Abstract
Picrorhiza kurroa Royle ex Benth, Kutki (P.kurroa) is an important medicinal plant, traditionally recommended and used in Ayurveda for millennia, with certain cautions. There has been a significant revival of keen interest in its pharmacology, pharmacognosy, and phytochemistry for the last few decades. The evidence of its hepatoprotective activity, in experimental and clinical studies, accelerated the correlation of the specific phytochemical constituents of P.kurroa with precise pharmacological activities. Iridoid glycosides, particularly picrosides, emerged as the active molecules. For effective translation of traditional remedies into modern therapy, value addition by mechanistic understanding of molecular actions, drug targets, the degrees of efficacy and safety as well as convenient dosage forms is needed. Reverse pharmacology approach and phytopharmaceutical drug category facilitate such a translation. The present review illustrates how a potential translation of traditional practices of using P.kurroa into a phytochemically standardized, clinically targeted natural product for global unmet medical needs viz. Fatty liver disease can be attained.
Keywords: Picrorhiza kurroa, Reverse pharmacology, Hepatoprotectives, Picrosides, Non-alcoholic fatty liver disease, Ayurveda
1. Introduction
Picrorhiza kurroa Royle ex Benth, Kutki- (P.kurroa) finds mention for its clinical uses in an ancient and classical treatise of Ayurveda- Charaka-samhita [1]. Its application for diverse clinical conditions has continued even now since the medieval period [[4], [5], [6], [7]]. Its clinical usefulness, with some precautions, has consensual validity in the contemporary Asian medicine practices [[8], [9], [10]]. Since the second half of the last century until today, P. Kurroa has generated a lot of interest in amongst modern biomedical scientists, as evident through the scientific publications [[11], [12], [13], [14]]. The evidence of its hepatoprotective activity, in experimental and clinical studies accelerated the correlation of the specific phytochemical constituents of P.kurroa with precise pharmacological activities [15]. Fascinatingly, its potential utility in SARS CoV2 through in-silico studies has also been postulated [16] and clinical studies in combination with other herbs has been explored in COVID-19 [17].
Paradoxically, ever-increasing Ayurvedic usage demand and interest in the P.kurroa-Kutki has led to it's over harvesting, and the plant which has a limited natural habitat mainly at high altitude of sub-Himalayan region is now listed in the category of ‘endangered species’ [18]. Interestingly, plant tissue culture and genetic engineering techniques are being explored for micropropagation and to enhance the yield of its phytoactives [[19], [20], [21]]. Iridoid glycosides viz. Kutkins, kutkosides and picrosides, cucurbitacin, triterpenes and simple phenols such as apocynin are the most explored phytoactives of P.kurroa for their biological activity such as hepatoprotective [[22], [23], [24]], anti-inflammatory [25,26], anti-arthritic [27], anti-diabetic [28], anti-asthmatic [29], collagen synthesis-promoting and collagenase inhibitory [30], hyaluronidase inhibitory [31], anti-fibrotic [32], anti-oxidant [33,34], immunomodulatory [35,36], anti-carcinogenic [[37], [38], [39], [40]], anti-microbial [41,42], anti-leishmanial [43] etc.
Classically, P.kurroa-Kutki has been indicated for diverse clinical conditions such as anorexia, burning sensation, fevers, especially intermittent fevers, pre-diabetic and diabetic states, asthma, cough, haemorrhoids, dermatoses, helminthiasis etc. Some of these uses have been investigated (vide supra). The plant properties, classically described are adipolysis, excretion of wastes, orexigenic, laxative, anti-inflammatory, hepatoprotective, and diuretic activities; it is favourable to the heart action [44]. However, in traditional clinical practice P.kurroa-Kutki is predominantly used for hepatobiliary and gastrointestinal disorders.
The present review covers the classical literature and clinical traditions of usage of the plant, its pharmacology with an eye to develop a phytopharmaceutical for the focussed therapeutic indication of non-alcoholic fatty liver disease (NAFLD), by application of Reverse Pharmacology approach to P.kurroa for hepatoprotection, based on its specific phytoactives.
2. Classical literature and clinical tradition of P.kurroa-Kutki usage
P.kurroa-Kutki, as a medicinal plant, finds mention in all the three major ancient treatises of Ayurveda viz. Charak-samhita, Sushrut-samhita and Ashtang-hrudaya [[1], [2], [3]]. Moreover, uninterrupted, continued tradition of clinical use of P.kurroa-Kutki is evident by its mention in majority of the Nighantus compiled through medieval period like Bhavprakash Nighantu, Madanpal Nighantu, Kaiyadev Nighantu, Raj Nighantu and many more [[4], [5], [6], [7]]. Practicing vaidyas of the recent centuries have recorded in their personal notes their clinical experiences using P.kurroa-Kutki [[45], [46], [47], [48], [49]]. Ayurvedic volumes of periodicals also find articles on different clinical uses of P.kurroa-Kutki. Currently, Ayurvedic practitioners use P.kurroa-Kutki as a single plant formulation or in combination with other herbs or herbo-mineral complex formulations. Picrorhiza plant species has also been used for diverse healthcare problems by many other Asian traditional systems of medicine [8,9].
P.kurroa-Kutki is a shrub which grows naturally at high altitude of sub-Himalayan regions. The roots and rhizomes of this plant have major medicinal value, that now we know possess major phytoactives. Ayurveda classics give importance to the Desh (place of collection), Kala (time of collection), Prayojyanga (part of the plant) etc as important factors for collecting the plants for medicinal purpose [50]. Now, it has been well documented that the altitude, part of the plant and time and light are the determining factors for the phytoactive contents of P.kurroa-Kutki [[51], [52], [53]]. The classical literature enlists Kutki by different synonyms such as in Bhavprakash Nighantu [54]. These synonyms explain either typical characteristics or biological activity viz. Katumbhara (full of bitter principles), Ashoka (relieves from grief), Rohini (protects the organs by regenerative ability), Krushnabheda (potential to cause black stools) etc. To illustrate a few biological effects and clinical indications attributed to Kutki and relevant to this review are Lekhaneeya (adipolysis), Bhedaneeya (piercing through and expelling of waste metabolites), Aruchihara (improving appetite), Deepaniya (facilitate appetite), Rechani (laxative action), Yakrutdalyodar-nashak (alleviating liver enlargement), Shotha-nashak (anti-inflammatory), Kamala-nashak (alleviating jaundice), Jalodarnashan (useful in ascites), Pramehaghna (useful in pre-diabetic and diabetic states), and Hrudya (favourable for the heart).
Conventionally, for most of the clinical indications P.kurroa-Kutki rhizome powder is used in a dose range of 300 mg–500 mg, two to three times a day, for an adult [44]. For laxative purpose the dose used is 2 gms to 4 gms as a single dose [44]. Although P.kurroa- Kutki is also considered as a detoxicating agent (Vishapaha), it has been reported to cause toxicity if not used in an appropriate dose for the correct indication [10]. As per our clinical experience of using P.kurroa-Kutki and its traditional products, it is observed to have developed adverse effects such as increased bowel frequency, diarrhoea, abdominal gurgling, abdominal colic etc.
Traditional practitioners more often prefer multi-ingredient formulations over single plant-based prescriptions. Arogyavardhini, Punarnavashtak kwath, Mahatiktaka Ghruta, Sarivadyasava, MahayograjGuggulu, Sudarshan choorna, Katukadyavaleha, Tiktadi kwath, are some of the commonly prescribed multi-ingredient traditional formulations containing P.kurroa-Kutki as one of the ingredients which may be in a major proportion or minor proportion. Although intricacy of pharmacodynamics of these multi-ingredient formulations is beyond the scope of this article the Table 1 takes an account of diverse indications of P.kurroa-Kutki as stated in different Nighantus. Arogyavardhini in a tablet form is one of the most frequently used complex herbo-mineral multi-ingredient formulation, where P.kurroa-Kutki the major ingredient is in an equal proportion of the other 12 ingredients [55]. Arogyavardhini is commonly used in traditional practice for liver disorders, hepato-splenomegaly, ascites, dyslipidaemia, obesity, skin disorders, as an anti-inflammatory, for improving appetite, digestion, and laxation [56]. Table 2 summarizes the clinical trials reported with P.kurroa-Kutki (alone) and P.kurroa-Kutki in combination with other agents in liver related and other disorders.
