Abstract
Cyathostomins are the most common and highly prevalent parasites of horses worldwide. Historically, the control of cyathostomins has mainly relied on the routine use of anthelmintic products. Increasing reports on anthelmintic resistance (AR) in cyathostomins are concerning. A potential method proposed for detecting emerging AR in cyathostomins has been estimating the egg reappearance period (ERP). This paper reviews the data available for the ERP of cyathostomins against the three major classes of anthelmintics, macrocyclic lactones, tetrahydropyrimidines, and benzimidazoles. Published peer-reviewed original research articles were obtained from three databases (PubMed, CAB Direct and Web of Science) and were evaluated for their inclusion in a systematic review. Subsets of articles were then subjected to a review of ERP data. A total of 54 (of 134) studies published between 1972 and 2022 met the criteria for inclusion in the systematic review. Until the beginning of 2022, there was no agreed definition of the ERP; eight definitions of ERP were identified in the literature, complicating the comparison between studies. Additionally, potential risk factors for the shortening of the ERP, including previous anthelmintic use and climate, were frequently not described. Reports of shortened ERP for moxidectin and ivermectin are frequent: 20 studies that used comparable ERP definitions reported shortened moxidectin and ivermectin ERPs of 35 and 28 days, respectively. It is unclear whether the ERPs of these anthelmintics reduced to such levels are due to the development of AR or some biological factors related to horses, cyathostomin species, and/or the environment. The ERPs for other anthelmintics, such as fenbendazole and pyrantel, were frequently not reported due to established resistance against these drugs. Future research in horses is required to understand the mechanism(s) behind the shortening of ERP for cyathostomins. Based on this systematic review, we propose recommendations for future ERP studies.
Keywords: Egg reappearance period, Cyathostomins, Anthelmintics, Anthelmintic resistance, Strongylid, Horse
Graphical abstract
Highlights
-
•
Is the egg reappearance period (ERP) an early indicator of anthelmintic resistance?
-
•
A summary of cyathostomins ERP for equine registered anthelmintics from 1972 to 2022.
-
•
Recent developments on the definition of ERP.
-
•
Proposed recommendations for future ERP studies.
1. Introduction
Horses can be infected with a variety of gastrointestinal nematodes, and cyathostomins (Strongylida: Cyathostominae) are by far the most common parasites of horses worldwide, with 40 species within 14 genera and coinfections with 15–25 species being commonplace (Lichtenfels et al., 2008; Saeed et al., 2019; Bellaw and Nielsen, 2020). The majority of horses infected with adult cyathostomins do not show clinical disease, even with burdens ranging from a few thousand to hundreds of thousands of worms (Love et al., 1999). However, clinical manifestations such as weight loss, colic, pyrexia, diarrhea, subcutaneous oedema accompanied by marked hypoproteinemia, and even death can be associated with a synchronous emergence of fourth-stage larvae from the intestinal wall, giving rise to the syndrome called larval cyathostominosis (Reid et al., 1995; Love et al., 1999).
Adult cyathostomins are unlikely to cause a significant disease (Love et al., 1999) as these stages feed on luminal contents and mucosal plugs, leaving small erosions of the mucosa (Cullinane et al., 2006). However, the pathogenicity of the adult stages is not well understood (Nielsen et al., 2014) as the individual horses could markedly differ in their susceptibility to strongylid infections (Morgan et al., 2005). High adult worm burdens could contaminate the pastures heavily with their eggs passed in the manure (Matthews, 2008); therefore, current worm control practices should be aimed at reducing the risk of pasture contamination and minimising the chances of larval cyathostominosis (Matthews, 2008, 2011) using strategic and/or targeted treatments based on faecal egg counts (FECs), and assessment of the efficacy of routinely used anthelmintics (Saeed et al., 2019). Three major classes of anthelmintics are available for chemotherapeutic control of nematodes in horses: macrocyclic lactones (MLs; abamectin (ABM), ivermectin (IVM) and moxidectin (MOX)), tetrahydropyrimidines (THPs; pyrantel (PYR) and morantel MOR)) and benzimidazoles (BZs; fenbendazole (FBZ), oxfendazole (OXF), oxibendazole (OXB), and albendazole (ALB)) (Matthews, 2014; Peregrine et al., 2014). Of these, FBZ and MOX have reported efficacy against encysted larvae at a dose of 7.5 mg/kg once daily for five consecutive days and 0.4 mg/kg, respectively (Xiao et al., 1994; Monahan et al., 1995, 1996; Duncan et al., 1998). Although most horses globally are managed using a rotational deworming program involving the administration of anthelmintics at regular intervals during the year (Nielsen et al., 2018, 2019), targeted parasite control programs involving periodic evaluation of FECs are preferred (Kaplan and Nielsen, 2010; Nielsen et al., 2014). Unfortunately, the uptake of targeted parasite programs has been variable (Matthews, 2014; Cain et al., 2019; Silva et al., 2019).
The interval-based deworming program is a major predisposing factor for developing anthelmintic resistance (AR) (Kaplan, 2004a, b). Currently, resistance to BZs and THPs is well established in cyathostomins and is reported worldwide (Kaplan, 2004a; Matthews, 2014; Peregrine et al., 2014; Nielsen, 2022), while resistance to MLs is emerging. Although there were a few early reports suggesting reduced efficacy of IVM against cyathostomins (von Samson-Himmelstjerna et al., 2007; Traversa et al., 2009), the first confirmed report of ML resistance was published in 2020 (Nielsen et al., 2020) where resistance to IVM and MOX was found by retesting the population. Subsequent reports of either IVM or MOX resistance have been published from various parts of the world in the last two years (Flores et al., 2020; Abbas et al., 2021; Lignon et al., 2021; Martins et al., 2021; Nielsen et al., 2022a). Of further concern, recent studies have found evidence of reduced larvicidal efficacy of the only drugs registered with activity against encysted cyathostomins (i.e., FBZ and MOX) (Reinemeyer et al., 2015; Bellaw et al., 2018). Therefore, mechanism(s)/indicator(s) that can detect the early development of AR are desirable, so that timely action can be taken to slow down the emergence of resistance against a particular drug, particularly in the absence of novel anthelmintic classes for use in horses in the future.
Previously, the determination of egg reappearance period (ERP) following anthelmintic treatment has been suggested as a useful indicator for the early identification of AR in cyathostomins (Sangster, 1999). The ERP is defined as the time between the administration of an effective anthelmintic and the recommencement of shedding of parasite eggs in faeces (Nielsen et al., 2019a). Numerous experimental definitions of the ERP have been used, complicating the comparisons of data from studies estimating ERP. There are numerous studies in which the ERP is used as a surveillance tool for the early detection of AR in cyathostomins and/or to identify the treatment interval for a calendar-based deworming strategy. Many of the studies conducted in the past two decades have reported reduced ERP following treatment with MLs and suggested that resistance to these compounds would be more common in the near future; indeed, this is now evident from the results of recent studies where resistance to IVM and MOX has been reported globally (Flores et al., 2020; Abbas et al., 2021; Lignon et al., 2021; Martins et al., 2021). The concept of a shortened ERP as an indicator of anthelmintic resistance was based mainly on a greater selection pressure for the development of AR on some species or reduced efficacy of an anthelmintic against luminal stages of cyathostomins (Sangster, 1999). However, a recent terminal study that investigated the efficacy of IVM and MOX against all parasitic stages of cyathostomins suggested that reduced cyathostomin ERP may not be linked with the emergence of AR (Nielsen et al., 2022b). The authors of this study found that the faecal egg count reduction (FECR) of both drugs was >99% and >97% at 2, 3 and 4 weeks post-treatment but was reduced sharply to <75% at 5 weeks post-treatment. Similarly, a decrease in cyathostomin FECR of MOX and a combination of ABM and MOR has been recorded at 5 weeks following treatment (Abbas et al., 2021). Previously, the decrease in ERP was thought to be due to selection pressure on larval stages of cyathostomins, luminal L4s (LL4s) which survived the anthelmintic treatment and completed the last moult and started laying eggs and/or the reduced efficacy of MOX against encysted stages of cyathostomins (Lyons et al., 2009). Recently, Nielsen et al. (2022b) suggested that shortened ERP may not be attributed to surviving LL4s, as the low numbers of LL4s found two weeks post-treatment did not correspond with the thousands of adult stages recovered in both treated groups at the time of ERP, while the larvicidal efficacy of MOX was still within historical ranges. While it remains unknown whether the LL4s survived treatment or appeared after treatment and contributed to reduced ERP, there are likely other reasons for an increased number of adult worms in the 5th week compared to the 2nd week post-treatment reported by Nielsen et al. (2022b). These findings provide further insights into the dynamics of the larval stages of cyathostomins following treatment and possible links with ERP. Although a study by Nielsen et al. (2022b) was conducted on a single horse population, at one location during one season, it warrants further investigations to determine the plausible reason(s) responsible for the shortening of ERP (e.g., factors related to horse or cyathostomin species).
The aims of this systematic review are to (i) identify and describe current knowledge on the ERP of cyathostomins after treatment with BZs, MLs and THPs in horses, including the assessment of various ERP definitions, (ii) perform a qualitative analysis of available ERP data based on comparable ERP definitions and (iii) identify various factors that can potentially affect ERP estimates, thereby proposing recommendations for future ERP studies.
2. Material and methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were utilised to conduct this systematic review (Moher et al., 2009).
2.1. Literature search
An electronic literature search was conducted using PubMed, CAB Direct and Web of Science databases from 1972 to 2022 (accessed on 22nd September 2022). The population in this systematic review was defined as a horse of any age and sex, including foals, and any data on donkeys, mules and zebras were excluded. The intervention addressed was administering anthelmintic drugs, including IVM, MOX, ABM, doramectin (DRM), FBZ, MOR, OXF, OXB, and PYR. The outcome of interest was the ERP of cyathostomins. Any additional papers identified through full-text analyses of relevant papers were eligible for inclusion and subjected to the same criteria as papers identified through the initial search.
