Highlights
-
•
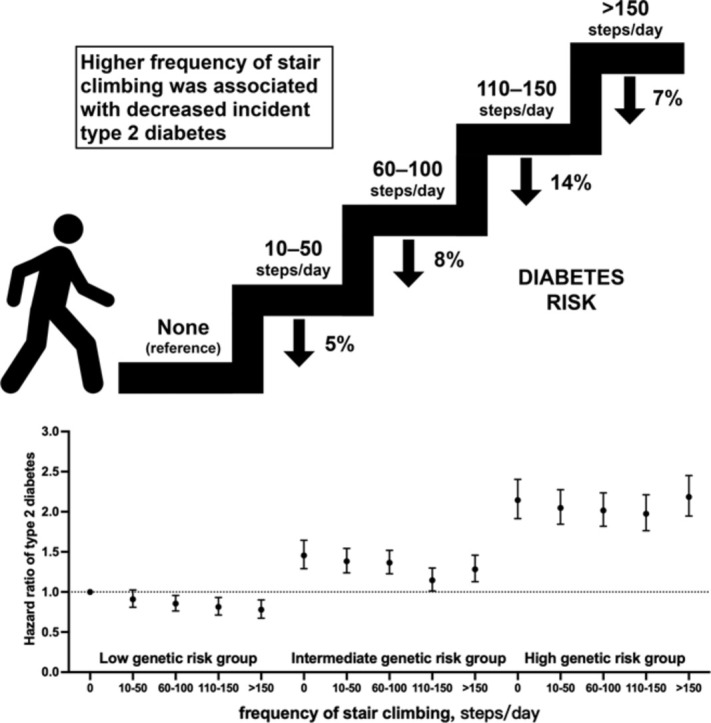
In this 12.1-year follow-up of a cohort study in UK Biobank, those participants who climbed stairs regularly showed a lower risk of incident type 2 diabetes than those who reported no stair climbing.
-
•
The association was modified by genetic background, with the overall risk showing a downward trend in subjects with low genetic risk; those who reported stair climbing activity of 110–150 steps/day appear to have the lowest overall risk of type 2 diabetes among those with intermediate to high genetic risk.
-
•
These findings highlight how stair climbing offers a simple and low-cost complement to public health interventions for diabetes prevention.
Keywords: Cohort, Genetic risk score, Stair climbing, Type 2 diabetes, UK Biobank
Abstract
Background
Cross-sectional evidence and small-scale trials suggest positive effects of stair climbing on cardiometabolic disease and glucose regulation. However, few studies have examined the long-term association between stair climbing and the incidence of type 2 diabetes (T2D). We aimed to prospectively evaluate the association of stair climbing with T2D and assess modifications by genetic predisposition to T2D.
Methods
We included 451,699 adults (mean age = 56.3 ± 8.1 years, mean ± SD; 55.2% females) without T2D at baseline in the UK Biobank and followed up to March 31, 2021. Stair climbing information was collected through the touchscreen questionnaire. Genetic risk score for T2D consisted of 424 single nucleotide polymorphisms.
Results
During a median follow up of 12.1 years, 14,896 T2D cases were documented. Compared with participants who reported no stair climbing, those who climbed stairs regularly had a lower risk of incident T2D (10–50 steps/day: hazard ratio (HR) = 0.95, 95% confidence interval (95%CI): 0.89–1.00; 60–100 steps/day: HR = 0.92, 95%CI: 0.87–0.98; 110–150 steps/day: HR = 0.86, 95%CI: 0.80–0.91; >150 steps/day: HR = 0.93, 95%CI: 0.87–0.99, p for trend = 0.0007). We observed a significant interaction between stair climbing and genetic risk score on the subsequent T2D risk (p for interaction = 0.0004), where the risk of T2D showed a downward trend in subjects with low genetic risk and those who reported stair climbing activity of 110–150 steps/day appeared to have the lowest overall T2D risk among those with intermediate to high genetic risk.
Conclusion
A higher number of stairs climbed at home was associated with lower T2D incidence risk, especially among individuals with a low genetic predisposition to T2D. These findings highlight that stair climbing, as incidental physical activity, offers a simple and low-cost complement to public health interventions for T2D prevention.
Graphical abstract
1. Introduction
Diabetes is a global pandemic affecting 537 million adults in 2021. This number is predicted to rise to 643 million worldwide by 2030 and to 783 million by 2045.1 The International Diabetes Federation reported that diabetes caused 6.7 million deaths and more than 966 billion dollars in health expenditure in 2021,1 some of which could have been avoided via improved lifestyle behaviors, such as increased physical activity. Physical activity is recognized as one of the primary strategies for diabetes prevention, with the American Diabetes Association currently recommending at least 150 min/week of moderate intensity physical activity.2 However, more than a quarter of adults worldwide (27.5%) report insufficient physical activity, with the greatest prevalence in high-income countries over time (from 31.6% in 2001 to 36.8% in 2016).3 One of the most common barriers to physical activity is lack of time due to work and family responsibility.4 Thus, it is important for individuals to incorporate simple and effective physical activity into their daily lives, especially for those who are unable to meet current recommendations for physical activity.
Stair climbing is an easy and accessible way to incorporate physical activity into daily life. As a kind of moderate-to-vigorous intensity activity, stair climbing can achieve a workload 4.0–8.8 metabolic equivalent tasks (METs), depending on the pace of climbing, and it involves more muscle strength than walking on flat ground or down stairs.5 The proposed health benefits of stair climbing include enhanced cardiovascular fitness, improved lipoprotein profiles, decreased body fat, a reduction in fasting blood glucose, and increased strength in the lower limbs.6, 7, 8, 9 Additionally, several observational studies suggest positive effects of stair climbing on metabolic syndrome, cardiovascular disease (CVD), cancer mortality, and all-cause mortality.10, 11, 12, 13, 14, 15 However, few studies have examined stair climbing and the incidence of type 2 diabetes (T2D). Preliminary evidence from small-scale trials indicates that a short bout of stair climbing might be a clinically useful modality for efficient amelioration of postprandial hyperglycemia because it can be performed without excessive physical effort and it reduces postprandial blood glucose levels efficiently in normal weight individuals, those with impaired glucose tolerance, and T2D patients.16, 17, 18 Given the potential cardiometabolic benefits of daily stair climbing, we hypothesized that daily stair climbing may be associated with a reduced risk of T2D. However, we have not observed any longitudinal evidence in this regard.
