Abstract
Dengue is a major global public health problem requiring a safe and efficacious vaccine as the foundation of a comprehensive countermeasure strategy. Despite decades of attempts, the world has a single dengue vaccine licensed in numerous countries, but restrictions and conditions of its use have deterred uptake. Recently, clinical efficacy data has been revealed for two additional dengue vaccine candidates and the data appears encouraging. In this perspective I discuss dengue, the complexities of dengue vaccine development, early development setbacks, and how the latest data from the field may be cause for measured optimism. Finally, I provide some perspectives on evaluating dengue vaccine performance and how the pursuit of the perfect dengue vaccine may prevent advancement of vaccines which are good enough.
Subject terms: Biologics, Drug development
Introduction
Dengue is caused by infection with any of the four dengue viruses (DENV-1–4) and represents a significant global public health burden1. Dengue not only causes morbidity and mortality in the infected but also consumes scarce resources for infection prevention, caring for the ill, and missed work and school2,3. The DENVs are transmitted in tropical and subtropical regions by infected Aedes mosquito species as they take a blood meal from a susceptible host. Hundreds of millions of people are infected every year and an estimated 96 million infections are clinically apparent4,5.
Clinically relevant dengue is characterized by fever, headache, bone and muscle pain, eye discomfort, fatigue, and the development of rash. Gastrointestinal and respiratory complaints may also be common depending on the age of the infected individual6,7. Severe dengue manifests with plasma leakage, intravascular volume depletion, and reduced organ perfusion (shock). Disruption of coagulation is also possible and may result in significant hemorrhage contributing to shock8.
Individuals are at greatest risk for severe dengue when they experience two sequential DENV infections with two different DENV types separated in time by more than 18 months9,10. Additional risk factors under exploration include genetic background, pre-existing medical conditions (obesity, renal and cardiovascular disease, diabetes), and female sex11–18. The contributions of human–vector–virus interactions and the potential evolution, and co-evolution, of human immunity, vector competence, and changes in virus genotype/lineage are also being studied19–24.
The exact immunopathogenic mechanisms of sequential heterotypic DENV infections are incompletely understood, but considerable evidence points to humoral and cellular adaptive immune responses occurring in response to the first infection facilitating increased DENV replication during the second infection which, in turn, drives pro-inflammatory cytokine secretion25–31. The exact number of annual dengue fatalities is not known but the estimates range between 5000 and 40,000 with many deaths occurring in children32,33.
Supportive treatment (antipyretics, judicious intravascular volume repletion) delivered by clinicians with experience treating dengue is very effective with low case fatality rates. Unfortunately, variance in clinical care exists and dengue has high morbidity and mortality in many endemic countries34. Currently, no anti-DENV antivirals or immune-based (monoclonal antibodies) prophylactics or therapeutics are approved for use, but promising efforts are underway35,36.
Reducing human arboviral infections through mosquito control strategies has had intermittent success. The widely held opinion that mosquito control is a necessary component of a comprehensive dengue control strategy requires the expanded study of available and novel approaches37,38. The recent development and deployment of mosquito control methods using genetic- or microbial-based alterations to mosquito populations offers the potential for improved outcomes39,40.
Vaccines
Vaccination has long been recognized as the required foundation of a multi-pronged approach to reducing the global dengue burden but developing a safe and effective dengue vaccine has been very difficult. For more than 75 years, scientists and product developers have attempted to design and advance safe and efficacious dengue vaccine candidates, but the challenges have been substantial and formidable (Box 1)41,42. Although numerous different approaches are being explored, only live attenuated virus vaccines have achieved licensure or reached advanced clinical development43.
Box 1. Dengue vaccine development challenges.
Existence of four DENV types (1–4), each capable of causing infection, disease, and death
No validated immune correlate of protection
Animal models do not comprehensively recapitulate the human dengue infection/disease experience
Immunologic assays are unable to precisely define DENV type-specific (homotypic) immune responses
Requirement for very large efficacy trials to demonstrate benefit across diverse populations and clinical endpoints
Dengvaxia®
Sanofi Pasteur licensed the first dengue vaccine (Dengvaxia®) in Mexico in 2015, and more than 20 countries thereafter, based on the safety and efficacy demonstrated in two phase III trials and a single season of disease surveillance. Unfortunately, the optimisim that a dengue vaccine was finally available quickly became disappointment when a safety signal was observed in vaccine recipients who were dengue non-immune at the time of vaccine administration44,45. In the third year of the phase III clinical trial, the youngest, non-immune vaccine recipients experienced increased rates of hospitalized and severe dengue compared to their unvaccinated peers46.
Many hypotheses were offered to explain this occurrence including the idea that imbalanced homotypic and heterotypic immunity across the four DENV types primed dengue naive (serostatus negative) vaccine recipients for antibody-dependant enhancement (ADE) when they encountered their first natural infection47. Others postulated that the absence of DENV non-structural proteins in the vaccine construct prevented the formation of protective cellular immunity and/or anti-NS1 antibodies48. Younger age was also proposed as an independent risk factor for clinically apparent and more severe dengue. Unfortunately, the phase III trials’ study design and limited blood sampling at baseline did not allow for a stratified analysis of vaccine safety and efficacy by baseline dengue serostatus. Instead, Sanofi tested volunteers one month after their last vaccine dose using an anti-NS1 antibody assay. The idea was that dengue serostatus negative vaccine recipients would be without NS1 antibodies because the vaccine does not contain NS1 proteins, in contrast to serostatus positive recipients who would have been naturally infected and exposed to NS149,50.
