Abstract
Surgical treatment of carotid restenosis and radiation-induced occlusive disease is challenging because of the high morbidity and mortality associated with this procedure. Carotid stenting has been proposed as an alternative approach.
We report a series of 8 patients who were treated via the percutaneous approach for either carotid restenosis (n = 4) or radiation-induced occlusive disease (n = 4). Technical success was achieved in all of the cases. There have been no deaths or strokes during the periprocedural or follow-up period. After dilation of the extracranial vessel, 1 patient experienced severe intracranial internal carotid arterial spasm that required stent placement. Wallstents® were used in 6 patients and S.M.A.R.T.™ stents were used in the remaining 2. Restenosis occurred in 2 patients and was treated successfully with redilation or restenting.
Carotid stenting appears to be a feasible and safe alternative to surgery for restenosis after carotid endarterectomy and for radiation-induced occlusive disease.
Key words: Blood vessel prosthesis; carotid arteries/surgery; carotid stenosis/surgery; cerebrovascular disorders/prevention & control; endarterectomy, carotid; radiotherapy/adverse effects; stent
The management of atherosclerotic extracranial carotid arterial disease was revolutionized in the early 1990s, when publication of the North American Symptomatic Carotid Endarterectomy Trial (NASCET) 1 established carotid endarterectomy as the treatment of choice. The results of this trial revealed an impressive 51% relative risk reduction for any stroke or death and a 65% relative risk reduction for any ipsilateral stroke. The incidences of adverse outcomes were as follows: stroke (5.8%); stroke and death (5.8%); major stroke and death (2.1%); and fatality (0.6%).
In 1989, the American Heart Association (AHA) issued guidelines for assessing the risk of stroke or death associated with carotid endarterectomy. 2 These guidelines state that the perioperative risk should be less than 3% for asymptomatic patients, less than 5% for patients with transient ischemic attacks (TIAs), less than 7% for patients with a recent stroke, and less than 10% for patients who have restenotic lesions. The last guideline reflects the known increased risk associated with surgical interventions in this subset of patients, namely the increased morbidity and mortality from both stroke and cranial nerve palsies. 3,4 Patients who develop occlusive disease in the extracranial carotid artery after radiation therapy for head and neck malignancies also have increased perioperative morbidity during surgical management of the stenosis that arises from the distortion of the subcutaneous tissue and scar formation. 5–8 This has led several investigators to use an endovascular approach for these lesions. 9–12 We present a series of 8 patients—4 with restenotic lesions and 4 with radiation-induced occlusive disease—who underwent stenting of the carotid artery.
Patients
Between September 1998 and December 1999, 8 patients who had either restenotic post-endarterectomy obstructions or radiation-induced occlusive disease of the carotid arteries received endovascular therapy at our institution. These patients were enrolled in a protocol approved by our Institutional Review Board (IRB) to investigate the feasibility of carotid arterial stenting. All patients gave written informed consent.
Patients' characteristics are summarized in Table I. The mean age was 62.6 ± 8.5 years. The group comprised 3 men and 5 women, and the following comorbidities were present: hypertension (7 patients), diabetes (2 patients), and hyperlipidemia (5 patients). Coronary artery disease was present in 4 patients, and peripheral vascular disease was noted in 2. Five patients had obstructions in the right carotid: 4 involving only the right internal carotid artery (RICA), and 1 involving both the RICA and the right common carotid artery. There were 3 stenoses in the left carotid artery, 2 in the left internal carotid artery (LICA), and 1 in the left common carotid artery.
Table I. Patients' Characteristics, Procedure, and Follow-up
All patients had carotid stenosis of at least 60%, and all patients underwent a complete neurologic evaluation by a staff neurologist before intervention. Neurologic symptoms were present in 4 patients (Table I). Presenting symptoms were amaurosis fugax in 3 patients and dizziness in 1. All patients underwent extracranial carotid, vertebral, and cerebral digital subtraction angiography before the intervention. These angiograms were examined by a team that included an interventional radiologist, a neurologist, and a cardiologist. After stent placement, patients were examined clinically and by Doppler duplex imaging to assess vascular patency.
