Abstract
Introduction
Necrotizing crescentic glomerulonephritis is a major contributor to morbidity and mortality in Antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV). Because therapy relies on immunosuppressive agents with potentially severe adverse effects, a reliable noninvasive biomarker of disease activity is needed to guide treatment.
Methods
We used flow cytometry to quantify T cell subsets in blood and urine samples from 95 patients with AAV and 8 controls to evaluate their biomarker characteristics. These were compared to soluble markers, monocyte chemoattractant protein-1 (MCP-1), soluble CD163 (sCD163), soluble CD25 (sCD25), and complement C5a (C5a), measured using multiplex analysis. Available kidney biopsies (n = 21) were classified according to Berden.
Results
Patients with active renal AAV (rAAV) showed significantly higher urinary cell counts than those in remission, or those with extrarenal manifestation, or healthy controls. Urinary T cells showed robust discrimination of disease activity with superior performance compared to MCP-1 and sCD163. Patients whose kidney biopsies had been classified as “crescentic” according to Berden classification showed higher urinary T cell counts. Discordant regulatory T cells (Treg) proportions and CD4+/CD8+ ratio in blood and urine suggested that urinary cells reflect tissue migration rather than mere micro-bleeding. Furthermore, urinary Treg and T helper cells (TH17) patterns were associated with clinical response and risk of renal relapse.
Conclusion
Urinary T cells reflect the renal inflammatory milieu in AAV and provide further insights into the pathogenesis of this chronic condition. Their promising potential as noninvasive diagnostic and prognostic biomarkers deserves further exploitation.
Keywords: ANCA, TH, Treg, vasculitis
ANCA cause systemic life-threatening AAV. AAV affects any organ but most prominently the kidneys, causing pauci-immune necrotizing crescentic glomerulonephritis.1,2 With renal involvement being a major contributor to mortality, preventing kidney damage is a main objective of therapy.3 However, most therapy regimens rely on immunosuppressive agents with considerable side effects potentially contributing to morbidity and mortality. Clinicians are therefore challenged to find the right balance between overtreatment and undertreatment.4 To aid decision making an adequate assessment of renal involvement, disease activity, and progression is much needed.
Kidney biopsy remains the gold standard for establishing the diagnosis of pauci-immune necrotizing crescentic glomerulonephritis and offers prognostic value.5 However, its invasive nature, cost, and sampling error render renal biopsy unsuitable for serial monitoring of disease activity.6 Assuming the urine space represents a “window into the kidney” because of its proximity to renal pathology, urinary biomarkers could be ideal for noninvasive and cost-effective serial monitoring of disease activity. Urinalysis is widely used by clinicians, yet its implications remain controversial.7, 8, 9, 10, 11, 12 Recently, MCP-1, sCD163, and sCD25 have been shown to be promising urinary biomarkers of renal disease activity in AAV.13, 14, 15, 16 Surprisingly, little is known about urinary leukocyte populations in AAV and whether they reflect disease activity, even though this has been shown in other conditions such as lupus nephritis. In lupus nephritis, urinary leukocytes directly reflect interstitial infiltration, and T cells represent a reliable biomarker.17, 18, 19, 20 Only 3 studies of urinary leukocytes have so far been reported in AAV, and they focused on CD4+ T effector cells and macrophages. These were found to reflect disease activity and histopathological extent of disease; however, their suitability as diagnostic or prognostic biomarkers was never assessed.21, 22, 23
We hypothesized that urinary leukocyte populations reflect renal vasculitis and can be used as biomarkers in the assessment of disease activity. In particular, we focused on Treg and IL-17 producing TH17, which have never been studied in urine despite their emerging role in the pathogenesis of AAV.24, 25, 26, 27
We provide evidence that active renal vasculitis causes distinct leukocyte subpopulations to migrate into the urine space representing a noninvasive prognostic biomarker for rAAV.
Methods
Patients
Patients with AAV, based on Chapel Hill Consensus Conference criteria,28 were recruited in the Department of Nephrology and Medical Intensive Care, Charité-Universitätsmedizin Berlin, Germany; Department of Nephrology, Helios Klinikum Berlin-Buch, Berlin, Germany; Department of Nephrology and Endocrinology/Diabetology, Ernst von Bergmann Klinikum, Potsdam, Germany; and Department of Renal Medicine, University College London Royal Free Hospital, London, United Kingdom. Based on disease activity, assessed by Birmingham Vasculitis Activity Score Version 3 (BVAS)29 and histopathological examination of kidney biopsy, patients were stratified into 3 groups as follows: (i) active vasculitis with renal involvement (rAAV) (histologic proof of pauci-immune necrotizing crescentic glomerulonephritis within 2 weeks and/or BVAS renal subcategory ≥ 10), (ii) active vasculitis without renal involvement nonrenal AAV (nrAAV) (BVAS ≥ 1 and renal subcategory = 0), and (iii) remission with previous renal involvement (Rem) (BVAS = 0). Both relapses and first manifestations of AAV were included, whereas patients with anuria, malignancy, menses, or bladder pathology (2 cases of bladder cancer) were excluded. In addition, healthy controls (HC) were recruited. Clinical and routine laboratory data were collected. Abiding by the Declaration of Helsinki and the Declaration of Istanbul, local ethics committees approved the study (EA2_103_17), and all participants gave written, informed consent.
Between November 1, 2017 and December 31, 2019, a total of 112 subjects were recruited. After the completion of the recruitment process, 17 participants had to be excluded from the final analysis because of technical issues (e.g., flow cytometer clogging, n = 6; technical failure rate 5 %) and diagnosis of a concomitant kidney condition after initial inclusion (n = 11, e.g., proof of concomitant membranous nephropathy, minimal change, etc., on kidney biopsy). The remaining 95 patients (Berlin n = 67, London n = 28) were split into an inception cohort (between November 1, 2017 and December 31, 2018, n = 65) and a validation cohort (between January 1, 2019 and December 31, 2019, n = 30). The population demographics are shown in Table 1; an overview of the collected samples are provided in Supplementary Table S1.
Table 1.
