Abstract
Introduction
Complement plays an important role in the pathogenesis of glomerulonephritis (GN). Even though the underlying etiology of GN might be different, complement activation with subsequent glomerular deposition of complement proteins result in glomerular injury and progression of the lesions. Routine immunofluorescence microscopy (IF) includes staining for only complement factors C3c and C1q. Therefore, with regard to evaluation of the complement pathways, routine kidney biopsy provides only limited information.
Methods
In this study, using laser microdissection of glomeruli followed by mass spectrometry, complement proteins and pathways involved in GN were analyzed.
Results
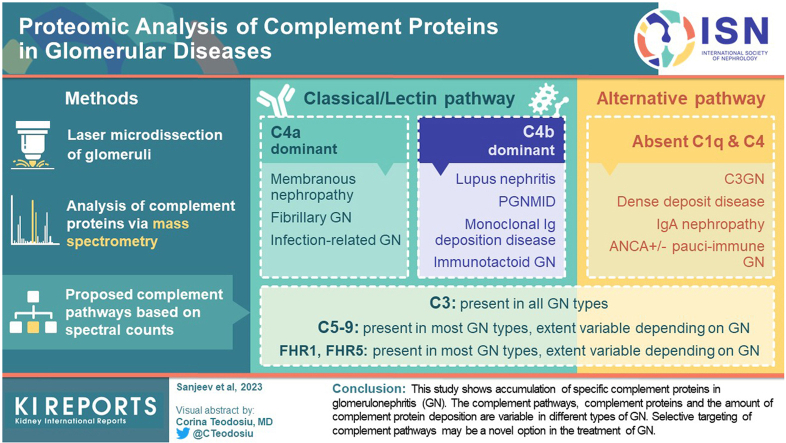
We found that C3 followed by C9 are the most abundant complement proteins in GN, indicating activation of classical or lectin or alternative, and terminal pathways, either exclusively or in a combination of pathways. Furthermore, depending on the type of GN, C4A and/or C4B were also present. Therefore, membranous nephropathy (MN), fibrillary GN, and infection-related GN showed C4A dominant pathways, whereas lupus nephritis (LN), proliferative GN with monoclonal Ig deposits, monoclonal Ig deposition disease (MIDD), and immunotactoid glomerulopathy showed C4B dominant pathways. Significant deposition of complement regulatory proteins, factor H-related protein-1 (FHR-1) and factor H-related protein-5 (FHR-5), were also detected in most GN.
Conclusions
This study shows accumulation of specific complement proteins in GN. The complement pathways, complement proteins, and the amount of complement protein deposition are variable in different types of GN. Selective targeting of complement pathways may be a novel option in the treatment of GN.
Keywords: complement, glomerulonephritis, kidney, laser microdissection, mass spectrometry
Graphical abstract
GN results from glomerular injury because of varying etiologies. GN is now classified based on the etiology and pathogenesis instead of the traditional pattern-based classification. Therefore, GN is broadly classified into immune-complex GN, antiglomerular basement membrane GN, antineutrophil cytoplasmic antibody (ANCA) GN, C3 glomerulopathy, and monoclonal immunoglobulin-associated renal diseases.1 Although, the underlying etiology and pathogenesis is different, the glomerular injury and progression of the lesions in these heterogeneous groups of diseases may result from activation of the complement pathways. A kidney biopsy is required to confirm the diagnosis of a specific GN. Routine IF includes staining for only complement factors C3c and C1q. Therefore, with regard to evaluation of the complement pathways routine, kidney biopsy provides only limited information.
In this study, complement proteins and pathways involved in GN were analyzed using laser microdissection of glomeruli followed by mass spectrometry (MS/MS). Minimal change disease and MN, as well as classical forms of noninflammatory glomerular diseases were also analyzed for comparison. The complement findings on routine biopsy IF was compared with that detected on MS/MS.
