Abstract
Background
Irritable bowel syndrome (IBS) is one of the clinically challenging disorders. It has a significant effect on health, cost and quality of life. Ayurveda management through whole system approach in IBS is explored.
Objective
To evaluate the efficacy of whole system Ayurveda approach in IBS.
Methods
The present trial is a randomized controlled parallel group study. 48 patients diagnosed as IBS (Rome IV Criteria) between the age group of 20–60 yrs were recruited in the study. Patients were randomly divided into 2 groups. KC group intervened with Kalingadi Churna 3 gm twice a day, before food with buttermilk. WS group intervened with whole system ayurveda protocol (WSAP). Duration of intervention was 60 days with follow up on every 15th day. Assessments were through various clinical measures like IBS Symptom Severity Score (IBS-SSS), IBS Adequate Relief (IBS-AR), Gastrointestinal symptom rating scale (GSRS), IBS-VAS, Complete Spontaneous Bowel Movements (CSBMs), Bristol Stool Form (BSF), Hamilton Anxiety Rating Scale (HARS), Hamilton Depression Rating Scale (HDRS), IBS quality of life (IBS-QoL) at every follow up. Hemoglobin, Erythrocyte sedimentation rate and stool examination was conducted at pre and post study.
Results
Study showed that WS group had significant improvement compared to KC group in IBS-SSS, IBS-AR, IBS-VAS, CSBM, BSF-Diarrhea and BSF-Constipation. Both groups were comparable in GSRS, HARS, HDRS and IBS-QOL. Blood and stool parameters assessments showed comparable improvements in both the groups. Within group significant improvements in all the clinical assessment scales were observed in both the groups.
Conclusion
WSAP was effective in management of IBS (IBS constipation and IBS diarrhea). Improvements were observed in abdominal pain, stool frequency, consistency and adequate relief.
Keywords: Irritable bowel syndrome, Kalingadi churna, Whole system research ayurveda protocol
1. Introduction
Irritable bowel syndrome (IBS) remains a clinical challenge in the 21st century. IBS is one of the functional Gastrointestinal Disease (FGID) having high population prevalence. It is characterized by abdominal pain, change in frequency and form of stool. The symptoms occur with no structural pathology of the Gastrointestinal tract [1]. Rome IV describes IBS with four subtypes namely constipation predominant (IBS-C), diarrhea predominant (IBS-D), mixed or alternating pattern (IBS-M) and IBS-U (unclassified) [2]. IBS diagnosis is based on clinical symptoms and the exclusion of somatic diseases [3]. The severity of symptoms of IBS varies from very mild to incapacitating. The prevalence of moderate and severe cases may be underestimated [4]. IBS has significant health care cost and burden, in USA cost of IBS management per year is more than US $ 1 billion and indirect costs are more than US $ 200 million [5]. A threefold higher rate of cholecystectomy, a two fold higher rate of appendectomy and hysterectomy, approximately 50% higher rate of back surgery have been recorded in IBS patients compared to those without IBS [6]. IBS impairs health related quality of life, possibly even increasing the risk for suicidal behaviors [7].
IBS prevalence has high variability between different countries. Pooled prevalence of IBS with ROME III criteria from 53 studies showed 9.2% and with ROME IV criteria from 6 studies showed prevalence as 3.8%. Indian studies with ROME IV criteria showed 0.2% and with ROME III as 0.4% prevalence. IBS-M subtype was the most in ROME III criteria and IBS-D with ROME IV criteria. Differences could be due to a more restrictive in ROME IV than ROME III criteria [8]. However population statistics in many African and Asian countries are scarce. These could be due to inability to differentiate between infectious diarrhea and IBS in countries with poor health care systems. IBS is a complex multifactorial disease. Interaction of genetic and epigenetic factors are involved in etiopathogenesis of IBS. It involves the derangement in various systems like nervous, immune, digestive, microbiota and the environment. They form complex non liner reciprocal interactions. It include central and peripheral mechanisms. Various biological abnormalities like gut epithelium, immune system, neuroendocrine mechanisms, brain structure and function, affective, cognitive, gene polymorphism, gut microbiome have been reported. Dietary and intestinal pathogens also play an important role [9]. Health status and clinical outcome of IBS patients are affected by psychosocial factors [10]. A number of risk factors for IBS have been identified including female gender, psychological problems, stress, food intolerance and bacterial overgrowth of the small intestine.
Disease Grahani roga explained in Ayurveda has similar presentations as IBS. Grahani roga is the disease caused due to derangements in dosha (body humors) and various systems (Srotas). Systems involved are annavaha (gastro intestinal), purishavaha (excretory), manoavaha (psychological), vatavaha (neurological), ahara (dietary), vihara (behavioral), agni (major metabolic factor), and kostha (gut health). These components plays a complex, interconnecting, reciprocal role and this warrants a systems approach. Manifestations include derangements in bowel like constipation or loose stools, change in frequency, associated with pain or burning, foul smelling etc. Other symptoms include bloating, loss of appetite, debility etc [11].
Conventional management of IBS includes serotonin (5-HT) 5-HT3 receptor antagonist, alosetron, the 5-HT4 agonist, tegaserod, guanylate cyclase agonist, linaclotide and chloride channel blocker lubiprostone [12]. In spite of huge number of studies, IBS management still remains a challenge due to the lack of effective treatment options [13].
Ayurveda managements and medications remains to be explored in the management of IBS. Previous studies have highlighted the role of various Ayurveda medications like Kalingadi churna [14], Bilvadileha [15] and Lavanbhaskar Churna [16] in management of IBS. Clinical research in Complementary and Alternative medicine (CAM) including Ayurveda is predominantly through the gold standard western biomedicine model of randomized controlled trial in which a single active ingredient or drug is studied. However the management principles of Ayurveda does not match this approach but of a stage wise, customized, personalized approach. Hence newer design for CAM is being explored including whole system research (WSR) approach [[17], [18], [19]]. WSR aims to use appropriate research design and method that can assess a whole system within its unique explanatory model [20]. Grahani (IBS) is formed of derangements in complex non linear dynamic systems and needs a system approach. Accordingly need a complex multi modal treatment regimens. Considering these limitations in IBS management and Ayurveda research, current study was planned to explore the efficacy of Whole System Ayurveda protocol (WSAP) in IBS.
2. Materials and methods
The study was approved by the Institute ethics committee (Protocol Id- BMK/16/PG/KC/02, KLEU BMK Ayurveda Mahavidyalaya Belagavi, Date of Approval-21.07.2017. CTRI Registration Number-CTRI/2018/01/011628). A pilot study was conducted on 4 patients each from both the group. This was to look into feasibility of administering Kalingadi Churna 3gm twice a day in KC group and Whole system Ayurveda Protocol in WS group. Pilot study helped in fine tuning the interventions and showed good acceptance from the patients.
Patients attending outpatient department of the institute were recruited for the study. The CONSORT statement guidelines [21] were followed in reporting the outcomes of the study.
2.1. Patients
48 Patients diagnosed as IBS as per Rome IV Criteria [2,22] were recruited from KLE Shri B.M.K. Ayurveda Hospital, Shahapur, Belagavi Karnataka India.
2.1.1. Inclusion criteria
Patients between the age group of 20–60 years of either sex were included in the study.