It is widely accepted that the development of T2D is the result of complex interactions between lifestyle and genetic factors. Both high genetic risk and low physical activity contribute to an augmented risk of T2D.2,19 Current evidence suggests that the association between physical activity and T2D risk is modified by genetic variants.20 Whether the association between stair climbing and T2D is affected by genetic predisposition for T2D remains unclear. Our study aimed to clarify whether daily stair climbing at home is related to a lower risk of T2D and to examine how the genetic risk of T2D might modify such an association.
2. Methods
2.1. Study design and population
The UK Biobank is a large prospective cohort consisting of more than 500,000 participants aged 40–69 years across 22 centers within the UK between 2006 and 2010. The details of the study design and execution have been described elsewhere.21 The study was approved by the North West–Haydock Research Ethics Committee (16/NW/0274). All participants gave informed consent.
Participants were excluded from withdrawing their information (n = 46). Those with pre-existing diabetes at baseline (n = 31,414), those who reported inability to walk (n = 1498), those who had absent data on stair climbing (n = 7034), and those who had no genetic data or a mismatch between self-reported and genetic sex (n = 10,814) were further excluded. Finally, 451,699 individuals were included in the present analysis (Supplementary Fig.1).
2.2. Exposure
Stair climbing data was collected through the touchscreen questionnaire by asking participants this question: “At home, during the last 4 weeks, about how many times a day do you climb a flight of stairs? (approximately 10 steps),” followed by these options: “none”, “1–5 times/day”, “6–10 times/day”, “11–15 times/day”, “16–20 times/day”, and “more than 20 times/day”. Long-term reliability of stair climbing was validated in a subset of UK Biobank participants with repeated assessment (mean 4.2 years after baseline) using a moderate quadratic weighed kappa coefficient (0.62).15
2.3. Ascertainment of T2D
The definitions for prevalent diabetes and incident T2D cases are presented in Supplementary Table 1. Prevalent diabetes was identified based on the modified UK Biobank algorithms by Eastwood et al.22 via hospital inpatient records, self-reported medical history and medication, and biochemical examination for blood glucose and glycated hemoglobin. Incident T2D was diagnosed by International Classification of Diseases, 10th edition, code E11 in hospital inpatient records from the Hospital Episode Statistics for England, the Scottish Morbidity Record data for Scotland, and the Patient Episode Database for Wales. For this analysis, hospital admission data were available until March 31, 2021.
2.4. Covariates
Covariates, including age, sex, ethnicity, educational attainment, household income, current employment status, type of accommodation, smoking status, alcohol consumption, dietary, physical activity, and health and medical history, were acquired using touchscreen questionnaires at the baseline recruitment. Socioeconomic deprivation was measured by Townsend deprivation index scores, and higher Townsend scores indicated higher levels of socioeconomic deprivation.23 Alcohol consumption was calculated based on the frequency and alcohol equivalent of different drinks consumed in a typical day, week, or month. We defined a healthy diet score with reference to the dietary priorities for CVD, diabetes, and obesity,24 as described in a previous UK Biobank study.25 Definitions of each component of a healthy diet score—which considers higher intake of fruits, vegetables, whole grains, fish, dairy, and vegetable oils and lower intake of refined grains, unprocessed meats, processed meats, and sugar-sweetened beverages—are described in Supplementary Table 2. Diets were scored on a scale of 1–10, with a higher score indicating healthier diet habits. Height and weight were measured by a trained nurse during the initial assessment visit. Body mass index (BMI) was calculated as weight divided by the square of height in meters. Self-reported long-standing illness, disability or infirmity, depression, hypertension, CVD, dyslipidemia, antihypertensive medication use, lipid-lowering treatment, and aspirin use were collected as part of the participant's health and medical history at baseline recruitment. Physical activity was assessed using the International Physical Activity Questionnaire,26 which asks about the frequency, intensity, duration of walking and other moderate and vigorous activity in the last 4 weeks, and is scored according to the protocol to estimate total MET minutes expended. Sedentary behavior was calculated by adding the hours spent on driving, using the computer, and watching TV. Self-reported walking pace was described as slow pace (<3 miles/h), steady average pace (3–4 miles/h), and fast pace (>4 miles/h).
2.5. Polygenic Risk Score for T2D
Genotype calling in the UK Biobank was performed on 2 closely related purpose-designed arrays, UK BiLEVE Axiom and UK Biobank Axiom. Detailed information about genotyping, imputation, and quality control has been described elsewhere.27 We calculated a genetic risk score (GRS) for T2D using 424 single-nucleotide polymorphisms (SNPs), which were reported to be associated with T2D in the largest genome-wide multiethnic meta-analysis.28 Details concerning 424 selected SNPs are shown in Supplementary Table 3. Each participant's GRS was calculated by summing the number of risk alleles at each genetic variant, which were weighted by the respective allelic effect sizes on T2D. The formula is as follows: GRS = (β1 × SNP1 + β2 × SNP2 + … + β424 × SNP424) × (424/sum of the β-coefficients), where SNP i (i = 1, 2,..., 424) is the risk allele number of each SNP.29 A higher GRS indicates a higher genetic predisposition to T2D. Distribution of T2D-GRS in the UK Biobank is shown in Supplementary Fig. 2.
2.6. Statistical analysis
Cox proportional hazard regression models considering competing risks by using the cause-specific hazard function model30 were used to evaluate the association between daily stair climbing at home and T2D events, and the results were presented as hazard ratios (HRs) with 95% confidence intervals (95%CIs). The time to events was calculated from the date of baseline recruitment to the date of T2D diagnosis, lost to follow-up, death, or the censoring date (March 31, 2021), whichever occurred first. The proportional hazards assumption was tested by the Schoenfeld residual method, and no violation was observed. Daily stair climbing at home was assessed as a categorical variable (none, 10–50 steps/day, 60–100 steps/day, 110–150 steps/day, or >150 steps/day), and the category of “none” (no stair climbing) was set as the referent in each model.