Procedure
In the interventional suite, a vascular sheath was inserted via the femoral approach. An initial 5,000-unit bolus of unfractionated heparin was then administered intravenously and supplemented as needed to maintain an activated clotting time between 200 and 250 seconds. A 5-F diagnostic catheter was advanced to the aortic arch over a J-tipped 0.035-inch hydrophilic guidewire. Angiography was performed in at least 2 orthogonal views to display the area of occlusive disease.
A 0.035-inch Glidewire® (Boston Scientific Corp.; Natick, Mass) was then directed to the external carotid artery, and the diagnostic catheter was advanced over this wire. The Glidewire was then exchanged for a 0.035-inch Amplatz Super Stiff™ wire (Boston Scientific) to support the introduction of an 80- to 90-cm Flexor® sheath (Cook Inc.; Bloomington, Ind) into the common carotid artery. The Amplatz wire was then replaced with a 0.018-inch guidewire—either the Roadrunner® (Cook) or the Steelcore™ (Guidant Corp.; Indianapolis, Ind)—which was advanced through the lesion and into the internal carotid artery below the carotid siphon.
Predilation was accomplished with the use of a 4.0- × 40-mm coronary angioplasty balloon. After removal of the 0.018-inch guidewire-compatible coronary angioplasty balloon, the stent was advanced through the stenosis and deployed at the dilated lesion. At first, we used Wallstents® (Boston Scientific), but we began using the S.M.A.R.T.™ stent (Cordis Corporation, a Johnson & Johnson company; Warren, NJ) when this device became available. Both Wallstents and S.M.A.R.T. stents are self-expanding. After deployment, the stent was dilated with a balloon of appropriate size, usually 5.0 to 6.0 mm in diameter. Final angiography was then obtained to assess adequacy of the results. An 8-F Prostar® XL device (Perclose Inc.; Menlo Park, Calif) was used to achieve hemostasis after completion of the procedure. All patients were placed on aspirin and clopidogrel, and these medications were continued for 30 days thereafter.
Results
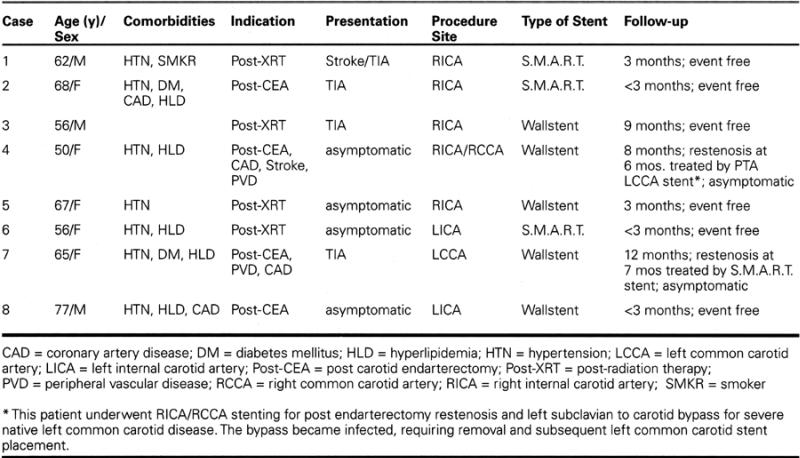
A total of 10 stents were deployed in 8 patients. Six Wallstents were used in 5 carotid lesions, and 4 S.M.A.R.T. stents in 3 lesions. Two patients required multiple stents, 1 for a severe, long post-radiation stenosis (Fig. 1), and the other for separate lesions in the common and internal carotid arteries. Success, defined as a residual stenosis of less than 30%, was obtained in all cases.
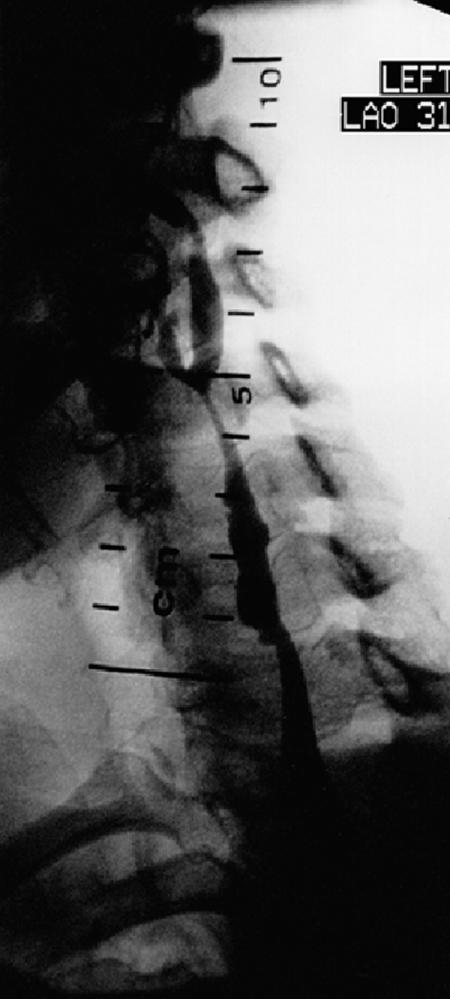
Fig. 1A Angiography in the left anterior oblique view shows the left common carotid artery in a patient with radiation-induced occlusive disease. Extensive disease is present in both the common and internal carotid arteries.
