Summary
Background
The UK government reclassified gabapentin and pregabalin as ‘controlled drugs’ from April 2019. This study aimed to describe the trends in gabapentinoid prescribing before and immediately after reclassification, in the UK Clinical Practice Research Datalink, an electronic primary care health record broadly representative of the UK.
Methods
Separately for gabapentin and pregabalin, we calculated annual incident and prevalent prescribing rates from year of UK approval (April 1997 and 2004 respectively) to September 2019, and monthly incident and prevalent prescribing rates (October 2017–September 2019). Significant changes in temporal trends were determined using joinpoint regression. We also described potential prescribing indications, prior pain-related prescribing, and co-prescribing with potentially interacting medicines.
Findings
Incident gabapentin prescribing increased annually, peaking in 2016–17, at 625/100,000 patient years before falling steadily to 2019. Incident pregabalin prescribing peaked at 329/100,000 patient years in 2017–18 and did not fall significantly until 2019. Prevalent gabapentin and pregabalin prescribing increased annually to 2017–18 and 2018–19 respectively, before plateauing. Gabapentinoids were commonly co-prescribed with opioids (60%), antidepressants (52%), benzodiazepines (19%), and Z-drugs (10%).
Interpretation
Following a dramatic rise, incident gabapentinoid prescribing has started to fall but the specific impact of reclassification on prescribing rates remains unclear. Limited change in prevalent gabapentinoid prescribing during the 6 months following their reclassification as controlled drugs suggests little immediate impact on continued gabapentinoid prescribing for existing users.
Funding
National Institute for Health and Care Research (NIHR) Research for Patient Benefit Programme. NIHR Applied Research Collaboration West Midlands. NIHR School for Primary Care Research.
Keywords: Pharmacoepidemiology, Pregabalin, Gabapentin, Gabapentinoids, Primary care, Prescriptions
Research in context.
Evidence before this study
We searched PubMed for articles published to April 28, 2022 using the search terms (“gabapentin” OR “pregabalin” OR “gabapentinoid”) AND (“prescription” OR “prescribing”) AND (“trend” OR “pattern”) without language restrictions. Our search yielded studies, largely from government or insurer prescribing data, reporting rising trends in gabapentinoid prescriptions, over varying periods between 2002 and 2016 across a number of countries worldwide including the United States (US), Canada, Australia, UK and Ireland. In addition, published studies using the UK Clinical Practice Research Datalink (CPRD) reported a tripling in the rate of patients starting gabapentinoids between 2007 and 2017 and a three-fold increase in incident gabapentinoid prescriptions among patients with osteoarthritis between 2005 and 2015. Alongside this, reported cases of gabapentinoid misuse and dependence were rising progressively, with gabapentinoids increasingly implicated in overdose deaths, often in combination with opioids and other drugs with central nervous system (CNS) depressant effects. Published studies report co-prescribing of gabapentinoids with benzodiazepines in around 20% and co-prescribing with opioids in 15–70% of patients, depending on the patient cohort, healthcare setting and definition of co-prescribing used.
Added value of this study
This is the first study investigating UK trends in gabapentinoid prescribing during the period immediately before and after their reclassification as controlled drugs in April 2019. Our study indicates that the dramatic rise in incident gabapentinoid prescribing has peaked, but we observed different temporal trends for gabapentin and pregabalin. Incident gabapentin prescribing peaked in 2016–17, before falling steadily during the period before and immediately after reclassification. Incident pregabalin prescribing peaked later, in 2017–18, and then plateaued, with no significant annual downward trend was identified prior to 2019. Prevalent gabapentin and pregabalin prescribing also appears to have plateaued, but has not fallen substantially. In addition, we report on potential prescribing indications, gabapentinoid co-prescribing with potentially interacting central nervous system (CNS) depressant medicines, including antidepressants and Z-drug hypnotics, and describe analgesic prescribing in the twelve months prior to starting gabapentinoids.