Table 1.
Diverse clinical indications of P.kurroa-Kutki noted from Ayurveda Nighantus’ (Compendia of Ayurvedic medicinal plants).
| Sr.No | Roghaghnata (Indications) | B.N [1]. | K.N [2]. | M.N [3]. | R.N [4]. | D.N [5]. | A.N [6]. | V.N [7]. | P.N [8]. | V.G [9]. | DG.H [10]. | V.V [11]. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Malabhedan/Rehan (Piercing through and expelling of waste metabolites) | + | + | + | + | + | ||||||
| 2 | Krumighna (Anti-helminthic) | + | + | + | + | + | + | |||||
| 3 | Dahanashaka (Useful in burning) | + | + | + | + | + | + | + | ||||
| 4 | Jwraghna (Antipyretic) | + | + | + | + | + | + | + | + | + | + | |
| 5 | Kamala nashak (Alleviating jaundice) | + | + | + | + | + | ||||||
| 6 | Raktavikar nashak (Useful in blood disorders) | + | + | + | + | + | + | + | ||||
| 7 | Sheetapittaghna (Anti-urticarial) | + | + | + | ||||||||
| 8 | Kaphaghna (Pacifying vitiated Kapha) | + | + | + | + | + | + | + | + | + | ||
| 9 | Pittaghna (Pacifying vitiated Pitta) | + | + | + | + | + | + | + | ||||
| 10 | Aruchinashaka (Improving distaste) | + | + | + | + | |||||||
| 11 | Kushthaghna (useful in skin diseases) | + | + | + | + | + | ||||||
| 12 | Vishaghna (Detoxicant) | + | ||||||||||
| 13 | Rajayakshmanivarini (Alleviating Tuberculosis) | + | + | |||||||||
| 14 | Hridrogaharana/Hrudya (Useful in Heart diseases) | + | + | + | + | |||||||
| 15 | Pramehanashaka (Anti-diabetic) | + | + | + | + | + | ||||||
| 16 | Kasahara/Shwasahara (Anti-tussive/Anti-asthmatic) | + | + | + | + | + | ||||||
| 17 | Chardinashana (Anti-emetic) | + | ||||||||||
| 18 | Hikkanashana (Useful in Hiccups) | + | ||||||||||
| 19 | Pandunashaka (Helpful in Anemia) | + | ||||||||||
| 20 | Kantharoga (Useful in throat disorder) | + | ||||||||||
| 21 | Raktashodhaka (Blood purifier) | + | + | + | + | + | ||||||
| 22 | Agnimandya nashaka/Deepani (Anti-anorexic/Appetizer) | + | + | |||||||||
| 23 | Anaha nivarana (Relieving painful Blotting) | + | ||||||||||
| 24 | Yakrutdalyudara pleehavruddhi har (Useful in Ascites & Hepato- splenomegaly) | + | + | |||||||||
| 25 | Shothanashaka (Anti-inflammatory) | + |
(B.N. – Bhavaprakasha Nighantu, K.N.-Kaiyadeva Nighantu, M.N.-Madanapala Nighantu, R.N.-Raja Nighantu, D.N.- Dhanvantari Nighantu, A.N.-Adarsha Nighantu, V.N.-Vanaushadhi Nighantu, P.N.-Priya Nighantu, V.G.-Vanaushadhi Gunadarsha, DG.H.-Dravyaguna Hastamalaka, V.V.-Vanaspati Vijnana).
Table 2.
Summary of clinical trials of Picrorhiza kurruoa (alone) and Picrorhiza kurroa in combination with other agents in liver related and other disorders.
| Formulation and Study Design | Dose | Duration and No of subjects | Adversity | Activity | Reference |
|---|---|---|---|---|---|
| Arogyavardhini &Punarnavadi (Open label) | 500 mg x 3 & 30 ml x 2 |
3 weeks N = 24 |
No ADR | Acute Viral Hepatitis | [57] |
| Arogyavardhini (Double Blind) | 750 mg x 3 | 2 weeks N = 20 |
No ADR | Acute Viral Hepatitis | [58] |
| Kutaki (Double Blind) | 375 mg x 3 | 2 weeks N = 15 |
No ADR | Acute Viral Hepatitis | [15] |
| Kutaki + Methoxasalen (Oral & Topical) | 200 mg x 2 20 mg once |
3months N = 30 |
No ADR | Vitiligo | [59] |
| Kutaki with Sita (Two arms) | One gm x 2 | 3 weeks N = 30 (15 in each group) |
No ADR | Amlapitta | [60] |
| Arogyavardhini &Triphala Guggulu and Pathya (Two arms) | 250 mg x 2 | 3months N = 21 |
No ADR | NAFLD | [61] |
| Phalatrikadi Kwath (Open Label) | 40 ml x 2 | 6months N = 59 |
No ADR | HbsAg + ve | [62] |
| Kutaki processed in Guduchi with Atorvastatin (Open Label) |
Atovarstatin: 20 mg twice daily+2 gm Katukai (P. Kurroa) processed in Guduchi twice daily | 3months N = 32 |
No ADR | Hepatoprotective | [63] |
| Elastographic liver evaluation of Katukyadi churna in the management of Non-Alcoholic Steatohepatitis (NASH) – A single arm clinical trial | 6 gm (Sachets) twice a day with water. Katukyadichurna comprises 1 part each of Katuki + Nimba + Amrita + Bhringaraj + Bhumyamalki |
180 days | 11 patients participated in the study. Two patients suffered from loose stools 2–3 times/day for the first 8 days. | NASH | [64] |
3. Reverse pharmacology approach to Kutki (P. kurroa) for hepatoprotection
Continued tradition of clinical use of P.kurroa-Kutki and Kutki-based multi-ingredient formulations, attracted attention of contemporary bio-medical investigators to investigate this plant. Major clinical and experimental studies of P.kurroa-Kutki were undertaken at BHU by Pandey VN, as a post-graduate thesis work in mid 1960s [65] and later submitted Ph.D. thesis on “The effect of indigenous drugs in the management of certain liver disorder” in 1979 [66]. Meanwhile Dhar, ML [67], Das, PK [68] and Kanitkar, SV [69] investigated this drug for its biological activity and hepatoprotective activity in early 70's. Tiwari, NS and Jain, PC investigated Arogyavardhini (Kutki-50%) clinically for its hypocholesterolaemic activity with special reference to obesity [70]. Vohora, SB and colleagues investigated P.kurroa-Kutki with special reference to its choleretic and antimicrobial properties [12]. The plant was also taken up by CDRI, Lucknow for extensive investigations and came out with a hepatoprotective product ‘Picroliv’ in 1990s. Ramesh Chander et al. [13], Dhawan BN and group [71] extensively worked on ‘Picroliv’ to investigate its hepato–protective activity whereas Shukla B et al. [72] investigated the choleretic effect of ‘Picroliv’. Saraswat B, investigated protective effect of Picroliv, active constituent of P.kurroa, against oxytetracycline induced hepatic damage [73]. Sporadically clinical trials of P.kurroa-Kutki (alone) and P.kurroa-Kutki in combination with other agents in liver related and other disorders has been investigated with variable outcomes [[59], [60], [61], [62], [63]].