2.2. Selection criteria
To meet the selection criteria for this systematic review, a publication had to be a peer-reviewed primary research paper and contain evidence of an estimation of the ERP for cyathostomins in the title, abstract and/or keywords. Publications published in any language were considered eligible if the full text was available in English. The title and abstract of all publications identified by the final search string were screened by two authors (SLM and GA). If unsure whether a paper should be included or excluded based on the title and abstract alone, a further evaluation was performed and any publications that appeared relevant following a more in-depth evaluation were included in the review. Following initial selection, papers were then evaluated for inclusion in the assessment of the ERP data. Publications were selected for further investigation if they defined the ERP as (i) the time when the FECR was <90% for MLs or <80% for BZs and PYR, and/or (ii) the time when post-treatment FEC was >10% of pre-treatment FEC. However, both mentioned definitions of the ERP have the same meaning; hence, we will use only one of these subsequently.
2.3. Data extraction
Data extractions were completed in two phases. Firstly, papers using any definition of the ERP were evaluated to investigate the range of research available assessing ERP. The following information was extracted from all papers using any definition of the ERP: title, author(s), year of publication, study design, location, parasite type, anthelmintic drug(s), dose and route of administration, the ERP definition used, calculated ERP value(s), animal characteristics (such as age, breed, use (e.g., racehorse), housing), sample size, source of infection, study duration, and frequency of FEC measurement. A study was categorised as:
-
a)
a comparative study if intervention was introduced by researchers and pre-intervention FEC values were used as a control for comparison of the post-intervention FEC;
-
b)
a randomised controlled or clinical trial if intervention was introduced by researchers and study population randomly allocated to control, placebo or treatment group(s);
-
c)
a retrospective study if intervention was not introduced by researchers and it included analysis of historical data on intervention and outcome; and
-
d)
an observational field study if research in which intervention was not introduced by researchers but sampling and outcome measurements were performed by researchers.
Secondly, additional data for the evaluation and comparison of ERP values were collected from the two groups of publications that used comparable ERP definitions. Where available, the following additional information was extracted from the publications: grazing pattern (rotational vs. continuous); history of anthelmintic use, including length of history provided, drug rotation, treatment schedule (interval or targeted) and treatment intervals, the timing of the last anthelmintic used; any previous history of AR in parasites detected using the same animals; climate; method of collecting the faecal sample; method of measuring bodyweight; method of administering anthelmintics; threshold of FEC for the inclusion criteria; FEC detection method and multiplication factor; method of calculating FECR; and if larval culture was performed.
2.4. Evaluation of bias
The 21 publications included for the evaluation of ERP values were also evaluated for selection bias, performance bias, detection bias, attrition bias and reporting bias, as previously described by Higgins et al. (2019). To assess selection bias, papers were evaluated for the detailed description of the random allocation of participants (i.e., horses) to study (i.e., treatment) groups. Studies were assessed for performance and detection bias by evaluating the description of blinding of study personnel and outcome assessors to intervention allocations, respectively. The potential for the influence of the loss of study participants on study results was evaluated to assess attrition bias. Last, the risk of reporting bias was assessed by evaluating the completeness of the reporting of the study findings. For each type of bias, the risk of bias was assessed as either low, some concerns, high, or no information. The criteria for (i) low risk of bias was if the trial was judged to be at low risk of bias for all domains for this result; (ii) some concerns were reported if the trial was judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain; and (iii) high risk of bias was reported if the trial was judged to be at high risk of bias in at least one domain for this result or the trial was judged to have some concerns for multiple domains in a way that substantially lowers confidence in the result (Higgins et al., 2019). Subsequently, an overall risk of bias judgement was made by the review authors.
3. Results
3.1. Literature search
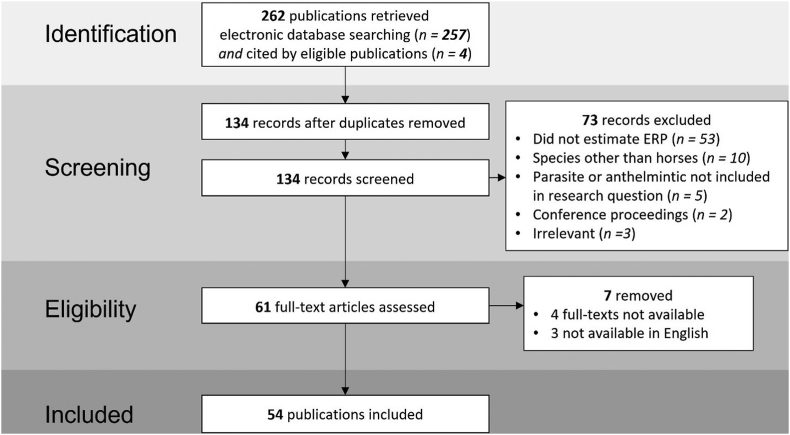
A total of 54 publications were included in the qualitative synthesis of the data (Fig. 1). However, only 21 (out of 54) were eligible for inclusion for a direct comparison of the ERP (Table 1).
Fig. 1.
Flow diagram presenting article identification and selection process for the systematic review. This flow diagram is made in accordance with the PRISMA guidelines.
Table 1.
History of anthelmintic use, housing, minimum FEC for inclusion, method of FEC calculation and method of calculation of FECR for publications eligible for evaluation of the ERP (n = 21).
| Anthelmintic(s) | Housing | History of anthelmintic use |
Minimum FEC for inclusion | FEC method (detection limit) | Method of calculation of FECR | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Length of history | Drug rotation | Treatment schedule (interval) | Timing of last anthelmintic dose | ||||||
| IVM | Stabled | No information | No information | No information | No information | Not specified | Modified McMaster (50 epg) | Not specified | Borgsteede et al. (1993) |
| IVM | Not specified | Not specified | PYR, BZs, IVM, MOX | Interval (1-12x/year) | No information | >150 epg | Modified McMaster (50 epg) | Arcsine | von Samson-Himmelstjerna et al. (2007) |
| IVM | Pasture | Most recent anthelmintic | IVM | No information | 60–180 days prior | Not specified | Modified Wisconsin (1 epg) | WAAVP | McFarlane et al. (2010) |
| IVM | Not specified | ≥2 years | IVM | Targeted | No information | >200 epg | Modified McMaster (50 epg) | WAAVP | Larsen et al. (2011) |
| IVM | Pasture | Not specified | Not specified | Interval (2x/year) | >8 weeks prior | >20 epg | Modified McMaster (20 epg) | Not specified | Zak et al. (2017) |
| IVM | Rotational grazing | Most recent anthelmintic to one year | MLs, BZs, THPs, PZQ | Interval (every 5–8 weeks) or targeted | >6–12 weeks prior | ≥25 epg | Modified McMaster (25 epg) | WAAVP | Rosanowski et al. (2017) |
| IVM | Pasture | No information | IVM, MOX | Targeted | 10 weeks prior | >75 epg | Centrifugal flotation (1 epg) | WAAVP | Molena et al. (2018) |
| IVM | Not specified | Last one year | IVM, MOX, PYR, FBZ, PZQ | Interval, targeted or both | 8 weeks prior | ≥200 epg | Mini-FLOTAC (5 epg) | Bayesian hierarchical model | Butler et al. (2021) |
| MOX | Rotational grazing | Not specified | IVM, MOX, FBZ | Interval (not specified) or targeted | No information | Not specified | Modified salt flotation (1 epg) | WAAVP | Tzelos et al. (2017) |
| MOX | Continuous grazing | Last 5 years | IVM, ABM, OFZ/PYR, ABM/MOR | Targeted | 8–10 weeks prior | ≥45 epg | Modified McMaster (15 epg) | WAAVP | Abbas et al. (2021) |
| IVM, MOX | Stabled | No information | No information | No information | No information | >100 epg | Modified McMaster (25–50 epg) | Not calculated | (van Doorn et al., 2014) |
| IVM, MOX | Not specified | One year | MLs | Interval (≥2x/year) | No information | 100-150 epg | Modified McMaster (25 epg) | WAAVP | Geurden et al. (2014) |
| IVM, MOX | No information | No information | No information | No information | >90 days prior | >150 epg | Modified McMaster (25 epg) | Not specified | Daniels and Proudman (2016) |
| IVM, MOX | Not specified | Not specified | Varied | Interval (≥2x/year) | No information | ≥150 epg | Modified McMaster (25 epg) | Arcsine | Kooyman et al. (2016) |
| IVM, MOX | Pasture | Not specified | Not specified | No information | 180 days prior | Paracount-EPG Kit | WAAVP/AAEP | Johnson and Biddle (2021) | |
| MOX, ABM | Pasture | No information | No information | No information | No information | Not specified | Modified McMaster (20 epg) | Geometric mean FEC | Holm-Martin et al. (2005) |
| MOX, FBZ | Pasture or stabled | Not specified | FBZ, PYR, IVM, MOX | Interval (not specified) | 4 months prior | Not specified | Double centrifugation (not specified) | WAAVP | Rossano et al. (2010) |
| IVM, MOX, ABM | Continuous grazing | Past decade | MLs | Not specified | 12–16 weeks prior | >150 epg | Modified McMaster (<10 epg) | WAAVP | Beasley et al. (2017) |
| IVM, MOX, PYR, FBZ | Continuous grazing | One year | MLs | Not specified | At least 6–12 weeks prior | ≥40 epg | Modified salt flotation (1 epg) | WAAVP, Arcsine and non-parametric bootstrapping | Relf et al. (2014) |
| IVM, MOX, PYR, FBZ | Pasture or stabled | Not specified | No information | Interval (1-2x/year) | >3 months prior | >150 epg | Modified McMaster (not specified) | WAAVP | Sanna et al. (2016) |
| PYR | Pasture | No information | No information | No information | No information | Not specified | McMaster (50 epg) | Not specified | Boersema et al. (1995) |
Abbreviations: ERP, egg reappearance period; FEC, faecal egg count; FECR; faecal egg count reduction; epg, eggs per gram of faces; ABM, abamectin; BZs, benzimidazoles; FBZ, fenbendazole; IVM, ivermectin; MOR, morantel; MOX, moxidectin; PYR, pyrantel; THPs, tetrahydropyrimidines; PZQ, praziquantel; WAAVP, World Association for the Advancement of Veterinary Parasitology.