The safety signal in year three became less pronounced over time but the damage was done. Sanofi had already decided to seek an indication only for older children (9 years and above) and regulators forced the company to modify the vaccine’s label stating that only individuals previously infected by a DENV should be vaccinated. There was an outcry in the Philippines as hundreds of thousands of children had been vaccinated between the time of licensure and acknowledgement of the safety signal. The country subsequently revoked the vaccine’s license.
The World Health Organization Strategic Advisory Group of Experts on Immunization (SAGE) modified its original endorsement of Dengvaxia® recommending it only be used in dengue immune individuals51. Although Dengvaxia was proven safe and efficacious in dengue immune recipients, especially against more severe forms of disease, and remains licensed in many countries, including the U.S., vaccination implementation and uptake has been low52–54. There has been little information on the outcomes, good or bad, of over 800,000 children who were vaccinated with Dengvaxia®, including hundreds of thousands who received only a single dose when the vaccination program was shut down55.
The next generation of dengue vaccines
As expected, every dengue vaccine candidate following Dengvaxia® is being stringently reviewed for safety and efficacy in dengue immunes and non-immunes, across a broad age range of recipients, and for their ability to protect against the full spectrum of disease outcomes caused by infection with any DENV type. There is also a requirement for demonstrating safety and efficacy across more than one dengue season56–58.
Two new live attenuated dengue vaccines have now completed phase III efficacy trials and there is room for cautious optimism once again. Takeda recently received approval from Indonesia, European Commission, and Brazilian regulators for use of their two-dose vaccine (TAK-003) in people 4 years of age and older, regardless of baseline dengue immune status. Approval was based on safety, immunogenicity, and efficacy data from 19 phase I, II, and III trials with more than 28,000 participants spanning a broad age range. Dengue surveillance in the phase III trial extended for 4.5 years.
The primary study endpoint for the phase III trial was efficacy against any dengue, of any severity, caused by any DENV type in either dengue immune or non-immune recipients. Within 12 months of the second dose vaccine efficacy was 80.2%59. At the 18-month timepoint, vaccine efficacy against all dengue in dengue immune recipients was 76.1% and 66.2% in dengue non-immune recipients. Efficacy against hospitalized dengue was 90.4% and 85.9% against dengue hemorrhagic fever (DHF) (WHO, 1997 criteria). DENV type-specific efficacy was 69.8% for DENV-1, 95.1% for DENV-2, and 48.9% for DENV-3 with variable confidence intervals60. At 54 months, overall vaccine efficacy had waned to 61.2% with efficacy of 64.2% in dengue immune recipients and 53.5% in dengue non-immunes. Efficacy against hospitalized dengue was 84.1%. DENV type specific efficacy in dengue non-immunes was 78.4% for DENV-1, 100% for DENV-2, there was no efficacy for DENV-3, and not enough DENV-4 cases to calculate a value. DENV-3 efficacy in dengue immunes was 74%. Efficacy against DHF caused by any DENV type was 70.0% and against severe dengue (determined by an adjudication committee) was 70.2%. These data have not been presented in the peer-reviewed scientific literature but are accessible from the sponsor’s Summary of Product Characteristics (https://www.takeda.com/siteassets/system/newsroom/2022/qdenga/ema-combined-h-5155-en.pdf) (accessed 21 January 2023).
The dengue working group of the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) recently (February 23, 2023) reviewed TAK-003 performance indicating; (1) the vaccine protected seropositive recipients against all dengue and hospitalized dengue caused by infection with any serotype; (2) the vaccine protected seronegative recipients against all and hospitalized dengue due to DENV-1 and -2 infection; (3) the vaccine did not protect seronegative recipients against all dengue and hospitalized dengue due to DENV-3; and (4) the vaccine’s performance against DENV-4 infection outcomes in seronegative children could not be conclusively determined due to low event numbers. The lack of a defined immune correlate of protection made the significance of the presented immunogenicity data unclear.
More recently, the Instituto Butantan, U.S. NIH, and Merck (MSD) reported the first results from a phase III trial in Brazil with over 16,000 participants and at least two years of disease surveillance. The vaccine (Butantan-DV) was made using materials licensed from the U.S. NIH and is analogous to the NIH’s TV003 formulation tested previously61,62. MSD joined the collaboration when they entered into a co-development and licensing agreement in 2018. The phase III trial was initiated in 2016 and included participants ranging in age from 2 to 59 years who received a single dose of vaccine and were followed for any dengue, of any severity, caused by any DENV type. Dengue immune and non-immune participants were included in the trial.
Overall efficacy was 79.6% with dengue immunes having higher efficacy (89.2%) compared to dengue non-immunes (75.3%). Efficacy data is only available for DENV-1 (89.5%) and DENV-2 (69.6%) due to the low circulation of types DENV-3 and -4 during the trial. DENV type specific data by dengue immune status reveals higher efficacy against DENV-1 in dengue immunes (96.8%) compared to non-immunes (85.5%) and similar findings for DENV-2 (immune 83.6%, non-immune 57.9%). There were no severe cases or cases with clinical warning signs reported. The trial will continue until 2024 leaving open the possibility there will be sufficient cases caused by DENV-3 and -4 to gain a clearer view of vaccine performance against these types. These data have not been published in the peer reviewed scientific literature but are accessible from the Butantan website (https://butantan.gov.br/noticias/butantan%27s-dengue-vaccine-has-79.6-efficacy-partial-results-from-2-year-follow-up-show) (accessed 21 January 2023).