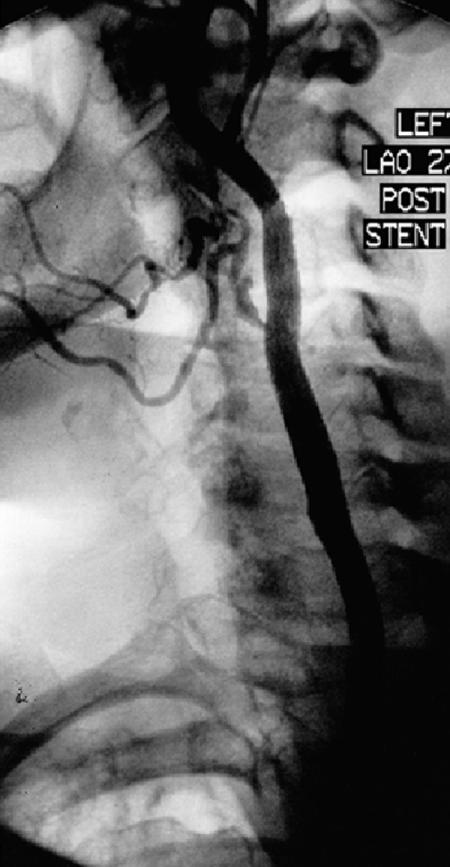
Fig. 1B Angiogram of same patient shows relief of stenosis in the left common and internal carotid arteries after deployment of 2 overlapped S.M.A.R.T.™ stents.
One patient developed severe, asymptomatic intracranial carotid artery spasm that was refractory to nitroglycerine, nicardipine, and balloon dilation. The spastic segment required the deployment of a 4.0 × 25-mm coronary NIR® Stent (Boston Scientific). Pre-procedure diagnostic film review had revealed no stenosis in that region. No patients sustained strokes or TIAs during or after the procedure.
During a mean follow-up of 4.5 ± 4.6 months (range, 0 to 12 months), there were no neurologic events. Restenosis, detected by serial Doppler evaluations, occurred in 2 patients, 1 at 6 months and the other at 8 months after the procedure. The first patient was treated with balloon redilation alone; the other patient required redilation and placement of a S.M.A.R.T. stent. Both restenotic lesions occurred in areas that had been treated with a Wallstent.
Discussion
The treatment of atherosclerotic carotid vascular disease has advanced tremendously in the last 10 years. The number of carotid endarterectomy procedures has increased dramatically since the publication of the NASCET, 1 ECST, 13 and ACAS 14 trials in the early 1990s. Between 1991 and 1996, the increase was 94%. 15 This would be expected to lead to an increase in the incidence of post-endarterectomy restenosis. However, there is limited support for this expectation in the surgical literature. In a review of 29 studies, Frericks and coauthors 16 found that the cumulative Weibull incidence curve for restenosis rose steeply to 10% at 1 year and flattened gradually to 20% at 10 years. They also noted significant heterogeneity among these studies. For example, restenosis rates at an average follow-up of 2 and 3 years varied widely between 1.9% and 22.1%.
The cause of early (i.e., within the first 3 postoperative years) recurrent disease after endarterectomy is thought to be myointimal hyperplasia from collagenous proliferation of the medial layer of the arterial wall. 17 In later follow-up years, there appears to be progression of the atherosclerotic process. Known risk factors such as hyperlipidemia, hypertension, diabetes, and smoking are thought to influence recurrent disease. Although there has been controversy regarding the risk of stroke with recurrent carotid artery disease, Ricotta and others 18,19 have found that long-term follow-up shows these patients to be at increased risk for ischemic events. This finding supports the aggressive management of patients who have severe restenotic disease.