Implications of all the available evidence
Whilst UK gabapentinoid prescribing may have peaked, the immediate impact of their reclassification as controlled drugs on the prevalence of gabapentinoid prescribing has been limited. This is concerning, given the lack of evidence for their effectiveness outside clearly defined licensed indications and their potential to cause harm, particularly as they are commonly co-prescribed with other central nervous system depressants. Next steps include investigating the risk of gabapentinoid-related adverse events and factors associated with poor outcome, to inform interventions aimed at improving the appropriateness and safety of gabapentinoid prescribing.
Introduction
Gabapentin and pregabalin (gabapentinoids) are approved in the UK and European Union for epilepsy and neuropathic pain. Pregabalin is also approved for generalised anxiety disorder. Gabapentinoid prescribing has risen dramatically, in the UK and globally,1, 2, 3, 4 despite limited evidence supporting their effectiveness outside the licensed indications. Gabapentinoids benefit around 20% of people with neuropathic pain, with most evidence for their effectiveness in neuropathic pain derived from trials in populations with post-herpetic neuralgia and painful diabetic neuropathy. The evidence for their effectiveness in other chronic pain conditions is lacking.5 Nevertheless, widespread ‘off-label’ gabapentinoid prescribing, mostly for non-neuropathic chronic pain, is suspected.4, 5, 6 Furthermore, a growing body of evidence highlights the misuse potential of gabapentinoids, particularly pregabalin.7 Cases of gabapentinoid misuse and dependence reported to the European Medicines Agency have risen progressively8 and gabapentinoids are increasingly implicated in post-mortem toxicology reports, often in combination with opioids.8, 9, 10 UK deaths involving gabapentinoids increased from 12 in 2012 to 170 in 2016.10 Concerns about the abuse potential of gabapentinoids, the associated risk of addiction and the potential for illegal diversion led to the UK Advisory Council on the Misuse of Drugs recommending that gabapentin and pregabalin were controlled under the Misuse of Drugs Regulations. Following a formal consultation on proposals to reclassify gabapentinoids as controlled drugs (November 2017–January 2018) the UK government announced, in October 2018, that gabapentinoids would be reclassified in the UK as scheduled 3 (class C) controlled drugs from April 2019.11 Thereafter, there are stronger controls on gabapentinoid prescribing to reduce the likelihood of gabapentin and pregabalin falling into the wrong hands or being stockpiled by patients.
To date, much of the published UK data regarding gabapentinoids focus on trends in community prescribing, which may reflect changes in the number of patients prescribed gabapentinoids and/or the amount prescribed to existing users and tells us little about the patients receiving gabapentinoids. Published studies of anonymised primary care data using the UK Clinical Practice Research Datalink (CPRD GOLD) reported a tripling in the rate of incident gabapentin prescribing between 2007 and 2017,4 with 50% attributed to an unlicensed indication in 2017, more than three times higher than in 20054; and a three-fold increase in incident gabapentinoid prescriptions among patients with osteoarthritis between 2005 and 2015.6 Montastruc et al. also reported that, over time, rates of gabapentinoid co-prescribing with opioids rose in proportion to the rise in gabapentinoid prescribing,4,12 with 21.8% of patients newly treated with gabapentin and 24.1% newly treated with pregabalin receiving a same-day prescription for opioids and/or benzodiazepines. These studies only investigated incident prescribing and there are no primary care studies investigating UK gabapentinoid prescribing trends beyond 2017.
We hypothesised that reclassification of gabapentinoids as controlled drugs in April 2019, and the preceding consultation and associated publicity regarding the risks of prescribing gabapentinoids (2016–2019), would reverse the rising trend in UK gabapentinoid prescribing. Building on previous work, this study aimed to investigate trends and patterns in gabapentinoid prescribing in a larger CPRD dataset comprising GOLD and AURUM databases, during the period before and immediately after gabapentinoid reclassification in April 2019. Our main objective was to examine changes in incident and prevalent prescribing, separately for gabapentin and pregabalin, from the year of their UK approval for neuropathic pain (1997 and 2004 respectively) to 30 September 2019. Prevalent prescribing was included to reflect the impact of treatment continuation on prescribing trends. Additional objectives were to describe the population with an incident gabapentinoid prescription, including recorded diagnoses that may represent prescribing indications, pain-related prescribing in the 12 months prior to starting gabapentinoids, and rates of co-prescribing with potentially interacting medicines, including antidepressants and Z-drug hypnotics (zopiclone and zolpidem), in addition to opioids and benzodiazepines.