2.1.2. Exclusion criteria
Patients with Carcinoma Colon, ulcerative colitis, functional abdominal bloating, functional constipation, functional diarrhea, bile acid diarrhea and celiac disease were excluded. Patients suffering from major systemic illness necessitating long term drug treatment like rheumatoid arthritis, tuberculosis, etc were excluded. Those patients on any intervention for IBS in the period of last 4 weeks, alcohol or drug dependency, pregnant and lactating women were not included in the study.
2.1.3. Screening methods
All the patients recruited in the study were subjected to thorough clinical evaluation and their data was recorded. Recordings on the basis of various subjective and objective parameters were done. All the laboratory investigations like complete blood count, stool examination were carried out at Clinical Laboratory, KAHER's BMK Ayurveda Mahavidyalaya Belagavi at baseline and 60th day of intervention.
2.2. Research design
The study was randomized clinical study. Block randomization with block size of 2 was done. Computer generated random numbers were utilized to generate allocation sequence. Allocation of patients into control and trial groups were in 1:1 ratio. Those involved in randomization, distribution and administration of the study articles were independent from the investigators. A pilot study was conducted on 4 patients each on two groups before the study initiation. Assessment were done through IBS Symptom Severity Score (IBS-SS). The sample size was 24 in each group under 5% alpha error and 80% power of the test.
2.2.1. Intervention
All the patients were randomly divided into 2 interventional groups. KC Group was intervened with Kalingadi Churna 3 g twice a day, before food with buttermilk as a vehicle. The ingredients of Kalingadi churna were procured from authentic distributors and powder was prepared in GMP approved KLE Ayurveda Pharmacy, Belagavi as per the standard procedures.
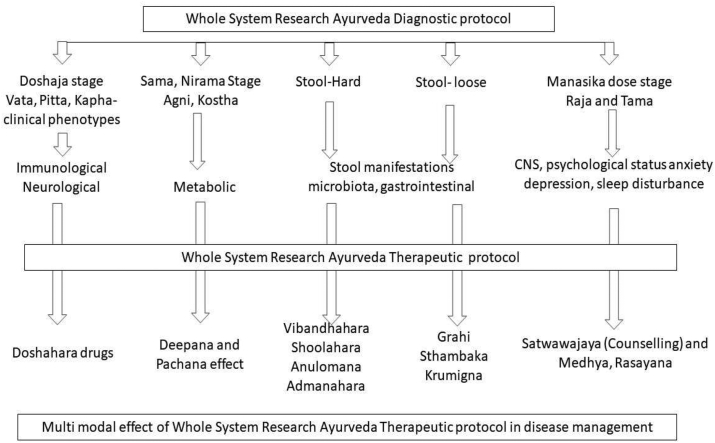
In WS Group, intervention was with whole system Ayurveda Protocol (WSAP). WSAP was designed after a thorough review of the literature, discussion and inputs from various experts both within the department, institute and outside experts. Both diagnostic and treatment protocols were refined till the consensus was reached among the experts. Treatment protocol was limited to oral internal medications and no panchakarma methods were incorporated. This was done considering the limited resources and costs involved in the study. Whole System Ayurveda Protocol for IBS (Grahani) had diagnostic and treatment protocols. Diagnostic protocol of Grahani is through identification of components of disease. Components of Grahani includes Sama and nirama stage [23,24], hard or loose bowels, manifestations of dosha symptomatology (Sahreeriaka and Manasika) [25]. Clinical presentations are through different combinations of these diagnostic components. Major components include strength of agni, Sama and Nirama vata, Sama and Nirama pitta, Sama and Nirama kapha, hard or loose bowels. Manasika dosha derangements like raja and tama. Treatment protocol had medicaments for all these subcomponents. In moderate and severe degrees of anxiety and depression, interventions were through both counseling and pharmacotherapy (Annexure 1). Duration of intervention was 60 days with follow up on every 15th day.
Intervention in WS group was administered by a senior ayurveda clinician, which was similar to a real time clinical practice at OPD level. Major factors involved in treatment algorithm were 1. agni, sama-nirama stage along with shareerika dosha involvement, 2. Loose and hard stools, 3. mansika dosha. 4. Symptomatic managements. These assessments were done at every visit and medications were planned accordingly. Number of drugs administered at every visit ranged from 2 to 3. Interventions included drugs increasing agni and amapachaka (grahi) on the basis of sharerika dosha involvement. Initially in sama state of different dosha corresponding ama pachaka drugs were given. Example in vata dosha agnitundi vati was administered, Chitrakadi vati in kapha dosha and in pitta dosha shunti churna was administered. On Nirama and agni increased state, in vata dosha changeri ghruta, in pitta dosha pravala panchamruta rasa and in kapha dosha mustakarista were administered.
Second factor included was status of bowels like loose or hard stools. Dosha state association was evaluated. Few examples are, hard stools with vata dosha were intervened with hareetaki churna, pitta dosha with avipattikar churna, kapha dosha with triphala churna. Loose stools with vata dosha was intervened with jeerakarista, pitta and kapha with kutajarista, Mustakarista. Majority of hard stools (IBS-C) were associated with nirama vata dosha, Raja dosha involvement. Loose stools (IBS-D) were associated sama kapha and sama pitta dosha, tama dosha involvement.
Third factor was manasika dosha assessment. Raja was associated with bhaya (fear), irsha (envy), krodha (anger), dwesha (hatred) forming the components of anxiety state. Tama with alasya (laziness), tandra (drowsiness), atiraga (desire), atinidra (excess sleep) forming the components of depressive states. In Raja dosha, drugs like kalynaka ghruta, brahmi vati were administered. In Tamasika dosha, vacha churna, mustakarista were intervened. Moderate and severe stages assessed through HARS and HDRS were considered for counseling sessions. They included education, assurance, life style management through dinacharya (daily regimens) and achara rasayana (code of conducts) and other counseling techniques. Sessions were conducted till scores reduced to mild levels.
Symptomatic managements were followed during treatment on case by case basis. In flatulence, hareetaki powder, lavanbhaskara powder etc were administered. In abdominal pain drugs like lashunadi vati or hingwastak choorna were preferred. Avipattikara or pravala panchamruta rasa was considered in abdominal burning sensation. Abdominal heaviness was managed with trikatu or panchakola powder.
The nature and study design were explained and informed consent was obtained from the participants. Data collection was from August 2017 to June 2019. During study patients were asked to adhere to the treatment protocol and report any of the adverse events to the investigators at the earliest. Any manifestations either existing or new during the course of intervention that cause considerable distress were screened for possible adverse events.
2.3. Criteria of assessment
2.3.1. Primary outcome criteria
-
1.
IBS Symptom Severity Score (IBS-SSS) [27] – Total Score ranges from 0 to 500. Higher score indicate worse condition. Mild IBS (<175), Moderate IBS (175–300) and Severe IBS (>300). A decrease of 50 points correlated with improvement in clinical symptoms.
-
2.
IBS Adequate Relief (IBS-AR) [28,29] – the patients were asked a dichotomous single item “Over the past week have you had adequate relief of your IBS symptoms?” (YES/NO)
Food and drug administration (FDA) has accepted IBS-AR as primary end point. It is responsive, reproducible, it measures the outcome in the same direction as other measures. ROME III has encouraged use of IBS-AR in clinical trials [30].
2.3.2. Secondary outcome criteria
-
1.
Gastrointestinal Symptom Rating Scale (GSRS) [31].
- 2.
-
3.
Complete Spontaneous Bowel Movements (CSBMs) [34].
-
4.
Bristol Stool Form (BSF) [35].
-
5.
Hamilton Anxiety Rating Scale [36].