Surgical therapy for restenotic disease is fraught with problems, notably increased stroke rates 3,4 and cranial nerve palsies. Dissection through scarred tissue may lead to injury of the vagus, hypoglossal, and glossopharyngeal nerves. Cranial nerve injury rates as high as 10% have been reported. 3 Frequently, standard endarterectomy is not feasible and interposition grafting is necessary. For this reason, some authors have proposed percutaneous endovascular management of this entity. 9,10,12
The feasibility and safety of carotid angioplasty in the setting of recurrent disease has been previously reported. 9,10,12 Yadav and colleagues 9 reported their series of 22 patients whose recurrent disease was managed with angioplasty and stenting. In their series the technical success rate was 100%, and only 1 minor perioperative stroke occurred, resulting in a complication rate of 4%. At 6 months, angiographic follow-up revealed no significant restenotic lesions.
More recently, Lanzino and colleagues 12 reported their series of 21 patients who had 25 lesions treated either with angioplasty or with angioplasty and stent placement. There was only 1 minor periprocedural stroke in their series. Their initial experience of angioplasty alone in 5 patients yielded suboptimal results: mean post-procedural stenosis was 40%, and stenosis recurred in 3 patients. This prompted the routine use of stents in the remaining 18 patients, after which only 1 restenotic episode was reported.
It is worth noting that 2 of our 4 patients with post-endarterectomy lesions developed substantial (≥50%) restenosis after stenting. Both were women who had hypertension, hyperlipidemia, diffuse peripheral vascular disease, and coronary artery disease. Their obstructions were long and involved the common carotid artery, leading us to use a Wallstent (Fig. 2).
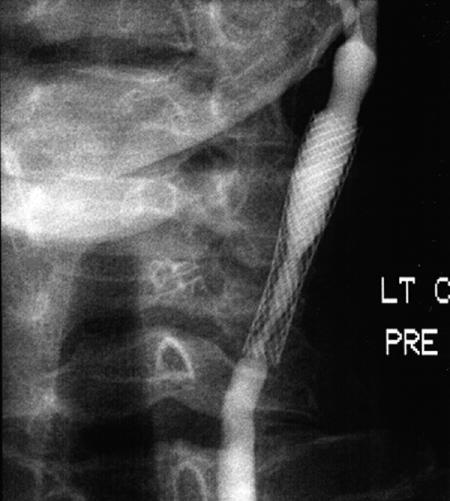
Fig. 2A Angiogram taken 7 months postoperatively for abnormal follow-up Doppler study shows a Wallstent® in the left common carotid. There is severe in-stent restenosis. The patient was asymptomatic.
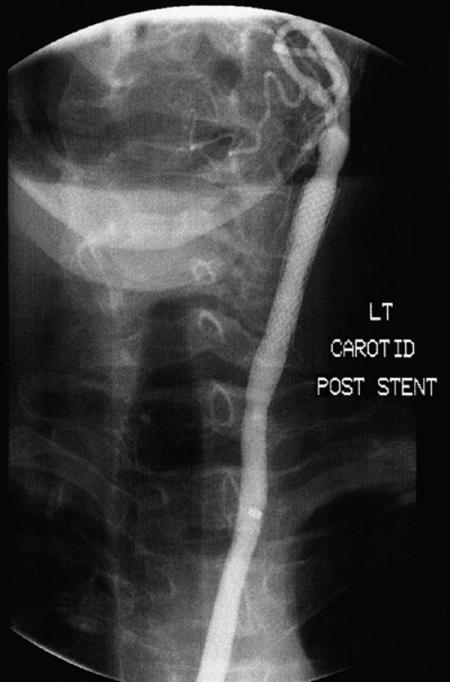
Fig. 2B Angiogram shows the same lesion after dilation and placement of a S.M.A.R.T.™ stent.
So far, no clear predictors of restenosis after carotid stenting for restenotic disease have been identified. A larger number of patients with similar risk factors should be evaluated to determine whether the characteristics seen in our patients reflect an actual increase in risk.