Methods
This was an observational database study performed in CPRD, a high-quality database of de-identified, coded primary care records for use in health research. CPRD comprises two datasets (GOLD and AURUM) that capture diagnoses, symptoms, prescriptions, referrals, and tests from general practices. The GOLD and AURUM datasets contain data contributed by practices using different clinical systems. The data structure is therefore slightly different, and they are available as separate datasets. At the time of the study, both the GOLD and AURUM datasets were broadly representative of the population in terms of age, sex, ethnicity, and body mass index. Median follow-up time in the AURUM dataset for all individuals included since 1995 is estimated to be 4.2 years.13 We would expect this to be similar for individuals in GOLD. As the geographical spread of practices is not representative within GOLD, and AURUM does not contain data from Wales and Scotland, we used both datasets to accurately investigate the whole UK population. The Independent Scientific Advisory Committee (ISAC) provided permission for data access and related analysis (ISAC protocol 19_214A). Details of all definitions used are provided as a supplement (see Supplementary Materials). All code lists are freely available from the Keele University repository (http://doi.org/10.21252/k6bc-ys67).
Gabapentinoid prescribing population included
Gabapentin and pregabalin were considered separately. Gabapentin was approved in the UK for neuropathic pain in 1997 and pregabalin in 2004. We defined gabapentinoid users as individuals aged 18 years and over who were issued at least one gabapentin prescription between April 1, 1997 and September 30, 2019 or at least one pregabalin prescription between April 1, 2004 and September 30, 2019. Drug code lists to define gabapentinoids were established by a clinical academic pain specialist and an academic general practitioner.
Patterns of use
We formed a cohort of adult patients aged 18 years and over with an incident gabapentinoid prescription (see Supplement) and at least 12 months of up-to-standard records in CPRD before the prescription. For each patient, we considered multiple treatment periods and calculated average daily dose and duration of gabapentinoid therapy within each treatment period. Considering the first treatment period of each individual, we extracted information regarding recorded diagnoses that are licensed indications for gabapentinoids (epilepsy, neuropathic pain, and (for pregabalin only) anxiety) using lists of clinical codes from a range of previous studies using electronic health records. All codes were reviewed, and modified as necessary, by an academic pain specialist and an academic GP. General practitioners (GPs) in the UK are encouraged to record new diagnoses (using clinical codes) in the electronic medical record and all prescriptions are issued electronically and automatically recorded. However, there is no direct link between each prescription and the indication for prescribing and GPs do not typically repeatedly enter the diagnostic code for existing conditions. As epilepsy, neuropathic pain, and anxiety are usually chronic conditions, and other approved treatments may have been trialled prior to gabapentinoids, the diagnostic code for these conditions may not be recorded around the time of gabapentinoid prescription. Therefore, we extracted information on a diagnosis of epilepsy, neuropathic pain, and anxiety if this was ever recorded before the end of the individual's final gabapentinoid treatment period. We also extracted data on conditions associated with chronic pain that may represent unlicensed indications namely: fibromyalgia, chronic back pain (without radiculopathy), chronic neck pain, osteoarthritis, chronic headache, migraine, chronic abdominal pain and restless legs syndrome. These conditions were defined using lists of clinical codes that are from previous studies or have been agreed through expert consensus, and were reviewed and updated as necessary. We extracted information on these diagnoses if they occurred between 14 days before and 90 days after the first gabapentinoid prescription, to provide a temporal link between the diagnosis and first gabapentinoid prescription.14 To assess the sensitivity of our analyses to these assumptions, we repeated the analysis using a window of −30 to 90 days.