-
6.
Hamilton Depression Rating Scale [37].
-
7.
IBS quality of life (IBS-QoL) [38].
Assessments were done at baseline, 15th, 30th, 45th and 60th day of the intervention with the help of various assessment parameters. Laboratory parameters of complete blood count, stool examination was done at baseline and 60th day of intervention.
2.3.2. Statistical methods
Statistical analysis was carried out using SPSS Version 25.0. Homogeneity of the data across the groups was evaluated by the χ2 test. Comparison of between groups across different time point was assessed by repeated measure ANOVA test and within group comparison was carried out by Bonferroni post-hoc test. In objective parameters independent paired T test was applied. Values are reported as mean ± 1 standard deviation. Effect size was calculated by Partial Eta Square method. Effect of treatment was evaluated through outcome from baseline to 30th day and 60th day. Effect size interpretation was 0–0.2 minimal, 0.2–0.5 was small, 0.5 to 0.8 was medium and above 0.8 was large effect [39] size. All tests were considered statistically significant at p < 0.05.
3. Results
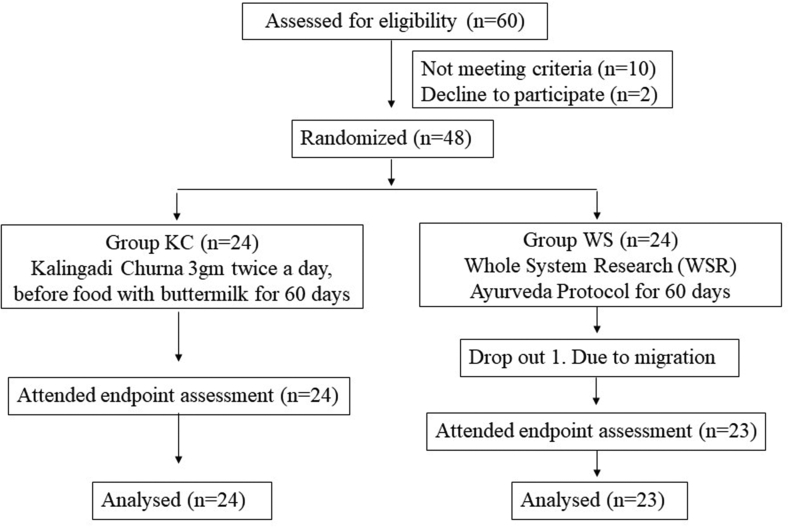
A total of 48 patients were recruited in the study. 1 patient from WS group dropped out due to migration to other place (Fig. 1).
Fig. 1.
Subject flow chart through the study.
3.1. Patient profiles
Mean age of participants was 35.31 yrs, mean duration of illness was 3.59 yrs, severity of the illness (IBS-SSS) in the patients was sever (79%) and moderate (29%), mean weight was 62.18 kg, mean BMI was 22.90, males were more in number (75%), moderate occupation was in 68% of participants, more patients were married (62.5%) and had graduate level education (75%). Gender (p = 0.5), education (p = 0.31), marital status (p = 0.55), duration of illness (p = 0.59), weight (p = 0.83), BMI (p = 0.2), sleep (p = 0.37) and life styles (p = 0.66) were comparable between groups (Table 1). Observations on Whole system diagnostic protocol showed maximum number of patients were nirama stage, predominant vata symptoms, had hard stools (60.41%) and raja dosha dominant (58.33%). Baseline profile of the patients on various clinical assessment scales like IBS-SSS, GSRS, IBS-VAS, CSBM, BSF-D, BSF-C, HARS, HDRS, IBS-QOL, Agni (p = 0.38), and Prakurti (p = 0.5) were comparable between the groups (Table 2).
Table 1.
Baseline data.
| Clinical profiles | KC (n = 24) | WS (n = 24) | p-value |
|---|---|---|---|
| Demographic data | |||
| Age (yrs) | 36 ± 10.41 | 34.63 ± 10.9 | 0.65 |
| Gender – male:female | 17:7 | 19:5 | 0.5 |
| Unmarried:married | 8:16 | 10:14 | 0.55 |
| Education – below graduation:graduation | 8:16 | 4:20 | 0.31 |
| Life style – hard:moderate:sedentary | 4:16:4 | 2:17:5 | 0.67 |
| Disease status | |||
| Weight (kg) | 61.87 ± 11 | 62.5 ± 10.12 | 0.83 |
| BMI | 3.54 ± 3.99 | 22.26 ± 2.76 | 0.202 |
| Duration of illness (years) | 2.68 ± 3.98 | 2.2 ± 1.7 | 0.59 |
| IBS-constipation | 14 | 15 | 0.76 |
| IBS-diarrhea | 10 | 9 | |
| IBS-severity – moderate:severe | 6:18 | 4:20 | 0.47 |
| Anxiety | |||
| Normal (HARS < 8) | 3 | 3 | 0.23 |
| Mild (HARS – 9 to 17) | 18 | 13 | |
| Moderate (HARS – 18 to 24) | 3 | 5 | |
| Sever (HARS > 24) | 0 | 3 | |
| Depression | |||
| Normal (HDRS < 7) | 2 | 2 | 0.22 |
| Mild (HDRS – 8 to 16) | 22 | 18 | |
| Moderate (HDRS – 17 to 24) | 0 | 3 | |
| Sever (HDRS > 25) | 0 | 1 | |
| Sleep – normal:disturbed | 16:8 | 13:11 | 0.37 |
Table 2.
Baseline characteristics of the clinical assessment scales in two groups. Expressed in mean and standard deviations (S.D.).
| S. no | Parameters | KC Group | WS Group | p |
|---|---|---|---|---|
| 1. | IBS-SSS | 337.73 ± 63.91 | 360.43±68.45 | 0.21 |
| 2. | GSRS | 15.68 ± 3.06 | 17.91 ± 4.42 | 0.09 |
| 3. | IBS-VAS | 300 ± 86.9 | 310.43 ± 69.83 | 0.39 |
| 4. | CSBM | 3.36 ± 1.09 | 2.91 ± 1.08 | 0.17 |
| 5. | BSF-D | 6.57 ± 0.53 | 6.42 ± 0.53 | 0.92 |
| 6. | BSF-C | 1.76 ± 0.43 | 1.53 ± 0.51 | 0.29 |
| 7. | HARS | 13.05 ± 3.9 | 15.57 ± 6.82 | 0.13 |
| 8. | HDRS | 11.45 ± 2.92 | 12.91 ± 5.22 | 0.34 |
| 9. | IBS-QOL | 96.55 ± 29.64 | 107.91 ± 27.45 | 0.21 |
| Ayurveda variables | ||||
| 10. | Prakurti – Vata | 4 | 1 | 0.5 |
| Pitta | 0 | 1 | ||
| Kapha | 2 | 1 | ||
| Vata Pitta | 12 | 13 | ||
| Vata Kapha | 4 | 7 | ||
| Pitta Kapha | 2 | 2 | ||
| 11. | Agni – Sama: Manda | 14:10 | 11:13 | 0.39 |
| 12. | Kostha – Madhyama:Mridu | 11:13 | 11:13 | 1 |
| 13. | Ama – Sama:Nirama | 10:14 | 9:15 | |
| 14. | Guna – Raja:Tama | 15:9 | 13:11 | |
| 15. | Bowels – hard:loose | 14:10 | 15:9 | |
| 16. | Counseling sessions (30 min each) | 9 | 21 | |
Assessment of diagnostic subgrouping were done in both the groups. In KC group patients, moderate agni and nirama stage (14) were more compared to agnimandya and sama state (10) and. Dosha wise assessment showed predominance of vata dosha (14) followed by kapha dosha (7) and pitta dosha (3). All vata dosha were nirama state and pitta and kapha had sama state. Bowel pattern showed more of hard (14) then loose (10). Manasika dosha assessments showed more of raja dosha (15) compared to tama dosha (9). Moderate and severe anxiety (HARS) was observed in 3 patients and none were with moderate or severe depression (HDRS). Post treatment all patients were of nirama state and showed improvements in shareerika dosha state, manasika dosha state and bowel pattern derangements. Counseling sessions conducted were 9. In WC group, more patients had moderate agni and nirama stage (15) compared to agnimandya and sama state (9). Dosha wise assessment showed predominance of vata dosha (15), kapha dosha (7) and pitta dosha (2). All vata doshaja were nirama. Pitta and kapha had sama state. Bowel pattern showed more of hard (15) then loose stools (9). Manasika dosha assessments showed predominance of raja dosha (13) compared to tama dosha (11). Post treatment all patients were of nirama state and showed improvements in shareerika dosha state, manasika dosha state and bowel pattern derangements. Moderate and severe grades of anxiety (HARS) was observed in 8 and depression (HDRS) was in 4 patients. Total counseling sessions conducted were 21.