Our research team has been consistently pursuing for the purpose of translational medicine on the path of reverse pharmacology [74,75]. Arogyavardhini formulation (50% P.kurroa-Kutki) was studied in acute viral hepatitis (HAV) on the basis of ‘clinical-usage-experience’. Since Arogyavardhini was often used along-with Punarnavadi-Kwath in traditional practices, the same was adhered-to, and studied in an exploratory open-labelled clinical trial in acute viral hepatitis. This study ensured activity and safety [57]. Taking cue from this exploratory study, a double-blind, placebo controlled, clinical study of Arogyavardhini was conducted. This study demonstrated early recovery of symptoms, faster reduction of liver transaminases, quicker clearance of bilirubin and a total reduction in disease duration [58]. Subsequent to this a placebo-controlled study was undertaken with the standardized extract of P.kurroa-Kutki the major ingredient of Arogyavardhini. The efficacy and safety of this extract (standardized for Picroside-I and II) in viral hepatitis was also established [15].
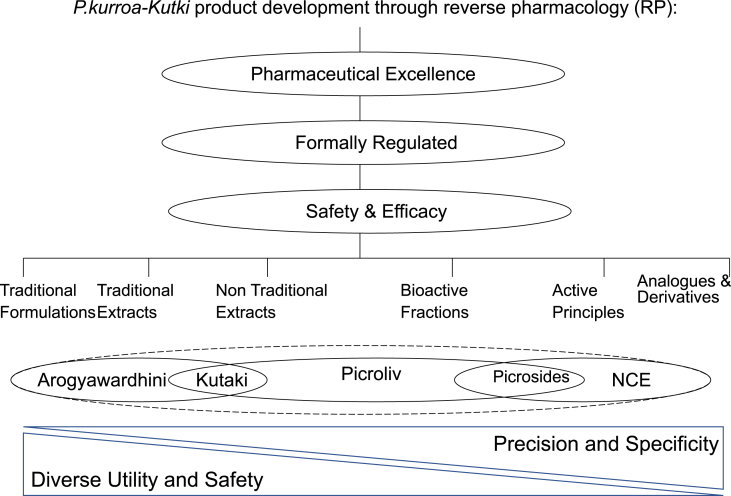
Later researchers at KHS-MRC pursued the activity of P.kurroa-Kutki in NAFLD (Non-Alcoholic Fatty Liver Diseases), the emerging major healthcare concern [76]. In the bedside to bench mode, a rat model for NAFLD was established which showed by histological tissue studies of the liver that oral administration of P.kurroa-Kutki extract protected the rats from accumulation of fat and from the tissue damage induced by NAFLD. P.kurroa-Kutki was better when compared with control group and also compared with silymarin. The serum alkaline phosphatase (ALP) levels decreased faster in the P.kurroa-Kutki group than in the silymarin group [77]. Further to comprehend the molecular mechanism in a targeted condition of NAFLD, an in-vitro study was undertaken. This experimental work in in-vitro NAFLD model has shown that Picroside-II, the active ingredient in P.kurroa-Kutki, reduces fatty acid accumulation in HepG2 cells via modulation of fatty acid uptake and synthesis [78]. Subsequent recent in-vitro study has further shown that Picroside-II reduced not only lipid accumulation, but also oxidative stress and mitochondrial dysfunction in in-vitro NAFLD-Hep G2cell model [79]. P.kurroa-Kutki product development through reverse pharmacology provide wide scope for developing standardized traditional formulation, traditional or non-traditional plant extract, bioactive fraction, single active principle, its analogues and derivatives. However, for a plant like P. kurroa-Kutki, pharmaceutical excellence, formal regulation, and ethical prescription is desired [Fig. 1].
Fig. 1.
Prerequisites being pharmaceutical excellence, formal regulation, and ethical prescription. RP provide wide scope for developing standardized traditional formulation, traditional or non-traditional plant extract, bioactive fraction, single active principle, its analogues and derivatives. However, the traditional formulations have diverse utility and better safety profile but the precision and specificity is relatively less. Whereas, actives and derivatives have more precision and specificity in the drug action but the safety and diversity are potentially compromised. Hence, the phytopharmaceutical product such as Picroliv is expected to strike this balance.
4. Phytoactives of P.kurroa-Kutki for the indication of fatty liver disease
Picrorhiza species has been explored since 1940s for its chemical composition [11]. Since then, several phytocompounds have been isolated from different parts of this plant species which have shown the presence of glycosides, aromatic esters, bis-iridoid, phenyl propenoids, alcoholic compounds and fatty acids [80]. Iridoid glycosides viz. kutkins, kutkosides and picrosides, cucurbitacins, triterpenes and simple phenols such as apocynin are the most explored phytoactives of P.kurroa for their biological activity and potential clinical applicability. The active principles of P.kurroa are the iridoid glycosides picrosides I, II and III. Kutkin is a mixture of picrosides I and II. P.kurroa also contains Picroside IV and V, verminoside, catapol, veronicoside, specioside 6- feruloylcatapol, pikuroside, aucibin and many more. P.kurroa also contains other active constituents such as apocynin, drosin, nine cucurbitacin glycosides, D-mannitol, phenolic acids and phenylethanoids. As has been reported by Sah & Varsheya, 132 chemical constituents belonging to different class of compounds from roots, rhizomes, seeds, stem and leaves of two Picrorhiza species (P. kurroa Royle ex Benth and Picrorhiza scrophulariiflora Pennel) are listed between 1949 and 2013 [81]. In the last decade or so, 53 more phytoconstituents have been identified [80]. This reflects the ever-increasing enthusiasm and interest of biomedical scientists in Picrorhiza species.
However, Picroside-II is one of the iridoid glycosides from P.kurroa which has been extensively studied for its pharmacological effects in both in vitro and in vivo models. In a recent review, S Ma et al., 2020, have discussed in details the therapeutic potential of picroside-II for organic ischemia and reperfusion injury (to organs and tissues such as cerebrum, myocardium, kidney, testes, skeletal muscles and erythrocytes), liver damage, inflammation, cancer metastasis and osteoclastogenesis. Although the effects of picroside-II are multiple and complex, with the intricate involvement of a number of pathways, the mechanisms of picroside-II appear to be mainly acting through anti-oxidant, anti-inflammatory and anti-apoptotic mechanisms [82].
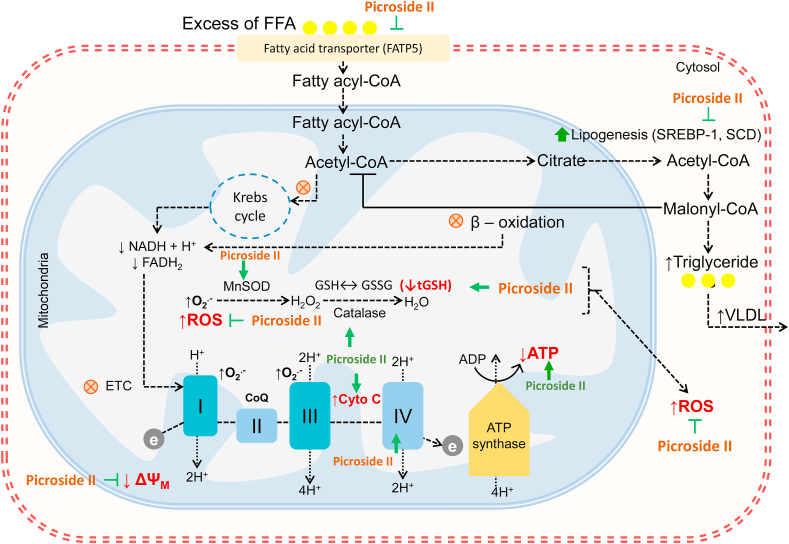
Recently our group has reported results of in-vitro studies that Picroside-II effectively attenuated fatty acid accumulation in Hep G2cells pre-treated with Picroside-II. In this study Picroside-II pre-treatment inhibited FFAs-induced lipid accumulation by attenuating the expression of fatty acid transport protein 5, sterol regulatory element binding protein 1 and stearoyl CoA desaturase. It was also observed that pre-treatment with Picroside-II decreased the expression of forkhead box protein O1 and phosphoenolpyruvate carboxykinase. These findings suggest that Picroside-II effectively attenuated fatty acid accumulation by decreasing FFAs uptake and lipogenesis. Picroside-II also decreased the expression of gluconeogenic genes [79,80]. The molecular mechanisms of Picroside-II in fatty liver are depicted in Fig. 2 [83]. Interestingly Hua Han et al., recently have reported many synthesized derivatives from picroside-II and demonstrated the hepatoprotective activity of the derivatives evaluated on SMMC-7721 cells [84].