Demographic and clinical information of participants
| Inception |
Validation |
||||||
|---|---|---|---|---|---|---|---|
| 65 |
30 |
||||||
|
n |
rAAV |
nrAAV |
Rem |
HC |
rAAV |
nrAAV |
Rem |
| n | 33 | 4 | 20 | 8 | 19 | 3 | 8 |
| Age: yr median (IQR) | 57 (48, 64) | 44 (38, 51) | 63 (55, 71) | 47 (40, 54) | 67 (56, 73) | 50 (44, 58) | 64 (52, 68) |
| Sex: females (%) | 13 (39%) | 1 (25%) | 10 (50%) | 5 (63%) | 5 (26%) | 0 | 4 (50%) |
| Diagnosis | |||||||
| GPA: n, (%) | 21 (64%) | 4 (100%) | 11 (55%) | 10 (53%) | 2 (67%) | 5 (63%) | |
| MPA: n, (%) | 12 (36%) | 0 | 9 (45%) | 9 (47%) | 1 (33%) | 3 (37%) | |
| ANCA | |||||||
| anti-MPO: n (%) | 16 (48%) | 1 (25%) | 9 (45%) | 9 (47%) | 1 (33%) | 3 (38%) | |
| anti-PR3: n (%) | 17 (52%) | 3 (75%) | 11 (55%) | 10 (53%) | 2 (67%) | 5 (62%) | |
| Histologically proven | 17 (52%) | 1 (25%) | 15 (75%) | 6 (32%) | 1 (33%) | 3 (38%) | |
| Number of relapses | |||||||
| First manifestation | 17 (52%) | 2 (50%) | 9 (45%) | 11 (58%) | 0 | 5 (62%) | |
| Median (IQR) | 0 (0, 2) | 0.5 (0, 1.25) | 1 (0, 1) | 0 (0, 1) | 1 (1, 1.5) | 0 (0, 1) | |
| Mo since first diagnosis: median (IQR) | 2 (0, 52) | 9 (4, 136) | 46 (35, 69) | 1 (0, 48) | 174 (105, 243) | 40 (20, 57) | |
| Berden class: count, (%) | |||||||
| Crescentic | 4 (31%) | 0 (0%) | |||||
| Focal | 2 (15%) | 4 (44%) | |||||
| Mixed | 5 (38%) | 2 (22%) | |||||
| Sclerotic | 0 (0%) | 1 (11%) | |||||
| Too few | 2 (15%) | 1 (11%) | |||||
| Treatment before sample acquisition | |||||||
| Naive | 16 (48%) | 9 (47%) | |||||
| Steroids | 17 (52%) | 10 (53%) | |||||
| BVAS: median (IQR) | 15 (12, 19) | 16 (10, 19) | 0 (0, 0) | 16 (14, 20) | 12 (11, 12) | 0 (0, 0) | |
| BVAS renal subcategory: median (IQR) | 10 (10, 12) | 0 (0, 0) | 0 (0, 0) | 12 (10, 12) | 0 (0, 0) | 0 (0, 0) | |
| Creatinine: [μmmol/l] median (IQR) | 161 (95, 300) | 81 (77, 89) | 111 (82, 164) | 291 (216, 382) | 83 (73, 87) | 116 (90, 173) | |
| CRP [mg/l]: median (IQR) | 20 (6, 46) | 17 (6, 30) | 3 (2, 6) | 12 (6, 27) | 15 (9, 28) | 4 (2, 7) | |
| Proteinuria [g/l]: median (IQR) | 1.21 (0.30, 2.10) | 0.11 (0.09, 0.14) | 0.18 (0.11, 0.47) | 0.75 (0.40, 1.29) | 0.13 (0.07, 0.16) | 0.21 (0.12, 0.51) | |
| Ery dipstick | 3 (3, 3) | 0 (0, 0) | 0 (0, 1) | 0 (0, 0) | 3 (3, 3) | 0 (0, 0) | 0 (0, 1) |
| Active sedimenta | 11 (33%) | 0 | 0 | 0 | 7 (36%) | 0 | 0 |
AAV, ANCA-associated vasculitis; ANCA, antineutrophil cytoplasmic antibodies; BVAS, Birmingham Vasculitis Activity Score; CRP, C-reactive protein; IQR, interquartile range; ery dipstick, erythrocytes on urinary dipstick analysis; GPA, granulomatosis with polyangiitis; HC, healthy control; MPA, microscopic polyangiitis; MPO, myeloperoxidase; nrAAV, nonrenal active AAV; PR3, proteinase 3; rAAV, active renal AAV; Rem, remission.
Presence of acanthocytes on phase-contrast microscopy of urinary sediment.
Materials
Phosphate-buffered saline (PBS) without Mg2+ and Ca2+ was procured from Biochrom GmbH (Berlin, Germany), antibodies CD3-PE/Cy5 (catalog number: 300310), CD4-PE/Cy7 (357410), CD8-APC/Cy7 (344714), CD127-BV510 (351332), FoxP3-FITC (320106), Tbet-APC (644814) were procured from BioLegend (San Diego, CA, USA), RORγt-PE (12-6988-82) was procured from Invitrogen by Thermo Fisher Scientific (San Diego, CA, USA), and CD25-APC/Cy7 (557753) was procured from BD Pharmingen (Franklin Lakes, NJ, USA). Live/dead staining was performed using ViobilityTM (Miltenyi Biotec, Bergisch Gladbach, Germany). Luminex Human Magnetic Assay (4-Plex) was used for MCP-1, sCD163, sCD25 and C5a (Luminex Corporation, Austin, Texas, USA).
Sample Processing and Flow Cytometry
Freshly voided urine samples with a volume of at least 20 ml were collected from all patients and within 6 hours, they were centrifuged at 2000 rpm for 8 minutes at 4 °C. Supernatant was stored at −70 °C for later detection of soluble biomarkers. Cell pellets were washed in PBS + 1% bovine serum albumin (BSA), centrifuged as above, resuspended in PBS, and transferred to 96-well plates. Venous blood was collected in lithium heparin tubes, 1 ml of which was lysed for 5 minutes in 10 ml ammonium chloride lysis buffer at 37 °C and centrifuged at 2000 rpm for 5 minutes at 4 °C. Subsequently, the pellet was resuspended in 1 ml PBS and 100 μl of the cell suspension were transferred to 96-well plates. The panel consisted of CD3-PE/Cy5, CD4-PE/Cy7, CD8-APC/Cy7, CD127-BV510, FoxP3-FITC, Tbet-APC, and RORγt-PE; and live/dead stained using ViobilityTM. In a subset of patients, CD25-APC/Cy7 was substituted for CD8-APC/Cy7.
Live/dead staining was performed using Viobility in 100 μl of PBS for 15 minutes on ice. After washing with 100 μl PBS-BSA 1% and centrifugation at 2000 rpm for 3 minutes at 4 °C, samples were blocked using Fc-block in PBS/BSA for 10 minutes on ice. Subsequently, they were stained for surface markers (CD3, CD4, CD8, and CD127) for 15 minutes on ice. Subsequently, they were washed, centrifuged twice (see above), fixed, and permeabilized (FoxP3 Transcription Factor Staining Buffer Kit, Invitrogen by Thermo Fisher Scientific, San Diego, CA, USA) and stained for intracellular markers (FoxP3, RORγt, and Tbet). CountBright Absolute Counting Beads (Invitrogen by Thermo Fisher Scientific, San Diego, CA, USA) were added, and samples were analyzed on BD FACSCanto II or BD LSR II (both BD Biosciences, Franklin Lakes, NJ, USA). FACS data were analyzed using FlowJo 10 (TreeStar, Ashland, OR, USA), and absolute cell counts were calculated based on bead count and sample size and normalized to 100 ml blood or urine.
Detection of Soluble Biomarkers
Frozen cell-free urine samples were thawed at room temperature, centrifuged, diluted 1:10 with PBS/BSA and analyzed with a Luminex Human Magnetic Assay (4-Plex) for MCP-1, sCD163, sCD25, and C5a (Luminex Corporation, Austin, Texas, USA). Multiplex assays were analyzed with a Bioplex reader and Bioplex software (Bio-Rad Laboratories, Hercules, CA, USA). When a given molecule was not detectable in a sample, the concentration was determined as half of the lowest detectable value. Protein concentrations were normalized as pg/g urinary creatinine. Creatinine concentration was determined by R&D Creatinine Parameter Assay Kit (R&D Systems Inc., Minneapolis, MN, USA).
Histopathological Analysis
Patients with active renal vasculitis who had received a kidney biopsy at the initial presentation were included for histopathological evaluation. A total of 21 samples were available for analysis by an expert in kidney histopathology and classified according to the Berden classification.5 Results were compared with urinary T cell counts.