Methods
Laser Microdissection and MS/MS
Kidney biopsy tissue from formalin fixed paraffin embedded material (FFPE) was used for MS/MS studies. The kidney biopsy tissue was sent to the renal pathology laboratory at the Mayo Clinic, Rochester, Minnesota, for diagnosis and interpretation. The study was approved by Mayo Clinic Institutional Review Board. An established method was utilized to analyze the proteomic content of the glomeruli in the FFPE tissue biopsies.2 In brief, 6μm-thick sections of FFPE tissue were mounted on Director slides (Expression Pathology), deparaffinized and stained with hematoxylin and eosin. Nonsclerotic glomeruli were identified using polarized light and resected with laser microdissection using a Leica LMD6000 laser-capture microscope. Multiple (2–3) independent microdissections were performed for each case and each microdissection was configured to capture an area of 125,000 μm2. FFPE fragments from each microdissection were collected in a cap containing a Tris-EDTA Zwittergent cell lysis buffer and analyzed individually. Proteins were extracted from the FFPE fragments using heat and denatured via sonication. Extracted proteins were digested overnight using trypsin (Promega, Madison WI) and the resulting peptide mixture was analyzed on a QExactive-Plus mass spectrometer (Thermo-Fisher, Bremen, Germany) coupled to a Dionex Ultimate 3000 RSLC nano-flow high performance liquid chromatography system. The resulting MS/MS spectra were analyzed using Mascot and X!Tandem. All search engines derived tryptic peptides from a reference database containing sequences for SwissProt canonical human proteins and common contaminants. Reversed protein sequence entries were appended to the database for estimating the protein and peptide false discovery rates. Resulting peptide identifications were combined and filtered using Scaffold software (Proteome Software Inc., Portland, OR) configured to treat raw files from replicate microdissections as biological replicates. Proteins with at least single confident unique peptide identification (probability > 0.9) and more than 5 spectral matches were considered for clinical interpretation. The cases that were used for this study served as controls for amyloid detection and discovery of DNAJB9 in fibrillary GN.3, 4, 5 The proteomic data on these cases were then analyzed for complement pathways for this study. The controls included greater than 100 cases with glomerular disease. Complement deposition based on total spectral counts were graded as very high (>50, ++++), high (16–50, +++), moderate (6–15, ++), low (2–5, +), and baseline (0–1).
Results
MS/MS of Complement Proteins in GN (Table 1)
Immune-Complex GN
Infection (Bacterial)-Related GN
Infection-related GN is characterized by diffuse proliferative GN that occurs in the setting of an infection. Infection-related GN may result from viral, bacterial, and fungal infections.6, 7, 8 Bacterial infections include Gram-positive bacterial such as Streptococcus and Staphylococcus, and Gram-negative bacteria such as Escherichia coli, Yersinia, Pseudomonas, and Hemophilus.
Kidney biopsy findings: IF microscopy in bacterial-related GN is dominated by bright capillary wall staining for C3. C1q may or may not be present.7
Proteomics: MS/MS of 4 cases (case 6 to 9) showed high spectral counts of C3 (41.8 ± 7.4), moderate spectral counts of C9 (6.75 ± 2.0), and low spectral counts for C4A (2.25 ± 1.7), C6, and C7. Complement regulatory proteins included low spectral counts of FHR-1 and FHR-5 (Figure 1a).
Figure 1.
Proteomic Identification of complement proteins in glomerular diseases: (a) Infection related GN. (b) Lupus nephritis. (c) IgA nephropathy. (d) Fibrillary glomerulonephritis. (e) Pauci-immune ANCA-negative and ANCA-positive (PR3 and MPO) GN. (f) C3 glomerulonephritis (C3GN). (g) Dense deposit disease (DDD). (h) Proliferative glomerulonephritis with monoclonal Ig deposits (PGNMID). (i) Immunotactoid glomerulopathy. (j) Heavy chain immunoglobulin deposition disease (HCDD). (k) Membranous nephropathy. Numbers in green boxes represent spectral counts of MS/MS matches to a respective protein. Protein grouping ambiguity (red star) indicates shared amino acid sequences for certain proteins.
Conclusion: significant complement activation is present in infection (bacterial)-related GN from C3- and C4A- based pathways. Terminal pathway complement proteins and regulatory proteins of the complement pathway are also present.
LN
LN is considered a classic immune-complex mediated GN and is defined by the presence of both immunoglobulins and complement factors. LN is classified into class I to VI based on light microscopy features.9,10
Kidney biopsy findings: IF in LN is characterized by staining for C3 and C1q. The intensity of staining is variable with C3 staining in general being brighter than C1q. The pattern of staining depends on the class (mesangial in class I and II, and capillary wall in class III, IV, and V).
Proteomics: MS/MS of 5 cases (case 40 to 44) showed high spectral counts of C3 (43.4 ± 44.9) in all cases. The spectral counts varied from 16 to 123. The variability may be explained by higher spectral counts in class IV diffuse proliferative lesions (case 40, 41, 43) versus class III or class IV with segmental lesions (case 42, 44). High spectral counts of C4B (22 ± 25.2) were detected in all cases, whereas 1 case showed both C4A and C4B. High C1q spectral counts were detected in 1 case and moderate to low counts were detected in 3 cases. Moderate spectral counts of C9 (8.8 ± 10.8) were detected in all 5 cases, whereas C6 was present in 4 cases. Low to moderate spectral counts FHR-1 and FHR-5 were detected in 3 cases (Figure 1b).
Conclusion: significant complement activation is present in LN from C3-based and C4B-based pathways. Terminal pathway complement proteins and regulatory proteins of the complement pathway are also present.
IgA Nephropathy
IgA nephropathy is a common glomerular disease that is characterized typically by mesangial proliferation resulting from mesangial deposits that stain for IgA.11 Endocapillary hypercellularity and crescents may be present.
Kidney biopsy findings: IF in IgA nephropathy shows variable staining for C3 (trace to 3+). C1q is typically absent.