3.2. Data extraction
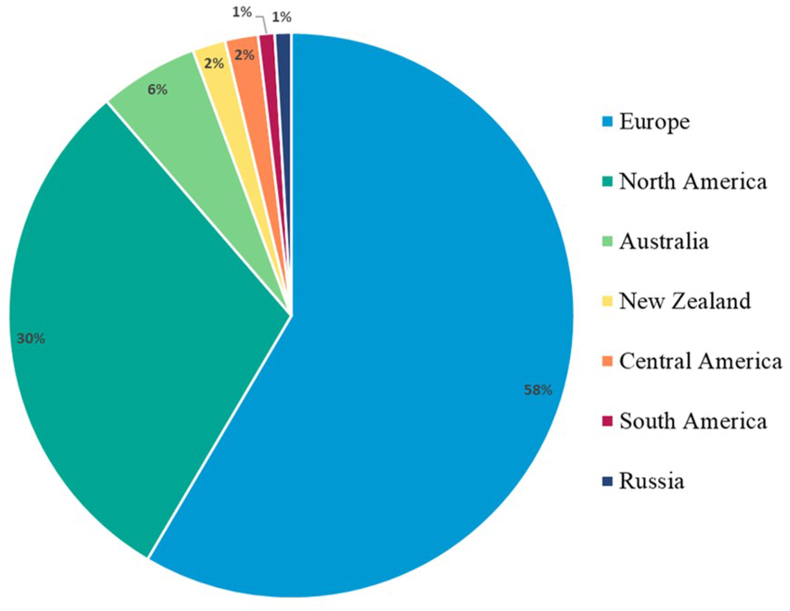
The included publications were published between 1978 and 2022 and all were available in English. Most of the studies were performed as comparative (number = 29) and randomised controlled trials (n = 19) whereas other studies utilised controlled clinical trials (n = 2), retrospective (n = 1), and observational field study designs (n = 3). To date, ERP studies have been reported from Australia (n = 4), Central America (n = 1), Europe (n = 30), New Zealand (n = 1), North America (n = 16), Russia (n = 1) and South America (n = 1) (Fig. 2). Other studies were conducted either in two (Australia and Brazil; Mercier et al., 2001) or three (Belgium, Italy, and The Netherlands) countries (Geurden et al., 2014).
Fig. 2.
Locations of studies evaluating the egg reappearance period (ERP) of equine cyathostomins.
Animal characteristics varied significantly between studies, with horses of all age groups, multiple breeds, their use and various housing environments. The sample size and study duration were highly variable between studies; the smallest sample size was three horses (Butler et al., 2021), and the largest was 428 horses (Fischer et al., 2015). All 54 studies determined FECs and the interval between the determination of FECs was variable; however, one study did not specify the interval between pre- and post-deworming FECs (Daniels and Proudman, 2016).
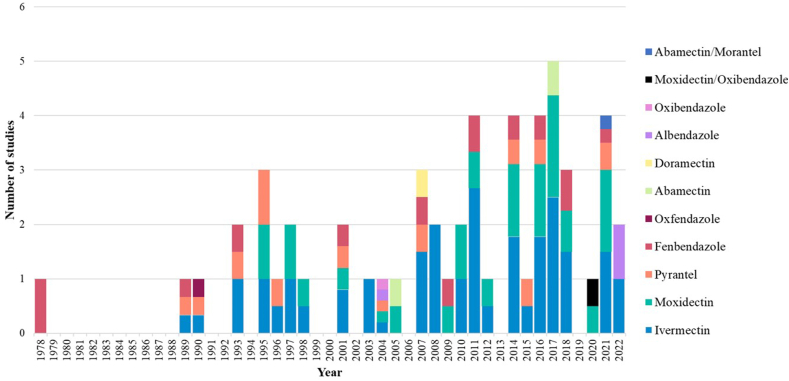
Ivermectin (n = 44) and MOX (n = 27) were the most frequently assessed anthelmintics while PYR (n = 13) and FBZ (n = 10) were investigated less frequently (Fig. 3). Furthermore, studies utilising DRM (Cırak et al., 2007), ABM (Holm-Martin et al., 2005; Beasley et al., 2017), albendazole (ALB) and oxibendazole (OXB) (Vyšniauskas et al., 2004; Baranova et al., 2022), OXF (Piché et al., 1990) and a combination of anthelmintics, MOX and OXB (Scare et al., 2020), and ABM and MOR (Abbas et al., 2021) were identified (Fig. 3). Detailed information of data extracted from 54 publications for this systematic review is presented in Supplementary Table 1.
Fig. 3.
Year and anthelmintic(s) assessed by publications evaluating the egg reappearance period (ERP) of small strongyles (n = 54).
3.3. Definitions of ERP
No agreed definition of the ERP was available in the literature as we identified eight different definitions (see Supplementary Table 2), including (i) the time when post-treatment FEC was >10% of the pre-treatment FEC or FECR <90%, (ii) the time when post-treatment FEC was >20% of the pre-treatment FEC, (iii) the time period between treatment and when FECR was <85%, (iv) the interval between treatment and the time when mean FEC exceeded 100 eggs per gram (epg) of faeces, (v) the interval between treatment and when mean FEC exceeded 200 epg, (vi) the time after treatment when over half the horses had a positive FEC and mean group FEC >200 epg, (vii) and the return of any positive FEC. We also found that two studies used two ERP definitions (Relf et al., 2014; Tzelos et al., 2017) and one study used three ERP definitions (Daniels and Proudman, 2016). In 2022, the World Association for the Advancement of Veterinary Parasitology (WAAVP) revised the guidelines for evaluating the efficacy of equine anthelmintics and defined the ERP for cyathostomins to address this lack of consensus on the definition of the ERP. It used the %FECR values and defined it as when the upper 95% confidence (or credible) limit falls below a threshold defined as the FECR determined two weeks post-treatment minus 10% (Nielsen et al., 2022c). In this manuscript, the published application of this new ERP definition where the method was used to calculate the ERP of cyathostomin against IVM and MOX (Nielsen et al., 2022b) is reported as the eighth definition.
3.4. Evaluation of ERP data with comparable definitions for MLs
Twenty publications that defined the ERP as the time when post-treatment FECR was <90% for MLs (n = 13) and/or the time when post-treatment FEC was >10% of pre-treatment FEC (n = 8) (Table 1), and only one study estimated the ERP using both cut-offs (Daniels and Proudman, 2016). Of these 20 publications, 16 estimated the ERP for IVM as ranging from 28 to 150 days, whereas twelve publications estimated the ERP for MOX as ranging from 35 to >150 days (Table 2).
Table 2.
Egg reappearance period (ERP) data of ivermectin and moxidectin from publications eligible for evaluation of the ERP.
| Anthelmintic | ERP in days (mean) | Reference |
|---|---|---|
| Ivermectin (IVM) | 63 | Borgsteede et al. (1993) |
| IVM | 35 to >56 | von Samson-Himmelstjerna et al. (2007) |
| IVM | 42 | McFarlane et al. (2010) |
| IVM | ≥42 | Larsen et al. (2011) |
| IVM | 49–112 | Zak et al. (2017) |
| IVM | 28 to >42 | Rosanowski et al. (2017) |
| IVM | 28–49 | Molena et al. (2018) |
| IVM | 42 | Relf et al. (2014) |
| IVM | 28–42a | (van Doorn et al., 2014) |
| IVM | 28 to >56 | Geurden et al. (2014) |
| IVM | 90–150 | Sanna et al. (2016) |
| IVM | 56–70 | Daniels and Proudman (2016) |
| IVM | ≥42a | Kooyman et al. (2016) |
| IVM | 42 | Beasley et al. (2017) |
| IVM | 49–63 | Butler et al. (2021) |
| IVM | 42 | Johnson and Biddle (2021) |
| Moxidectin (MOX) | >84 | Holm-Martin et al. (2005) |
| MOX | 35 | Rossano et al. (2010) |
| MOX | 42–84 | Tzelos et al. (2017) |
| MOX | 42–63 | Relf et al. (2014) |
| MOX | 42a | (van Doorn et al., 2014) |
| MOX | 42 | Geurden et al. (2014) |
| MOX | >150 | Sanna et al. (2016) |
| MOX | 77–91 | Daniels and Proudman (2016) |
| MOX | 42 to ≥56a | Kooyman et al. (2016) |
| MOX | 84 | Beasley et al. (2017) |
| MOX | 35 | Abbas et al. (2021) |
| MOX | 84 | Johnson and Biddle (2021) |
Papers with known shortened ERP prior to study.
In addition to estimating the ERPs for IVM and MOX, one, three and five studies respectively investigated ABM (Holm-Martin et al., 2005), FBZ (Rossano et al., 2010; Relf et al., 2014; Sanna et al., 2016) and PYR Boersema et al. (1995); Relf et al. (2014); Sanna et al. (2016); (Butler et al., 2021; Johnson and Biddle, 2021), respectively (Supplementary Table 1). Studies that aimed at estimating ERPs but found resistance at 2 weeks post-treatment are not discussed further in this paper. The only study to investigate FBZ, reported an ERP of 60 days when defining ERP as the time when <90% reduction in FEC occurred (Sanna et al., 2016). Five studies investigated pyrantel: the ERP was estimated to be 35–42 days in two studies (Boersema et al., 1995; Relf et al., 2014), 2–4 weeks when the ERP was defined as the time at which post-treatment FEC >10% pre-treatment FEC in one study (Butler et al., 2021), 90 days (Sanna et al., 2016) in one study, and 28–42 days, based on a cut-off of ≤80% reduction in FEC in one study (Johnson and Biddle, 2021). For ABM, one study estimated the ERP as 28 days (Holm-Martin et al., 2005).
