Abstract
Introduction
Diet, chronic kidney disease (CKD), and Apolipoprotein L1 (APOL1) (DCA) Study is examining the role of dietary factors in CKD progression and APOL1 nephropathy. We describe enrollment and retention efforts and highlight facilitators and barriers to enrollment and operational challenges, as well as accommodations made in the study protocol.
Methods
The DCA study is enrolling participants in 7 centers in West Africa. Participants who consented were invited to complete dietary recalls and 24-hour urine collections in year 1. We conducted focus groups and semistructured interviews among study personnel to identify facilitators and barriers to enrollment as well as retention and operational challenges in the execution of the study protocol. We analyzed emerging themes using content analyses.
Results
A total of 712 participants were enrolled in 18 months with 1256 24-hour urine and 1260 dietary recalls. Barriers to enrollment were the following: (i) a lack of understanding of research, (ii) the burden of research visits, and (iii) incorporating cultural and traditional nuances when designing research protocols. Factors facilitating enrollment were the following: (i) designing convenient research visits, (ii) building rapport and increased communication between the research team and participants, and (iii) cultural sensitivity – adapting research protocols for the populations involved. Offering home visits, providing free dietary counseling, reducing the volume of study blood collection, and reducing the frequency of visits were some changes made in the study protocol that increased participant satisfaction.
Conclusion
Adopting a participant-centered approach with accommodations in the protocol for cultural adaptability and incorporating participant feedback is vital for carrying out research in low-income and middle-income regions.
Keywords: 24hr-urine potassium & sodium, Africa, engagement, retention
Diet is a critical and modifiable determinant of health and disease, including in CKD. Dietary nutrients and other dietary factors are associated with the development of CKD, a disease that disproportionately impacts individuals of African descent.1, 2, 3, 4, 5 Higher dietary sodium and lower dietary potassium lead to higher blood pressure and increased risk of CKD and CKD progression.6, 7, 8, 9 In adition, adherence to healthy dietary patterns such as the Mediterranean diet and the Dietary Approaches to Stop Hypertension diet is associated with reduced incidence of CKD and CKD progression.1,10,11
CKD can effectively be a death sentence in sub-Saharan Africa because <2% of the population has access to life-sustaining renal replacement therapies like dialysis or transplantation.12 The greater prevalence of CKD among individuals of African descent is associated in large part to polymorphisms in the gene encoding Apolipoprotein L1 (APOL1).13,14 The prevalence of CKD is 15.8% in Africa and 19.8% in West Africa.15 Ghana and Nigeria have the highest prevalence of the APOL1 high-risk variants, as high as 40% among Yoruba and Igbo people in southwest Africa.16,17 The lowest prevalence of the APOL1 high-risk variants is in East Africa.13,17,18 In the US, approximately 50% of African Americans have at least 1 APOL1 risk allele and about 13% of the African Americans carry 2 APOL1 risk alleles.19
APOL1 is a unique genetic risk factor because of the relatively high prevalence of risk polymorphisms and the strong effect sizes observed with CKD risk. APOL1 high-risk alleles increase risk in a recessive manner, but it is thought to be due to a gain of function mutation.6,7 The incomplete penetrance of the APOL1 high-risk genotypes in Black individuals (i.e., the kidney disease manifests in some individuals and not in others) suggests that there are unknown “second hits” interacting with APOL1 genotypes to cause disease. Identifying factors that lead to manifestation of disease on the background of a high-risk genotype i.e., gene-by-environment interactions in APOL1 kidney disease is of great scientific and public health importance.14 Recent studies have implicated inflammation as a risk factor for enhancing APOL1 gene expression.20 Regular intake of an unhealthy diet may increase proinflammatory effects, leading to the development and progression of CKD and potentially an interaction with APOL1 genotypes.21,22
There are major gaps in the understanding of the role of dietary factors in the development and progression of CKD, particularly as they relate to more than 1 billion Africans at risk for CKD and end stage kidney disease, as follows: (i) previous studies were conducted in the USA or Europe and mostly in White populations; (ii) findings from dietary studies have inconsistent associations; and (iii) no dietary studies have investigated interactions with the APOL1 high-risk genotypes.
The overall goal of the DCA Study is to investigate the role of dietary factors in CKD and APOL1 kidney disease. The study is set in West Africa, where APOL1 prevalence may be higher than in the US and where the APOL1 risk polymorphisms likely originated.13 The DCA study is collecting 24-hour urine samples, dietary information, biological samples, and longitudinal follow-up data from over 700 study participants. We report the rationale and design of the DCA study and also highlight facilitators and barriers to conducting this study in low-income and middle-income settings.
Methods
Overall Design
The DCA study is an ancillary study to the Human Hereditary and Health in Africa (H3Africa) Kidney Disease Study, a prospective cohort study involving 3,300 participants with CKD in sub-Saharan Africa which started recruiting in 2016. Details of the inclusion and exclusion criteria for the H3Africa study were reported previously.23 Briefly, the inclusion criteria for the parent H3Africa kidney disease study includes the following: (i) age >15 years, (ii) there were 2 groups of patients, in the first group/CKD group, estimated glomerular filtration rate (eGFR) criteria was eGFR 20−59 ml/min per 1.73 m2 using the 2009 CKD-epidemiology collaboration equation without correction for Black race, and (iii) in the second group, the glomerulonephritis group, the eGFR criteria was eGFR >15 ml/min per 1.73 m2 and a biopsy-confirmed diagnosis of glomerulonephritis in the H3Africa cohort. Exclusion criteria include the following: (i) HIV positive individuals, (ii) kidney transplant recipients, institutionalized individuals, (iii) New York Heart Association Class III or IV, (iv) known cirrhosis, (v) pregnant or breast-feeding women, (vi) polycystic kidney disease, (vii) solid organ or bone marrow transplant, (viii) chronic obstructive uropathy, (ix) adult-onset monogenic forms of kidney disease, (x) mitochondrial diseases, (xi) maturity onset diabetes of the young, and (xii) individuals with kidney failure.