Fig. 2.
Molecular mechanisms of Picroside II in fatty liver. Proposed mechanism of action of Picroside II on FFA accumulation, oxidative stress and mitochondrial dysfunction: Picroside II attenuated FFA accumulation in HepG2 cells by downregulation of FATP5, SREBP-1 and SCD, hence decreasing fatty acid uptake and lipid synthesis. Picroside II decreases the production of ROS, increases the levels of antioxidants (glutathione, Manganese superoxide dismutase and Catalase). Picroside II improves mitochondrial functions by increasing ATP production, decreasing ΔΨM loss and increasing cytochrome c expression. (The figure is adopted and modified further from; Dhami-Shah H, Vaidya R, Talwadekar M, Shaw E, Udipi S, Kolthur-Seetharam U, Vaidya ADB. Intervention by Picroside II on FFAs induced lipid accumulation and lipotoxicity in HepG2 cells. J Ayurveda Integr Med. 2021 Jul–Sep; 12(3):465–473′). FFA, Free Fatty Acid; FATP5, Fatty Acid Transporter Protein 5; SREBP-1, Sterol Regulatory Element-Binding Protein-1; SCD, Stearoyl CoA Desaturase; ROS, Reactive Oxygen Species; ATP, Adenosine Triphosphate; ΔΨM, Mitochondrial Membrane Potential.
Bioavailability is an important pharmacokinetic parameter that is essential to be correlated with the clinical effect of most drugs. Some studies have been published of late regarding bioavailability of picrosides and its metabolites [[85], [86], [87], [88], [89]]. However, bioavailability of picrosides is variable or low, most probably due to primary metabolism through gut microbial flora and the hydrophilic nature of these iridoid glycosides [85]. However, information gained from Zahiruddin S et al. postulates that after oral administration of iridoid rich fraction the basic pharmacokinetic profiling of picroside I, II and apocynin as well as fate of other metabolites, demonstrates scientific basis of P.kurroa-Kutki's traditional use in Ayurveda [86]. Gao et al., have proposed four metabolic pathways for picroside-II viz. I) Picroside-II is de-glycosylated to generate the aglycone, which is isomerized to a dialdehyde-type intermediate. A series of metabolic reactions, including glucuronidation, subsequently occurs. II) Picroside-II is subjected to ester bond hydrolysis to form vanillic acid, which is further subjected to sulfate conjugation, glycine conjugation, glucuronidation, and demethylation. III) Picroside-II is directly conjugated with glucuronic acid to yield a predominant metabolite in plasma. IV) Picroside-II is directly conjugated with sulphate [87]. Interestingly, Zhu J et al. have demonstrated higher concentration of picroside-II in liver tissues after I.V. administration in Sprague Dawley rats [88]. This correlates well with the traditional clinical usage of P.kurroa-Kutki in hepatic disorders. Moreover, wide spectrum hepatoprotective activities of isolated chemical constituents and plant extracts of P.kurroa-Kutki such as anti-lipogenic [79,80], anti-inflammatory [25,26], anti-fibrotic [32], anti-oxidant [33,34], anti-hepatocellular carcinogenic [34], make this product applicable to the entire spectrum of alcoholic/non-alcoholic fatty liver disease [90].
5. Scope and challenges of P.kurroa-Kutki natural products/phytopharmaceutical
Founded on insights from the Ayurveda knowledge-base and traditional clinical usage-experiences a novel natural product of P.kurroa-Kutki is possible while integrating current scientific and experimental database. Such a natural product is expected to have targeted efficacy, wide safety profile and desired reproducibility. The new natural product category of phytopharmaceutical has formally been approved in India [91].
It offers an opportunity to develop P.kurroa-Kutki phytopharmaceutical, standardized for phytoactives, in a targeted clinical condition of NAFLD. This phytopharmaceutical should identify picroside-II as a main phytoactive for the indication of the fatty liver disease besides minimum three other phytomarkers [[92], [93], [94]]. Table 3 summarizes the structure and biological activity of picroside-II and other three relevant phytomarkers suitable for NFALD. Although pre-clinical studies have demonstrated ample evidence; clinical studies with the P.kurroa-Kutki phytopharmaceutical may face challenges of the bioavailability and biocompatibility of phytoactives.
Table 3.
Phytoconstituents of Picrorhiza kurroa and their hepatoprotective activity.
| Sr. | Phytoconstituent | Chemical structure | Biological Activity | References |
|---|---|---|---|---|
| 1 | Picroside I | 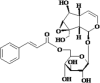 |
Hepato- protective, Antioxidant, Anti-inflammatory |
[26,39,92] |
| 2 | Picroside II | 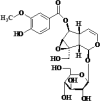 |
Hepato- protective, Antioxidant | [39,65,92] |
| 3 | Kutkoside | 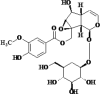 |
Anti- inflammatory Hepato-protective |
[26,93] |
| 4 | Apocynin | 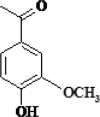 |
Neuro-protective, Anti- inflammatory | [94] |
The challenge of bioavailability of P.kurroa-Kutki phytoactives has been explored through the application of nanoencapsulation technique. Alcoholic extract of P.kurroa-Kutki was successfully encapsulated into pluronic-F-68-PLA nanoparticles by nanoprecipitation method. Encapsulation efficiency for picrosides I and II was determined 60.17 ± 2.8% and 67.2 ± 7.4% respectively [95]. The authors stated that the release dynamics profile suits intestinal absorption and uptake in humans and could be a promising approach for enhancing intestinal absorption, biocompatibility as well as bioavailability, making it a suitable form of administering the alcoholic extract. However, limited studies on oral bioavailability indicate the need for further work along with exploring the potential for encapsulation and controlled release of the preparations, followed by appropriate studies for safety and toxicity of the nano-encapsulates.
Another important challenge is of drug interactions with the P.kurroa-Kutki phytopharmaceutical when co-administered with conventional chemical drugs. Under experimental conditions hydro alcoholic extract of P. kurroa rhizome has displayed β-cell regeneration with enhanced insulin production and antihyperglycemic effects in streptozotocin induced rat model and in insulin producing Rin5f cells [96]. Also, Husain et al., have reported that standardized P.kurroa-Kutki extract increased the insulin-mediated translocation of GLUT-4 from cytosol to plasma membrane which resulted in better glucose uptake by skeletal muscles and improved glycaemic control in diabetic rats [28]. This certainly warrants an attention for potential interaction of P.kurroa-Kutki products with anti-diabetic drugs. Hence, concomitant administration of other herbal products and chemical drugs demands attention from the drug interaction perspective. Although no known major reports of drug interactions with P.kurroa-Kutki specifically are reported in literature [[97], [98], [99]]. It is possible to record such a potential drug interaction in an organized clinical study. Not all interactions are harmful, and some can be beneficial, and some insignificant. However, it is worthwhile to have data from post marketing studies and pharmaco-epidemiological studies for such a phytopharmaceuticals as has been recommended recently for Ayurvedic products [100].
The wisdom of using traditional formulations containing P.kurroa-Kutki in combination with other herbs and herbo-minerals also should be explored for understanding the synergistic activity. The unique processes involved in these formulations’ development needs to be comprehended to provide further insights through investigations. Although, P.kurroa-Kutki and its traditional products are sometimes known to lead to adverse effects such as increased bowel frequency, diarrhoea, abdominal gurgling, abdominal colic etc, vigilance about such side effects is desired during clinical trials of P.kurroa-Kutki products. Observations regarding compatibility and potentiation of P.kurroa-Kutki-products with certain food substances is also relevant. This should facilitate scheduling the time of oral administration of the P.kurroa-Kutki natural product.