Information on the previous use of anthelmintics on a farm is pivotal for the interpretation of ERP data, including consideration of association(s) between the type and number of products used and the estimated efficacy of the tested anthelmintic product. Additionally, the number of treatments used per year should be considered as this is a predisposing factor for the development of AR in cyathostomins (Nielsen et al., 2019b). In the current review, six (out of 21) studies did not provide any information on previous anthelmintic use (Table 1), while 8 (out of 21) did not report the duration of use of these anthelmintics on the properties. When these data were provided, the reported duration of previous use of the anthelmintics varied greatly between studies, with periods of 6 weeks to 1 year (n = 1), 1 year (n = 3), 2 years (n = 1), 5 years (n = 1), and 10 years (n = 1) reported (Table 1). Based on the previous use of anthelmintics on the farms studied, MLs were the most frequently reported anthelmintics (n = 12), with IVM most common (n = 7), followed by MOX (n = 6) and ABM (n = 2). Historical use of FBZ (n = 5), PYR (n = 4), and combinations of ABM and MOR (n = 1) and OXF and PYR (n = 1) was also described. The most commonly reported strategies for the use of anthelmintics were interval i.e., calendar-based (n = 7), targeted i.e., based on FEC results (n = 3), or interval and/or targeted (n = 3), while four studies did not provide such information. The most recent use of anthelmintic in horses reported was 90 days (n = 2), 60–180 days (n = 2), 6–12 weeks (n = 6), and 12–16 weeks (n = 1) prior to commencement of the ERP studies (Table 1). No study reported a previous diagnosis of AR in parasites of the study populations of horses; however, four publications included horses with a suspected or confirmed shortened ERP before the study (Table 2).
Stocking density and grazing patterns have also been proposed to influence the development of AR (Schumacher et al., 2009). The analyses of information on stocking density and grazing patterns in the ERP studies revealed that horses were either stabled (n = 2), stabled and grazed (n = 3), or pastured (n = 9). Seven studies did not provide information on animal housing. When pastured, horses were grazed rotationally (n = 2), continuously (n = 3) or not specified (n = 4). Few papers (n = 5) provided information on climate or season when the study was undertaken (Sanna et al., 2016; Beasley et al., 2017; Abbas et al., 2021; Butler et al., 2021; Johnson and Biddle, 2021). Such factors are important, especially in ERP studies, as season and climate can affect parasite epidemiology and influence ERP studies due to their longer length than a regular FECRT. For example, a simulation-based study found a direct effect of climate on parasitic infection dynamics and, along with seasonal variation, an influence on the development of AR in cyathostomins (Nielsen et al., 2019b).
One of the critical criteria before the commencement of an ERP study is to assess the FECs in the study population which confirms the patency of parasitic infection. Available data on pre-treatment worm egg count indicated that the minimum FEC for inclusion ranged between 20 and 200 epg; however, such data were not provided in six studies (Table 1). A modified McMaster technique was the most frequently used diagnostic method to determine the FEC (n = 13), with a minimum detection limit (MDL) ranging from 6.2 to 50 epg. Other studies used a modified McMaster centrifugation-enhanced method with an MDL of 20 epg (n = 1), a modified salt flotation method with an MDL of 1 epg (n = 2), a modified Wisconsin (a centrifugal floatation method) with an MDL of 1 epg (n = 3), Paracount-EPG kit with an MDL of 5 epg (n = 1), and Mini-FLOTAC with an MDL of 5 epg (n = 1). The most frequently used method for calculating FECR was according to the WAAVP guidelines (n = 13) (Coles et al., 1992). Alternative methods used to calculate FECR were arcsine transformed FEC data (n = 2) or by geometric mean FEC data (n = 1). One publication calculated FECR using three methods; arcsine transformed FECR data, according to WAAVP guidelines and non-parametric bootstrapping (Relf et al., 2014). The method of calculation of FECR was occasionally not specified (n = 3) or it was not calculated (n = 1).
3.5. Risk of bias
Six and three studies were considered to have low risk, and some concerns of selection bias, respectively (Table 3). Thirteen studies could not be assessed for the risk of selection bias. Performance bias was not applicable for comparative studies in which all participants received the same intervention (n = 8), while the remaining studies were judged as high risk (n = 1), with some concerns (n = 1) or no information (n = 9). Few papers provided sufficient information to make a judgement of detection bias (n = 3), with one and two studies judged as high and low risk, respectively (Table 3). Most studies had a low risk of attrition bias (n = 17), although two studies were judged as high risk (Rosanowski et al., 2017; Butler et al., 2021), and some concerns were identified for one study (Holm-Martin et al., 2005). No information was available for one study (Daniels and Proudman, 2016). The risk of reporting bias was frequently low (n = 12), although judgements of some concerns (n = 1), high risk (n = 1) or no information (n = 2) were made. Overall, most studies had a low risk of bias (n = 11), while six had some concerns of bias and four had a high risk (Rossano et al., 2010; Rosanowski et al., 2017; Zak et al., 2017; Butler et al., 2021).
Table 3.
Risk of bias assessment for publications eligible for evaluation of the ERP (n = 21).
| Anthelmintic(s) | Study design | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Risk of bias judgement | Reference |
|---|---|---|---|---|---|---|---|---|
| IVM | Comparative | N/A | N/A | No information | Low | Low | Low | Borgsteede et al. (1993) |
| IVM | Comparative | N/A | N/A | No information | Low | Some concerns | Some concerns | von Samson-Himmelstjerna et al. (2007) |
| IVM | Comparative | N/A | N/A | No information | Low | Low | Low | McFarlane et al. (2010) |
| IVM | Randomised controlled | Low | No information | No information | Low | Low | Low | Larsen et al. (2011) |
| IVM | Comparative | N/A | N/A | No information | Low | High | High | Zak et al. (2017) |
| IVM | Comparative | N/A | N/A | No information | High | Some concerns | High | Rosanowski et al. (2017) |
| IVM | Comparative | N/A | N/A | No information | Low | Low | Low | Molena et al. (2018) |
| IVM | Comparative | Some concerns | Low | No information | High | No information | High | Butler et al. (2021) |
| IVM, MOX | Comparative | Some concerns | No information | No information | Low | Low | Some concerns | Johnson and Biddle (2021) |
| MOX | Comparative | Low | Some concerns | No information | Low | Some concerns | Some concerns | Abbas et al. (2021) |
| IVM, MOX | Randomised controlled | No information | No information | No information | Low | Some concerns | Some concerns | (van Doorn et al., 2014) |
| IVM, MOX | Retrospective | N/A | Some concerns | No information | No information | Low | Some concerns | Daniels and Proudman (2016) |
| IVM, MOX, ABM | Comparative | No information | No information | No information | Low | Low | Low | Beasley et al. (2017) |
| IVM, MOX, PYR, FBZ | Comparative | No information | No information | No information | Low | Some concerns | Some concerns | Relf et al. (2014) |
| IVM, MOX | Randomised comparative | Low | No information | Low | Low | Low | Low | Geurden et al. (2014) |
| IVM, MOX, PYR, FBZ | Comparative | Low | No information | Low | Low | Low | Low | Sanna et al. (2016) |
| IVM, MOX | Comparative | Low | No information | No information | Low | Low | Low | Kooyman et al. (2016) |
| MOX | Comparative | N/A | N/A | No information | Low | Low | Low | Tzelos et al. (2017) |
| MOX, ABM | Randomised controlled | Low | No information | No information | Some concerns | Low | Some concerns | Holm-Martin et al. (2005) |
| MOX, FBZ | Observational field | N/A | High | High | Low | Some concerns | High | Rossano et al. (2010) |
| PYR | Comparative | N/A | N/A | No information | Low | No information | Low | Boersema et al. (1995) |
Abbreviations: ERP, egg reappearance period; N/A, not applicable; ALB, albendazole; ABM, abamectin; FBZ, fenbendazole; IVM, ivermectin; MOX, moxidectin; PYR, pyrantel.
4. Discussion
Reduced ERP of cyathostomins is considered to reflect emerging AR. However, recent research findings suggest there could be alternative explanations for reduced ERP (Nielsen et al., 2022b). Further, the lack of consensus on the definition of ERP complicates the interpretation and consideration of other factors that could contribute to reduced ERP. In our systematic review, eight different definitions of the ERP were identified: when more than one definition was used within a single study, this resulted in differing ERP estimates. Here we discuss the findings of the review and the relationship between reduced ERP and AR development, new developments in the definition of the ERP, and other factors that can potentially contribute to the reduction in the efficacy of anthelmintics. Finally, we suggest directions for future ERP studies.
4.1. Change in reported ERP over the review period
When MOX and IVM were first introduced, the ERPs reported for these drugs were 112–154 days and 63–91 days, respectively (Borgsteede et al., 1993; Boersema et al., 1996; Demeulenaere et al., 1997; DiPietro et al., 1997). These early studies followed a similar study design as of the recent studies where faecal samples were collected for weekly or biweekly egg counts for a variable time period following anthelmintic treatment but the criteria for defining ERP was vastly different which makes comparison complicated, while the manufacturers of MOX claimed its ERP to be at least 14 weeks in Australia (Anonymous, 2022). Subsequently, within range ERPs of both MOX (70–84 days) and IVM (42–56 days) were reported when the drugs were effective (Nielsen et al., 2019a). The mean ERP for MOX and IVM has significantly reduced from previous effective levels of 70–84 days and 42–56 days to 35 and 28 days, respectively. This change should be interpreted with caution due to the wide range of data points and small sample sizes in the available data especially when comparing the latest ERP values with the early studies where various ERP definitions were used (Borgsteede et al., 1993; Boersema et al., 1996; Demeulenaere et al., 1997; DiPietro et al., 1997). Evaluating the estimated prevalence of shortened ERP in multiple cyathostomin populations is of importance as it indicates temporal patterns in ERP values since the drug of interest was first registered. Shortening of the ERP has frequently been reported for IVM and/or MOX in studies included in this review. However, the possibility of overestimating the prevalence of shortened ERP must be considered, as some studies selected horses for a known shortened ERP (van Doorn et al., 2014; Kooyman et al., 2016), including one study (Kooyman et al., 2016) where the study population of horses identified to have a shortened ERP were also used in a previous study (Geurden et al., 2014).
