Abstract
Introduction
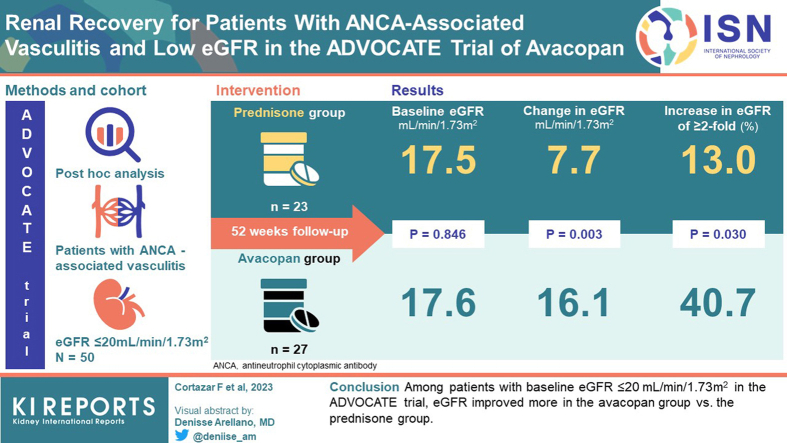
In the 330-patient ADVOCATE trial of avacopan for the treatment of antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis, in which 81% of patients had renal involvement, estimated glomerular filtration rate (eGFR) increased on average 7.3 ml/min per 1.73 m2 in the avacopan group and 4.1 ml/min per 1.73 m2 in the prednisone group (P = 0.029) at week 52. This new analysis examines the results in the patient subgroup with severe renal insufficiency at enrollment into the trial, i.e., eGFR ≤20 ml/min per 1.73 m2.
Methods
eGFR was determined at baseline and over the course of the trial. Changes in eGFR were compared between the 2 treatment groups.
Results
In ADVOCATE, 27 of 166 patients (16%) in the avacopan group and 23 of 164 patients (14%) in the prednisone group had a baseline eGFR ≤20 ml/min per 1.73 m2. At week 52, eGFR increased on average 16.1 and 7.7 ml/min per 1.73 m2 in the avacopan and prednisone groups, respectively (P = 0.003). The last eGFR value measured during the 52-week treatment period was ≥2-fold higher than baseline in 41% of patients in the avacopan group compared to 13% in the prednisone group (P = 0.030). More patients in the avacopan group versus prednisone group had increases in eGFR above 20, 30, and 45 ml/min per 1.73 m2, respectively. Serious adverse events occurred in 13 of 27 patients (48%) in the avacopan group and 16 of 23 patients (70%) in the prednisone group.
Conclusion
Among patients with baseline eGFR ≤20 ml/min per 1.73 m2 in the ADVOCATE trial, eGFR improved more in the avacopan group than in the prednisone group.
Keywords: ANCA-associated vasculitis, avacopan, complement, complement 5a receptor, low eGFR, renal recovery
Graphical abstract
ANCA-associated vasculitis often involves the kidneys. Fifteen percent to 38% of the patients develop end-stage kidney disease within 5 years,1, 2, 3, 4, 5, 6, 7 and once patients need dialysis, 29% to 72% die or are still on dialysis 3 to 6 months after initiation of dialysis.7, 8, 9, 10, 11, 12, 13 Therefore, effectively managing renal vasculitis and preventing patients from reaching dialysis have important consequences.
Avacopan, an orally administered, selective C5a receptor inhibitor, was approved in 2021 for the treatment of adults with ANCA-associated vasculitis. The phase 3 ADVOCATE trial enrolled patients with active granulomatosis with polyangiitis or microscopic polyangiitis and found that the avacopan group had superior rates of sustained remission at 52 weeks compared with the prednisone group.14 In ADVOCATE, 81% of enrolled patients had kidney involvement at baseline. In these patients, the eGFR increased on average at 7.3 ml/min per 1.73 m2 in the avacopan group and 4.1 ml/min per 1.73 m2 in the prednisone group (P = 0.029) at week 52.
The ADVOCATE trial excluded patients with an eGFR <15 ml/min per 1.73 m2. However, 50 patients with baseline eGFR ≤20 ml/min per 1.73 m2 were enrolled. The aim of this post hoc analysis was to evaluate the changes in kidney function in these patients over the course of the 52-week treatment period of ADVOCATE.
Methods
Study Design and Patients
The study design is presented elsewhere.15 Briefly, the original clinical trial was a multicenter, randomized, double-blind, active-controlled trial. The aim was to replace the standard oral glucocorticoid taper with avacopan without compromising efficacy or safety in treating patients with ANCA-associated vasculitis.
Avacopan 30 mg twice daily or matching placebo was administered for 52 weeks, with 8 weeks follow-up. Prednisone or a matching placebo was given in a tapering schedule for 20 weeks (60 mg per day tapered to 0 by week 21).
The main eligibility criteria were newly-diagnosed or relapsing granulomatosis with polyangiitis or microscopic polyangiitis, according to the Chapel Hill Consensus Conference definitions,16 for whom treatment with cyclophosphamide or rituximab was indicated, tested positive for antibodies to either proteinase-3 or myeloperoxidase, had an eGFR of at least 15 ml/min per 1.73 m2, had at least 1 major or 3 non-major items, or at least 2 items of hematuria and proteinuria on the Birmingham Vasculitis Activity Score version 3 (range 0–63 with higher scores indicating more disease activity).17 Complete inclusion and exclusion criteria are described elsewhere.14
The trial was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Ethics committees and institutional review boards at participating sites approved the research protocol. All patients or their parent/guardian gave written informed consent before entry. ChemoCentryx sponsored the trial and provided study medication.
This study was conducted in the patients from the original trial who had the lowest kidney function at baseline, i.e., those with eGFR ≤20 ml/min per 1.73 m2.
All authors attest to adherence of the trial to the protocol, accurate data analysis, and complete reporting of adverse events. All authors participated with the sponsor in data analysis and manuscript writing.
Randomization and Treatment
Patients were randomized in a 1:1 ratio to receive 30 mg avacopan twice daily orally plus prednisone-matching placebo, or a tapering oral regimen of prednisone plus avacopan-matching placebo in a double-dummy design. Randomization was stratified based on having newly-diagnosed or relapsing vasculitis, proteinase-3-ANCA or myeloperoxidase-ANCA, and treatment with cyclophosphamide or rituximab. All patients received either of the following: (i) cyclophosphamide i.v. 15 mg/kg up to 1.2 g on day 1 and weeks 2, 4, 7, 10, and 13; (ii) cyclophosphamide orally 2 mg/kg up to 200 mg per day for 14 weeks; or (3) i.v. rituximab 375 mg/m2/wk for 4 weeks. From week 15 onwards, cyclophosphamide was followed by oral azathioprine at a target dose of 2 mg/kg/d.
Patients, study personnel, sponsor, and sponsor representatives involved in trial conduct were masked to patient treatment allocation. All trial drugs had matching active and placebo capsules, provided to trial centers in identical bottles.
End Points
The endpoint for the current study was the change from baseline eGFR. eGFR was calculated using the Modification of Diet in Renal Disease equation.18 For Japanese patients, the Modification of Diet in Renal Disease equation was modified as follows: eGFR (ml/min per 1.73 m2) = 194 × (serum creatinine in mg/dl)−1.094 × (Age)−0.287 × (0.739 if female), and for adolescents, the modified Schwartz equation was used.19 This was a prespecified secondary end point in the original clinical trial. Analyses of change in eGFR in the subgroups of patients with baseline eGFR <30, 30 to 59, and >59 ml/min per 1.73 m2 were prespecified. Post hoc analyses of eGFR changes were conducted in patients who approached the dialysis threshold, i.e., those with baseline eGFR ≤20 ml/min per 1.73 m2. In addition, as exploratory endpoints, the proportion of patients whose last measured eGFR during the 52-week treatment period was >20, ≥30, ≥45, ≥60 ml/min per 1.73 m2, and those whose last measured eGFR was ≥2-fold the baseline eGFR value was evaluated in patients with baseline eGFR ≤20 ml/min per 1.73 m2. Percent change from baseline in urinary albumin-to-creatinine ratio (UACR) was also evaluated.