In summary, the three live attenuated dengue vaccines which have generated clinical endpoint efficacy data have all demonstrated; (1) higher efficacy in dengue immune recipients; (2) higher efficacy against more severe clinical phenotypes; (3) variance in DENV type specific efficacy, and (4) the challenge of capturing data for all desired clinical endpoints (any dengue, severe dengue, hospitalized dengue), across all DENV-1–4 types, in both dengue immune and non-immune recipients.
Assessing dengue vaccine performance
With new dengue vaccine efficacy data becoming available, regulators, public health officials, and scientists are grappling with how to assess the risk and benefit of imperfect dengue vaccines.
Safety
The local and systemic reactogenicity profile of a dengue vaccine must be acceptable and on par with other licensed vaccines. In addition, the rates of dengue and severe dengue cannot be greater in vaccine recipients compared to unvaccinated peers. Lack of benefit against a specific clinical outcome may be acceptable when taken in the larger context of all benefits, but associations between vaccination and developing the disease the vaccine is intended to prevent is not.
How to assess for the potential of vaccine-associated dengue is not straight forward. After two years of surveillance in the Butantan study there were no severe dengue cases nor cases with clinical warning signs. The Takeda experience, however, is more complex, and even though clinical and regulatory review committees for the European Commission and Brazil’s National Health Surveillance Agency (ANVISA) did not believe there was a safety signal in dengue non-immune recipients, this a point of contention63–65. Two issues may prevent achieving a consensus on the Takeda data: (1) low numbers of severe and DENV type-specific cases reducing the statistical power to make generalizable conclusions and (2) using hospitalization as a surrogate for severe disease.
One would think hospitalizing an individual is an accurate reflection of disease severity but differences in hospitalization practice across countries calls this into question. As a routine practice, trial sponsors defer to the local standards of medical care. This makes sense but presents opportunities for site-to-site variance and introduces potential confounders into data analysis. For example, some sites may admit all patients based on diagnosis alone while others only admit patients based on clinical necessity.
Markers of severe disease such as clinical signs or symptoms, or laboratory evidence of plasma leakage and/or hemorrhage would also appear to be a clear method to classify disease severity, but this approach has the potential to confound due to variance in diagnostic resources across trial sites. For example, some sites may have access to, and routinely use, ultrasound to detect fluid collections such as ascites or pleural effusions indicating the occurrence of plasma leakage. Other sites may lack these resources and must rely on less sensitive methods such as abdominal palpation or lung auscultation. Adding to the complexity of this issue is that documentation methods supporting clinical trials may not be nuanced enough to distinguish between the mere occurrence of a finding from the clinical relevance of a finding.
Even when the decision is made to utilize published classification systems of severe disease there is potential for variance. For example, vaccine developers may choose to utilize severity criteria contained within the WHO 1997 guidelines or choose the revised 2009 document66,67. Concern that these guidelines were designed to support clinical care and were not a good fit for use in research settings prompted the U.S. NIH to lead an effort to develop guidelines for use in interventional trials68,69. Sponsors may also commission external experts to develop additional criteria and guidelines like Takeda did with their dengue case adjudication committee65.
Expectations based on extrapolation
Differences in vaccine construct may translate into significant qualitative differences in immune responses following vaccination. These differences should be kept in mind when predicting vaccination outcomes with newer vaccine candidates using Dengvaxia® as the reference.
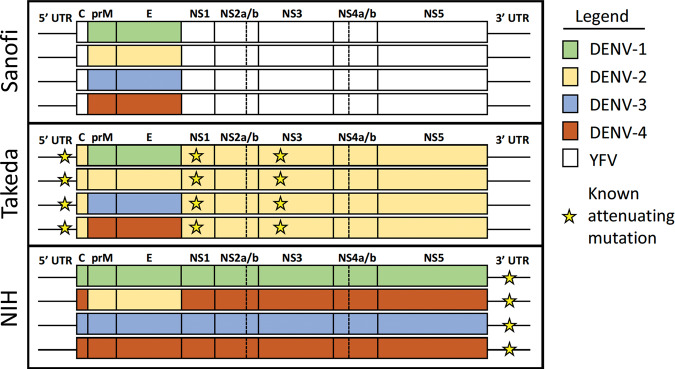
Most humoral immunity epitopes are located within the domains of the DENV envelop (E) protein while cellular immunity epitopes are located on the non-structural (NS) proteins27,70. As noted in Fig. 1, Dengvaxia is based on a Yellow Fever (YF) 17D backbone with DENV-1-4/YF chimeras made through the introduction of DENV prM and E genes and removal of the YF prM and E genes. The vaccine contains no DENV NS proteins. The Takeda vaccine uses a DENV-2 backbone to create DENV-1/-2, -3/-2, and -4/-2 chimeras and therefore has only DENV-2 NS proteins. The NIH/Butantan/MSD vaccine has full genome DENV-1, -3, and -4 components with a DENV-2/-4 chimera based on a DENV-4 backbone. This vaccine possesses NS proteins from DENV-1, -3, and -4. These important differences in vaccine construct should motivate a pause before trying to directly and broadly extrapolate the Dengvaxia® experience to all vaccines43.