We considered the following covariates in multivariable models sequentially: Model 1 adjusted for age (continuous) and sex (male, female). Model 2 additionally adjusted for ethnicity (Caucasian, Mixed, Asian, Negroid, Chinese, other, or unknown), educational attainment (college or university, vocational, upper secondary, lower secondary, other, or unknown), Townsend deprivation index (in quintiles), annual household income (<18,000 GBP; 18,000 to <30,999 GBP; 30,999 to <51,999 GBP; 51,999 to <100,000 GBP; or ≥100,000 GBP), employment status (yes, no), type of accommodation (house/bungalow, flat/maisonette/apartment, or other), and assessment center (22 categories). Model 3 further adjusted for smoking status (ever, former, or current smoker), alcohol intake (<0.1, 0.1 to <4.9 g/day, 4.9 to <14.9 g/day, 14.9 to <19.9 g/day, 9.9 to <29.9 g/day, or ≥29.9 g/day), physical activity (MET-h/week; in quintiles), healthy diet score (in categories), BMI (<18.5 kg/m2, 18.5 to <22.9 kg/m2, 22.9 to <24.9 kg/m2, 24.9 to <29.9 kg/m2, 29.9 to <34.9 kg/m2, or ≥34.9 kg/m2), sedentary behavior (in quintiles), and walking pace (slow, steady, or fast). Depression, dyslipidemia, hypertension, CVD, cancer, long-standing illness, disability or infirmity, lipid-lowering treatment, antihypertensive medication use, aspirin use at baseline, T2D-GRS (continuous), the first 10 primary components of ancestry, and genotype measurement batches were additionally included in the fully adjusted model (Model 4). Missing data were coded as a missing indicator category in regression models, if necessary.
We explored whether or not genetic predisposition is a potential effect modifier for daily stair climbing at home and T2D-GRS. To test the multiplicative interaction between daily stair climbing at home and genetic predisposition, we treated the group coded “none” for daily stair climbing at home and low genetic risk (the lowest tertile of T2D-GRS) as the reference group and calculated HRs for other groups. The p value for interaction was estimated using the joint test.31 We further examined the association between daily stair climbing at home and T2D events stratified by the tertiles of T2D-GRS.
Several secondary analyses were performed. First, we conducted the stratified analyses examining the associations of daily stair climbing at home and T2D events across age, sex, educational attainment, Townsend deprivation index, household income, employment status, smoking status, alcohol consumption, physical activity, healthy diet score, BMI, sedentary behavior time, walking pace, depression, dyslipidemia, hypertension, CVD, cancer, and long-standing illness, disability, or infirmity. The joint test was used to examine interactions between stair climbing and these subgroups.31 Second, we excluded those who were diagnosed with T2D or who died or were lost within 2 years of follow-up, and we reran the main analyses to minimize reverse causality. Third, sensitivity analyses were rerun on the primary analyses between daily stair climbing at home and T2D events by sequentially excluding participants with CVD, cancer, depression, and long-standing illness, disability, or infirmity at baseline in each replication. Fourth, we restricted the analyses to unrelated individuals based on genetic profiling. Fifth, we conducted an analysis of the sensitivity of association between stair climbing, GRS, and risk of T2D among individuals of European descent, and we used an alternative genetic instrument containing 112 SNPs in Europeans (Supplementary Table 4).32
Statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC, USA), and a p value of less than 0.05 was considered statistically significant (two-sided tests).
3. Results
3.1. Baseline characteristics
We included 451,699 participants (mean age 56.3 ± 8.1 years, mean ± SD; 55.2% females), of whom 39,805 reported no stair climbing, 89,840 reported 10–50 steps/day, 164,931 reported 60–100 steps/day, 85,122 reported 110–150 steps/day, and 72,001 reported more than 150 steps/day (Table 1). Compared with subjects who reported no stair climbing, those with a higher frequency of stair climbing were more likely to be younger, non-smokers, better educated, employed, higher income, more physically active, walking at a fast pace, less sedentary, lower BMI, and living in a house or bungalow. They were also less likely to have prevalent long-standing illness, disability, or infirmity, depression, dyslipidemia, hypertension, CVD, and cancer and were less likely to use antihypertensive medication, lipid-lowering treatment, and aspirin at baseline.
Table 1.
Baseline characteristics of 451,699 participants in UK Biobank by stair climbing.
| Variable | Frequency of stair climbing (steps/day) |
||||
|---|---|---|---|---|---|
| None | 10–50 | 60–100 | 110–150 | >150 | |
| No. of participants | 39,805 | 89,840 | 164,931 | 85,122 | 72,001 |
| Age (year) | 59.0 ± 7.7 | 55.9 ± 8.0 | 56.2 ± 8.0 | 56.2 ± 8.2 | 55.9 ± 8.2 |
| BMI (kg/m2) | 27.7 ± 4.9 | 28 ±5.1 | 27.2 ± 4.5 | 26.6 ±4.2 | 26.3 ± 4.1 |
| Male | 17,688 (44.4) | 42,964 (47.8) | 75,570 (45.8) | 36,706 (43.1) | 29,470 (40.9) |