The 2 primary efficacy end points of the original study were the proportion of patients in clinical remission at week 26, and in sustained remission at week 52.14 Results for these and other end points are reported elsewhere.14
Statistical Analysis
Changes from baseline in eGFR and UACR were analyzed using mixed effects models for repeated measures with treatment group, visit, and treatment-by-visit interaction as factors, and baseline as covariate. Patients were considered as repeated measure units over visits. Least squares means (LSMs), standard errors, and confidence intervals (CIs) are from the mixed effects models for repeated measures. UACR data were log-transformed before analysis because these data are typically not normally distributed.
The remission and sustained remission end points were analyzed using the stratified summary score test and estimate for the common difference in proportions, adjusting the randomization strata. The other categorical end points were analyzed by χ2 testing. No adjustment was made for multiplicity of the end points.
This subgroup analysis was exploratory, and the overall type 1 error was not controlled. P-values were nominal. The original study was registered with ClinicalTrials.gov (NCT02994927).
Results
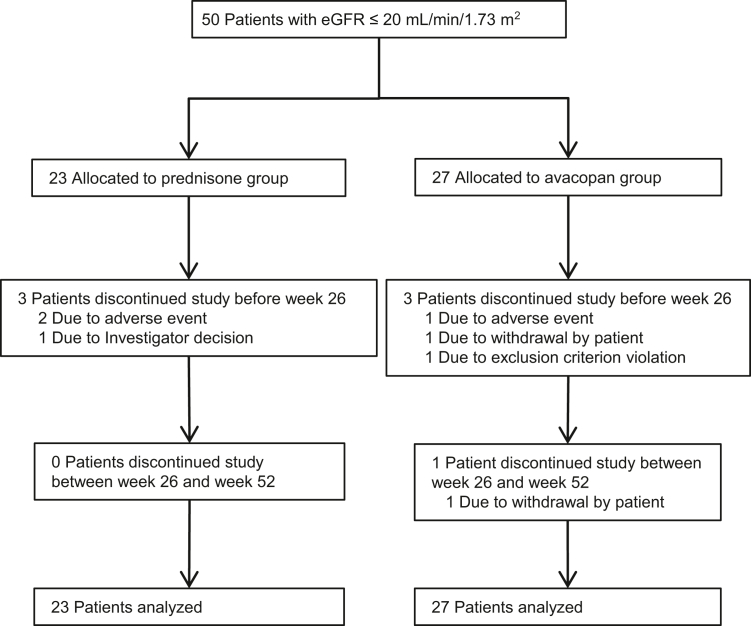
The ADVOCATE trial ran from March 15, 2017 (first patient enrolled) until November 1, 2019. Patient disposition for the main study is presented elsewhere.14 Twenty-seven of 166 patients (16%) in the avacopan group and 23 of 164 patients (14%) in the prednisone group had eGFR ≤20 ml/min per 1.73 m2 at baseline. Disposition of these patients is shown in Figure 1. The demographics and baseline characteristics were similar between the 2 groups (Table 1). The mean age in the 50 patients with low eGFR was comparable to the overall 330-patient population (66 vs. 61 years), but included a higher proportion of patients with newly diagnosed disease (88% vs. 69%), positivity for antimyeloperoxidase ANCA (84% vs. 57%), microscopic polyangiitis (72% vs. 45%), and use of cyclophosphamide (50% vs. 35%).14 One patient in the avacopan group had a baseline eGFR of 14 ml/min per 1.73 m2 and 1 patient in the prednisone group had a baseline eGFR of 12 ml/min per 1.73 m2. These were deviations from eligibility criteria of the study protocol that specified a baseline eGFR of ≥15 ml/min per 1.73 m2. Data from these patients are included in this analysis.
Figure 1.
Disposition among patients in the ADVOCATE trial with baseline eGFR ≤20 ml/min per 1.73 m2. Of the 50 patients with baseline eGFR ≤20 ml/min per 1.73 m2, 23 were in the prednisone group and 27 in the avacopan group. Three patients in the prednisone group and 4 in the avacopan group discontinued the study early, most within the first 26 weeks. eGFR, estimated glomerular filtration rate.
Table 1.
Demographics and clinical characteristics at baseline among patients with eGFR ≤20 ml/min per 1.73 m2 in the ADVOCATE Trial
| Category | Prednisone Group (N = 23) | Avacopan group (N = 27) | P-value for group comparisona |
|---|---|---|---|
| Age (yr), mean ± SD | 64.8 ± 17.22 | 67.1 ± 11.13 | 0.5612 |
| Sex, Male / Female (n) | 11 / 12 | 15 / 12 | 0.5856 |
| Race (n) | 0.9698 | ||
| Asian | 3 | 3 | |
| Other | 1 | 1 | |
| White | 19 | 23 | |
| Body mass index (kg/m2), mean ± SD | 26.8 ± 4.12 | 25.4 ± 5.67 | 0.3526 |
| Duration of ANCA-associated vasculitis (mo), median (range) | 0.10 (0-190.2) | 0.13 (0-339.9) | 0.6224 |
| Disease history | |||
| Newly diagnosed, n (%) | 21 (91.3) | 23 (85.2) | 0.5069 |
| Relapsed disease, n (%) | 2 (8.7) | 4 (14.8) | |
| ANCA type | |||
| Anti-proteinase 3 positive, n (%) | 3 (13.0) | 5 (18.5) | 0.5987 |
| Anti-myeloperoxidase positive, n (%) | 20 (87.0) | 22 (81.5) | |
| Background treatment | |||
| Rituximab i.v., n (%) | 13 (56.5) | 12 (44.4) | 0.6752 |
| Cyclophosphamide i.v., n (%) | 9 (39.1) | 13 (48.1) | |
| Cyclophosphamide oral, n (%) | 1 (4.3) | 2 (7.4) | |
| Disease type | |||
| Granulomatosis with polyangiitis, n (%) | 7 (30.4) | 7 (25.9) | 0.7234 |
| Microscopic polyangiitis, n (%) | 16 (69.6) | 20 (74.1) | |
| Disease assessment scores | |||
| Birmingham vasculitis activity score,b mean ± SD | 15.7 ± 3.80 | 17.8 ± 5.77 | 0.0913 |
| Vasculitis damage index mean ± SD | 0.2 ± 0.61 | 0.1 ± 0.46 | 0.0856 |
| Organ involvement (based on Birmingham Vasculitis Activity Score) | |||
| Renal, n (%) | 23 (100.0) | 27 (100.0) | |
| General, n (%) | 14 (60.9) | 18 (66.7) | 0.6704 |
| Ear, nose, and throat, n (%) | 5 (21.7) | 9 (33.3) | 0.3628 |
| Chest, n (%) | 6 (26.1) | 7 (25.9) | 0.9897 |
| Nervous system, n (%) | 2 (8.7) | 5 (18.5) | 0.3184 |
| Cutaneous, n (%) | 1 (4.3) | 4 (14.8) | 0.2188 |
| Mucous membranes/eyes, n (%) | 1 (4.3) | 2 (7.4) | 0.6498 |
| Cardiovascular, n (%) | 0 (0) | 1 (3.7) | 0.3512 |
| Abdominal, n (%) | 0 (0) | 0 (0) | |
| Renal aspects | |||
| Estimated glomerular filtration rate (ml/min per 1.73 m2),c mean ± SD (range) | 17.5 ± 2.04 (12-20) | 17.6 ± 1.86 (14-20) | 0.8460 |
| Hematuria ≥10 red blood cells per high power field, n (%) | 17 (73.9) | 22 (81.5) | 0.5196 |
| Urinary albumin-to-creatinine ratio (mg/g creatinine), geometric mean (range) | 739.7 (56-3516) | 593.6 (32-2830) | 0.5055 |
SD, standard deviation.