Fig. 1. LIve attenuated dengue vaccine constructs.
DENV genome components of Sanofi, Takeda, and NIH/Bhutantan/MSD dengue vaccine candidates with the location of known attenutating mutations.
However, when it comes to multi-component replicating dengue vaccines, construct alone may not be sufficient to explain variance in immunogenicity and efficacy. Both the Sanofi and Takeda vaccines appear to have a dominant single vaccine virus which replicates after administration (Sanofi—DENV-4, Takeda—DENV-2) despite having all four DENV types included in the vaccine71–73. In contrast, MSD’s formulation of the NIH vaccine induced replication of three or more vaccine viruses in 64% of flavivirus non-immune recipients74. How this will translate into efficacy across the five years of follow up and DENV type-specific efficacy remains to be seen.
Immunogenicity does not guarantee efficacy
It is unclear whether homotypic immunity to each DENV type is necessary to be protected against disease caused by infection with any DENV type. Sequential infections with two different DENV types appears to impart a mix of broadly protective homo- and heterotypic immunity, as evidenced by the very rare occurrence of clinically relevant third and fourth DENV infections75. Vaccine developers must pursue the development of tetravalent vaccine formulations containing antigens to each DENV type, but it has been difficult, especially with replicating vaccines, to avoid some element of immunodominance and an imbalance of homotypic immunity to the dominant DENV type and cross-reactive immunity to the others76–82. As a result, a major lesson learned when assessing dengue vaccine performance is that immunogenicity does not necessarily translate into clinical efficacy.
Sanofi learned this lesson following unblinding of its phase 2b efficacy trial results from Thailand83. The expectation was that generation of measurable neutralizing antibodies to a specific DENV type would portend a reasonable likelihood of having protection against disease if infected with the same type. But, despite having balanced geometric mean neutralizing antibody titers greater than 100 for all DENV types and high rates (>95%) of seropositivity after three vaccine doses, the trial failed to meet its primary efficacy endpoint. The immunogenicity and efficacy mismatch by DENV type would occur again during subsequent phase III testing of Dengvaxia® and Takeda’s vaccine46,59,60,65,84–88. Based on early efficacy data from the Butantan trial, the disconnect will likely persist based on review of the vaccine’s historic immunogenicity data and the recent disclosure of lower DENV-2 efficacy61,89.
A safe and good dengue vaccine is better than no vaccine
A perfect dengue vaccine would safely deliver benefit across a myriad of scenarios. The perfect vaccine would: (1) protect across a diverse age range, (2) prevent infection (ideally) and disease caused by any DENV type and possibly numerous genotypes within each type, (3) prevent all clinically relevant phenotypes of dengue, not only severe disease, (4) protect recipients regardless of their flavivirus immunity status at the time of vaccination, (5) disrupt transmission of virus between people and mosquitoes, and (6) have durable protection until the recipient transitioned out of the risk window by acquiring a profile of homo- and heterotypic immunity like what is observed after two natural DENV infections. Shared gaps in the performance of current vaccines include a reduced ability to protect the non-immune recipient from clinically relevant, but more mild disease, caused by any DENV type.
A dengue vaccine available only to dengue immune recipients may have clinical value but lack the necessary practical attributes to support meaningful uptake. A ‘test and vaccinate’ strategy, although feasible, could be very difficult to operationalize across the multitude of dengue endemic areas90–94. A good vaccine not used for vaccination delivers no benefit.
Less severe forms of dengue contribute substantially to the overall public health burden33,95–97. Prevention of milder forms of dengue would not only reduce morbidity but also the economic and other opportunity costs of missed school or work. However, a vaccine which reliably only prevents hospitalization or more severe forms of dengue still has the potential to make a major public health impact, especially during high-transmission outbreaks (epidemics). This is particularly true in low- and middle-income countries where resources for the critically ill are scarce or in locations where experience with treating severely ill dengue patients is lacking. In addition, when hospital beds are not occupied by dengue patients, these resources can be allocated towards other public health burdens such as respiratory or gastrointestinal diseases.
A dengue vaccine that is efficacious against some, but not all DENV types can still deliver value. In many dengue endemic areas, numerous DENV types co-circulate and infect populations98,99. A vaccine which does not increase the recipient’s risk of dengue and can reduce the risk of disease caused by even some of the DENV types would still deliver an overall public health net benefit. This is especially true for DENV types more commonly associated with disease (DENV-1, -2) and more severe clinical outcomes (DENV-2)11,100.
Conclusion
It is clear the perfect dengue vaccine is not on the immediate horizon, but the Sanofi, Takeda, and Butantan/NIH/Merck experiences do inform us that it is possible to effectively immunize some people against disease scenarios that constitute dengue’s burden. I would contend when it comes to dengue countermeasure development, safety is non-negotiable, but all other expectations must be managed and considered in the aggregate. Our challenges with effectively communicating coronavirus disease 2019 vaccine performance characteristics should be a cautionary tale in this regard. Pursuit of the perfect dengue vaccine is a laudable goal, but not at the cost of overlooking imperfect options that could safely deliver tangible, albeit smaller scale, public health benefit.
