Abstract
There is an increasing number of people with diabetes on peritoneal dialysis (PD) worldwide. However, there is a lack of guidelines and clinical recommendations for managing glucose control in people with diabetes on PD. The aim of this review is to provide a summary of the relevant literature and highlight key clinical considerations with practical aspects in the management of diabetes in people undergoing PD. A formal systematic review was not conducted because of the lack of sufficient and suitable clinical studies. A literature search was performed using PubMed, MEDLINE, Central, Google Scholar and ClinicalTrials.gov., from 1980 through February 2022. The search was limited to publications in English. This narrative review and related guidance have been developed jointly by diabetologists and nephrologists, who reviewed all available current global evidence regarding the management of diabetes in people on PD.We focus on the importance of individualized care for people with diabetes on PD, the burden of hypoglycemia, glycemic variability in the context of PD and treatment choices for optimizing glucose control. In this review, we have summarized the clinical considerations to guide and inform clinicians providing care for people with diabetes on PD.
Keywords: diabetes, kidney failure, peritoneal dialysis
Management of diabetes in people with kidney failure (KF) can pose a clinical challenge because of multiple factors, including high risk of hypoglycemia, impact of kidney replacement therapy on glucose levels, prevalence of multiple comorbidities, and contraindication of certain diabetes medications in KF. Even though there are several recent guidelines and review articles on the management of diabetes in people on hemodialysis, there is a gap in the literature and knowledge in this area for people receiving PD.1 The aim of this narrative review is to appraise the relevant literature and summarize the clinical considerations and practice points to guide clinicians looking after people with diabetes on PD. Our review includes recommendations from the recent Joint British Diabetes Societies Inpatient Care guideline on the management of adults with diabetes on dialysis that incorporates a chapter on management of diabetes in people undergoing PD.1
Search Strategy
In reviewing the literature evidence, we conducted a search for the literature from the last 42 years, from 1980 through February 2022 using PubMed, MEDLINE, Central, Google Scholar and ClinicalTrials.gov. The search was limited to publications in English. Because of the limited availability of studies related to diabetes in the PD population, all systematic reviews, meta-analysis, prospective observational studies of cross-sectional, case control, longitudinal cohort design or randomized studies, and case series were included. The terms “peritoneal dialysis,” “diabetes,” “chronic kidney disease,” “kidney failure, “icodextrin,” “glucose-based dialysate,” “insulin,” “hypoglycemia,” “nutrition,” “exercise,” “insulin,” and other relevant items in different combinations helped to identify relevant literature. Reference lists from relevant articles were hand-searched, and the search was further supplemented by key articles by the experts in the relevant fields.
The literature search yielded 1985 records and after excluding duplicates, 1234 titles were screened, and 134 relevant articles were selected for full text review, together with 8 additional articles from direct citation search, out of which 112 were finally included in the current review (Supplementary Figure).
Because of the dearth of evidence to support management decisions, we have developed a series of clinical practice points to inform and guide clinicians looking after people with diabetes on PD rather than making explicit recommendations (Table 1). Practice points represent the expert judgment of the writing group and may also be on the basis of limited evidence. Unlike recommendations, practice points are not graded for strength of recommendation or quality of evidence.
Table 1.
Summary practice points
| Summary practice points |
|---|
|
|
|
|
|
|
|
|
|
|
|
HbA1c, hemoglobin; PD, peritoneal dialysis.
Impact of Dialysis on the Glycemic Control and Metabolic Parameters in People Undergoing Peritoneal Dialysis
Diabetes remains the most common cause of KF globally, accounting for nearly 50% of KF cases worldwide.2,3 For many people, PD as a home-based therapy provides multiple advantages over hemodialysis, in terms of autonomy and independence. Current data suggest that PD accounts for 9% of all people receiving kidney replacement therapy and 11% of all undergoing dialysis.4,5 People with diabetes on PD have a higher risk of mortality and technique failure compared with those who do not have diabetes; they are also at significant risk for diabetes-related complications such as eye disease and foot disease.6,7
The role of PD in the care of people with KF and diabetes has previously caused debate. A considerable number of observational studies of varying sizes have examined the relationship between dialysis modality and survival. Widely differing outcomes have been reported, some favoring hemodialysis,8 others favoring PD,9 and still others reporting equivalence.10,11 These studies also report on significant modifiers of this relationship and suggest that hemodialysis confers survival benefits for older patients, patients with diabetes, and those on kidney replacement therapy for more than 2 years.12,13 A major limitation of these observational studies has been the inability to fully adjust for the substantial systematic differences between PD and hemodialysis cohorts, resulting in selection bias. More recent studies utilizing propensity score matching to adjust for these differences have given more reassuring and equivalent outcomes.14, 15, 16
The role of PD in the worsening of metabolic parameters remains unclear. Commercially available peritoneal dialysates contain 1 of 3 osmotic agents, namely glucose, icodextrin, or amino acids. Glucose-containing dialysates remain the most used because they are largely safe, effective, and inexpensive. The standard glucose-containing dialysate solutions consist of dextrose monohydrate in variable concentrations ranging from 1.36% to 4.25% and are available in different brands. As a result of its small molecular size, glucose is freely absorbed from the peritoneal cavity, resulting in a loss of the osmotic gradient and net absorption of glucose estimated at 100 to 300 g per 24 hours.17, 18, 19 The amount of glucose absorbed into the blood will depend on the tonicity and volume of the dialysate, transport characteristics of the peritoneal membrane, dwell time, and the individual’s blood glucose level.19
A study in 1981 involving 7 people on continuous ambulatory peritoneal dialysis (CAPD) demonstrated that the amount of glucose absorbed through the dialysate is directly proportional to the glucose concentration in the peritoneal dialysate, ranging from 1.5% to 4.25% glucose.20 A more recent study involving 8 people with either type 1 diabetes or type 2 diabetes with KF on CAPD demonstrated that the quality of glycemic control can be affected by the type of PD fluid being used.21 In this study, the mean 24-hour continuous glucose monitoring (CGM) glucose level was higher in the phase which used 1.36% and 3.86% dextrose-containing dialysate than in the phase which used 1.36% and icodextrin-containing dialysate.21
There is conflicting evidence with regard to new onset or worsening of hyperglycemia with glucose-containing PD solutions. Although new onset hyperglycemia and impaired glucose tolerance have been reported in people commenced on PD,22,23 subsequent epidemiologic studies and a recent meta-analysis indicated no difference in the risk of new onset hyperglycemia compared with their hemodialysis counterparts.24,25 In the early stages of time on PD, an improvement in insulin sensitivity as a result of reduced uremia may offset some of the additional glucose load.26
Small physiological studies have demonstrated increases in plasma glucose concentrations associated with glucose-containing dwells in people with diabetes.21,27, 28, 29 The Global Fluid Study reported higher random glucose levels associated with increasing dialysate glucose exposure in people without diabetes.30 Because people on dialysis have multiple risk factors for hyperglycemia, insulin resistance and metabolic derangement, the relative impact of peritoneally-absorbed glucose on short-term and long-term glycemic control remains unclear. As discussed below, quantifying the metabolic burden of PD is further complicated by the choice of an appropriate and accurate marker of glycemia in this population.
Diabetes in People on PD
A detailed description of the diagnosis of new onset diabetes in people on PD is out of the scope of this article and should be based on the standard diagnostic criteria as per the current available guidelines.31,32 Only a few studies are available with regard to the incidence of new onset diabetes in people on PD, and these show conflicting data with regard to the comparative incidence of new onset diabetes in people on PD compared to hemodialysis.33, 34, 35
In our opinion, when a person with diabetes is newly initiated on PD, close monitoring of blood glucose is required because of the known effects of PD on glycemic control. Blood glucose needs to be checked before and after each exchange to assess whether the PD per se has had an impact on the glycemic control. If the blood glucose values after the exchanges are persistently above 10 mmo/l (180 mg/dl), and if the person is on maximum doses of oral glucose-lowering therapies, a discussion with the diabetes team with regard to initiation of insulin may be warranted. If the person is already on insulin before PD, we advise discussions with the diabetes team for dose adjustments and please refer to the section on Clinical Considerations When Using Insulin Treatment in People With Diabetes on PD in this review for further information. In someone on diet and lifestyle control only for their diabetes, we would advise the same process as above and if values are persistently above 10 mmo/l (180 mg/dl), a discussion with the diabetes team on suitable oral treatment options, or insulin if overt hyperglycemic symptoms are present. Similarly, if a person with diabetes is newly initiated on automated peritoneal dialysis (APD), we recommend that monitoring blood glucose for the first week after initiation, to assess the impact of APD on the glycemic control as optimization of glucose-lowering therapies, will be required. Details on treatment options are described in more detail in the section on Treatment of Diabetes in People on PD.
