Introduction
Neural epidermal growth factor-like 1 (NELL1) is a recently described target antigen in membranous nephropathy (MN). NELL1-associated MN accounts for 1.5% to 8% of MN cases1, 2, 3,S1 and was reportedly associated with malignant diseases,2 lipoic acid supplementation,4 and consumption of traditional indigenous medicine containing mercury.5 Therefore, NELL1 may be an important antigen in secondary MN. Rheumatoid arthritis (RA) is an autoimmune inflammatory disease occasionally accompanied by MN. Complications of drugs, including gold salts, D-penicillamine, and bucillamine, are common causes of MN in patients with RA.6,S2-S5 Here, we report on 4 Japanese patients with NELL1-positive MN complicated by RA.
Results
Between January 2005 and December 2018, 221 patients with MN underwent NELL1 staining, and 10 (4.5%) were diagnosed with NELL1-positive MN. Among these, 6 (60%) patients had NELL1-positive MN with RA, including 4 with suspected drug-induced MN, 1 diagnosed with chronic hepatitis C virus infection–related MN at kidney biopsy, and 1 diagnosed with RA 4 years after MN diagnosis. In 4 patients with NELL1-positive MN without RA, MN was not associated with other underlying diseases at the time of kidney biopsy and was diagnosed as idiopathic MN. The clinical and pathologic characteristics of 4 patients having suspected drug-induced NELL1-positive MN with RA are shown in Table 1.
Table 1.
Clinical characteristics at the time of diagnostic kidney biopsy, histopathologic findings, and disease outcomes in patients with NELL1-positive MN complicated by RA
| Case | Clinical characteristics at kidney biopsy |
Kidney biopsy findings |
Clinical courses | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | Sex | Duration of RA (yr) | eGFR (ml/min /1.73 m2) |
UPCR (g/gCr) | Treatment for RA | Interval from BUC or ADA initiation to proteinuria (mo) | IgG | IgA | IgM | C3 | C1q | Distribution of deposits | Ehrenreich-Churg stage | Follow-up period (mo) | ||
| 1 | 61 | F | 4 | 120 | 1.4 | PSL, BUC | 3 | (+) | (−) | (+) | (−) | (+) | Seg | III | 51 | After BUC discontinuation, UPCR decreased to 0.98 g/gCr. |
| 2 | 54 | M | 1 | 71 | 3.4 | BUC | 6 | (+) | (−) | (+) | (−) | (+) | Diff | I | 24 | After BUC discontinuation, CS was initiated. UPCR decreased to 0.15 g/gCr. |
| 3 | 59 | M | 4 | 84 | 4.4 | ADA | 4 | (+) | (+) | (+) | (−) | (+) | Diff | III | 77 | After ADA discontinuation, UPCR decreased to 0.1 g/gCr. |
| 4 | 81 | M | 0.4 | 94 | 4.6 | BUC, mPSL | 3 | (+) | (−) | (−) | (−) | (+) | Seg | I | 5 | After BUC discontinuation, patient was transferred to an attending physician. |
ADA, adalimumab; BUC, bucillamine; Cr, creatinine; CS, corticosteroid; Diff, diffuse; eGFR, estimated glomerular filtration rate; F, female; M, male; MN, membranous nephropathy; mPSL, methylprednisolone; NELL1, neural epidermal growth factor-like 1; PSL, prednisolone; RA, rheumatoid arthritis; Seg, segmental; UPCR, urine protein/creatinine ratio.
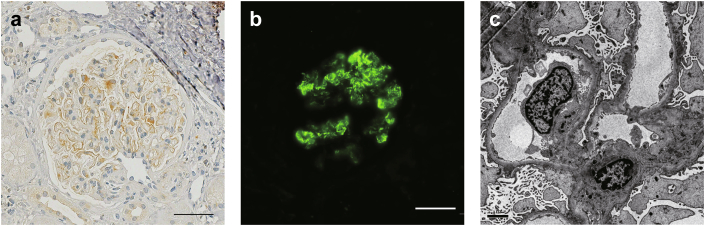
The mean patient age was 63 years. There were 3 men (75%) in the cohort. The time between the diagnosis of RA and MN ranged from 5 months to 4 years. Three patients (cases 2, 3, and 4) presented with nephrotic syndrome at the time of diagnosis. All patients had a history of drug initiation within 6 months before proteinuria onset, with 3 patients (cases 1, 2, and 4) receiving bucillamine and 1 patient (case 3) receiving adalimumab. All patients with drug-induced MN had discontinued the suspected drugs several months before MN diagnosis. Histopathologic examination showed granular immunostaining on glomerular capillary wall. All cases were positive for IgG and C1q, 1 (case 3) for IgA, and none for C3. In 2 cases (cases 1 and 4), glomerular subepithelial deposits had segmental distribution (Figure 1). Ehrenreich-Churg stage of MN was I in cases 2 and 4 and III in cases 1 and 3. All cases were negative for glomerular immunostaining of phospholipase A2 receptor antigen.