Competing interests
S.J.T. is an academic physician and scientist with federal grants and industry support for a wide variety of dengue research initiatives. He has/is supporting dengue countermeasure development efforts as a consultant, advisory board member, or blinded case adjudication committee member for GlaxoSmithKline, Sanofi, Takeda, and/or Merck and was/is compensated for his time. S.J.T. has a patent related to dengue vaccines and all rights were assigned to the U.S. government.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tian N, et al. Dengue incidence trends and its burden in major endemic regions from 1990 to 2019. Trop. Med. Infect. Dis. 2022;7:180. doi: 10.3390/tropicalmed7080180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castro MC, Wilson ME, Bloom DE. Disease and economic burdens of dengue. Lancet Infect. Dis. 2017;17:e70–e78. doi: 10.1016/S1473-3099(16)30545-X. [DOI] [PubMed] [Google Scholar]
- 3.Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. Lancet Infect. Dis. 2016;16:935–941. doi: 10.1016/S1473-3099(16)00146-8. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endy TP. Human immune responses to dengue virus infection: lessons learned from prospective cohort studies. Front Immunol. 2014;5:183. doi: 10.3389/fimmu.2014.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalayanarooj S, et al. Early clinical and laboratory indicators of acute dengue illness. J. Infect. Dis. 1997;176:313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 7.Endy TP, et al. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am. J. Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 8.Kalayanarooj S. Clinical manifestations and management of dengue/DHF/DSS. Trop. Med. Health. 2011;39:83–87. doi: 10.2149/tmh.2011-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson KB, et al. A shorter time interval between first and second dengue infections is associated with protection from clinical illness in a school-based cohort in Thailand. J. Infect. Dis. 2014;209:360–368. doi: 10.1093/infdis/jit436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montoya M, et al. Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl. Trop. Dis. 2013;7:e2357. doi: 10.1371/journal.pntd.0002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sangkaew S, et al. Risk predictors of progression to severe disease during the febrile phase of dengue: a systematic review and meta-analysis. Lancet Infect. Dis. 2021;21:1014–1026. doi: 10.1016/S1473-3099(20)30601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vejbaesya S, et al. TNF and LTA gene, allele, and extended HLA haplotype associations with severe dengue virus infection in ethnic Thais. J. Infect. Dis. 2009;199:1442–1448. doi: 10.1086/597422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vejbaesya S, et al. HLA class I supertype associations with clinical outcome of secondary dengue virus infections in ethnic Thais. J. Infect. Dis. 2015;212:939–947. doi: 10.1093/infdis/jiv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xavier Eurico de Alencar L, et al. HLA-B *44 is associated with dengue severity caused by DENV-3 in a Brazilian population. J. Trop. Med. 2013;2013:648475. doi: 10.1155/2013/648475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu YY, et al. The association of obesity and dengue severity in hospitalized adult patients. J. Microbiol. Immunol. Infect. 2023;56:267–273. doi: 10.1016/j.jmii.2022.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Zulkipli MS, et al. The association between obesity and dengue severity among pediatric patients: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2018;12:e0006263. doi: 10.1371/journal.pntd.0006263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kochel TJ, et al. Effect of dengue-1 antibodies on American dengue-2 viral infection and dengue haemorrhagic fever. Lancet. 2002;360:310–312. doi: 10.1016/S0140-6736(02)09522-3. [DOI] [PubMed] [Google Scholar]
- 18.Watts DM, et al. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet. 1999;354:1431–1434. doi: 10.1016/S0140-6736(99)04015-5. [DOI] [PubMed] [Google Scholar]
- 19.Tan CH, et al. Lineage replacement associated with fitness gain in mammalian cells and Aedes aegypti: a catalyst for dengue virus type 2 transmission. Microorganisms. 2022;10:1100. doi: 10.3390/microorganisms10061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor O, et al. Potential role of vector-mediated natural selection in dengue virus genotype/lineage replacements in two epidemiologically contrasted settings. Emerg. Microbes Infect. 2021;10:1346–1357. doi: 10.1080/22221751.2021.1944789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koo C, et al. Highly selective transmission success of dengue virus type 1 lineages in a dynamic virus population: an evolutionary and fitness perspective. iScience. 2018;6:38–51. doi: 10.1016/j.isci.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang AT, et al. Beneath the surface: amino acid variation underlying two decades of dengue virus antigenic dynamics in Bangkok, Thailand. PLoS Pathog. 2022;18:e1010500. doi: 10.1371/journal.ppat.1010500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salje H, et al. Reconstructing unseen transmission events to infer dengue dynamics from viral sequences. Nat. Commun. 2021;12:1810. doi: 10.1038/s41467-021-21888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katzelnick LC, et al. Antigenic evolution of dengue viruses over 20 years. Science. 