Introduction
Ketamine usage is encountered in clinical practice both as an illicit street drug as well as a pharmacotherapeutic. In recent years, its clinical use has gained traction for a variety of indications including mood disorders, anxiety, and chronic pain syndromes.1 Typically, these are administered as a series of i.v. infusions, or i.n. in the case of esketamine, the (S)-enantiomer of ketamine. Ketamine-induced uropathy is a well described phenomenon where individuals develop recurring aseptic cystitis, ulcerative, or interstitial cystitis,2 and lower urinary tract pain.3 Development of ureteral strictures, papillary necrosis, and hydronephrosis has been described4 as well as subsequent acute kidney injury because of obstructive uropathy.5 Sixty percent of rats that were fed illicit ketamine by Rajandram et al.,6 were found to have developed interstitial nephritis at necropsy. This report describes what we believe to be the first described case of severe acute kidney injury because of biopsy proven allergic interstitial nephritis in the context of serial ketamine infusions.
Case Presentation
The patient, a 61-year-old Caucasian woman, presented to the emergency department because of rash, fatigue, and decreased appetite over the preceding 7 days. Her past medical history was notable for hypertension, hypercholesterolemia, complex regional pain syndrome, lupus, and rheumatoid arthritis. She had no history of preexisting renal or urological disease. Home medications included levothyroxine, cetirizine, topiramate, tizanidine, and cholecalciferol. She had been receiving a prescribed course of 10 ketamine infusions over a 2-week span. They were administered under the direction of pain management specialists because she was suffering from severe left lower extremity pain following a motor vehicle accident some years prior. In total, she received 8 ketamine infusions, at 0.35 mg/kg. On presentation, she was found to be cooperative but lethargic. Her rash was maculopapular and largely confined to her trunk. She relayed that the exanthem started 1 day before her presentation. She had no dependent edema on exam. Initial labs were notable for BUN 71, creatinine 6.0 mg/dl, CO2 of 19 mmol/l and potassium of 3.1 mmol/l. Complete blood count with differential was notable for peripheral eosinophilia with eosinophils at 10.6%. Urinalysis revealed 2+ protein, 2+ blood, and on microscopic examination, there were many white blood cells in clumps as well as numerous urine eosinophils. Urinary biomarkers of tubular injury such as β-2 microglobulin, N-acetyl-β-D-glucosaminidase, or neutrophil gelatinase-associated lipocalin would have been useful, but unfortunately were not available at this institution.
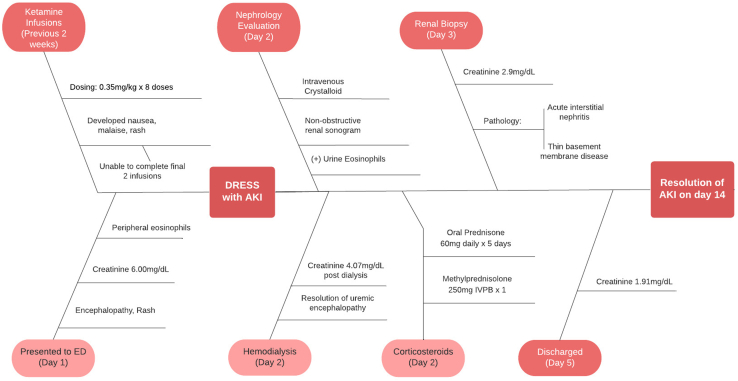
She was admitted to the medical intensive care unit with a presumptive diagnosis of drug reaction with eosinophilia and systemic symptoms. The inpatient nephrology service was promptly consulted to address her acute kidney injury. Retroperitoneal ultrasound confirmed normal appearing kidneys without hydronephrosis or bladder abnormalities. In addition to electrolyte replacement and crystalloid infusion, the decision to provide urgent hemodialysis was made because of her renal insufficiency and lethargy. After receiving 3 hours of hemodialysis, she became considerably more alert. Given the peripheral eosinophils and presence of urine eosinophils, a percutaneous renal biopsy was performed, and she was administered intravenous Solu-Medrol, 250 mg. Following further hydration, electrolyte replacement, and oral prednisone dosed 60 mg daily over the ensuing 5 days, her renal indices continued to improve without the need for continued dialytic support. Eventually, she was discharged home and was advised to forgo additional ketamine infusions until the renal pathology was available. A graphical summary of the patient’s clinical course is detailed in Figure 1.