Statistical Analysis
Statistical analysis and creation of graphs were performed in R (R Core Team, Vienna, Austria).30 Group medians were compared using Kruskal-Wallis and post hoc Dunn’s test to correct for multiple comparisons from the PMCMRplus package.31 Bonferroni corrected P-values < 0.05 were considered significant. Data were displayed as medians and interquartile range, adding 1 to visualize zeros on logarithmic scales, using the ggplot2 package.32 To compare results of a single cell population between blood and urine samples, paired Wilcoxon signed-rank test from the ggpubr package was used. Correlation analysis between urine cells and routine clinical parameters was performed using the ggstatsplot package. Pearson’s index with P-value correction for multiple comparisons using Holm’s method was used to identify statistically significant correlations. Biomarker abilities were assessed and compared using receiver operating characteristic curves (ROC) from the pROC and plotROC packages.33,34 Defining rAAV as cases and nrAAV and remission as controls, Youden’s index (i.e., the point on the curve with the maximum sum of sensitivity and specificity) was used to determine ideal cut off values for the diagnosis of active renal vasculitis for each marker. Survival plots were created using the survival and survminer packages and statistical significance was evaluated using logrank test.35
Results
Urinary T Cells are Elevated in Active Renal Vasculitis
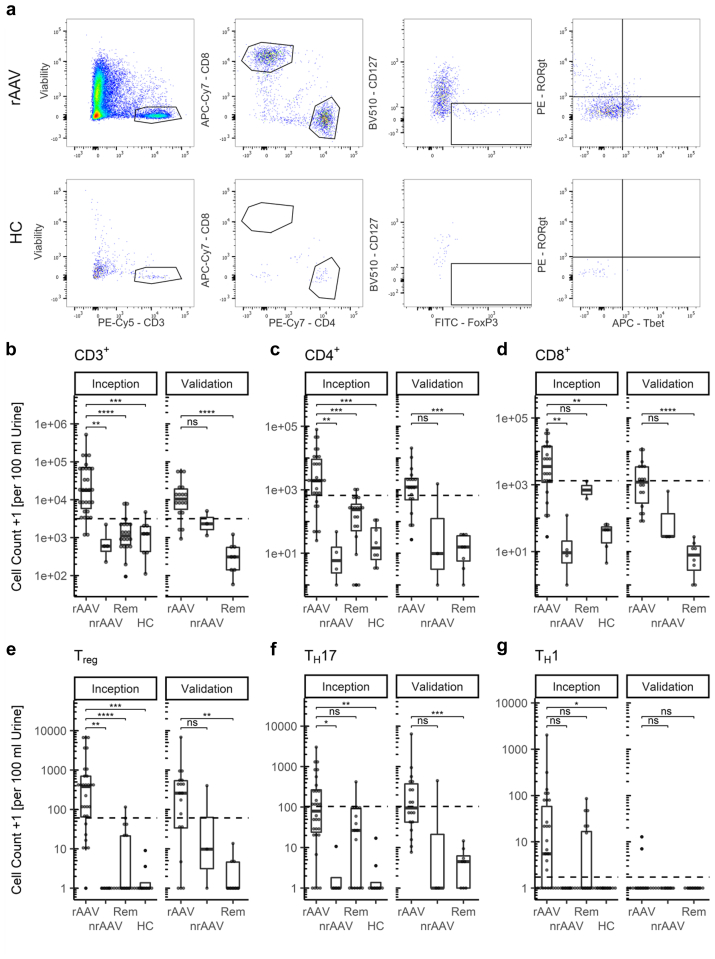
We recruited a total of 95 patients in 2 separate cohorts. The inception cohort comprised 65 subjects, distributed as follows: 33 active rAAV, 4 active nrAAV, 20 patients in stable remission with previous rAAV remission. In addition, 8 HCs were recruited. The validation cohort included 30 patients, distributed as follows: 19 rAAV, 3 nrAAV, and 8 remission. Samples were analyzed for the presence of CD3+ T cells by flow cytometry and normalized to 100 ml of blood or urine. Exemplary dot plots illustrating distinct urinary T cell populations found in active rAAV are shown in Figure 1a (see blood sample including fluorescence minus one controls for comparison in Supplementary Figure S1).
Figure 1.
T cells show distinct, highly elevated subpopulations in urine in renal ANCA-associated vasculitis (AAV). (a) Exemplary urinary flow cytometry dot plots of renal active AAV (rAAV) patient and HC, previous gating: even flow, singlets; (b–g) box plots of urinary T cell subpopulations: CD3+, CD4+, CD8+, Treg, TH17, and TH1 cells. Cell counts plotted +1 to visualize zeros on logarithmic scale, statistics were determined using Kruskal-Wallis and post hoc Dunn’s test, P-value summary: ns: P > 0.05, ∗: P = 0.05 – 0.01, ∗∗: P = 0.01 – 0.001, ∗∗∗: P < 0.001. AAV, ANCA-associated vasculitis; ANCA, antineutrophil cytoplasmic antibodies; HC, healthy control, dashed line indicates optimal cut off for identification of rAAV; nrAAV, nonrenal active AAV; rAAV, active renal AAV; Rem, remission; Treg, regulatory T cell; TH17, T helper 17; TH1, T helper 1.
We found a significant increase in absolute urinary CD3+, CD4+, and Treg cell counts in rAAV in comparison to most other groups in the inception cohort (Figure 1b, c, and e). The increase of CD8+ and TH17 cells seen in rAAV only reached significance compared to nrAAV and HC, whereas for TH1 the only significant difference was between rAAV and HC (Figure 1d, f, and g; Table 2). There were no significant differences when comparing centers, ANCA serotype (i.e., antimyeloperoxidase vs. Antiproteinase 3) or clinical syndrome (i.e., granulomatosis with polyangiits vs. microscopic polyangiitis) (Supplementary Figures S2–S4).
Table 2.
Summary of urinary T cell count
| Urinary T cell count [1/100 ml urine] median (IQR) | |||||||
|---|---|---|---|---|---|---|---|
| Inception cohort | |||||||
| rAAV | nrAAV | P-value | Rem | P-value | HC | P-value | |
| CD3+ | 17599 (5459, 72194) | 597 (383, 1436) | 0.0031 | 1116 (638–2376) | < 0.0001 | 1263 (406, 2064) | 0.0005 |
| CD4+ | 1917 (667, 9626) | 6 (1, 28) | 0.0014 | 236 (45, 400) | 0.0007 | 15 (5, 75) | 0.0001 |
| CD8+ | 3766 (1131, 14658) | 8 (3, 66) | 0.0061 | 829 (381, 1277) | 1.0000 | 44 (13, 55) | 0.0031 |
| Treg | 378 (60, 698) | 0 (0, 0) | 0.0026 | 0 (0, 21) | <0.0001 | 0 (0, 1) | 0.0001 |
| TH17 | 82 (22, 281) | 0 (0, 5) | 0.0320 | 26 (0, 92) | 0.2015 | 0 (0, 1) | 0.0012 |
| TH1 | 5 (0, 75) | 0 (0, 0) | 0.1637 | 0 (0, 17) | 0.2510 | 0 (0, 0) | 0.0189 |
| Validation cohort | |||||||
| CD3+ | 10519 (5137, 20206) | 2324 (1733, 3675) | 0.4463 | 311 (140, 554) | <0.0001 | - | - |
| CD4+ | 1233 (445, 2303) | 9 (4, 777) | 0.1369 | 15 (4, 35) | 0.0007 | - | - |
| CD8+ | 1215 (306, 3440) | 28 (27, 334) | 0.3797 | 7 (20, 14) | <0.0001 | - | - |
| Treg | 259 (33, 535) | 9 (4, 205) | 0.9543 | 0 (0, 4) | 0.0065 | - | - |
| TH17 | 97 (41, 403) | 0 (0, 224) | 0.2861 | 4 (0, 6) | 0.0009 | - | - |
| TH1 | 0 (0, 0) | 0 (0, 0) | 1.0000 | 0 (0, 0) | 0.9327 | - | - |
AAV, ANCA-associated vasculitis; ANCA, antineutrophil cytoplasmic antibodies; HC, healthy control; IQR, interquartile range; nrAAV, nonrenal active AAV; rAAV, active renal AAV; Rem, remission.