2021;374:999–1004. doi: 10.1126/science.abk0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katzelnick LC, et al. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salje H, et al. Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature. 2018;557:719–723. doi: 10.1038/s41586-018-0157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 28.Srikiatkhachorn A, Mathew A, Rothman AL. Immune-mediated cytokine storm and its role in severe dengue. Semin. Immunopathol. 2017;39:563–574. doi: 10.1007/s00281-017-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halstead SB, Nimmannitya S, Cohen SN. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J. Biol. Med. 1970;42:311–328. [PMC free article] [PubMed] [Google Scholar]
- 30.Halstead SB, O’Rourke EJ. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977;265:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 31.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 2013;158:1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Quam MBM, Zhang T, Sang S. Global burden for dengue and the evolving pattern in the past 30 years. J. Travel Med. 2021;28:taab146. doi: 10.1093/jtm/taab146. [DOI] [PubMed] [Google Scholar]
- 33.Zeng Z, Zhan J, Chen L, Chen H, Cheng S. Global, regional, and national dengue burden from 1990 to 2017: a systematic analysis based on the global burden of disease study 2017. EClinicalMedicine. 2021;32:100712. doi: 10.1016/j.eclinm.2020.100712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalayanarooj S, Rothman AL, Srikiatkhachorn A. Case management of dengue: lessons learned. J. Infect. Dis. 2017;215:S79–S88. doi: 10.1093/infdis/jiw609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaptein SJF, et al. A pan-serotype dengue virus inhibitor targeting the NS3-NS4B interaction. Nature. 2021;598:504–509. doi: 10.1038/s41586-021-03990-6. [DOI] [PubMed] [Google Scholar]
- 36.Troost B, Smit JM. Recent advances in antiviral drug development towards dengue virus. Curr. Opin. Virol. 2020;43:9–21. doi: 10.1016/j.coviro.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Bowman LR, Donegan S, McCall PJ. Is dengue vector control deficient in effectiveness or evidence?: systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2016;10:e0004551. doi: 10.1371/journal.pntd.0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Achee NL, et al. A critical assessment of vector control for dengue prevention. PLoS Negl. Trop. Dis. 2015;9:e0003655. doi: 10.1371/journal.pntd.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dufault SM, et al. Disruption of spatiotemporal clustering in dengue cases by wMel Wolbachia in Yogyakarta, Indonesia. Sci. Rep. 2022;12:9890. doi: 10.1038/s41598-022-13749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Utarini A, et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N. Engl. J. Med. 2021;384:2177–2186. doi: 10.1056/NEJMoa2030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabin AB, Schlesinger RW. Production of immunity to dengue with virus modified by propagation in mice. Science. 1945;101:640–642. doi: 10.1126/science.101.2634.640. [DOI] [PubMed] [Google Scholar]
- 42.Thomas SJ, Rothman AL. Trials and tribulations on the path to developing a dengue vaccine. Am. J. Prev. Med. 2015;49:S334–S344. doi: 10.1016/j.amepre.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Waickman AT, Newell K, Endy TP, Thomas SJ. Biologics for dengue prevention: up-to-date. Expert Opin. Biol. Ther. 2023;23:73–87. doi: 10.1080/14712598.2022.2151837. [DOI] [PubMed] [Google Scholar]
- 44.Thomas SJ. Preventing dengue-is the possibility now a reality? N. Engl. J. Med. 2015;372:172–173. doi: 10.1056/NEJMe1413146. [DOI] [PubMed] [Google Scholar]
- 45.Simmons CP. A candidate dengue vaccine walks a tightrope. N. Engl. J. Med. 2015;373:1263–1264. doi: 10.1056/NEJMe1509442. [DOI] [PubMed] [Google Scholar]
- 46.Hadinegoro SR, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 47.Halstead SB. Licensed dengue vaccine: public health conundrum and scientific challenge. Am. J. Trop. Med. Hyg. 2016;95:741–745. doi: 10.4269/ajtmh.16-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guy B, Jackson N. Dengue vaccine: hypotheses to understand CYD-TDV-induced protection. Nat. Rev. Microbiol. 2016;14:45–54. doi: 10.1038/nrmicro.2015.2. [DOI] [PubMed] [Google Scholar]
- 49.Nascimento EJM, et al. Development of an anti-dengue NS1 IgG ELISA to evaluate exposure to dengue virus. J. Virol. Methods. 2018;257:48–57. doi: 10.1016/j.jviromet.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Thomas SJ, Yoon IK. A review of Dengvaxia(R): development to deployment. Hum. Vaccin. Immunother. 2019;15:2295–2314. doi: 10.1080/21645515.2019.1658503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilder-Smith A, et al. Deliberations of the Strategic Advisory Group of Experts on Immunization on the use of CYD-TDV dengue vaccine. Lancet Infect. Dis. 2019;19:e31–e38. doi: 10.1016/S1473-3099(18)30494-8. [DOI] [PubMed] [Google Scholar]