4.2. ERP definitions and the impact of interpretation of data
Irrespective of the ERP definition used, if a study calculates the %FECR at 2 weeks post-treatment and finds no eggs, it means that the anthelmintic used is 100% effective and that the reappearance of eggs at any time point following the second week would be apparently the ERP but when it would be declared as shortened, was the main point of variation and various studies interpreted it differently. Different methods for determination of the ERP and interpretation of the ERP thresholds are described ranging from the first appearance of eggs following treatment to complex calculations. In an attempt to address the lack of consensus on/standardisation, the WAAVP has defined the ERP for strongylids of horses as the post-treatment week at which the upper 95% confidence limit (UCL) of calculated %FECR falls below the %FECR calculated at 2 weeks post-treatment minus 10% (Nielsen et al., 2022c). Recently, this new ERP definition was used to evaluate the retrospective data on ERP for IVM and MOX and found evidence of increasingly reduced ERP values for both drugs over the course of three decades (Nielsen, 2022). The results of several studies indicate that ERP values for IVM and MOX have shortened since the introduction of these drugs (von Samson-Himmelstjerna et al., 2007; Geurden et al., 2014; van Doorn et al., 2014; Molena et al., 2018; Nielsen et al., 2022a). Of considerable concern is the finding that ERP values are often the same (i.e., 4–5 weeks) for both drugs (van Doorn et al., 2014; Rossano et al., 2010; Abbas et al., 2021; Nielsen et al., 2022b). For some of the previously published studies reviewed by Nielsen (2022), insufficient data were available to implement the ERP definition described by the WAAVP, and the author used mean FECR instead of UCL. To facilitate consistency and ability to compare results of studies, future studies should use the ERP definition given by WAAVP.
4.3. Association between the active ingredient and the ERP
Moxidectin is one of only two equine anthelmintics with documented efficacy against all encysted cyathostomins (Bellaw et al., 2018) and has a longer half-life than all other anthelmintics (Cobb and Boeckh, 2009). Cyathostomin resistance to IVM has been reported recently after almost four decades of intensive use globally, while MOX is a comparatively newer drug and was expected to remain effective for at least the same duration as IVM. However, it was also suggested that resistance would develop quickly against MOX due to larvicidal efficacy and greater lipophilicity (Sangster, 1999), a phenomenon now becoming obvious when resistance to MOX has been reported from various parts of the world (Flores et al., 2020; Nielsen et al., 2020; Abbas et al., 2021). Another possibility for resistance in cyathostomins to multiple ML drugs is the phenomenon of co-resistance (if two drugs share a common mode of action and resistance to one drug has already developed, it is likely to be developed in the other drug) among MLs which has been reported previously in sheep nematodes using laboratory experiments (Conder et al., 1993; Ménez et al., 2016). Reports of multiple ML resistance in Parascaris equorum has been published based on field studies (Nielsen, 2022) that could be due to phenomenon of co-resistance, but no experimental study has been conducted to demonstrate such development. While it is unclear yet whether shortened ERP indicates early development of AR, many studies have evaluated the efficacy of both IVM and MOX, although only three have illustrated a shortened ERP for both anthelmintics on the same farm (Geurden et al., 2014; van Doorn et al., 2014; Nielsen et al., 2022b).
Regarding FBZ, AR was reported more frequently than reduced ERP (Parry et al., 1993; Tarigo-Martinie et al., 2001; Lind et al., 2007; Rossano et al., 2010; Relf et al., 2014; Bellaw et al., 2018). This was consistent with reports on well-established resistance to this anthelmintic (Matthews, 2014; Nielsen, 2022). The high prevalence of resistance of cyathostomins to BZs and PYR suggests future research estimating the ERPs for these anthelmintics would be of limited value, as once resistance to a particular drug is established, reversion to parasiticide efficacy is unlikely (Jackson and Coop, 2000; Lyons et al., 2007). However, Leathwick et al. (2015) showed that reversion is possible in sheep nematodes under appropriate resistance management programmes, but the use of such strategies with cyathostomins requires further investigation.
4.4. Mechanism for reduced ERP
The underlying mechanism of shortening the ERP for MLs remains unknown. One of the most widely suggested explanations is the reduced susceptibility of luminal L4 cyathostomins to MLs (Lyons et al., 2009; van Doorn et al., 2014; Kooyman et al., 2016; Bellaw et al., 2018). In addition, it is also proposed that accelerated maturation of the surviving luminal L4s could contribute to the shortened ERPs (Lyons et al., 2009; Geurden et al., 2014). However, in one study, a shortened ERP for MOX (42 days) in a group of horses that were subsequently treated with pyrantel at 84 days post-treatment and determined to have an ERP within the expected range for pyrantel was reported (Kooyman et al., 2016). The authors proposed that if the shortening of the ERP for MOX was attributable to the accelerated maturation of luminal stages, it would be observed independently of the anthelmintic used; hence, decreased MOX susceptibility was the most likely cause of a shortened ERP (Kooyman et al., 2016).
Larval culture and molecular methods have identified cyathostomin species with shortened ERPs, including the predominance of Cylicocyclus sp., Cyathostomum catinum and Cylicostephanus longibursatus (van Doorn et al., 2014; Kooyman et al., 2016; Bellaw et al., 2018; Molena et al., 2018; Nielsen et al., 2022b), leading to the suggestion that the shortening of the ERP may be due to the selection of cyathostomin species with a shorter life cycle (Sangster, 1999; Geurden et al., 2014). However, this is unlikely as the population structure of cyathostomins in horses with normal and shortened ERPs has been found to be similar before anthelmintic treatment but species compositions differ significantly following anthelmintic treatment (van Doorn et al., 2014; Kooyman et al., 2016). Another study demonstrated that the ERPs of cyathostomin species were variable following IVM and MOX treatments as almost all the species present in IVM pre-treatment samples were also detected at week 4 post-treatment whereas the species reduced 100% by MOX for longer time points (Cylicocyclus sp. and Cyathostomum sp.) appeared more rapidly (Johnson and Biddle, 2021) than others. Variations in the appearance of species following treatment were either attributed to various environmental factors relating to the pasture grazed during the study period or the possibility of the emergence of encysted larvae from the mucosa (Johnson and Biddle, 2021). However, the results of this study should be interpreted cautiously given the low sample size and bias concerns. Recently, a comprehensive study (Nielsen et al., 2022b) that used high throughput DNA metabarcoding techniques coupled with necropsy of the horses at two-time points (2 weeks and 5 weeks post-treatment) reported findings consistent with Johnson and Biddle (2021) whereby the numbers of adult cyathostomins were significantly different at 2 weeks post-treatment in control, IVM and MOX treated groups, but similar, high burdens of adults were found in both treated groups at 5 weeks. In addition, the study did not find any major shift in cyathostomin species composition (Nielsen et al., 2022b) following IVM and MOX treatment, and the most abundant species reported (Cylicocyclus nassatus, Cylicostephanus longibursatus, and Cyathostomum catinatum) were the same as those that have been reported in the last four decades (Bellaw and Nielsen, 2020).
Resumed development of encysted larval stages has also been proposed as a cause of shortened ERP (Borgsteede et al., 1993), but comprehensive evidence to support this hypothesis has not been reported to date. However, the removal of adult cyathostomin burdens from the intestinal lumen has been shown to result in the emergence of encysted stages quickly, allowing a rapid replacement of the adult worm population with larvae (Love and Duncan, 1992). A further consideration is a variation in the prepatent periods of cyathostomin species which are subjected to changes with time, season, region, previous parasitic exposure, and age of the horse (Round, 1969; Love and Duncan, 1992; Matthews, 2011). It is likely that anthelmintic treatment would select the cyathostomin species with shorter pre-patent periods to resume development, leading to reduced ERP for a particular drug. Additional studies are required to investigate possible associations between the resumption of encysted larval development, cyathostomin species composition and reduced ERP.
Another explanation for reduced ERP was greater selection pressure on cyathostomin species with shorter prepatent periods (Sangster, 1999; Schumacher and Taintor, 2008). This hypothesis was linked to the efficacy of a drug against encysted stages of a particular species and age of the horse: the higher efficacy of a drug against mucosal stages and horses of younger age was speculated to be favourable for selection pressure (Sangster, 1999). Currently, it is not possible to determine associations between shortened ERPs in specific cyathostomin species or different host age categories (e.g., younger vs adult horses) from the published data due to complexities including variable definition used for the ERP evaluation, variable study designs, limited data on host variables (e.g., horse age) and environmental variable (e.g., season, climate), and the lack of information on cyathostomin species detected following treatments. Additionally, in the only study where such information was available, no major shift in species composition was observed (Nielsen et al., 2022b).
Another study proposed that reduced ERP could be due to quick reinfection with infective L3s following anthelmintic treatment where horses are exposed to infective larvae whilst grazing pasture (Johnson and Biddle, 2021). However, this will require further investigations as a shortened ERP has been observed in horses stabled for the entire study period where reinfection was unlikely (Borgsteede et al., 1993; Monahan et al., 1995, 1996; van Doorn et al., 2014).
4.5. Association between shortened ERP and AR
To date, no study has reported repeated examination of host (horse) populations with known shortened ERP to examine the further progression of the shortening and to observe the possible development of AR. Thus, there is no clear link between shortened ERP and the development of AR and whether a shortened ERP will lead to faster resistance development. Further avenues for investigation of ERP include monitoring cyathostomins ERP in different age categories of horses in various seasons located in different regions of the world.
4.6. Association between treatment strategy and AR
Interval programs involving frequent use of anthelmintics may contribute to AR and the shortening of the cyathostomin ERP (Kaplan, 2004a, b; Matthews, 2014). Unfortunately, few studies evaluating ERP provided thorough information on previous anthelmintic use in the study horse population(s). Shortened IVM and MOX ERPs have been reported on farms with a high frequency of anthelmintic use (von Samson-Himmelstjerna et al., 2007; Geurden et al., 2014), including farms where predominantly MLs were previously used (Geurden et al., 2014). In contrast, ERPs within the expected range have been reported on farms using interval treatment where horses received less frequent treatments (von Samson-Himmelstjerna et al., 2007), suggesting that frequency of treatment has more important consequences for development of AR than simply treatment program method. However, no study has reported a direct comparison of ERP in horses with different historical anthelmintic regimens. If the factors responsible for the development of AR also contribute to the shortening of ERP, targeted anthelmintic programs could be a better option in controlling the loss of anthelmintic efficacy which reduces the selection pressure for AR by promoting maintenance of refugia (whereby a susceptible population of worms unexposed to anthelmintics is preserved) in untreated horses and thus reducing the selection pressure for AR (Kaplan and Nielsen, 2010; Matthews, 2014; Nielsen et al., 2014).