| Caucasian ethnicity | 38,073 (95.6) | 83,348 (92.8) | 157,574 (95.5) | 81,717 (96.0) | 68,215 (94.7) |
| Townsend deprivation index | –2.0 (–3.6 to 1.3) | –1.5 (–3.4 to 1.6) | –2.3 (–3.7 to 0) | –2.4 (–3.8 to –0.2) | –2.3 (–3.7 to 0.1) |
| Education | |||||
| College or university | 8806 (22.1) | 26,960 (30.0) | 55,274 (33.5) | 31,477 (37.0) | 27,060 (37.6) |
| Vocational | 5291 (13.3) | 11,086 (12.3) | 19,368 (11.7) | 9241 (10.9) | 7445 (10.3) |
| Upper secondary | 3602 (9.0) | 9468 (10.5) | 18,880 (11.4) | 10,354 (12.2) | 8678 (12.1) |
| Lower secondary | 10,517 (26.4) | 23,759 (26.4) | 45,408 (27.5) | 22,660 (26.6) | 18,522 (25.7) |
| Other | 11,026 (27.7) | 17,558 (19.5) | 24,625 (14.9) | 10,732 (12.6) | 9545 (13.3) |
| Unknown | 563 (1.4) | 1009 (1.1) | 1376 (0.8) | 658 (0.8) | 751 (1.0) |
| Household income (GBP) | |||||
| <18,000 | 12,606 (31.7) | 19,780 (22.0) | 27,305 (16.6) | 13,034 (15.3) | 11,404 (15.8) |
| ≥18,000 to <30,999 | 9398 (23.6) | 19,359 (21.5) | 35,965 (21.8) | 18,490 (21.7) | 15,034 (20.9) |
| ≥30,999 to <51,999 | 6399 (16.1) | 19,260 (21.4) | 39,600 (24.0) | 20,967 (24.6) | 16,674 (23.2) |
| ≥51,999 to <100,000 | 3400 (8.5) | 14,500 (16.1) | 31,982 (19.4) | 17,192 (20.2) | 14,159 (19.7) |
| ≥100,000 | 693 (1.7) | 3935 (4.4) | 8449 (5.1) | 4477 (5.3) | 4239 (5.9) |
| Unknown | 7309 (18.4) | 13,006 (14.5) | 21,630 (13.1) | 10,962 (12.9) | 10,491 (14.6) |
| Employment status | 16,151 (40.6) | 56,431 (62.8) | 102,540 (62.2) | 49,396 (58.0) | 264,855 (56.0) |
| Type of accommodation | |||||
| House or bungalow | 27,374 (68.8) | 69,689 (77.6) | 156,837 (95.1) | 82,468 (96.9) | 69,243 (96.2) |
| Flat, maisonette, or apartment | 11,443 (28.7) | 19,311 (21.5) | 7510 (4.6) | 2405 (2.8) | 43,137 (3.4) |
| Other | 988 (2.5) | 840 (0.9) | 584 (0.4) | 249 (0.3) | 2951 (0.4) |
| Smoking status | |||||
| Never | 19,554 (49.1) | 46,820 (52.1) | 91,736 (55.6) | 48,737 (57.3) | 42,170 (58.6) |
| Former | 14,735 (37.0) | 30,851 (34.3) | 56,580 (34.3) | 28,758 (33.8) | 23,111 (32.1) |
| Current | 5293 (13.3) | 11,812 (13.1) | 16,095 (9.8) | 7394 (8.7) | 6474 (9) |
| Unknown | 223 (0.6) | 357 (0.4) | 520 (0.3) | 233 (0.3) | 246 (0.3) |
| Alcohol intake (g/day) | 10.2 (3.2 to 20.8) | 10.3 (3.4 to 22.2) | 11.9 (5.1 to 22.3) | 11.9 (5.1 to 22.2) | 11.4 (5.1 to 22.1) |
| Healthy diet score | 3.1 ± 1.4 | 2.9 ± 1.4 | 3.0 ± 1.4 | 3.1 ± 1.4 | 3.2 ± 1.4 |
| Physical activity (MET-h/week) | 28.9 (12.3 to 59.5) | 24.2 (10.4 to 51.5) | 28.6 (13.5 to 56.4) | 32.2 (15.6 to 62.2) | 39.3 (19.3 to 75.1) |
| Sedentary (h/day) | 4.7 ± 2.7 | 4.7 ± 2.7 | 4.5 ± 2.5 | 4.4 ± 2.5) | 4.3 ± 2.5 |
| Walking pace | |||||
| Slow | 5640 (14.2) | 11,382 (12.7) | 9166 (5.6) | 3276 (3.8) | 2395 (3.3) |
| Steady | 20,870 (52.4) | 49,071 (54.6) | 89,938 (54.5) | 43,026 (50.5) | 33,329 (46.3) |
| Brisk | 12,986 (32.6) | 28,979 (32.3) | 65,564 (39.8) | 38,710 (45.5) | 36,123 (50.2) |
| Unknown | 309 (0.8) | 408 (0.5) | 263 (0.2) | 110 (0.1) | 154 (0.2) |
| Long-standing illness, disability, or infirmity | 15,390 (38.7) | 30,029 (33.4) | 44,478 (27.0) | 21,616 (25.4) | 18,028 (25.0) |
| Antihypertensive medication use | 9695 (24.4) | 17,661 (19.7) | 29,584 (17.9) | 14,095 (16.6) | 11,504 (16.0) |
| Lipid-lowering treatment | 8255 (20.7) | 13,937 (15.5) | 23,167 (14.0) | 11,133 (13.1) | 8800 (12.2) |
| Aspirin use | 6303 (15.8) | 11,444 (12.7) | 19,091 (11.6) | 9304 (10.9) | 7631 (10.6) |
| Depression | 5098 (12.8) | 10,914 (12.1) | 17,687 (10.7) | 9016 (10.6) | 7653 (10.6) |
| Dyslipidemia | 22,891 (57.5) | 49,132 (54.7) | 84,513 (51.2) | 41,064 (48.2) | 33,109 (46.0) |
| Hypertension | 24,627 (61.9) | 49,920 (55.6) | 88,753 (53.8) | 43,901 (51.6) | 36,710 (51) |
| Cardiovascular disease | 4064 (10.2) | 6634 (7.4) | 9549 (5.8) | 4284 (5.0) | 3489 (4.9) |
| Cancer | 5164 (13) | 9641 (10.7) | 17,577 (10.7) | 9353 (11.0) | 7939 (11.0) |
Notes: Data are presented as mean ± SD, n (%), or median (IQR). Some percentages do not sum to 100% because of rounding.
Abbreviations: BMI = body mass index; IQR = interquartile range; MET = metabolic equivalent.
3.2. Association between stair climbing and risk of T2D
During a median follow up of 12.1 years (5,300,144 person-years), 14,896 T2D cases were documented. The incidence of T2D per 1000 person-years for the sequential increase in frequency of stair climbing was 4.36, 3.66, 2.67, 2.08, and 2.10, respectively. In Cox regression analyses, we observed that stair climbing was negatively associated with incident T2D (Table 2). In age- and sex-adjusted models, compared with the reference (no stair climbing), the HRs were 0.94 (95%CI: 0.89–1.00), 0.68 (95%CI: 0.64–0.71), 0.53 (95%CI: 0.50–0.57), 0.55 (95%CI: 0.51–0.58) for those who climbed 10–50, 60–100, 110–150, and >150 steps/day, respectively (p for trend <0.0001). In the fully adjusted model (Model 4), compared with the reference (no stair climbing), the hazards of stair climbing on T2D were attenuated but remained significant: 10–50 steps/day: HR = 0.95 (95%CI: 0.89–1.00); 60–100 steps/day: HR = 0.92 (95%CI: 0.87– 0.98); 110–150 steps/day: HR = 0.86 (95%CI: 0.80–0.91); >150 steps/day: HR = 0.93 (95%CI: 0.87–0.99), p for trend = 0.0007.
Table 2.