Figure 1.
Fishbone diagram illustrating the clinical course of this case in a temporal fashion, including the treatment and creatinine levels. AKI, acute kidney injury; ED, emergency department.
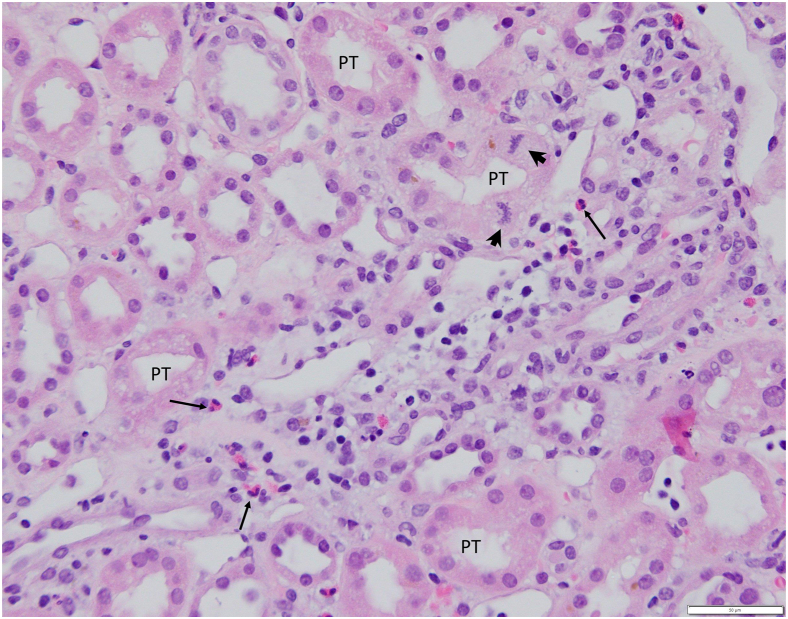
The patient returned to the renal clinic after 2 weeks. Oral corticosteroids had been completed as directed, and she had resumed all her previous outpatient medications. The previously noted rash had also completely dissipated. The follow-up laboratory results were notable for a creatinine of 0.8 mg/dl with normal electrolytes, urinalysis with trace blood, and a bland urinary sediment. Renal pathology revealed moderate acute interstitial nephritis, with accompanying acute tubular necrosis. The interstitial infiltrates were composed of mononuclear cells, neutrophils, as well as eosinophils (See Figure 2). The biopsy included kidney cortex and outer medulla; 29 glomeruli were present, 5 showed global sclerosis. The viable glomeruli revealed normal cellularity without signs of active glomerulitis or thickening of the basement membranes. Approximately 20% of the cortex reveal tubular atrophy and interstitial fibrosis. Small arteries and arterioles showed moderate sclerosis of the walls resulting in mild focal narrowing of the lumen; there were no signs of active vasculitis. The immunofluorescence microscopy did not disclose immune deposits along the peripheral glomerular capillary walls, along the tubular basement membranes, or in the interstitium. Trace granular C3 deposits were present in some glomeruli, and scattered fibrin deposits were seen in the interstitium. On electron microscopy she was noted to have thin basement membranes with harmonic mean thickness of 224 nm. Electron dense deposits were not seen in the glomeruli or along the tubular basement membranes. Based on these findings in conjunction with her clinical improvement, she was advised to list ketamine as an allergy and avoid this agent henceforth.
Figure 2.
Light microscopy findings in the outer medulla. The focal interstitial inflammatory infiltrate consists mostly of lymphocytes and includes several eosinophils (arrows) and isolated plasma cell. The straight segments of the proximal tubules show mild distention of the lumen, focal vacuolization of the cytoplasm, loss of brush border, and occasional mitotic figures (arrowheads). (Hematoxylin and eosin, 40× objective). PT, proximal tubule.