3.2. Primary outcomes
3.2.1. IBS symptom severity score (IBS-SSS)
Effect of interventions on IBS Symptom Severity Score (IBS-SSS) showed significant difference (p = 0.002). Post hoc bonferroni analysis showed that WS group produced significant difference at different time points like 30th (p = 0.007), 45th (p < 0.001)and 60th day (p < 0.001). Within group analysis showed that in WS group improvements were in all time points of 15th, 30th, 45th and 60th day (p < 0.001). In KC Group improvements were from 30th, 45th and 60th day (p < 0.001). Effect size was extremely large. Severity reduced from severe to moderate in KC group and from severe to mild in WS group (Table 3).
Table 3.
Effect of interventions on various clinical assessment scales. Expressed in mean and standard deviations (S.D.). ∗ p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
| Clinical variables | Groups | Baseline | 30th day | 60th day | BL–30th day | 30th–60th day | BL–60th day | p value | Effect size (0–60 days) |
|---|---|---|---|---|---|---|---|---|---|
| IBS-SSS | KC | 337.73 ± 63.91 | 289.09 ± 79.63 | 237.73 ± 96.06 | <0.001∗∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | 0.002∗∗ | 2.09 |
| WS | 360.43 ± 68.45 | 221.74 ± 75.05 | 74.35 ± 49.06 | <0.001∗∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | |||
| IBS-AR | KC | 0a | 0.05 ± 0.22 | 0.45 ± 0.5 | 0.31 | 0.005∗∗ | 0.003∗∗ | <0.001∗∗∗ | 1.54 |
| WS | 0a | 0.25 ± 0.44 | 1 ± 0 | 0.008∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | |||
| GSRS | KC | 15.68 ± 3.06 | 12.14 ± 2.85 | 8.95 ± 2.4 | <0.001∗∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | 0.07 | 2.02 |
| WS | 17.91 ± 4.42 | 10.22 ± 3.13 | 4.04 ± 1.74 | <0.001∗∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | |||
| IBS-VAS | KC | 300 ± 86.9 | 353.18 ± 75.36 | 395.45 ± 80.75 | <0.001∗∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | 3.82 |
| WS | 310.43 ± 69.83 | 472.61 ± 66.07 | 605.65 ± 52.81 | <0.001∗∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | |||
| CSBM | KC | 3.36 ± 1.09 | 4.73 ± 1.03 | 5.18 ± 1.4 | <0.001∗∗∗ | 0.25 | <0.001∗∗∗ | <0.001∗∗∗ | 3.03 |
| WS | 2.91 ± 1.08 | 7.3 ± 1.49 | 10.26 ± 2.56 | <0.001∗∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | |||
| BSF-D | KC | 6.57 ± 0.53 | 6.57 ± 0.53 | 5.71 ± 0.48 | 0.31 | 0.004∗ | 0.004∗∗ | <0.001∗∗∗ | 1.84 |
| WS | 6.42 ± 0.53 | 5.57 ± 0.53 | 4.14 ± 0.37 | 0.008∗∗ | 0.009∗∗ | 0.008∗∗ | |||
| BSF-C | KC | 1.76 ± 0.43 | 1.84 ± 0.37 | 2.15 ± 0.37 | 0.317 | 0.04∗ | 0.02∗ | <0.001∗∗∗ | 1.62 |
| WS | 1.53 ± 0.51 | 2.46 ± 0.51 | 3.3 ± 0.63 | 0.004∗∗ | 0.001∗∗ | 0.001∗∗ | |||
| HARS | KC | 13.05 ± 3.9 | 10.59 ± 3.67 | 7.82 ± 3.5 | 0.003∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | 0.34 | 1.81 |
| WS | 15.57 ± 6.82 | 8.52 ± 4.79 | 3.13 ± 2.86 | <0.001∗∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | |||
| HDRS | KC | 11.45 ± 2.92 | 8.5 ± 3.05 | 6.41 ± 2.5 | <0.001∗∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | 0.441 | 1.18 |
| WS | 12.91 ± 5.22 | 7.43 ± 3.35 | 3.7 ± 2.49 | <0.001∗∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | |||
| IBS-QOL | KC | 96.55 ± 29.64 | 78.18 ± 27.49 | 62.55 ± 25.92 | <0.001∗∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | 0.168 | 2.02 |
| WS | 107.91 ± 27.45 | 68.04 ± 20.77 | 33.43 ± 12.66 | <0.001∗∗∗ | <0.001∗∗∗ | <0.001∗∗∗ | |||
| Hb (g/dl) | KC | 12.64 ± 1.34 | – | 13.07 ± 0.9 | – | – | <0.001∗∗∗ | 0.31 | – |
| WS | 13.07 ± 1.13 | – | 13.36 ± 0.98 | – | – | <0.001∗∗∗ | |||
| ESR (mm/h) | KC | 19.59 ± 12.14 | – | 14.34 ± 6.75 | – | – | 0.94 | 0.84 | – |
| WS | 14.91 ± 8.94 | – | 13.22 ± 8.11 | – | – | <0.001∗∗∗ | |||
| Presence of mucus in stool (number of patients) | KC | 8 | – | 3 | – | – | – | 0.75b | – |
| WS | 4 | – | 1 | – | – | – | |||
| Undigested food in stools (number of patients) | KC | 11 | – | 8 | – | – | – | 0.85b | – |
| WS | 11 | – | 9 | – | – | – |
15th day assessment.
Chi square test.
3.2.2. IBS adequate relief (IBS-AR)
Effect of interventions on IBS Adequate Relief (IBS-AR) showed significant difference (p < 0.001) between groups. Post hoc assessment with wilcoxon matched paired test analysis showed that WS group produced significant difference at 45th and 60th day of assessment (p = 0.001). Within group analysis showed that in WS group improvements were in time points of 30th (p < 0.001) and 60th day (p < 0.001). In KC Group improvements was only at 60th day (p = 0.003). Effect size was extremely large (Table 3).