P-values are derived from the χ2 tests for categorical variables and t-tests for the continuous variables.
The Birmingham vasculitis activity score version 3 was used to capture vasculitis disease activity. The score ranges from 0 to 63 with higher scores denoting more severe disease activity.17
Estimated glomerular filtration rate based on Modification of Diet in Renal Disease equation derived from serum creatinine.18
Data on eGFR are summarized in Table 2. Eleven of 27 patients (41%) in the avacopan group had a ≥2-fold increase in eGFR versus 3 of 23 patients (13%) in the prednisone group (P = 0.030). Numerically, more patients in the avacopan group had increases in eGFR above 20, 30, and 45 ml/min per 1.73 m2, respectively (Table 2; P-values 0.055, 0.203, and 0.069, respectively). eGFR in 1 patient in the avacopan group increased to 65 ml/min per 1.73 m2 at week 52 (baseline 17 ml/min per 1.73 m2). Four patients in each group had decreases from baseline eGFR, among whom 2 of the 4 patients in the avacopan group and none of the 4 in the prednisone group had relapsing disease at baseline. One patient in the avacopan group received dialysis during the 52-week treatment period compared to 2 in the prednisone group. These were single sessions in 1 patient in each of the 2 groups, and an unknown number of sessions for the other patient in the prednisone group.
Table 2.
Renal function results among patients with eGFR ≤20 ml/min per 1.73 m2 in the ADVOCATE Trial
| Renal outcome | Prednisone group (N = 23) | Avacopan group (N = 27) | P-value for treatment group comparison |
|---|---|---|---|
| Baseline eGFR (ml/min per 1.73 m2), mean (SD) | 17.5 (2.04) | 17.6 (1.86) | 0.846a |
| LSM change in eGFR at week 26, mean (SEM)b | 6.1 (2.00) | 11.9 (1.85) | 0.037d |
| LSM change in eGFR at week 52, mean (SEM)c | 7.7 (2.01) | 16.1 (1.88) | 0.003d |
| Laste eGFR ≥2-fold the baseline eGFR, n (%) | 3 (13.0%) | 11 (40.7%) | 0.030f |
| Last eGFR >20 ml/min per 1.73 m2, n (%) | 13 (56.5%) | 22 (81.5%) | 0.055f |
| Last eGFR ≥30 ml/min per 1.73 m2, n (%) | 7 (30.4%) | 13 (48.1%) | 0.203f |
| Last eGFR ≥45 ml/min per 1.73 m2, n (%) | 1 (4.3%) | 6 (22.2%) | 0.069f |
| Last eGFR ≥60 ml/min per 1.73 m2, n (%) | 0 (0%) | 1 (3.7%) | Not calculablef |
| Last eGFR lower than baseline, n (%) | 4 (17.4%) | 4 (14.8%) | 0.804f |
| Requiring dialysis during 52-week periodg | 2 (8.7%) | 1 (3.7%) | 0.459f |
| Baseline urinary albumin:creatinine ratio, geometric mean (range) (mg/g) | 740 (56-3516) | 594 (32-2830) | 0.506a |
| LSM % change in urinary albumin:creatinine ratio from baseline to: | |||
| week 4 | +66% | −16% | 0.011b |
| week 13 | +20% | −35% | 0.024b |
| week 26 | −40% | −55% | 0.310b |
| week 52 | −62% | −62% | 0.965b |
eGFR, estimated glomerular filtration rate; LSM, least squares mean.
t-test.
The sample size in the prednisone and avacopan groups at week 26 was 19 and 24, respectively.
The sample size in the prednisone and avacopan groups at week 52 was 20 and 23, respectively.
P-values are from mixed effects models for repeated measures with treatment group, visit, and treatment-by-visit interaction as factors and baseline as a covariate.
Last = last eGFR measurement during the 52-week treatment period.
χ2 test.
One patient in each group had a single dialysis session; the number of dialysis sessions in the second patient in the prednisone group is unknown.
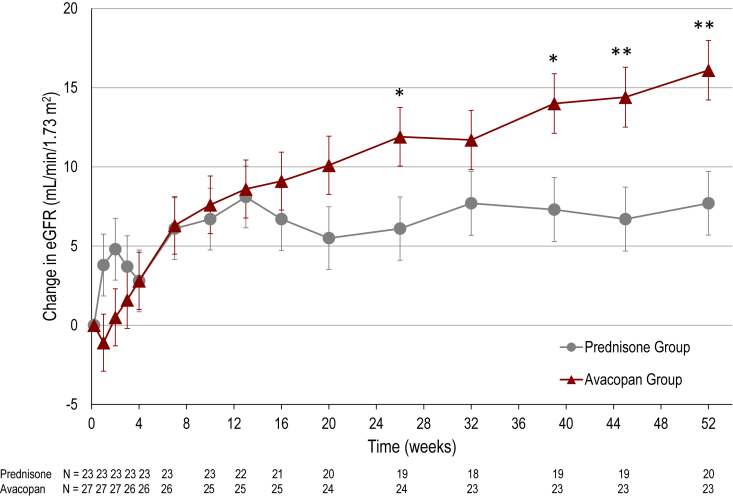
On average, eGFR increased from 17.6 ml/min per 1.73 m2 at baseline to 33.9 ml/min per 1.73 m2 at 52 weeks in the avacopan group, and from 17.5 ml/min per 1.73 m2 to 26.7 ml/min per 1.73 m2 in the prednisone group. The LSM change from baseline data for the 2 treatment groups are shown in Figure 2. At 26 weeks, there was a mean increase from baseline in eGFR of 11.9 ml/min per 1.73 m2 in the avacopan group compared to 6.1 ml/min per 1.73 m2 in the prednisone group (difference 5.8 ml/min per 1.73 m2; 95% CI 0.4, 11.2; P = 0.037). At 52 weeks, there was a mean increase from baseline in eGFR of 16.1 ml/min per 1.73 m2 in the avacopan group compared to 7.7 ml/min per 1.73 m2 in the prednisone group (difference 8.4 ml/min per 1.73 m2; 95% CI 2.9, 13.8; P = 0.003). A paired t-test for the eGFR data at week 52 compared to week 26 indicates a significant difference in the avacopan group (P < 0.001), but not in the prednisone group (P = 0.369).
Figure 2.
Change in kidney function among patients in the ADVOCATE trial with baseline eGFR ≤20 ml/min per 1.73 m2. Least squares mean (± SEM) change from baseline in eGFR by treatment group over the 52-week treatment period. ∗P < 0.05, ∗∗P < 0.01 for comparison of the avacopan group to prednisone group by mixed effects model for repeated measures analysis with treatment group, study visit, and treatment-by-visit interaction as factors, and baseline as covariate. eGFR, estimated glomerular filtration rate.
