Abstract
Two new species of Polyalthiopsis (Annonaceae), P. nigra Y.H. Tan & Bin Yang from Guangxi and Yunnan Provinces and P. xui Y.H. Tan & Bin Yang from Yunnan Province, are described and illustrated. P. nigra is morphologically similar to P. chinensis in having narrowly elliptic-oblong, lemon to yellowish green petals, but differs by having obovoid monocarps, a higher number of leaf secondary veins, leaf blades usually widest above the middle, and a lower ratio of leaf blade length to width. P. xui is morphologically similar to P. floribunda in having axillary inflorescences, 1–3(–4) flowers, elliptic leaves, and elliptic-ovate petals, but differs in the numbers of carpels per flower and ovules per carpel. The molecular phylogenetic analysis using five plastid markers confirm that the two new species belong to the genus Polyalthiopsis and show clear interspecific divergences between P. nigra and P. xui and between them and other species in the genus. Detailed descriptions, colored photographs, and habitat and distribution data for the two new species are provided. In addition, the fruit morphology of P. chinensis is described for the first time, based on living collections. Geographical distributions and a diagnostic key for all Polyalthiopsis species are also presented.
Keywords: Annonaceae, Morphology, Polyalthia, Polyalthiopsis, Southeast Yunnan
Highlights
-
•
Two new species of Polyalthiopsis from China are described and illustrated based on morphological characters and phylogenetic analysis.
-
•
The genus Polyalthiopsis was extensively sampled, with each species represented by 2–3 samples collected from different locations.
-
•
The fruit morphology of P. chinensis is described for the first time, based on living collections.
1. Introduction
Annonaceae is a large pantropical flowering plant family that consists of 107 genera and ca. 2430 species (Chatrou et al., 2012, 2018; Chaowasku et al., 2012, 2018a, 2018b; Xue et al., 2012, 2018; Guo et al., 2017; Chaowasku, 2020; Bangkomnate et al., 2021). A collection of about 100 Annonaceae plants have been maintained at Xishuangbanna Tropical Botanical Garden (XTBG) as part of the Tropical Plant Resource Conservation and Sustainable Use project, which collected over 7000 tropical plant species from China and adjacent countries from 2000 to 2004. Several species have been identified from these collections by monitoring flowering and fruiting (Xue et al., 2017, 2021; Yang et al., 2020).
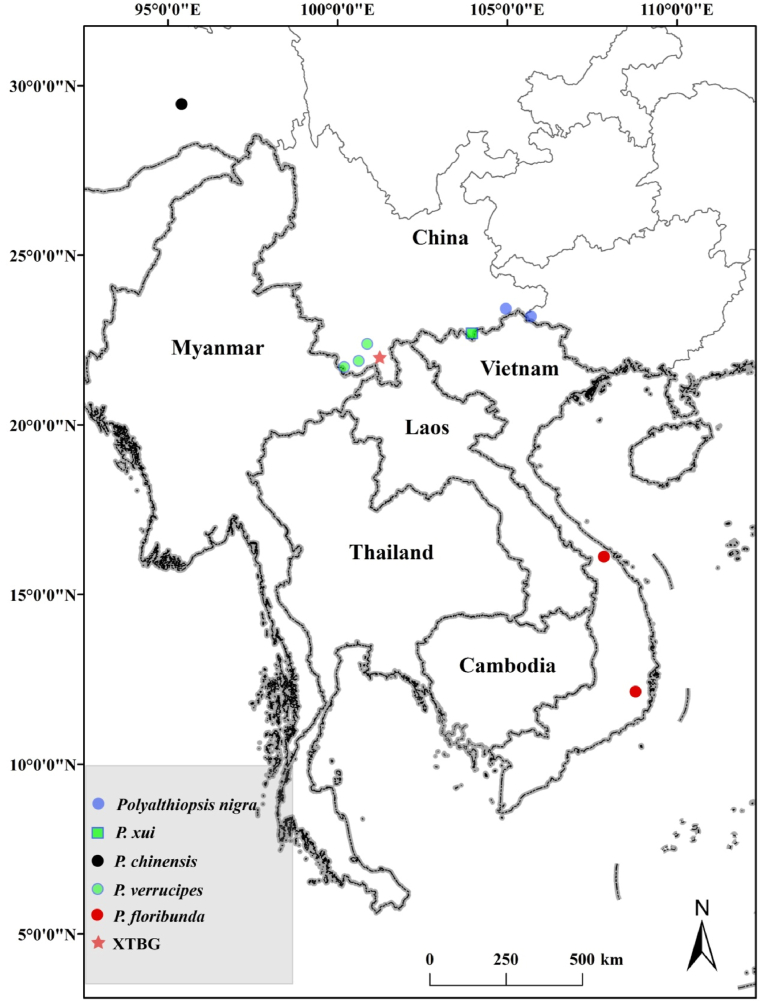
Recently, observations of flower morphology in the Annonaceae collection at XTBG has led to the identification of three unknown species belonging to the genus Polyalthiopsis Chaowasku. Polyalthiopsis, which was recently segregated from Polyalthia (Chaowasku et al., 2018b), consists of three previously described species distributed in SW China and Vietnam (Xue et al., 2020; Fig. 1). The genus is characterized by axillary inflorescences, a raised midrib on the adaxial leaf surface, petioles with transverse striations when dry, and foliar glands on the dried leaf surface (Chaowasku et al., 2018b; Xue et al., 2020). Two of the unknown species were introduced from southeastern Yunnan in 2000 and from Napo, Guangxi, in 2002, respectively, but without any information on their locations or habitats. Fortunately, the authors, Yun-Hong Tan and Bin Yang, identified the locations and in April 2021 found these species in the wild.
Fig. 1.
Distributions of Polyalthiopsis xui, P. nigra, P. chinensis, P. verrucipes and P. floribunda.
Here, morphological comparisons and phylogenetic analyses based on five chloroplast regions indicate that these plants represent two undescribed species, which we describe and name as Polyalthiopsis nigra Y.H. Tan & Bin Yang and Polyalthiopsis xui Y.H. Tan & Bin Yang. The third species, Polyalthiopsis chinensis (S.K. Wu ex P.T. Li) B. Xue & Y.H. Tan (Xue et al., 2020), was originally published as Polyalthia chinensis based only on flowering material (Li, 1976). We provide a full description of P. chinensis, which was introduced from Mêdog in 2003.
2. Material and methods
2.1. Morphological studies
Measurements and morphological character assessments of the two putative new species were carried out on living plants in the field and on herbarium specimens. Related species were chosen for morphological comparisons and phylogenetic results (Chaowasku et al., 2018b; Xue et al., 2020), with the morphological features of these species culled from specimens and descriptions in previous studies (Li, 1976; Chaowasku et al., 2018b; Xue et al., 2020; Table 1). All voucher specimens have been deposited in the herbarium of the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (HITBC).
Table 1.
Morphological comparisons of Polyalthiopsis nigra, P. chinensis, P. xui, P. floribunda and P. verrucipes.
| Character | P. nigra | P. chinensis | P. xui | P. floribunda | P. verrucipes |
|---|---|---|---|---|---|
| Petiole | 4–7 mm | 4–6 mm | 3–7 mm | 3–5 mm | 3–7 mm |
| Leaf blades | obovate-elliptic, oblong-elliptic to oblong, 8–18.5 × 3.2–8.3 cm | oblong-elliptic to obovate-lanceolate, 7.9–13 × 2.2–3.8 cm | elliptic, oblong-elliptic, 8.5–19.8 × 2.7–6.3 cm | oblong-elliptic to oblong, 10–15 × 3.5–5 cm | oblong to oblong-lanceolate, 10–17 × 2.5–5 cm |
| Length/width ratio of leaf | 2.0–3.0 | 3.2–4.5 | 2.3–3.8 | 2.5–3.2 | 3.0–4.5 |
| Number of secondary veins on leaf | 15–25 pairs | 8–15 pairs | 8–14 pairs | 10–15 pairs | 15–18 pairs |
| Inflorescences | 1–4 flowers | 1–2 flowers | 1–3(–4) flowers | 1–5 flowers | solitary flower |
| Pedicel | 3–7 mm | 4–7 mm | 5–7 mm | 8–10 mm | 1–2 mm |
| Outer petals | lemon to yellowish green, narrowly elliptic-oblong, 13–16 × 3–4 mm | lemon to yellowish green, narrowly elliptic-oblong, 12–13 × 3.5–4.5 mm | lemon to yellowish green; elliptic-ovate, 7–10 × 3–5 mm | color unknown, elliptic-ovate, ca. 6–8 × 3–4 mm | white, narrowly elliptic-oblong, 16 × 3–5 mm |
| Number of carpels per flower | 9–15 | 8–10 | 8–12 | 7 | 12–16 |
| Number of ovules per carpel | 2 | 2 | 2 | 1 | 2 |
| Monocarps (fresh) | obovoid, ca. 1.4 × 0.9 cm (immature) | ellipsoid to rhomboid, 2.7–3.8 × 1.3–2.6 cm | ellipsoid to rhomboid, 2–2.9 × 1.6–2.5 cm | cylindrical, ca. 2.8 × 1.5 cm | ellipsoid to rhomboid, 2.5–3.2 × 1.5–2.3 cm |
| Seeds (fresh) | ellipsoid, ca. 8 × 2.5–3 mm (immature) | cylindrical, 2.2–3.2 × 1.0–1.3 cm | ellipsoid, 1.9–2.4 × 0.9–1.1 cm | cylindrical, ca. 2.4 × 1.2 cm | ellipsoid to cylindrical, 1.6–2.5 × 0.9–1.2 cm |
Flowers of P. chinensis, P. nigra and P. xui were collected from XTBG and fixed in formalin acetic alcohol (FAA: 70% alcohol, formaldehyde and glacial acetic acid in a ratio of 90:5:5). Pollen grains were collected from mature and undehisced stamens. The fixed stamens were dehydrated in an ascending ethyl alcohol series, critical-point dried. The pollen sacs of these mature stamens were opened carefully with a tweezer and needle in order to release the pollen grains from the sacs. For scanning electron microscopic (SEM) observations, pollen grains were mounted on copper stubs with double-sided adhesive tape, coated with 20 nm Pt–Pd using a Q150R S Auto Sputter Coater and examined using a ZEISS EVO LS10. Further pollen terminology follows Punt et al. (2007).