3.3. Secondary outcomes
3.3.1. Gastrointestinal symptom rating scale (GSRS)
Effect of interventions on Gastrointestinal symptom rating scale (GSRS) showed significant difference (p = 0.01). Post hoc bonferroni analysis showed that WS group produced significant difference at different time points like 30th (p = 0.008), 45th (p < 0.001)and 60th day (p < 0.001) of assessment. Within group analysis showed that in WS group improvements were in all time points of 15th, 30th, 45th and 60th day (p < 0.001). In KC Group improvements were from 30th, 45th and 60th day (p < 0.001). Effect size was extremely large (see Table 4).
Table 4.
Diagnostic details and the effect of whole system ayurveda protocol.
| S. no | Diagnostic work up details | Effect of WSAP |
|---|---|---|
| 1. | Prakurti – Predominant was Vatapittaj-52%, VataKaphaja-22.9% |
Treatment responders vatapittaja (53.3%), Vatakaphaja (27%) |
| 2. | Nirama stage (60.4%), Sama (39.58%) | 25% improvement in group II and in group I was 16.6%. |
| 3. | Dosha type of Grahani – Vataja (60.41%), pittaja (10.41%), kaphaja (29.16%) | Symptoms reduced. |
| 4. | Raja (58.33%) tama (41.66%). And sleep derangement (39.5%) |
Treatment responders Rajasika (55%), Tamasika (45%) |
| 5. | Mild anxiety (64.5%) and moderate (16.66%) anxiety. | Significant improvement. Effect size was very large |
| 6. | Mild depression (83.33%) and moderate (6.25%) | Significant improvement. Effect size was very large |
| 7. | Abdominal pain related variables like IBS AR, IBS SS and IBS VAS | Significant improvement. Effect size was very large |
| 8. | IBS-C (60.41%), IBS-D (39.58%). Hard stools (60.41%) and loose stools (39.58%). Stool related variables like CSBM and BSF scale which includes both stool frequency and consistency | Significant improvement. Effect size was very large |
| 9. | Quality of life | Significant improvement. Effect size was very large |
| 10. | Adequate relief | Significant improvement. Effect size was very large |
GSRS has 15 commonly encountered symptoms of IBS. Assessment of interventions on each symptom showed that ‘urgent need for defecation’ had significant (p = 0.01) difference in between group comparison. WS Group was better than KC group at 30th day (p = 0.04), 45th day (p = 0.003), 60th day (p = 0.004) of intervention. Other symptoms had comparable effect. Post hoc analysis showed the significant difference at 60th day (heart burn), 45th and 60th day (abdominal pain, nausea and vomiting, abdominal distention, feeling of incomplete evacuation). No change between groups and post hoc assessments were observed on eructation, increased flatus, borborygmus, sucking sensation in epigastrium and acid regurgitation. Within group assessment showed improvement of all symptoms in both the groups (Table 3).
3.3.2. IBS-VAS
Effect of interventions on IBS- VAS showed significant difference (p < 0.001) between groups. Post hoc bonferroni analysis showed that WS group produced significant difference at different time points like 15th day (p = 0.01), 30th (p = 0.007), 45th (p < 0.001) and 60th day (p < 0.001) of assessment. Within group analysis showed that in both KC group and WS group had improvements at all time points of 15th, 30th, 45th and 60th day (p < 0.001). Effect size was extremely large (Table 3).
3.3.3. Complete soft bowel movement (CSBM)-stool frequency-numbers/day
Effect of interventions on CSBM showed significant difference (p = 0.001). Post hoc bonferroni analysis showed that WS group produced significant difference at different time points like 15th (p = 0.004), 30th (p = 0.008), 45th (p < 0.001) and 60th day (p < 0.001) of assessment. Within group analysis showed that in WS group improvements were in all time points of 15th, 30th, 45th and 60th day (p < 0.001). In KC Group improvements were at 15th day (p = 0.005) and all other time points 30th, 45th and 60th day (p < 0.001). Effect size was extremely large (Table 3).
3.3.4. Bristol stool form-BSF-diarrhea (score 5–7)-(stool consistency)
Effect of interventions on BSF-Diarrhea showed significant difference (p < 0.001) favoring WS group. Effect size was extremely large (Table 3).
3.3.5. Bristol stool form-BSF-constipation (score 1–3)-(stool consistency)
Effect of interventions on BSF-constipation showed significant difference (p < 0.001) favoring WS group. Effect size was extremely large (Table 3).
3.3.6. Hamilton anxiety rating scale (HARS)
Effect of interventions on HARS showed non significant difference (p = 0.28). Within group analysis showed that in WS group improvements were in all time points of 15th, 30th, 45th and 60th day (p < 0.001). In KC Group improvements were from 30th 45th and 60th day (p < 0.001). Effect size was extremely large (Table 3).
3.3.7. Hamilton depression rating scale (HDRS)
Effect of interventions on HDRS showed non significant difference (p = 0.25). Within group analysis showed that in KC group and WS group improvements were in all time points. At 15th day in WS group (p = 0.04) and KC group (p = 0.01) improvements were significant. Both groups at 30th, 45th and 60th day had significant (p < 0.001) improvements. Effect size was extremely large (Table 3).
3.3.8. IBS quality of life (IBS-QoL)
Effect of interventions on IBS quality of life (IBS-QoL) showed non significant difference (p = 0.28). Within group analysis showed that in WS group improvements were in all time points of 15th, 30th, 45th and 60th day (p < 0.001). In KC group improvements were at 15th day (p = 0.04) and all other time points 30th, 45th and 60th day (p < 0.001). Effect size was extremely large (Table 3).
3.3.9. Laboratory parameters
Hemoglobin showed improvement in both the groups (p < 0.001) and were comparable (p = 0.31). ESR changes between groups were comparable (p = 0.84), however within group decrease was only in WS group (p < 0.001).
Stool examination showed derangements in few patients. Mucus in stool (25%), undigested food (45.83%) and pus cells (4.1%) were deranged. No abnormality in ova, cyst, frank blood, occult blood, epithelial cells, fat globules, helminths, trophozoites, crystals, bacteria was observed in any patients. Between group assessment showed that improvements were comparable (Table 3).
4. Discussion
Study demonstrated that WS Ayurveda protocol for IBS was effective compared to Kalingadi choorna in irritable bowel syndrome. WSAP showed efficacy in both primary assessment parameters (IBS-AR, IBS-SSS) and secondary parameters like IBS-VAS, CSBM, BSF-D and BSF-C. Both groups were comparable in GSRS, HARS, HDRS and IBS-QOL. Blood and stool parameters assessments showed comparable improvements in both the groups. Both the groups produced improvement in hemoglobin levels. No adverse events were reported in both the groups.
WSR has the model validity principle of traditional, complementary, and integrative medicine including Ayurveda science. WSR method tries to balance principles and practices of these sciences [40]. Ayurveda has integrated approaches of patient's physical, mental, emotional, psychosocial and patient's preferences. A study incorporating WSR approach with Ayurveda and yoga in weight management has shown beneficial effect [41]. In Ayurveda, conceptual models, diagnostic approaches and management strategies are distinct from or in addition to biomedical science. Treatments in Ayurveda are complex involving multiple synergistic treatment modalities or components tailored to the specific patient. WSAP in current study had two components namely diagnostic and management protocols.