Data on co-prescribing of potentially interacting medicines with gabapentinoids were extracted. Specifically, data were extracted regarding prescriptions for opioids, benzodiazepines, Z-drug hypnotics (zopiclone and zolpidem), and antidepressants during a period of gabapentinoid prescribing (i.e., calculated periods of potentially interacting drug use overlapped within any gabapentinoid treatment period) (see Supplement). We also extracted data on the pain-related prescribing pathway to gabapentinoids (see Supplement). Specifically, data were extracted on prescriptions during the 12 months prior to first gabapentinoid prescription for: simple analgesics (paracetamol and nefopam), opioids, non-steroidal anti-inflammatories (NSAIDs), topical neuropathic pain treatments (lidocaine plasters and capsaicin cream), and other medicines that are recommended in the UK National Institute for Health and Care excellence (NICE) Guideline on neuropathic pain,15 namely antidepressants (amitriptyline and duloxetine) and carbamazepine, a non-gabapentinoid anticonvulsant.
Statistical analyses
For each dataset, we calculated annual rates of incident gabapentin and pregabalin prescriptions and 95% confidence intervals per 100,000 patient years in CPRD. Incidence stratified by age group (18–24, 25–34, 35–44, 45–54, 55–64, 65–74, ≥75 years, based on previous studies16) and sex is also reported. Prevalence of gabapentin and pregabalin prescriptions was calculated separately in each year (any therapy in the year April to March) using the mid-year population on 1st July as the denominator.
Monthly incidence and prevalence were calculated in the same way, using the 15th of the month as the denominator for the prevalence calculation for the period October 2017–September 2019. For each treatment period, we calculated median daily dose (mg) and median therapy duration (days) with corresponding interquartile range (IQR). Therapy duration was defined as the number of treatment days recorded by a GP or by dividing the quantity prescribed by the numeric daily dose (see Supplement). We calculated the proportions of incident gabapentin and pregabalin users prescribed medicines on the pain-related prescribing pathway to gabapentinoids during the 12 months prior to first gabapentinoid prescription and the proportions of patients co-prescribed opioids, benzodiazepines, Z-drugs, and antidepressants with gabapentinoids (i.e., overlapping treatment periods. All diagnoses and prescriptions were assumed to have been recorded if they occurred.
Analyses were completed separately for the AURUM and GOLD datasets, removing migrating practices from the GOLD dataset to ensure mutually exclusive datasets (see Supplement). They were then combined using a two-stage individual participant data (IPD) meta-analysis using a fixed-effects model with an inverse variance approach to pool estimates, as there were no clinical or methodological differences in data recording conditions or classification.17 All IPD meta-analysis results are presented as effect estimate with 95% confidence intervals (95% CIs) for a given outcome, except for median daily dose and duration of treatment, which due to extremely skewed distributions, are presented as median of medians for each prescription.
Significant changes in temporal trends were determined by calculating the mean annual/monthly percentage change (APC/MPC) and 95% confidence interval (CI) using Joinpoint Regression.18 Joinpoint analyses were performed using Joinpoint Program 4.9.1.0 (available at https://surveillance.cancer.gov/joinpoint/). When interpreting Joinpoint regression, a positive value of the APC/MPC suggests an increasing trend and a negative value suggests a decreasing trend. Optimal models (≤5 Joinpoints) were selected using a permutation test with 4500 permutations and a significance level of p < 0.05 (further detail regarding the permutation test in Joinpoint regression is provided in the Supplementary Materials).