6. Conclusion and prospects
Starting from bedside with the hint from observational therapeutics of classical Ayurveda treatment for hepatitis and further, well organized clinical studies, has given convincing clinical lead for the hepatoprotective potential of P.kurroa-Kutki standardized product. However, extensive phytochemicals identification, and isolation of phytoactives has given further boost to the experimental in-vitro and in-vivo studies of P.kurroa-Kutki plant molecules and products. Well organized, multicentric controlled-clinical trial, with a standardized phytopharmaceutical for phytoactives in fatty liver disease are likely to be undertaken soon. However, in the last couple of decades scientists have unravelled many more phytoactives from P.kurroa-Kutki with biological activities at different organ systems and molecular targets. These experimental bench-side findings encourage clinical scientists to go back to bedside and traditional clinical practices to get further hints and leads to undertake the clinical studies with P.kurroa-Kutki phytopharmaceuticals exploring clinical targets beyond hepatology.
Authors contribution
Ashwinikumar Raut: Conceptualization, writing original draft, writing – review & editing, visualization, supervision; Hiteshi Dhami-Shah: Writing – review & editing, visualization; Aashish Phadke: Writing – review & editing, visualization; Anand Shindikar: Writing – review & editing, visualization; Shobha Udipi: Writing – review & editing, supervision; Jayashree Joshi: Writing – review & editing, supervision; Rama Vaidya: Conceptualization, writing – review & editing, supervision; Ashok DB Vaidya: Conceptualization, writing – review & editing, supervision.
Source of Funding
None.
Conflict of interest
The authors do not have conflict of interest. Although AAR is a member of the J-AIM Editorial Board, he was not involved in peer review process and editorial decisions related to this paper.
Acknowledgements
We acknowledge Indian Council of Medical Research (ICMR) for awarding the Product Development Centre (PDC) to KHS-MRC (Kasturba Health Society’s Medical Research Centre), (ICMR-PDC Project 65/11/2018/PD/ICMR/BMS/Centre-4), which includes a senior research fellowship. We also thank President, KHS Shri. Dhirubhai Mehta and Secretary, KHS, Dr B.S. Garg for providing the organizational support to KHS-MRC.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Sharma R.K., Dash Bhagvan, editors. Agnivesha, Charaka samhita, chakrapanidatta's Ayurveda dipika. 2nd ed. vol. I. Choukhambha Sanskrit Series Office; Varanasi: 1983. [Google Scholar]
- 2.Acharya Y.T., editor. Sushruta, sushruta samhita, nibandhasangraha commentary by dalhana. Chaukhambha Surbharati Prakashan; Varanasi: 1994. [Google Scholar]
- 3.Garde G.K., editor. (Marathi translation) 5th ed. SarthVagbhata, Ashtangahrudaya, Aryabhushan Mudranalaya; Pune: 1963. [Google Scholar]
- 4.Chunekar K.C. Reprint; 1999. Sri bhavamishra, Bhavaprakasha Nighantu ,commentary by chaukhamba bharati academy; pp. 69–71. [Google Scholar]
- 5.Pandit Trivedi Harihar Prasad . Acharya madanapala, madanapala Nighantu, varanasi. Chaukhambha Krishnadas Academy; 2009. [Google Scholar]
- 6.Acharya Kaiyadev, Nighantu Kaiyadev. In: 2nd ed. Sharma P.V., editor. Chaukhambha Orientalia; Varanasi: 2006. [Google Scholar]
- 7.Pandita Narhari, Raja Nighantu Dr. In: 4th ed. Tripathi Indradeva., editor. Vol. 5. Chowkhambha Krishnadas Academy; Varanasi: 2006. [Google Scholar]
- 8.Ranjit Roy Chaudhury &Uton Muchtar Rafei . Traditional medicine in asia, world health organization regional office for south-east asia New Delhi. SEARO Regional Publications No. 39 © World Health Organization; 2001. [Google Scholar]
- 9.Wang H., Zhao W., Choomuenwai V., Andrews K.T., Quinn R.J., Feng Y. Chemical investigation of an antimalarial Chinese medicinal herb Picrorhiza scrophulariiflora. Bioorg Med Chem Lett. 2013 Nov 1;23(21):5915–5918. doi: 10.1016/j.bmcl.2013.08.077. Epub 2013 Aug 27. PMID: 24035096. [DOI] [PubMed] [Google Scholar]
- 10.Desai V.G. 1st ed. 1975. Oshadhisangraha, gajanan book depot; pp. 86–87. Dadar. [Google Scholar]
- 11.Rastogi R.P., Sharma V.N., Siddiqui S. Chemical examination of picrorhiza kurroa benth. J Sci Ind Res B. 1949;8:173–178. [Google Scholar]
- 12.Vohora S.B., Kumar I., Naqvi S., Afaq S.H. Pharmacological investigation on picrorhiza kurroa roots with special reference to its choleretic and antimicrobial properties. Indian J Pharmacol. 1972;34:17–19. [Google Scholar]
- 13.Chander R., Dwivedi Y., Rastogi R., Sharma S.K., Garg N.K., Kapoor N.K., et al. Evaluation of hepatoprotective activity of picroliv (from Picrorhiza kurroa) in Mastomysnatalensis infected with Plasmodium berghei. Indian J Med Res. 1990 Feb;92:34–37. PMID: 2189829. [PubMed] [Google Scholar]
- 14.Rawat G., Bameta A., Singh B.R., Bains G., Rawat D.S., Gaur A.K. Himalayan diversity: an ethnopoetic medicinal plant. J Pharmacogn Phytochem. 2020;9(2):1911–1919. [Google Scholar]
- 15.Vaidya A.B., Antarkar D.S., Doshi J.C., Bhatt A.D., Ramesh V., Vora P.V., et al. Picrorrhiza kurroa (Kutaki) Royle Ex. Benth as a hepatoprotective agent- experimental & clinical studies. J Postgrad Med. 1996;42(4):105–108. [PubMed] [Google Scholar]
- 16.Ram T.S., Munikumar M., Raju V.N., Devaraj P., Boiraju N.K., Hemalatha R., et al. In silico evaluation of the compounds of the ayurvedic drug, AYUSH-64, for the action against the SARS-CoV-2 main protease [published online ahead of print, 2021 Feb 25] J Ayurveda Integr Med. 2022;13(1) doi: 10.1016/j.jaim.2021.02.004. 10.1016/j.jaim.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AYUSH 64 found useful in the treatment of mild to moderate COVID-19 infection. https://pib.gov.in/PressReleasePage.aspx?PRID=1714815 Randomized clinical study demonstrated the safety and efficacy of AYUSH 64 in mild-moderate Covid-19: Dr Arvind Chopra. Posted On: 29 APR 2021 1:53PM by PIB Delhi accessed on 25th May 2021.