Proteomics: MS/MS of 4 cases (case 84 to 87) showed high spectral counts of C3 (28.3 ± 10.9) and moderate spectral counts of C9 (6.5 ± 4.5) in all cases. Moderate spectral counts FHR-1 (17 ± 12.2) were detected in 3 cases (Figure 1c).
Conclusion: significant complement activation is present in IgA nephropathy from C3 pathways. The absence of any significant C4 and C1 suggests activation of the alternative pathway of complement. Terminal pathway complement proteins and regulatory proteins of the complement pathway are also present.
Fibrillary GN
Fibrillary GN is a rare glomerular disease that is characterized by proliferative GN resulting from mesangial and capillary wall fibrillary deposits that stain for IgG.12 The fibrillary deposits are typically negative on Congo red staining but are positive for DNAJB9.5
Kidney biopsy findings: IF in fibrillary GN shows bright staining for C3. C1q (trace to 1+) may be present.12
Proteomics: MS/MS of 5 cases (Case 24 to 28) showed very high spectral counts of C3 (72 ± 14.5) in all cases. High spectral counts of C4A (29.4± 7.7) were also present in all 5 cases and 2 cases also showed C4B. Moderate spectral counts of C9 (13.4 ± 6.1) were detected in all cases. High spectral counts of FHR-1 (23.0 ± 13.8) and FHR-5 (20.4 ± 15.7) were present in all cases (Figure 1d).
Conclusion: significant complement activation is present in fibrillary GN from C3-based and C4A-based pathways. Terminal pathway complement proteins and regulatory proteins of the complement pathway are also present.
Pauci-Immune ANCA-Associated GN and ANCA-Negative GN
ANCA-associated GN is characterized by a pauci-immune necrotizing and crescentic GN and comprises of 3 heterogeneous multisystem disorders as follows: (i) granulomatosis with polyangiitis, (ii) microscopic polyangiitis, and (iii) eosinophilic granulomatosis with polyangiitis.13 The autoantigens proteinase 3 (PR3) or myeloperoxidase (MPO) are present in most ANCA-associated GN. One-third of the pauci-immune necrotizing and crescentic GN are negative for MPO and PR3-ANCA and are referred to as ANCA-negative pauci-immune GN. Complement activation plays an important role in the pathophysiology ANCA vasculitis,14 although it remains unclear which pathway may be activated.15
Kidney biopsy findings: IF in ANCA-GN often shows coarse segmental C3 in the glomeruli involved by the necrotizing and crescentic lesions. The uninvolved glomeruli may show minimal or no C3 (0-1+), C1q is absent.15
Proteomics: MS/MS of 5 cases of ANCA-negative GN (70–74) showed moderate to high spectral counts of C3 (15.8 ± 8.2), whereas both MPO-associated and PR3-associated GN (MPO-associated case 75 to 78, PR3-associated case 79 to 83) showed much lower spectral counts of C3 (3.5 ± 1.9 and 3.4 ± 4.9, respectively). Similarly, moderate spectral counts of C9 (11.2 ± 7.3) were present in ANCA-negative GN compared to MPO-associated (0.5 ± 1) and PR3-associated GN (0.6 ± 1.3). C4 was minimal or absent in ANCA-negative GN. Moderate spectral counts of FHR-1 (8.4 ± 5.6) and low spectral counts of FHR-5 (3.4 ± 3.4) were present in ANCA-negative GN and they were essentially absent in ANCA-associated GN (Figure 1e).
Conclusion: glomeruli that are not involved by necrotizing and crescentic lesions were dissected. In these glomeruli, significant complement activation is present in pauci-immune ANCA-negative GN from C3-based pathways. Terminal pathway complement proteins and regulatory proteins of the complement pathway are also present. Glomeruli without crescents show lesser complement activation in MPO/PR3-associated GN compared to ANCA-negative pauci-immune GN. On the other hand, glomeruli with crescents and necrotizing lesions in MPO/PR3-associated GN show large spectral counts of C3 and C5b-9 (data not shown).16
C3 Glomerulopathy
C3 glomerulopathy is characterized by bright C3 staining on IF along with the absence or minimal Ig staining. It can be further classified into C3 GN (C3GN), and dense deposit disease (DDD) based on ultrastructural findings.
Kidney biopsy findings: IF in C3GN and DDD shows bright mesangial and capillary wall staining for C3. C1q is typically absent.17
Proteomics: MS/MS of 9 cases (case 10 to 18) of C3GN showed very high spectral counts of C3 (129 ± 92.8) and high spectral counts of C9 (24.5 ± 37.1) in all cases. Moderate spectral counts of C6, C7, and C8 were also present. High spectral counts of FHR-1 (23.0 ± 13.8) and FHR-5 (20.4 ± 15.7) were detected (Figure 1f).
MS/MS of 5 cases (case 19 to 23) of DDD showed very high spectral counts of C3 (75.8 ± 51.9) and C9 (45.6 ± 23.7) in all cases. Moderate spectral counts of C6, C7, and C8 were also present. High spectral counts of FHR-1 (16.2± 23.4) and moderate spectral counts of FHR-5 (11.8 ± 9.75) were detected (Figure 1g).