The DCA Study
The DCA study is recruiting a convenience sample of more than 700 participants from 7 centers in Nigeria and Ghana. Participants enrolled will contribute multiple 24-hour urine collections and 24-hour dietary recalls. Participating centers include College of Medicine, University of Lagos; Lagos State University Teaching Hospital; University of Ghana; College of Medicine, University of Nigeria, Ituku-Ozalla; Obafemi Awolowo University, Ile-Ife; and University of Ibadan, Ibadan. Inclusion criteria for the DCA study are age >15 years, enrollment in the parent H3Africa kidney disease study, as well as willingness to provide 24-hour urine samples and 24-hour dietary recalls. Individuals on dialysis are excluded from enrollment. Institutional review board approval was obtained from the DCA coordinating center (Boston Medical Center) and all participating centers. Enrollment started in February 2021 and will continue for 2 years; follow-up visits started in February 2022, and we plan for at least 3 years of annual follow-up. As of August 2022, 712 participants were enrolled. We are using the Research Electronic Data Capture for data collection.24
Recruitment Plan
Study personnel contacted eligible participants in the parent study to gauge their interest using a phone script. Those who agreed to participate were invited to the clinical center for a screening visit. For participants who were not reachable, coordinators used alternative contacts, such as family members or sending messages via WhatsApp.
Participant Management Toolkit
Because centers lacked electronic medical records, we created a participant management tool using Research Electronic Data Capture. The management tool provides a systematic method for patient contact, appointment scheduling, as well as visit monitoring and tracking. The toolkit records and tracks outcomes of participant contact with recorded reasons for nonenrollment (e.g., if participants could not be reached, declined participation, had started dialysis, had died, or had relocated). The participant management tool is a useful resource for both enrollment and retention and to ensure that participant visits are tracked through screening and study visits for urine collection, annual follow-up visits to study conclusion.
Study Protocol
The study protocol involved a screening visit, baseline visits for 24-hour urine collection, and then up to 2 other urine collections in the first year, dietary recalls, and annual follow-up visits (Table 1). Trained study coordinators informed participants about how to perform the urine collections. Participants were invited to submit up to 3 24-hour urine samples in the first year of the study. Ideally these visits were to occur between 0 to 6 months; however, we had to give up to 12 months for the urine collections to be completed. Collecting up to 3 urine samples enabled us to calculate the average excretion rates, given the known within-person variability of urinary sodium, potassium, and other metabolites.25 We later had to reduce the number of collections to a maximum of 2 to reduce participant burden.
Table 1.
Study Procedures for screening, enrollment, and study visits for the diet, chronic kidney disease and apolipoprotein L1 (APOL1) study
| Study visit | Study tasks | Duration |
|---|---|---|
| Screening/baseline visit | 1. Description of study and what the study entails | 60 min |
| 2. Sign additional consent forms | ||
| 3. Train on 24h urine collection | ||
| 4. Train on completing 24h dietary forms | ||
| 5. Participants receive the 24h collection containers | ||
| 6. Determine date of 24h urine collection | ||
| 7. Inform family members if present to remind of urine collection day | ||
| 8. Coordinator provides signs and pins to remind participants of date of 24h urine collection | ||
| 9. The day before the 24h urine collection, coordinator calls participants to remind about the urine collection | ||
| Study visit 1 | 1. Return of 24h urine | 90–120 min |
| 2. In-depth dietary interview for 24h dietary recalls using the forms given to participants | ||
| 3. Blood draw for serum creatinine measurement and plasma for future studies | ||
| 4. Measure of height, weight, and blood pressure | ||
| 5. Schedule time for next 24h urine collection and provide materials for next visit | ||
| Study visits 2 and 3 | 1. Return of second 24h urine specimen | 90–120 min |
| 2. In-depth 24h dietary recall interview administered by study coordinator/research nurse | ||
| 3. Blood draw for serum creatinine | ||
| 4. . Measure of height, weight, and blood pressure | ||
| 5. Schedule time for the next 24h urine collection and provide materials for next visit | ||
| Annual follow-up visit | 1. Update contact information | |
| 2. CRFs on physical activity | ||
| 3. Measure of height, weight, and blood pressure | ||
| 4. Blood draw for serum creatinine measurement and plasma for future studies |
CRF, case report form.
Dietary Recalls
Trained dieticians conduct 24-hour dietary recalls at visit 1, and then at the second or third visits, where applicable, concurrent with the 24-hour urine collections. Before the dietary recall, participants are asked to note the foods eaten on the designated day for the dietary recall, on a form given to the participant during the screening visit. Thereafter, during the visits, trained coordinators and dieticians conduct standardized interviews asking in-depth questions about mealtimes, brands of food eaten, and the number of consumed meals.