- 52.Forrat R, et al. Analysis of hospitalized and severe dengue cases over the 6 years of follow-up of the tetravalent dengue vaccine (CYD-TDV) efficacy trials in Asia and Latin America. Clin. Infect. Dis. 2021;73:1003–1012. doi: 10.1093/cid/ciab288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salje H, et al. Evaluation of the extended efficacy of the Dengvaxia vaccine against symptomatic and subclinical dengue infection. Nat. Med. 2021;27:1395–1400. doi: 10.1038/s41591-021-01392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flasche S, Wilder-Smith A, Hombach J, Smith PG. Estimating the proportion of vaccine-induced hospitalized dengue cases among Dengvaxia vaccinees in the Philippines. Wellcome Open Res. 2019;4:165. doi: 10.12688/wellcomeopenres.15507.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ylade M, et al. Effectiveness of a single-dose mass dengue vaccination in Cebu, Philippines: a case-control study. Vaccine. 2021;39:5318–5325. doi: 10.1016/j.vaccine.2021.07.042. [DOI] [PubMed] [Google Scholar]
- 56.Edelman R, Hombach J. “Guidelines for the clinical evaluation of dengue vaccines in endemic areas”: summary of a World Health Organization Technical Consultation. Vaccine. 2008;26:4113–4119. doi: 10.1016/j.vaccine.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 57.Vannice KS, et al. Clinical development and regulatory points for consideration for second-generation live attenuated dengue vaccines. Vaccine. 2018;36:3411–3417. doi: 10.1016/j.vaccine.2018.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hombach J. Guidelines for clinical trials of dengue vaccine in endemic areas. J. Clin. Virol. 2009;46:S7–S9. doi: 10.1016/S1386-6532(09)70287-2. [DOI] [PubMed] [Google Scholar]
- 59.Biswal S, et al. Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N. Engl. J. Med. 2019;381:2009–2019. doi: 10.1056/NEJMoa1903869. [DOI] [PubMed] [Google Scholar]
- 60.Biswal S, et al. Efficacy of a tetravalent dengue vaccine in healthy children aged 4-16 years: a randomised, placebo-controlled, phase 3 trial. Lancet. 2020;395:1423–1433. doi: 10.1016/S0140-6736(20)30414-1. [DOI] [PubMed] [Google Scholar]
- 61.Kallas EG, et al. Safety and immunogenicity of the tetravalent, live-attenuated dengue vaccine Butantan-DV in adults in Brazil: a two-step, double-blind, randomised placebo-controlled phase 2 trial. Lancet Infect. Dis. 2020;20:839–850. doi: 10.1016/S1473-3099(20)30023-2. [DOI] [PubMed] [Google Scholar]
- 62.Durbin AP. Historical discourse on the development of the live attenuated tetravalent dengue vaccine candidate TV003/TV005. Curr. Opin. Virol. 2020;43:79–TV003/TV087. doi: 10.1016/j.coviro.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biswal S, Patel SS, Rauscher M. Safety of dengue vaccine? Clin. Infect. Dis. 2022;76:771–772. doi: 10.1093/cid/ciac808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Silva A. Safety of dengue vaccine? Clin. Infect. Dis. 2022;76:371–372. doi: 10.1093/cid/ciac690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rivera L, et al. Three-year efficacy and safety of Takeda’s dengue vaccine candidate (TAK-003) Clin. Infect. Dis. 2022;75:107–117. doi: 10.1093/cid/ciab864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control 2nd edn. (WHO, 1997). [PubMed]
- 67.World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control (WHO, 2009). [PubMed]
- 68.Tomashek KM, et al. Development of standard clinical endpoints for use in dengue interventional trials. PLoS Negl. Trop. Dis. 2018;12:e0006497. doi: 10.1371/journal.pntd.0006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaenisch T, et al. Development of standard clinical endpoints for use in dengue interventional trials: introduction and methodology. BMC Med. Res. Methodol. 2018;18:134. doi: 10.1186/s12874-018-0601-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinheiro JR, et al. Comparison of neutralizing dengue virus B cell epitopes and protective T cell epitopes with those in three main dengue virus vaccines. Front. Immunol. 2021;12:715136. doi: 10.3389/fimmu.2021.715136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tricou V, et al. Safety and immunogenicity of a single dose of a tetravalent dengue vaccine with two different serotype-2 potencies in adults in Singapore: a phase 2, double-blind, randomised, controlled trial. Vaccine. 2020;38:1513–1519. doi: 10.1016/j.vaccine.2019.11.061. [DOI] [PubMed] [Google Scholar]
- 72.Torresi J, et al. Replication and excretion of the live attenuated tetravalent dengue vaccine CYD-TDV in a flavivirus-naive adult population: assessment of vaccine viremia and virus shedding. J. Infect. Dis. 2017;216:834–841. doi: 10.1093/infdis/jix314. [DOI] [PubMed] [Google Scholar]
- 73.Henein S, et al. Dissecting antibodies induced by a chimeric yellow fever-dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) in naive and dengue-exposed individuals. J. Infect. Dis. 2017;215:351–358. doi: 10.1093/infdis/jiw576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russell KL, et al. A phase I randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability, and immunogenicity of a live-attenuated quadrivalent dengue vaccine in flavivirus-naive and flavivirus-experienced healthy adults. Hum. Vaccin. Immunother. 2022;18:2046960. doi: 10.1080/21645515.2022.2046960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gibbons RV, et al. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am. J. Trop. Med. Hyg. 2007;77:910–913. doi: 10.4269/ajtmh.2007.77.910. [DOI] [PubMed] [Google Scholar]
- 76.Anderson KB, et al. Interference and facilitation between dengue serotypes in a tetravalent live dengue virus vaccine candidate. J. Infect. Dis. 2011;204:442–450. doi: 10.1093/infdis/jir279. [DOI] [PubMed] [Google Scholar]