- 18.Arya D., Bhatt D., Kumar Ravi, Tewari L.M., Kishor Kamal, Joshi G.C. Studies on natural resources, trade and conservation of kutki (picrorhiza kurroa royle ex benth., scrophulariaceae) from kumaun himalaya. Academic Journals Scientific Research and Essays. 11 April, 2013;8(14):575–580. DOI 10.5897/SRE12.495 ISSN 1992-2248 © 2013. [Google Scholar]
- 19.Kharb A., Chauhan R.S. Complexity of gene paralogues resolved in biosynthetic pathway of hepatoprotective iridoid glycosides in a medicinal herb, Picrorhiza kurroa through differential NGS transcriptomes. Mol Genet Genom. 2021 doi: 10.1007/s00438-021-01787-w. Published online. [DOI] [PubMed] [Google Scholar]
- 20.Thakur K., Partap M., Dinesh Kumar, Warghat A.R. Enhancement of picrosides content in Picrorhiza kurroa Royle ex Benth. mediated through nutrient feeding approach under aeroponic and hydroponic system. Ind Crop Prod. July 2019;133:160–167. [Google Scholar]
- 21.Chawla A., Amit Kumar, Warghat A., Singh S., Shashi Bhushan, Sharma R.K., et al. Approaches for conservation and improvement of Himalayan plant genetic resources. Chapter 18, in Advancement in Crop Improvement Techniques. 2020:297–317. [Google Scholar]
- 22.Visen P.K., Shukla B., Patnaik G.K., Dhawan B.N. Prevention of galactosamine induced hepatic damage by picroliv: study on bile flow and isolated hepatocytes (ex vivo) Planta Med. 1993;59(1):37–41. doi: 10.1055/s-2006-959600. [DOI] [PubMed] [Google Scholar]
- 23.Rastogi R., Srivastava A.K., Srivastava M., Rastogi A.K. Hepatocurative effect of picroliv and silymarin against aflatoxin B1 induced hepatotoxicity in rats. Planta Med. 2000;66(8):709–713. doi: 10.1055/s-2000-9907. [DOI] [PubMed] [Google Scholar]
- 24.Verma P.C., Basu V., Gupta V., Saxena G., Rahman L.U. Pharmacology and chemistry of a potent hepatoprotective compound Picroliv isolated from the roots and rhizomes of Picrorhiza kurroa royle ex benth. (kutki) Curr Pharmaceut Biotechnol. 2009;10(6):641–649. doi: 10.2174/138920109789069314. [DOI] [PubMed] [Google Scholar]
- 25.Sharma M.L., Rao C.S., Duda P.L. Immunostimulatory activity of Picrorhiza kurroa leaf extract. J Ethnopharmacol. 1994;41(3):185–192. doi: 10.1016/0378-8741(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 26.Singh G.B., Bani S., Singh S., Khajuria A., Sharma M.L., Gupta B.D., et al. Antiinflammatory activity of the iridoids kutkin, picroside-1 and kutkoside from Picrorhiza kurrooa. Phytother Res. 1993;7(6):402–407. [Google Scholar]
- 27.Kumar R., Gupta Y.K., Singh S., Arunraja S. Picrorhiza kurroa inhibits experimental arthritis through inhibition of pro-inflammatory cytokines, angiogenesis and MMPs. Phytother Res. 2016 Jan;30(1):112–119. doi: 10.1002/ptr.5509. Epub 2015 Nov 11. PMID: 26556014. [DOI] [PubMed] [Google Scholar]
- 28.Husain G.M., Rai R., Rai G., Singh H.B., Thakur A.K., Kumar Vikas. Potential mechanism of anti-diabetic activity of Picrorhiza kurroa. TANG. 2014;4(4):e27. [Google Scholar]
- 29.Sehgal R., Chauhan A., Gilhotra U.K., AindryGilhotra in-vitro and in-vivo evaluation of antiasthmatic activity of picrorhiza kurroa plant. IJPSR. 2013;4(9):3440–3443. [Google Scholar]
- 30.Morikawa T., Yusuke N., Yoshiaki N., Hideyuki M., Okino K., Hamasaki S., et al. Collagen synthesis-promoting and collagenase inhibitory activities of constituents isolated from the rhizomes of Picrorhiza kurroa Royle ex Benth. Fitoterapia. June 2020;143 doi: 10.1016/j.fitote.2020.104584. 104584. [DOI] [PubMed] [Google Scholar]
- 31.Morikawaa T., Nakanishia Y., Inouea H., Mansea Y., Matsuuraa H., Hamasakia S., et al. Acylated iridoid glycosides with hyaluronidase inhibitory activity from the rhizomes of Picrorhiza kurroa Royle ex Benth. Phytochemistry. 2020;169:112–185. doi: 10.1016/j.phytochem.2019.112185. [DOI] [PubMed] [Google Scholar]
- 32.Joy K.L., Ramadasankuttan Inhibition by picrorrhiza kurroa extract of oxygen free radical reactions and hepatic fibrosis in rats. J Clin Biochem Nutr. 1999;27:9–17. [Google Scholar]
- 33.Navya K., Kumar G.P., Chandrasekhar Y., Kumar A. Evaluation of potassium dichromate (K2Cr2O7)-induced liver oxidative stress and ameliorative effect of Picrorhiza kurroa extract in Wistar Albino Rats. Biol Trace Elem Res. 2018;184(1):154–164. doi: 10.1007/s12011-017-1172-2. [DOI] [PubMed] [Google Scholar]
- 34.Rajkumar V., Gunjan Guha, Ashok Kumar. Food and chemical toxicology. Antioxidant and anti-neoplastic activities of Picrorhiza kurroa extracts. February 2011;49(2):363–369. doi: 10.1016/j.fct.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Hussain A., Shadma W., Maksood A., Ansari S.H. Protective effects of Picrorhiza kurroa on cyclophosphamide-induced immunosuppression in mice. Pharma Res. 2013;5(1):30–35. doi: 10.4103/0974-8490.105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagarathna P.K.M., Reena K., Reddy S., Wesley J. Review on immunomodulation and immunomodulatory activity of some herbal plants. Int J Pharmaceut Sci Rev Res. 2013;22(1):223–230. [Google Scholar]
- 37.Soni D., Grover A. Picrosides” from Picrorhiza kurroa as potential anti-carcinogenic agents. Biomed Pharmacother. 2019;109:1680–1687. doi: 10.1016/j.biopha.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 38.Lou C., Zhu Z., Xu X., Zhu R., Sheng Y., Zhao H. Picroside II, an iridoid glycoside from Picrorhiza kurroa, suppresses tumor migration, invasion, and angiogenesis in vitro and in vivo. Biomed Pharmacother. December 2019;120:109494. doi: 10.1016/j.biopha.2019.109494. [DOI] [PubMed] [Google Scholar]
- 39.Mallick M.N., Singh M., Parveen R., Khan W., Ahmad S., Najm M.Z., et al. HPTLC analysis of bioactivity guided anticancer enriched fraction of hydroalcoholic extract of picrorhiza kurroa. BioMed Res Int. 2015:18. doi: 10.1155/2015/513875. Article ID 513875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rathee D., ThankiM, Bhuva S., Anandjiwala S., Agrawal R. Iridoid glycosides-Kutkin, Picroside I, and Kutkoside from Picrorrhiza kurroa Benth inhibits the invasion and migration of MCF-7 breast cancer cells through the down regulation of matrix metalloproteinases. Arabian Journal of Chemistry. 2013;6(1):49–58. [Google Scholar]
- 41.Rathee D., Rathee P., Rathee S., Rathee D. Phytochemical screening and antimicrobial activity of Picrorrhiza kurroa, an Indian traditional plant used to treat chronic diarrhoea. Arabian Journal of Chemistry. 2016;9(2):1307–S1313. Supplement 2. [Google Scholar]
- 42.Mohammed R., Mohammed U., Yamgar S., Gadgoli C., Salunkhe D. Preliminary screening and antimicrobial activity of picrorhiza kurroa royle ethanolic extracts. Int J Pharmaceut Sci Rev Res. 2012;14(1):73–76. No. 15. [Google Scholar]
- 43.Guilherme Arrache´ Gonc¸alves . Vera Lucia Eifler-Lima . Gilsane Lino von Poser Revisiting nature: a review of iridoids as a potential antileishmanial class. Phytochemistry Rev. 2021 doi: 10.1007/s11101-021-09750-8. Published online: 16 March 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gogate V.M. 1st ed. 1982. Dravyagunavidnyana, continental prakashan for Maharashtra vidyapeeth granthanirmiti mandal, pune 411030; pp. 261–263. [Google Scholar]
