndow N, Collins N, Vicenzino B, Tucker K, Crossley K. Is there a biomechanical link between patellofemoral pain and osteoarthritis? A narrative review. Sport Med. 2016;46:1797–1808. doi: 10.1007/s40279-016-0545-6. [DOI] [PubMed] [Google Scholar]
- 9.Lack S, Neal B, De Oliveira Silva D, Barton C. How to manage patellofemoral pain—understanding the multifactorial nature and treatment options. Phys Ther Sport. 2018;32:155–166. doi: 10.1016/j.ptsp.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Maclachlan LR, Collins NJ, Matthews MLG, Hodges PW, Vicenzino B. The psychological features of patellofemoral pain: A systematic review. Br J Sports Med. 2017;51:732–742. doi: 10.1136/bjsports-2016-096705. [DOI] [PubMed] [Google Scholar]
- 11.Powers CM, Witvrouw E, Davis IS, Crossley KM. Evidence-based framework for a pathomechanical model of patellofemoral pain: 2017 patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester, UK: part 3. Br J Sports Med. 2017;51:1713–1723. doi: 10.1136/bjsports-2017-098717. [DOI] [PubMed] [Google Scholar]
- 12.Neal BS, Lack SD, Lankhorst NE, Raye A, Morrissey D, van Middelkoop M. Risk factors for patellofemoral pain: A systematic review and meta-analysis. Br J Sports Med. 2019;53:270–281. doi: 10.1136/bjsports-2017-098890. [DOI] [PubMed] [Google Scholar]
- 13.Collins NJ, Bierma-zeinstra SM, Crossley KM, van Linschoten RL, Vicenzino B, van Middelkoop M. Prognostic factors for patellofemoral pain: A multicentre observational analysis. Br J Sports Med. 2013;47:227–233. doi: 10.1136/bjsports-2012-091696. [DOI] [PubMed] [Google Scholar]
- 14.Lankhorst NE, van Middelkoop M, Crossley KM, et al. Factors that predict a poor outcome 5–8 years after the diagnosis of patellofemoral pain: A multicentre observational analysis. Br J Sports Med. 2016;50:881–886. doi: 10.1136/bjsports-2015-094664. [DOI] [PubMed] [Google Scholar]
- 15.Hart HF, Barton CJ, Khan KM, Riel H, Crossley KM. Is body mass index associated with patellofemoral pain and patellofemoral osteoarthritis? A systematic review and meta-regression and analysis. Br J Sports Med. 2017;51:781–790. doi: 10.1136/bjsports-2016-096768. [DOI] [PubMed] [Google Scholar]
- 16.Kastelein M, Luijsterburg PA, Heintjes EM, et al. The 6-year trajectory of non-traumatic knee symptoms (including patellofemoral pain) in adolescents and young adults in general practice: A study of clinical predictors. Br J Sports Med. 2015;49:400–405. doi: 10.1136/bjsports-2014-093557. [DOI] [PubMed] [Google Scholar]
- 17.Sowers MF, Yosef M, Jamadar D, Jacobson J, Karvonen-Gutierrez C, Jaffe M. BMI vs. body composition and radiographically-defined osteoarthritis of the knee in women: A 4-year follow-up study. Osteoarthr Cartil. 2008;16:367–372. doi: 10.1016/j.joca.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis HC, Blue MNM, Hirsch KR, et al. Body composition is associated with physical performance in individuals with knee osteoarthritis. J Clin Rheumatol. 2020;26:109–114. doi: 10.1097/RHU.0000000000000967. [DOI] [PubMed] [Google Scholar]