The incidence of carotid artery stenosis is significantly higher in patients who receive neck radiation. Moritz and colleagues 20 used duplex scanning to study a group of patients who had advanced neck carcinomas. In this cohort, 30% of patients who had undergone neck radiation had developed stenotic carotid artery lesions, while only 6% of patients in a non-irradiated control group had developed such lesions. The successful treatment of head and neck malignancies by means of high-dose radiation and radical cervical dissection has increased the long-term survival of patients with these conditions. Consequently, radiation-induced carotid artery disease has become a more common clinical problem. 5
Histologically, radiation-induced lesions in the vessel wall are similar to those produced by atherosclerosis; angiographically, they may be indistinguishable. However, radiation-induced stenoses are usually long, multiple, or circumferential, and have unusual proximal or distal distribution. 11 This is accompanied by severe fibrosis and atrophy of the cutaneous tissues overlying the stenotic segment, which complicates the surgical management of this disease entity. 6,8
Surgical management of radiation-induced occlusive disease is plagued with many of the same difficulties encountered in restenotic carotid disease. Radiation causes fibrosis and obliteration of normal tissue planes. The resulting arteritis can cause either adventitial atrophy or scarring and leave an irregular post-endarterectomy surface that would likely be thrombogenic. 6 This frequently leads to difficulty in performing standard endarterectomy and may require patch closure or interposition grafts. 5–8 When severe atrophy of the overlying subcutaneous tissues exists, dermal grafting or rotation flaps are sometimes necessary for wound closure. 6 These risks make percutaneous endovascular therapy an attractive option for patients in this subgroup.
Al-Mubarak's group 11 published the 1st large series of patients treated with angioplasty and stenting for radiation-induced occlusive disease. They reported a series of 14 patients with 15 severe carotid lesions, all of whom underwent stenting. Technical success was achieved in all patients. One patient had a minor stroke after the procedure and recovered fully within 2 days. Six-month angiography or duplex scanning in 9 of the 14 patients showed no restenosis. At 18 ± 2 months of follow-up, 3 patients had died of unrelated causes. No neurologic events occurred during follow-up.
Our 4 patients with radiation-induced stenosis show promising preliminary results (Fig. 3). We have had no neurologic events during or after the procedure, and we have found no restenotic lesions on serial Doppler assessment.
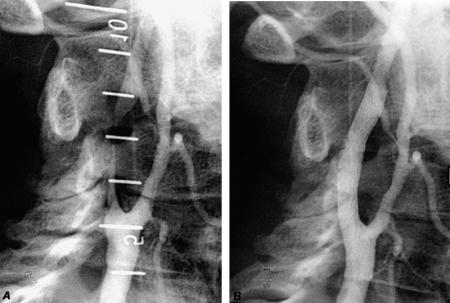
Fig. 3 A) This angiogram shows a long, critical right internal carotid lesion (from 5.5 cm to 8.0 cm on the angiographic ruler) in a patient who had undergone radiation therapy for a head and neck malignancy. B) A S.M.A.R.T.™ stent was deployed from the bifurcation of the internal and external carotid arteries to the internal carotid artery.
Conclusion
In this report we have presented a small series of 8 patients who underwent successful percutaneous endovascular therapy for radiation-induced occlusive disease or stenosis after endarterectomy. This study highlights the safety and low morbidity associated with this procedure, in contrast with the surgical approach. Patients who undergo this procedure can be discharged the next day and require little or no convalescent period, which leads to greater patient satisfaction. Recent technological advances such as distal protection devices and smaller-profile stent-delivery devices will add to the safety of this procedure. 21 Given the complexity of the surgical approach for both of these conditions, it is our opinion that endovascular therapy should be considered a primary approach for their management. Randomized clinical trials will help determine whether carotid stenting is as safe as carotid endarterectomy for the management of atherosclerotic disease.