Contextually, for the overall trial in the 268 patients with renal disease, at 26 weeks there was a mean increase from baseline in eGFR of 5.8 ml/min per 1.73 m2 in the avacopan group compared to 2.9 ml/min per 1.73 m2 in the prednisone group (difference 2.9 ml/min per 1.73 m2; 95% CI 0.1, 5.8; P = 0.046). At 52 weeks, there was a mean increase from baseline in eGFR of 7.3 ml/min per 1.73 m2 in the avacopan group compared to 4.1 ml/min per 1.73 m2 in the prednisone group in the overall trial population (difference 3.2 ml/min per 1.73 m2; 95% CI 0.3, 6.1; P = 0.029). In the prespecified subgroups of patients with baseline eGFR <30, 30 to 59, and >59 ml/min per 1.73 m2, the LSM (SEM) change from baseline to week 52 in eGFR was 13.7 (1.37), 10.5 (1.53), and −5.9 (2.70) ml/min per 1.73 m2, respectively, in the avacopan group and 8.2 (1.42), 7.8 (1.42), and −7.5 (2.62) ml/min per 1.73 m2, respectively, in the prednisone group. Differences between treatment groups were statistically significant for the <30 ml/min per 1.73 m2 subgroup (P = 0.005), but not for the other 2 subgroups (P = 0.212 and 0.672, respectively).
After the 8-week follow-up period, during which patients did not receive any avacopan or avacopan-matching placebo treatment, the difference in eGFR between the 2 treatment groups in patients with eGFR ≤20 ml/min per 1.73 m2 largely remains, as follows: at week 60, the LSM (SEM) change from baseline was 16.5 (2.64) ml/min per 1.73 m2 in the avacopan group and 8.8 (2.84) in the prednisone group; the LSM (SEM) difference between groups was 7.7 (3.88), 95% CI −0.1, 15.6 (P = 0.053).
UACR levels for the eGFR ≤20 ml/min per 1.73 m2 subgroup improved more rapidly in the avacopan group versus the prednisone group. At 4 weeks, there was a mean decrease of 16% in UACR in the avacopan group compared to an increase of 66% in the prednisone group (difference −50%; 95% CI −70%, −15%; P = 0.011). At 13 weeks, the decrease was 35% in the avacopan group compared to an increase of 20% in the prednisone group (difference −46%; 95% CI −68%, −8%; P = 0.024). At 26 weeks, the decrease was 55% in the avacopan group compared to a decrease of 40% in the prednisone group (difference not statistically significant), and at 52 weeks, both treatment groups showed a mean decrease of 62% in UACR. These results on UACR are generally similar to those reported for the overall study population.14
At 26 weeks, remission, defined as a Birmingham Vasculitis Activity Score of 0 and not taking glucocorticoids for ANCA-associated vasculitis within 4 weeks before week 26, was achieved in 19 of 27 patients (70.4%) in the avacopan group and 14 of 23 patients (60.9%) in the prednisone group (estimate of common difference 13.4%; 95% CI −10.1, 36.9; P-value for noninferiority = 0.003 [one-sided]; P-value for superiority = 0.13). At 52 weeks, sustained remission, defined as achieving remission at week 26 and week 52 and having no relapse between week 26 and 52, was achieved in 18 of 27 patients (66.7%) in the avacopan group and 14 of 23 patients (60.9%) in the prednisone group (estimate of common difference 13.4%; 95% CI −10.1, 36.9; P-value for noninferiority = 0.003 [one-sided]; P-value for superiority = 0.13). These results are generally comparable to the overall trial population, except that in the full ADVOCATE trial population, statistical superiority was achieved for the avacopan group compared to the prednisone group for sustained remission at week 52.14 Two of 27 patients (7.4%) in the avacopan group relapsed during the treatment period compared to 4 of 23 patients (17.4%) in the prednisone group (P = 0.279).
Consistent with the overall study results, within this subgroup analysis of 50 patients, the median total glucocorticoid dose (prednisone-equivalent) was lower in the avacopan group, 580 mg (mean 1376 mg), compared to 3040 mg (mean 3875 mg) in the prednisone group.
Safety results for the overall study population have been published elsewhere.14 Safety results for the 50 patients with baseline eGFR are summarized in Table 3. Serious adverse events occurred in 13 of 27 patients (48.1%) in the avacopan group, with 25 events, and 16 of 23 patients (69.6%) in the prednisone group, with 45 events. There was 1 death because of bronchopneumonia in the avacopan group and 1 death because of a pleural empyema in the prednisone group. The number of adverse events, serious adverse events, and infections were lower in the avacopan compared to the prednisone groups. One patient in each treatment group had a serious adverse event of increase in liver function tests.
Table 3.
Safety results among patients with eGFR ≤20 ml/min per 1.73 m2 in the ADVOCATE trial
| Event | Prednisone group (N = 23) | Avacopan group (N = 27) |
|---|---|---|
| Any adverse event, n (%) | 23 (100%) | 27 (100%) |
| Number of events | 405 | 332 |
| Any serious adverse event,an (%) | 16 (69.6%) | 13 (48.1%) |
| Number of events | 45 | 25 |
| Any infection, n (%) | 21 (91.3%) | 21 (77.8%) |
| Number of events | 63 | 41 |
| Any serious infection, n (%) | 7 (30.4%) | 6 (22.2%) |
| Number of events | 10 | 6 |
n = number of patients; % = n/N x 100
Serious adverse events were defined as any adverse event that resulted in death, was immediately life threatening, required or prolonged hospitalization, resulted in persistent or significant disability or incapacity, was a birth defect, or was an important event that might jeopardize the patient or might have required intervention to prevent any of the above.
Discussion
The ADVOCATE trial showed that the avacopan treatment group was superior to the prednisone group in sustaining remission at 52 weeks.14 The ADVOCATE study also demonstrated greater improvements in kidney function, measured by eGFR, in the avacopan group compared to the prednisone group over the course of the study.
The analysis presented here shows that avacopan successfully reverses the decline in kidney function to a greater extent than a standard prednisone taper in patients with the most severely impaired kidney function, i.e., those with baseline eGFR ≤20 ml/min per 1.73 m2 in whom the kidney prognosis is worst and there is the greatest need to rescue kidney function.
Another important finding of this analysis was that the kidney function appeared to continue to improve between the 26-week and 52-week timepoints in the avacopan group, but not in the prednisone group. In the avacopan group, the LSM change from baseline to week 26 was 11.9 ml/min per 1.73 m2 and to week 52 16.1 ml/min per 1.73 m2, whereas in the prednisone group the changes were 6.1 and 7.7 ml/min per 1.73 m2, respectively. As shown, there was a significant difference between the eGFR at week 52 and week 26 in the avacopan group, but not in the prednisone group. This suggests that the effect of avacopan cannot be explained by a transient hemodynamic effect, but that there are sustained benefits on kidney inflammation and repair through 12 months that might be extended beyond this time period.
Complement activation is involved in neutrophil attraction and activation, and the neutrophil is the key cellular component driving glomerular necrosis. Complement activation also compromises the integrity of Bowman’s capsule in the glomeruli and stimulates infiltration of M2 macrophages that are involved in promotion of fibrosis.20,21 Blocking the effects of complement with avacopan may help to maintain the integrity of Bowman’s capsule, and reduce glomerular inflammation and interstitial fibrosis.
Albuminuria improved faster in the avacopan group compared to the prednisone group, even though the ultimate magnitude of improvement was the same in both groups. Both albuminuria and hematuria are markers of glomerular inflammation and injury and this reduction in albuminuria represents a biomarker for glomerular pathology and integrity, as discussed above.
Efficacy in the avacopan group was achieved in the context of an 81% reduction in median overall total glucocorticoid dose (and a 64% reduction in the mean dose) compared to the prednisone group. These results are consistent with the overall study results.14 There was numerically a lower incidence of serious adverse events in the avacopan compared to the prednisone group, and a lower number of adverse events and infections, likely related to the reduced glucocorticoid exposure.