Discussion
The effects of oral and parenteral ketamine on the urogenital tract are well described, particularly in the context of illicit use as a street drug. In some studies, up to 30% of ketamine users reported experiencing lower urinary tract symptoms as a result.7 The use of ketamine and its enantiomers are becoming more widespread in clinical practice as a pharmaceutical agent for a constellation of psychiatric and analgesic indications. However, its relationship to the development of urogenital pathology is less clear. Chronic interstitial cystitis, ureteral stenosis, hydronephrosis, and papillary necrosis have been described both in human as well as mice. Investigators found that mice injected with ketamine over the course of several months developed an infiltration of mononuclear cells surrounding the glomeruli as well as renal tubules. The aggregation of these cells also extended to the renal pelvis and ureters.8 Although the precise mechanism of injury is unclear, immunoglobulin-E-mediated inflammation, and nitric oxide synthase-mediated inflammation have been implicated in lower urinary tract pathology.9
Conclusion
The implications in our case suggest that in addition to lower urinary tract pathology, ketamine also appears to result in upper urinary tract disease in humans. We describe a patient who developed drug reaction with eosinophilia and systemic symptoms manifesting as acute kidney after receiving a series of prescribed ketamine infusions. Her injury was severe enough to warrant acute hemodialysis and was found to have evidence of acute interstitial nephritis on pathology. Regardless of the underlying mechanism, ketamine cessation is the cornerstone to successful treatment. Table 1 summarizes the teaching points highlighted in this article.
Table 1.
Teaching points
| Therapeutic ketamine use is increasing in prevalence and is often encountered in clinical practice. |
| Lower urinary tract pathology has been well described as an adverse effect. |
| Use linked to recurring aseptic cystitis, chronic interstitial cystitis, ureteral stenosis, hydronephrosis, and papillary necrosis. |
| Interstitial nephritis has been identified in rats fed ketamine. |
| Ketamine infusions were attributed to DRESS syndrome and AKI in this case. |
| Acute interstitial nephritis was identified as the cause for AKI. |
| Immunoglobulin-E-mediated inflammation, as well as nitric oxide synthase-mediated inflammation are potential pathogenic mechanisms. |
| Avoiding further ketamine exposures would be advisable. |
AKI, acute kidney injury; DRESS, drug reaction with eosinophilia and systemic symptoms.
Disclosure
All of the authors declared no competing interests. Author contributions were evenly divided, and included but not limited to patient care, research, writing the manuscript, and subsequent revisions.
References
- 1.Sanacora G., Frye M.A., McDonald W., et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74:399–405. doi: 10.1001/jamapsychiatry.2017.0080. [DOI] [PubMed] [Google Scholar]
- 2.Chiew Y.W., Yang C.S. Disabling frequent urination in a young adult. Ketamine-associated ulcerative cystitis. Kidney Int. 2009;76:123–124. doi: 10.1038/ki.2009.139. [DOI] [PubMed] [Google Scholar]
- 3.Lamers G., Van Dyck J., Schapmans S., et al. Ketamine-induced uropathy: a diagnostic pitfall in an increasing healthcare issue in youngsters. Urol Case Rep. 2022;42 doi: 10.1016/j.eucr.2022.102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang P., Meng E., Cha T., et al. ‘Walking-stick ureters’ in ketamine abuse. Kidney Int. 2011;80:895. doi: 10.1038/ki.2011.242. [DOI] [PubMed] [Google Scholar]
- 5.Selby N.M., Anderson J., Bungay P., et al. Obstructive nephropathy and kidney injury associated with ketamine abuse. NDT Plus. 2008;1:310–312. doi: 10.1093/ndtplus/sfn054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajandram R., Yap N.Y., Ong T.A., et al. Oral ketamine induced pathological changes of the urinary tract in a rat model. Malays J Pathol. 2017;39:47–53. [PubMed] [Google Scholar]
- 7.Chu P.S., Ma W.K., Wong S.C., et al. The destruction of the lower urinary tract by ketamine abuse: a new syndrome? BJU Int. 2008;102:1616–1622. doi: 10.1111/j.1464-410X.2008.07920.x. [DOI] [PubMed] [Google Scholar]
- 8.Yeung L.Y., Rudd J.A., Lam W.P., et al. Mice are prone to kidney pathology after prolonged ketamine addiction. Toxicol Lett. 2009;191:275–278. doi: 10.1016/j.toxlet.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Jhang J.F., Hsu Y.H., Kuo H.C. Possible pathophysiology of ketamine-related cystitis and associated treatment strategies. Int J Urol. 2015;22:816–825. doi: 10.1111/iju.12841. [DOI] [PubMed] [Google Scholar]