- 19.Raud B, Gay C, Guiguet-auclair C, et al. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci Rep. 2020;10:3601. doi: 10.1038/s41598-020-60587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Culvenor AG, Felson DT, Niu J, et al. Thigh muscle specific-strength and the risk of incident knee osteoarthritis: The influence of sex and greater body mass index. Arthritis Care Res. 2017;69:1266–1270. doi: 10.1002/acr.23182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batsis JA, Zbehlik AJ, Barre LK, Bynum JP, Pidgeon D, Bartels SJ. Impact of obesity on disability, function and physical activity: Data from the Osteoarthritis Initiative. Scand J Rheumatol. 2015;44:495–502. doi: 10.3109/03009742.2015.1021376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtça MP, Aslan UB, Senol H. Is functional level related with body composition in patients with knee osteoarthritis. Osteoarthr Cartil. 2019;27:S447. doi: 10.1016/j.joca.2019.02.482. [DOI] [Google Scholar]
- 23.Hussain SM, Tan MC, Stathakopoulos K, et al. How are obesity and body composition related to patellar cartilage? A systematic review. J Rheumatol. 2017;44:1071–1082. doi: 10.3899/jrheum.151384. [DOI] [PubMed] [Google Scholar]
- 24.Ding C, Parameswaran V, Cicuttini F, et al. Association between leptin, body composition, sex and knee cartilage morphology in older adults: The Tasmanian older adult cohort (TASOAC) study. Ann Rheum Dis. 2008;67:1256–1261. doi: 10.1136/ard.2007.082651. [DOI] [PubMed] [Google Scholar]
- 25.Sowers M, Karvonen-Gutierrez CA, Jacobson JA, Jiang Y, Yosef M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am. 2011;93:241–251. doi: 10.2106/JBJS.I.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim N, Browning RC, Lerner ZF. The effects of pediatric obesity on patellofemoral joint contact force during walking. Gait Posture. 2019;73:209–214. doi: 10.1016/j.gaitpost.2019.07.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riddle DL, Stratford PW. Body weight changes and corresponding changes in pain and function in persons with symptomatic knee osteoarthritis: a cohort study. Arthritis Care Res (Hoboken) 2013;65:15–22. doi: 10.1002/acr.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messier SP, Resnik AE, Beavers DP, et al. Intentional weight loss for overweight and obese knee osteoarthritis patients: Is more better. Arthritis Care Res (Hoboken) 2018;70:1569–1575. doi: 10.1002/acr.23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atukorala I, Makovey J, Lawler L, Messier SP, Bennell K, Hunter DJ. Is there a dose-response relationship between weight loss and symptom improvement in persons with knee osteoarthritis? Arthritis Care Res (Hoboken) 2016;68:1106–1114. doi: 10.1002/acr.22805. [DOI] [PubMed] [Google Scholar]
- 30.Henriksen M, Christensen R, Danneskiold-samsøe B, Bliddal H. Changes in lower extremity muscle mass and muscle strength after weight loss in obese patients with knee osteoarthritis: A prospective cohort study. Arthritis Rheum. 2012;64:438–442. doi: 10.1002/art.33394. [DOI] [PubMed] [Google Scholar]
- 31.Barton CJ, Crossley KM, Macri EM. Should we consider changing traditional physiotherapy treatment of patellofemoral pain based on recent insights from the literature? Br J Sports Med. 2018;52:1546–1547. doi: 10.1136/bjsports-2017-098695. [DOI] [PubMed] [Google Scholar]
- 32.Urquhart DM, Berry P, Wluka AE, et al. Increased fat mass is associated with high levels of low back pain intensity and disability. Spine (Phila Pa 1976) 2011;36:1320–1325. doi: 10.1097/BRS.0b013e3181f9fb66. [DOI] [PubMed] [Google Scholar]
- 33.Hussain SM, Urquhart DM, Wang Y, et al. Fat mass and fat distribution are associated with low back pain intensity and disability: Results from a cohort study. Arthritis Res Ther. 2017;19:26. doi: 10.1186/s13075-017-1242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoo JJ, Cho NH, Lim SH, Kim HA. Relationships between body mass index, fat mass, muscle mass, and musculoskeletal pain in community residents. Arthritis Rheumatol. 2014;66:3511–3520. doi: 10.1002/art.38861. [DOI] [PubMed] [Google Scholar]