Statistics: Kruskal-Wallis and post hoc Dunn’s test with Bonferroni correction for multiple comparisons.
Urinary T Cell Populations Differ From Blood T Cell Populations
In parallel, T cell subpopulations were analyzed in the peripheral blood. Here, we found no significant difference in CD3+, CD4+, Treg, TH17, or TH1 T cell subpopulations between the different groups. Only patients in remission in the validation cohort showed a slight increase in CD8+ cells compared to rAAV (Supplementary Figure S5a–f).
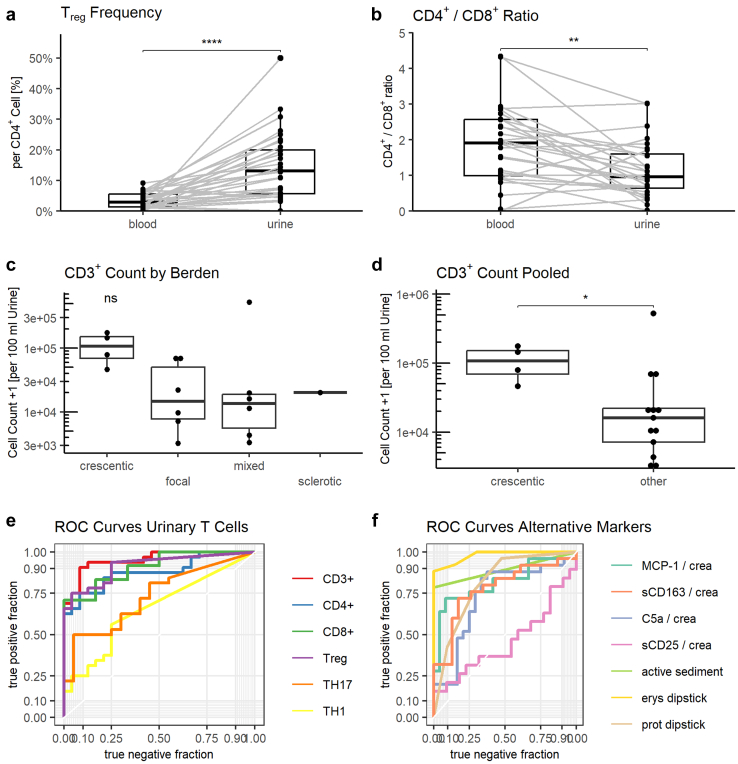
To assess whether the finding of higher urinary T cells in rAAV patients was merely a surrogate of transglomerular bleeding, we compared T cell subset proportions in the peripheral blood and urine. Matched comparison revealed a significantly higher frequency of Treg per CD4+ cell in urine than in blood (medians: 13.4% vs. 2.9%, P < 0.0001) unique to the rAAV group (Figure 2a). A similar, albeit statistically insignificant increase was found for TH17 cells (6.1% vs. 2.5%, P = 0.2) (Supplementary Figure 6b). Conversely, the CD4+/CD8+ ratio was significantly lower in urine than in blood in rAAV patients (0.96 vs. 1.91, P = 0.0008) (Figure 2b).
Figure 2.
Urinary T cells show an altered phenotype between blood and urine, reflect the Berden classification, and show robust biomarker characteristics. Patients with active renal ANCA-associated vasculitis (AAV) show significantly higher urinary regulatory T cell (Treg) frequency (a) and decreased CD4+/CD8+ ratio in urine (b). Frequency of chemokine receptor 6 (CCR6) positive CD4+ and Treg is significantly higher in urine than in blood (c and d) in patients with active renal AAV. (e) Urinary CD3+ cells trend higher in patients classified as crescentic according to Berden classification. (f) Urinary CD3+ cells are significantly elevated when comparing crescentic class to pooled other classes. (g−h) receiver operator characteristic (ROC) curves for the diagnosis of active renal AAV of urinary T cells, soluble markers and traditional markers. Statistics: (a−d): Paired Wilcoxon signed-rank test, (e): Kruskal-Wallis, F: Mann-Whitney-Wilcoxon test, P-value summary: ns: not significant, ∗: 0.05 – 0.01, ∗∗: P = 0.01 – 0.001, ∗∗∗: P = 0.001 – 0.0001, ∗∗∗∗: P < 0.0001. CCR6, chemokine receptor 6; Erys dipstick, erythrocytes on urinary dipstick analysis; HC, healthy control; nrAAV, nonrenal active AAV; Prot dipstick, protein on urinary dipstick analysis; rAAV, active renal AAV; Rem, remission; ROC, receiver operator characteristics; Treg, regulatory T cell.
Urinary T Cell Count Correlated With Serum C-reactive Protein and Proteinuria
Analysis of urinary cells and routine clinical data in the rAAV group showed a strong correlation between C-reactive protein with urinary CD4+ cell count (Pearson’s r = 0.72, P = 0.0015), and Treg/TH17 ratio and ANCA titer (r = 0.60, P = 0.032). Correlations between CD3+, CD4+, and Treg were disregarded because they reflect the gating strategy. All other correlations were statistically insignificant after correction for multiple testing using Holm’s method (Supplementary Figure S7).
Urinary T Cells Represent a Reliable Biomarker for Active Renal Vasculitis
Using the inception cohort data, we generated ROC curves to examine the ability of urinary T cells to identify active renal vasculitis (Figure 2g). Patients with renal active AAV were compared to those with nonrenal active AAV and those in remission. In short, CD3+ and Treg cells represent the most reliable biomarkers with an area under the curve (AUC) of 0.95 and 0.92, respectively. We defined ideal cut off values (i.e., the point on the curve with maximum sum of sensitivity and specificity) of 3149 CD3+ cells per 100 ml of urine (sensitivity: 90%, specificity: 92%) and 60 Treg (74%, 96%) (Figure 2g, Table 3). These cut-offs were then validated in an independent validation cohort of 30 patients, yielding comparable biomarker characteristics (Table 3). The composition of combined markers i.e., Treg/TH17 ratio did not add any benefit (area under the curve: 0.73) (Supplementary Figure S8).
Table 3.