2.2. Phylogenetic analyses
To assess the phylogenetic position of the two putative new species, we sampled five plastid regions (psbA-trnH, matK, trnL-trnF, rbcL and ndhF) of 41 species from Annonaceae. The ingroup consisted of 36 species of Miliuseae, plus Dendrokingstonia nervosa (Hook. f. & Thomson) Rauschert and Maasia discolor (Diels) Mols P. J. A. Kessler & Rogstad (Malmeoideae), whereas the outgroups consisted of two species from the Annonoideae and one species from the Anaxagoreoideae. The genus Polyalthiopsis was extensively sampled, and each species was represented by 2–3 samples collected from different locations. Most sequences of the tribe Miliuseae and the genus Dendrokingstonia and Maasia were retrieved from previous data sets (Appendix A; Chaowasku et al., 2018b; Xue et al., 2018, 2020) and downloaded from GenBank to complement our own data. Voucher information and GenBank accession numbers for all sequences are listed in Appendix A.
Genomic DNA of seven samples was extracted from silica gel-dried leaves using a modified cetyl trimethyl ammonium bromide (CTAB) method (Doyle and Doyle, 1987). The five chloroplast regions were amplified and sequenced using the same protocol and primer sets as Xue et al. (2020). The newly generated sequences were checked and assembled using Geneious v.9.1.7 (Kearse et al., 2012). The five combined regions were aligned through MAFFT v.7.450 (Katoh and Standley, 2013), then manually edited using Geneious v.9.1.7. Gaps were treated as missing data. The best-fitting model of nucleotide substitutions for the combined plastid data matrix was determined according to the Akaike Information Criterion in jModelTest2 (Darriba et al., 2012). Bayesian inference (BI) analysis was performed using MrBayes v.3.2.7, under the substitution model of GTR + I + G (Ronquist et al., 2012). Two independent runs and four chains using a Markov chain Monte Carlo algorithm were run for two million generations, with every 100 generations sampled and the first 25% of trees discarded as burn-in. The convergence of the two runs was assessed by Tracer 1.6 (Rambaut et al., 2014), with an effective sample size (ESS) > 200. Phylogenetic relationships are considered well-resolved when clades have posterior probabilities (PP) > 0.9. Maximum likelihood (ML) analysis was performed using W-IQ-tree (Trifinopoulos et al., 2016) with 1000 ultrafast bootstrap permutations and 1000 replicates of SH-aLRT single branch test. The supermatrix was partitioned into five subsets (matK: 1–770 bp, ndhF: 771–2654 bp, psbA-trnH: 2655–3044 bp, rbcL: 3045–4338 bp, trnL-trnF: 4339–5128 bp). The best-fit substitution model was determined under the corrected Akaike information criterion. The robustness of ML phylogenetic trees was well supported with SH-aLRT support (sh-alrt > 80%) and ultrafast bootstrap (UFBoot) values > 95%.
3. Results
3.1. Morphology
Polyalthiopsis nigra is morphologically similar to P. chinensis in that they both have lemon to yellowish green petals and a short pedicel (< 7 mm long). P. xui is morphologically similar to P. floribunda as both have axillary inflorescences, 1–3(–4) flowers, elliptic leaves, and elliptic-ovate petals. However, they are easily distinguished from each other by several morphological characters (Table 1).
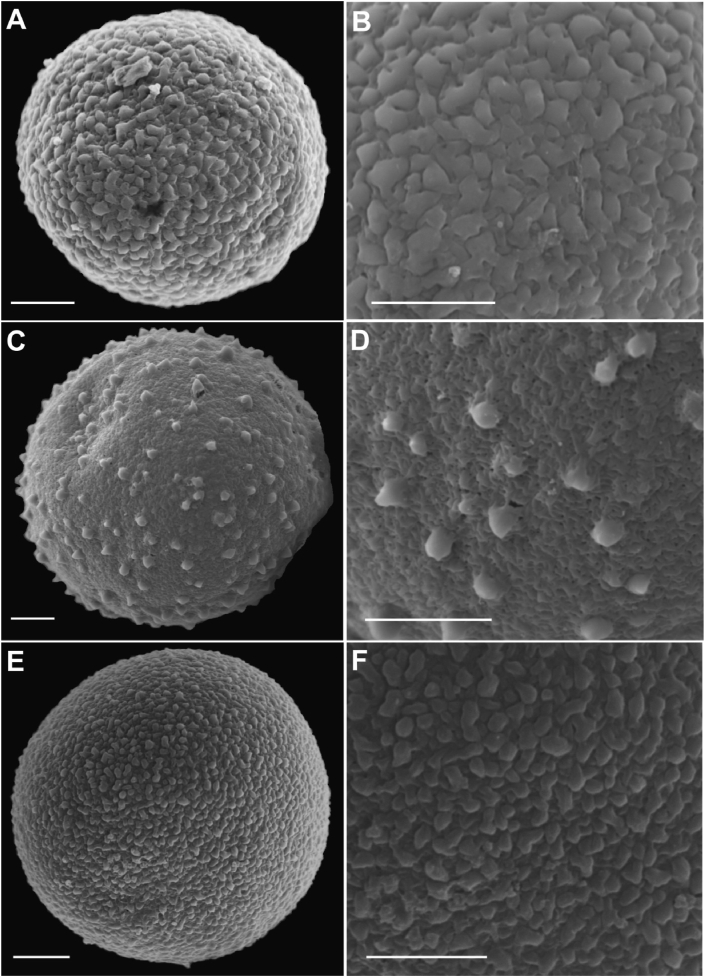
The pollen grains of Polyalthiopsis nigra are spheroidal monads (Fig. 2A), and the exine ornamentation is rugulate-verrucate (Fig. 2B), ca. 24 μm in diameter. The pollen grains of P. xui are spheroidal monads (Fig. 2C), and the exine ornamentation is echinate ornamentation (Fig. 2D), ca. 32 μm in diameter. The pollen grains of P. chinensis are spheroidal monad (Fig. 2E), and the exine ornamentation is scabrate (Fig. 2F), ca. 30 μm in diameter.
Fig. 2.
SEM micrographs of pollen grains (A, C, E) and ornamentation of exine (B, D, F). (A, B) Polyalthiopsis nigra, spheroidal monad (A) with rugulate-verrucate ornamentation (B); (C, D) Polyalthiopsis xui, spheroidal monad (C) with echinate ornamentation (D); (E, F) Polyalthiopsis chinensis, spheroidal monad (E) with scabrate ornamentation (F). Scale bars: 5 μm.
3.2. Phylogenetic analysis
The aligned matrix for the combined plastid data set was 5128 nucleotides in length and possessed 456 parsimony-informative sites with gaps treated as missing data.