Monitoring of Glucose Control in People With Diabetes on PD
Assessing Long-term Glycemic Control
Please refer to Table 2 for a tabulated summary of advantages and disadvantages of available glycemic monitoring methods in people on PD. Overall, HbA1c is currently the best evidence-based measure of long-term glycemic control in people with diabetes without KF and reflects average glycemia over approximately 8 to 12 weeks, equivalent to the red cell lifespan.36 There is a well-established relationship between HbA1c, estimated average glucose levels, and the risk of diabetes-related morbidity and mortality.37,38
Table 2.
Monitoring glycemic control in people on peritoneal dialysis
| Modality | Pros | Cons |
|---|---|---|
| HbA1c |
|
|
| Fructosamine |
|
|
| Glycated albumin |
|
|
| Capillary blood glucose (CBG) monitoring |
|
|
| Intermittent scanned or real-time continuous glucose monitoring systems |
|
|
CBG, capillary blood glucose; KF, kidney failure; KT, kidney transplant; PD, peritoneal dialysis; TIR, time in range.
Because of multiple factors including anemia, and its treatment with iron and erythropoietin, HbA1c can be less reliable in people with advancing chronic kidney disease (CKD), particularly those with CKD stages 4 and 5.39, 40, 41 Modern HbA1c assays are, however, less likely to be influenced by uremia or hemoglobinopathies.42,43
There is limited observational evidence for a J-shaped relationship between HbA1c and mortality in hemodialysis cohorts.44, 45, 46, 47 Similar studies in purely PD cohorts are scarce and have produced conflicting results17,48 Therefore, unlike in the non-dialysis diabetes population there is no compelling evidence linking improvements in HbA1c with measurable benefits in clinical outcomes.
Other proposed alternatives for assessment of long-term glycemic control include fructosamine and glycated albumin (GA). Establishing a relationship between these markers and mortality or other clinical outcomes has also been challenging.
GA is formed when glucose is covalently bound to a free amino group on albumin. Because of the shorter half-life of albumin, it is thought to represent glycemic levels in the preceding 3 weeks. Because the half-life of albumin is not affected by uremia, in people on dialysis, GA has been proposed as a more accurate marker of glycemia and more predictive of outcomes compared with HbA1c; however the single study suggesting this was in a population predominantly composed of people on hemodialysis.49 There have been concerns about the utility of GA in people on PD given the high prevalence of hypoalbuminemia, as a result of both proteinuria and albumin losses into PD fluid.50 However, there are some observational data suggesting a predictive relationship between GA and mortality in people on PD.48
When addressing how accurately these markers reflect glycemic control, the challenge is establishing what the comparative marker should be. Several small studies using CGM have suggested moderate correlations between mean interstitial glucose concentrations and both HbA1c (r = 0.51) and fructosamine (r = 0.45).51, 52, 53 Preliminary results from The Glycaemic Indices in Dialysis Evaluation study showed moderate correlations between all 3 markers and random glucose levels.54 Although this study had only a small proportion of people on PD (16%), it still represents the largest prospective study (282 people) of glycemia and diabetes-related outcomes in people on PD .
Overall, there are potential drawbacks to all 3 methods and the results need to be interpreted with caution and in the context of individual patient characteristics (Table 2). There are insufficient data to recommend one measurement assay over others in the context of PD; however in our opinion HbA1c is generally preferred because of the wide availability of a standardized assay and longer duration of experience.
Assessing Glycemic Variability
Self-Monitoring of Capillary Blood Glucose
Capillary blood glucose monitoring is the most used method of assessing day-to-day glycemic variability in diabetes. Glucometer and strips in people on PD should be chosen with caution because of certain types of glucometers giving rise to false readings.
There are 2 key components of a glucometer, namely an enzyme reaction and a detector. Three types of enzymatic reactions are currently being utilized: glucose oxidase, glucose dehydrogenase (GDH), and hexokinase. GDH-based glucometers use 3 types of coenzymes; GDH and GDH-PQQ, GDH and coenzyme nicotine adenine dinucleotide, and GDH and coenzyme flavin adenine dinucleotide. Out of these, GDH-PQQ is not glucose specific and can react with other sugars including maltose, galactose, and xylose, thereby giving rise to falsely elevated glucose readings, resulting in insulin overdose and hypoglycemia.55
This becomes a particular issue in people on PD with icodextrin-based dialysate, because icodextrin metabolizes to maltose, which can cross react with the GDH-PQQ based glucometer system. Adverse events, including severe hypoglycemia and death have been reported with this assay being used in the care of people with diabetes on icodextrin-based PD.55, 56, 57, 58 Consequently GDH-PQQ based systems should always be avoided in people on PD. Clinicians and patients are recommended to review the labels of both the glucometer and the test strips used, or if in doubt, contact the manufacturers to ensure the type of enzymatic method being used. Furthermore, maltose metabolites produced after PD with icodextrin take at least 2 weeks to return to baseline; therefore, the glucometer assay interference may persist for some time even after cessation of icodextrin dialysate use.59
Intermittent Scanned and Real-Time CGM
Intermittent scanned (flash) and real-time CGM systems are rapidly evolving technologies which are being increasingly used in the care of people with diabetes for over a decade. By measuring interstitial glucose concentrations at regular intervals throughout the day and night, they allow assessment of glycemic variability and detection of asymptomatic hypoglycemia and hyperglycemia. Newer models are being used as replacements for self-monitoring of blood glucose and have the advantages of providing data on glycemic variability, estimated HbA1c, percentage time in range, hyperglycemia, and hypoglycemia; and along with low alerts are extremely useful in detecting asymptomatic hypoglycemia. However, their utility in the care of people on dialysis is still being defined.60
Studies with small numbers of participants were encouraging with regard to accuracy compared to venous glucose measurements.21 However, the accuracy of newer models has not been rigorously assessed in PD cohorts, and therefore, the potential for interference by physiological states such as hypoxia and uremia and exogenous substances such as maltose has not been assessed. Of note, most commercially available intermittent scanned and real-time CGM systems use glucose oxidase-based enzymatic reactions, which are not known to be affected by icodextrin metabolites.60
Small-scale studies in people on PD have demonstrated that self-monitoring of blood glucose as routinely carried out by people with diabetes can miss many hours of high readings and therefore underestimate actual levels of glycemia,61 thereby proving the utility of intermittent scanned and real-time CGM systems. They may also prove useful in broadening our understanding of the impact of peritoneally-absorbed glucose on overall glycemic control. These small-scale studies, one involving 8 people with insulin-treated diabetes on CAPD (both type 1 diabetes and type 2 diabetes), and the other involving 25 people with diabetes on PD (details on diabetes type or treatments were not reported) have demonstrated modest to reasonably good correlations of CGM systems with venous self-monitoring of blood glucose measurements, as well as fructosamine, and HbA1c levels.21,51
Studies have demonstrated significantly different effects of APD and CAPD on the 24-hour glycemic profiles, which would not be appreciated by traditional metabolic markers such as HbA1c, fasting plasma glucose, or markers of insulin resistance.62,63
Although the use and benefits of CGM systems is yet to be tested in large scale trials among people on PD, data from the above small studies, and larger studies in hemodialysis cohorts and clinical real world experience suggest that this technology can be beneficial in people with diabetes on PD, especially with regard to minimizing glycemic variability and hypoglycemia.64,65 How CGM generated metrics such as time in range (defined as the time spent between glucose levels 3.9 to 10 mmol/l (70−180 mg/dl]), and glycemic variability correlate to overall diabetes-related complications, morbidity, and mortality is yet to be elucidated.