Summary of receiver operator characteristics
| Inception cohort | ||||||||
|---|---|---|---|---|---|---|---|---|
| Urinary marker | AUC | cut off | sens | spec | ppv | npv | LR+ | LR− |
| CD3+ | 0.95 | 3149 | 0.94 | 0.92 | 0.94 | 0.92 | 11.23 | 0.07 |
| CD4+ | 0.88 | 664 | 0.74 | 0.92 | 0.92 | 0.73 | 8.90 | 0.28 |
| CD8+ | 0.91 | 1321 | 0.71 | 1.00 | 1.00 | 0.46 | Inf | 0.29 |
| Treg | 0.92 | 60 | 0.74 | 0.96 | 0.96 | 0.74 | 17.81 | 0.27 |
| TH17 | 0.73 | 103 | 0.48 | 0.95 | 0.94 | 0.54 | 9.68 | 0.54 |
| TH1 | 0.66 | 1 | 0.58 | 0.75 | 0.75 | 0.58 | 2.32 | 0.56 |
| Erys dipstick | 0.98 | 3+ | 0.88 | 1.00 | 1.00 | 0.87 | Inf | 0.12 |
| Prot dipstick | 0.81 | 1+ | 0.96 | 0.52 | 0.71 | 0.92 | 2.02 | 0.07 |
| Active sedimenta | 0.89 | y | 0.79 | 1.00 | 1.00 | 0.62 | Inf | 0.21 |
| sCD163/crea | 0.78 | 40.88 | 0.72 | 0.83 | 0.82 | 0.73 | 4.14 | 0.34 |
| MCP1/crea | 0.82 | 0.44 | 0.72 | 0.92 | 0.90 | 0.76 | 8.64 | 0.31 |
| sCD25/crea | 0.54 | 0.52 | 0.42 | 0.77 | 0.62 | 0.61 | 1.85 | 0.75 |
| C5a/crea | 0.73 | 2.93 | 0.84 | 0.67 | 0.72 | 0.80 | 2.52 | 0.24 |
| Validation cohort | ||||||
|---|---|---|---|---|---|---|
| sens | spec | ppv | npv | LR+ | LR- | |
| CD3+ | 0.82 | 0.89 | 0.93 | 0.73 | 7.41 | 0.20 |
| CD4+ | 0.65 | 0.89 | 0.92 | 0.57 | 5.82 | 0.40 |
| CD8+ | 0.47 | 1.00 | 1.00 | 0.50 | Inf | 0.53 |
| Treg | 0.65 | 0.89 | 0.92 | 0.57 | 5.82 | 0.40 |
| TH17 | 0.47 | 0.89 | 0.89 | 0.47 | 4.24 | 0.60 |
| TH1 | 0.12 | 1.00 | 1.00 | 0.38 | Inf | 0.88 |
AUC, area under the curve; C5a, complement C5a (pg/g creatinine); Erys dipstick, erythrocytes on urinary dipstick analysis; LR−, negative likelihood ratio; LR+, positive likelihood ratio; MCP-1, monocyte chemoattractant protein-1 (pg/g creatinine); npv, negative predictive value; ppv, positive predictive value; Prot dipstick, proteinuria on urinary dipstick analysis; sCD163, soluble CD163 (pg/g creatinine); sCD25, soluble CD25 (pg/g creatinine); Sens, sensitivity; Spec, specificity; TH1, T helper 1; TH17, T helper 17; Treg, T regulatory cell;
Ideal cut off was determined in the inception cohort using Youden’s index (i.e. point on the ROC curve with maximum sum of sensitivity and specificity).
Presence of acanthocytes in urinary sediment.
Next, the diagnostic performance of urinary T cells was compared to established markers, such as hematuria, proteinuria, phase-contrast microscopy, and recently reported urinary biomarkers for active rAAV, namely urinary sCD163, sCD25, MCP-1, and complement C5a. Traditional dipstick markers showed robust diagnostic performance (hematuria: ideal cut off: 3+, area under the curve 0.98, sensitivity: 88%, specificity 100%, proteinuria: 1+, 0.81, 96%, 52%), whereas presence of acanthocytes on phase-contrast microscopy performed weakly (0.89, 79%, 100%). sCD163 (0.78), MCP-1 (0.82), and C5a (0.73) showed modest diagnostic accuracy, whereas sCD25 (0.59) performed poorly (Figure 2h, Table 3).
Urinary T Cells Correspond With Berden Classification
To determine if urinary T cells specifically reflect renal inflammation, available kidney biopsies were classified according to the Berden classification. From all 52 patients included as active rAAV, 21 kidney biopsies were available for evaluation. In the others, no biopsy had been performed, mainly because of previously established clinical diagnosis. Berden classes were as follows (crescentic: 4/21, focal: 6/21, mixed 7/21, and sclerotic 1/21) and 3 of 21 samples contained too few glomeruli to be classified. Urinary CD3+ counts trended higher for the crescentic class (medians: 111,859; focal: 15,843; mixed: 13,765; sclerotic: 20,206; global P = 0.15) (Figure 2e). Similar but statistically insignificant differences were also found for urinary CD4+, CD8+, Treg, and TH17 counts (Supplementary Figure S9 and Supplementary Table S2). Because of the small sample numbers, we compared crescentic to all other classes pooled. Both urinary CD3+ cell count (crescentic: 111,859 vs. other: 16,142, P = 0.023) and CD4+ (crescentic: 10,747 vs. other: 667, P = 0.032) were significantly elevated in the crescentic class (Supplementary Figure S10B). Differences were not significant for CD8+, Treg and TH17 (Supplementary Tables S2 and S3).
Urinary Treg and TH17 Cells Predict Clinical Response
Having demonstrated that urinary T cells reflect active renal disease and histopathological classification, we next asked whether the concentration of urinary T cells could predict disease outcome. All patients with active rAAV were pooled and follow-up data were available for 30 participants. Routine clinical (BVAS, renal BVAS [i.e., sum of BVAS renal subcategory]) and laboratory parameters were determined 2, 4, and 6 months after the initial assessment; renal remission was defined as a renal BVAS of 0.29
Only a single patient (1/30) had not reached complete remission after 6 months (i.e., nonresponder). Out of the responders 18 of 29 had reached a renal BVAS of 0 after 2 months and were considered early responders as opposed to 11 of 29 late responders. Early responders showed higher urinary T cell counts for CD3+, CD4+, Treg, and TH17 cells, which did not reach statistical significance (Supplementary Table S4). Interestingly, hematuria (>1+) was still present in 15 of 29 responders at 2 months follow-up and persisted even after 6 months in 11 of 29 patients.
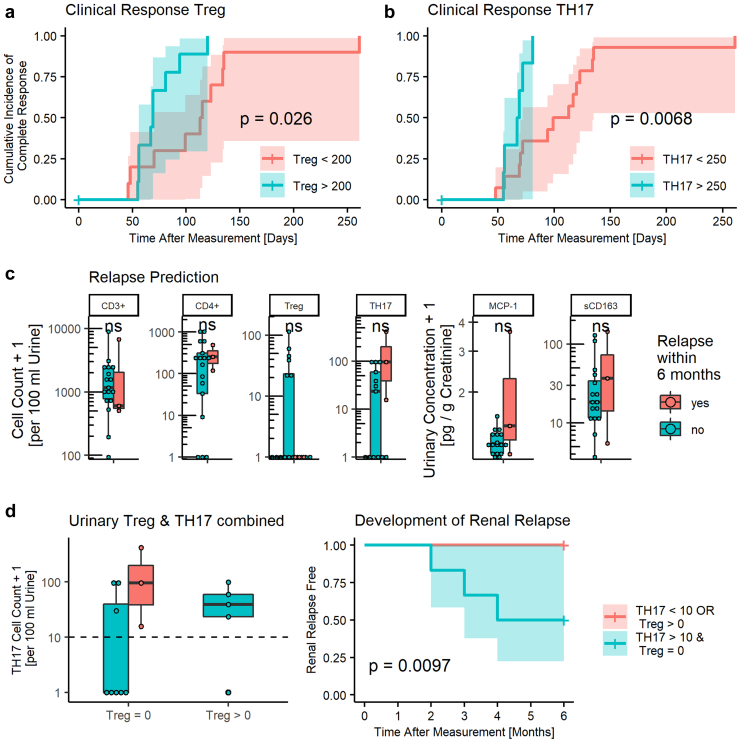
Next, we created Kaplan-Meier curves for urinary T cells based on automatically generated cut off values set between these 2 medians and selected those curves with the best stratification based on logrank test. TH1 cells were disregarded because of the low urinary cell count. CD3+, CD4+, and CD8+ showed no significant difference in cumulative incidence of full response. Conversely, we identified a cut off of 200 Treg cells and 250 TH17 cells per 100 ml of urine, which significantly predicted early response behavior (P-value: 0.026 and 0.0068, respectively) (Figure 3a and b).