Conclusions: both C3GN and DDD show significant complement activation through C3-based pathways. Terminal pathway complement proteins and regulatory proteins of the complement pathway are also present. However, although both C3GN and DDD show high spectral counts of C9, the counts appear higher in DDD compared to C3GN.
Monoclonal Immunoglobulin-Associated Glomerular Disease
Deposition of monoclonal immunoglobulins (MIg) can result in diseases involving the glomeruli, interstitium, and vessels.18 Larger molecular weight MIg molecules consisting of heavy and light chain are unlikely to pass the glomerular filtration barrier resulting in glomerular deposition of the MIg with ensuing glomerular inflammation. These include proliferative GN with monoclonal Ig deposition and immunotactoid glomerulopathy. In addition, MIg that interact with other proteins such as matrix proteins can result in glomerular disease such MIDD.
Proliferative GN With Monoclonal Ig Deposition (PGNMID)
The kidney biopsy is characterized by a proliferative GN with glomerular MIg deposits on IF studies and granular (nonorganized) electron dense deposits by electron microscopy. The most common MIg is IgG, and the most common IgG subtype is IgG3.19,20
Kidney biopsy findings: IF in PGNMID shows bright glomerular capillary wall staining C3 (2-3+) and mild staining for C1q (1–2+, in 64% of cases).
Proteomics: MS/MS of 5 cases of PGNMID (Case 65 to 69) showed very high spectral counts of C3 (63.8 ± 38.0), high spectral counts of C4B (24.8 ± 17.2), and moderate spectral counts of C4A (11.8 ± 12.8) and C9 (9.0 ± 9.9). FHR-1 (14.4 ± 18.0) and FHR-5 (8.2 ± 10.2) were present in all cases (Figure 1h).
Conclusion: significant complement activation is present in PGNMID from C3-based and C4B-based pathways. Terminal pathway complement proteins and regulatory proteins of the complement pathway are also present.
Immunotactoid Glomerulopathy
Immunotactoid glomerulopathy is a very rare glomerular disease that is characterized by the deposition of Congo red-negative organized microtubular glomerular deposits that stain for monotypic IgG. Most immunotactoid GN are associated with a circulating MIg and an underlying hematologic malignancy.21
Kidney biopsy findings: IF in immunotactoid glomerulopathy shows bright glomerular capillary wall staining C3 (2–3+) and mild staining for C1q (1–2+, in 63% of cases).
Proteomics: MS/MS of 2 cases of immunotactoid glomerulopathy (case 38 and 39) showed very high spectral counts of C3 (107.0 ± 26.8) and C4B (51.5 ± 21.9) and moderate spectral counts of C9 (7.0 ± 5.6). One case showed high spectral counts of C4A in addition to C4B. Variable moderate to high spectral counts of FHR-1 (25.5 ± 26.0) and FHR-5 (15 ± 9.8) were present in both cases (Figure 1i).
Conclusion: significant complement activation is present in immunotactoid from C3-based and C4B-based pathways. Terminal pathway complement proteins and regulatory proteins of the complement pathway are also present.
MIDD
MIDD is a renal disease that is characterized by the deposition of Congo red-negative punctate mesangial, glomerular, and tubular basement membrane deposits that stain for monoclonal light chains, monoclonal heavy chains, and light and heavy chains. Most MIDD are associated with a circulating MIg and an underlying hematologic malignancy.22
Kidney biopsy findings: IF in MIDD showed C3 (2–3+) staining in only 6 of 64 cases and weak staining for C1q in only 3 patients.
Proteomics: MS/MS of 4 cases of monoclonal Ig heavy chain deposition disease (case 29, 30, 33, and 39) showed very high spectral counts of C3 (73.0 ± 40.2), C4B (67.8 ± 47.2), and high spectral counts of C9 (18.3 ± 10.1). Moderate spectral counts of FHR-1 (13.8 ± 15.3) and FHR-5 (8.6 ± 9.8) were present in all cases (Figure 1j).
Conclusion: significant complement activation is present in MIDD from C3-based and C4B-based pathways. Terminal pathway complement proteins and regulatory proteins of the complement pathway are also present.
MS/MS of Complement Proteins in Noninflammatory Glomerular Diseases
Minimal Change Disease
Minimal change disease is a common cause of nephrotic syndrome and is seen in children and accounts for 20% of cases in adults.
Kidney biopsy findings: IF is commonly negative for all immunoglobulins and complement factors. Trace mesangial C3 stain may occasionally be present.
Proteomics: MS/MS from 3 cases of minimal change did not show any significant complement factor spectral counts.
Conclusion: Although minimal change disease results from podocyte injury because of circulating or permeability factors, the kidney biopsy and MS/MS findings suggest that complement deposition does not play a significant role in the pathophysiology of minimal change disease.