Data Collection and Entry for 24-Hour Dietary Recall
We built a dietary database using the West African Food Composition Table and the Research Electronic Data Capture database,24,26 including food groups, amounts consumed, as well as snacks and drinks consumed as entered by trained dieticians. After consulting with dieticians with expertise in local foods, we included a list of food items not originally present in the food tables and made provision for additional free text. Dieticians either came from, trained in, or resided in the local communities so that they were conversant with the foods eaten in the areas. They were able to review the available food list and added additional foods based on the local consumption. We created a new list of food items not in the food tables and the nutrition content of these foods were determined by consensus between the dieticians in Africa and the research dietician at Penn State University.
Physical Measurements
After the participants returned the 24-hour urine samples, study coordinators measured height, weight, waist circumference, and blood pressure. Study coordinators measured blood pressure once using the Omron HEM-907XL pro blood pressure monitor when the participant was seated.
Home Visits
In May 2021, because of the ongoing COVID-19 pandemic, we expanded the protocol to include home visits because of the hesitation of participants for in-person visits to the clinical centers. Other participants felt embarrassed to take public transport with containers filled with urine from their homes to the clinical centers. The procedures for the home visits, observing COVID-19 precautions to ensure the safety of the research staff and participants are shown in Table 2. Additional training on standard operating procedures for home visits was conducted for staff on the home visit or mobile team. The home visit team consisted of at least 2 people: a dietician, and a laboratory personnel or clinical research coordinator. Overall, home visits increased participant engagement and reduced the burden of the research visits on the participants. Since February 2021, the DCA study has completed 125 home visits.
Table 2.
Study procedures for home visits in the diet, chronic kidney disease and APOL1 study in Africa
| Preparation for home visits |
| 1. Participants were called to schedule appointments for home visits |
| 2. Participants were consented before the start of the home visit |
| 3. Site study team clustered participants living in similar areas to manage transportation costs for visits |
| 4. All sample collection tubes and 24h urine jugs were prelabelled |
| On the day of the visit |
| 1. Study coordinators ensured that the check list for the home visit was available |
| 2. Study coordinators ensured that the specimen collection ice box had sufficient ice |
| 3. Study coordinators ensured availability of blood pressure cuffs in all sizes |
| 4. Study coordinators preprinted labels for all planned/consented participants |
| 5. Study coordinators offered masks to participants who were agreeable |
| 6. Skilled laboratory personnel on the home visit team collected samples and they were placed on ice after collection |
| Participant check-out |
| 1. Study coordinators gave participant reimbursement |
| 2. Study coordinators confirmed all future research appointments for the participant and gave appointment cards to the participants |
| Home visit close of day activities |
| 1. Study personnel ensured that specimens were collected and stored on ice |
| 2. Two individuals checked all laboratory samples and certified that all samples were labeled and well collected |
| 3. The team cleaned and disinfected all the equipment after each visit |
| 4. Case report forms were stored in a safe locked bag before getting to the site |
| 5. Team ensured that all participants’ information were kept confidential |
Sample Processing
Adequacy of Urine Collection
We defined inadequate urine collections as urine collection volumes <500 ml and timed collections <22 hours or >26 hours.27 We discarded samples where urine volume <500 ml/d or urine creatinine excretion <7 mg/kg/d or >100 mg/kg/d, and asked participants for repeat urine collections where feasible.28 We further assessed adequacy by calculating expected 24h urine creatinine excretion and excluding collection amounts below and above the 5th and 97.5th percentiles, as estimated by an equation that incorporates sex, weight, height, and age to derive population-based limits of urine creatinine excretion.29
Processing of Urine Samples
Once participants returned the samples to the clinical centers, the volume was measured using a graduated cylinder. The entire 24-hour urine samples were sent to processing laboratories in Nigeria and Ghana, where the measurements of urine sodium, potassium, protein, and creatinine are performed. The remaining samples were aliquoted, centrifuged, and stored for future studies.
Laboratory Measurements
Urinary sodium, potassium, and creatinine were measured at Synlab Nigeria Limited in Lagos, Nigeria, and Ghana. Urine creatinine was measured using the modified Jaffe kinetic method,30 and urine sodium and potassium were measured using an ion selective electrode on the Beckman Coulter AU 480 and 680 series.
Exposures
24-Hour Urine Sodium and Potassium
We used mean values of 2 (or 3) collections to minimize the effects of short-term within-person biological variability. We analyzed samples collected only before individuals had reach the end point of 40% decline in eGFR or initiation of renal replacement therapy or death. We will examine how 24-hour urine sodium and potassium vary across CKD stages and albuminuria and over the average 24-hour urine collections. We included only 24-hour urines collected before the participants achieved outcomes of interest.
Dietary Pattern
We will identify major dietary patterns using principal component analysis (PCA) and known food groups.31,32 Food from the 24-hour recalls will be classified into food groups using the INFOODS West African Food Composition Tables.26 Principal component analysis will identify dietary patterns based on correlations between food groups. An optimal pattern will be defined as higher intakes of fruits, vegetables, whole grains, fish, and poultry. A suboptimal pattern will be characterized by higher intakes of processed food and red meats, refined grains, sweets, and desserts. We will treat dietary patterns both as continuous and categorical variables (quartiles).