It has been shown that treatment of ANCA-associated vasculitis with rituximab plus glucocorticoids or cyclophosphamide/azathioprine plus glucocorticoids have similar effects on eGFR.22 Patients with the lowest baseline eGFR (<30 ml/min per 1.73 m2) in the RAVE clinical trial had a change in the mean eGFR from 24.4 at baseline to 28.1 ml/min per 1.73 m2 (an increase of 3.7 ml/min per 1.73 m2) at 12 months in the rituximab group, and from 25.5 to 30.5 ml/min per 1.73 m2 (an increase of 5.0 ml/min per 1.73 m2) in the cyclophosphamide group.22 In patients with the same baseline eGFR (<30 ml/min per 1.73 m2) in ADVOCATE, mean eGFR changed from 21.1 at baseline to 35.2 ml/min per 1.73 m2 (an increase of 14.1 ml/min per 1.73 m2) at 12 months in the avacopan group (N = 52), and from 21.6 to 30.8 ml/min per 1.73 m2 (an increase of 9.2 ml/min per 1.73 m2) in the prednisone group (N = 48; P = 0.005 for LSM difference between treatment groups). These results indicate that treatment with avacopan improves kidney function more than just treatment with rituximab or cyclophosphamide/azathioprine plus glucocorticoids as used in these trials.
The study has limitations. It is a post hoc analysis with associated potential biases. The relatively small sample size and multiplicity of testing increase the probability of a type I error. Nevertheless, the results from this subgroup analysis in patients with low eGFR are strong, and consistent with the overall study results from prespecified analyses. The sample size is relatively small, potentially limiting the generalizability of the findings.
Results from this study raise the question of whether use of avacopan could benefit patients presenting with an eGFR below 15 ml/min per 1.73 m2, many having an imminent requirement for dialysis. These patients have the highest risk for end-stage kidney disease and mortality, and are in need of effective therapies that reduce these risks and their downstream consequences. The data presented here further support the need to study this more severe subgroup who may have much to gain from avacopan.
In conclusion, among patients with ANCA-associated vasculitis with baseline eGFR ≤20 ml/min per 1.73 m2 in the ADVOCATE trial, kidney function as measured by eGFR improved more in the avacopan versus prednisone group. The improvement in eGFR continues throughout the 52-week treatment period and was particularly striking between weeks 26 and 52. Avacopan may be helpful in preventing or at least delaying dialysis in these patients.
Disclosure
FBC received consulting fees from ChemoCentryx, Aurinia Pharmaceuticals, Travere Therapeutics and ValenzaBio. JLN received consulting and investigator fees from ChemoCentryx and investigator fees from Alexion. DRWJ received consulting and investigator fees from ChemoCentryx, and research grants and consulting fees from Roche/Genentech, a grant from Sanofi/Genzyme, and investigator and consulting fees from Boehringer-Ingelheim and Medimmune, he is supported by the NIHR Cambridge Biomedical Research Centre. PAM reports receiving funds for the following activities in the past 2 years: Consulting: AbbVie, AstraZeneca, Boehringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, CSL Behring, Dynacure, EMDSerono, Forbius, Genentech/Roche, GlaxoSmithKline, Immagene, InflaRx, Jannsen, Kiniksa, Kyverna, Magenta, MiroBio, Mitsubishi, Neutrolis, Novartis, NS Pharma, Pfizer, Q32, Regeneron, Sparrow, Takeda. Research Support: AbbVie, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, Eicos, Electra, Forbius, Genentech/Roche, GlaxoSmithKline, InflaRx, Sanofi, Takeda. Stock options: Kyverna. Royalties: UpToDate. AB received consulting and investigator fees from AstraZeneca, Bayer, ChemoCentryx, Fresenius, Merck/MSD, and Vifor. HY and TJS are employees, and shareholders of ChemoCentryx, the sponsor of this study. PB is a consultant and shareholder of ChemoCentryx.
Acknowledgments
The authors thank all the many study coordinators, investigators, and patients involved for their valuable contributions. The Clinical Trials identifier for this study is NCT02994927. This study was funded by ChemoCentryx, Inc., San Carlos, CA, USA.
Data Sharing Statement
Because of the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data are not available.
Contributor Information
Frank B. Cortazar, Email: frankcortazar24@gmail.com.
the ADVOCATE Study Group:
C. Au Peh, A. Chakera, B. Cooper, J. Kurtkoti, D. Langguth, V. Levidiotis, G. Luxton, P. Mount, D. Mudge, E. Noble, R. Phoon, D. Ranganathan, A. Ritchie, J. Ryan, M. Suranyi, A. Rosenkranz, K. Lhotta, A. Kronbichler, N. Demoulin, C. Bovy, R. Hellemans, J. Hougardy, B. Sprangers, K. Wissing, C. Pagnoux, S. Barbour, S. Brachemi, S. Cournoyer, L. Girard, L. Laurin, P. Liang, D. Philibert, M. Walsh, V. Tesar, R. Becvar, P. Horak, I. Rychlik, W. Szpirt, H. Dieperink, J. Gregersen, P. Ivarsen, E. Krarup, C. Lyngsoe, C. Rigothier, J. Augusto, A. Belot, D. Chauveau, D. Cornec, N. Jourde-Chiche, M. Ficheux, A. Karras, A. Klein, F. Maurier, R. Mesbah, O. Moranne, A. Neel, T. Quemeneur, D. Saadoun, B. Terrier, P. Zaoui, M. Schaier, U. Benck, R. Bergner, M. Busch, J. Floege, F. Grundmann, H. Haller, M. Haubitz, B. Hellmich, J. Henes, B. Hohenstein, C. Hugo, C. Iking-Konert, F. Arndt, T. Kubacki, I. Kotter, P. Lamprecht, T. Lindner, J. Halbritter, H. Mehling, U. Schönermarck, N. Venhoff, V. Vielhauer, O. Witzke, I. Szombati, G. Szucs, G. Garibotto, F. Alberici, E. Brunetta, L. Dagna, S. De Vita, G. Emmi, A. Gabrielli, L. Manenti, F. Pieruzzi, D. Roccatello, C. Salvarani, H. Dobashi, T. Atsumi, S. Fujimoto, N. Hagino, A. Ihata, S. Kaname, Y. Kaneko, A. Katagiri, M. Katayama, Y. Kirino, K. Kitagawa, A. Komatsuda, H. Kono, T. Kurasawa, R. Matsumura, T. Mimura, A. Morinobu, Y. Murakawa, T. Naniwa, T. Nanki, N. Ogawa, H. Oshima, K. Sada, E. Sugiyama, T. Takeuchi, H. Taki, N. Tamura, T. Tsukamoto, K. Yamagata, M. Yamamura, P. van Daele, A. Rutgers, Y. Teng, R. Walker, I. Chua, M. Collins, K. Rabindranath, J. de Zoysa, M. Svensson, B. Grevbo, S. Kalstad, M. Little, M. Clarkson, E. Molloy, I. Agraz Pamplona, J. Anton, V. Barrio Lucia, S. Ciggaran, M. Cinta Cid, M. Diaz Encarnacion, X. Fulladosa Oliveras, M. Jose Soler, H. Marco Rusinol, M. Praga, L. Quintana Porras, A. Segarra, A. Bruchfeld, M. Segelmark, I. Soveri, E. Thomaidi, K. Westman, T. Neumann, M. Burnier, T. Daikeler, J. Dudler, T. Hauser, H. Seeger, B. Vogt, D. Jayne, J. Burton, R. Al Jayyousi, T. Amin, J. Andrews, L. Baines, P. Brogan, B. Dasgupta, T. Doulton, O. Flossmann, S. Griffin, J. Harper, L. Harper, D. Kidder, R. Klocke, P. Lanyon, R. Luqmani, J. McLaren, D. Makanjuola, L. McCann, A. Nandagudi, S. Selvan, E. O'Riordan, M. Patel, R. Patel, C. Pusey, R. Rajakariar, J. Robson, M. Robson, A. Salama, L. Smyth, J. Sznajd, J. Taylor, P. Merkel, A. Sreih, E. Belilos, A. Bomback, J. Carlin, Y. Chang Chen Lin, V. Derebail, S. Dragoi, A. Dua, L. Forbess, D. Geetha, P. Gipson, R. Gohh, G.T. Greenwood, S. Hugenberg, R. Jimenez, M. Kaskas, T. Kermani, A. Kivitz, C. Koening, C. Langford, G. Marder, A. Mohamed, P. Monach, N. Neyra, G. Niemer, J. Niles, R. Obi, C. Owens, D. Parks, A. Podoll, B. Rovin, R. Sam, W. Shergy, A. Silva, U. Specks, R. Spiera, J. Springer, C. Striebich, A. Swarup, S. Thakar, A. Tiliakos, Y. Tsai, D. Waguespack, and M. Chester Wasko
Appendix
List of Collaborators From the ADVOCATE Trial
(Presented by country, with National Coordinating Center followed by participating centers, in alphabetical order by principal investigator.)