Role of funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
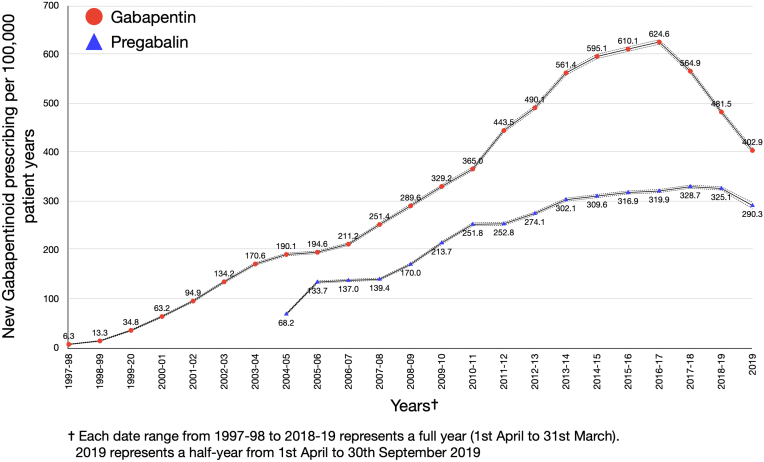
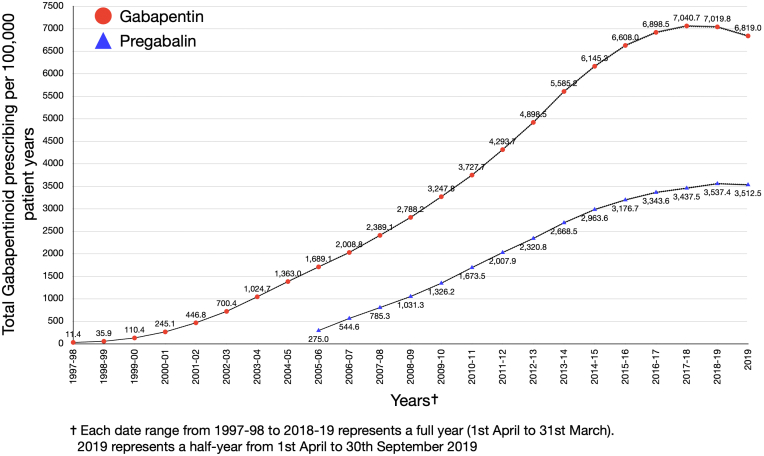
Between April 1, 1997 and September 30, 2019, 34.7 million patients contributed data to the CPRD. Incident gabapentin prescriptions were found in the records of 778,080 people and pregabalin in 419,387. Across the two drugs and GOLD and AURUM datasets, between 61 and 63% of those with a gabapentin prescription were female. The rate (95% CI) of incident gabapentin prescribing per 100,000 person years increased annually from 6.30 (5.73, 6.92) in 1997–98 until 2016–17, peaking at 624.57 (620.07, 629.10) (Fig. 1, Table 1). In 2016–17 there is a significant change, marking the start of a steady downward trend in incident gabapentin prescribing, with an annual percent change of −13.4 (95% CI −18.5 to −7.9) (Fig. 1, Table 1). The joinpoint analysis of monthly incident prescribing in the period immediately before and after reclassification (October 2017–September 2019) shows a steady downward trend, with a monthly percent change of −1.5% (95% CI −1.9 to −1.0) (Table 1). Prevalent gabapentin prescribing (95% CI) per 100,000 person years increased annually from 11.42 (10.61, 12.22) in 1997–98, peaking at 7040.68 (7026.5, 7054.86) in 2017–18 before falling slightly to 7019.75 (7005.69, 7033.80) in 2018–19 (Fig. 2, Supplementary Table S2). No significant change points in the annual trend in prevalent gabapentin prescribing were identified in the joinpoint analysis (Table 1). In the joinpoint analysis of monthly prevalent gabapentin prescribing for the period immediately before and after reclassification (October 2017–September 2019) a significant change to a slight downward trend is identified in September 2018 with a monthly percentage change of −0.2% (95% CI −0.2 to −0.3) to July 2019 and −2.2% (95% CI −2.9 to −1.8) from July 2019 to September 2019 (Table 1).
Fig. 1.
Annual rates of incident gabapentinoid prescribing (1997–2019) per 100,000 patient years.
Table 1.
Joinpoint analysis: Identifying changes in the incidence and prevalence of gabapentinoid prescribing over time.