- 45.Gune G.G. 6th ed. Part 2. 1987. pp. 27–35. (AyurvedeeyaAushadhiGunadharmashastra). [Google Scholar]
- 46.Shankar Dajishastri Pade. 2nd ed. 1980. Aushadhi bad Part 1 to 3 , shree gajanan book depot. Dadar. [Google Scholar]
- 47.Vishwanath Gokhale Bhaskar, Chikitsapradeep DhanwantariPratishthan. Pune Reprint. 1990:108. [Google Scholar]
- 48.Appashastri Sathe. Gharaguti aushadhe. Ganesh printers; Pune: 1998. Kutki; pp. 61–62. 15th Edition (1st Edn1922) [Google Scholar]
- 49.Nrusinhacharya Bagewadikar, Anubhavmruta – Pratyakshavara Adharlela Ayurvediya Chikitsa Granth . 1971. Yakrutdaludara , 1stEdn; p. 203. [Google Scholar]
- 50.Sharma R.K., Dash Bhagvan. 2nd ed. vol. II. Choukhambha Sanskrit Series Office; Varanasi: 1985. (Agnivesha, Charaka samhita, chakrapanidatta's Ayurveda dipika). Vimansthana, [Chapter 8]/87. [Google Scholar]
- 51.Katoch M., Fazli I.S., Suri K.A., Ahuja A., Quazi G.N. Effect of altitude on picroside content in core collections of Picrorhiza kurrooa from the north western Himalayas. J Nat Med. 2011;1 65:578–582. doi: 10.1007/s11418-010-0491-9. [DOI] [PubMed] [Google Scholar]
- 52.Pandit S., Shitiz K., Sood H., Naik P.K., Chouhan R.S. Expression pattern of fifteen genes of non-mevalonate (MEP) and mevalonate (MVA) pathways in different tissues of endangered medicinal herb Picrorhiza kurroa with respect to picrosides content. Mol Biol Rep. 2013;40:1053–1063. doi: 10.1007/s11033-012-2147-1. [DOI] [PubMed] [Google Scholar]
- 53.Kawoosa T., Singh H., Kumar A., Sharma S.K., Devi K., Datta S., et al. Light and temperature regulated terpene biosynthesis: hepatoprotective monoterpene picroside accumulation in Picrorhiza kurrooa. FunctIntegr Genomics. 2010;10:393–404. doi: 10.1007/s10142-009-0152-9. [DOI] [PubMed] [Google Scholar]
- 54.Chunekar K.C. Chaukhamba Bharati Academy; Reprint: 1999. Commentary on sri bhavamishra's Bhavaprakasha Nighantu; pp. 69–71. [Google Scholar]
- 55.Acharya Yadavji Trikamji , Siddhayogasangraha, Yakrut – Pleeha _Udar _ ShothaRogadhikar . 7th ed. Publisher –Shree Baidyanath Ayurved Bhavan Ltd.; Nagpur: 1979. pp. 65–66. [Google Scholar]
- 56.Velankar V., Arogyavardhini, ‘Arogyavardhini vaparatanaghyavayachiKalaji ‘ . 1st ed. 1993. Ayurved mahasammelan ,dombivali branch; p. 92. [Google Scholar]
- 57.Antarkar D.S., Tathed P.S., Vaidya A.B. A pilot phase II trial with Arogyavardhini and Punarnavadi-kwath in Viral hepatitis. Panminerva Med. 1978;20(3):157–160. [PubMed] [Google Scholar]
- 58.Antarkar D.S., Vaidya A.B., Doshi J.C., Athavale A.S., Vinchoo K.S., Natekar M.R., et al. A double-blind clinical trial of Arogyavardhini an Ayurvedic drug in acute viral hepatitis. Indian J Med Res. 1980;72:588–593. [PubMed] [Google Scholar]
- 59.Bedi K.L., Zutshi U., Chopra C.L., Amla V. Picrorhiza kurroa, an ayurvedic herb, may potentiate photochemotherapy in vitiligo Jammu 1989. J Ethnopharmacol. 1989;27:347–352. doi: 10.1016/0378-8741(89)90009-3. [DOI] [PubMed] [Google Scholar]
- 60.Shilpa A. Clinical study to evaluate effect of kutaki (picroriza kurroa royle ex benth) in amlapitta. J Hormoniz Med. Res. And Hlth. Sci. 2014;1(1):75–82. [Google Scholar]
- 61.Singhal P., Nesari T., Gupta G.S. Efficacy of herbomineral compounds and pathya (Ayurvedic dietary regime and physical exercise) in the management of YakritRoga (Non-alcoholic fatty liver disease) Ancient Sci Life. 2015;34:216–222. doi: 10.4103/0257-7941.160866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumar N., Singh A.K., Ghildiyal S. Potent hepatoprotective phaltrikadi kwath: a clinical study. SM J Pharmac Ther. 2015;1(1):1005. [Google Scholar]
- 63.Harbans, et al. Clinical evaluation of kutaki (picroriza kurroa royle ex benth) processed in guduchi (tinospora cordifolia wild) miers in patients receiving lipid lowering drugs (statins) Indian Journal of Traditional Knowledge. 2011;10(4):657–660. [Google Scholar]
- 64.Tarapure S., Tubaki B.R., Khot S. Elastographic liver evaluation of Katukyadichurna in the management of Non-Alcoholic Steatohepatitis (NASH) - a single arm clinical trial. Jan-Mar. 2021;12(1):136–142. doi: 10.1016/j.jaim.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pande V.N. B.H.U.; Varanasi: 1966. Clinical and experimental studies on certain liver disease with special reference to an indigenous drug, Kutki (Picrorhiza kurroa)in the treatment of Jaundice (Kamala Roga). Thesis submitted for the degree of Doctor of Ayurvedic Medicine. [Google Scholar]
- 66.Pande V.N. B.H.U.; Varanasi: 1979. Effect of indigenous drugs in the management of certain liver disorder. Thesis submitted for Doctor of Philosophy. [Google Scholar]
- 67.Dhar M.L., Dhar M.M., Dhawan B.N., Mehrortra B.N., Srimal R.C., Tandon J.S., et al. Screening of Indian Plants for biological activities,Pt.IV. Indian J.Experi.Biol. 1973;11(1):43–54. [PubMed] [Google Scholar]
- 68.Das P.K., Tripathi R.M., Agarwal V.K., Sanyal A.K., et al. Pharmacology of Kutkin and its two organic constituents, cinnamic and vanillic acids. Indian J.Experi.Biol. 1976;14:456–458. [PubMed] [Google Scholar]
- 69.Kanitkar S.V., Bramhe G.N., Phadke A.R., Joglekar G.V. Effect of alcoholic extract of P. kurroa on chronic carbon tetrachloride induced hepatotoxicity in rats. J Res Indian Med Yoga Homoeopath. 1976;11(3):112–114. [Google Scholar]
- 70.Tiwari N.S., Jain P.C. Clinical evaluation of Arogyavardhini as a hypocholestrerloaemic agent with special reference to obesity. J.Res.Ayur.Siddha. 1980;1(1):121–132. [Google Scholar]
- 71.Tripathi S.C., Patnaik G.K., DhawanBN Hepatoprotective activity of picroliv against alcohol – carbon tetrachloride induced damage in rats. IndianJ.of Pharmacology. 1991;23(3):143–148. [Google Scholar]
- 72.Shukla B., et al. Choleretic effect of picroliv , the hepatoprotective principle of picrorhiza kurroa. Planta Med. 1991;57(1):29–33. doi: 10.1055/s-2006-960010. [DOI] [PubMed] [Google Scholar]
- 73.Saraswat B., Visen P.K., Patnaik G.K., Dhawan B.N. Protective effect of picroliv, active constituent of Picrorhiza kurroa, against oxytetracycline induced hepatic damage. Ind.J.Experi. Biol. 1997;37(12):1302–1305. [PubMed] [Google Scholar]
- 74.Vaidya A.D.B. Reverse pharmacological correlates of Ayurvedic drug actions. Indian J Pharmacol. 2006;38:311–315. [Google Scholar]
- 75.Raut A.A., Chorghade M.S., Vaidya A.B.D. Academic Press; 2017. Reverse pharmacology; chapter 4 in innovative approaches in drug discovery- ethnopharmacology, systems biology and holistic targeting; pp. 89–126. [Google Scholar]
- 76.Press Information Bureau Government of India. Dr. Harsh vardhan launches operational guidelines for integration of non-alcoholic fatty liver disease (NAFLD) with NPCDCS. https://pib.gov.in/PressReleseDetailm.aspx?PRID=1699904 accessed on 17th June 2021.