Genotyping for APOL1
APOL1 genotyping is performed by the parent study. APOL1 G1 risk allele is defined by the presence of the G allele at both rs73885319 (S342G) and rs60910145 (I384M), and the G2 risk allele by the presence of a 6 base pair deletion (rs71785313).13 All participants are categorized into 2 groups according to their APOL1 genotyping results as follows: (i) APOL1 high-risk genotype are individuals with 2 APOL1 risk alleles (G1G1, G2G2, or G1G2) and (ii) APOL1 low-risk genotype are individuals with 0 or 1 APOL1 risk alleles (G1G0, G2G0, or G0G0).
Study Outcomes
Participants return for annual follow-up visits where blood is collected for measurement of serum creatinine and eGFR by the CKD-epidemiology collaboration equation 2009 equation without correction for Black race similar to the parent study.28, 33,34 We will perform sensitivity analyses with the CKD-epidemiology collaboration 2021 equation although its performance has not been validated in African populations.33,35 Primary time-to-event outcome is a 40% decrease in eGFR or a diagnosis of kidney failure. In exploratory analyses, we will also examine eGFR slope over time. For eGFR slope analyses, the choice of eGFR equation is not as critical.
Statistical Analyses
We will calculate the crude and adjusted event rates of CKD progression by quartiles of urinary sodium and potassium, and dietary patterns by estimating the survival function and through Cox proportional hazards models. We will compare the association between each predictor variable and the outcome using log-rank tests. For exploratory analyses of eGFR slope, we will employ a linear mixed model, which will include time varying covariates to indicate the eGFR of an individual at a certain time. In addition, we will adjust for each center by adding center dependent random effects to the models. Our multivariable adjustment strategy is based on classical epidemiologic principles and biological or clinical plausibility.36,37
We will test for gene-by-diet interactions by examining interaction terms (urinary sodium, potassium, or dietary patterns with APOL1 genotype) and stratifying by diet quartiles to assess effect estimates if significant interaction is found.
Statistical Power
A sample size of 1000 participants will provide 90% power at α 0.05 to detect a hazard ratio as low as approximately 1.21, for each standard deviation increase in the risk factor. For gene-by-diet interactions, assuming a 25% to 30%38 prevalence of APOL1 high-risk genotypes, a sample size of 1000 will provide 80% power at alpha 0.05 to detect a hazard ratio of approximately 2.2 in high dietary sodium and high-risk APOL1 genotype compared to those with high dietary sodium and low-risk APOL1 genotype.
Project Management
Our study team consisted of study coordinators, dieticians, investigators, a project manager in sub-Saharan Africa, a monitoring and evaluation unit, and a data analyst. The monitoring and evaluation team was essential to ensure that study procedures were uniform across sites. We had 5 in-person site initiation visits and 2 remote site initiation visits (because of the pandemic restrictions) before starting enrollment at the sites. Monitoring visits have continued throughout the course of the study.
Community Advisory Board
We convened an advisory board comprising key community members. The advisory board consisted of 5 professional community members who have extensive experience with community engagement in their respective vocations and are leaders in the community, and 2 medical professionals who were not investigators in the study. The entire board provides guidance on the sociopolitical and local challenges surrounding the research study and reviewed study procedures from the perspective of a potential participant. The advisory board was critical in ensuring the study did not pose an undue burden to participants. Meetings were held quarterly in person or via Zoom and are ongoing. The community advisory board members did not interact with participants but provided knowledge based on local norms and the study.
Barriers and Facilitators to Participant Engagement and Retention in the DCA Study
To elucidate barriers and facilitators to participant engagement and retention in cohort studies of this type in low-income and middle-income settings, we performed semistructured interviews and focus group discussions with our study team. We performed 2 focus group discussions each with 4 research coordinators or dieticians and 5 semistructured interviews with investigators. Discussions and interviews were recorded and transcribed by coauthors JC and MA and analyzed for emerging themes using content analysis.
Results
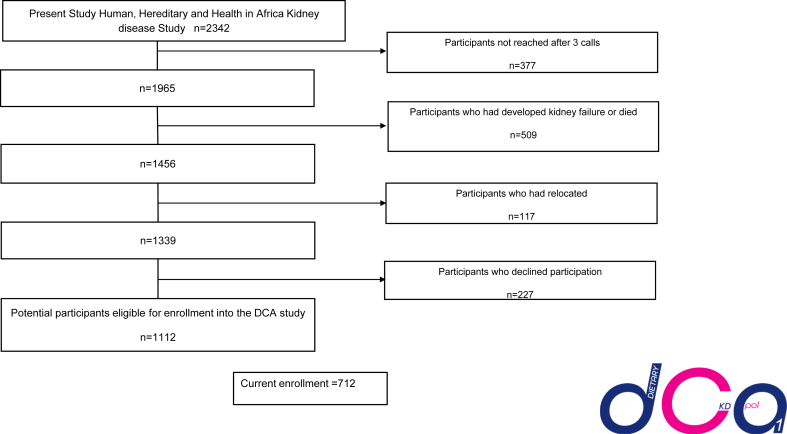
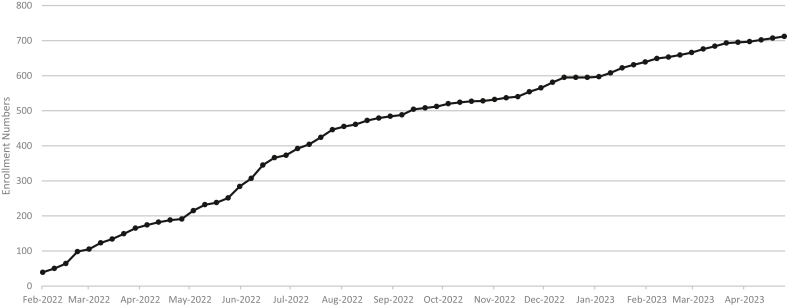
Screened participants in the DCA study using the participant management toolkit are shown in Figure 1. We screened 2342 participants from the parent study and were unable to reach 377 (16.1%) of participants. Of the screened participants, 227 (9.7%) declined participation. Another 413 (21.6%) were ineligible because of death or kidney failure after screening (n = 96), and some participants had relocated (n = 117). This left 1112 potential participants. Cumulative enrollment to date is 712 participants in the first 18 months of the study (Figure 2).