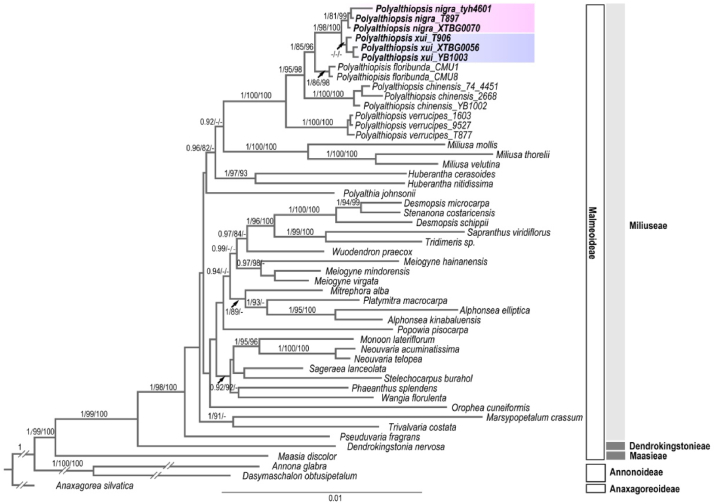
Both Bayesian Inference and Maximum Likelihood confirmed the phylogenetic affiliation of Polyalthiopsis nigra and P. xui to the genus Polyalthiopsis (Fig. 3). The monophyly of the genus Polyalthiopsis and most of the Polyalthiopsis species were strongly supported (PP = 1, sh-alrt > 80% and UFBoot > 95%). Polyalthiopsis was grouped as sister to Miliusa, and both genera were grouped together as sister to Huberantha.
Fig. 3.
Phylogeny inferred from five plastid (psbA-trnH, matK, trnL-trnF, rbcL and ndhF) DNA sequences. BI posterior probabilities (PP > 0.90), ML SH-aLRT support (sh-alrt > 80%) and ultrafast bootstrap support (UFBoot > 95%) are presented above branches.
P. nigra forms a monophyletic clade (PP = 1, sh-alrt = 81% and UFBoot = 99%), with one accession (XTBG0070) from an individual cultivated in XTBG and the other two accessions (T897 & tyh4601) from the wild in Malipo, southeastern Yunnan, and Napo, Guangxi, respectively. Samples of P. xui formed a cluster that lacked high support values (PP < 0.90, sh-alrt < 80% and UFBoot < 95%), among which were two accessions (XTBG0056 & YB1003) from different individuals cultivated at XTBG, and one accession (T906) from the wild in southeastern Yunnan. These two new species form a clade as closely related sister groups with strong support values (PP = 1, sh-alrt = 98% and UFBoot = 100). The branch lengths are short within this clade.
The relationships of the two new species with Polyalthiopsis floribunda, P. verrucipes and P. chinensis were well resolved (Fig. 3). These two new species group together as sister to P. floribunda (PP = 1, sh-alrt = 85% and UFBoot = 96%). The entire clade, consisting of these three species, is sister to P. chinensis (PP = 1, sh-alrt = 95% and UFBoot = 98%). P. verrucipes is sister to the rest of genus (PP = 1, sh-alrt = 100% and UFBoot = 100%) (Fig. 3).
4. Discussion
4.1. Morphology
Polyalthiopsis contains five species: P. nigra, P. xui, P. floribunda, P. chinensis and P. verrucipes. All Polyalthiopsis species have axillary inflorescences, a raised midrib on the adaxial leaf surface, petioles with transverse striations when dry, and foliar glands on the dried leaf surface (Chaowasku et al., 2018b; Xue et al., 2020). Our field collection and recently published literature indicate that P. verrucipes is morphologically distinct from the other four species by having white flowers (Xue et al., 2020).
Polyalthiopsis xui and P. nigra are easily distinguished from P. floribunda by several morphological characters. They differ in the number of carpels per flower and ovules per carpel, as well as the morphology of the monocarps (Table 1 and Key). Both P. xui and P. floribunda have oblong-elliptic leaf blades, and elliptic-ovate outer petals, but P. xui differs from P. floribunda by having 2 ovules per carpel, so that the carpels develop into a monocarp with rhomboid shape and having 8–12 (vs. 7) carpels per flower (Table 1).
These two new species can be distinguished from each other by several morphological characters. Polyalthiopsis xui has 8–14 pairs of secondary veins, whereas P. nigra has 15–25 pairs; the outer petals of P. xui are 7–10 mm long and elliptic-ovate, whereas those of P. nigra are 13–16 mm long and narrowly elliptic-oblong; also, the monocarps of P. xui are ellipsoid to rhomboid, whereas those of P. nigra are obovoid. The size of pollen grains of P. nigra is smaller than that of P. xui, and the difference in exine ornamentation of the two species is obvious (Fig. 2).
Both Polyalthiopsis nigra and P. chinensis have lemon to yellowish green, narrowly elliptic-oblong petals. Although P. nigra resembles P. chinensis in having oblong-elliptic leaf blades, they can readily be distinguished by the number of lateral veins and the length/width ratio of leaf blades (Table 1). The most notable difference between them is the shape of the monocarps, which are obovoid in P. nigra, but ellipsoid to rhomboid in P. chinensis (Table 1). The size of pollen grains of P. nigra is smaller than that of P. chinensis, and the exine ornamentation of the two species are also different (Fig. 2).
Our observations indicate that the pollen grains of Polyalthiopsis are spheroidal monads (Fig. 2), with a shape that strongly resembles that of Miliusa and Huberantha in the tribe Miliuseae (Chaowasku et al., 2008; Shao and Xu, 2018). Most species of Miliusa and Huberantha have verrucate or scabrate exine ornamentation (Chaowasku et al., 2008; Shao and Xu, 2018). Pollen grains are smaller in Polyalthiopsis species than in Miliusa species (24–32 μm vs. 36–54 μm in diameter) (Chaowasku et al., 2008). Pollen grains of the three species of Polyalthiopsis differ in exine ornamentation and size. Micromorphological characteristics also support the designation of P. nigra and P. xui as two independent species.
4.2. Phylogenetic relationships
Our unresolved backbone of the phylogeny of Miliuseae is consistent with previous studies for most groups (e.g., Xue et al., 2020). Polyalthiopsis has been shown to be a sister group to Miliusa (Chaowasku et al., 2018b, 2020; Xue et al., 2020). Polyalthiopsis differs from Miliusa mainly by having a dilated truncate connective apex of the stamens and outer petals that are much larger than the sepals (Chaowasku et al., 2018b). However, Polyalthiopsis and Miliusa share more morphological similarities. Specifically, previous studies and our own observations note that species in Polyalthiopsis and Miliusa have lamelliform endosperm rumination (Chaowasku et al., 2014; Xue et al., 2020). Our confirmation that these two genera share a sister relationship (PP = 0.92, but sh-alrt < 80% and UFBoot < 95%) (Fig. 3), and that Huberantha is sister to the clade Polyalthiopsis-Miliusa (PP = 0.96, sh-alrt = 82% but UFBoot < 95%) is consistent with previous studies (Chaowasku et al., 2018b, 2020).
The clade with these two new species diverges from the monophyletic clade of Polyalthiopsis floribunda with longer branch lengths, indicating that these two species are phylogenetically distinct from the latter. The monophyly of P. nigra is strongly supported by our study with high statistic values. The clade of P. xui exhibits low support values, suggesting molecular markers with more phylogenetically informative sites should be used in further studies. However, phylogenetic analysis revealed a degree of distinctness between P. nigra from P. xui. Even though the two new species have narrow and adjacent distribution ranges in southeastern Yunnan, they occur at different elevations in limestone areas. P. nigra grows in limestone forest at elevations from 1100 to 1200 m, whereas P. xui grows at between 300 and 400 m. The divergence in elevations may hamper gene flow between the two species and facilitate their genetic differentiation.