Figure 3.
Urinary T cells predict clinical response and early relapses. (a, b) Active renal AAV patients with higher urinary Treg and TH17 T cells show faster full response (i.e., renal BVAS of 0). (c) Patients in remission who develop a renal flare within 6 months of measurement show no difference in urinary T cell counts or monocyte chemoattractant protein-1, soluble CD163. (d) A combined predictor of elevated TH17 and absent Treg predicts renal relapse. Dashed line indicates suggest cut off value of 10 TH17 cells per 100 ml urine. Curve of Relapse free survival based on combined predictor TH17 > 10/100 ml urine and Treg = 0/100 ml urine. Cell counts and concentrations plotted +1 to visualize zeros on logarithmic scale. Statistics: logrank test, 95% confidence interval indicated by colored area, Mann-Whitney-Wilcoxon test, ns: P > 0.05. Treg, regulatory T cell; TH17, T helper 17; TH1, T helper 1.
Urinary T Cells Predict AAV Renal Flares
Finally, we tested whether elevated urinary T cells in patients with AAV who are in clinical remission reflects ongoing occult renal inflammation and could therefore predict renal flares in these patients. To this end, we followed patients in remission from the inception cohort, focusing on the first 6 months after initial measurement because of infeasible prediction beyond this time point. Not accounting for therapy adjustment, 3 of 28 patients developed a renal relapse in this period (median time to relapse was 3 months).
Interestingly, all 3 patients with early relapses showed markedly elevated urinary TH17 counts and a complete absence of urinary Treg cells. Other T cell subpopulations did not show such a marked difference (Figure 3c). Comparison of soluble markers revealed an insignificant increase in urinary MCP-1 and sCD163 concentration in patients with subsequent renal flare (Figure 3c). Of note, all 3 patients who flared within 6 months showed positive erythrocytes on dipstick analysis and 2 patients without flare were also positive (positive predictive value = 60%, negative predictive value = 100%). Only 1 patient showed ANCA above the reference range and did not develop a relapse within the follow-up period, whereas all 3 patients with early relapse were ANCA negative at the time of sampling.
Next, we performed ROC analysis to identify cut off values with maximum positive predictive value i.e., risk of renal relapse without missing any relapses within the first 6 months. TH17 cells appeared to be the best predictor, with a risk of renal relapse of 30% at a threshold of 10 cells per 100 ml urine. Because patients with equally elevated urinary TH17 cells as relapsing patients but simultaneously detectable urinary Treg cells showed 0% risk of relapse, we combined these 2 markers. These patients showed a 50% risk of relapse. On this basis, we created a combined predictor of TH17 greater 10 and Treg count of 0 cells per 100 ml urine (Figure 3d). This combination identified all renal flares within 6 months with a positive predictive value of 50% and negative predictive value of 100% (P-value = 0.0097) (Figure 3d). However, this finding is strongly limited because of the small number of events available for this analysis.
Discussion
We studied patients with AAV from 4 centers using flow cytometry to show that T cells are highly elevated in urine in active renal vasculitis and reflect the Berden classification. Specific migration into the kidney and subsequent release into the urine space, rather than mere transglomerular bleeding, cause this increase. There was no difference in blood T cell subpopulations, and only few correlations with routine clinical parameters were present. Particularly CD3+, CD4+, and Treg T cells showed robust biomarker characteristics outperforming previously described soluble markers such as sCD163 or MCP-1 and traditional markers, such as hematuria and proteinuria. Finally, we found that Treg and TH17 cells even hold prognostic value in predicting clinical outcome of patients with active disease and potentially even renal relapse in patients in remission up to 6 months before relapse.
Our findings extend emerging evidence that urinary T cells reflect the renal inflammatory milieu in various inflammatory kidney diseases.18,36,37 To our knowledge, this is the first report on urinary T cell subpopulations, particularly Treg and TH17, and their biomarker performance in rAAV. Urinary cell counts reflect the clinical and histopathological disease state. Furthermore, we observed an increased frequency of urinary Treg cells, discordant CD4+/CD8+ ratios in urine and blood. Therefore, we hypothesize, that urinary T cells are not merely the product of transglomerular bleeding because of ruptured capillaries but reflect an active selective process. Therefore, it seems viable to use the urine space as a window into the inflammatory milieu of the kidney. However, we cannot distinguish whether these immigrating cells are the cause of renal immunopathogenesis or merely attracted by the ongoing inflammation.
Although CD3+, CD4+, and Treg urinary T cells showed a robust discrimination between active and remission state, there was no significant difference between these groups for CD8+ and TH17 T cells. At this point, we speculate that the apparent increase of CD8+ and TH17 urinary T cells in remission compared to HC reflects occult renal inflammation and renal vasculitis activity in patients with AAV, who are considered as clinically inactive. Therefore, the biomarker characteristics may underestimate the true predictive capacity of our T cell biomarkers.
This concept is further supported by our observation that remission patients with increased urinary TH17 cell counts showed a higher risk of future renal flare. This risk was decreased if patients showed an elevated urinary Treg cell count. These findings are strongly limited by the small number of events and further studies are needed to confirm this. However, this might give further insight into the dual nature of T cell subpopulations, which has been postulated before. Although Treg cells seem protective over the entire course of AAV, TH17 cells seem to exhibit proinflammatory effects in the early phase of inflammation and protective effects during the subsequent course.38 Notably, although we only observed 3 renal flares within the first 6 months of follow-up, thus limiting these results, we cannot rule out that clinicians altered remission therapy regimens thereby preventing additional relapses. Despite this caveat, these observations not only reflect experimental results in humans in vivo but also underline that we were not measuring an epiphenomenon of inflammation. Finally, increased urinary Treg and TH17 cell counts were associated with earlier clinical response in active rAAV patients in our study. These hypotheses are supported by various animal models demonstrating the important anti-inflammatory role of Treg cells and damage promoting effects of TH17 cells in crescentic glomerulonephritis and AAV.24, 25, 26
Interestingly, we did not find a difference in blood Treg frequency between active and remission AAV. There are contradicting reports on this subject, which are likely caused by varying definitions of the Treg subset.39
The traditional biomarker, hematuria on dipstick analysis, showed robust biomarker characteristics, particularly a high area under the ROC curve. In addition, we found that erythrocytes were also able to predict renal relapse with high positive predictive value, as previously described.9 However, we argue this strong performance might be skewed because of the inclusion of hematuria in the definition of active disease and renal relapse. Furthermore, we cannot rule out that some remission patients with persistent hematuria who were considered as “grumbling disease,” were never considered for this study. Although the significance and origin of persistent hematuria in AAV are still highly disputed, it is difficult to differentiate hematuria caused by scarring from active vasculitis.9,40 Along these lines, acanthocytes have been traditionally used to detect active renal vasculitis in AAV. However, the detection and quantification of acanthocytes is characterized by high interobserver variability and requires rapid processing of samples. This leads to suboptimal test performance in the real world.41 Conversely, we showed that urinary T cells accurately reflect clinical course with cell counts following clinical response closely. Furthermore, distinct subpopulation patterns in urine enable the differentiation between mere bleeding caused by scarring or active immunologic activity.