Figure 1.
Schematic diagram of Screening and Enrollment into the diet, chronic kidney disease and apolipoproteinL1 (DCA) Study in the first 18 months of enrollment. Schematic diagram showing the number of eligible participants in the parent human, hereditary and health in Africa kidney disease study and how we arrived at the current participants eligible for enrollment (N = 1112) of these, 712 have been enrolled into the study. DCA, Diet, CKD, and Apolipoprotein L1 study.
Figure 2.
Figure showing the enrollment for the diet, chronic kidney disease and apolipoprotein L1 (DCA) study from February 2021 to April 2022. As of April 2022, 712 individuals have been enrolled into the DCA study and completed 24-hour dietary recalls and 24-hour urine collections.
From 560 follow-up visits completed thus far, in the first year of follow-up, before the follow-up visit, 4.6% (n = 26) participants developed kidney failure requiring dialysis, 5.5% (n = 31), achieved a 40% decline in eGFR, and 7.5% (n = 42) died. Therefore, for the CKD progression outcome (40% decline in eGFR or incident kidney failure), there was a combined event rate of 9.1%; and for a composite event of CKD or death, the event rate is 14.8%.
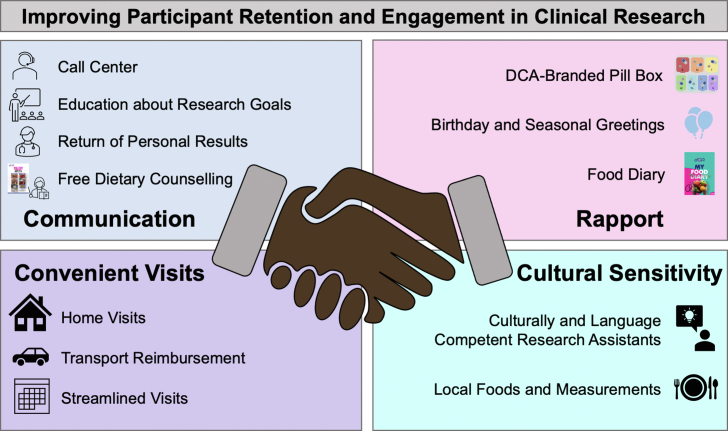
We employed several enrollment and retention strategies, utilizing a participant-centered approach (Figure 3). This included improving communication and rapport between participants and research staff, making research visits more convenient for participants, and prioritizing cultural sensitivity to the populations involved. In addition, there was constant feedback from the participants that necessitated adjustments to the study protocol for participant convenience.
Figure 3.
Various strategies employed for participant retention and engagement in the Diet, CKD and ApolipoproteinL1 (DCA) study. (a) Improved communication with participants. (b) Improved rapport between research staff and participants. (c) Making research visits more convenient for participants. (d) Prioritizing cultural sensitivity in dealing with populations. DCA, Diet, CKD, and Apolipoprotein L1 study.
Considerations made over the course of the study include, obtaining Institutional Review Board approval for revised transportation costs, and ensuring that transportation was appropriately adjusted to cover mileage. Most participants were unable to afford the cost of an additional clinic visit or the cost of losing a day of work and paying to come to the clinic. Thus, the protocol was revised to cover mileage to clinic rather than just a blanket reimbursement for patients. Cultural considerations and adaptability were crucial in carrying out the study. First, the coordinators had to be able to explain things in a culturally appropriate manner, translate food items to local dialect, and ensure privacy so that participants felt comfortable disclosing food amounts. In addition, planned study procedures to store urine in the fridge was not included in the protocol because of the cultural inappropriateness of placing waste products like urine in the refrigerator. Rather, we proposed that participants store urine in a cool place out of the sunshine or in a shaded room.
Although we did not collect data directly from the participants, we received feedback from study coordinators across all study sites, research personnel, and investigators consistently. The consistent feedback of the research personnel on the reception of the study by participants resulted in several recurrent issues which resulted in protocol changes. We found that the return of results was extremely important to the participants. They were more satisfied when they could take ownership of the results of the 24-hour urine tests or serum creatinine. Whenever this opportunity was missed, participants appeared less engaged and gave feedback to the study sites on how the lack of return of results made them less interested. Having the primary providers explain results to the participants was also key for the return of results. With genetic results, which will be available later in the studies, return of results may pose a challenge in low-resource regions in West Africa because of the dearth of genetic counselors.