5. Taxonomic treatment
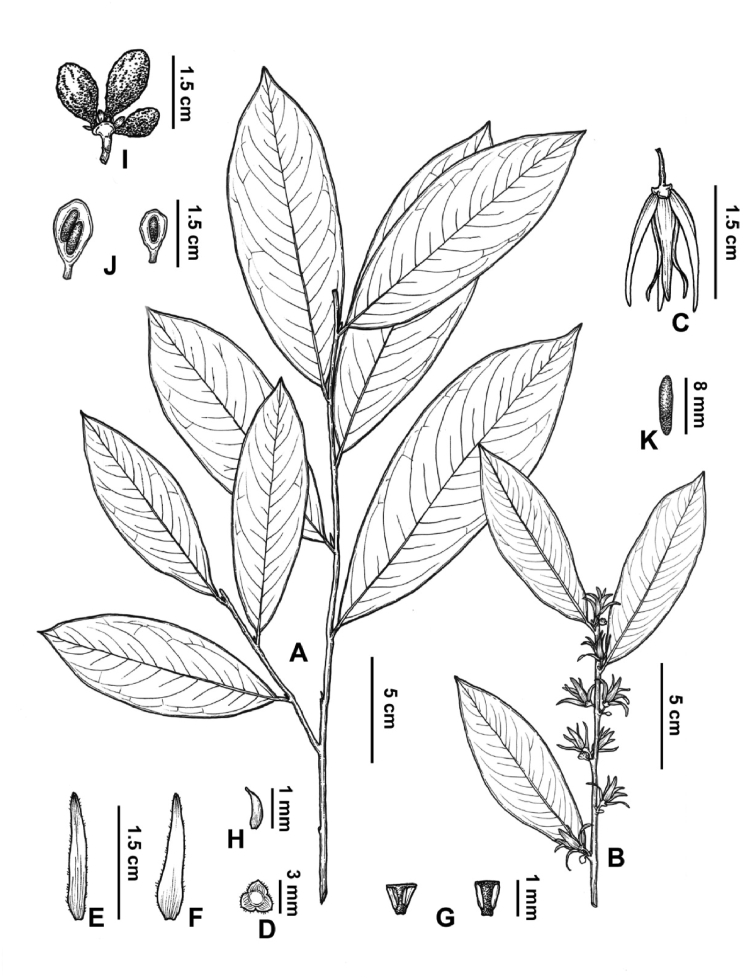
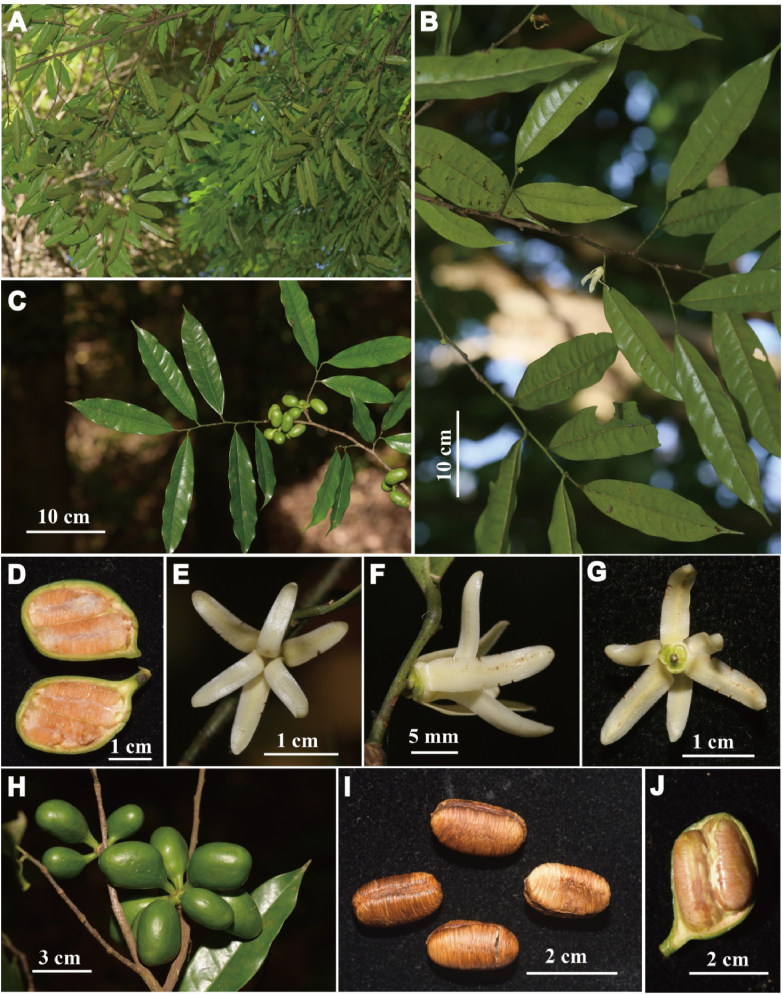
5.1. Polyalthiopsis nigra Y.H. Tan & Bin Yang, sp. nov. (Fig. 4, Fig. 5)
Fig. 4.
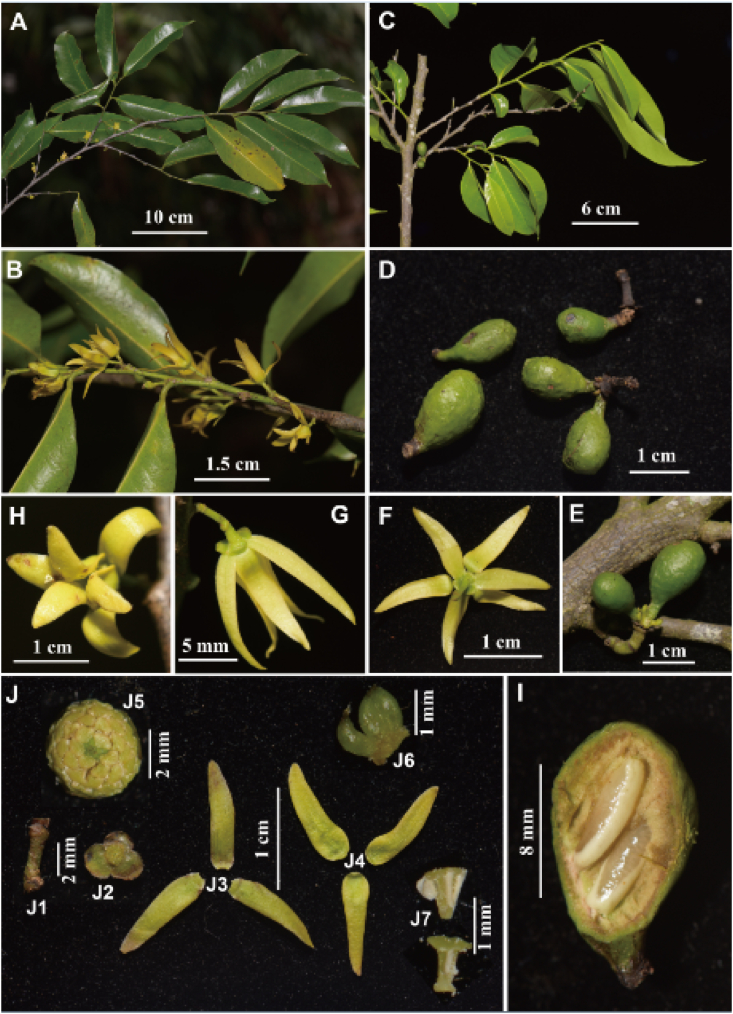
Polyalthiopsis nigra Y.H. Tan & Bin Yang, sp. nov. (A) Flowering branch; (B) Part of flowering branch (showing flowers); (C) Fruiting branch; (D) Monocarps; (E) Fruit; (F) Flower (back view); (G) Flower (lateral view); (H) Flower (front view); (I) Longitudinal section of monocarp (showing two young seeds); (J) Dissection of a flower (J1. Pedicel; J2. Sepals; J3. Outer petals; J4. Inner petals; J5. Flower with the petals removed, showing the sepals, stamens, and carpels; J6. Carpels; J7. Stamens).
Fig. 5.
Polyalthiopsis nigra Y.H. Tan & Bin Yang, sp. nov. (A) Branch; (B) Flowering branch; (C) Flower; (D) Sepals; (E) Outer petal; (F) Inner petal; (G) Stamens; (H) Carpel; (I) Fruit; (J) Longitudinal section of monocarp. Drawing: Yifan Li.
Diagnosis: — Polyalthiopsis nigra is morphologically similar to P. chinensis (S.K. Wu ex P.T. Li) B. Xue & Y.H. Tan with lemon to yellowish green petals and < 7 mm long pedicel, but clearly differs by having obovoid (vs. ellipsoid to rhomboid) monocarps and the pericarp of the monocarps not secreting (vs. secreting) white liquid when injured, 15–25 (vs. 8–15) pairs of secondary veins, the widest part of leaf blades usually above the middle (vs. in the middle), leaf blades 2.0–3.0 (vs. 3.2–4.5) times as long as wide, and flowering from February to April (vs. August).
Type: — CHINA. Yunnan Province, Malipo County, Liuhe Township, Wangjiawan Village, 23°22′14.42″ N, 104°52′58.54″ E, 1104 m, limestone forest near roadside, 3 April 2021, Y.H. Tan & B. Yang T897 (holotype: HITBC; isotypes: HITBC).