Figure 1.
Glomerular immunostaining in NELL1-positive membranous nephropathy in case 1. (a) NELL1 staining of paraffin-embedded sections. An incomplete and segmental distribution of NELL1 granular positivity was observed (original magnification ×400, scale bar = 50 μm). (b) Immunofluorescence staining of NELL1 (original magnification ×200, scale bar = 50 μm). (c) Electron microscopy image of a glomerulus showed incomplete distribution of subepithelial deposits with Ehrenreich-Churg stage III (original magnification ×6000, scale bar = 2 μm). NELL1, neural epidermal growth factor-like 1.
The clinical course after MN diagnosis was as follows. In 2 patients (cases 1 and 3), proteinuria decreased with drug discontinuation without requirement of additional therapy. Case 2 was treated with corticosteroids after bucillamine discontinuation and achieved proteinuria remission. Case 4 was transferred to an attending physician and was not followed up in our hospital.
Of the 221 patients with MN, another 3 had RA diagnosis (1.4% of patients with NELL1-negative MN). These patients were treated with bucillamine before MN diagnosis and were suspected of bucillamine-induced MN at kidney biopsy. Regarding clinical and histopathologic features of cases with NELL1-positive and NELL1-negative MN with bucillamine-induced RA, patients with NELL1-negative MN had longer RA history and 2 of them had longer interval from bucillamine initiation to proteinuria onset. In addition, patients with NELL1-negative MN showed diffuse distribution of glomerular subepithelial deposits (Supplementary Table S1).
Discussion
In this study, 60% of Japanese patients with NELL1-positive MN had a history of RA, and 66% of cases with NELL1-positive MN with RA were drug-associated (mostly bucillamine) MN, indicating that Japanese patients with NELL1-positive MN were frequently complicated with RA and bucillamine-associated MN. Renal involvement is relatively common in patients with RA, and MN is a frequently observed pathologic finding.6 The most common form of MN related to RA is drug-associated nephropathy caused by disease-modifying antirheumatic drugs, such as gold salts, D-penicillamine, and bucillamine, and rarely anti–tumor necrosis factor-α agents.6,7,S6-S12 Although less frequent, cases directly associated with RA have been reported.8,S13 In our study, 3 patients were suspected of bucillamine-induced MN based on their clinical course. Bucillamine has a chemical structure similar to D-penicillamine and was widely used in Japan. Similar to D-penicillamine, many cases with bucillamine-induced MN have been reported. Bucillamine-related MN most often occurs during the first year of therapy, and proteinuria resolves within 1 year after therapy discontinuation. Therefore, the clinical courses of our 3 patients were consistent with that of bucillamine-induced MN.
Morphologically, bucillamine-induced MN shows occasional segmental distribution of subepithelial electron-dense deposits with relatively early stages of MN as defined by electron microscopy. Notably, segmental distribution of immune deposits is a rare MN variant (2.5% of cases with MN).9 In addition, approximately 30% of segmental MN variants had positive NELL1 staining.9 Among 3 patients associated with bucillamine intake, 2 showed segmental distribution of immune deposits. Case 3 developed nephrotic syndrome during treatment with adalimumab (anti–tumor necrosis factor-α agent). With several previous reports of new onset of MN in patients with RA receiving anti–tumor necrosis factor-α agents,7 adalimumab may be one causal agent of drug-induced MN.
Among our patients with NELL1-negative MN, 3 (1.4%) had RA. All patients were suspected of bucillamine-associated MN at kidney biopsy. Regarding clinical background, NELL1-negative MN complicated by RA had longer RA history as compared with NELL1-positive MN with bucillamine use. In addition, all cases with NELL1-negative MN complicated by RA showed diffuse distribution of immune deposits. Thus, bucillamine initiation time might affect NELL1 positivity in MN with RA. Although it remains unclear which additional factors contribute to the development of NELL1-positive MN in patients with RA, the prevalence of RA and bucillamine use among patients with NELL1-positive MN was higher than that of patients with NELL1-negative MN (30% vs. 1.4%). Therefore, our results suggest that NELL1 plays an important role as a causative antigen in bucillamine-associated MN.