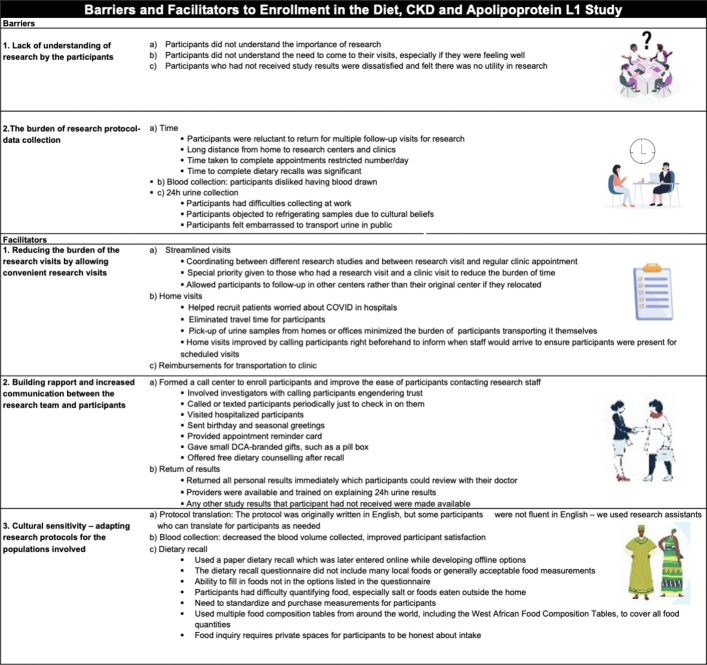
Based on the focus group discussions and semistructured interviews with the research personnel and investigators, several themes regarding the barriers and facilitators to clinical research emerged (Figure 4). Barriers to research included the following: (i) lack of understanding of research by the participants; (ii) the burden of research protocol-data collection, including transportation costs of getting to clinical centers, time spent on research visits, and the perceptions surrounding both blood and urine for participants; and (iii) challenges of incorporating local cultural and traditional nuances into the research protocol to be adaptable to the participants. Three themes emerged as facilitators to increasing participant retention and engagement in this study as follows: (i) reducing the burden of the research visits by allowing convenient research visits; (ii) building rapport and increased communication between the research team and participants; and (iii) cultural sensitivity – adapting research protocols for the populations involved (Figure 4). Specific quotes from research personnel and investigators from the focus group discussions are shown in Supplementary Table 1.
Figure 4.
Barriers and facilitators to enrollment and retention in the diet, and CKD and apolipoprotein L1 Study (themes from focus group discussion and key informant interviews). CKD, chronic kidney disease.
Discussion
The roles of genetics and environmental factors (e.g., diet, other comorbid conditions, poverty, etc.) in CKD have not been systematically investigated in sub-Saharan Africa. Identifying modifiable risk factors for CKD and its progression in sub-Saharan Africa would have substantial public health importance, because dialysis and transplantation are unaffordable for most individuals. Many sub-Saharan Africa countries lack national dialysis programs, and countries like Nigeria and Ghana are only able to provide dialysis for 2 to 8 persons per million population.39 Furthermore, even where available, dialysis care is marked by a huge financial burden, high rates of infrequent dialysis sessions, higher rates of mortality in the first 3 months from cardiovascular disease, and other complications of kidney failure.39,40
Each of the dietary risk factors investigated in this study – sodium, potassium, and dietary patterns–have interesting potential intersections relevant to APOL1 kidney disease. Mechanisms involved in APOL1 kidney toxicity are not fully known, but recent studies have implicated inflammation as a potent risk factor for enhancing APOL1 gene expression. Proinflammatory dietary patterns may therefore act synergistically to contribute to CKD by increasing APOL1 gene expression. Some dietary modifications are cheap and could be tested in interventional trials to see if the onset of chronic dialysis therapy or renal transplantation can be delayed.41
A main limitation of dietary assessment is the limited availability of food included in the West African Food Composition Table. For foods not in the West African Food Composition Table, our dietician analysts used the Nutrition Data System for Research, Food Central (U.S Department of Agriculture's food and nutrient data) and corresponding internet searches. For foods not in any of the existing databases, we found similar foods that could be used to help account for the food nutrients. We also reviewed specific foods not listed at consensus meetings held between dieticians in Nigeria, Ghana, and the US. For studies on diet, cultural awareness is critical to ensure that the nutrients are assessed correctl.
While conducting the study, we made some adjustments in the study protocol based on research personnel and participant feedback and to enable execution in the settings in West Africa (Figure 4). One of the initial modifications before the onset of the study was to increase the number of enrollment site clinical centers from 4 to 7 to ensure we meet enrollment target and to accommodate the high mortality rate of CKD in West Africa (See Figure 1). The number of eligible participants for the study drastically reduced because of death and/or dialysis. Some of these adjustments may have implications in interpreting results and may be useful for those embarking on future research in low-income and middle-income settings in Africa. First, we prioritized a participant-centered approach to address concerns raised by participants and the advisory board. For instance, participants expressed concerns about blood volume collected during phlebotomy. There was hesitation to give blood samples because many thought that it would worsen their anemia, and many participants in the study sites in Africa were not comfortable with the concept of blood collection for research or clinical tests. Therefore, we reduced the total volume of blood collected during year 1 study visits from 15 ml to 9 ml; for yearly follow-up visits, the blood volume was further reduced from 6 ml to 3 ml. This change was done in direct response to participant feedback and should not impact the number of analyses planned but will limit the storage volume for future analyses on stored biospecimens. We also ensured synchronized follow-up visits with the parent study and regular appointments to reduce participant burden, and scheduling was made at the participant’s convenience.