MN
MN is the most common cause of nephrotic syndrome in the adult Caucasian population. MN is characterized by bright granular staining for IgG and C3 along the capillary walls. MN is usually divided into primary MN, of which 60% to 70% are phospholipase A2 receptor (PLA2R)-positive; and secondary MN, of which 30% are exostosin 1 (EXT1/EXT2) positive and are seen in patients with autoimmune disease.23
Kidney biopsy findings: IF in primary PLA2R-positive MN shows bright capillary wall staining for C3 in primary MN; C1q is typically negative.
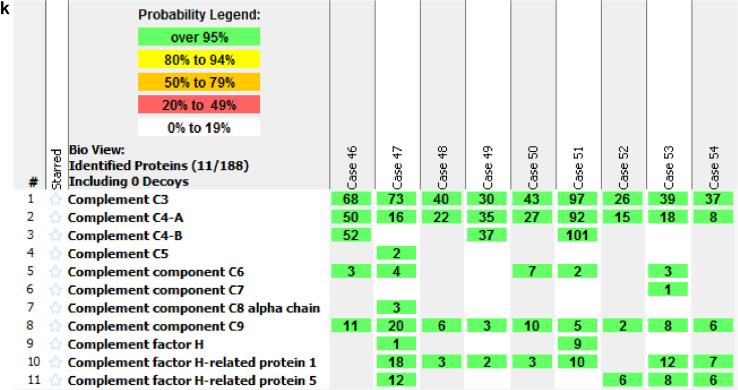
Proteomics: MS/MS of 9 cases of MN (Case 46 to 54) showed very high spectral counts of C3 (50.3 ± 23.6), and high spectral counts of C4A (31.4 ± 25.9), and moderate spectral counts of C9 (7.8 ± 5.4). Moderate spectral counts of FHR-1 (6.1 ± 6.1) and low spectral counts of FHR-5 (3.5 ± 4.5) were also detected (Figure 1k).
On the other hand, using a different methodology, MS/MS showed high spectral counts of C3, C4, C5, C6, C7, C8, and C9, in PLA2R-associated and EXT1/EXT2-associated MN. In addition, low spectral counts of C1 were present in EXT1/EXT2-positive MN. Complement regulatory factors included high spectral counts of complement factor H, FHR-1, FHR-5, clusterin, and vitronectin, with lower spectral counts of factor H-related protein-3, factor H-related protein-4, and CD59. Low spectral counts of factor B and properdin, key components of the alternative pathway were also detected (data not shown).24
Conclusion: significant complement activation is present in MN as evidenced by large spectral counts of complement proteins from C3-based and C4A-based pathways, including regulatory proteins of the complement pathways.
Discussion
Complement pathways play an important role in triggering, maintaining, and in the resolving phase of GN.25 It is likely that accumulation of large amounts activated complement proteins play an even greater role in pathogenesis of GN than the original inciting trigger such as immunoglobulins (e.g., in immune-complex GN and monoclonal Ig-associated renal diseases), which may not be cumulative because the inciting injury may be limited (e.g., infection-related GN). Unlike the original injury, the complement pathway is a process driven by amplification and thus can result in glomerular accumulation of large amounts of complement proteins. A systematic study of complement proteins and pathways can therefore shed important information of the pathogenesis of the various types of GN. Unfortunately, whereas the routine kidney biopsy is typically geared toward diagnosis and determining the underlying etiology of GN, it falls short in assessing extent and type of complement proteins involved in various types of GN. It is of paramount importance that we study the complement system in detail in all forms of GN. This information is particularly valuable because newer drugs targeting specific pathways of complement are likely to play an important role in treating GN.
In this manuscript, we studied the complement proteins using laser microdissection and mass spectrometry (MS/MS) in GN. MS/MS allows for a semiquantitative analysis of proteins analyzed. We found that C3 was the most dominant complement protein present in GN (Table 1). This is easily explained because not only is C3 the converging point of all complement pathways but it is also involved in the amplification loop. As expected, the highest spectral counts of C3 were present in C3GN and DDD. However, interestingly, very high spectral counts for C3 were also present in GN associated with monoclonal immunoglobulin-associated renal lesions and MN. High spectral counts of C3 were also present in immune-complex mediated GN, although it was less than that seen in C3GN or monoclonal immunoglobulin-associated renal lesions, keeping in mind that the immune-complex mediated GN is a heterogenous group of diseases.
Table 1.
Complement proteins in glomerulonephritis
| Complement proteins | IRG | LN | IgAN | FG | ANCA + GN | ANCA - GN | C3GN | DDD | PGNMID | ITG | MIDD | MN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C3 | +++ | +++ | +++ | ++++ | + | +++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| C4A | + | − | − | +++ | +/− | +/− | − | ++ | − | +++ | ||
| C4B | − | +++ | − | +++ | +++ | ++++ | − | |||||
| C9 | ++ | ++ | ++ | ++ | − | − | +++ | +++ | ++ | ++ | +++ | ++ |
| FHR-1 | + | ++ | +++ | +++ | − | − | +++ | +++ | ++ | +++ | ++ | ++ |
| FHR-5 | + | ++ | +/− | +++ | − | − | +++ | ++ | ++ | ++ | ++ | + |
ANCA, anti-neutrophil cytoplasmic antibodies; DDD, dense deposit disease; FG, fibrillary glomerulonephritis; GN, glomerulonephritis; IRG, infection-related GN; IgAN, IgA nephropathy; ITG, immunotactoid glomerulopathy; LN, lupus nephritis; MIDD, monoclonal immunoglobulin deposition disease; MN, membranous nephropathy; PGNMID, proliferative GN with monoclonal immunoglobulin deposits.