Treatment of Diabetes in People on PD
Glycemic Targets
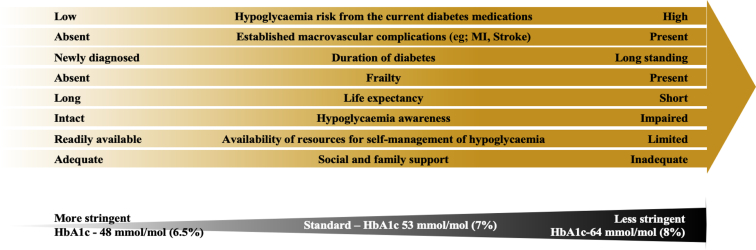
Considering that no large studies have been conducted that examined the relationship between glycemic control and outcomes in people on PD, current guidelines are extrapolated from studies in people with normal kidney function and some studies in people on hemodialysis. From the data from other CKD populations, it is evident that both inadequately controlled and excessively lowered glycemia can be associated with adverse health outcomes.45,66 Therefore, as per the current guidelines, less stringent HbA1c targets than the standard target of 53 mmol/mol (7.0%) may need to be considered in these people because of the higher risk of hypoglycemia.67, 68, 69 As outlined in Figure 1, these individuals may also have other risk factors for hypoglycemia in addition to KF, therefore supporting the need for less stringent HbA1c targets. The main goals of treating diabetes in people on PD should be to maintain euglycemia particularly during the dwell time, to prevent postprandial hyperglycemia, and to avoid hypoglycemia, although achieving all of these goals may be challenging. Treatment considerations in managing diabetes in people on PD are summarized in Table 3.
Figure 1.
Individualizing HbA1c targets; factors to be taken into consideration in the person with diabetes on peritoneal dialysis. MI, myocardial infarction.
Table 3.
Treatment considerations in managing diabetes in people on peritoneal dialysis
Oral anti-diabetic drugs and Glucagon-like peptide receptor agonists in peritoneal dialysis
|
Insulin regimen
|
Route of insulin
|
Hypoglycemia detection and management
|
| Changes to dialysate prescription Any changes to dialysate prescription with changes of glucose load should lead to changes of insulin prescription accordingly to avoid hypoglycemia or increased glycaemic variability.Any changes to dialysate prescription with changes of glucose load should lead to changes of insulin prescription accordingly to avoid hypoglycemia or increased glycemic variability. |
Insulin pumps
|
APD, automated peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; DPP-4, dipeptidyl peptidase-4; SGLT2i, Sodium glucose cotransporter-2 inhibitors; PD, peritoneal dialysis.
For people on intermittently scanned or real-time CGM systems, the time in range (TIR) target (3.9–10 mmol/l or 70−180 mg/dl) will depend on the age, comorbidities, and the risk of hypoglycemia as described in recent recommendations.70 However, there is lack of consensus for the TIR targets in people with PD because of insufficient evidence. In general, for older people on PD with multiple comorbidities and frailty with a high risk of hypoglycemia, a lower TIR target of 50% to 70% is preferred, with a target time <3.9 mmol/l (or <70 mg/dl) of below 1%. For younger people with diabetes on PD, we recommend a TIR target of 70% or above, with a time <3.9 mmol/l (or <70 mg/dl) of less than 4%. These more stringent goals (TIR >70%) in our opinion should also be considered in people with coexisting diabetes microvascular complications such as retinopathy or those being prepared for kidney transplantation who do not have additional risk factors for severe hypoglycemia (Table 2).
Oral Glucose-Lowering Therapies and Glucagon-Like Peptide-1 Receptor Agonists
Options for oral glucose-lowering agents in people with diabetes and advanced CKD are often limited (Table 3). For those with KF, treatment options include dipeptidyl peptidase-4 inhibitors (or “gliptins”) and pioglitazone. A study involving 36 people with both diabetes and nondiabetes (out of which 10 had type 2 diabetes), and high triglycerides on PD demonstrated marked improvement of parameters of dysmetabolism, including fasting plasma glucose, markers of insulin resistance and surrogate markers of inflammation, whereas no significant changes in HbA1c was observed after 12 weeks of pioglitazone treatment at 15 mg daily dose.71 Although pioglitazone can be used in KF, in our opinion, it may be less preferred because of known adverse effects, including worsening of fluid retention, higher risk of fractures, and diabetic macular edema.72 Sulphonylureas and metformin are contraindicated in KF. Glucagon-like peptide-1 receptor agonists are also not licensed for the use in KF. Dipeptidyl peptidase-4 inhibitors are not associated with an increased risk of hypoglycemia. Linagliptin can be used at 5 mg dose across a range of CKD stages, and other agents in the dipeptidyl peptidase-4 inhibitors class can be used in KF but with appropriate dose adjustments as per their license.72
The glucose-lowering mechanism of the sodium-glucose-transporter-2 inhibitors (SGLT-2i) depends on the estimated glomerular filtration rate, and is therefore limited in people with KF.73 Overall, glycemic benefit of SGLT-2i is low in people with estimated glomerular filtration rate <45 ml/min per 1.73 m2 with no significant reduction of HbA1c.72 However, cardiovascular and kidney outcome trials have demonstrated benefits of SGLT-2i for slowing the progression of CKD and improving cardiovascular outcomes and all-cause mortality even in those with advanced CKD stages, irrespective of the presence or absence of diabetes.74, 75, 76, 77, 78 Whether these effects extend into dialysis populations remains unclear. It has been suggested that there may be a role for SGLT-2i in preserving residual kidney function and protecting the peritoneal membrane although this is yet to be rigorously studied.79
SGLT-2 receptors are also expressed on human peritoneal mesothelial cells resulting in interest in a potential use for SGLT-2i to reduce peritoneal glucose absorption and thus maintain the osmotic gradient for longer while reducing the additional metabolic load. The 3-pore model dictates that glucose transport is predominantly paracellular and the result of diffusion; therefore the effect of blocking transport of glucose into cells is likely to be minimal. Animal models have produced conflicting results.80,81 A study in humans is currently underway (ClinicalTrials.gov Identifier: NCT05250752).82
Insulin-Clinical Considerations When Using Insulin Treatment in People With Diabetes on PD
Route of Administration
Intraperitoneally administered insulin has previously been advocated to mitigate the additional glucose load associated with PD.83 However, multiple studies have reported adverse effects, including abnormal lipid profile, hepatic subcapsular steatosis, and increased risk of peritonitis.84, 85, 86 Therefore, we recommend that insulin only be administered via the subcutaneous route.
Insulin dose adjustments
It is well known that the kidney plays an important role in the metabolism of exogenous insulin, accounting for up to 80%, because it is not subjected to first pass metabolism in the liver.87 As the individual progresses to KF, they are more prone to hypoglycemia because of reduced kidney clearance of insulin. Considerations in managing insulin treatment for people with diabetes on PD are summarized in Table 3.
Although some studies have suggested that additional insulin would be needed to account for increases in dialysate glucose load,88 there is sparse evidence to guide recommendations on specific dose adjustments and as discussed above, the impact of peritoneally-absorbed glucose on glycemia varies between patients and is dependent on multiple factors, including individual membrane characteristics.89 We therefore suggest that an adjustment in insulin dose should be considered if dialysate glucose load is changed and should be individualized.
Considering that evidence with regard to the insulin dose adjustments in people on PD are sparse, the following suggestions are based on the clinical opinions and the evidence from non-PD populations.
In our opinion, for insulin-treated people on PD, a multiple-daily-injection regime with long-acting (basal) analog insulin and meal-time short-acting insulin is preferred if this is feasible and the person is agreeable to such a regime. A study in 47 people with diabetes (4 had type 1 diabetes) on PD demonstrated a reduction in mean HbA1c of 0.74%, and 52% of the cohort achieved HbA1c <7.5% after a 3-month intervention consisting of an educational program combined with multiple daily injections of analog basal and meal-time short-acting insulin. Importantly, this improvement in HbA1c was not associated with an increased risk of hypoglycemia. Of note, most of the cohort (44 of 47) were already on a multiple daily injections before being enrolled in the intervention.90 The authors reported that they initiated multiple-daily-injection regime at 0.5 units of insulin per kg using 50% of dose as basal insulin and 50% of dose as premeal bolus insulin with titration of basal insulin every 3 days, aiming for fasting glucose target between 5.5 to 7.2 mmol/l (100–130 mg/dl). Once this fasting target was achieved, premeal bolus insulin was titrated by 2 unit increments to ensure 2-hour postmeal glucose levels were <10 mmol/l (<180 mg/dl).90
Multiple-daily-injection regime enables more flexible dose adjustments to help mitigate glycemic variability related to glucose load in dialysate when compared to twice daily premixed insulin regime and is comparatively less likely to cause hypoglycemia. Consideration should be given to when the peritoneal glucose load is likely to be maximal. In our opinion, giving basal insulin in the morning can help to minimize the risk for night-time hypoglycemia in some people. However, modern basal analog insulin provides 24 hours or longer cover and can be dosed at night. An individualized approach based on characteristics such as the type and timing of PD, type of insulins that is available, hypoglycemia awareness, and technology to mitigate this risk will guide this decision. For people on APD, basal insulin at night time may be preferred.