4.1. Whole system ayurveda diagnostic protocol
Diagnostic components includes assessment of agni, sama and nirama stage, hard and loose stool, sharirika dosha stage (vata, pitta, kapha), manasika dosha stage (raja, tama). At baseline diagnostic sub components in patients were sama (39.58%) and nirama (60.41%). Sharirika dosha involved were vataja (60.41%), pittaja (10.41%), kaphaja (29.16%). Consistency of stools was hard stools (60.41%) and loose stools (39.58%) (Table 2). Majority of patients were of IBS-C (60.41%) followed by IBS-D (39.58%) and non were of IBS-M and IBS-U. Manasika dosha involved were raja (58.33%) tama (41.66%). More number of patients had mild anxiety (64.5%) and moderate (16.66%) anxiety. Predominant depressive state was mild depression (83.33%) and moderate (6.25%). Previous study [42] has shown high level of anxiety and depressive symptoms in patients of IBS. Laboratory parameters (Blood and stools) were deranged only in few patients. Severity of IBS was sever in 79.1% as assessed through IBS-SSS. Sleep was deranged in 39.5% patients.
4.2. Whole system ayurveda therapeutic protocol
WSAP treatment protocol included management of different diagnostic protocol components through oral medicaments. Ayurveda psychological counseling techniques [43] were used in the rajasika (anxious predominant, HARS > 18) and tamasika (depression predominant states, HDRS > 17). Counseling sessions were 9 (3 patients) in KC group and 21 (8 patients) in WS group. Total number of medicines enlisted were 41. Total number medications used were 27. At any time 2 or 3 medications were administered looking into components of WSAP diagnostic protocol. Treatments in WSAP was administered by a physician with 20 years of clinical experience. Sama stage reduction in patients was more in WS group (25%) than KC group (16.6%). WSAP was effective in pain related variables like IBS AR, IBS SSS and IBS VAS. Effect size (IBS VAS) was extremely large. Pain is one of the important variable in IBS symptoms. WSAP shown significant effect on stool related variables like CSBM and BSF scale which includes both stool frequency and consistency. Effect size was extremely large. WSAP intervention was effective in both the criteria of BSF like Diarrhea and constipation in bringing stool to normal form and effect size was extremely large. Psychological symptoms of anxiety and depression showed significant improvement with a large effect size. Quality of life improvement was also with large effect size. Comprehensively WSAP brought better adequate relief (IBS-AR).
Previous studies have also used the clinical parameters of the current study for demonstrating the interventional effect in IBS. A study [44] demonstrated the beneficial effect of probiotic supplementation in patients of IBS with celiac disease through clinical parameters IBS Severity Scoring System (IBS-SSS); Gastrointestinal Symptom Rating Scale (GSRS); Bristol Stool Form Scale (BSFS); and IBS Quality of Life Questionnaire (IBS-QOL). Acupuncture [45] intervention did not show superiority over sham acupuncture assessed through IBS Global Improvement Scale (IBS-GIS), IBS Symptom Severity Scale (IBS-SSS), the IBS Adequate Relief (IBS-AR), and the IBS Quality of Life (IBS-QOL). A study [46] evaluated the effect of open label placebo in IBS through parameters like IBS Symptom Severity Scale (IBS-SSS), IBS Adequate Relief (IBS-AR) and IBS Quality of Life (IBS-QoL).
Kalingadi churna and WSAP treatment approaches produced significant improvements. This could be due to the actions of their ingredients. In WSAP group, the medicines used in hard stools were Hareetaki churna, Shunthi churna, Abhayarishta, Lavanabhaskara churna, Trikatu churna, Avipattikara churna, Triphala churna, Draksharishta, Manibhardra guda etc. in accordance with other pathological components. Hareetaki churna (Terminalia chebula Retz) possess significant intestinal motility-enhancing effect, local stimulant effect and has Anulomana [47] effect. It is one of the ingredient in the formulations like Abhayarista, A. churna, Triphala churna, Manibadra guda etc. Lavana bhaskara churna has deepana (stimulant for digestive fire), amapachana (metabolizes undigested substrates) and vatanulomana (downward movement of vata) effect. Triphala churna has antidiarrheal and gastro intestinal protective and anti-inflammatory activity [48]. Medicines used in loose stools were Agnitundi vati, Chitrakadi vati, Lashunadi vati, Kutajarishta, Anada bhairava rasa, Kutaja Ghana vati, Kutajarishta, Dadimashtaka choorna, Lashunadi vati, Jeerakarishta etc. Agnitundi vati is deepana, pachana, decreases Kapha and vata, aamahara, grahi (absorbent with hot potency), sthambaka (stops watery secretions) effects. Kutaja (Holarrhena antidysenterica (L.) Wall. ex A. DC.) has antidiarrheal activity and gives protection in multiple stages of diarrhea [49]. Musta (Cyperus rotundus L. syn. C. hexastachyos Rottb) has antidiarrheal activity, central nervous system depressant activity, analgesic, anticonvulsant and nootropic activity [48]. Psychotherapy and psychotropic medications were administered in patients with predominant anxiety and depression. Psychotherapy included behavioral, emotional and cognitive modifications (Table 2, Fig. 2). Physical exercise and psychological treatments such as stress management, relaxation, meditation, cognitive behavioral therapy, functional relaxation, mindfulness, hypnotherapy and body awareness therapy have shown to be helpful in IBS [50]. Meta-analysis showed beneficial effects of antidepressants on IBS symptoms [51]. Antidepressants are beneficial in chronic pain disorders and act on intestinal transit time. Kalingadi churna contains medicines like Ativisha (Aconitum heterophyllum Wall. Cat.), Hareetaki [T. chebula Retz] etc. Hareetaki can act on hard stools (IBS-C) and Ativisha (A. heterophyllum) on IBS-D, as it has antidiarrheal, anti-inflammatory, immunomodulatory, anthelmintic activity [52].
Fig. 2.
Whole System Ayurveda Diagnostic and Therapeutic Protocol – Probable mode of action.
Other studies have also shown favorable outcomes in IBS. Ayurveda medications like Bilvadileha [15], Lavanbhaskar Churna [16] and Takrarista [17] have shown beneficial effect. However these studies lacks assessment through standard clinical assessment measures. Iberogast (STW-5, is a mixture of diverse extracts of flower, leaves, fruit, root, and herbs with antispasmodic effects on gastrointestinal smooth muscle) showed improvement in global symptoms and abdominal pain scores of IBS [53]. Metanalysis showed that prebiotics have an overall beneficial effect in IBS and the greatest impact was on abdominal pain, bloating, flatulence but not on bowel urgency or bowel function [54]. Yoga is helpful in reducing the severity of IBS, somatic symptoms and walking improves overall gastrointestinal symptoms, negative affect and anxiety [55].
The present study is the first study to evaluate WS Ayurveda approach in IBS (Grahani). The randomization of the clinical trail is the main strength of the study. This study evaluated a wide spectrum of clinical assessment parameters like pain, gastro intestinal symptoms, severity, stool forms, assessing both IBS-C and IBS-D, anxiety, depression, quality of life and over all relief. The limitations of the study include absence of blinding procedure and a smaller sample size. Biological assessments with fecal short-chain fatty acids, granins and breath analysis would have added more evidence.
5. Conclusion
The present study shows that WSAP has significantly beneficial effect in IBS. There were significant improvements in reducing the severity, abdominal pain, gastrointestinal symptoms, bowels frequency, bowel consistency, anxiety, depression and quality of life. WSAP showed beneficial effect compared to Kalingadi churna in reducing the severity of symptoms, over all adequate relief, pain, bowel frequency and consistency. Both the interventions were comparable in terms of quality of life, anxiety, depression and gastro intestinal symptoms improvements.