4.7. Association between non-chemical parasite control and ERP
Rotational grazing has been recommended to reduce parasitic infection by decreasing pasture contamination (Francisco et al., 2012). Interestingly, two studies specifying a rotational grazing pattern illustrated shortened ERPs for MLs (Rosanowski et al., 2017; Tzelos et al., 2017). Attrition and calculation of the ERP on properties where resistance was identified, conferred a high risk of bias for one study as some breeding farms discontinued the study in the follow-up periods starting from 14, 28 and 35 days post-treatment (Rosanowski et al., 2017), while another study included cohorts which had highly variable pasture management (Tzelos et al., 2017). Climate and seasonal variations between countries and regions are considerable as they significantly influence the pasture conditions and parasite burden (Nielsen et al., 2007). However, relevant climatic factors have rarely been reported in studies evaluating the ERP. In future, ERP research should describe the climatic conditions in which the study is undertaken.
4.8. Association between host factors and ERP
Younger horses are more susceptible to parasitism and often have higher FECs (Eysker et al., 2008; Nielsen et al., 2019b). However, the impact of age on the ERP is unclear. Some studies reported no significant difference in the ERP for IVM in foals, yearlings and adults (Boersema et al., 1996; von Samson-Himmelstjerna et al., 2007), while other studies reported earlier ERPs for IVM and MOX in younger horses (Herd and Gabel, 1990; Demeulenaere et al., 1997; Eysker et al., 2008). These findings are difficult to interpret where the age distribution or the number of horses in each age group is not provided in some of the studies included in this review (Demeulenaere et al., 1997; von Samson-Himmelstjerna et al., 2007), or where the age groups evaluated were considerably different (i.e., more adults than young horses) (Eysker et al., 2008).
4.9. Study weaknesses
We acknowledge some weaknesses of this systematic review. Most importantly, it would be ideal to perform a quantitative analysis of ERP data. However, this was not performed due to the small sample size of included studies and the variability of data reported in ERP studies. Although the risk of bias was assessed using criteria described by Higgins et al. (2019), the overall risk of bias interpretation was made by the review authors and could contribute to judgement bias.
5. Conclusions and future directions
From the 1990s to mid-2022, ERP was widely accepted as an early indicator of AR development (Sangster, 1999) and was supported by a few investigations that found reduced efficacy of anthelmintics against luminal larval stages leads to reduced EPR (Lyons et al., 2009; Lyons and Tolliver, 2013). Recently, Nielsen et al. (2022b) demonstrated that the luminal larval stages surviving the anthelmintic treatment do not equate at two- and five-week post-treatment and suggested that ERP might not be a true indicator of the early development of resistance, rather it could be a performance indicator for anthelmintic products. However, these findings of Nielsen et al. (2022b) study require further investigations, given the study was conducted at one location in one season in a population of young (≤5 years) horses. Despite the lack of consensus on the definition of the ERP, a comparable definition was found in the 21 studies included in this review, the ERP for MOX and IVM has reduced remarkably from previously reported periods of 70–84 days and 42–56 days–35 days and 28 days, respectively. Reasons for the substantial reductions in ERP remain unclear; however, possible explanations considered by the research community have been:
-
i.
increased selection pressure on larval stages;
-
ii.
reduced efficacy against luminal stages;
-
iii.
the rapid development of luminal stages that survive treatment;
-
iv.
decreased efficacy of MOX and FBZ against encysted larval stages;
-
v.
rapid release of encysted larvae and the development to successive stages;
-
vi.
reinfection rate variation among cyathostomin species;
-
vii.
reduced prepatent periods of some species;
-
viii.
continuous ingestion of fresh infective larvae from the pastures;
-
ix.
horse and farm level factors; and
-
x.
environmental and regional factors, including season, climate, temperature, rainfall etc.
Furthermore, numerous studies estimating the ERP for cyathostomins reported incomplete information on previous anthelmintic use, horse age groups, grazing, season and climate, precluding assessment of risk factors that can promote the shortening of the ERP. Recently, the WAAVP revised the guidelines for evaluating the efficacy of equine anthelmintics and redefined the ERP for strongyles to address the lack of consensus on the definition of the ERP; it is expected that future ERP studies will follow the new definition, facilitating direct comparisons between studies globally which would further help to probe factors associated with shortening of ERP. Future research should also provide information on factors including the location of the study, climatic zones, season, farm type, horse breed, age of the selected horses, sample size, method of randomisation, stocking density, history of AR or reduced ERP in the study population/farm, and bias (if any) in the study design. Last, due to widespread resistance in cyathostomins, the estimation of the ERP for FBZ and PYR is likely to be of limited use. Resources could be better allocated to prolonging the efficacy of MLs and the development of new anthelmintics for horses.
Funding
Financial assistance was provided by AgriFutures Australia, Thoroughbred Breeders Australia and Boehringer Ingelheim, Australia. Boehringer Ingelheim did not have any role in the design or content of this manuscript.
Declaration of competing interest
The authors are members of the Australian Equine Parasitology Advisory Panel supported by AgriFutures Australia and Boehringer Ingelheim Animal Health Australia. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The first draft of the review was completed by SLM as a final year research project for the Doctor of Veterinary Medicine at The University of Melbourne.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2022.12.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abbas G., Ghafar A., Hurley J., Bauquier J., Beasley A., Wilkes E.J.A., Jacobson C., El-Hage C., Cudmore L., Carrigan P., Tennent-Brown B., Gauci C.G., Nielsen M.K., Hughes K.J., Beveridge I., Jabbar A. Cyathostomin resistance to moxidectin and combinations of anthelmintics in Australian horses. Parasites Vectors. 2021;14:597. doi: 10.1186/s13071-021-05103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous . 2022. Equest®plustape, Overview.https://www.zoetis.com.au/product-class/parasiticide-products/equest-plus-tape.aspx [Google Scholar]
- Baranova M.V., Panova O.A., Polukhina D.N., Panova D.S. Reduction of the nematode egg reappearance period in horses after anthelmintic therapy. Vet. World. 2022;15:1530–1534. doi: 10.14202/vetworld.2022.1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley A.M., Kotze A.C., Allen K., Coleman G.T. A survey of macrocyclic lactone efficacy in Australian cyathostomin populations. Vet. Parasitol. Reg. Stud. Rep. 2017;8:127–132. doi: 10.1016/j.vprsr.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Bellaw J.L., Krebs K., Reinemeyer C.R., Norris J.K., Scare J.A., Pagano S., Nielsen M.K. Anthelmintic therapy of equine cyathostomin nematodes - larvicidal efficacy, egg reappearance period, and drug resistance. Int. J. Parasitol. 2018;48:97–105. doi: 10.1016/j.ijpara.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Bellaw J.L., Nielsen M.K. Meta-analysis of cyathostomin species-specific prevalence and relative abundance in domestic horses from 1975–2020: emphasis on geographical region and specimen collection method. Parasites Vectors. 2020;13:509. doi: 10.1186/s13071-020-04396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersema J.H., Borgsteede F.H.M., Eysker M., Saedt I. The reappearance of strongyle eggs in faeces of horses treated with pyrantel embonate. Vet. Q. 1995;17:18–20. doi: 10.1080/01652176.1995.9694524. [DOI] [PubMed] [Google Scholar]
- Boersema J.H., Eysker M., Maas J., Aar W.M.v.d. Comparison of the reappearance of strongyle eggs in foals, yearlings, and adult horses after treatment with ivermectin or pyrantel. Vet. Q. 1996;18:7–9. doi: 10.1080/01652176.1996.9694602. [DOI] [PubMed] [Google Scholar]
- Borgsteede F.H.M., Boersema J.H., Gaasenbeek C.P.H., van der Burg W.P.J. The reappearance of eggs in faeces of horses after treatment with ivermectin. Vet. Q. 1993;15:24–26. doi: 10.1080/01652176.1993.9694363. [DOI] [PubMed] [Google Scholar]
- Butler A.J., Greenbank H., Parrish R., Nielsen M.K., Stoughton W.B. Prevalence of anthelmintic resistant cyathostomins in prince edward island, Canada. Vet. Parasitol. Reg. Stud. Rep. 2021;26 doi: 10.1016/j.vprsr.2021.100629. [DOI] [PubMed] [Google Scholar]
- Cain J.L., Foulk D., Jedrzejewski E., Stofanak H., Nielsen M.K. The importance of anthelmintic efficacy monitoring: results of an outreach effort. Parasitol. Res. 2019;118:2877–2883. doi: 10.1007/s00436-019-06423-6. [DOI] [PubMed] [Google Scholar]
- Cırak V.Y., Güleğen E., Yıldırım F., Durmaz M. A field study on the efficacy of doramectin against strongyles and its egg reappearance period in horses. Deut. tierarztl. Wschr. 2007;114:64–66. [PubMed] [Google Scholar]
- Cobb R., Boeckh A. Moxidectin: a review of chemistry, pharmacokinetics and use in horses. Parasites Vectors. 2009;2:S5. doi: 10.1186/1756-3305-2-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles G.C., Bauer C., Borgsteede F.H.M., Geerts S., Klei T.R., Taylor M.A., Waller P.J. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992;44:35–44. doi: 10.1016/0304-4017(92)90141-u. [DOI] [PubMed] [Google Scholar]