+ to ++++: −negative or baseline (0–2), + (low) spectral counts between 2–5, ++ (moderate) spectral counts between 6–15, +++ (high) spectral counts between 16–50, ++++ (very high) spectral counts over 50; ANCA + and ANCA- are pauci-immune crescentic GN with positive and negative ANCA titers, respectively.
C4 was also detected in most Ig or immune complex-associated and monoclonal Ig-associated renal diseases. C4B was the dominant C4 protein in monoclonal Ig-associated renal diseases and LN whereas C4A proteins were detected in MN, fibrillary GN, and infection-related GN (Figure 2). The C4A and C4B isotypes are defined by 4 specific amino acid residues at position 1101, 1102, 1105, and 1106 encoded by exon 26 of C4 gene.26 C4A is thought to be important in solubilization, immunoclearance, and opsonization of immune complexes whereas C4B is important for the propagation of classical and lectin pathways.27,28 The significance of dominant C4A versus C4B and vice-versa in GN is not known and needs further investigation. It is worth pointing out that the identification of C4A and C4B by MS/MS is made difficult by the small differences in the amino acids sequence of C4A and C4B.
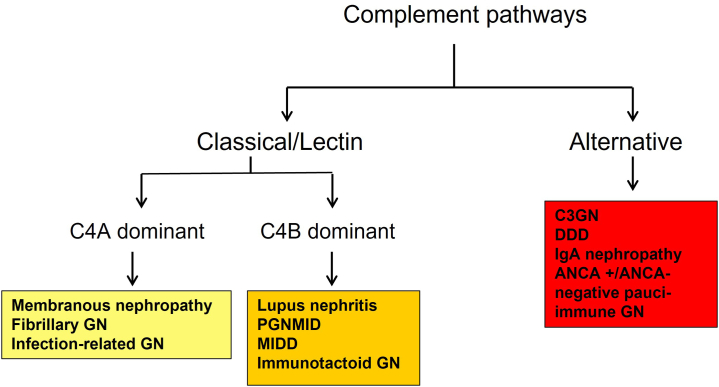
Figure 2.
Proposed complement pathways in glomerulonephritis. ANCA, antineutrophil cytoplasmic antibody; DDD, dense deposit disease; MIDD, monoclonal immunoglobulin deposition disease.
C9 was detected in all GN except ANCA-associated GN (see below) and was the highest in C3GN and DDD. The presence of C9 indicates that the presence of terminal complex (membrane attack complex) in GN. Even though the counts of C9 are not as high as C3, likely explained by lack of amplification loop, the moderate spectral counts of C9 detected in most GN indicate a role of the membrane attack complex in causing disruption of GBM architecture.
The ratio of C3:C9 spectral count is intriguing in C3GN. The C3 counts are very high in C3GN whereas the counts of C9 are only moderate with a ratio of almost 5:1. In contrast, both C3 and C9 counts are high in DDD, giving a ratio of only 1:1.6. These findings suggest greater activation of terminal pathway of complement in DDD compared to C3GN.2,29
The detection of complement in pauci-immune necrotizing and crescentic GN was variable. There were only low spectral counts of C3 present in ANCA-positive (both MPO and PR3)-associated GN whereas there were high spectral counts of C3 in ANCA-negative GN. The high spectral counts of C3 and the absence or very low spectral counts of C4 suggests a role of alternative pathway of complement in ANCA-negative GN.15 The glomeruli dissected in pauci-immune GN did not include those with necrotizing and crescentic lesions and this may account for the lack of high spectral counts of C3 in ANCA-positive GN. We have recently completed a study comparing the complement profile in normal appearing glomeruli versus crescentic glomeruli in MPO-associated or PR3-associated GN and find significantly increased complement factors including C3 and C5b−9 in crescentic glomeruli compared to normal appearing glomeruli (in press).16 Similarly, moderate spectral counts of C3 and low counts of C9 were present in IgA nephropathy in the absence of C1q and C4, suggesting a role for alternative and terminal pathways.30,31
Complement regulating proteins FHR-1 and FHR-5, which are fluid phase regulators, were also detected in moderate to high spectral counts in all GN except pauci-immune-GN and infection-related GN. Although the precise function of these proteins is not known, it likely that they play a key role in modulating complement activation in GN.