Author contributions
B R Tubaki: Conceptualization, Methodology, Writing - Original draft preparation, Writing - Reviewing and Editing, Statistical analysis. Teja D Naik: Supervision, Data curation, Writing - Reviewing and Editing. Devayani S Patankar: Visualization, Writing - Reviewing and Editing.
Source of funding
No funding received.
Declaration of competing interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2022.100592.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Enck P., Aziz Q., Barbara G., Farmer A.D., Fukudo S., Mayer E.A., et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.14. PMID: 27159638; PMCID: PMC5001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmulson M.J., Drossman D.A. What is new in Rome IV. J Neurogastroenterol Motil. 2017;23(2):151–163. doi: 10.5056/jnm16214. PMID: 28274109; PMCID: PMC5383110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson P.K., DiPalma J.A. Diagnosing irritable bowel syndrome: a changing clinical paradigm. South Med J. 2011;104:195–199. doi: 10.1097/SMJ.0b013e31820bfb6c. PMID: 21297534. [DOI] [PubMed] [Google Scholar]
- 4.Lembo A., Ameen V.Z., Drossman D.A. Irritable bowel syndrome: toward an understanding of severity. Clin Gastroenterol Hepatol. 2005;3:717–725. doi: 10.1016/s1542-3565(05)00157-6. [PMID: 16233998] [DOI] [PubMed] [Google Scholar]
- 5.Inadomi J.M., Fennerty M.B., Bjorkman D. Systematic review: the economic impact of irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18(7):671–682. doi: 10.1046/j.1365-2036.2003.t01-1-01736.x. [DOI] [PubMed] [Google Scholar]
- 6.Longstreth G.F., Yao J.F. Irritable bowel syndrome and surgery: a multivariable analysis. Gastroenterology. 2004;126:1665–1673. doi: 10.1053/j.gastro.2004.02.020. [PMID: 15188159] [DOI] [PubMed] [Google Scholar]
- 7.Spiegel B., Schoenfeld P., Naliboff B. Systematic review: the prevalence of suicidal behaviour in patients with chronic abdominal pain and irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:183–193. doi: 10.1111/j.1365-2036.2007.03357.x. [PMID: 17593064] [DOI] [PubMed] [Google Scholar]
- 8.Oka P., Parr H., Barberio B., Black C.J., Savarino E.V., Ford A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(10):908–917. doi: 10.1016/S2468-1253(20)30217-X. Epub 2020 Jul 20. Erratum in: Lancet Gastroenterol Hepatol. 2020 Dec;5(12):e8. PMID: 32702295. [DOI] [PubMed] [Google Scholar]
- 9.Mayer E.A., Labus J.S., Tillisch K., Cole S.W., Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol. 2015;12(10):592–605. doi: 10.1038/nrgastro.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho H.S., Park J.M., Lim C.H., Cho Y.K., Lee I.S., Kim S.W., et al. Anxiety, depression and quality of life in patients with irritable bowel syndrome. Gut Liver. 2011;5(1):29–36. doi: 10.5009/gnl.2011.5.1.29. Epub 2011 Mar 16. PMID: 21461069; PMCID: PMC3065090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agnivesha Charaka Samhita, ayrvedaDipikaAyushi Hindi Commentary . In: Part 2, traslated by Acharya Siddhinandan Mishra. Kushawaha Vd Harishchandra Singh, ChoukhambaOrientalia, editors. 2009. Varanasi, ChikitsaSthana 15/53-54, Pg 392. [Google Scholar]
- 12.Barboza J.L., Talley N.J., Moshiree B. Current and emerging pharmacotherapeutic options for irritable bowel syndrome. Drugs. 2014;74(16):1849–1870. doi: 10.1007/s40265-014-0292-7. PMID: 25260888. [DOI] [PubMed] [Google Scholar]
- 13.Hungin A.P.S., Chang L., Locke G.R., Dennis E.H., Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21(11):1365–1375. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 14.Sorathiya A.P., Vyas S.N., Bhat P.S. A clinical study on the role of ama in relation to Grahani Roga and its management by Kalingadi Ghanavati and Tryushnadi Ghrita. Ayu. 2010;31(4):451–455. doi: 10.4103/0974-8520.82041. PMID: 22048538; PMCID: PMC3202250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiwari R., Pandya D.H., Baghel M.S. Clinical evaluation of Bilvadileha in the management of irritable bowel syndrome. Ayu. 2013;34(4):368–372. doi: 10.4103/0974-8520.127717. PMID: 24696573; PMCID: PMC3968698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wange M. From the proceedings of Insight Ayurveda 2013, Coimbatore. 24th and 25th May 2013. PA03.22. Importance of Agni Chikitsa in the management of Grahani Disease w.s.r to Lavanabhaskara Churna. Anc Sci Life. 2013;32(Suppl 2):S91. doi: 10.4103/0257-7941.123920. [DOI] [Google Scholar]
- 17.Witt C.M., Michalsen A., Roll S., Morandi A., Gupta S., Rosenberg M., et al. Comparative effectiveness of a complex Ayurvedic treatment and conventional standard care in osteoarthritis of the knee--study protocol for a randomized controlled trial. Trials. 2013 23;14:149. doi: 10.1186/1745-6215-14-149. PMID: 23701973; PMCID: PMC3664613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel M.V., Patel K.B., Gupta S., Michalsen A., Stapelfeldt E., Kessler C.S. A complex multiherbal regimen based on ayurveda medicine for the management of hepatic cirrhosis complicated by ascites: nonrandomized, uncontrolled, single group, open-label observational clinical study. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/613182. Epub 2015 Aug 3. PMID: 26339267; PMCID: PMC4539059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bensoussan A., Talley N.J., Hing M., Menzies R., Guo A., Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280(18):1585–1589. doi: 10.1001/jama.280.18.1585. PMID: 9820260. [DOI] [PubMed] [Google Scholar]
- 20.Verhoef M.J., Lewith G., Ritenbaugh C., Boon H., Fleishman S., Leis A. Complementary and alternative medicine whole systems research: beyond identification of inadequacies of the RCT. Complement Ther Med. 2005;13(3):206–212. doi: 10.1016/j.ctim.2005.05.001. PMID: 16150375. [DOI] [PubMed] [Google Scholar]
- 21.Schulz K.F., Altman D.G., Moher D., CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palsson Olafur S., Whitehead William E., van Tilburg Miranda A.L., Chang Lin, et al. Development and validation of the Rome IV diagnostic questionnaire for adults. Gastroentrology. 2016;150(6):1481–1491. [Google Scholar]
- 23.Agnivesha . 2009. Charaka Samhita, traslated by Acharya Siddhinandan Mishra and Vd. Harishchandra Singh Kushawaha,ChoukhambaOrientalia. Varanasi, ChikitsaSthana, chapter 15, verse 53-54, Pg 392. [Google Scholar]
- 24.Samhita Sushruta, Atrideva Dr. 2002. Shri BhaskarGhanekar, MotilalBanarasidas. Uttarsthana, chapter 40, verse 17, pg 698. [Google Scholar]
- 25.Arundatta and Hemadri commentary, Vagbhata, Ashtangahridayam, collated by Dr. Anna MoreswarKunte and Krishna RamchandraShastriNavre, edited by Bhishagacharya Vaidya, Krishnadas Academy, Choukhamba press, Varanasi, Sutrasthana, chapter 13, verse27, Pg 216
- 27.Francis C.Y., Morris J., Whorwell P.J. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. PMID: 9146781. [DOI] [PubMed] [Google Scholar]