Association between stair climbing and risk of T2D.
| Frequency of stair climbing (steps/day) |
p for trenda | |||||
|---|---|---|---|---|---|---|
| None | 10–50 | 60–100 | 110–150 | >150 | ||
| Cases/person-years | 1999/458,767 | 3832/1,045,642 | 5191/1,940,655 | 2091/1,004,844 | 1783/850,236 | |
| Incident rate per 1000 person-years | 4.36 | 3.66 | 2.67 | 2.08 | 2.10 | |
| Model 1 | 1.00 (reference) | 0.94 (0.89, 1.00) | 0.68 (0.64, 0.71) | 0.53 (0.50, 0.57) | 0.55 (0.51, 0.58) | <0.0001 |
| Model 2 | 1.00 (reference) | 0.97 (0.92, 1.03) | 0.78 (0.74, 0.83) | 0.64 (0.60, 0.68) | 0.64 (0.60, 0.69) | <0.0001 |
| Model 3 | 1.00 (reference) | 0.92 (0.87, 0.98) | 0.89 (0.84, 0.94) | 0.82 (0.77, 0.88) | 0.88 (0.83, 0.95) | <0.0001 |
| Model 4 | 1.00 (reference) | 0.95 (0.89, 1.00) | 0.92 (0.87, 0.98) | 0.86 (0.80, 0.91) | 0.93 (0.87, 0.99) | 0.0007 |
Linear trend was tested by treating the classification of daily stair climbing at home as a continuous variable.
Model 1 is adjusted for age and sex.
Model 2 is adjusted for Model 1 plus race, educational attainment, Townsend deprivation index, household income, employment status, type of accommodation, and assessment center.
Model 3 is adjusted for Model 2 plus smoking status, alcohol intake, physical activity, a healthy diet score, body mass index, sedentary, and walking pace.
Model 4 is adjusted for Model 3 plus depression; dyslipidemia; hypertension; cardiovascular disease; cancer; long-standing illness, disability, or infirmity; lipid-lowering treatment; antihypertensive medication use; aspirin use at baseline; T2D-GRS; the first 10 primary components of ancestry; and genotype measurement batches.
Abbreviations: GRS = genetic risk score; T2D = type 2 diabetes.
3.3. Interaction between stair climbing and GRS on T2D incidence
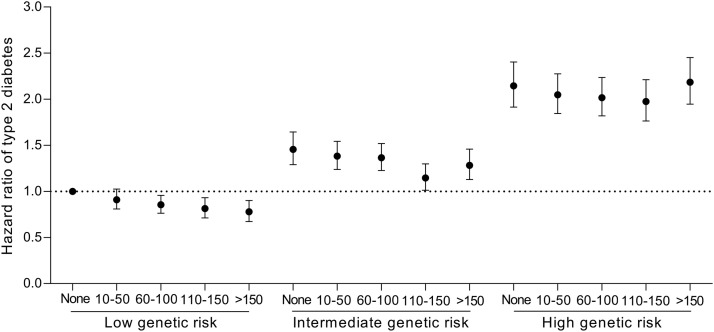
Compared with the low T2D-GRS group, the adjusted HRs for T2D were 1.53 (95%CI: 1.46–1.61) and 2.36 (95%CI: 2.26–2.46) for those with the intermediate and high T2D-GRS, respectively. Each standard deviation increase in T2D-GRS was associated with a 48% (95%CI: 46%–51%) augmented T2D risk (Supplementary Table 5). We observed a significant interaction between stair climbing and T2D-GRS on the subsequent T2D risk in the fully adjusted model (p for interaction = 0.0004, Table 3). Compared with the reference (no stair climbing), those who climbed 110–150 steps/day or >150 steps/day were associated with 13% (95%CI: 0.1%–25%) and 17% (95%CI: 3%–29%) lower risk of T2D in low T2D-GRS individuals, respectively. Compared with the reference (no stair climbing), we observed that climbing 110–150 steps/day was associated with only a 19% (95%CI: 9%–28%) lower risk of T2D in intermediate T2D-GRS individuals. However, among the high T2D-GRS individuals, climbing 60–100 steps/day was associated with a 9% (95%CI: 1%–16%) lower risk of T2D, and climbing 110–150 steps/day was associated with a 11% (95%CI: 3%–19%) lower risk of T2D, but a null association was observed in individuals who climbed >150 steps/day (Table 3). In terms of the joint association between stair climbing and T2D-GRS tertiles (Fig. 1), we observed that as the frequency of stair climbing increased, the risk of T2D showed a downward trend in those with low genetic risk, while those who climbed 110–150 steps/day appear to have the lowest T2D risk among those with intermediate and high genetic risk.
Table 3.
Association between stair climbing, genetic risk score, and risk of type 2 diabetes.
| Frequency of stair climbing (steps/day) |
p for trenda | p for interaction | |||||
|---|---|---|---|---|---|---|---|
| None | 10–50 | 60–100 | 110–150 | >150 | |||
| Low genetic risk score | 0.0004 | ||||||
| Cases/person-years | 441/153,865 | 751/342,669 | 1025/653,677 | 423/339,903 | 312/284,638 | ||
| Incident rate per 1000 person-years | 2.87 | 2.19 | 1.57 | 1.24 | 1.1 | ||
| Hazard ratio (95%CI) | 1.00 (reference) | 0.91 (0.81, 1.03) | 0.89 (0.79, 1.01) | 0.87 (0.75, 1.00) | 0.83 (0.71, 0.97) | 0.0165 | |
| Intermediate genetic risk score | |||||||
| Cases/person-years | 634/153,396 | 1192/346,484 | 1650/645,920 | 611/337,301 | 534/286,155 | ||
| Incident rate per 1000 person-years | 4.13 | 3.44 | 2.55 | 1.81 | 1.87 | ||
| Hazard ratio (95%CI) | 1.00 (reference) | 0.95 (0.86, 1.05) | 0.96 (0.87, 1.05) | 0.81 (0.72, 0.91) | 0.91 (0.80, 1.02) | 0.0060 | |
| High genetic risk score | |||||||
| Cases/person-years | 924/151,507 | 1889/356,491 | 2516/641,059 | 1057/327,642 | 937/279,445 | ||
| Incident rate per 1000 person-years | 6.10 | 5.3 | 3.92 | 3.23 | 3.35 | ||
| Hazard ratio (95%CI) | 1.00 (reference) | 0.95 (0.88, 1.03) | 0.91 (0.84, 0.99) | 0.89 (0.81, 0.97) | 0.98 (0.89, 1.08) | 0.3068 | |
Linear trend was tested by treating the classification of daily stair climbing at home as a continuous variable.