- Conder G.A., Thompson D.P., Johnson S.S. Demonstration of co-resistance of Haemonchus contortus to ivermectin and moxidectin. Vet. Rec. 1993;132:651–652. doi: 10.1136/vr.132.26.651. [DOI] [PubMed] [Google Scholar]
- Cullinane A.A., Barr B., Bernard W., Duncan J.L., Mulcahy G., Smith I.M., Timoney J.F. In: The Equine Manual. second ed. Higgins A.J., Snyder J.R., editors. W.B. Saunders; Edinburgh: 2006. Chapter 1 - infectious diseases; pp. 1–111. [Google Scholar]
- Daniels S.P., Proudman C.J. Shortened egg reappearance after ivermectin or moxidectin use in horses in the UK. Vet. J. 2016;218:36–39. doi: 10.1016/j.tvjl.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Demeulenaere D., Vercruysse J., Dorny P., Claerebout E. Comparative studies of ivermectin and moxidectin in the control of naturally acquired cyathostome infections in horses. Vet. Rec. 1997;141:383–386. doi: 10.1136/vr.141.15.383. [DOI] [PubMed] [Google Scholar]
- DiPietro J.A., Hutchens D.E., Lock T.F., Walker K., Paul A.J., Shipley C., Rulli D. Clinical trial of moxidectin oral gel in horses. Vet. Parasitol. 1997;72:167–177. doi: 10.1016/s0304-4017(97)01108-4. [DOI] [PubMed] [Google Scholar]
- Duncan J.L., Bairden K., Abbott E.M. Elimination of mucosal cyathostome larvae by five daily treatments with fenbendazole. Vet. Rec. 1998;142:268–271. doi: 10.1136/vr.142.11.268. [DOI] [PubMed] [Google Scholar]
- Eysker M., Bakker J., Berg M.V.D., van Doorn D.C.K., Ploeger H.W. The use of age-clustered pooled faecal samples for monitoring worm control in horses. Vet. Parasitol. 2008;151:249–255. doi: 10.1016/j.vetpar.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Fischer J.K., Hinney B., Denwood M.J., Traversa D., von Samson-Himmelstjerna G., Clausen P.H. Efficacy of selected anthelmintic drugs against cyathostomins in horses in the federal state of Brandenburg, Germany. Parasitol. Res. 2015;114:4441–4450. doi: 10.1007/s00436-015-4685-7. [DOI] [PubMed] [Google Scholar]
- Flores A.G., Osmari V., Ramos F., Marques C.B., Ramos D.J., Botton A.S., Vogel F.S.F., Sangioni L.A. Multiple resistance in equine cyathostomins: a case study from military establishments in Rio Grande do Sul, Brazil. Braz. J. Vet. Parasitol. 2020;29 doi: 10.1590/S1984-29612020086. [DOI] [PubMed] [Google Scholar]
- Francisco R., Paz-Silva A., Francisco I., Javier Cortiñas F., Miguélez S., Suárez J., Cazapal-Monteiro C.F., Suárez J.L., Arias M.S., Sánchez-Andrade R. Preliminary analysis of the results of selective therapy against strongyles in pasturing horses. J. Equine Vet. Sci. 2012;32:274–280. [Google Scholar]
- Geurden T., van Doorn D., Claerebout E., Kooyman F., De Keersmaecker S., Vercruysse J., Besognet B., Vanimisetti B., di Regalbono A.F., Beraldo P., Di Cesare A., Traversa D. Decreased strongyle egg re-appearance period after treatment with ivermectin and moxidectin in horses in Belgium, Italy and The Netherlands. Vet. Parasitol. 2014;204:291–296. doi: 10.1016/j.vetpar.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Herd R., Gabel A.J. Reduced efficacy of anthelmintics in young compared with adult horses. Equine Vet. J. 1990;22:164–169. doi: 10.1111/j.2042-3306.1990.tb04237.x. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. John Wiley & Sons; 2019. Cochrane Handbook for Systematic Reviews of Interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm-Martin M., Levot G., Dawson K. Control of endoparasites in horses with a gel containing moxidectin and praziquantel. Vet. Rec. 2005;156:835–838. doi: 10.1136/vr.156.26.835. [DOI] [PubMed] [Google Scholar]
- Jackson F., Coop R.J.P. The development of anthelmintic resistance in sheep nematodes. Parasitology. 2000;120:95–107. doi: 10.1017/s0031182099005740. [DOI] [PubMed] [Google Scholar]
- Johnson A.C.B., Biddle A.S. The use of molecular profiling to track equine reinfection rates of cyathostomin species following anthelmintic administration. Animals. 2021;11:1345. doi: 10.3390/ani11051345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., Klei T.R., Lyons E.T., Lester G., Courtney C.H., French D.D., Tolliver S.C., Vidyashankar A.N., Zhao Y. Prevalence of anthelmintic resistant cyathostomes on horse farms. J. Am. Vet. Med. Assoc. 2004;225:903–910. doi: 10.2460/javma.2004.225.903. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., Nielsen M.K. An evidence-based approach to equine parasite control: it ain't the 60s anymore. Equine Vet. Educ. 2010;22:306–316. [Google Scholar]
- Kooyman F., van Doorn D., Geurden T., Mughini-Gras L., Ploeger H.W., Wagenaar J. Species composition of larvae cultured after anthelmintic treatment indicates reduced moxidectin susceptibility of immature Cylicocyclus species in horses. Vet. Parasitol. 2016;227:77–84. doi: 10.1016/j.vetpar.2016.07.029. [DOI] [PubMed] [Google Scholar]
- Larsen M.L., Ritz C., Petersen S.L., Nielsen M.K. Determination of ivermectin efficacy against cyathostomins and Parascaris equorum on horse farms using selective therapy. Vet. J. 2011;188:44–47. doi: 10.1016/j.tvjl.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Lichtenfels J.R., Kharchenko V.A., Dvojnos G.M. Illustrated identification keys to strongylid parasites (Strongylidae: nematoda) of horses, zebras and asses (Equidae) Vet. Parasitol. 2008;156:4–161. doi: 10.1016/j.vetpar.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Lignon J.S., Gonçalves N.F., Cunha L.L., Antunes T.A., Leão M.S., Camassola J.L.T., Pellegrin T.G., Ripoll P.K., Pappen F.G., Pinto D.M. Anthelmintic resistance in creole horses in the south of rio grande do sul, Brazil. Arq. Bras. Med. Vet. Zootec. 2021;73:598–604. [Google Scholar]
- Lind E.O., Kuzmina T., Uggla A., Waller P.J., Höglund J. A field study on the effect of some anthelmintics on cyathostomins of horses in Sweden. Vet. Res. Commun. 2007;31:53–65. doi: 10.1007/s11259-006-3402-5. [DOI] [PubMed] [Google Scholar]
- Love S., Duncan J.L. Development of cyathostome infection of helminth-naive foals. Equine Vet. J. 1992;24:93–98. [Google Scholar]
- Love S., Murphy D., Mellor D. Pathogenicity of cyathostome infection. Vet. Parasitol. 1999;85:113–122. doi: 10.1016/s0304-4017(99)00092-8. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Collins S.S. Study (1991 to 2001) of drug-resistant Population B small strongyles in critical tests in horses in Kentucky at the termination of a 40-year investigation. Parasitol. Res. 2007;101:689–701. doi: 10.1007/s00436-007-0535-6. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Collins S.S. Probable reason why small strongyle EPG counts are returning “early” after ivermectin treatment of horses on a farm in Central Kentucky. Parasitol. Res. 2009;104:569–574. doi: 10.1007/s00436-008-1231-x. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C. Further indication of lowered activity of ivermectin on immature small strongyles in the intestinal lumen of horses on a farm in Central Kentucky. Parasitol. Res. 2013;112:889–891. doi: 10.1007/s00436-012-3098-0. [DOI] [PubMed] [Google Scholar]
- Martins N.S., Pinto D.M., da Cunha L.L., Lignon J.S., dos Santos T.C., Evaristo T.A., Pappen F.G., Nizoli L.Q. Assessment of the efficacy of commercial anthelmintics in horses naturally infected with gastrointestinal nematodes. Med. Vet. UFRPE. 2021;15:28–32. [Google Scholar]
- Matthews J.B. An update on cyathostomins: anthelmintic resistance and worm control. Equine Vet. J. 2008;20:552–560. doi: 10.1111/j.2042-3306.2011.00397.x. [DOI] [PubMed] [Google Scholar]
- Matthews J.B. Facing the threat of equine parasitic disease. Equine Vet. J. 2011;43:126–132. doi: 10.1111/j.2042-3306.2010.00356.x. [DOI] [PubMed] [Google Scholar]
- Matthews J.B. Anthelmintic resistance in equine nematodes. Int. J. Parasitol. Drugs Drug Resist. 2014;4:310–315. doi: 10.1016/j.ijpddr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane D., Hale G.H., Johnson E.M., Maxwell L.K. Fecal egg counts after anthelmintic administration to aged horses and horses with pituitary pars intermedia dysfunction. J. Am. Vet. Med. Assoc. 2010;236:330–334. doi: 10.2460/javma.236.3.330. [DOI] [PubMed] [Google Scholar]
- Ménez C., Alberich M., Kansoh D., Blanchard A., Lespine A. Acquired tolerance to ivermectin and moxidectin after drug selection pressure in the nematode Caenorhabditis elegans. Antimicrob. Agents Chemother. 2016;60:4809–4819. doi: 10.1128/AAC.00713-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier P., Chick B., Alves-Branco F., White C.R. Comparative efficacy, persistent effect, and treatment intervals of anthelmintic pastes in naturally infected horses. Vet. Parasitol. 2001;99:29–39. doi: 10.1016/s0304-4017(01)00453-8. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- Molena R.A., Peachey L.E., Cesare A.D., Traversa D., Cantacessi C. Cyathostomine egg reappearance period following ivermectin treatment in a cohort of UK Thoroughbreds. Parasites Vectors. 2018;11:61. doi: 10.1186/s13071-018-2638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan C.M., Chapman M.R., French D.D., Taylor H.W., Klei T.R. Dose titration of moxidectin oral gel against gastrointestinal parasites of ponies. Vet. Parasitol. 1995;59:241–248. doi: 10.1016/0304-4017(94)00762-2. [DOI] [PubMed] [Google Scholar]
- Monahan C.M., Chapman M.R., Taylor H.W., French D.D., Klei T.R. Comparison of moxidectin oral gel and ivermectin oral paste against a spectrum of internal parasites of ponies with special attention to encysted cyathostome larvae. Vet. Parasitol. 1996;63:225–235. doi: 10.1016/0304-4017(95)00910-8. [DOI] [PubMed] [Google Scholar]