Therefore, complement pathways play roles in all the GN studied although the extent and type of complement proteins can be variable depending on the GN. C3 is the dominant complement protein with the highest spectral counts in C3GN, PGNMID, fibrillary GN, immunotactoid glomerulopathy, MIDD, and MN. This is likely because of the persistent activation of complement pathway in these disease entities. Similarly, large spectral counts of C5b−9 are present in C3GN and MIDD, with moderate spectral counts in other types of GN. It is also tempting to speculate the complement pathways involved based on the presence of C3, C4, and C9 in the different types of GN (Figure 2).
There are limitations to this study because some disease entities are rare and MS/MS was done in only 2 to 3 cases. It should also be pointed out that the complement activation and subsequent deposition of complement proteins is dependent on the location (fluid vs. tissue) and the phase (active vs. chronic) of the disease process, and this can lead to variable findings. In addition, further studies by immunohistochemistry are required to confirm our findings. Finally, we studied mostly abundant complement proteins and did not search for other complement proteins with low abundance such as complement factor B, CD59, etc (see next section on methodology).
Regarding the methodology of MS/MS, 2 extraction and subsequent MS/MS protocols were utilized in this study. The cohort outlined above utilized a smaller cubic volume of glomeruli (∼125,000 μm³) and the MS/MS methods were tailored toward detection of higher abundance proteins by utilization of a lower dynamic exclusion time and maximum ionization times. Therefore, the finding of C3, C4A, C4B, C9, FHR-1, and FHR-5 in dissections suggests abundance of these proteins. On the other hand, our recently published studies on detection of novel membranous antigens, such as exostosin, utilized a greater cubic volume of glomeruli (∼23-fold greater) and the MS/MS methods were tailored toward a more discovery methodology aimed at detection of lower abundance proteins by utilizing a longer gradient, larger dynamic exclusion window, and lower maximum ionization times. Therefore, the spectral counts of proteins using greater cubic volume are higher when compared to dissection of smaller cubic volumes. Both methodologies enable the detection of higher abundance proteins such as complement proteins but will differ in their abilities to detect low abundance biomarkers such as exostosin (EXT proteins), neural epidermal growth factor-like 1 protein (NELL-1), and semaphorin 3B (Sema3B) and complement proteins of lower abundance such as factor B, properdin, and CD59.23,24,32,33
To summarize, C3 followed by C9 are most abundant complement proteins in GN, indicating activation of classical or lectin or alternative, and terminal pathways. Depending on the type of GN, C4A and/or C4B are also present. The C3:C9 ratio in C3GN suggests increased accumulation of terminal pathway complement in DDD compared to C3GN. The presence of FHR-1 and FHR-5 indicate regulatory mechanisms are also in play. Therefore, activation of complement pathways with deposition of complement proteins plays an important role in pathogenesis of GN. Selective targeting of different complement pathways in the treatment of GN is currently undergoing evaluation in a number of clinical trials.
Disclosure
All the authors declared no competing interests.
Author Contributions
SS designed the study, interpreted the MS/MS data and wrote the manuscript; LP and FCF contributed to designing the study, creating figures, and critically read the manuscript, JT performed the MS/MS studies.
References
- 1.Sethi S., Haas M., Markowitz G.S., et al. Mayo Clinic/renal pathology society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol. 2016;27:1278–1287. doi: 10.1681/ASN.2015060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi S., Gamez J.D., Vrana J.A., et al. Glomeruli of Dense Deposit Disease contain components of the alternative and terminal complement pathway. Kidney Int. 2009;75:952–960. doi: 10.1038/ki.2008.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vrana J.A., Gamez J.D., Madden B.J., Theis J.D., Bergen H.R., Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry based proteomic analysis in clinical biopsy specimens. Blood. 2009;114:4957–4959. doi: 10.1182/blood-2009-07-230722. [DOI] [PubMed] [Google Scholar]
- 4.Sethi S., Vrana J.A., Theis J.D., et al. Laser microdissection and mass spectrometry-based proteomics aids the diagnosis and typing of renal amyloidosis. Kidney Int. 2012;82:226–234. doi: 10.1038/ki.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasari S., Alexander M.P., Vrana J.A., et al. DnaJ heat shock protein family B Member 9 is a novel biomarker for fibrillary GN. J Am Soc Nephrol. 2018;29:51–56. doi: 10.1681/ASN.2017030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nadasdy T., Hebert L.A. Infection-related glomerulonephritis: understanding mechanisms. Semin Nephrol. 2011;31:369–375. doi: 10.1016/j.semnephrol.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Nasr S.H., Radhakrishnan J., D’Agati V.D. Bacterial infection-related glomerulonephritis in adults. Kidney Int. 2013;83:792–803. doi: 10.1038/ki.2012.407. [DOI] [PubMed] [Google Scholar]