Cox proportional hazards models adjusted for age; sex; race; educational attainment; Townsend deprivation index; household income; employment status; type of accommodation; assessment center; smoking status; alcohol intake; physical activity; a healthy diet score; body mass index; sedentary; walking pace; depression; dyslipidemia; hypertension; cardiovascular disease; cancer; long-standing illness, disability, or infirmity; lipid-lowering treatment; antihypertensive medication use; aspirin use at baseline; the first 10 primary components of ancestry; and genotype measurement batches.
Abbreviation: 95%CI = 95% confidence interval.
Fig. 1.
Hazard ratio of type 2 diabetes according to joint categories of stair climbing and genetic risk score. Cox proportional hazards models adjusted for age; sex; race; educational attainment; Townsend deprivation index; household income; employment status; type of accommodation; assessment center; smoking status; alcohol intake; physical activity; a healthy diet score; body mass index; sedentary; walking pace; depression; dyslipidemia; hypertension; cardiovascular disease; cancer; long-standing illness, disability, or infirmity; lipid-lowering treatment; antihypertensive medication use; aspirin use at baseline; the first 10 primary components of ancestry; and genotype measurement batches. The category of “none” (no stair climbing) in the low genetic risk score group was set as the reference group.
3.4. Secondary analyses
In stratified analyses, we observed that the association between stair climbing and T2D risk was stronger in men (p for interaction = 0.0317) and those without depression (p for interaction = 0.0083) (Supplementary Table 6). In sensitivity analyses, which sequentially excluded participants diagnosed with T2D or who died or were lost within 2 years of follow-up, as well as those with CVD, cancer, depression and long-standing illness, disability or infirmity at baseline, the effect of stair climbing on T2D remained robust (Supplementary Table 7). When we restricted the analyses to unrelated individuals based on genetic profiling, individuals of European descent, or used an alternative genetic instrument containing 112 SNPs in Europeans, the interaction regarding stair climbing and T2D-GRS was still significant (Supplementary Tables 8, 9, and 10).
4. Discussion
In this large study of 451,699 individuals from UK Biobank, we examined the association between stair climbing, genetic predisposition, and the risk of incident T2D. Compared with participants who reported no stair climbing, those who did regular stair climbing at home had a 5% to 14% lower risk of T2D after adjusting for potential confounders. The association showed a significant downward trend among individuals with low T2D-GRS, while those who climbed 110–150 steps per day appeared to have the lowest T2D risk among those with intermediate and high T2D-GRS.
To the best of our knowledge, this is the first large-scale study concerning the longitudinal association between stair climbing and T2D risk. Our observations were consistent with existing evidence supporting the positive effects of stair climbing on metabolic syndrome, CVD, cancer mortality, and all-cause mortality.10, 11, 12, 13, 14, 15 A cross-sectional study of 782 participants found that no daily stair climbing was associated with increased risk for metabolic syndrome (adjusted odds ratio = 1.72, 95%CI: 1.12–2.64, p = 0.01), with the strongest association between high blood glucose and no stair climbing (adjusted odds ratio = 1.73, 95%CI: 1.12–2.66, p = 0.01),14 which is a known risk factor for T2D. In a prospective cohort study of Harvard University male alumni (Harvard Alumni Health Study), stair climbing was U-shaped in relation to stroke risk and negatively associated with heart attack.10,11 Additionally, the Harvard Alumni Health Study reported that higher amounts of stair climbing were inversely associated with all-cause mortality12,13 but not CVD mortality risk.13 Another study of 280,423 participants (median follow-up 11.1 years) indicated that climbing more than 50 steps per day at home was associated with a 10% to 12% lower risk of all-cause and cancer mortality, but not cardiovascular mortality.15 These studies above highlighted the health benefits of stair climbing as a simple and effective physical activity, but there is still insufficient evidence to support the breadth and scope of purported health benefits. To date, no longitudinal population-based studies have reported long-term association between stair climbing and incident T2D. We first reported that a higher frequency of stair climbing at home was associated with lower T2D risk, and such association remained robust after controlling for a wealth of covariates, including sociodemographic variables, health behavioral factors, health and medical history, medication use, and genetic predisposition to diabetes. Although few studies have reported on stair climbing and T2D, several recent longitudinal studies have examined the relationship between daily steps and T2D. Those studies found that a higher daily step count and greater step intensity, as measured by a wearable accelerometer, were strongly associated with a lower risk of incident diabetes.33, 34, 35 While the number of daily steps can be counted by an accelerometer, stair climbing can only be self-reported. However, both measures emphasize the importance and necessity of incorporating simple exercise into daily life. Our findings reinforce public health recommendations to incorporate routine stair climbing as an accessible way to increase physical activity levels and provide health benefits.
Our study observed a significant interaction between stair climbing and T2D-GRS on incident T2D. We found the relationship between stair climbing and the risk of T2D was weaker in those with higher T2D-GRS compared to those with lower T2D-GRS. This finding was consistent with those from a prospective cohort study with 8101 participants, in which 821 cases of incident T2D in the Atherosclerosis Risk in Communities showed a significant interaction between physical activity and the T2D-GRS on T2D incidence, suggesting a weaker protective effect of physical activity in those at high genetic risk.36 This study suggests that for individuals who already have a high genetic susceptibility to T2D, the effect of behavioral change on their risk may be smaller. Our study also found the relationship between stair climbing and the risk of developing T2D was observed only among participants without depression. Depression is a mood disorder that causes a persistent feeling of sadness and loss of interest, and it has been recognized as a risk factor for T2D, increasing the risk of developing T2D up to 37%.37 In addition, depression is associated with lower levels of physical activity in our study (median 27.9 vs. 30.0 MET-h/week, p < 0.001). A meta-analysis of prospective cohort studies showed that physical activity was independently and significantly associated with reduced risk of T2D in both men and women.38 When men and women were compared separately, the effects appeared to be more pronounced in women, with a pooled relative risk (RR) of 0.83 (95%CI: 0.77–0.90), as opposed to men, who have a pooled RR of 0.89 (95%CI: 0.86–0.93) per 10 MET-h/week.38 This appears to be inconsistent with our observation that the association between stair climbing and T2D risk was stronger in men. However, the San Antonio Heart Study showed that the protective effect of leisure-time physical activity on diabetes may be seen in men only.39 Hitherto, we have not found the underlying mechanism to explain these results. However, the evidence above indicates that the association between physical activity and diabetes incidence may differ for the 2 sexes. In our study, there is a higher prevalence of T2D in men as compared to women at this age. In the fully-adjusted model, compared with women, the HR of T2D for men was 1.50 (95%CI: 1.44–1.55) (data not shown). Importantly, we observed that the association between stair climbing and T2D risk was stronger in men, implying that daily stair climbing may be an effective intervention for reducing T2D in men at this age.