- 77.Shetty S.N., Mengi S., Vaidya R., Vaidya A.D. A study of standardized extracts of Picrorhiza kurroa Royle ex Benth in experimental nonalcoholic fatty liver disease. J Ayurveda Integr Med. 2010;1:203–210. doi: 10.4103/0975-9476.72622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dhami-Shah Hiteshi, Vaidya Rama, Udipi Shobha, Raghavan Srividhya, Abhijit Shiny, Mohan Viswanathan, et al. Picroside II attenuates fatty acid accumulation in HepG2 cells via modulation of fatty acid uptake and synthesis. Clin Mol Hepatol. 2018;24:77–87. doi: 10.3350/cmh.2017.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dhami-shah Hiteshi, Vaidya Rama, Talwadekar Manasi, Shaw Eisha, Udipi Shobha, UllasKolthur-seetharam, et al. Picroside ii reduces lipid accumulation, oxidative stress and mitochondrial dysfunction in in vitro NAFLD HePG2 cell model. Journal of Clinical and Experimental Hepatology. July 2018;8:S40–S41. [Google Scholar]
- 80.Prakash V., Kumari Anjana, Kaur H., Kumar M., Gupta S., Bala R. Chemical constituents and biological activities of genus picrorhiza: an update. Indian J Pharmaceut Sci. July-August 2020:562–577. [Google Scholar]
- 81.Sah J.N., Varshney V.K. Chemical constituents of Picrorhiza genus: a review. American Journal of Essential Oils and Natural Products. 2013;1(2):22–37. [Google Scholar]
- 82.Ma S., Wang X., Lai F., Lou C. The beneficial pharmacological effects and potential mechanisms of picroside II: evidence of its benefits from in vitro and in vivo. Biomed Pharmacother. 2020;130:110421. doi: 10.1016/j.biopha.2020.110421. [DOI] [PubMed] [Google Scholar]
- 83.Dhami-Shah H., Vaidya R., Talwadekar M., Shaw E., Udipi S., Kolthur-Seetharam U., et al. Intervention by picroside II on FFAs induced lipid accumulation and lipotoxicity in HepG2 cells. J Ayurveda Integr Med. 2021 Jul-Sep;12(3):465–473. doi: 10.1016/j.jaim.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han H., Li Z.Q., Gao Z.L., Yin X., Dong P.L., Yang B.Y., et al. Synthesis and biological evaluation of picroside derivatives as hepatoprotective agents. Nat Prod Res. 2019;33(19):2845–2850. doi: 10.1080/14786419.2018.1508143. 10.1080/14786419.2018.1508143 [DOI] [PubMed] [Google Scholar]
- 85.Upadhyay D., Anandjiwala S., Padh H., Nivsarkar M. In vitro - in vivo metabolism and pharmacokinetics of picroside I and II using LC-ESI-MS method. Chem Biol Interact. 2016;254:83–92. doi: 10.1016/j.cbi.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 86.Zahiruddin S., Khan W., Nehra R., Alam Md J., MallickMd N., Parveen R., et al. Pharmacokinetics and comparative metabolic profiling of iridoid enriched fraction of Picrorhiza kurroa - an Ayurvedic Herb. J Ethnopharmacol. 2017;197:157–164. doi: 10.1016/j.jep.2016.07.072. [DOI] [PubMed] [Google Scholar]
- 87.Gao T., Sheng T., Zhang T., Han H. Characterization of picroside II metabolites in rats by ultra-high-performance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Pharm Biomed Anal. 2016;128:352–359. doi: 10.1016/j.jpba.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 88.Zhu J., Xue B., Ma B., Zhang Q., Liu M., Liu L., et al. A pre-clinical pharmacokinetic study in rats of three naturally occurring iridoid glycosides, Picroside-I, II and III, using a validated simultaneous HPLC-MS/MS assay. J Chromatogr B AnalytTechnol Biomed Life Sci. 2015;993–994:47–59. doi: 10.1016/j.jchromb.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 89.Yang F.C., Yang S.L., Xu L.Z. Determination of picroside II in dog plasma by HPLC and its application in a pharmacokinetics study. Biomed Chromatogr. 2005;19(4):279–284. doi: 10.1002/bmc.453. [DOI] [PubMed] [Google Scholar]
- 90.Baliga M.S., Shivashankara A.R., Venkatesh S., Bhat H.P., Palatty P.L., Bhandari G., et al. Chapter 7 - phytochemicals in the prevention of ethanol-induced hepatotoxicity: a revisit, dietary interventions in liver disease. Foods, Nutrients, and Dietary Supplements. 2019:79–89. [Google Scholar]
- 91.Bhatt A. Phytopharmaceuticals: a new drug class regulated in India. Perspect Clin Res. 2016;7(2):59–61. doi: 10.4103/2229-3485.179435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ansari R.A., Tripathi S.C., Patnaik G.K., Dhawan B.N. Antihepatotoxic properties of picroliv: an active fraction from rhizomes of Picrorhiza kurrooa. J Ethnopharmacol. 1991 Aug;34(1):61–68. doi: 10.1016/0378-8741(91)90189-k. [DOI] [PubMed] [Google Scholar]
- 93.Dwivedi Y., Rastogi R., Garg N.K., Dhawan B.N. Picroliv and its components kutkoside and picroside I protect liver against galactosamine- induced damage in rats. Pharmacol Toxicol. 1992;71:383–387. doi: 10.1111/j.1600-0773.1992.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 94.Engels F., Renirie B.F., Hart B.A., Labadie R.P., Nijkamp F.P. Effects of apocynin, a drug isolated from the roots of Picrorhiza kurroa, on arachidonic acid metabolism. FEBS Lett. 1992 Jul 6;305(3):254–256. doi: 10.1016/0014-5793(92)80680-f. [DOI] [PubMed] [Google Scholar]
- 95.Jia D., Barwal I., Thakur S., Yadav S.C. Methodology to nanoencapsulate hepatoprotective components from Picrorhiza kurroa as food supplement. Food Biosci. 2015;9(1):28–35. [Google Scholar]
- 96.Kumar S., Patial V., Soni S., Sharma S., Pratap K., Kumar D., et al. Picrorhiza kurroa enhances β-cell mass proliferation and insulin secretion in streptozotocin evoked β-cell damage in rats. Front Pharmacol. 2017 Aug 22;8:537. doi: 10.3389/fphar.2017.00537. PMID: 28878669; PMCID: PMC5572391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Awortwe C., Makiwane M., Reuter H., Muller C., Louw J., Rosenkranz B. Critical evaluation of causality assessment of herb-drug interactions in patients. Br J Clin Pharmacol. 2018 Apr;84(4):679–693. doi: 10.1111/bcp.13490. Epub 2018 Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Borse S.P., Singh D.P., Nivsarkar M. Understanding the relevance of herb-drug interaction studies with special focus on interplays: a prerequisite for integrative medicine. Porto Biomed. J. 2019;4:e15. doi: 10.1016/j.pbj.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Babos M.B., Heinan M., Redmond L., Moiz F., Souza-Peres J.V., Samuels V., et al. Herb-drug interactions: worlds intersect with the patient at the center. Medicines (Basel) 2021 Aug 5;8(8):44. doi: 10.3390/medicines8080044. PMID: 34436223; PMCID: PMC8401017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vaidya R.A., Vaidya A.D.B., Patwardhan B., Tillu G., Rao Y. Ayurvedic pharmacoepidemiology: a proposed new discipline. J Assoc Phys India. 2003;51:528. [PubMed] [Google Scholar]