The DCA study experienced various challenges, including the COVID-19 pandemic. However, with a focus on a participant-centered approach, we mitigated some of the challenges to enrollment and retention. Findings from this study may identify additional gene-by-diet interactions in CKD and could provide important scientific information on the interrelationships between dietary factors, APOL1 genotype, and clinical outcomes.42
Disclosure
TI, BO, DA, EK, FA, RB, UO, TA, and YR receive funds from National Institute of Diabetes and Digestive and Kidney Diseases. AS, MM, and TU receive funds from National Institute of Diabetes and Digestive and Kidney Diseases and Human, Heredity and Health in Africa (H3Africa); Cohort and renewal studies. EE received funds for renal nutrition from Apetamin, Astymin, Astyfer, Neutrosec and Hapenz and consults for Sahel Health, Africa.
Acknowledgments
We would like to thank Grace Mfon, Morenikeji Akinpelu, Jillian Wilson, Motunrayo Sholarin, Ajibola Olokode, Olaitan Bobade, Rosemary Mamudu, Portia Antwi, Daniel Ntow, Tunji Akobi, Ijeoma Nnoli, Chinyere Okwara, and the entire H3Africa kidney disease study Network for their invaluable contribution to this project. We would like to thank Jillian Wilson for her invaluable contribution to this work. We would also like to thank members of the ∗community advisory board for their efforts and contributions to this project. We would also like to acknowledge all the participants who participated in the DCA study and provided feedback to the study team. We would like to thank the community advisory board members for their input in this this study. Finally, we would like to acknowledge our funders, the NIDDK and Boston University School of Medicine.
Funding
TI is funded by the National Institute of Diabetes and Digestive and Kidney Diseases K23DK119542 and the Department of Medicine, Boston Medical Center.
Footnotes
Table S1. Quotes indicating barriers and facilitators to participant engagement in the diet, CKD and apolipoprotein L1 study from qualitative studies.
Supplementary Material
Table S1. Quotes indicating barriers and facilitators to participant engagement in the diet, CKD and apolipoprotein L1 study from qualitative studies.
References
- 1.Rebholz C.M., Crews D.C., Grams M.E., et al. DASH (dietary approaches to stop hypertension) diet and risk of subsequent kidney disease. Am J Kid Dis. 2016;68:853–861. doi: 10.1053/j.ajkd.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutierrez O.M. Contextual poverty, nutrition, and chronic kidney disease. Adv Chronic Kidney Dis. 2015;22:31–38. doi: 10.1053/j.ackd.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mun K.H., Yu G.I., Choi B.Y., et al. Association of dietary potassium intake with the development of chronic kidney disease and renal function in patients with mildly decreased kidney function: the Korean multi-rural communities cohort study. Medi Sci Monit. 2019;25:1061–1070. doi: 10.12659/MSM.913504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crews D.C., Kuczmarski M.F., Miller E.R., 3rd, et al. Dietary habits, poverty, and chronic kidney disease in an urban population. J Renl Nutr. 2015;25:103–110. doi: 10.1053/j.jrn.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Udler M.S., Nadkarni G.N., Belbin G., et al. Effect of genetic African ancestry on eGFR and kidney disease. J Am Soc Nephrol. 2015;26:1682–1692. doi: 10.1681/ASN.2014050474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kieneker L.M., Bakker S.J., de Boer R.A., et al. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease. Kid Int. 2016;90:888–896. doi: 10.1016/j.kint.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 7.He J., Mills K.T., Appel L.J., et al. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol. 2016;27:1202–1212. doi: 10.1681/ASN.2015010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araki S., Haneda M., Koya D., et al. Urinary potassium excretion and renal and cardiovascular complications in patients with Type 2 diabetes and normal renal function. Clin J Am Soc Nephrol CJASN. 2015;10:2152–2158. doi: 10.2215/CJN.00980115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogata S., Akashi Y., Sakusabe T., et al. A multiple 24-hour urine collection study indicates that kidney function decline is related to urinary sodium and potassium excretion in patients with chronic kidney disease. Kidney Int. 2022;101:164–173. doi: 10.1016/j.kint.2021.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Bach K.E., Kelly J.T., Palmer S.C., et al. Healthy dietary patterns and incidence of CKD: a meta-analysis of cohort studies. Clin J Am Soc Nephrol. 2019;14:1441–1449. doi: 10.2215/CJN.00530119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee T., Crews D.C., Tuot D.S., et al. Poor accordance to a DASH dietary pattern is associated with higher risk of ESRD among adults with moderate chronic kidney disease and hypertension. Kid Int. 2019;95:1433–1442. doi: 10.1016/j.kint.2018.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ojo A. Addressing the global burden of chronic kidney disease through clinical and translational research. Trans Am Clin Climatol Assoc. 2014;125:229–243. [PMC free article] [PubMed] [Google Scholar]