The is no clinical data regarding the use of short-acting insulin to correct hyperglycemia in PD. In our opinion, for people on multiple-daily-injection regimes, the use of ‘correction’ doses of short-acting insulin (e.g., insulin aspart, insulin lispro), starting at 2 to 4 units can be given at the start of dialysis if high blood glucose levels (>15mmol/l or >270 mg/dl) are noted within 2 to 4 hours after each exchange. This dose of insulin can be up-titrated if blood glucose levels do not improve and remain raised. This would be distinct to the usual short-acting insulin dose given with meals. There has to be a gap of at least 4 hours in between each short-acting insulin dose.89,91 In the scenario where people are eating meals at the time of exchange and are taking a premeal short-acting insulin dose, the above suggested short-acting insulin dose (starting at 2–4 units) can be added to the usual premeal short-acting insulin dose if high glucose values are observed. This dose can then be subsequently increased if required to further improve glucose control.
If a multiple-daily-injection insulin regime is not preferred, a premixed insulin regime such as 70/30 or 75/25 insulin can be considered, with the timing of dosing overlapped to the start of the PD if glucose excursions are significant with exchange. This may be suitable if degree of glucose excursions with PD is greater than with food (meal) intake. In patients who may require a further dose of premixed insulin (to cover meal-related rise and background basal insulin requirements) a 12-hour gap between doses is recommended. An individualized approach is required for insulin management in people on PD with advice, input, and support of the diabetes multidisciplinary team.
The insulin dose adjustments for the PD-free rest days need to be individualized. In our opinion, capillary blood glucose should be monitored at least 3 times daily premeals, and if the blood glucose is noted to be <5 mmol/l (90 mg/dl), a specific PD-free day insulin regime or dose change will need to be considered, often with lower doses of insulin after discussion with diabetes or endocrine specialist team. The use of dipeptidyl peptidase-4 inhibitors or pioglitazone can be continued without any dose adjustments on PD-free days because they do not increase the risk of hypoglycemia.
Any changes to the glucose concentration of the PD prescription can lead to increased glycemic variability or hypoglycemia, therefore should be discussed with the diabetes team for the insulin dose to be correctly adjusted, especially in people with multiple risk factors for hypoglycemia or hypoglycemia unawareness. A person on PD transferred to hemodialysis may require insulin dose adjustments accordingly to prevent any hypoglycemia because of sudden withdrawal of glucose-containing PD regime.89,92
Use of Continuous Subcutaneous Insulin Infusion
Our literature search did not identify any clinical trials or large case series of people with type 1 diabetes on PD treated with continuous subcutaneous insulin infusion. In general, increased basal rates especially overnight, to counter the increased glucose load in people on APD, as well as adjustments of insulin-to-carbohydrate ratios to avoid postprandial hyperglycemia will be required. Similarly, a reduction in basal rates or basal insulin dose if on a multiple-daily-injection regime is required after any reduction of the glucose concentration of the dialysate, to avoid hypoglycemia. Closed loop systems may be a better way forward in optimizing diabetes management in people with type 1 diabetes and KF. A recent study demonstrated improved glycemic control and reduced hypoglycemia with fully automated closed loop system compared to standard insulin therapy in people with diabetes on dialysis.93 However, this study included only 1 person on PD out of 27 total participants, indicating the need for further studies in this cohort.
Hypoglycemia Management
People with diabetes having KF are at a higher risk of developing hypoglycemia because of the reduced clearance of insulin and other glucose-lowering medications, reduced gluconeogenesis in kidneys, and overall blunting of counter-regulatory responses to hypoglycemia.94 Significant levels of hypoglycemia, both symptomatic and asymptomatic, have been recognized in hemodialysis cohorts. The burden of hypoglycemia in PD cohorts may be less but the incidence is poorly characterized.89
A retrospective study involving 60 people on PD on a CGM system showed that 3 of 15 patients even with HbA1c >75 mmol/mol (9%), experienced significant hypoglycemia.53 Studies have shown an increased risk of severe hypoglycemia in people with KF transitioning to dialysis, and a higher risk has been observed with people on hemodialysis compared to PD in the first year after transitioning, as well as in people on insulin compared to noninsulin treatment.95 A recent clinical audit carried out in 3 large hospitals in the United Kingdom found that 15% of people with diabetes on PD have impaired hypoglycemia awareness, and self-reported hypoglycemic events at least once a month were reported in 21%.96 Noninsulin therapies such as dipeptidyl peptidase-4 inhibitors or pioglitazone are not known to be associated with hypoglycemia when used on their own. Currently, there is no clinical data or studies that have compared hypoglycemia burden with different types of dialysates or different dialysis modalities such as APD versus CAPD.
People with diabetes on PD treated with insulin require education on avoiding hypoglycemia and management of hypoglycemia should this occur. Supplementary Material 2 summarizes the initial management of hypoglycemia.1,97
Objective assessment of hypoglycemia awareness by clinicians, using validated questionnaires such as Gold and Clarke (please see Supplementary Material 3)98,99 should be performed in people on insulin who are on PD. It is crucial for the treating team to be aware of the local or national guidance on initial hypoglycemia management and advice from local diabetes team should be sought if the hypoglycemia is either difficult to manage or recurrent. The precipitating factors for the hypoglycemia should be identified and corrected. An example of a patient education leaflet on hypoglycemia from diabetes.org.uk can be accessed on https://www.diabetes.org.uk/guide-to-diabetes/complications/hypos/having-a-hypo.
We suggest regular reinforcement of this advice and guidance by the diabetes multidisciplinary team. People with diabetes on PD should be advised to keep appropriate treatment for hypoglycemia with them at all times should they require treatment.
Diet and Lifestyle Modifications in People With Diabetes on PD
The treating clinicians should be aware that dietary management for type 1 diabetes and type 2 diabetes are different and this must be considered when giving dietary advice to these people.1,100,101 In people on PD, current guidelines recommend a minimum dietary protein intake of 1.0 to 1.2 g/kg/d, matched to the ideal body weight to account for the protein losses during dialysis and for maintenance of a good nutritional status.1,102,103 The recommended energy requirement for people on PD is less than that of people on hemodialysis to account for the calories provided through PD solutions and is usually 30 to 35 kcal/kg body weight.1,102
One needs to consider several potential limitations while discussing exercise with people having KF. These include decreased aerobic capacity, slow gait speed, and reduced strength of lower limbs.104 In contrast to people on hemodialysis, there is limited data on the impact of exercise in people on PD. In general, aerobic exercise as tolerated including walking, swimming at well-maintained facilities, with care taken to protect the PD catheter, as well as core strength training exercises are recommended. However, activities that may increase the intra-abdominal pressure should be delayed for 2 to 6 weeks after PD catheter insertion, depending on the technique of insertion.105 An individualized approach to lifestyle modification is advised considering comorbidities, as well as background diabetes treatment such as insulin and patient preferences.
Impact of Adjusting Dialysate Prescription on Diabetes Control
The role of adjusting the dialysate prescription to improve glycemic control continues to be debated. There is no strong evidence to support one PD modality over another (APD versus CAPD), with regard to improving glycemic control.
Glucose-based solutions remain the most widely used. Alternative osmotic agents have been explored to circumvent some of the drawbacks of traditional glucose-based solutions. Osmosis can also be induced with colloidal agents. Icodextrin, the most used colloidal agent, is a mixture of starch-derived high molecular weight (1638−4500 kDa) glucose polymers, with a structure similar to that of glycogen. Icodextrin, unlike glucose, has a net reflection coefficient approaching 1 and therefore provides an almost constant colloid osmotic pressure, which can sustain ultrafiltration for up to 16 hours even in high transporters. Icodextrin is currently only licensed for a single exchange daily and because of its sustained ultrafiltration properties, it is best suited to the long exchange.