Australia—National Coordinating Center: Royal Adelaide Hospital, Adelaide SA (C. Au Peh); Sir Charles Gairdner Hospital, Nedlands, WA (A. Chakera); Royal North Shore Hospital, St Leonards (B. Cooper); Griffith University, Southport (J. Kurtkoti); Wesley Medical Research, Auchenflower (D. Langguth); Western Health, St. Albans Victoria (V. Levidiotis); Prince of Wales Hospital, Randwick NSW (G. Luxton); Austin Health, Heidelberg Victoria (P. Mount); Princess Alexandra Hospital, Woolloongabba, QLD (D. Mudge); Sunshine Coast University Hospital, Birtinya (E. Noble); Westmead Hospital, Westmead NSW (R. Phoon); Royal Brisbane and Women’s Hospital, Herston QLD (D. Ranganathan); Concord Repatriation General Hospital, Concord (A. Ritchie); Monash Medical Centre, Clayton Victoria (J. Ryan); Liverpool Hospital, Liverpool, NSW (M. Suranyi).
Austria—National Coordinating Center: Medizinische Universitaet Graz, Graz (A. Rosenkranz); Landeskrankenhaus Feldkirch, Feldkirch (K. Lhotta); Medical University of Innsbruck, Innsbruck (A. Kronbichler).
Belgium—National Coordinating Center: Cliniques Universitaires Saint-Luc, Brussels (N. Demoulin); Centre Hospitalier Universitaire (CHU) de Liege, Liege (C. Bovy); Antwerp University Hospital (UZA), Edegem (R. Hellemans); Universite Libre de Bruxelles (ULB) -Hopital Erasme, Brussels (J. Hougardy); University Hospital (UZ) Leuven, Leuven (B. Sprangers); University Hospital Brussels, Brussels (K. Wissing).
Canada—National Coordinating Center: University of Toronto, Toronto (C. Pagnoux); St. Paul Hospital, Vancouver (S. Barbour); Centre de Recherche du Centre Hospitalier de l'Université de Montréal, Montreal (S. Brachemi); CISSS de la Monteregie-Centre – Hopital Charles LeMoyne, Greenfield Park (S. Cournoyer); University of Calgary, Calgary (L. Girard); Hospital Maisonneuve-Rosemont, Montreal (L. Laurin); Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke (P. Liang); CHUQ-L'Hotel-Dieu de Quebec, Quebec City (D. Philibert); St. Josephs Healthcare, Hamilton (M. Walsh).
Czech Republic—Department of Nephrology, General University Hospital, Prague (V. Tesar); Rheumatology Institute, Prague (R. Becvar); University Hospital Olomouc, Olomouc (P. Horak); University Hospital Vinohrady, Prague (I. Rychlik).
Denmark—National Coordinating Center: Copenhagen University Hospital, Copenhagen (W. Szpirt); Odense University Hospital, Odense (H. Dieperink); Aalborg University Hospital, Aalborg (J. Gregersen); Aarhus University Hospital - Skejby, Aarhus (P. Ivarsen); Herlev Hospital, Herlev (E. Krarup); Sjaellands Universitetshospital Roskilde, Roskilde (C. Lyngsoe).
France—National Coordinating Center: CHU Bordeaux - Hospital Pellegrin, Bordeaux (C. Rigothier); CHU Angers, Angers (J. Augusto); CHU Lyon- Hopital Femme- Mere-Enfant, Bron (A. Belot); CHU de Toulouse - Hospital Rangueil, Toulouse (D. Chauveau); CHU de Brest -Hopital de la Cavale Blanche, Brest (D. Cornec); APHM - Hopital de la Conception, Marseille (N. Jourde-Chiche); CHU de Caen, Caen (M. Ficheux); Hopital Europeen Georges Pompidou, Paris (A. Karras); Hopitaux Civils de Colmar, Colmar (A. Klein); Hopitaux Prives de Metz, Metz (F. Maurier); Centre Hospitalier Boulogne sur Mer, Boulogne sur Mer (R. Mesbah); CHU Nimes – Hopital Caremeau, Nimes (O. Moranne); CHU Nantes Medicine Interne, Nantes (A. Neel); Centre Hospitalier de Valenciennes, Valenciennes (T. Quemeneur); Hospital Pitie Salpetriere, Paris (D. Saadoun); Hopital Cochin, Paris (B. Terrier); CHU de Grenoble, Grenoble Isere Cedex (P. Zaoui).
Germany—National Coordinating Center: University Clinic Heidelberg, Heidelberg (M. Schaier); University Clinic Mannheim, Mannheim (U. Benck); Clinic of Ludwigshafen am Rhein, Ludwigshafen (R. Bergner); University Clinic Jena, Jena (M. Busch); University Clinic Aachen, Aachen (J. Floege); University Clinic Cologne, Cologne (F. Grundmann); Medizinische Hochschule Hannover, Hannover (H. Haller); Klinikum Fulda, Fulda (M. Haubitz); Medius Clinic Kirchheim, Kirchheim-unter-Teck (B. Hellmich); University Hospital Tuebingen, Tuebingen (J. Henes); Nephrological Center Villingen-Schwenningen, Villingen-Schwenningen (B. Hohenstein); University Clinic Carl Gustav Carus, Dresden (C. Hugo); Klinikum Bad Bramstedt GmbH, Bad Bramstedt (C. Iking-Konert and F. Arndt); Asklepios Kinik, Hamburg (T. Kubacki and I. Kotter); University Clinic Schleswig-Holstein, Luebeck (P. Lamprecht); University Clinic Leipzig, Leipzig (T. Lindner and J. Halbritter); Charité - Universitaetsmedizin Berlin, Berlin (H. Mehling); Universität München – Großhadern, Munich (U. Schönermarck); University Clinic Freiburg, Freiburg (N. Venhoff); University Clinic Munich, Munich (V. Vielhauer); University Clinic Essen, Essen (O. Witzke).
Hungary—Qualiclinic Kft, Budapest (I. Szombati); DEOEC Rheumatology Faculty, Debrecen (G. Szucs).
Italy—National Coordinating Center: IRCCS Azienda Ospedaliera Universitaria San Martino, Genova (G. Garibotto); ASST Santi Paolo e Carlo-Presidio Ospedale San Carlo, Milan (F. Alberici); Istituto Clinico Humanitas, Rozzano (E. Brunetta); IRCCS Ospedale San Raffaele, Milan (L. Dagna); Azienda Sanitaria Universitaria Integrata di Udine, Udine (S. De Vita); Azienda Ospedaliero-Universitaria Careggi, Florence (G. Emmi); AOU Ospedali Riuniti di Ancona, Torrette Ancona (A. Gabrielli); Azienda Ospedaliero Universitaria di Parma, Parma (L. Manenti); ASST di Monza-Ospedale San Gerardo, Monza (F. Pieruzzi); ASL Città di Torino -Ospedale San Giovanni Bosco, Torino (D. Roccatello); Azienda Unità Sanitaria Locale di Reggio Emilia, Reggio Emilia (C. Salvarani).