Footnotes
Address for reprints: Zvonimir Krajcer, MD, 6624 Fannin Street, Suite 2780, Houston, TX 77030
References
- 1.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991;325:445–53. [DOI] [PubMed]
- 2.Beebe HG, Clagett GP, DeWeese JA, Moore WS, Robertson JT, Sandok B, et al. Assessing risk associated with carotid endarterectomy. A statement for health professionals by an Ad Hoc Committee on Carotid Surgery Standards of the Stroke Council, American Heart Association. Circulation 1989;79:472–3. [DOI] [PubMed]
- 3.Hertzer NR, O'Hara PJ, Mascha EJ, Krajewski LP, Sullivan TM, Beven EG. Early outcome assessment for 2228 consecutive carotid endarterectomy procedures: the Cleveland Clinic experience from 1989 to 1995. J Vasc Surg 1997;26:1–10. [DOI] [PubMed]
- 4.Piepgras DG, Sundt TM Jr, Marsh WR, Mussman LA, Fode NC. Recurrent carotid stenosis. Results and complications of 57 operations. Ann Surg 1986;203:205–13. [DOI] [PMC free article] [PubMed]
- 5.Seabrook GR, Towne JB. Nonatherosclerotic cerebrovascular disease. In: Haimovici H, Ascer E, Hollier LH, Strandness DE, Towne JB, editors. Haimovici's vascular surgery principles and techniques. 4th ed. Cambridge: Blackwell Science, 1996:966–81.
- 6.Rockman CB, Riles TS, Fisher FS, Adelman MA, Lamparello PJ. The surgical management of carotid artery stenosis in patients with previous neck irradiation. Am J Surg 1996;172:191–5. [DOI] [PubMed]
- 7.Melliere D, Becquemin JP, Berrahal D, Desgranges P, Cavillon A. Management of radiation-induced occlusive arterial disease: a reassessment. J Cardiovasc Surg (Torino) 1997; 38:261–9. [PubMed]
- 8.Phillips GR 3d, Peer RM, Upson JF, Ricotta JJ. Late complications of revascularization for radiation-induced arterial disease. J Vasc Surg 1992;16:921–5. [PubMed]
- 9.Yadav JS, Roubin GS, King P, Iyer S, Vitek J. Angioplasty and stenting for restenosis after carotid endarterectomy. Initial experience. Stroke 1996;27:2075–9. [DOI] [PubMed]
- 10.Hobson RW 2nd, Goldstein JE, Jamil Z, Lee BC, Padberg FT Jr, Hanna AK, et al. Carotid restenosis: operative and endovascular management. J Vasc Surg 1999;29:228–38. [DOI] [PubMed]
- 11.Al-Mubarak N, Roubin GS, Iyer SS, Gomez CR, Liu MW, Vitek JJ. Carotid stenting for severe radiation-induced extracranial carotid artery occlusive disease. J Endovasc Ther 2000;7:36–40. [DOI] [PubMed]
- 12.Lanzino G, Mericle RA, Lopes DK, Wakhloo AK, Guterman LR, Hopkins LN. Percutaneous transluminal angioplasty and stent placement for recurrent carotid artery stenosis. J Neurosurg 1999;90:688–94. [DOI] [PubMed]
- 13.MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. European Carotid Surgery Trialists' Collaborative Group. Lancet 1991;337:1235–43. [PubMed]
- 14.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421–8. [PubMed]
- 15.Chassin MR. Appropriate use of carotid endarterectomy. N Engl J Med 1998;339:1468–71. [DOI] [PubMed]
- 16.Frericks H, Kievit J, van Baalen JM, van Bockel JH. Carotid recurrent stenosis and risk of ipsilateral stroke: a systematic review of the literature. Stroke 1998;29:244–50. [DOI] [PubMed]
- 17.Hertzer NR. Postoperative management and complications following carotid endarterectomy. In: Rutherford RB, editor. Vascular surgery. 5th edition. Philadelphia: WB Saunders Co., 2000:1881–1906.
- 18.Ricotta JJ, O'Brien MS, DeWeese JA. Natural history of recurrent and residual stenosis after carotid endarterectomy: implications for postoperative surveillance and surgical management. Surgery 1992;112:656–63. [PubMed]
- 19.Ricotta JJ, O'Brien-Irr MS. Conservative management of residual and recurrent lesions after carotid endarterectomy: long-term results. J Vasc Surg 1997;26:963–72. [DOI] [PubMed]
- 20.Moritz MW, Higgins RF, Jacobs JR. Duplex imaging and incidence of carotid radiation injury after high-dose radiotherapy for tumors of the head and neck. Arch Surg 1990; 125:1181–3. [DOI] [PubMed]
- 21.Henry M, Amor M, Henry I, Klonaris C, Chati Z, Masson I, et al. Carotid stenting with cerebral protection: first clinical experience using the PercuSerge GuardWire system. J Endovasc Surg 1999;6:321–31. [DOI] [PubMed]