| Outcomes by gabapentinoid prescribing | Joinpoint | Start | End | APC/MPC (95% CI) | Test Statistic (t) | p-value |
|---|---|---|---|---|---|---|
| Incidence (annual) | ||||||
| Gabapentin | 0 | 1997–98 | 1999–20 | 164.2 (−2.4 to 614.8) | 2.2 | 0.055 |
| Gabapentin | 1 | 1999–20 | 2003–04 | 52.0 (12.1–106.0) | 3.1 | 0.012 |
| Gabapentin | 2 | 2003–04 | 2013–14 | 13.6 (12.4–14.8) | 26.3 | <0.001 |
| Gabapentin | 3 | 2013–14 | 2016–17 | 4.7 (−4.5 to 14.7) | 1.1 | <0.001 |
| Gabapentin | 4 | 2016–17 | 2019 | −13.4 (−18.5 to −7.9) | −5.3 | 0.010 |
| Pregabalin | 0 | 2004–05 | 2010–11 | 19.0 (12.0–26.5) | 6.3 | |
| Pregabalin | 1 | 2010–11 | 2019 | 3.4 (1.0–5.9) | 3.1 | |
| Incidence (monthly) | ||||||
| Gabapentin | 0 | October-17 | September-19 | −1.5 (−1.9 to −1.0) | −6.3 | <0.001 |
| Pregabalin | 0 | October-17 | January-19 | 0.5 (−0.5 to 1.4) | 1.0 | 0.326 |
| Pregabalin | 1 | January-19 | September-19 | −3.4 (−5.6 to −1.1) | −3.1 | 0.005 |
| Prevalence (annual) | ||||||
| Gabapentin | 0 | 1997–98 | 2001–02 | 127.5 (93.2–167.9) | 11.4 | <0.001 |
| Gabapentin | 1 | 2001–02 | 2005–06 | 36.6 (29.6–43.6) | 14 | <0.001 |
| Gabapentin | 2 | 2005–06 | 2013–14 | 15.6 (14.8–16.4) | 47.9 | <0.001 |
| Gabapentin | 3 | 2013–14 | 2016–17 | 7.2 (3.6–11.0) | 4.6 | 0.001 |
| Gabapentin | 4 | 2016–17 | 2019 | −0.6 (−2.2 to 1.0) | −0.9 | 0.407 |
| Pregabalin | 0 | 2005–06 | 2009–10 | 41.7 (29.5–55.0) | 9.2 | <0.001 |
| Pregabalin | 1 | 2009–10 | 2014–15 | 16.5 (11.6–21.6) | 8.4 | <0.001 |
| Pregabalin | 2 | 2014–15 | 2019 | 3.1 (0.7–5.5) | 3.1 | 0.017 |
| Prevalence (monthly) | ||||||
| Gabapentin | 0 | October-17 | January-18 | −0.2 (−0.5 to 0.1) | −1.5 | 0.166 |
| Gabapentin | 1 | January-18 | September-18 | 0.1 (0.1–0.2) | 4.1 | 0.001 |
| Gabapentin | 2 | September-18 | July-19 | −0.2 (−0.3 to −0.2) | −9.7 | <0.001 |
| Gabapentin | 3 | July-19 | September-19 | −2.4 (−2.9 to −1.8) | −8.7 | <0.001 |
| Pregabalin | 0 | October-17 | February-18 | 0.0 (−0.2 to 0.3) | 0.1 | 0.907 |
| Pregabalin | 1 | February-18 | September-18 | 0.5 (0.3–0.6) | 7.5 | <0.001 |
| Pregabalin | 2 | September-18 | July-19 | 0.0 (−0.1 to 0.0) | −0.9 | 0.404 |
| Pregabalin | 3 | July-19 | September-19 | −4.2 (−5.0 to −3.4) | −11.6 | <0.001 |
APC: annual percent change; CI: confidence interval; MPC: monthly percent change.
Fig. 2.
Annual rates of prevalent gabapentinoid prescribing (1997–2019) per 100,000 patient years.
The rate (95% CI) per 100,000 person years of incident pregabalin prescribing increased annually from 68.15 (66.57, 69.78) in 2004–05, peaking at 328.74 (325.54, 331.97) in 2017–18 before falling slightly to 325.11 (321.93, 328.32) during 2018–19 (Fig. 1, Supplementary Table S1). Prevalent pregabalin prescribing (95% CI) per 100,000 person years increased annually from 275.04 (271.87, 278.22) in 2004–05, peaking at 3537.37 (3527.20, 3547.54) in 2018–19 (Fig. 2, Supplementary Table S2). No significant change points in the annual trends for incident or prevalent pregabalin prescribing were identified in the joinpoint analysis (Table 1). In the joinpoint analysis of monthly pregabalin prescribing for the period immediately before and after reclassification (October 2017–September 2019) there was a significant change to a downward trend in incident pregabalin prescribing in January 2019 with a monthly percent change of −3.4% (95% CI −5.6 to −1.1) from January to September 2019. The first significant change to a downward trend in monthly prevalent pregabalin prescribing occurred in July 2019, with a monthly percent change of −4.2% (95% CI −5.0 to −3.4) during July to September 2019.