Japan—National Coordinating Investigator: Prof. M. Harigai, Tokyo Women’s Medical University, Tokyo; Kagawa University Hospital, Kagawa (H. Dobashi); Hokkaido University Hospital, Hokkaido (T. Atsumi); University of Miyazaki Hospital, Miyazaki (S. Fujimoto); Teikyo University Chiba Medical Center, Chiba (N. Hagino); National Hospital Organization Yokohama Medical Center, Yokohama (A. Ihata); Kyorin University Hospital, Tokyo (S. Kaname); Keio University Hospital, Tokyo (Y. Kaneko); Juntendo University Shizuoka Hospital, Shizuoka (A. Katagiri); Nagoya Medical Center, Aichi (M. Katayama); Yokohama City University Hospital, Kanagawa (Y. Kirino); National Hospital Organization Kanazawa Medical Center, Ishikawa (K. Kitagawa); Akita University Hospital, Akita City (A. Komatsuda); Teikyo University Hospital, Tokyo (H. Kono); Saitama Medical Center, Saitama (T. Kurasawa); National Hospital Organization Chiba East Hospital, Chiba (R. Matsumura); Saitama Medical University Hospital, Saitama (T. Mimura); Kobe University Hospital, Hyogo (A. Morinobu); Shimane University Hospital, Shimane (Y. Murakawa); Nagoya City University Hospital, Aichi (T. Naniwa); Toho University Omori Medical Center, Tokyo (T. Nanki); Hamamatsu University Hospital, Shizuoka (N. Ogawa); National Hospital Organization Tokyo Medical Center, Tokyo (H. Oshima); Okayama University Hospital, Okayama (K. Sada); Hiroshima University Hospital, Hiroshima (E. Sugiyama); Osaka Medical College Hospital, Osaka (T. Takeuchi); Toyama University Hospital, Toyama (H Taki); Juntendo University Hospital, Tokyo (N. Tamura); Tazuke Kofukai Medical Research Institute Kitano Hospital, Osaka (T. Tsukamoto); University of Tsukuba Hospital, Ibaraki (K. Yamagata); Okayama Saiseikai General Hospital, Okayama (M. Yamamura).
The Netherlands—Erasmus MC, Rotterdam (P. van Daele); Groningen Universitair Medisch Centrum, Groningen (A. Rutgers); Leids Universitair Medisch Centrum, Leiden (Y. Teng).
New Zealand—National Coordinating Center: Dunedin Hospital, Dunedin (R. Walker); Christchurch Clinical Studies Trust, Christchurch (I. Chua); Auckland City Hospital, Auckland (M. Collins); Waikato Hospital, Hamilton (K. Rabindranath); North Shore Hospital, Takpuna, Auckland (J. de Zoysa).Norway— National Coordinating Center: Akershus Universitetssykehus, Nordbyhagen (M. Svensson); Oslo Universitessykkehus, Oslo (B. Grevbo); University Hospital of North Norway, Tromso (S. Kalstad).
Republic of Ireland—National Coordinating Center: Beaumont Hospital, Dublin (M. Little); Cork University Hospital, Cork (M. Clarkson); St. Vincent's University Hospital, Dublin (E. Molloy).
Spain—Hospital Vall D Hebron, Barcelona (I. Agraz Pamplona); Hospital Sant Joan de Deu, Barcelona (J. Anton); Hospital Universitario Infanta Sofia, San Sebastian de los Reyes, Madrid (V. Barrio Lucia); Hospital Da Costa, Burela (S. Ciggaran); Hospital Clinic Barcelona – Autoimmune Diseases Department, Barcelona (M. Cinta Cid); Fundacio Puigvert, Barcelona (M. Diaz Encarnacion); Hospital Universitari de Bellvitge, Barcelona (X. Fulladosa Oliveras); Hospital del Mar, Barcelona (M. Jose Soler); Hospital Germans Trias i Pujol, Badalona (H. Marco Rusinol); Hospital 12 de Octubre, Madrid (M. Praga); Hospital Clinic Barcelona, Barcelona (L. Quintana Porras); Hospital Universitari Arnau de Vilanova, Lleida (A. Segarra).
Sweden—National Coordinating Center: Karolinska University Hospital, Stockholm (A. Bruchfeld); Linköping University, Linköping (M. Segelmark); Uppsala University Hospital, Uppsala (I. Soveri); Örebro University Hospital, Örebro (E. Thomaidi); Skane University Hospital, Malmo (K. Westman).
Switzerland—National Coordinating Center: Kantonsspital St. Gallen, St. Gallen (T. Neumann); CHUV Lausanne, Lausanne (M. Burnier); University Hospital Basel, Basel (T. Daikeler); Hôpital Fribourgeois, Fribourg (J. Dudler); Immunologie- Zentrum Zürich, Zürich (T. Hauser); Universitätsspital Zürich, Zürich (H. Seeger); Inselspital, Universitätsspital Bern, Bern (B. Vogt).
United Kingdom—National Coordinating Center: Addenbrooke’s Hospital - Cambridge University Hospitals, Cambridge (D. Jayne); Leicester General Hospital, Leicester (J. Burton and R. Al Jayyousi); Leeds Childrens Hospital, Leeds (T. Amin); Leeds Teaching Hospitals NHS Trust, Leeds (J. Andrews); Freeman Hospital, Newcastle upon Tyne (L. Baines); Great Ormond Street Hospital for Children, London (P. Brogan); Southend University Hospital, Westcliff on Sea (B. Dasgupta); Kent and Canterbury Hospital, Canterbury – Kent (T. Doulton); Royal Berkshire Hospital, Reading, Berkshire (O. Flossmann); University Hospital of Wales, Cardiff (S. Griffin); Royal Liverpool University Hospital, Liverpool (J. Harper); University of Birmingham, Birmingham (L. Harper); University Aberdeen, Aberdeen (D. Kidder); Russells Hall Hospital, Dudley (R. Klocke); Queens Medical Centre, Nottingham (P. Lanyon); Nuffield Orthopaedic Centre, Oxford (R. Luqmani); Whytemans Brae Hospital, Fife (J. McLaren); St Helier Hospital, Carshalton (D. Makanjuola); Alder Hey Children's NHS Foundation Trust, Liverpool (L. McCann); Basildon University Hospital, Basildon (A. Nandagudi and S. Selvan); Salford Royal NHS Foundation Trust Manchester, Salford (E. O'Riordan); University of Manchester, Manchester Royal Infirmary, Manchester (M. Patel); Queen Elizabeth University Hospital, Glasgow (R. Patel); Imperial College Healthcare NHS Trust, London (C. Pusey); The Royal London Hospital, London (R. Rajakariar); Bristol Royal Infirmary, Bristol (J. Robson); Guy’s and St Thomas’s NHS Foundation Trust, London (M. Robson); UCL Centre for Nephrology Royal Free, London (A. Salama); Royal Devon and Exeter Hospital, Exeter (L. Smyth); Raigmore Hospital, Inverness (J. Sznajd); Dorset County Hospital, Dorchester (J. Taylor).