The other commercially available nonglucose-based dialysate contains a 1.1% solution of amino acids. This solution provides similar ultrafiltration potential to 1.36% glucose-based dialysate but has the advantage of no exposure to absorbed glucose or glucose degradation products. Its main role is in enhancing nutrition in hypoalbuminemic patients but its use is limited to a single daily exchange because of concerns regarding the potential for symptomatic uremia and acidosis.106
The combined results of 2 large, multinational, interventional studies in people with diabetes on PD demonstrated the potential systemic benefits of reduced dialysate glucose exposure.107,108 During a 6-month study period, participants were randomized to treatment with either a glucose-sparing regime (using icodextrin and amino acid-based dialysate for 2 of the daily exchanges) or standard all-glucose-based dialysate.107 In an intention to treat analysis, HbA1c fell in the intervention group but remained unchanged in the control group (0.5% difference between groups, 95% confidence interval 0.1% to 0.8%, P = 0.006).107 The mean HbA1c separation between the 2 groups was observed as early as 3 months and persisted to the 6-month study end-point. This corresponded with a reduction in very low-density lipoprotein cholesterol and serum triglycerides in the intervention group. However, this glucose minimizing approach appeared to compromise good fluid balance because the study reported more adverse events, especially uncontrolled hypertension and heart failure, in the intervention group.107
The results of a recent systematic review and meta-analysis, enriched with previously unpublished data do not support the use of a single daily icodextrin exchange alone as a strategy for improving glycemic control.109 This analysis of 19 randomized control trials included people both with and without diabetes and compared icodextrin for the long dwell versus glucose only solutions. The study, which reported no difference in fasting plasma glucose or HbA1c between groups despite a reduction in glucose exposure and absorption equivalent to 45 g per day, however showed an overall reduced mortality rate in the icodextrin group, possibly because of improved fluid balance. This review and the preceding Cochrane review report significantly lower rates of uncontrolled fluid overload in the group prescribed a daily icodextrin exchange.110
Icodextrin in combination with an amino acid solution as part of a glucose minimizing regime can result in improved glycemic control.107,109 In people on PD having diabetes, it is reasonable to use icodextrin for the long exchange with the aim of reduced glucose exposure and improved ultrafiltration.109 There are several glucose-sparing osmotic agents such as taurine, polyglycerol, carnitine, and xylitol that are currently in the preclinical research stage.111 Their impact on glycemic control is yet to be determined.
Areas for Future Research
Research evidence to guide and inform the management of glycemia in people with diabetes on PD is limited. Our literature search and narrative review highlight several key areas to for future research. These include:
-
1.
Need for studies to evaluate the efficacy of different insulin types and insulin regimes in people with diabetes on PD.
-
2.
Further studies assessing the clinical utility and benefits of CGM and their related metrics such as time in range and glycemic variability to guide insulin management.
-
3.
Research to evaluate if there are clinically relevant differences on glycemic indices between APD and CAPD
-
4.
The impact of different dialysates on glycemic control in people with diabetes on PD
Conclusion
A significant proportion of people with diabetes and KF are on PD across the world. There is a dearth of high quality research studies focused on optimizing diabetes care and control in people with diabetes on PD. There is an urgent need for such studies to inform clinical practice. Considering that PD can have a significant impact on glycemic control, for monitoring of glucose and treatment choices, a collaborative management approach between different health care professionals is required. We hope this review and the practice points will help guide clinicians and improve care of people with diabetes receiving PD.
Disclosure
SB has received honoraria, teaching, and research sponsorship or grants from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Roche, and Sanofi Aventis. He has also received funding for the development of educational programs from Elsevier, OmniaMed, and Medscape. He is a partner in Glycosmedia, which carries sponsorship declared on its website. ID has previously received research grants from Medtronic and Sanofi. He has been a member of advisory committees and received educational grants from AstraZeneca, Sanofi, GSK, and Vifor Pharmaceuticals. JK has received honoraria for delivering educational meetings and/or attending advisory boards of Boehringer Ingelheim, AstraZeneca, Sanofi, and Novo Nordisk; and research grants from AstraZeneca and Sanofi. AF has received research grants from Boehringer Ingelheim. He has been a member of advisory committees and received educational grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Vifor pharmaceuticals, and NAPP UK. PW, JW, MW, ML, and TAC report no competing interests.
Footnotes
Supplementry Material S1. Summary of the search strategy.
Supplementry Material S2. Initial management of hypoglycemia.
Supplementry Material S3. Assessment of hypoglycemia awareness – Gold and Clarke scores
Contributor Information
Janaka Karalliedde, Email: j.karalliedde@kcl.ac.uk.
The Association of British Clinical Diabetologists (ABCD) and UK Kidney Association (UKKA) Diabetic Kidney Disease Clinical Speciality Group:
Steve Bain, Indranil Dasgupta, Tahseen A. Chowdhury, Mona Wahba, Andrew H. Frankel, and Janaka Karalliedde
Appendix
Members of The Association of British Clinical Diabetologists (ABCD) and UK Kidney Association (UKKA) Diabetic Kidney Disease Clinical Speciality Group
Steve Bain, Indranil Dasgupta, Tahseen A. Chowdhury, Mona Wahba, Andrew H. Frankel, Janaka Karalliedde
Supplementary Material
Supplementry Material S1. Summary of the search strategy.
Supplementry Material S2. Initial management of hypoglycemia.
Supplementry Material S3. Assessment of hypoglycemia awareness – Gold and Clarke scores
References
- 1.Management of adults with diabetes on dialysis: August 2022. Joint British Diabetes Society. https://abcd.care/sites/abcd.care/files/site_uploads/JBDS_Guidelines_Current/JBDS_11_Renal_Guide_August_2022.pdf
- 2.Reutens A.T., Prentice L., Atkins R.C. In: The Epidemiology of Diabetes Mellitus. 2nd ed. Ekoé J.M., Rewers M., Williams R., Zimmet P., editors. Wiley; 2008. The epidemiology of diabetic kidney disease; pp. 499–517. [DOI] [Google Scholar]
- 3.UK Renal Registry 24th Annual Report The Renal Association. https://ukkidney.org/audit-research/annual-report
- 4.Bello A.K., Levin A., Tonelli M., et al. Assessment of global kidney health care status. JAMA. 2017;317:1864–1881. doi: 10.1001/jama.2017.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pecoits-Filho R., Okpechi I.G., Donner J.A., et al. Capturing and monitoring global differences in untreated and treated end-stage kidney disease, kidney replacement therapy modality, and outcomes. Kidney Int Suppl (2011) 2020;10:e3–e9. doi: 10.1016/j.kisu.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Yi C., Liu X., et al. Clinical outcome and risk factors for mortality in Chinese patients with diabetes on peritoneal dialysis: a 5-year clinical cohort study. Diabetes Res Clin Pract. 2013;100:354–361. doi: 10.1016/j.diabres.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Chen J.H., Johnson D.W., Wong G., et al. Associations between diabetes and sex with peritoneal dialysis technique and patient survival: results from the Australia and New Zealand Dialysis and Transplant Registry cohort study. Perit Dial Int J Int Soc Perit Dial. 2021;41:57–68. doi: 10.1177/0896860820918708. [DOI] [PubMed] [Google Scholar]