- 77.Guy B, et al. Evaluation of interferences between dengue vaccine serotypes in a monkey model. Am. J. Trop. Med. Hyg. 2009;80:302–311. doi: 10.4269/ajtmh.2009.80.302. [DOI] [PubMed] [Google Scholar]
- 78.Edelman R, et al. Phase I trial of 16 formulations of a tetravalent live-attenuated dengue vaccine. Am. J. Trop. Med. Hyg. 2003;69:48–60. doi: 10.4269/ajtmh.2003.69.48. [DOI] [PubMed] [Google Scholar]
- 79.DeMaso CR, et al. Specificity and breadth of the neutralizing antibody response to a live attenuated tetravalent dengue vaccine. J. Infect. Dis. 2022;226:1959–1963. doi: 10.1093/infdis/jiac272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.White LJ, et al. Defining levels of dengue virus serotype-specific neutralizing antibodies induced by a live attenuated tetravalent dengue vaccine (TAK-003) PLoS Negl. Trop. Dis. 2021;15:e0009258. doi: 10.1371/journal.pntd.0009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nivarthi UK, et al. A tetravalent live attenuated dengue virus vaccine stimulates balanced immunity to multiple serotypes in humans. Nat. Commun. 2021;12:1102. doi: 10.1038/s41467-021-21384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henein S, et al. Dengue vaccine breakthrough infections reveal properties of neutralizing antibodies linked to protection. J.Clin. Investig. 2021;131:e147066. doi: 10.1172/JCI147066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sabchareon A, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 84.Villar L, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N. Engl. J. Med. 2015;372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 85.Capeding MR, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384:1358–1365. doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]
- 86.Lopez-Medina E, et al. Efficacy of a dengue vaccine candidate (TAK-003) in healthy children and adolescents 2 years after vaccination. J. Infect. Dis. 2022;225:1521–1532. doi: 10.1093/infdis/jiaa761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rivera L, et al. Three years efficacy and safety of Takeda’s dengue vaccine candidate (TAK-003) Clin. Infect. Dis. 2022;75:107–117. doi: 10.1093/cid/ciab864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lopez-Medina E, et al. Efficacy of a dengue vaccine candidate (TAK-003) in healthy children and adolescents two years after vaccination. J. Infect. Dis. 2022;225:1521–1532. doi: 10.1093/infdis/jiaa761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kirkpatrick BD, et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci. Transl. Med. 2016;8:330ra336. doi: 10.1126/scitranslmed.aaf1517. [DOI] [PubMed] [Google Scholar]
- 90.Fongwen N, et al. Implementation strategies for the first licensed dengue vaccine: a meeting report. Vaccine. 2021;39:4759–4765. doi: 10.1016/j.vaccine.2021.06.083. [DOI] [PubMed] [Google Scholar]
- 91.Echegaray F, et al. Adapting rapid diagnostic tests to detect historical dengue virus infections. Front. Immunol. 2021;12:703887. doi: 10.3389/fimmu.2021.703887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Savarino SJ, et al. Accuracy and efficacy of pre-dengue vaccination screening for previous dengue infection with a new dengue rapid diagnostic test: a retrospective analysis of phase 3 efficacy trials. Lancet Microbe. 2022;3:e427–e434. doi: 10.1016/S2666-5247(22)00033-7. [DOI] [PubMed] [Google Scholar]
- 93.DiazGranados CA, et al. Accuracy and efficacy of pre-dengue vaccination screening for previous dengue infection with five commercially available immunoassays: a retrospective analysis of phase 3 efficacy trials. Lancet Infect. Dis. 2021;21:529–536. doi: 10.1016/S1473-3099(20)30695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thommes E, et al. Public health impact and cost-effectiveness of implementing a ‘pre-vaccination screening’ strategy with the dengue vaccine in Puerto Rico. Vaccine. 2022;40:7343–7351. doi: 10.1016/j.vaccine.2022.10.071. [DOI] [PubMed] [Google Scholar]
- 95.Hung TM, Wills B, Clapham HE, Yacoub S, Turner HC. The uncertainty surrounding the burden of post-acute consequences of dengue infection. Trends Parasitol. 2019;35:673–676. doi: 10.1016/j.pt.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 96.Zeng W, Halasa-Rappel YA, Durand L, Coudeville L, Shepard DS. Impact of a nonfatal dengue episode on disability-adjusted life years: a systematic analysis. Am. J. Trop. Med. Hyg. 2018;99:1458–1465. doi: 10.4269/ajtmh.18-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anderson KB, et al. Burden of symptomatic dengue infection in children at primary school in Thailand: a prospective study. Lancet. 2007;369:1452–1459. doi: 10.1016/S0140-6736(07)60671-0. [DOI] [PubMed] [Google Scholar]
- 98.Guo C, et al. Global epidemiology of dengue outbreaks in 1990-2015: a systematic review and meta-analysis. Front. Cell. Infect. Microbiol. 2017;7:317. doi: 10.3389/fcimb.2017.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamashita A, et al. DGV: Dengue Genographic Viewer. Front. Microbiol. 2016;7:875. doi: 10.3389/fmicb.2016.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yuan K, Chen Y, Zhong M, Lin Y, Liu L. Risk and predictive factors for severe dengue infection: a systematic review and meta-analysis. PLoS ONE. 2022;17:e0267186. doi: 10.1371/journal.pone.0267186. [DOI] [PMC free article] [PubMed] [Google Scholar]