United States of America—University of Pennsylvania, Philadelphia (P. Merkel and A. Sreih); Winthrop University Hospital, Mineola (E. Belilos); Columbia University Medical Center, New York (A. Bomback); Virginia Mason Medical Center, Seattle (J. Carlin); University of South Florida, Tampa (Y. Chang Chen Lin); University of North Carolina Hospitals, Chapel Hill (V. Derebail); MedStar Georgetown University Hospital, Washington (S. Dragoi); University of Chicago Medical Center Rheumatology, Chicago (A. Dua); Cedars-Sinai Medical Center, Los Angeles (L. Forbess); Johns Hopkins Bayview Medical Center, Baltimore (D. Geetha); University of Michigan, Ann Arbor (P. Gipson); Rhode Island Hospital, Providence (R. Gohh); Brookview Hills Research Associates, Winston-Salem (G. T. Greenwood); Indiana University Nephrology, Indianapolis (S. Hugenberg); Western Washington Arthritis Clinic, Bothell (R. Jimenez); Northwest Louisiana Nephrology, Shreveport (M. Kaskas); University of California, Los Angeles, Santa Monica (T. Kermani); Altoona Center for Clinical Research, Duncansville (A. Kivitz); University of Utah, Salt Lake City (C. Koening); Cleveland Clinic, Cleveland (C. Langford); Northwell Health, Great Neck (G. Marder); University of Kentucky Medical Center, Lexington (A. Mohamed); Boston University, Boston (P. Monach); Arizona Kidney Disease and Hypertension Center Flagstaff, Flagstaff (N. Neyra); Articularis Healthcare Group, Charleston (G. Niemer); Massachusetts General Hospital, Boston (J. Niles); East Carolina University, Greenville (R. Obi); Renal Disease Research Institute, Dallas (C. Owens); Washington University School of Medicine, St. Louis (D. Parks); Colorado Kidney Care, Denver (A. Podoll); Ohio State University, Columbus (B. Rovin); San Francisco General Hospital Dialysis Center, San Francisco (R. Sam); Rheumatology Associates of North Alabama, Huntsville (W. Shergy); Boise Kidney & Hypertension, PLLC–Meridian, Caldwell (A. Silva); Mayo Clinic - Division of Pulmonary & Critical Care Medicine, Rochester (U. Specks); Hospital for Special Surgery, New York (R. Spiera); University of Kansas Medical Center, Kansas City (J. Springer); University of Colorado Denver-School of Medicine, Aurora (C. Striebich); Arizona Arthritis & Rheumatology Research, Phoenix (A. Swarup); University of Minnesota, Minneapolis (S. Thakar); Emory University School of Medicine, Atlanta (A. Tiliakos); Arthritis, Autoimmune and Allergy LLC, Daytona Beach (Y. Tsai); University of Texas Health Sciences Center, Houston (D. Waguespack); Allegheny General Hospital, Pittsburgh (M. Chester Wasko)
References
- 1.Jayne D. Evidence-based treatment of systemic vasculitis. Rheumatology (Oxford) 2000;39:585–595. doi: 10.1093/rheumatology/39.6.585. [DOI] [PubMed] [Google Scholar]
- 2.Koldingsnes W., Nossent H. Predictors of survival and organ damage in Wegener’s granulomatosis. Rheumatology (Oxford) 2002;41:572–581. doi: 10.1093/rheumatology/41.5.572. [DOI] [PubMed] [Google Scholar]
- 3.Corral-Gudino L., Borao-Cengotita-Bengoa M., del Pino-Montes J., Lerma-Márquez J.L. Overall survival, renal survival and relapse in patients with microscopic polyangiitis: a systematic review of current evidence. Rheumatology (Oxford) 2011;50:1414–1423. doi: 10.1093/rheumatology/ker112. [DOI] [PubMed] [Google Scholar]
- 4.Takala J.H., Kautiainen H., Finne P., Leirisalo-Repo M. Wegener’s granulomatosis in Finland in 1981–2000: risk of dialysis-dependent renal disease. Scand J Rheumatol. 2011;40:283–288. doi: 10.3109/03009742.2010.533693. [DOI] [PubMed] [Google Scholar]
- 5.Flossmann O., Berden A., de Groot K., et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70:488–494. doi: 10.1136/ard.2010.137778. [DOI] [PubMed] [Google Scholar]
- 6.Mohammad A.J., Segelmark M. A Population-based Study Showing Better Renal Prognosis for proteinase 3 antineutrophil cytoplasmic antibody (ANCA)–associated nephritis versus myeloperoxidase ANCA–associated nephritis. J Rheumatol. 2014;41:1366–1373. doi: 10.3899/jrheum.131038. [DOI] [PubMed] [Google Scholar]
- 7.Lionaki S., Hogan S.L., Jennette C.E., et al. The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int. 2009;76:644–651. doi: 10.1038/ki.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayne D.R.W., Gaskin G., Rasmussen N., et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180–2188. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 9.De Joode A.A.E., Sanders J.S.F., Stegeman C.A. Renal survival in proteinase 3 and myeloperoxidase ANCA-associated systemic vasculitis. Clin J Am Soc Nephrol. 2013;8:1709–1717. doi: 10.2215/CJN.01020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z.Y., Gou S.J., Chen M., Zhao M.H. Predictors for outcomes in patients with severe ANCA-associated glomerulonephritis who were dialysis-dependent at presentation: a study of 89 cases in a single Chinese center. Semin Arthritis Rheum. 2013;42:515–521. doi: 10.1016/j.semarthrit.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Pepper R.J., Chanouzas D., Tarzi R., et al. Intravenous cyclophosphamide and plasmapheresis in dialysis-dependent ANCA-associated vasculitis. Clin J Am Soc Nephrol. 2013;8:219–224. doi: 10.2215/CJN.03680412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee T., Gasim A., Derebail V.K., et al. Predictors of treatment outcomes in ANCA-associated vasculitis with severe kidney failure. Clin J Am Soc Nephrol. 2014;9:905–913. doi: 10.2215/CJN.08290813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah S., Hruskova Z., Segelmark M., et al. Treatment of severe renal disease in ANCA positive and negative small vessel vasculitis with rituximab. Am J Nephrol. 2015;41:296–301. doi: 10.1159/000431336. [DOI] [PubMed] [Google Scholar]
- 14.Jayne D.R.W., Merkel P.A., Schall T.J., Bekker P., ADVOCATE Study Group Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med. 2021;384:599–609. doi: 10.1056/NEJMoa2023386. [DOI] [PubMed] [Google Scholar]
- 15.Merkel P.A., Jayne D.R., Wang C., Hillson J., Bekker P. Evaluation of the safety and efficacy of avacopan, a C5a receptor inhibitor, in patients with antineutrophil cytoplasmic antibody–associated vasculitis treated concomitantly with rituximab or cyclophosphamide/azathioprine: protocol for a randomized, double-blind, active-controlled, phase 3 trial. JMIR Res Protoc. 2020;9 doi: 10.2196/16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenette J.C., Falk R.J., Bacon P.A., et al. Revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 17.Mukhtyar C., Lee R., Brown D., et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3) Ann Rheum Dis. 2009;68:1827–1832. doi: 10.1136/ard.2008.101279. [DOI] [PubMed] [Google Scholar]
- 18.Levey A.S., Coresh J., Greene T., et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz G., Muñoz A., Schneider M.F., et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye L., Liu Y., Duan T., et al. Digital special profiling of individual glomeruli from patients with anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.831253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.L’Imperio V., Vischini G., Pagni F., Ferraro P.M. Bowman’s capsule rupture on renal biopsy improves the outcome prediction of ANCA-associated glomerulonephritis classifications. Ann Rheum Dis. 2022;81:e95. doi: 10.1136/annrheumdis-2020-217979. [DOI] [PubMed] [Google Scholar]
- 22.Geetha D., Specks U., Stone J.H., et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis with renal involvement. J Am Soc Nephrol. 2015;26:976–985. doi: 10.1681/ASN.2014010046. [DOI] [PMC free article] [PubMed] [Google Scholar]