- 8.Termorshuizen F., Korevaar J.C., Dekker F.W., et al. Hemodialysis and peritoneal dialysis: comparison of adjusted mortality rates according to the duration of dialysis: analysis of the Netherlands cooperative study on the adequacy of Dialysis 2. J Am Soc Nephrol. 2003;14:2851–2860. doi: 10.1097/01.ASN.0000091585.45723.9E. [DOI] [PubMed] [Google Scholar]
- 9.Wong B., Ravani P., Oliver M.J., et al. Comparison of patient survival between hemodialysis and peritoneal dialysis among patients eligible for both modalities. Am J Kidney Dis Off J Natl Kidney Found. 2018;71:344–351. doi: 10.1053/j.ajkd.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Mircescu G., Stefan G., Gârneata L., Mititiuc I., Siriopol D., Covic A. Outcomes of dialytic modalities in a large incident registry cohort from Eastern Europe: the Romanian Renal Registry. Int Urol Nephrol. 2013;46:443–451. doi: 10.1007/s11255-013-0571-3. [DOI] [PubMed] [Google Scholar]
- 11.Mehrotra R., Chiu Y.-W., Kalantar-Zadeh K., Bargman J., Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med. 2011;171:110–118. doi: 10.1001/archinternmed.2010.352. [DOI] [PubMed] [Google Scholar]
- 12.McDonald S.P., Marshall M.R., Johnson D.W., Polkinghorne K.R. Relationship between dialysis modality and mortality. J Am Soc Nephrol. 2009;20:155–163. doi: 10.1681/ASN.2007111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vonesh E.F., Snyder J.J., Foley R.N., Collins A.J. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int Suppl. 2006;103:S3–S11. doi: 10.1038/sj.ki.5001910. [DOI] [PubMed] [Google Scholar]
- 14.Liu H., He Z., Hu X., et al. Propensity-matched comparison of mortality between peritoneal dialysis and hemodialysis in patients with type 2 diabetes. Int Urol Nephrol. 2022;54:1373–1381. doi: 10.1007/s11255-021-03026-y. [DOI] [PubMed] [Google Scholar]
- 15.Sanabria R.M., Vesga J.I., Johnson D.W., et al. Dialysis outcomes in a middle-income country: an updated comparison of patient mortality between hemodialysis and peritoneal dialysis. Blood Purif. 2022;51:780–790. doi: 10.1159/000520518. [DOI] [PubMed] [Google Scholar]
- 16.Elsayed M.E., Morris A.D., Li X., Browne L.D., Stack A.G. Propensity score matched mortality comparisons of peritoneal and in-centre hemodialysis: systematic review and meta-analysis. Nephrol Dial Transplant. 2020;35:2172–2182. doi: 10.1093/ndt/gfz278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duong U., Mehrotra R., Molnar M.Z., et al. Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin J Am Soc Nephrol. 2011;6:1041–1048. doi: 10.2215/CJN.08921010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies S.J., Phillips L., Naish P.F., Russell G.I. Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J Am Soc Nephrol. 2001;12:1046–1051. doi: 10.1681/ASN.V1251046. [DOI] [PubMed] [Google Scholar]
- 19.Rivara M.B., Mehrotra R. New-onset diabetes in peritoneal dialysis patients - which predictors really matter? Perit Dial Int. 2016;36:243–246. doi: 10.3747/pdi.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grodstein G.P., Blumenkrantz M.J., Kopple J.D., Moran J.K., Coburn J.W. Glucose absorption during continuous ambulatory peritoneal dialysis. Kidney Int. 1981;19:564–567. doi: 10.1038/ki.1981.53. [DOI] [PubMed] [Google Scholar]
- 21.Marshall J., Jennings P., Scott A., Fluck R.J., Mcintyre C.W. Glycemic control in diabetic CAPD patients assessed by continuous glucose monitoring system (CGMS) Kidney Int. 2003;64:1480–1486. doi: 10.1046/j.1523-1755.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- 22.Szeto C.C., Chow K.M., Kwan B.C.H., Chung K.Y., Leung C.B., Li P.K.T. New-onset hyperglycemia in nondiabetic Chinese patients started on peritoneal dialysis. Am J Kidney Dis Off J Natl Kidney Found. 2007;49:524–532. doi: 10.1053/j.ajkd.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Dong J., Yang Z.-K., Chen Y. Older age, higher body mass index and inflammation increase the risk for new-onset diabetes and impaired glucose tolerance in patients on peritoneal dialysis. Perit Dial Int. 2016;36:277–283. doi: 10.3747/pdi.2015.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue C., Gu Y.-Y., Cui C.-J., et al. New-onset glucose disorders in peritoneal dialysis patients: a meta-analysis and systematic review. Nephrol Dial Transplant. 2020;35:1412–1419. doi: 10.1093/ndt/gfz116. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y., Cai J., Shi C., Liu C., Li Z. Incidence and mortality of new-onset glucose disorders in peritoneal dialysis patients in China: a meta-analysis. BMC Nephrol. 2020;21:152. doi: 10.1186/s12882-020-01820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortes P.C., de Moraes T.P., Mendes J.G., Stinghen A.E., Ribeiro S.C., Pecoits-Filho R. Insulin resistance and glucose homeostasis in peritoneal dialysis. Perit Dial Int J Int Soc Perit Dial. 2009;29(suppl 2):S145–148. doi: 10.1177/089686080902902S28. [DOI] [PubMed] [Google Scholar]
- 27.Selby N.M., Fialova J., Burton J.O., McIntyre C.W. The haemodynamic and metabolic effects of hypertonic-glucose and amino-acid-based peritoneal dialysis fluids. Nephrol Dial Transplant. 2007;22:870–879. doi: 10.1093/ndt/gfl654. [DOI] [PubMed] [Google Scholar]
- 28.da Silva D.R., Figueiredo A.E., Antonello I.C., Poli de Figueiredo C.E., d’Avila D.O. Solutes transport characteristics in peritoneal dialysis: variations in glucose and insulin serum levels. Ren Fail. 2008;30:175–179. doi: 10.1080/08860220701810307. [DOI] [PubMed] [Google Scholar]
- 29.Oba I., Mori T., Chida M., et al. Glucose and insulin response to peritoneal dialysis fluid in diabetic and nondiabetic peritoneal dialysis patients. Adv Perit Dial. 2015;31:11–16. [PubMed] [Google Scholar]
- 30.Lambie M., Chess J., Do J.-Y., et al. Peritoneal dialysate glucose load and systemic glucose metabolism in non-diabetics: results from the GLOBAL fluid cohort study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Type 1 diabetes in adults: diagnosis and management National Institute of Clinical Excellence. https://www.nice.org.uk/guidance/ng17
- 32.When should I suspect type 2 diabetes in an adult? Diabetes Type;2, Published online 2021:7-9. National Institute of Clinical Excellence. https://cks.nice.org.uk/topics/diabetes-type-2/diagnosis/diagnosis-in-adults/
- 33.Woodward R.S., Schnitzler M.A., Baty J., et al. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transpl. 2003;3:590–598. doi: 10.1034/j.1600-6143.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 34.Tien K.-J., Lin Z.-Z., Chio C.-C., et al. Epidemiology and mortality of new-onset diabetes after dialysis: Taiwan national cohort study. Diabetes Care. 2013;36:3027–3032. doi: 10.2337/dc12-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou C.Y., Liang C.C., Kuo H.L., et al. Comparing risk of new onset diabetes mellitus in chronic kidney disease patients receiving peritoneal dialysis and hemodialysis using propensity score matching. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Little R.R., Rohlfing C., Sacks D.B. The national glycohemoglobin standardization program: over 20 years of improving hemoglobin A(1c) measurement. Clin Chem. 2019;65:839–848. doi: 10.1373/clinchem.2018.296962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathan D.M., Kuenen J., Borg R., et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stratton I.M., Adler A.I., Neil H.A.W., et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hahr A.J., Molitch M.E. Management of diabetes mellitus in patients with chronic kidney disease. Clin Diabetes Endocrinol. 2015;1:2. doi: 10.1186/s40842-015-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ly J., Marticorena R., Donnelly S. Red blood cell survival in chronic renal failure. Am J Kidney Dis. 2004;44:715–719. doi: 10.1016/S0272-6386(04)00951-5. [DOI] [PubMed] [Google Scholar]
- 41.Smith W.G., Holden M., Benton M., Brown C.B. Glycosylated and carbamylated haemoglobin in uraemia. Nephrol Dial Transplant. 1989;4:96–100. [PubMed] [Google Scholar]
- 42.Dolscheid-Pommerich R.C., Kirchner S., Weigel C., et al. Impact of carbamylation on three different methods, HPLC, capillary electrophoresis and TINIA of measuring HbA1c levels in patients with kidney disease. Diabetes Res Clin Pract. 2015;108:15–22. doi: 10.1016/j.diabres.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 43.Weykamp C.W., Miedema K., de Haan T., Doelman C.J. Carbamylated hemoglobin interference in glycohemoglobin assays. Clin Chem. 1999;45:438–440. doi: 10.1093/clinchem/45.3.438. [DOI] [PubMed] [Google Scholar]