Because kidney biopsy remains the gold standard for diagnostic and prognostic evaluation in patients with AAV, we compared urinary T cells to the established and validated Berden classification.5 We found that patients classified as crescentic showed significantly higher urinary CD3+ and CD4+ cells. This finding is strongly limited by the small number of available biopsies (21 compared to 51 active renal patients). This number was mainly limited by the large number of patients with relapse, in which a re-biopsy is not clinical standard. Although this may not be sufficient to validate urinary T cells as liquid biopsy, we argue it is sufficient additional evidence to support a robust clinical biomarker. Although urinary T cells are the first leukocyte biomarker in AAV, there are several described soluble markers relying on enzyme-linked immunoassays for monocyte proteins such as sCD163 and MCP-1.13,14 In direct comparison, urinary CD3+ and Treg outperformed these in diagnosing active rAAV. In addition, we identified urinary Treg and TH17 patterns as predictors of treatment response and renal relapse in our cohort, which has not been shown for soluble markers. This might help identify subsets of disease and also enable tailored treatment such as IL-17 blockade in patients where early TH17 cells still drive inflammation.
This study is limited by the small number of patients included. Particularly regarding the small number of relapses and the number of available kidney biopsies. Therefore, it is important to stress that this study does not allow definitive conclusions on the prognostic value and the use as liquid biopsy. However, we see this study as proof of concept that urinary T cells can be used as biomarkers in the assessment of AAV disease activity. Further, larger studies are needed to confirm some of the promising and interesting signals that we found, particularly regarding the prediction of renal relapse.
Besides these caveats, there are technical limitations to the use of urinary T cells in real world practice. It is important to stress that this biomarker is not designed to diagnose AAV specifically rather than assess disease activity of established AAV. We envision a scenario where urinary T cells are quantified as part of the routine follow up in patients with confirmed diagnosis. Until further studies validate the AAV specific nature of this biomarker, confounders such as urinary tract infection, bladder pathology, and bladder catheterization immediately before sample acquisition need to be excluded. Although this study required immediate processing of fresh urine samples, we have developed a protocol which could make this a more realistic tool for routine monitoring of outpatients and use in clinical studies. Using a cup with an extended formaldehyde releaser to which fresh urine is added allows analysis at later time points.42 One frequent critique against using urinary T cells as biomarker is, that flow cytometry is too complicated, arguing for enzyme-linked immunoassays or similar tests. However, flow cytometry is widely available in most hospitals in high income countries, is already routinely used by other clinical disciplines like hematology, and it offers the advantage of unprecedented, detailed information on the “state” of inflammatory renal diseases.
In summary, urinary T cells directly reflect the renal inflammatory milieu and show reliable biomarker characteristics, outperforming soluble markers. Furthermore, they may even reveal prognostic information, which warrants further study. If confirmed in further validation studies, urinary T cells would represent the first noninvasive biomarker to: (i) assess disease activity, (ii) enable prognosis of clinical response, (iii) predict future flares, and (iv) identify patients who might benefit from tailored treatment strategies such as IL-17 blockade. These characteristics could make urinary T cells a perfect tool for a personalized treatment of ANCA-associated glomerulonephritis.
Disclosure
The authors JS, JK, MB, AR, KE, SE, SP, SB, RK, ADS, and AS declare no conflicts of interest. PE declares that he has submitted a patent using flow cytometry for diagnosing renal transplant rejection and other inflammatory renal disease (EP17190719.9) and a patent for a conservation method for urinary cells (PCT/EP2019/086433).
Acknowledgments
We extend our sincere gratitude to Sylvia Lucke and Tanja Filipowski for performing experiments.
Funding
Funding for this project was received from “Translational kidney research–from physiology to clinical application” (TRENAL), Berlin Institute of Health and Deutscher Akademischer Austauschdienst (DAAD). This work was supported by grant SCHR 771/8-1 from the Deutsche Forschungsgemeinschaft to AS, grant 394046635–SFB 1365 from the Deutsche Forschungsgemeinschaft to AS, and an ECRC grant to AS.
Author Contributions
All authors meet the journal’s criteria for authorship.
Footnotes
Figure S1. Blood T cells and fluorescence minus one.
Figure S2. Urinary T cell count by center.
Figure S3. Urinary T cell count by ANCA serotype.
Figure S4. Urinary T cell count by clinical diagnosis.
Figure S5. Blood T cell counts.
Figure S6. T cell subpopulations in blood and urine.
Figure S7. Correlation matrix.
Figure S8. Treg/TH17 ratio.
Figure S9. Urinary T cell counts by Berden classification.
Figure S10. Urinary T cell counts by pooled Berden classification.
Table S1. Summary of collected samples.
Table S2. Summary of Urinary T cell counts according to Berden classification.
Table S3. Summary of Urinary T cell counts crescentic vs. Other Berden classes.
Table S4. Summary of Urinary T cell counts by response behavior.
Supplementary Material
Figure S1. Blood T cells and fluorescence minus one.
Figure S2. Urinary T cell count by center.
Figure S3. Urinary T cell count by ANCA serotype.
Figure S4. Urinary T cell count by clinical diagnosis.
Figure S5. Blood T cell counts.
Figure S6. T cell subpopulations in blood and urine.
Figure S7.Correlation matrix.
Figure S8. Treg/TH17 ratio.
Figure S9. Urinary T cell counts by Berden classification.
Figure S10. Urinary T cell counts by pooled Berden classification.
Table S1. Summary of collected samples.
Table S2. Summary of Urinary T cell counts according to Berden classification.
Table S3. Summary of Urinary T cell counts crescentic vs. Other Berden classes.
Table S4. Summary of Urinary T cell counts by response behavior.
References
- 1.Hutton H.L., Holdsworth S.R., Kitching A.R. ANCA-associated vasculitis: pathogenesis, models, and preclinical testing. Semin Nephrol. 2017;37:418–435. doi: 10.1016/j.semnephrol.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Hilhorst M., van Paassen P., Tervaert J.W., Limburg Renal Registry Proteinase 3-ANCA Vasculitis versus myeloperoxidase-ANCA Vasculitis. J Am Soc Nephrol. 2015;26:2314–2327. doi: 10.1681/ASN.2014090903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukhtyar C., Flossmann O., Hellmich B., et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism systemic vasculitis task force. Ann Rheum Dis. 2008;67:1004–1010. doi: 10.1136/ard.2007.071936. [DOI] [PubMed] [Google Scholar]
- 4.Little M.A., Nightingale P., Verburgh C.A., et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis. 2010;69:1036–1043. doi: 10.1136/ard.2009.109389. [DOI] [PubMed] [Google Scholar]
- 5.Berden A.E., Ferrario F., Hagen E.C., et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–1636. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- 6.Dhaun N., Bellamy C.O., Cattran D.C., Kluth D.C. Utility of renal biopsy in the clinical management of renal disease. Kidney Int. 2014;85:1039–1048. doi: 10.1038/ki.2013.512. [DOI] [PubMed] [Google Scholar]
- 7.Fujita T., Ohi H., Endo M., et al. Level of red blood cells in the urinary sediment reflects the degree of renal activity in Wegener’s granulomatosis. Clin Nephrol. 1998;50:284–288. [PubMed] [Google Scholar]