- Morgan E.R., Hetzel N., Povah C., Coles G.C. Prevalence and diagnosis of parasites of the stomach and small intestine in horses in south‐west England. Vet. Rec. 2005;156:597–600. doi: 10.1136/vr.156.19.597. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K. Equine anthelmintic resistance: current status and emerging trends. Int. J. Parasitol. Drugs Drug Resist. 2022;20:76–88. doi: 10.1016/j.ijpddr.2022.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M., Pfister K., von Samson-Himmelstjerna G. Selective therapy in equine parasite control—application and limitations. Vet. Parasitol. 2014;202:95–103. doi: 10.1016/j.vetpar.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Banahan M., Kaplan R.M. Importation of macrocyclic lactone resistant cyathostomins on a US Thoroughbred farm. Int. J. Parasitol. Drugs Drug Resist. 2020;14:99–104. doi: 10.1016/j.ijpddr.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M.K., Branan M.A., Wiedenheft A.M., Digianantonio R., Scare J.A., Bellaw J.L., Garber L.P., Kopral C.A., Phillippi-Taylor A.M., Traub-Dargatz J.L. Anthelmintic efficacy against equine strongyles in the United States. Vet. Parasitol. 2018;259:53–60. doi: 10.1016/j.vetpar.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Kaplan R.M., Thamsborg S.M., Monrad J., Olsen S.N. Climatic influences on development and survival of free-living stages of equine strongyles: implications for worm control strategies and managing anthelmintic resistance. Vet. J. 2007;174:23–32. doi: 10.1016/j.tvjl.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Littman B.A., Orzech S.W., Ripley N.E. Equine strongylids: ivermectin efficacy and fecal egg shedding patterns. Parasitol. Res. 2022;121:1691–1697. doi: 10.1007/s00436-022-07509-4. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Mittel L., Grice A., Erskine M., Graves E., Vaala W., Tully R.C., French D.D., Bowman R., Kaplan R.M. American Association of Equine Practitioners; Lexington: 2019. AAEP Parasite Control Guidelines.https://aaep.org/document/internal-parasite-control-guidelines [Google Scholar]
- Nielsen M.K., Sauermann C.W., Leathwick D.M. The effect of climate, season, and treatment intensity on anthelmintic resistance in cyathostomins: a modelling exercise. Vet. Parasitol. 2019;269:7–12. doi: 10.1016/j.vetpar.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Steuer A.E., Anderson H.P., Gavriliuc S., Carpenter A.B., Redman E.M., Gilleard J.S., Reinemeyer C.R., Poissant J. Shortened egg reappearance periods of equine cyathostomins following ivermectin or moxidectin treatment: morphological and molecular investigation of efficacy and species composition. Int. J. Parasitol. 2022 doi: 10.1016/j.ijpara.2022.09.003. (in press) [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., von Samson-Himmelstjerna G., Kuzmina T.A., van Doorn D.C.K., Meana A., Rehbein S., Elliott T., Reinemeyer C.R. World association for the advancement of veterinary parasitology (WAAVP): third edition of guideline for evaluating the efficacy of equine anthelmintics. Vet. Parasitol. 2022;303 doi: 10.1016/j.vetpar.2022.109676. [DOI] [PubMed] [Google Scholar]
- Parry J., Fisher M., Grimshaw W., Jacobs D. Anthelmintic dosing intervals for horses: comparison of three chemical groups. Vet. Rec. 1993;133:346–347. doi: 10.1136/vr.133.14.346. [DOI] [PubMed] [Google Scholar]
- Peregrine A.S., Molento M.B., Kaplan R.M., Nielsen M.K. Anthelmintic resistance in important parasites of horses: does it really matter? Vet. Parasitol. 2014;201:1–8. doi: 10.1016/j.vetpar.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Piché C.A., Kennedy M.J., Bauck S.W., Goonewardene L. Comparison of three anthelmintics in the control of intestinal nematodes in young horses on fall and winter pasture. Can. Vet. J. 1990;31:841. [PMC free article] [PubMed] [Google Scholar]
- Reid S.W.J., Mair T.S., Hillyer M.H., Love S. Epidemiological risk factors associated with a diagnosis of clinical cyathostomiasis in the horse. Equine Vet. J. 1995;27:127–130. doi: 10.1111/j.2042-3306.1995.tb03048.x. [DOI] [PubMed] [Google Scholar]
- Reinemeyer C.R., Prado J., Nielsen M.K. Comparison of the larvicidal efficacies of moxidectin or a five-day regimen of fenbendazole in horses harboring cyathostomin populations resistant to the adulticidal dosage of fenbendazole. Vet. Parasitol. 2015;214:100–107. doi: 10.1016/j.vetpar.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Relf V.E., Lester H.E., Morgan E.R., Hodgkinson J.E., Matthews J.B. Anthelmintic efficacy on UK Thoroughbred stud farms. Int. J. Parasitol. 2014;44:507–514. doi: 10.1016/j.ijpara.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Rosanowski S.M., Bolwell C.F., Scott I., Sells P.D., Rogers C.W. The efficacy of Ivermectin against strongyles in yearlings on Thoroughbred breeding farms in New Zealand. Vet. Parasitol. Reg. Stud. Rep. 2017;8:70–74. doi: 10.1016/j.vprsr.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Rossano M.G., Smith A.R., Lyons E.T. Shortened strongyle-type egg reappearance periods in naturally infected horses treated with moxidectin and failure of a larvicidal dose of fenbendazole to reduce fecal egg counts. Vet. Parasitol. 2010;173:349–352. doi: 10.1016/j.vetpar.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Round M.C. The prepatent period of some horse nematodes determined by experimental infection. J. Helminthol. 1969;43:185–192. doi: 10.1017/s0022149x00004016. [DOI] [PubMed] [Google Scholar]
- Saeed M.A., Beveridge I., Abbas G., Beasley A., Bauquier J., Wilkes E., Jacobson C., Hughes K.J., El-Hage C., O'Handley R., Hurley J., Cudmore L., Carrigan P., Walter L., Tennent-Brown B., Nielsen M.K., Jabbar A. Systematic review of gastrointestinal nematodes of horses from Australia. Parasites Vectors. 2019;12:188. doi: 10.1186/s13071-019-3445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster N. Pharmacology of anthelmintic resistance in cyathostomes: will it occur with the avermectin/milbemycins? Vet. Parasitol. 1999;85:189–204. doi: 10.1016/s0304-4017(99)00099-0. [DOI] [PubMed] [Google Scholar]
- Sanna G., Pipia A.P., Tamponi C., Manca R., Varcasia A., Traversa D., Scala A. Anthelmintics efficacy against intestinal strongyles in horses of Sardinia. Italy. Parasit. Epi. Cont. 2016;1:15–19. doi: 10.1016/j.parepi.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scare J.A., Leathwick D.M., Sauermann C.W., Lyons E.T., Steuer A.E., Jones B.A., Clark M., Nielsen M.K. Dealing with double trouble: combination deworming against double-drug resistant cyathostomins. Int. J. Parasitol. Drugs Drug Resist. 2020;12:28–34. doi: 10.1016/j.ijpddr.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J., Livesey L., DeGraves F., Blagburn B., Ziska S., Caldwell M., Brock K. Efficacy of moxidectin against cyathostomins after long‐term use in a large herd of draught horses with a high stocking density. Vet. Rec. 2009;164:652–654. doi: 10.1136/vr.164.21.652. [DOI] [PubMed] [Google Scholar]
- Schumacher J., Taintor J. A review of the use of moxidectin in horses. Equine Vet. Educ. 2008;20:546–551. [Google Scholar]
- Silva P.A., Cernea M., Carvalho L.M.D. Anthelmintic resistance in equine nematodes – a review on the current situation, with emphasis in Europe. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca. 2019;76:133–142. [Google Scholar]
- Tarigo-Martinie J.L., Wyatt A.R., Kaplan R.M. Prevalence and clinical implications of anthelmintic resistance in cyathostomes of horses. J. Am. Vet. Med. Assoc. 2001;218:1957–1960. doi: 10.2460/javma.2001.218.1957. [DOI] [PubMed] [Google Scholar]
- Traversa D., von Samson-Himmelstjerna G., Demeler J., Milillo P., Schurmann S., Barnes H., Otranto D., Perrucci S., di Regalbono A.F., Beraldo P., Boeckh A., Cobb R. Anthelmintic resistance in cyathostomin populations from horse yards in Italy, United Kingdom and Germany. Parasites Vectors. 2009;2(Suppl. 2):S2. doi: 10.1186/1756-3305-2-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzelos T., Barbeito J.S., Nielsen M.K., Morgan E.R., Hodgkinson J.E., Matthews J.B. Strongyle egg reappearance period after moxidectin treatment and its relationship with management factors in UK equine populations. Vet. Parasitol. 2017;237:70–76. doi: 10.1016/j.vetpar.2017.02.018. [DOI] [PubMed] [Google Scholar]
- van Doorn D.C., Ploeger H.W., Eysker M., Geurden T., Wagenaar J.A., Kooyman F.N. Cylicocyclus species predominate during shortened egg reappearance period in horses after treatment with ivermectin and moxidectin. Vet. Parasitol. 2014;206:246–252. doi: 10.1016/j.vetpar.2014.10.004. [DOI] [PubMed] [Google Scholar]
- von Samson-Himmelstjerna G., Fritzen B., Demeler J., Schürmann S., Rohn K., Schnieder T., Epe C. Cases of reduced cyathostomin egg-reappearance period and failure of Parascaris equorum egg count reduction following ivermectin treatment as well as survey on pyrantel efficacy on German horse farms. Vet. Parasitol. 2007;144:74–80. doi: 10.1016/j.vetpar.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Vyšniauskas A., Kaziūnaitė V., Kaminskaitė I., Petkevičius S., Pereckienė A., Craven B.J. The role of extense efficacy in the evaluation of anthelmintic resistance in horse strongyles. Helminthologia. 2004;41:73–80. [Google Scholar]
- Xiao L.H., Herd R.P., Majewski G.A. Comparative efficacy of moxidectin and ivermectin against hypobiotic and encysted cyathostomes and other equine parasites. Vet. Parasitol. 1994;53:83–90. doi: 10.1016/0304-4017(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Zak A., Siwinska N., Slowikowska M., Borowicz H., Kubiak K., Hildebrand J., Popiolek M., Niedzwiedz A. Searching for ivermectin resistance in a Strongylidae population of horses stabled in Poland. BMC Vet. Res. 2017;13:210. doi: 10.1186/s12917-017-1133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.