- 8.Satoskar A.A., Parikh S.V., Nadasdy T. Epidemiology, pathogenesis, treatment and outcomes of infection-associated glomerulonephritis. Nat Rev Nephrol. 2020;16:32–50. doi: 10.1038/s41581-019-0178-8. [DOI] [PubMed] [Google Scholar]
- 9.Bajema I.M., Wilhelmus S., Alpers C.E., et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93:789–796. doi: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Weening J.J., D’Agati V.D., Schwartz M.M., et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 11.Cattran D., Coppo R., Cook H., et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 12.Nasr S.H., Valeri A.M., Cornell L.D., et al. Fibrillary glomerulonephritis: a report of 66 cases from a single institution. Clin J Am Soc Nephrol. 2011;6:775–784. doi: 10.2215/CJN.08300910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jennette J.C., Falk R.J., Bacon P.A., et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 14.Kallenberg C.G.M., Heeringa P. Complement system activation in ANCA vasculitis: a translational success story? Mol Immunol. 2015;68:53–56. doi: 10.1016/j.molimm.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Sethi S., Zand L., De Vriese A.S., et al. Complement activation in pauci-immune necrotizing and crescentic glomerulonephritis: results of a proteomic analysis. Nephrol Dial Transplant. 2017;32:i139–i145. doi: 10.1093/ndt/gfw299. [DOI] [PubMed] [Google Scholar]
- 16.Sethi A., Grande J., Specks U., Fervenza F.C. Proteomic profile of uninvolved versus crescentic glomeruli in MPO-ANCA-associated vasculitis. Clin Kidney J. 2023:sfad030. doi: 10.1093/ckj/sfad030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravindran A., Fervenza F.C., Smith R.J.H., De Vriese A.S., Sethi S. C3 glomerulopathy: ten years’ experience at Mayo Clinic. Mayo Clin Proc. 2018;93:991–1008. doi: 10.1016/j.mayocp.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sethi S., Rajkumar S.V., D’Agati V.D. The complexity and heterogeneity of monoclonal immunoglobulin–associated renal diseases. J Am Soc Nephrol. 2018;29:1810–1823. doi: 10.1681/ASN.2017121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasr S.H., Satoskar A., Markowitz G.S., et al. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20:2055–2064. doi: 10.1681/ASN.2009010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sethi S., Zand L., Leung N., et al. Membranoproliferative glomerulonephritis secondary to monoclonal gammopathy. Clin J Am Soc Nephrol. 2010;5:770–782. doi: 10.2215/CJN.06760909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasr S.H., Fidler M.E., Cornell L.D., et al. Immunotactoid glomerulopathy: clinicopathologic and proteomic study. Nephrol Dial Transplant. 2012;27:4137–4146. doi: 10.1093/ndt/gfs348. [DOI] [PubMed] [Google Scholar]
- 22.Nasr S.H., Valeri A.M., Cornell L.D., et al. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin J Am Soc Nephrol. 2012;7:231–239. doi: 10.2215/CJN.08640811. [DOI] [PubMed] [Google Scholar]
- 23.Sethi S., Madden B.J., Debiec H., et al. Exostosin 1/exostosin 2–associated membranous nephropathy. J Am Soc Nephrol. 2019;30:1123–1136. doi: 10.1681/ASN.2018080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravindran A., Madden B., Charlesworth M.C., et al. Proteomic analysis of complement proteins in membranous nephropathy. Kidney Int Rep. 2020;5:618–626. doi: 10.1016/j.ekir.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaartinen K., Safa A., Kotha S., Ratti G., Meri S. Complement dysregulation in glomerulonephritis. Semin Immunol. 2019;45 doi: 10.1016/j.smim.2019.101331. [DOI] [PubMed] [Google Scholar]
- 26.Rupert K.L., Moulds J.M., Yang Y., et al. The molecular basis of complete complement C4A and C4B deficiencies in a systemic lupus erythematosus patient with homozygous C4A and C4B mutant genes. J Immunol. 2002;169:1570–1578. doi: 10.4049/jimmunol.169.3.1570. [DOI] [PubMed] [Google Scholar]
- 27.Walport M.J. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 28.Reid K.B.M., Porter R.R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- 29.Sethi S., Vrana J.A., Fervenza F.C., et al. Characterization of C3 in C3 glomerulopathy. Nephrol Dial Transplant. 2016;32:459–465. doi: 10.1093/ndt/gfw290. [DOI] [PubMed] [Google Scholar]
- 30.Medjeral-Thomas N.R., Lomax-Browne H.J., Beckwith H., et al. Circulating complement factor H–related proteins 1 and 5 correlate with disease activity in IgA nephropathy. Kidney Int. 2017;92:942–952. doi: 10.1016/j.kint.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tortajada A., Gutiérrez E., Goicoechea de Jorge E., et al. Elevated factor H–related protein 1 and factor H pathogenic variants decrease complement regulation in IgA nephropathy. Kidney Int. 2017;92:953–963. doi: 10.1016/j.kint.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 32.Sethi S., Debiec H., Madden B., et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020;97:163–174. doi: 10.1016/j.kint.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Sethi S., Debiec H., Madden B., et al. Semaphorin 3B–associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int. 2020;98:1253–1265. doi: 10.1016/j.kint.2020.05.030. [DOI] [PubMed] [Google Scholar]