- 44.Kalantar-Zadeh K., Kopple J.D., Regidor D.L., et al. A1C and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30:1049–1055. doi: 10.2337/dc06-2127. [DOI] [PubMed] [Google Scholar]
- 45.Hoshino J., Larkina M., Karaboyas A., et al. Unique hemoglobin A1c level distribution and its relationship with mortality in diabetic hemodialysis patients. Kidney Int. 2017;92:497–503. doi: 10.1016/j.kint.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Rhee J.J., Zheng Y., Montez-Rath M.E., Chang T.I., Winkelmayer W.C. Associations of glycemic control with cardiovascular outcomes among US hemodialysis patients with diabetes mellitus. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill C.J., Maxwell A.P., Cardwell C.R., et al. Glycated hemoglobin and risk of death in diabetic patients treated with hemodialysis: a meta-analysis. Am J Kidney Dis Off J Natl Kidney Found. 2014;63:84–94. doi: 10.1053/j.ajkd.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 48.Abe M., Hamano T., Hoshino J., Wada A., Nakai S., Masakane I. Glycemic control and survival in peritoneal dialysis patients with diabetes: a 2-year nationwide cohort study. Sci Rep. 2019;9:3320. doi: 10.1038/s41598-019-39933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freedman B.I., Andries L., Shihabi Z.K., et al. Glycated albumin and risk of death and hospitalizations in diabetic dialysis patients. Clin J Am Soc Nephrol. 2011;6:1635–1643. doi: 10.2215/CJN.11491210. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe Y., Ohno Y., Inoue T., Takane H., Okada H., Suzuki H. Blood glucose levels in peritoneal dialysis are better reflected by HbA1c than by glycated albumin. Adv Perit Dial. 2014;30:75–82. [PubMed] [Google Scholar]
- 51.Lee S.Y., Chen Y.C., Tsai I.C., et al. Glycosylated hemoglobin and albumin-corrected fructosamine are good indicators for glycemic control in peritoneal dialysis patients. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0057762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bomholt T., Feldt-Rasmussen B., Butt R., et al. Hemoglobin A1c and fructosamine evaluated in patients with type 2 diabetes receiving peritoneal dialysis using long-term continuous glucose monitoring. Nephron. 2022;146:146–152. doi: 10.1159/000519493. [DOI] [PubMed] [Google Scholar]
- 53.Qayyum A., Chowdhury T.A., Oei E.L., Fan S.L. Use of continuous glucose monitoring in patients with diabetes mellitus on peritoneal dialysis: correlation with glycated hemoglobin and detection of high incidence of unaware hypoglycemia. Blood Purif. 2016;41:18–24. doi: 10.1159/000439242. [DOI] [PubMed] [Google Scholar]
- 54.Williams M.E., Mittman N., Ma L., et al. The Glycemic Indices in Dialysis Evaluation (GIDE) study: comparative measures of glycemic control in diabetic dialysis patients. Hemodial Int. 2015;19:562–571. doi: 10.1111/hdi.12312. [DOI] [PubMed] [Google Scholar]
- 55.Frias J.P., Lim C.G., Ellison J.M., Montandon C.M. Review of adverse events associated with false glucose readings measured by GDH-PQQ-based glucose test strips in the presence of interfering sugars. Diabetes Care. 2010;33:728–729. doi: 10.2337/dc09-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kroll H.R., Maher T.R. Significant hypoglycemia secondary to icodextrin peritoneal dialysate in a diabetic patient. Anesth Analg. 2007;104:1473–1474. doi: 10.1213/01.ane.0000264007.46873.0f. table of contents. [DOI] [PubMed] [Google Scholar]
- 57.Tsai C.-Y., Lee S.-C., Hung C.-C., et al. False elevation of blood glucose levels measured by GDH-PQQ-based glucometers occurs during all daily dwells in peritoneal dialysis patients using icodextrin. Perit Dial Int. 2010;30:329–335. doi: 10.3747/pdi.2008.00285. [DOI] [PubMed] [Google Scholar]
- 58.Disse E., Thivolet C. Hypoglycemic coma in a diabetic patient on peritoneal dialysis due to interference of icodextrin metabolites with capillary blood glucose measurements. Diabetes Care. 2004;27:2279. doi: 10.2337/diacare.27.9.2279. [DOI] [PubMed] [Google Scholar]
- 59.Silver S.A., Harel Z., Perl J. Practical considerations when prescribing icodextrin: a narrative review. Am J Nephrol. 2014;39:515–527. doi: 10.1159/000363417. [DOI] [PubMed] [Google Scholar]
- 60.Ling J., Ng J.K.C., Chan J.C.N., Chow E. Use of continuous glucose monitoring in the assessment and management of patients with diabetes and chronic kidney disease. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.869899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwing W.D., Erhard P., Newman L.N., et al. Assessing 24-hour blood glucose patterns in diabetic paitents treated by peritoneal dialysis. Adv Perit Dial. 2004;20:213–216. [PubMed] [Google Scholar]
- 62.Okada E., Oishi D., Sakurada T., Yasuda T., Shibagaki Y. A comparison study of glucose fluctuation during automated peritoneal dialysis and continuous ambulatory peritoneal dialysis. Adv Perit Dial. 2015;31:34–37. [PubMed] [Google Scholar]
- 63.Williams J., Gilchrist M., Strain W.D., Fraser D., Shore A. 24-h glycemic profiles in peritoneal dialysis patients and non-dialysis controls with advanced kidney disease. Perit Dial Int. 2022;42:497–504. doi: 10.1177/08968608211047787. [DOI] [PubMed] [Google Scholar]
- 64.Joubert M., Fourmy C., Henri P., Ficheux M., Lobbedez T., Reznik Y. Effectiveness of continuous glucose monitoring in dialysis patients with diabetes: the DIALYDIAB pilot study. Diabetes Res Clin Pract. 2015;107:348–354. doi: 10.1016/j.diabres.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 65.Képénékian L., Smagala A., Meyer L., et al. Continuous glucose monitoring in hemodialyzed patients with type 2 diabetes: a multicenter pilot study. Clin Nephrol. 2014;82:240–246. doi: 10.5414/CN108280. [DOI] [PubMed] [Google Scholar]
- 66.Currie C.J., Peters J.R., Tynan A., et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481–489. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 67.Frankel A.H., Kazempour-Ardebili S. Management of adults with diabetes on the hemodialysis unit. http://www.diabetologists-abcd.org.uk/JBDS/JBDS_RenalGuide_2016.pdf
- 68.American Diabetes Association Professional Practice Committee 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S83–S96. doi: 10.2337/dc22-S006. [DOI] [PubMed] [Google Scholar]
- 69.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4S):S1–S115. doi: 10.1016/j.kint.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 70.Battelino T., Danne T., Bergenstal R.M., et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y., Xie Q., You H., et al. Twelve weeks of pioglitazone therapy significantly attenuates dysmetabolism and reduces inflammation in continuous ambulatory peritoneal dialysis patients--a randomized crossover trial. Perit Dial Int. 2012;32:507–515. doi: 10.3747/pdi.2011.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karalliedde J., Winocour P., Chowdhury T.A., et al. Clinical practice guidelines for management of hyperglycaemia in adults with diabetic kidney disease. Diabet Med. 2022;39 doi: 10.1111/dme.14769. [DOI] [PubMed] [Google Scholar]
- 73.Fioretto P., Zambon A., Rossato M., Busetto L., Vettor R. SGLT2 inhibitors and the diabetic kidney. Diabetes Care. 2016;39(suppl 2):S165–S171. doi: 10.2337/dcS15-3006. [DOI] [PubMed] [Google Scholar]
- 74.Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 75.Neal B., Perkovic V., Matthews D.R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099. doi: 10.1056/NEJMc1712572. [DOI] [PubMed] [Google Scholar]
- 76.Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 77.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 78.Anker S.D., Butler J., Filippatos G., et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR-reduced trial. Circulation. 2021;143:337–349. doi: 10.1161/CIRCULATIONAHA.120.051824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borkum M, Jamal A, Suneet Singh R, Levin A. The rationale for the need to study sodium-glucose co-transport 2 inhibitor usage in peritoneal dialysis patients. Perit Dial Int. Published online May 2, 2022. doi:10.1177/08968608221096556 [DOI] [PubMed]