- 13.Genovese G., Friedman D.J., Ross M.D., et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsa A., Kao W.H., Xie D., et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaze A.D., Ilori T., Jaar B.G., Echouffo-Tcheugui J.B. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol. 2018;19:125. doi: 10.1186/s12882-018-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulasi, Tzur S., Wasser W.G., et al. High population frequencies of APOL1 risk variants are associated with increased prevalence of non-diabetic chronic kidney disease in the Igbo people from south-eastern Nigeria. Nephron Clin Pract. 2013;123:123–128. doi: 10.1159/000353223. [DOI] [PubMed] [Google Scholar]
- 17.Limou S., Nelson G.W., Kopp J.B., Winkler C.A. APOL1 kidney risk alleles: population genetics and disease associations. Adv Chronic Kidney Dis. 2014;21:426–433. doi: 10.1053/j.ackd.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behar D.M., Kedem E., Rosset S., et al. Absence of APOL1 risk variants protects against HIV-associated nephropathy in the Ethiopian population. Am J Nephrol. 2011;34:452–459. doi: 10.1159/000332378. [DOI] [PubMed] [Google Scholar]
- 19.Gutiérrez O.M., Limou S., Lin F., et al. APOL1 nephropathy risk variants do not associate with subclinical atherosclerosis or left ventricular mass in middle-aged black adults. Kidney Int. 2018;93:727–732. doi: 10.1016/j.kint.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols B., Jog P., Lee J.H., et al. Innate immunity pathways regulate the nephropathy gene apolipoprotein L1. Kidney Int. 2015;87:332–342. doi: 10.1038/ki.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tonelli M., Sacks F., Pfeffer M., et al. Biomarkers of inflammation and progression of chronic kidney disease. Kid Int. 2005;68:237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 22.Nettleton J.A., Steffen L.M., Mayer-Davis E.J., et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2006;83:1369–1379. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osafo C., Raji Y.R., Burke D., et al. Human heredity and health (H3) in Africa Kidney Disease Research Network: a focus on methods in sub-Saharan Africa. Clin J Am Soc Nephrol. 2015;10:2279–2287. doi: 10.2215/CJN.11951214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.How REDCap is being used in response to COVID-19. REDCap. https://www.project-redcap.org Accessed January 3, 2023.
- 25.Cobb L.K., Anderson C.A.M., Elliott P., et al. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes a science advisory from the American Heart Association. Circulation. 2014;129:1173–1186. doi: 10.1161/CIR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 26.Stadlmayr B., Charrondière U.R., Burlingame B. Development of a regional food composition table for West Africa. Food Chem. 2013;140:443–446. doi: 10.1016/j.foodchem.2012.09.107. [DOI] [PubMed] [Google Scholar]
- 27.Mills K.T., Chen J., Yang W., et al. Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA. 2016;315:2200–2210. doi: 10.1001/jama.2016.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kestenbaum B., Ix J.H., Gansevoort R., et al. Population-based limits of urine creatinine excretion. Kidney Int Rep. 2022;7:2474–2483. doi: 10.1016/j.ekir.2022.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers G.L., Miller W.G., Coresh J., et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 31.Osborne J.W., Fitzpatrick D.C. Replication analysis in exploratory factor analysis: what it is and why it makes your analysis better. Pract Assess Res Eval. 2012;17:1–8. [Google Scholar]
- 32.Hu F.B. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Bukabau J.B., Yayo E., Gnionsahé A., et al. Performance of creatinine- or cystatin C-based equations to estimate glomerular filtration rate in sub-Saharan African populations. Kid Int. 2019;95:1181–1189. doi: 10.1016/j.kint.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 34.Eastwood J.B., Kerry S.M., Plange-Rhule J., et al. Assessment of GFR by four methods in adults in Ashanti, Ghana: the need for an eGFR equation for lean African populations. Nephrol Dial Transplant. 2010;25:2178–2187. doi: 10.1093/ndt/gfp765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luque-Fernandez M.A., Schomaker M., Redondo-Sanchez D., et al. Educational Note: paradoxical collider effect in the analysis of non-communicable disease epidemiological data: a reproducible illustration and web application. Int J Epidemiol. 2019;48:640–653. doi: 10.1093/ije/dyy275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang W., Xie D., Anderson A.H., et al. Association of kidney disease outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kid Dis. 2014;63:236–243. doi: 10.1053/j.ajkd.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kopp J.B., Nelson G.W., Sampath K., et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barsoum R.S., Khalil S.S., Arogundade F.A. Fifty years of dialysis in Africa: challenges and progress. Am J Kid Dis. 2015;65:502–512. doi: 10.1053/j.ajkd.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Ashuntantang G., Osafo C., Olowu W.A., et al. Outcomes in adults and children with end-stage kidney disease requiring dialysis in sub-Saharan Africa: a systematic review. Lancet Glob Health. 2017;5:e408–e417. doi: 10.1016/S2214-109X(17)30057-8. [DOI] [PubMed] [Google Scholar]
- 41.Kalantar-Zadeh K., Moore L.W., Tortorici A.R., et al. North American experience with Low protein diet for Non-dialysis-dependent chronic kidney disease. BMC Nephrol. 2016;17:90. doi: 10.1186/s12882-016-0304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilori T.O., Liu J., Rodan A.R., et al. Apolipoprotein L1 genotypes and the association of urinary potassium excretion with CKD progression. Clin J Am Soc Nephrol. 2022;17:1477–1486. doi: 10.2215/CJN.02680322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.