Trees to 15 m tall. Branches greyish-black, glabrous. Petiole 4–7 mm long, 1–1.5 mm in diameter, glabrous, with transverse striations when dry; leaf blades obovate-elliptic, oblong-elliptic to oblong, 8–18.5 × 3.2–8.3 cm, 2.0–3.0 times as long as wide, base broadly cuneate to obtuse, apex acuminate, the widest part usually above the middle, both surfaces glabrous, thinly leathery, densely verrucate with foliar glands when dry; upper surface of midrib raised when fresh, becoming flat or slightly raised when dry, lower surface of midrib raised; secondary veins 15–25 on each side of midrib, delicate and prominent on both surfaces; tertiary veins reticulate. Inflorescences axillary, with 1–4 flowers. Peduncle 0.5–1 mm. Pedicel 3–7 mm long, puberulous to puberulent, with one ovate bracteole at middle or above middle, 3–5 ovate bracteoles at base. Sepals semi-orbicular or reniform, 2 × 1.5 mm, slightly puberulent to glabrous, margin ciliate, sometimes slightly reflexed. Petals 6, valvate, free, in 2 whorls; lemon to yellowish green, narrowly elliptic-oblong, apex acute to attenuate, both whorls subequal, 13–16 × 3–4 mm, glabrous, slightly ciliate or puberulous along margin. Stamens 40–50 per flower, ca. 1 mm long; connective truncate. Carpels 9–15 per flower, oblong, slightly puberulent to glabrous; stigma ovoid, puberulent; ovary ca. 1 mm; ovules 2 per ovary, lateral, arranged vertically in 1 row. Fruiting pedicel becoming longer and thicker, 4–6 mm long, ca. 2 mm in diameter; immature monocarp stipes 3–4 mm long; immature monocarps obovoid, ca. 1.4 cm long, 0.9 cm in diameter. Immature seeds 1 or 2 per monocarp, ellipsoid, ca. 8 mm long, 2.5–3 mm in diameter.
Distribution and habitat: — Polyalthiopsis nigra is known only from Napo County in Guangxi Zhuang Autonomous Region, and Malipo County in Yunnan Province, Southwest China. It usually grows in the limestone forest, at elevations of 1100–1200 m.
Phenology: — Flowering from February to April, fruiting expected from April to July.
Etymology: — The species epithet “nigra” refers to its black bark, the local people called it as “Hēi pí shù (黑皮树)”.
Vernacular name: — Chinese Mandarin: hēi pí yóu yè mù (黑皮疣叶木).
Conservation status: — There are only three known distribution sites for this species. Only one mature individual was found in Malipo County during our field work, near a village and close to the road, so it is vulnerable to disturbance by human activity and could be destroyed by road construction. Therefore, the new species is assigned a preliminary status of Critically Endangered (CR) according to the IUCN Red List Categories (IUCN Standards and Petitions Committee, 2019). However, since very few details exist about its natural distribution, a detailed investigation of the same habitats may identify more populations and individuals of this species. Moreover, we do not have adequate information on its natural distribution in Napo County, so the lack of sufficient data currently does not allow a final risk evaluation and the species can be regarded as data deficient (DD) (IUCN Standards and Petitions Committee, 2019).
Additional specimens examined (paratypes): — CHINA. Guangxi Zhuang Autonomous Region, Napo County, Baisheng Township, 22 July 2015, Y.H. Tan tyh4601 (HITBC); Nonglong, voucher from a cultivated plant at the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, 24 February 2020, B. Yang XTBG-0070 (HITBC); 8 May 2020, B. Yang XTBG-0110 (fr.) (HITBC); 3 March 2021, B. Yang XTBG-0211 (HITBC); 13 February 2015, L.L. Zhen C250509 (fl.) (HITBC).
Notes: — The floral morphology of the new species most resembles Polyalthiopsis chinensis, especially the color and the size, but P. nigra has inflorescences 1–4-flowered, whereas P. chinensis has inflorescences 1–2-flowered (Table 1). They can be distinguished from each other by several vegetative characters: the sepals of P. sinensis are often reflexed and the margin rarely ciliate, whereas the sepals of P. nigra are rarely reflexed and the margin often ciliate; the leaf base of P. nigra is often broadly cuneate to obtuse, and with more than 15 lateral veins on each side of the midrib (Table 1), the widest part of leaf blades is usually above the middle, and the leaves usually wider, whereas the leaf base of P. chinensis is often attenuate to cuneate, and with less than 15 lateral veins on each side of the midrib, the widest part of leaf blades usually in the middle, and the leaves are usually narrow. Moreover, the flowering times of the two species are quite different. The monocarp of P. chinensis is further different in having a small, prominent, beaklike protuberance at the apex and the pericarp of the monocarps secreting white liquid when injured.
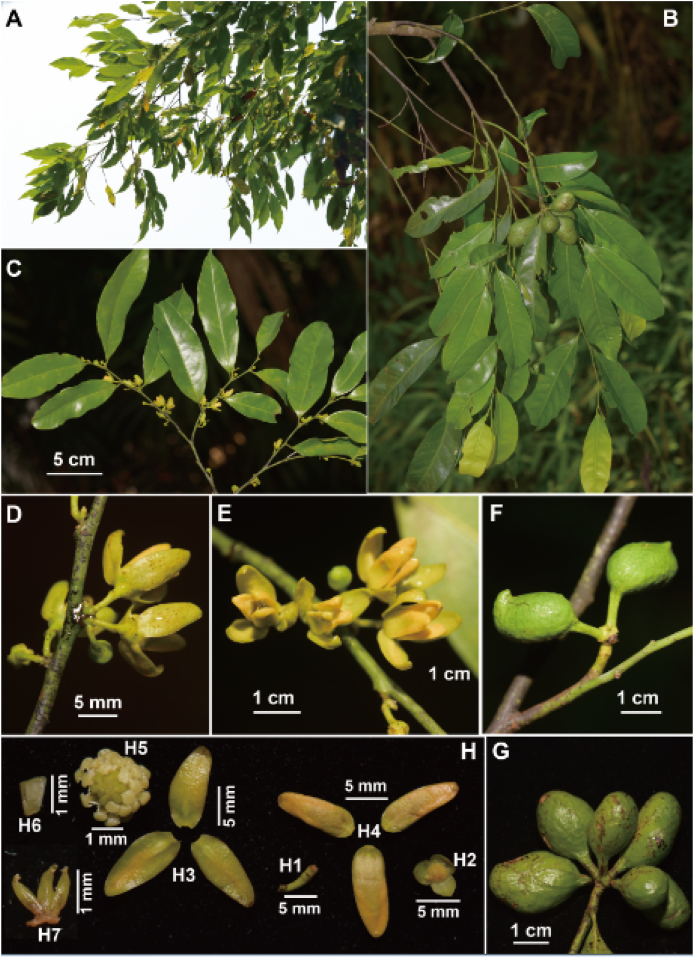
5.2. Polyalthiopsis xui Y.H. Tan & Bin Yang, sp. nov. (Fig. 6, Fig. 7)
Fig. 6.
Polyalthiopsis xui Y.H. Tan & Bin Yang, sp. nov. (A) Branches; (B) Part of fruiting branch; (C) Flowering branch; (D) Flowers (back view); (E) Flowers (front view); (F, G) Fruit; (H) Dissection of a flower (H1. Pedicel; H2. Sepals; H3. Outer petals; H4. Inner petals; H5. Stamens and carpels; H6. Stamen; H7. Carpels).
Fig. 7.
Polyalthiopsis xui Y.H. Tan & Bin Yang, sp. nov. (A) Flowering branch; (B) Close-up of leaf petiole, showing the transverse striations on dried petiole; (C) Flower; (D) Sepals; (E) Outer petal; (F) Inner petal; (G) Side view of inner petal; (H) Stamens; (I) Carpel; (J) Fruit; (K) Longitudinal sections of monocarps of different shapes; (L) Seeds. Drawing: Yifan Li.
Diagnosis: — Polyalthiopsis xui is morphologically similar to P. floribunda (Jovet-Ast) Chaowasku with inflorescences axillary, 1–3(–4) flowers, elliptic leaves, petals elliptic-ovate, but it clearly differs by having carpels 7–12 per flower, ovules 2 per carpel.
Type: — CHINA. Yunnan Province, Maguan County, Gulinqin Township, near Ma'anshan Village, 22°42′23.19″ N, 103°58′1.95″ E, 384 m, tropical rainforest of limestone areas, 4 April 2021, Y.H. Tan & B. Yang T906 (holotype: HITBC; isotypes: HITBC).