- 28.Mangel A.W., Hahn B.A., Heath A.T., et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res. 1998;26:76–81. doi: 10.1177/030006059802600203. [DOI] [PubMed] [Google Scholar]
- 29.Mangel A.W., Fehnel S. Adequate relief in IBS treatment trials: corrections to errors stated by Whitehead et al. Am J Gastroenterol. 2006;101(12):2884–2885. doi: 10.1111/j.1572-0241.2006.00867_1.x. author reply 2885 – 7. [DOI] [PubMed] [Google Scholar]
- 30.Camilleri M., Mangel A.W., Fehnel S.E., Drossman D.A., Mayer E.A., Talley N.J. Primary endpoints for irritable bowel syndrome trials: a review of performance of endpoints. Clin Gastroenterol Hepatol. 2007;5(5):534–540. doi: 10.1016/j.cgh.2007.03.004. Epub 2007 Apr 11. PMID: 17428741. [DOI] [PubMed] [Google Scholar]
- 31.Svedlund J., Sjödin I., Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33(2):129–134. doi: 10.1007/BF01535722. PMID: 3123181. [DOI] [PubMed] [Google Scholar]
- 32.Bodil Ohlsson. Practical Evaluation and handling of patients with Irritable Bowel Syndrome. EMJ Gastroenterol. 2013;1:48–53. [Google Scholar]
- 33.Bengtsson M., Hammar O., Mandl T., Ohlsson B. Evaluation of gastrointestinal symptoms in different patient groups using the visual analogue scale for irritable bowel syndrome (VAS-IBS) BMC Gastroenterol. 2011;11:122. doi: 10.1186/1471-230X-11-122. PMID: 22073983; PMCID: PMC3248355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Food and Drug Administration . 2010. Guidance for industry: Irritable bowel syndrome-clinical evaluation of products for treatment.http://www.fdanews.com/ext/resources/files/archives/i/IBS.pdf Accessed on. [Google Scholar]
- 35.Lewis S.J., Heaton K.W. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920–924. doi: 10.3109/00365529709011203. PMID: 9299672. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton M. Hamilton Anxiety Rating Scale (HAM-A), The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 37.Worboys M. The hamilton rating scale for depression: the making of a "gold standard" and the unmaking of a chronic illness, 1960-1980. Chronic Illn. 2013;9(3):202–219. doi: 10.1177/1742395312467658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groll D., Vanner S.J., Depew W.T., DaCosta L.R., Simon J.B., Groll A., Roblin N., Paterson W.G. The IBS-36: a new quality of life measure for irritable bowel syndrome. Am J Gastroenterol. 2002;97(4):962–971. doi: 10.1111/j.1572-0241.2002.05616.x. PMID: 12003433. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J. 2 ed. L. Erlbaum Associates; 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 40.Ijaz N., Rioux J., Elder C., Weeks J. Whole systems research methods in health care: a scoping review. J Altern Complement Med. 2019;25(S1):S21–S51. doi: 10.1089/acm.2018.0499. PMID: 30870019; PMCID: PMC6447996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rioux J., Thomson C., Howerter A. A pilot feasibility study of whole-systems Ayurvedic medicine and yoga therapy for weight loss. Glob Adv Health Med. 2014;3:28–35. doi: 10.7453/gahmj.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palsson O.S., Drossman D.A. Psychiatric and psychological dysfunction in irritable bowel syndrome and the role of psychological treatments. Gastroenterol Clin North Am. 2005;34:281–303. doi: 10.1016/j.gtc.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Tubaki B.R., Chandake S., Sarhyal A. Ayurveda management of major depressive disorder: a case study. J Ayurveda Integr Med. 2021;12(2):378–383. doi: 10.1016/j.jaim.2021.03.012. Epub 2021 May 20. PMID: 34024690; PMCID: PMC8186000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Francavilla R., Piccolo M., Francavilla A., Polimeno L., Semeraro F., Cristofori F., et al. Clinical and microbiological effect of a multispecies probiotic supplementation in celiac patients with persistent ibs-type symptoms: a randomized, double-blind, placebo-controlled, multicenter trial. J Clin Gastroenterol. 2019;53(3):e117–e125. doi: 10.1097/MCG.0000000000001023. PMID: 29688915; PMCID: PMC6382041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lembo A.J., Conboy L., Kelley J.M., Schnyer R.S., McManus C.A., Quilty M.T., et al. A treatment trial of acupuncture in IBS patients. Am J Gastroenterol. 2009;104(6):1489–1497. doi: 10.1038/ajg.2009.156. PMID: 19455132; PMCID: PMC2694961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaptchuk T.J., Friedlander E., Kelley J.M., Sanchez M.N., Kokkotou E., Singer J.P., et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0015591. PMID: 21203519; PMCID: PMC3008733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jirankalgikar Y.M., Ashok B.K., Dwivedi R.R. A comparative evaluation of intestinal transit time of two dosage forms of Haritaki [Terminalia chebula Retz] Ayu. 2012;33(3):447–449. doi: 10.4103/0974-8520.108866. PMID: 23723658; PMCID: PMC3665098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baliga M.S., Meera S., Mathai B., Rai M.P., Pawar V., Palatty P.L. Scientific validation of the ethnomedicinal properties of the Ayurvedic drug Triphala: a review. Chin J Integr Med. 2012;18(12):946–954. doi: 10.1007/s11655-012-1299-x. Epub 2012 Dec 13. PMID: 23239004. [DOI] [PubMed] [Google Scholar]
- 49.Daswani P.G., Birdi T.J., Antarkar D.S., Antia N.H. Investigation of antidiarrhoeal activity of Holarrhena antidysentrica. Int J Pharm Res. 2012;164–7 [Google Scholar]
- 50.Leahy A., Epstein O. Non-pharmacological treatments in the irritable bowel syndrome. World J Gastroenterol. 2001;7(3):313–316. doi: 10.3748/wjg.v7.i3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ford A.C., Quigley E.M., Lacy B.E., et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(9):1350–1365. doi: 10.1038/ajg.2014.148. [PubMed: 24935275] [DOI] [PubMed] [Google Scholar]
- 52.Nagarajan M., Kuruvilla G.R., Kumar K.S., Venkatasubramanian P. Pharmacology of Ativisha, Musta and their substitutes. J Ayurveda Integr Med. 2015;6(2):121–133. doi: 10.4103/0975-9476.146551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madisch A., Holtmann G., Plein K., Hotz J. Treatment of irritable bowel syndrome with herbal preparations: results of a double-blind, randomized, placebo-controlled, multi¬centre trial. Aliment Pharmacol Ther. 2004;19(3):271–279. doi: 10.1111/j.1365-2036.2004.01859.x. [PubMed: 14984373] [DOI] [PubMed] [Google Scholar]
- 54.Ford A.C., Quigley E.M., Lacy B.E., et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547–1561. doi: 10.1038/ajg.2014.202. [PubMed: 25070051] [DOI] [PubMed] [Google Scholar]
- 55.Shahabi L., Naliboff B.D., Shapiro D. Self-regulation evaluation of therapeutic yoga and walking for patients with irritable bowel syndrome: a pilot study. Psychol Health Med. 2016;21(2):176–188. doi: 10.1080/13548506.2015.1051557. [PubMed: 26086986] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.