Although the exact pathological mechanisms underlying the association between stair climbing and glucose homeostasis and T2D risks are still unknown, the observed association between lower T2D risk and stair climbing is biologically plausible. Stair climbing is a combination of aerobic and resistance exercise, which has additional benefits on the reduction of abdominal adiposity compared to aerobic or resistance exercise alone.40 Thus, routine practice of stair climbing may represent a more effective type of activity for improving glycemic control and insulin sensitivity. Blood glucose is primarily used by relevant muscle cells during leg exercise, therefore regular leg exercise may partly contribute to improved insulin action and glucose disposal.41,42 In fact, stair climbing as a short bout of high intensity physical activity led to favorable changes in glucose metabolism. Some short-term stair climbing small-scale intervention studies showed that stair climbing could reduce fasting blood glucose8 and improve postprandial glycemic control in healthy adult participants16,43 and sedentary middle-aged men with impaired glucose tolerance,17 which helps reduce the risk of T2D. Stair climbing may also reduce the risk of T2D by improving blood lipid profiles,6,8 improving insulin sensitivity,43,44 reducing fat mass,44 and reducing body weight,8 which are factors known to be associated with T2D.
The current study has several strengths. The prospective design, large sample size, long-term follow up (12 years), and high-quality data from UK Biobank may be favorable in that it allows us to consider a vast array of confounders and provides sufficient statistical power. In addition, GRS for T2D were calculated using 424 SNPs recently reported in the largest genome-wide multi-ethnic meta-analysis related to T2D, allowing us to make accurate genetic risk predictions. Importantly, stair climbing is an easy exercise to perform, and it maintains intensity at the moderate-to-vigorous level required to raise body mass against gravity. Unlike formal exercise, stair climbing does not require any equipment, special skills, or special clothing, and it even people who are unfamiliar with exercise can successfully perform the activity. Finally, there are no time restrictions related to stair climbing, which is a common cause of poor participation in other sports activities.4
Several limitations of the current study should be considered. First, information on stair climbing frequency was collected by questionnaire rather than on actigraphy-based measurement, which is susceptible to reporting bias. To the best of our knowledge, current actigraphy-based measurements do not accurately record and distinguish the movement behavior of stair climbing, so we were unable to elaborate on this relationship in this manuscript. However, the long-term reliability of stair climbing in UK Biobank was validated by repeated assessment using a quadratic weighed kappa with a coefficient of 0.62 (moderate reliability).15 Second, we only asked about at-home stair climbing and did not include questions about stair climbing in the workplace or elsewhere. However, it was found that the relationship between stair climbing at home and incident T2D was not affected by current employment status. Third, although we considered a large number of confounders and performed several sensitivity analyses, the possibility of residual confounding and potential bias may exist because of the nature of observational studies. Fourth, because GRS is a genetic instrument developed in a trans-continental sample and then applied to a predominantly European population, it may be weaker when applied to populations of different ancestry. Fifth, participants from the UK were mostly of European continental ancestry, and thus more studies in other ethnic and racial groups need to be conducted.
5. Conclusion
Our study demonstrated a high frequency of stair-climbing activity performed at home was associated with lower T2D risk, especially among individuals with a low genetic predisposition to T2D. These findings highlight stair climbing at home as a low-cost intervention strategy for the prevention of T2D, which has important public health implications.
Availability of data and materials
Data from the UK Biobank are available to all researchers upon submission of application. This research has been conducted using the UK Biobank Resource under Application 63454.
Acknowledgments
The authors acknowledge all UK Biobank participants and staff for their contributions to the health of mankind. This study is supported by the National Key Research and Development Program of China (grant number 2020YFC2006300) and the Young Scientists Fund of the National Natural Science Foundation of China (grant number 82103835). The funders of the study had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors’ contributions
LC conceived and designed the study, analyzed and interpreted data, and revised the manuscript; YW analyzed and interpreted data, drafted the manuscript, and revised the manuscript; ML and XT revised it critically. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.10.002.
Supplementary materials
References
- 1.International Diabetes Federation. IDF Diabetes Atlas, 10th ed, 2021. Available at: https://diabetesatlas.org/en/. [accessed 02.11.2021]. [PubMed]
- 2.American Diabetes Association Prevention or delay of Type 2 diabetes: Standards of medical care in diabetes-2021. Diabetes care. 2021;44(Suppl.1):S34–S39. doi: 10.2337/dc21-S003. [DOI] [PubMed] [Google Scholar]
- 3.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health. 2018;6:e1077–e1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Overcoming barriers to physical activity. Available at: https://www.cdc.gov/physicalactivity/basics/adding-pa/barriers.html. [accessed 02.11.2021].