Trees to 25 m tall. Branches greyish-black, glabrous. Petiole 3–7 mm long, 1–1.5 mm in diameter, glabrous, with transverse striations when dry; leaf blades elliptic, oblong-elliptic, 8.5–19.8 × 2.7–6.3 cm, 2.3–3.8 times as long as wide, base cuneate, apex attenuate to acuminate, both surfaces glabrous, papery, verrucate with foliar glands when dry; upper surface of midrib raised when fresh, becoming flat or slightly sunken when dry, lower surface of midrib raised; secondary veins 8–14 on each side of midrib, prominent on both surfaces; tertiary veins reticulate. Inflorescences axillary, with 1–3(4) flowers. Peduncle ca.1 mm. Pedicel 5–7 mm long, sparsely puberulent to glabrous, with one ovate bracteole at upper part above middle, 3–5 ovate bracteoles at base. Sepals semi-orbicular, connate at base, 1.5–2 × 2 mm, glabrous, margin ciliate sometimes. Petals 6, valvate, free, in 2 whorls, lemon to yellowish green; outer petals elliptic-ovate, 7–10 × 3–5 mm, internally thickened in the middle of the lower part, apex acute to obtuse, slightly inflexed, glabrous, slightly ciliate; inner petals slightly narrower than outer petals, ovate-oblong or narrowly elliptic-ovate, 7–10 × 2–4 mm, glabrous, slightly ciliate on the upper part, apex acute to attenuate, inflexed to form a beak, puberulous. Stamens 30–40 per flower, ca. 1 mm long; connective truncate. Carpels 8–12 per flower, cylindrical, glabrous; stigma ovoid, puberulent; ovary ca. 1 mm; ovules 2 per ovary, lateral, arranged vertically in 1 row. Fruiting pedicel becoming longer and thicker, 10–11 mm long, 1–2 mm in diameter; monocarp stipes 5–9 mm long; monocarps ellipsoid to rhomboid, 2–2.9 cm long, 1.6–2.5 cm in diameter when fresh, 1.7–2.6 × 1.1–1.7 cm when dry, sometimes apex with a small prominent beaklike protuberance. Seeds 1 or 2 per monocarp, ellipsoid, 1.9–2.4 cm long, 0.9–1.1 cm in diameter when fresh, 1.5–2.1 × 0.7–1.0 cm when dry, endosperm rumination lamelliform.
Distribution and habitat: — Polyalthiopsis xui is known only from the type locality in Maguan County, southeastern Yunnan Province, Southwest China. It usually grows in the tropical rainforest of limestone areas, at elevations of 300–400 m.
Phenology: — Flowering from March to April, fruiting from May to July.
Etymology: — The species is named after Professor Zai-Fu Xu, who was the former director and leader of the project named ‘Tropical Plant Resource Conservation and Sustainable Use’ from 2000 to 2004 in XTBG, for his contributions to plant introduction and ex situ conservation.
Vernacular name: — Mandarin Chinese: xŭ shì yóu yè mù (许氏疣叶木).
Conservation status: — This species is known only from the type locality in southeastern Yunnan. Although its habitat is under protection near Yunnan Daweishan National Nature Reserve, the forests are under severe pressure from agricultural expansion, and the population and mature individuals are near the roadside. We suggest the species be assessed as Vulnerable (VU) (IUCN Standards and Petitions Committee, 2019).
Additional specimens examined (Paratypes): — CHINA. Yunnan Province. Voucher from a cultivated plant at the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, 2 May 2019, B. Yang XTBG-0056 (HITBC); 13 March 2020, Y. H. Tan & H. B. Ding XTBG-0076 (HITBC); 9 May 2020, B. Yang XTBG-0113 (HITBC); 15 July 2020, B. Yang XTBG-0167 (fr.) (HITBC); 20 March 2021, B. Yang XTBG-0216 (HITBC); 29 May 2021 B. Yang YB1003 (fr.) (HITBC); 27 February 2022, B. Yang YB1135 (HITBC, PE); 25 April 2014, H. Lai C06011 (fl.) (HITBC); 7 April 2016, Z.P. Jing C120361 (fl.) (HITBC); Maguan County, Gulinqin township, near Ma'anshan Village, tropical rainforest of limestone areas, 22 July 2021, B. Yang YB1040 (fr.) (HITBC).
Notes: — Both Polyalthiopsis xui and P. chinensis have a monocarp apex with a small, prominent, beaklike protuberance, but they can be distinguished from each other by their petal shape and size. Both have elliptic-ovate petals, but P. xui differs in having 2 ovules per carpel so that the carpels develop into a monocarp with rhomboid shape (Table 1).
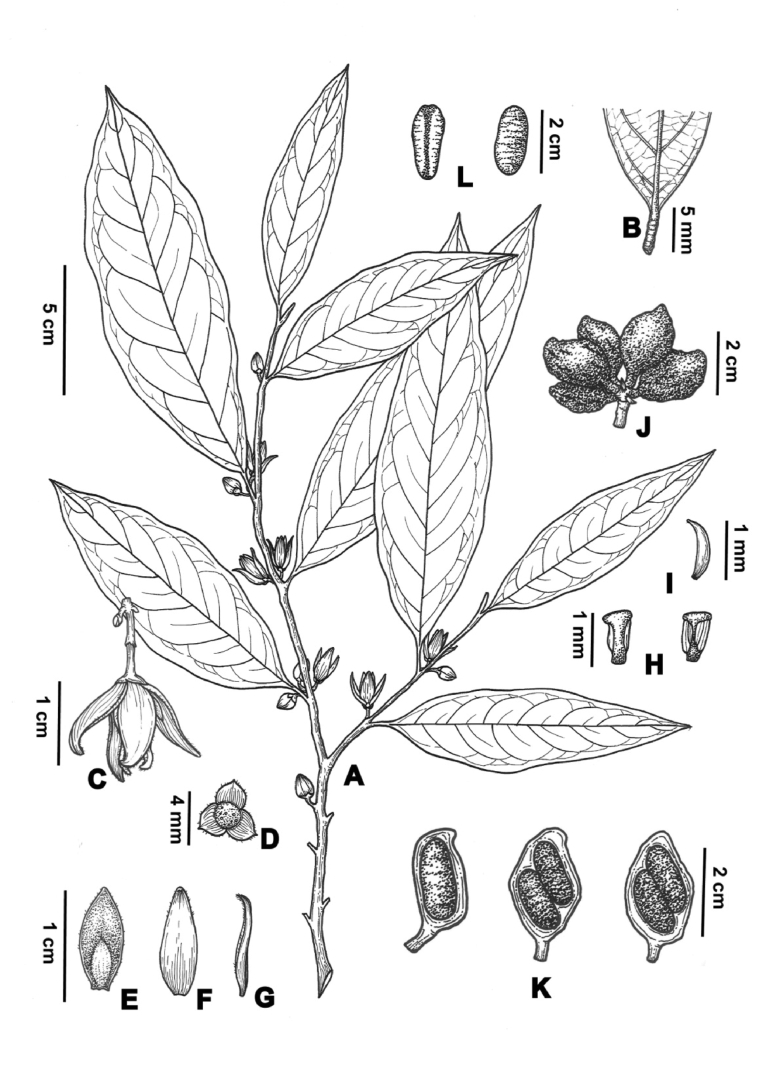
5.3. Polyalthiopsis chinensis (S.K. Wu ex P.T. Li) B. Xue & Y.H. Tan, PhytoKeys, 148: 82, 2020. (Fig. 8)
Fig. 8.
Polyalthiopsis chinensis (S.K. Wu ex P.T. Li) B. Xue & Y.H. Tan. (A) Branches; (B) Part of fruiting branch; (C) Part of flowering branch; (D) Immature fruit; (E) Fruit; (F) Longitudinal section of monocarps; (G) Flowers (side view); (H) Flowers (front view); (I) Dissection of a flower (I1. Flower with the petals removed; I2. Outer petals; I3. Inner petals; I4. Stamen; I5. Carpel).
Basionym: Polyalthia chinensis S.K. Wu & P.T. Li, Acta Phytotax. Sin. 14 (1): 108, t. 4. 1976. Type: — CHINA. Xizang, Mêdog, 20 August 1974, Qinghai-Xizang Exped. 74-4451 (holotype: PE01187290; isotypes: PE01187291, PE01187292, PE01187293, KUN0677650).
Trees to 15 m tall. Branches greyish-black, glabrous. Petiole 4–6 mm long, ca. 1 mm in diameter, glabrous, with transverse striations when dry; leaf blades oblong-elliptic to obovate-lanceolate, 7.9–13 × 2.2–3.8 cm, 3.2–4.5 times as long as wide, base attenuate to cuneate, apex acuminate to shortly caudate, the widest part usually in the middle, both surfaces glabrous, thinly papery, densely verrucate with foliar glands when dry; upper surface of midrib raised when fresh, becoming flat or slightly sunken when dry, lower surface of midrib raised; secondary veins 8–15 on each side of midrib, prominent on both surfaces; tertiary veins reticulate, abaxially slightly raised. Inflorescences axillary, with 1–2 flower(s). Peduncle ca.1 mm long. Pedicel 4–7 mm long, with one ovate bracteole at middle or above middle, 2–3 ovate bracteoles at base, ciliate. Sepals ovate, ca. 3.5 × 2 mm, slightly reflexed. Petals 6, valvate, free, in 2 whorls; lemon to yellowish green, narrowly elliptic-oblong, apex acute to obtuse, both whorls subequal, 12–13 × 3.5–4.5 mm, glabrous, slightly ciliate. Stamens ca. 50 per flower, ca. 1 mm long; connective truncate. Carpels 8–10 per flower, glabrous; stigma ovoid, puberulent; ovary ca. 1 mm long, with 1 or 2 line(s) of hairs; ovules 2 per ovary, lateral, arranged vertically in 1 row. Fruiting pedicel becoming longer and thicker, 8–11 mm long, 4–5 mm in diameter when fresh, 2–3 mm in diameter when dry; monocarp stipes 7–11 mm long, 3.5–5 mm in diameter when fresh, 2–3 mm in diameter when dry; monocarps ellipsoid to rhomboid, 2.7–3.8 cm long, 1.3–2.6 cm in diameter when fresh; 2.4–3.3 cm long, 1.2–2.3 cm in diameter when dry, apex with a small prominent beaklike protuberance and the coat of fresh monocarps secreting white juice when injured. Seeds 1 or 2 per monocarp, cylindrical, 2.2–3.2 cm long, 1.0–1.3 cm in diameter when fresh; 2.0–2.7 cm long, 0.9–1.2 cm in diameter when dry, endosperm rumination lamelliform.