Most cohort studies conducted in other countries on NELL1-associated MN did not report RA prevalence. One report from the United States showed that 1% of cases with NELL1-positive MN were complicated by RA.2 In contrast, a single-center retrospective study from Japan showed that NELL1-positive MN accounted for 3.8% of Japanese patients with MN (n = 104), and 2 of 4 patients who were NELL1-positive (50%) had RA.3 Several reports have suggested an association between the human leukocyte antigen and drug-induced MN or MN in patients with RA.8,S14-S18 Thus, differences in the genetic background may affect susceptibility to NELL1-positive MN in the presence of RA.
NELL1, a secreted protein with 810 amino acids, was first reported to be overexpressed during cranial suture closure in patients with craniosynostosis.S19 Thereafter, its osteogenic, prochondrogenic, and anti-inflammatory properties in osteoarthritis models have been reported.S20,S21 As such, the osteogenic properties of NELL1 in the joint involvement of patients with RA may cause immune antigenicity against MN.
A limitation of this study is that the frequency of RA might be underestimated because of the relatively large number of earlier cases included in this study and the extraction of information from old medical records. Because immunohistochemistry results for immunoglobulins and complements were based on paraffin-embedded tissue, their positivity might be different from that of frozen tissue. However, our overall results suggest a high prevalence of drug-induced MN, particularly bucillamine-associated MN, in Japanese patients with NELL1-positive MN. Therefore, a larger collection of cases and genetic background studies are warranted in the future.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We sincerely thank Ms. Ishida Moeno for her outstanding technical support in immunofluorescence staining of the kidney specimens of the patients. This research was supported by The Jikei University Research Fund. Institutions had no role in the study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
Author Contributions
RM and HU contributed to the research idea and study design; RM, HU, AH, AK, AS, and MO contributed to data acquisition; RM, HU, AS, and MO contributed to data analysis/interpretation; and KJ, NT, YM, MI, and TY supervised the study. Each author has contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
Supplementary Methods.
Supplementary References.
Table S1. Comparison of clinical characteristics at the time of diagnostic kidney biopsy and histopathologic findings between NELL1-positive and NELL1-negative cases suspected with bucillamine-induced MN.
Supplementary Material
Supplementary Methods.
Supplementary References.
Table S1. Comparison of clinical characteristics at the time of diagnostic kidney biopsy and histopathologic findings between NELL1-positive and NELL1-negative cases suspected with bucillamine-induced MN.
References
- 1.Sethi S., Debiec H., Madden B., et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020;97:163–174. doi: 10.1016/j.kint.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Caza T.N., Hassen S.I., Dvanajscak Z., et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021;99:967–976. doi: 10.1016/j.kint.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwakura T., Ema C., Isobe S., et al. Prevalence of neural epidermal growth factor-like 1- and exostosin 1/exostosin 2-associated membranous nephropathy: a single-center retrospective study in Japan. Sci Rep. 2022;12:2967. doi: 10.1038/s41598-022-07037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spain R.I., Andeen N.K., Gibson P.C., et al. Lipoic acid supplementation associated with neural epidermal growth factor-like 1 (NELL1)–associated membranous nephropathy. Kidney Int. 2021;100:1208–1213. doi: 10.1016/j.kint.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurien A.A., Prema Ks J., Walker P.D., Caza T.N. Traditional indigenous medicines are an etiologic consideration for NELL1-positive membranous nephropathy. Kidney Int. 2022;102:1424–1426. doi: 10.1016/j.kint.2022.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Nakano M., Ueno M., Nishi S., et al. Analysis of renal pathology and drug history in 158 Japanese patients with rheumatoid arthritis. Clin Nephrol. 1998;50:154–160. [PubMed] [Google Scholar]
- 7.Stokes M.B., Foster K., Markowitz G.S., et al. Development of glomerulonephritis during anti-TNF-α therapy for rheumatoid arthritis. Nephrol Dial Transplant. 2005;20:1400–1406. doi: 10.1093/ndt/gfh832. [DOI] [PubMed] [Google Scholar]
- 8.Honkanen E., Törnroth T., Pettersson E., Skrifvars B. Membranous glomerulonephritis in rheumatoid arthritis not related to gold or D-penicillamine therapy: a report of four cases and review of the literature. Clin Nephrol. 1987;27:87–93. [PubMed] [Google Scholar]
- 9.Kudose S., Santoriello D., Debiec H., et al. The clinicopathologic spectrum of segmental membranous glomerulopathy. Kidney Int. 2021;99:247–255. doi: 10.1016/j.kint.2020.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.