- 35.Collins NJ, Barton CJ, van Middelkoop M, et al. 2018 Consensus statement on exercise therapy and physical interventions (orthoses, taping and manual therapy) to treat patellofemoral pain: Recommendations from the 5th International Patellofemoral Pain Research Retreat, Gold Coast, Australia, 2017. Br J Sports Med. 2018;52:1170–1178. doi: 10.1136/bjsports-2018-099397. [DOI] [PubMed] [Google Scholar]
- 36.von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Br Med J. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crossley KM, Bennell KL, Cowan SM, Green S. Analysis of outcome measures for persons with patellofemoral pain: Which are reliable and valid? Arch Phys Med Rehabil. 2004;85:815–822. doi: 10.1016/s0003-9993(03)00613-0. [DOI] [PubMed] [Google Scholar]
- 38.Karachalios T, Hantes M, Zibis AH, Zachos V, Karantanas AH, Malizos KN. Diagnostic accuracy of a new clinical test (the Thessaly test) for early detection of meniscal tears. J Bone Joint Surg Am. 2005;87:955–962. doi: 10.2106/JBJS.D.02338. [DOI] [PubMed] [Google Scholar]
- 39.Benjaminse A, Gokeler A, van der Schans CP. Clinical diagnosis of an anterior cruciate ligament rupture: A meta-analysis. J Orthop Sport Phys Ther. 2005;36:267–288. doi: 10.2519/jospt.2006.2011. [DOI] [PubMed] [Google Scholar]
- 40.Rathleff MS, Rathleff CR, Crossley KM, Barton CJ. Is hip strength a risk factor for patellofemoral pain? A systematic review and meta-analysis. Br J Sports Med. 2014;48:1088. doi: 10.1136/bjsports-2013-093305. [DOI] [PubMed] [Google Scholar]
- 41.Boling MC, Nguyen AD, Padua DA, Cameron KL, Beutler A, Marshall SW. Gender-specific risk factor profiles for patellofemoral pain. Clin J Sport Med. 2019 doi: 10.1097/JSM.0000000000000719. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pazzinatto MF, de Oliveira Silva D, Barton C, Rathleff MS, Briani RV, de Azevedo FM. Female adults with patellofemoral pain are characterized by widespread hyperalgesia, which is not affected immediately by patellofemoral joint loading. Pain Med. 2016;17:1953–1961. doi: 10.1093/pm/pnw068. [DOI] [PubMed] [Google Scholar]
- 43.Vasold KL, Parks AC, Phelan DML, Pontifex MB, Pivarnik JM. Reliability and validity of commercially available low-cost bioelectric impedance analysis. Int J Sport Nutr Exerc Metab. 2019;29:406–410. doi: 10.1123/ijsnem.2018-0283. [DOI] [PubMed] [Google Scholar]
- 44.Durall CJ, Greene PF, Kernozek TW. A comparison of two isometric tests of trunk flexor endurance. J Strength Cond Res. 2012;26:1939–1944. doi: 10.1519/JSC.0b013e318237ea1c. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira AS, de Oliveira Silva D, Barton CJ, et al. Impaired isometric, concentric, and eccentric rate of torque development at the hip and knee in patellofemoral pain. J Strength Cond Res. 2021;35:2492–2497. doi: 10.1519/JSC.0000000000003179. [DOI] [PubMed] [Google Scholar]
- 46.Nunes GS, de Oliveira Silva D, Pizzari T, Serrão FV, Crossley KM, Barton CJ. Clinically measured hip muscle capacity deficits in people with patellofemoral pain. Phys Ther Sport. 2019;35:69–74. doi: 10.1016/j.ptsp.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Obesity WHO. preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 48.Field A. SAGE Publications Ltd; London: 2009. Discovering Statistics Using SPSS. 3rd ed. [Google Scholar]
- 49.Cohen J. Routledge Academic; New York, NY: 1988. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. [Google Scholar]