Distribution and habitat: — Only known from Mêdog in Xizang, growing in subtropical rainforests, at elevations of 800–1000 m.
Phenology: — Flowering in August (Li, 1976). Fruiting from March to July at XTBG.
Vernacular name: — Mandarin Chinese: xī zàng yóu yè mù (西藏疣叶木).
Conservation status: — This species is only known from its type locality in Mêdog and we have no other information on its natural distribution. We temporarily assess it as Data Deficient (DD) (IUCN Standards and Petitions Committee, 2019). A thorough survey may provide more information on its distribution and population status.
Additional specimens examined: — CHINA. Yunnan, Mengla County, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, introduced from Mêdog County, Tibet (Xizang), cultivated, 20 March 2021, B. Yang XTBG-0215 (HITBC); 29 May 2021, B. Yang YB1002 (fr.) (HITBC), 3 July 2021, B. Yang YB1009 (fr.) (HITBC).
Notes: — Although Polyalthiopsis chinensis was flowering in August based on the type materials, there are only two collections of this species from Mêdog, Xizang, China (Xue et al., 2020). Thus, phenological information for this species is still limited. The living collections of P. chinensis in XTBG were propagated from 18 seeds collected from Mêdog County in 2003. We found one of the individuals fruiting in March, and it gave us the chance to observe its fruiting period continuously.
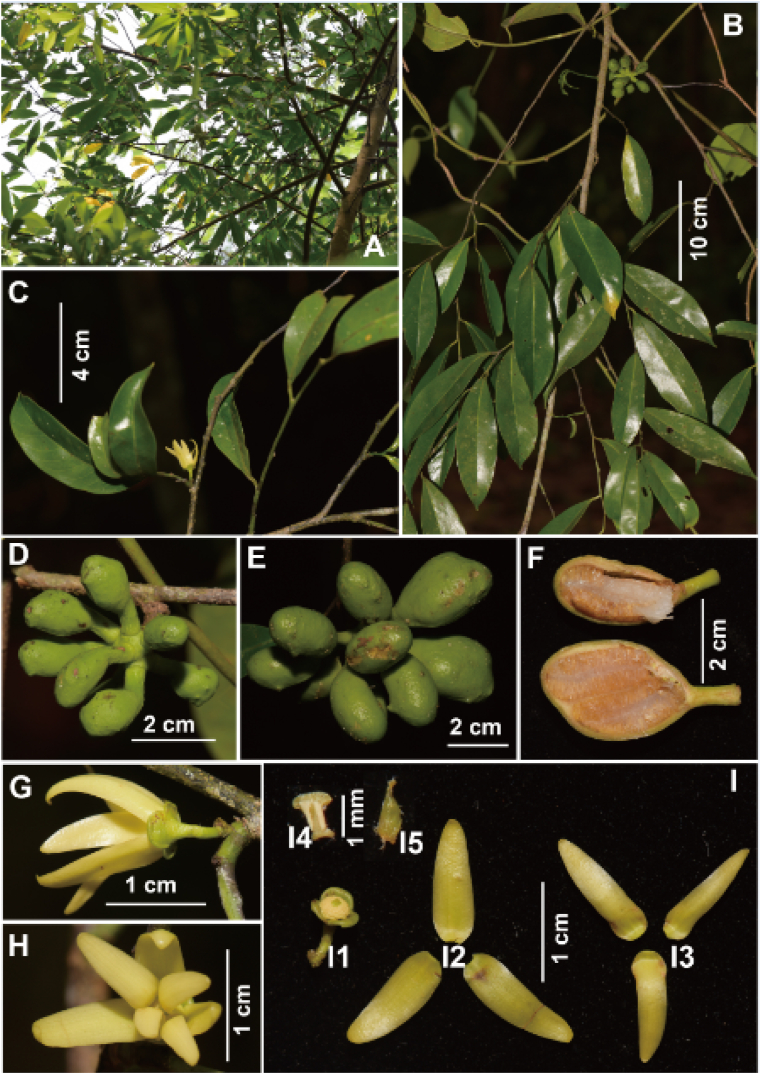
5.4. Polyalthiopsis verrucipes (C.Y. Wu ex P.T. Li) B. Xue & Y.H. Tan, PhytoKeys, 148: 82, 2020. (Fig. 9)
Fig. 9.
Polyalthiopsis verrucipes (C.Y. Wu ex P.T. Li) B. Xue & Y.H. Tan. (A) Branches; (B) Flowering branch; (C) Fruiting branch; (D) Longitudinal section of monocarp; (E) Flowers (front view); (F) Flowers (side view); (G) Flowers (bottom view); (H) Fruits; (I) Seeds; (J) Monocarp with longitudinal removal of half pericarp, showing the two seeds.
Basionym: Polyalthia verrucipes C.Y. Wu ex P.T. Li, Acta Phytotax. Sin., 14: 110. 1976. Type: — CHINA. Yunnan Province, Menghai County, Heilongtan, July 1936, C.W. Wang 76321 (lectotype: designated by Xue et al., PhytoKeys, 148: 82, 2020. IBSC0003386; isolectotypes: A00039580, IBSC0003386, PE01187287, PE01187470, NAS00321991).
Phenology: — Polyalthiopsis verrucipes was observed flowering in February to March, fruiting from April to September.
Distribution and habitat: — Known from several localities in Yunnan Province, growing in mountain evergreen forests, at elevations of 1300–1800 m.
Vernacular name: — Mandarin Chinese: yóu yè mù (疣叶木).
Conservation status: — This species was regarded as endangered (EN) and listed as a plant species with extremely small populations in Yunnan Province (Sun, 2021).
Additional specimens examined: — CHINA. Yunnan Province, Menghai County, Daluo Town, Manxi, 9 June 2020, B. Yang & P.Y. Wang MH414 (HITBC), MH416 (fr.) (HITBC); 10 August 2020, B. Yang, P.Y. Wang, G.Z. Yang & Z.B. Yang MH504 (HITBC), 4 March 2021, B. Yang & D.L. Quan T877 (fl.) (HITBC); 13 September 2021, B. Yang & X.D. Zeng YB1083 (fr.) (HITBC, PE); Jinghong, Menglong Town, Mengsong, April 1998, H. Wang 85 (HITBC).
Notes: — A detailed description of P. verrucipes is given by Xue et al. (2020). Our observations and collections indicate that the white flowers of this species are unique in this genus.
5.5. Polyalthiopsis floribunda (Jovet-Ast) Chaowasku, Ann. Bot. Fennici, 55: 130, 2018
Basionym: Polyalthia floribunda Jovet-Ast, Notul. Syst., 9: 75, 1940. Huberantha floribunda (Jovet-Ast) I.M.Turner, Webbia, 71: 229, 2016. Type: — VIETNAM. Phanrang Province, Tra Ca, 10 March 1924, Poilane 10052 (holotype: P00411080; isotypes: A00351290, BO?, CMUB, HN, K000608178, L3728819, P00411081, P00411082).
Phenology: — Flowering in March based on the type collection. The fruit falls in July and the clear fruiting period is unknown (Chaowasku et al., 2018b).
Distribution and habitat: — P. floribunda is known to occur in two localities from Thừa Thiên-Huế and Ninh Thuận Provinces of Vietnam (Chaowasku et al., 2018b), growing in evergreen forests.
Conservation status: — The conservation status of Polyalthiopsis floribunda was regarded as critically endangered (CR) in Chaowasku et al. (2018b).
Notes: — A comparison between this species and its congeners is made in Table 1.
Key to species ofPolyalthiopsis
-
1a
Inflorescences with solitary flower; pedicel 1–2 mm long; flowers white ................................................................P. verrucipes
-
1b
Inflorescences 1–4-flowered; pedicel 3–7 mm long; flowers lemon to yellowish green (P. floribunda is unclear) ..................2.