- 80.Zhou Y., Fan J., Zheng C., et al. SGLT-2 inhibitors reduce glucose absorption from peritoneal dialysis solution by suppressing the activity of SGLT-2. Biomed Pharmacother. 2019;109:1327–1338. doi: 10.1016/j.biopha.2018.10.106. [DOI] [PubMed] [Google Scholar]
- 81.Martus G., Bergling K., de Arteaga J., Öberg C.M. SGLT2 inhibition does not reduce glucose absorption during experimental peritoneal dialysis. Perit Dial Int. 2021;41:373–380. doi: 10.1177/08968608211008095. [DOI] [PubMed] [Google Scholar]
- 82.Reduction of peritoneal glucose uptake with use of SGLT2 in humans undergoing peritoneal dialysis treatment (PRESERVE). ClinicalTrials.gov identifier: NCT05250752. https://clinicaltrials.gov/ct2/show/NCT05250752
- 83.Quellhorst E. Insulin therapy during peritoneal dialysis: pros and cons of various forms of administration. J Am Soc Nephrol. 2002;13(suppl 1):S92–S96. doi: 10.1681/ASN.V13suppl_1s92. [DOI] [PubMed] [Google Scholar]
- 84.Almalki M.H., Altuwaijri M.A., Almehthel M.S., Sirrs S.M., Singh R.S. Subcutaneous versus intraperitoneal insulin for patients with diabetes mellitus on continuous ambulatory peritoneal dialysis: meta-analysis of non-randomized clinical trials. Clin Invest Med. 2012;35:E132–E143. doi: 10.25011/cim.v35i3.16589. [DOI] [PubMed] [Google Scholar]
- 85.Torun D., Oguzkurt L., Sezer S., et al. Hepatic subcapsular steatosis as a complication associated with intraperitoneal insulin treatment in diabetic peritoneal dialysis patients. Perit Dial Int. 2005;25:596–600. doi: 10.1177/089686080502500617. [DOI] [PubMed] [Google Scholar]
- 86.Nevalainen P.I., Lahtela J.T., Mustonen J., Pasternack A. Subcutaneous and intraperitoneal insulin therapy in diabetic patients on CAPD. Perit Dial Int. 1996;16(suppl 1):S288–S291. doi: 10.1177/089686089601601S54. [DOI] [PubMed] [Google Scholar]
- 87.Iglesias P., Díez J.J. Insulin therapy in renal disease. Diabetes Obes Metab. 2008;10:811–823. doi: 10.1111/j.1463-1326.2007.00802.x. [DOI] [PubMed] [Google Scholar]
- 88.Szeto C.C., Chow K.M., Leung C.B., et al. Increased subcutaneous insulin requirements in diabetic patients recently commenced on peritoneal dialysis. Nephrol Dial Transplant. 2007;22:1697–1702. doi: 10.1093/ndt/gfl834. [DOI] [PubMed] [Google Scholar]
- 89.Blaine E., Tumlinson R., Colvin M., Haynes T., Whitley H.P. Systematic literature review of insulin dose adjustments when initiating hemodialysis or peritoneal dialysis. Pharmacotherapy. 2022;42:177–187. doi: 10.1002/phar.2659. [DOI] [PubMed] [Google Scholar]
- 90.Gómez A.M., Vallejo S., Ardila F., et al. Impact of a basal-bolus insulin regimen on metabolic control and risk of hypoglycemia in patients with diabetes undergoing peritoneal dialysis. J Diabetes Sci Technol. 2018;12:129–135. doi: 10.1177/1932296817730376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dhatariya K., James J., Kong M.-F., Berrington R. Joint British Diabetes Society (JBDS) for Inpatient Care Group and guidelines writing group. Diabetes at the front door. A guideline for dealing with glucose related emergencies at the time of acute hospital admission from the Joint British Diabetes Society (JBDS) for Inpatient Care Group. Diabet Med. 2020;37:1578–1589. doi: 10.1111/dme.14304. [DOI] [PubMed] [Google Scholar]
- 92.Katla V., Khyalappa R. Hemodialysis and effect of corrective measures to prevent hypoglycemia. J Assoc Phys India. 2022;70:11–12. [Google Scholar]
- 93.Boughton C.K., Tripyla A., Hartnell S., et al. Fully automated closed-loop glucose control compared with standard insulin therapy in adults with type 2 diabetes requiring dialysis: an open-label, randomized crossover trial. Nat Med. 2021;27:1471–1476. doi: 10.1038/s41591-021-01453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahmad I., Zelnick L.R., Batacchi Z., et al. Hypoglycemia in people with type 2 diabetes and CKD. Clin J Am Soc Nephrol. 2019;14:844–853. doi: 10.2215/CJN.11650918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsiao C.C., Tu H.T., Lin C.H., Chen K.H., Yeh Y.H., See L.C. Temporal trends of severe hypoglycemia and subsequent mortality in patients with advanced diabetic kidney diseases transitioning to dialysis. J Clin Med. 2019;8:420. doi: 10.3390/jcm8040420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.eid H., Onyema M., Haboosh S., et al. Abstr EASD; 2022. Burden of hypoglycemia and impaired awareness of hypoglycemia in people with diabetes on peritoneal dialysis. Published online 2022. [Google Scholar]
- 97.The hospital management of hypoglycaemia in adults with diabetes mellitus. Joint British Diabetes Societies for in patient care. https://diabetes-resources-production.s3.eu-west-1.amazonaws.com/resources-s3/public/2022-03/JBDS%2001%20Hypo%20Guideline%20March%202022.pdf Revised March 2022.
- 98.Gold A.E., MacLeod K.M., Frier B.M. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994;17:697–703. doi: 10.2337/diacare.17.7.697. [DOI] [PubMed] [Google Scholar]
- 99.Clarke W.L., Cox D.J., Gonder-Frederick L.A., Julian D., Schlundt D., Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18:517–522. doi: 10.2337/diacare.18.4.517. [DOI] [PubMed] [Google Scholar]
- 100.Dyson P.A., Twenefour D., Breen C., et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541–547. doi: 10.1111/dme.13603. [DOI] [PubMed] [Google Scholar]
- 101.KDOQI KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 suppl 2):S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 102.Ikizler T.A., Burrowes J.D., Byham-Gray L.D., et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76(3 suppl 1):S1–S107. doi: 10.1053/j.ajkd.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Naylor H.L., Jackson H., Walker G.H., et al. British Dietetic Association evidence-based guidelines for the protein requirements of adults undergoing maintenance hemodialysis or peritoneal dialysis. J Hum Nutr Diet Off J Br Diet Assoc. 2013;26:315–328. doi: 10.1111/jhn.12052. [DOI] [PubMed] [Google Scholar]
- 104.Bennett P.N., Hussein W.F., Matthews K., et al. An exercise program for peritoneal dialysis patients in The United States: a feasibility study. Kidney Med. 2020;2:267–275. doi: 10.1016/j.xkme.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bennett P.N., Bohm C., Harasemiw O., et al. Physical activity and exercise in peritoneal dialysis: International Society for Peritoneal Dialysis and the Global Renal Exercise Network practice recommendations. Perit Dial Int. 2021;42:8–24. doi: 10.1177/08968608211055290. [DOI] [PubMed] [Google Scholar]
- 106.Lindholm B., Park M.S., Bergström J. Supplemented dialysis: amino acid-based solutions in peritoneal dialysis. Contrib Nephrol. 1993;103:168–182. doi: 10.1159/000422285. [DOI] [PubMed] [Google Scholar]
- 107.Li P.K.T., Culleton B.F., Ariza A., et al. Randomized, controlled trial of glucose-sparing peritoneal dialysis in diabetic patients. J Am Soc Nephrol. 2013;24:1889–1900. doi: 10.1681/ASN.2012100987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li P.K.T., Dorval M., Johnson D.W., et al. The benefit of a glucose-sparing PD therapy on glycemic control measured by serum fructosamine in diabetic patients in a randomized, controlled trial (IMPENDIA) Nephron. 2015;129:233–240. doi: 10.1159/000371554. [DOI] [PubMed] [Google Scholar]
- 109.Goossen K., Becker M., Marshall M.R., et al. Icodextrin versus glucose solutions for the once-daily long dwell in peritoneal dialysis: an enriched systematic review and meta-analysis of randomized controlled trials. Am J Kidney Dis. 2020;75:830–846. doi: 10.1053/j.ajkd.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 110.Htay H., Johnson D.W., Wiggins K.J., et al. Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst Rev. 2018;10:CD007554. doi: 10.1002/14651858.CD007554.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonomini M., Zammit V., Divino-Filho J.C., et al. The osmo-metabolic approach: a novel and tantalizing glucose-sparing strategy in peritoneal dialysis. J Nephrol. 2021;34:503–519. doi: 10.1007/s40620-020-00804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.