- 50.Glaviano NR, Baellow A, Saliba S. Physical activity levels in individuals with and without patellofemoral pain. Phys Ther Sport. 2017;27:12–16. doi: 10.1016/j.ptsp.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Wertli MM, Held U, Campello M, Schecter Weiner S. Obesity is associated with more disability at presentation and after treatment in low back pain but not in neck pain: Findings from the OIOC registry. BMC Musculoskelet Disord. 2016;17:140. doi: 10.1186/s12891-016-0992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willy RW, Hoglund LT, Barton CJ, et al. Patellofemoral pain: clinical practice guidelines linked to the international classification of functioning, disability and health from the academy of orthopaedic physical therapy of the American Physical Therapy Association. J Orthop Sport Phys Ther. 2019;49 CPG1–57. [Google Scholar]
- 53.Barton CJ, Lack S, Hemmings S, Tufail S, Morrissey D. The “Best Practice Guide to Conservative Management of Patellofemoral Pain”: incorporating level 1 evidence with expert clinical reasoning. Br J Sports Med. 2015;49:923–934. doi: 10.1136/bjsports-2014-093637. [DOI] [PubMed] [Google Scholar]
- 54.Bollinger LM. Potential contributions of skeletal muscle contractile dysfunction to altered biomechanics in obesity. Gait Posture. 2017;56:100–107. doi: 10.1016/j.gaitpost.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Pedroso MG, de Almeida AC, Aily JB, de Noronha M, Mattiello SM. Fatty infiltration in the thigh muscles in knee osteoarthritis: A systematic review and meta-analysis. Rheumatol Int. 2019;39:627–635. doi: 10.1007/s00296-019-04271-2. [DOI] [PubMed] [Google Scholar]
- 56.Kumar D, Karampino DC, MacLeod TD, et al. Quadriceps intramuscular fat fraction rather than muscle size is associated with knee osteoarthritis. Osteoarthr Cartil. 2014;22:226–234. doi: 10.1016/j.joca.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conroy MB, Kwoh CK, Krishnan E, et al. Muscle strength, mass, and quality in older men and women with knee osteoarthritis. Arthritis Care Res (Hoboken) 2012;64:15–21. doi: 10.1002/acr.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maly MR, Calder KM, Macintyre NJ, Beattie KA. Relationship of intermuscular fat volume in the thigh relates to knee extensor strength and physical performance in women at risk for or with knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65:44–52. doi: 10.1002/acr.21868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Oliveira Silva D, Pazzinatto MF, Rathleff MS, et al. Patient education for patellofemoral pain: A systematic review. J Orthop Sport Phys Ther. 2020;50:388–396. doi: 10.2519/jospt.2020.9400. [DOI] [PubMed] [Google Scholar]
- 61.Hulens M, Vansant G, Lysens R, Claessens AL, Muls E. Assessment of isokinetic muscle strength in women who are obese. J Orthop Sport Phys Ther. 2002;32:347–356. doi: 10.2519/jospt.2002.32.7.347. [DOI] [PubMed] [Google Scholar]
- 62.Hulens M, Vansant G, Lysens R, Claessens AL, Muls E, Brumagne S. Study of differences in peripheral muscle strength of lean versus obese women: An allometric approach. Int J Obes Relat Metab Disord. 2001;25:676–681. doi: 10.1038/sj.ijo.0801560. [DOI] [PubMed] [Google Scholar]
- 63.Barber Foss KD, Hornsby M, Edwards NM, Myer GD, Hewett TE. Is body composition associated with an increased risk of developing anterior knee pain in adolescent female athletes. Phys Sportsmed. 2012;40:13–19. doi: 10.3810/psm.2012.02.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.