- 8.Magrey M.N., Villa-Forte A., Koening C.L., et al. Persistent hematuria after induction of remission in Wegener granulomatosis: a therapeutic dilemma. Med (Baltim) 2009;88:315–321. doi: 10.1097/MD.0b013e3181c101cc. [DOI] [PubMed] [Google Scholar]
- 9.Rhee R.L., Davis J.C., Ding L., et al. The utility of urinalysis in determining the risk of renal relapse in ANCA-associated vasculitis. Clin J Am Soc Nephrol. 2018;13:251–257. doi: 10.2215/CJN.04160417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T.K., Murakami C., Manno R.L., Geetha D. Hematuria duration does not predict kidney function at 1 year in ANCA-associated glomerulonephritis. Semin Arthritis Rheum. 2014;44:198–201. doi: 10.1016/j.semarthrit.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa J., Hoshino J., Sekine A., et al. Clinical and pathological features of ANCA-associated vasculitis in patients with minor urinary abnormalities. Nephrol (Carlton) 2018;23:1007–1012. doi: 10.1111/nep.13157. [DOI] [PubMed] [Google Scholar]
- 12.Geetha D., Seo P., Ellis C., et al. Persistent or new onset microscopic hematuria in patients with small vessel vasculitis in remission: findings on renal biopsy. J Rheumatol. 2012;39:1413–1417. doi: 10.3899/jrheum.111608. [DOI] [PubMed] [Google Scholar]
- 13.Tam F.W., Sanders J.S., George A., et al. Urinary monocyte chemoattractant protein-1 (MCP-1) is a marker of active renal vasculitis. Nephrol Dial Transplant. 2004;19:2761–2768. doi: 10.1093/ndt/gfh487. [DOI] [PubMed] [Google Scholar]
- 14.O’Reilly V.P., Wong L., Kennedy C., et al. Urinary soluble CD163 in active renal vasculitis. J Am Soc Nephrol. 2016;27:2906–2916. doi: 10.1681/ASN.2015050511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dekkema G.J., Abdulahad W.H., Bijma T., et al. Urinary and serum soluble CD25 complements urinary soluble CD163 to detect active renal anti-neutrophil cytoplasmic autoantibody-associated vasculitis: a cohort study. Nephrol Dial Transplant. 2018;34:234–242. doi: 10.1093/ndt/gfy018. [DOI] [PubMed] [Google Scholar]
- 16.Moran S.M., Monach P.A., Zgaga L., et al. Urinary soluble CD163 and monocyte chemoattractant protein-1 in the identification of subtle renal flare in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant. 2020;35:283–291. doi: 10.1093/ndt/gfy300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolff S., Abdulahad W.H., van Dijk M.C., et al. Urinary T cells in active lupus nephritis show an effector memory phenotype. Ann Rheum Dis. 2010;69:2034–2041. doi: 10.1136/ard.2009.124636. [DOI] [PubMed] [Google Scholar]
- 18.Enghard P., Humrich J.Y., Rudolph B., et al. CXCR3+CD4+ T cells are enriched in inflamed kidneys and urine and provide a new biomarker for acute nephritis flares in systemic lupus erythematosus patients. Arthritis Rheum. 2009;60:199–206. doi: 10.1002/art.24136. [DOI] [PubMed] [Google Scholar]
- 19.Enghard P., Rieder C., Kopetschke K., et al. Urinary CD4 T cells identify SLE patients with proliferative lupus nephritis and can be used to monitor treatment response. Ann Rheum Dis. 2014;73:277–283. doi: 10.1136/annrheumdis-2012-202784. [DOI] [PubMed] [Google Scholar]
- 20.Dolff S., Abdulahad W.H., Arends S., et al. Urinary CD8+ T-cell counts discriminate between active and inactive lupus nephritis. Arthritis Res Ther. 2013;15:R36. doi: 10.1186/ar4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakatsume M., Xie Y., Ueno M., et al. Human glomerulonephritis accompanied by active cellular infiltrates shows effector T cells in urine. J Am Soc Nephrol. 2001;12:2636–2644. doi: 10.1681/ASN.V12122636. [DOI] [PubMed] [Google Scholar]
- 22.Abdulahad W.H., Kallenberg C.G., Limburg P.C., Stegeman C.A. Urinary CD4+ effector memory T cells reflect renal disease activity in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2009;60:2830–2838. doi: 10.1002/art.24747. [DOI] [PubMed] [Google Scholar]
- 23.de Souza A.W., Abdulahad W.H., Sosicka P., et al. Are urinary levels of high mobility group box 1 markers of active nephritis in anti-neutrophil cytoplasmic antibody-associated vasculitis? Clin Exp Immunol. 2014;178:270–278. doi: 10.1111/cei.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gan P.Y., Steinmetz O.M., Tan D.S., et al. Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J Am Soc Nephrol. 2010;21:925–931. doi: 10.1681/ASN.2009070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghali J.R., Wang Y.M., Holdsworth S.R., Kitching A.R. Regulatory T cells in immune-mediated renal disease. Nephrol (Carlton) 2016;21:86–96. doi: 10.1111/nep.12574. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber A., Rousselle A., Klocke J., et al. Neutrophil gelatinase-associated lipocalin protects from ANCA-induced GN by inhibiting T(H)17 immunity. J Am Soc Nephrol. 2020;31:1569–1584. doi: 10.1681/ASN.2019090879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krebs C.F., Paust H.J., Krohn S., et al. Autoimmune renal disease is exacerbated by S1P-Receptor-1-Dependent intestinal Th17 cell migration to the kidney. Immunity. 2016;45:1078–1092. doi: 10.1016/j.immuni.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jennette J.C., Falk R.J., Bacon P.A., et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 29.Mukhtyar C., Lee R., Brown D., et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3) Ann Rheum Dis. 2009;68:1827–1832. doi: 10.1136/ard.2008.101279. [DOI] [PubMed] [Google Scholar]
- 30.R: a language and environment for statistical computing. R Core Team (2020). The R Foundation for Statistical Computing. http://www.r-project.org/index.html
- 31.Pohlert T. Calculate pairwise multiple comparisons of mean rank sums extended. PMCMRplus. https://cran.r-project.org/web/packages/PMCMRplus/PMCMRplus.pdf Published 2020.
- 32.Wickham H. Springer; 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- 33.Sachs M.C. plotROC: A tool for plotting ROC curves. J Stat Softw. 2017;79:2. doi: 10.18637/jss.v079.c02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robin X., Turck N., Hainard A., et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Therneau TM. Survival analysis in R Version 2.38. Published 2015. https://cran.microsoft.com/snapshot/2015-06-27/web/packages/survival/survival.pdf
- 36.Arazi A., Rao D.A., Berthier C.C., et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat Immunol. 2019;20:902–914. doi: 10.1038/s41590-019-0398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Doesum W.B., Abdulahad W.H., van Dijk M.C., et al. Characterization of urinary CD4⁺ and CD8⁺ T cells in kidney transplantation patients with polyomavirus BK infection and allograft rejection. Transpl Infect Dis. 2014;16:733–743. doi: 10.1111/tid.12273. [DOI] [PubMed] [Google Scholar]
- 38.Odobasic D., Gan P.Y., Summers S.A., et al. Interleukin-17A promotes early but attenuates established disease in crescentic glomerulonephritis in mice. Am J Pathol. 2011;179:1188–1198. doi: 10.1016/j.ajpath.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyara M., Gorochov G., Ehrenstein M., et al. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744–755. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Mahoney S.L., Nachman P.H. Persistent hematuria in ANCA vasculitis: ominous or innocuous? Clin J Am Soc Nephrol. 2018;13:201–202. doi: 10.2215/CJN.14101217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crop M.J., de Rijke Y.B., Verhagen P.C., et al. Diagnostic value of urinary dysmorphic erythrocytes in clinical practice. Nephron Clin Pract. 2010;115:c203–c212. doi: 10.1159/000313037. [DOI] [PubMed] [Google Scholar]
- 42.Freund P., Skopnik C.M., Metzke Ds, et al. Addition of formaldehyde releaser imidazolidinyl urea and MOPS buffer to urine samples enables delayed processing for flow cytometric analysis of urinary cells. medRxiv. Forthcoming. 2022 doi: 10.1101/2022.04.07.22273579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.