- 5.Ainsworth BE, Haskell WL, Herrmann SD, et al. Compendium of physical activities: A second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 6.Boreham CA, Kennedy RA, Murphy MH, Tully M, Wallace WF, Young I. Training effects of short bouts of stair climbing on cardiorespiratory fitness, blood lipids, and homocysteine in sedentary young women. Br J Sports Med. 2005;39:590–593. doi: 10.1136/bjsm.2002.001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boreham CA, Wallace WF, Nevill A. Training effects of accumulated daily stair-climbing exercise in previously sedentary young women. Prev Med. 2000;30:277–281. doi: 10.1006/pmed.2000.0634. [DOI] [PubMed] [Google Scholar]
- 8.Michael E, White MJ, Eves FF. Home-based stair climbing as an intervention for disease risk in adult females; A controlled study. Int J Environ Res Public Health. 2021;18:603. doi: 10.3390/IJERPH18020603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allison MK, Baglole JH, Martin BJ, Macinnis MJ, Gurd BJ, Gibala MJ. Brief intense stair climbing improves cardiorespiratory fitness. Med Sci Sports Exerc. 2017;49:298–307. doi: 10.1249/MSS.0000000000001188. [DOI] [PubMed] [Google Scholar]
- 10.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 11.Lee IM, Paffenbarger RS., Jr Physical activity and stroke incidence: The Harvard Alumni Health Study. Stroke. 1998;29:2049–2054. doi: 10.1161/01.str.29.10.2049. [DOI] [PubMed] [Google Scholar]
- 12.Lee IM, Paffenbarger RS., Jr Associations of light, moderate, and vigorous intensity physical activity with longevity. The Harvard Alumni Health Study. Am J Epidemiol. 2000;151:293–299. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- 13.Rey-Lopez JP, Stamatakis E, Mackey M, Sesso HD, Lee IM. Associations of self-reported stair climbing with all-cause and cardiovascular mortality: The Harvard Alumni Health Study. Prev Med Rep. 2019;15 doi: 10.1016/j.pmedr.2019.100938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whittaker AC, Eves FF, Carroll D, et al. Daily stair climbing is associated with decreased risk for the metabolic syndrome. BMC Public Health. 2021;21:923. doi: 10.1186/s12889-021-10965-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez-Lastra MA, Ding D, Dalene KE, Del Pozo Cruz B, Ekelund U, Tarp J. Stair climbing and mortality: A prospective cohort study from the UK Biobank. J Cachexia Sarcopenia Muscle. 2021;12:298–307. doi: 10.1002/jcsm.12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore J, Salmons H, Vinoskey C, Kressler J. A single one-minute, comfortable paced, stair-climbing bout reduces postprandial glucose following a mixed meal. Nutr Metab Cardiovasc Dis. 2020;30:1967–1972. doi: 10.1016/j.numecd.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Takaishi T, Imaeda K, Tanaka T, Moritani T, Hayashi T. A short bout of stair climbing-descending exercise attenuates postprandial hyperglycemia in middle-aged males with impaired glucose tolerance. Appl Physiol Nutr Metab. 2012;37:193–196. doi: 10.1139/h11-140. [DOI] [PubMed] [Google Scholar]
- 18.Honda H, Igaki M, Hatanaka Y, et al. Stair climbing/descending exercise for a short time decreases blood glucose levels after a meal in people with type 2 diabetes. BMJ Open Diabetes Res Care. 2016;4 doi: 10.1136/bmjdrc-2016-000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyer WR, Churilla JR, Ehrlich SF, Crouter SE, Hornbuckle LM, Fitzhugh EC. Protective role of physical activity on type 2 diabetes: Analysis of effect modification by race-ethnicity. J Diabetes. 2018;10:166–178. doi: 10.1111/1753-0407.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietrich S, Jacobs S, Zheng JS, Meidtner K, Schwingshackl L, Schulze MB. Gene-lifestyle interaction on risk of type 2 diabetes: A systematic review. Obes Rev. 2019;20:1557–1571. doi: 10.1111/obr.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eastwood SV, Mathur R, Atkinson M, et al. Algorithms for the capture and adjudication of prevalent and incident diabetes in UK Biobank. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster HME, Celis-Morales CA, Nicholl BI, et al. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: A prospective analysis of the UK Biobank cohort. Lancet Public Health. 2018;3:e576–e585. doi: 10.1016/S2468-2667(18)30200-7. [DOI] [PubMed] [Google Scholar]
- 24.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation. 2016;133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Said MA, Verweij N, van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank Study. JAMA Cardiol. 2018;3:693–702. doi: 10.1001/jamacardio.2018.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 27.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vujkovic M, Keaton JM, Lynch JA, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet. 2020;52:680–691. doi: 10.1038/s41588-020-0637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuang P, Liu X, Li Y, et al. Effect of diet quality and genetic predisposition on hemoglobin A(1c) and type 2 diabetes risk: Gene-diet interaction analysis of 357,419 individuals. Diabetes Care. 2021;44:2470–2479. doi: 10.2337/dc21-1051. [DOI] [PubMed] [Google Scholar]
- 30.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SAS Institute. The PHREG procedure: Type 3 tests and joint tests. Available at: https://support.sas.com/documentation/cdl/en/statug/67523/HTML/default/viewer.htm#statug_phreg_details32.htm. [accessed 30.10.2021].
- 32.Scott RA, Scott LJ, Mägi R, et al. An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes. 2017;66:2888–2902. doi: 10.2337/db16-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballin M, Nordström P, Niklasson J, et al. Daily step count and incident diabetes in community-dwelling 70-year-olds: A prospective cohort study. BMC Public Health. 2020;20:1830. doi: 10.1186/s12889-020-09929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuthbertson CC, Moore CC, Sotres-Alvarez D, et al. Associations of steps per day and step intensity with the risk of diabetes: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Int J Behav Nutr Phys Act. 2022;19:46. doi: 10.1186/s12966-022-01284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garduno AC, LaCroix AZ, LaMonte MJ, et al. Associations of daily steps and step intensity with incident diabetes in a prospective cohort study of older women: The OPACH Study. Diabetes Carev. 2022;45:339–347. doi: 10.2337/dc21-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klimentidis YC, Chen Z, Arora A, Hsu CH. Association of physical activity with lower type 2 diabetes incidence is weaker among individuals at high genetic risk. Diabetologia. 2014;57:2530–2534. doi: 10.1007/s00125-014-3380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 38.Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: A systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia. 2016;59:2527–2545. doi: 10.1007/s00125-016-4079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monterrosa AE, Haffner SM, Stern MP, Hazuda HP. Sex difference in lifestyle factors predictive of diabetes in Mexican-Americans. Diabetes Care. 1995;18:448–456. doi: 10.2337/diacare.18.4.448. [DOI] [PubMed] [Google Scholar]
- 40.Johannsen NM, Swift DL, Lavie CJ, Earnest CP, Blair SN, Church TS. Combined aerobic and resistance training effects on glucose homeostasis, fitness, and other major health indices: A review of current guidelines. Sports Med. 2016;46:1809–1818. doi: 10.1007/s40279-016-0548-3. [DOI] [PubMed] [Google Scholar]
- 41.Wahren J, Felig P, Ahlborg G, Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Invest. 1971;50:2715–2725. doi: 10.1172/JCI106772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 43.Moore J, Bartholomae EM, Ward K, Hooshmand S, Kressler J. Three minutes of moderate-intensity stair walking improves glucose and insulin but not insulin sensitivity or total antioxidant capacity. Nutr Metab Cardiovasc Dis. 2022;32:479–486. doi: 10.1016/j.numecd.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 44.Chow BC, Li S, Zhu X, et al. Effects of descending or ascending stair exercise on body composition, insulin sensitivity, and inflammatory markers in young Chinese women with obesity: A randomized controlled trial. J Sports Sci. 2021;39:496–502. doi: 10.1080/02640414.2020.1829362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the UK Biobank are available to all researchers upon submission of application. This research has been conducted using the UK Biobank Resource under Application 63454.