-
2a
Pericarp of fresh monocarps secreting white liquid when injured.................................................................................P. chinensis
-
2b
Pericarp of fresh monocarps not secreting white liquid when injured ....................................................................................................3.
-
3a
Carpels 7 per flower, ovules 1 per carpel, monocarps cylindrical .................................................................................P. floribunda
-
3b
Carpels 8–15 per flower, ovules 2 per carpel, monocarps obovoid or ellipsoid to rhomboid .................................................4.
-
4a
Secondary veins of leaf blades 8–14 pairs; outer petals elliptic-ovate, 7–10 mm long; monocarps ellipsoid to rhomboid .......................................................................................P. xui
-
4b
Secondary veins of leaf blades 15–25 pairs; outer petals narrowly elliptic-oblong, 13–16 mm long; monocarps obovoid .......................................................................................P. nigra
Author contributions
Y.H.T. first discovered the new species in XTBG, Y.H.T. and B.Y. collected the material in the field. B.Y., H.B.D. and C.F.X. monitor the phenological period of the new species in XTBG, J.Y.L., R.J.Y., Y.J.Z., M.D. carried out the molecular experiment and analyzed the data. B.Y., Y.H.T., and Y.J.Z. wrote and revised the manuscript. All authors contributed to the revision of the manuscript.
Declaration of competing interest
All the authors declare that there is no conflict of interest.
Acknowledgments
We are grateful to Prof. Richard T. Corlett for his kind help in improving the manuscript, and to Mr. Shi-Wei Guo and Jun Zhang for providing the habitat information of the two new species in the wild. We thank Dr. Wen-Bin Yu for guidance on phylogenetic analysis, Ms. Han Lai, Mr. Wen-Guang Wang and Hai-Bo Mo for their help during the fieldwork, Dr. Jian-Wen Zhang for critical comments on the manuscript, Mr. Lin Wang for preparing the distribution map, and Mr. Yi-Fan Li for the line drawings. We thank Mr. Ting Tang and Ms. Rui Zhang of the Institutional Center for Shared Technologies and the Facilities of Xishuangbanna Tropical Botanical Garden, CAS for their technical assistance in SEM observation. We also thank the editors and reviewers for their work on the manuscript. This research was funded by the National Natural Science Foundation of China (Grants Nos. 31900180 and 31970223), the Special Fund of Yunnan Fundamental Research Projects (Grant No. 202101AT070058), the project of the Yunnan Academy of Forestry and Grassland (Grant No. KFJJ21-01), the Project of National Plant Specimen Resource Center (NPSRC) (E0117G1001), the Biodiversity Investigation Observation and Assessment Program (2019–2023) of Ministry of Ecology and Environment of China and the project of the Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences (Grant No. Y4ZK111B01).
Footnotes
Peer review under responsibility of Editorial Office of Plant Diversity.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.05.001.
Contributor Information
Yun-Juan Zuo, Email: zuoyunjuan@xtbg.ac.cn.
Yun-Hong Tan, Email: tyh@xtbg.org.cn.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Bangkomnate R., Damthongdee A., Baka A., et al. Pyramidanthe and Mitrella (Annonaceae, Uvarieae) unified: molecular phylogenetic and morphological congruence, with new combinations in Pyramidanthe. Willdenowia. 2021;51:383–394. [Google Scholar]
- Chaowasku T. Toward a phylogenetic reclassification of the subfamily Ambavioideae (Annonaceae): establishment of a new subfamily and a new tribe. Acta Bot. Bras. 2020;34:522–529. [Google Scholar]
- Chaowasku T., Aongyong K., Damthongdee A., et al. Generic status of Winitia (Annonaceae, Miliuseae) reaffirmed by molecular phylogenetic analysis, including a new species and a new combination from Thailand. Eur. J. Taxon. 2020;659:1–23. [Google Scholar]
- Chaowasku T., Damthongdee A., Jongsook H., et al. Enlarging the monotypic Monocarpieae (Annonaceae, Malmeoideae): recognition of a second genus from Vietnam informed by morphology and molecular phylogenetics. Candollea. 2018;73:261–275. [Google Scholar]
- Chaowasku T., Damthongdee A., Jongsook H., et al. Genus Huberantha (Annonaceae) revisited: erection of Polyalthiopsis, a new genus for H. floribunda, with a new combination H. luensis. Ann. Bot. Fenn. 2018;55:121–136. [Google Scholar]
- Chaowasku T., Johnson D.M., van der Ham R.W.J.M., et al. Characterization of Hubera (Annonaceae), a new genus segregated from Polyalthia and allied to Miliusa. Phytotaxa. 2012;69:33–56. [Google Scholar]
- Chaowasku T., Mols J.B., van der Ham R.W.J.M. Pollen morphology of Miliusa and relatives (Annonaceae) Grana. 2008;47:175–184. [Google Scholar]
- Chaowasku T., Thomas D.C., van der Ham R.W.J.M., et al. A plastid DNA phylogeny of tribe Miliuseae: Insights into relationships and character evolution in one of the most recalcitrant major clades of Annonaceae. Am. J. Bot. 2014;101:691–709. doi: 10.3732/ajb.1300403. [DOI] [PubMed] [Google Scholar]
- Chatrou L.W., Turner I.M., Klitgaard B.B., et al. A linear sequence to facilitate curation of herbarium specimens of Annonaceae. Kew Bull. 2018;73:39. doi: 10.1007/s12225-018-9764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatrou L.W., Pirie M.D., Erkens R.H.J., et al. A new subfamilial and tribal classification of the pantropical flowering plant family Annonaceae informed by molecular phylogenetics. Bot. J. Linn. Soc. 2012;169:5–40. [Google Scholar]
- Doyle J.J., Doyle J.D. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987;19:11–15. [Google Scholar]
- Darriba D., Taboada G.L., Doallo R., et al. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Tang C.C., Thomas D.C., et al. A mega-phylogeny of the Annonaceae: taxonomic placement of five enigmatic genera and recognition of a new tribe, Phoenicantheae. Sci. Rep. 2017;7:7323. doi: 10.1038/s41598-017-07252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUCN Standards and Petitions Committee Guidelines for using the IUCN Red List Categories and Criteria. Version 14. 2019. http://www.iucnredlist.org/documents/RedListGuidelines.pdf Prepared by the Standards and Petitions Committee. Downloadable from:
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software Version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M., Moir R., Wilson A., et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.T. Some notes on the Annonaceae of China. Acta Phytotax. Sin. 1976;14:96–113. [Google Scholar]
- Punt W., Hoen P.P., Blackmore S., et al. Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 2007;143:1–81. [Google Scholar]
- Rambaut A., Suchard M.A., Xie D., et al. Tracer v1.6. 2014. http://beast.bio.ed.ac.uk/Tracer Available from:
- Ronquist F., Teslenko M., van der Mark P., et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y.Y., Xu F.X. Studies on pollen morphology of selected species of Annonaceae from Thailand. Grana. 2018;57:161–177. [Google Scholar]
- Sun W.B. Yunnan Science and Technology Press; Kunming: 2021. List of Yunnan Protected Plant Species with Extremely Small Populations (2021) [Google Scholar]
- Trifinopoulos J., Nguyen L.T., von Haeseler A., et al. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016;44:W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Ding H.B., Yao G., et al. From Polyalthia to Polyalthiopsis (Annonaceae): transfer of species enlarges a previously monotypic genus. PhytoKeys. 2020;148:71–91. doi: 10.3897/phytokeys.148.50929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Shao Y.Y., Saunders R.M.K., et al. Alphonsea glandulosa (Annonaceae), a new species from Yunnan, China. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Su Y.C.F., Thomas D.C., et al. Pruning the polyphyletic genus Polyalthia (Annonaceae) and resurrecting the genus Monoon. Taxon. 2012;61:1021–1039. [Google Scholar]
- Xue B., Tan Y.H., Thomas D.C., et al. A new Annonaceae genus, Wuodendron, provides support for a post-boreotropical origin of the Asian-Neotropical disjunction in the tribe Miliuseae. Taxon. 2018;67:250–266. [Google Scholar]
- Xue B., Shao Y.Y., Xiao C.F., et al. Meiogyne oligocarpa (Annonaceae), a new species from Yunnan, China. PeerJ. 2021;9 doi: 10.7717/peerj.10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Zhu R.B., Ding H.B., et al. In: Plant Diversity of Southeast Asia-II. Jin X.H., Xia N.H., Tan Y.H., editors. vol. 138. 2020. A new species and two new records of Goniothalamus (Annonaceae) from Lao PDR; pp. 17–25. (PhytoKeys). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.