Incident gabapentinoid prescribing rates were higher in females than males (56% higher for gabapentin and 63% for pregabalin) and in older age groups, with the highest rates in patients aged 75 years and over (Supplementary Table S3). The median daily dose prescribed was 977 mg (IQR 477, 1799 mg) for gabapentin and 186 mg (IQR 121, 374 mg) for pregabalin. The median duration of therapy was 2.81 (IQR 0.95, 13.12) months for gabapentin and 4.18 (IQR 0.92, 19.66) months for pregabalin (Table 2).
Table 2.
Gabapentinoid prescribing patterns and pathways 1997–2019, meta-analysis of CPRD GOLD and AURUM data.
| Gabapentin | Pregabalin | |
|---|---|---|
| Dose and treatment duration | ||
| Median (IQR) dose (mg) | 977 (477, 1799) | 186 (121, 374) |
| Median (IQR) treatment duration (months) | 2.81 (0.95, 13.12) | 4.18 (0.92, 19.66) |
| Prescribing pathway in 12 months prior to index date (percentage (95% CI)) | ||
| Simple analgesics (paracetamol, nefopam) | 25.9 (25.8, 26.0) | 27.6 (27.5, 27.8) |
| Opioids | 64.9 (64.8, 65.0) | 64.6 (64.5, 64.8) |
| NSAIDs | 47.1 (47.0, 47.2) | 45.4 (45.2, 45.5) |
| Topical neuropathic pain treatments | 2.0 (2.0, 2.1) | 2.9 (2.8, 2.9) |
| Duloxetine/Amitriptylinea | 33.7 (33.6, 33.8) | 34.5 (34.3, 34.6) |
| Carbamazepinea | 2.9 (2.8, 2.9) | 2.6 (2.5, 2.6) |
| Concurrent prescriptions (percentage (95% CI)) | ||
| Opioids | 61.0 (60.9, 61.1) | 61.1 (60.9, 61.2) |
| Benzodiazepines | 16.3 (16.2, 16.4) | 21.1 (21.0, 21.2) |
| Z-drugs | 8.2 (8.1, 8.2) | 11.8 (11.7, 11.9) |
| Antidepressants | 48.6 (48.5, 48.8) | 54.8 (54.6, 54.9) |
Recommended in the UK NICE Neuropathic Pain Guideline.15
Around half (51.4%) of patients prescribed pregabalin and just over a third (36.9%) of patients prescribed gabapentin had a recorded diagnosis of a licensed indication before the end of their final gabapentinoid treatment period. The commonest was neuropathic pain, recorded in around one-third of patients (See Supplementary Table S4). Recorded diagnoses of pain conditions for which gabapentinoids are not licensed were found within −14 to +90 days of the first gabapentinoid prescription in almost one-fifth of cases. Expanding the time window to within −30 days to +90 days of the first prescription increased the proportions with these diagnoses from 19.1% to 20.9% (gabapentin) and 17.5% to 18.9% (pregabalin). Chronic back pain was the condition coded in the majority of these cases (See Supplementary Table S4).
In the 12 months prior to starting gabapentinoids, other NICE-recommended neuropathic pain treatments15 (amitriptyline, duloxetine, and carbamazepine) were prescribed to 36.6% and 37.1% of patients prescribed gabapentin and pregabalin respectively (Table 2). A higher proportion of patients were prescribed other classes of analgesics. In the 12 months prior to starting gabapentin, 64.9% of patients were prescribed opioids, 47.1% NSAIDs, and 25.9% simple analgesics (paracetamol or nefopam). In the 12 months prior to starting pregabalin, 64.6% were prescribed opioids, 45.4% NSAIDs, and 27.6% simple analgesics (Table 2). Opioids were co-prescribed (had overlapping treatment periods) with gabapentin in 61.0% and pregabalin in 61.1% of patients. Co-prescribing of other potentially interacting medicines was slightly more common in patients receiving pregabalin (54.8% antidepressants, 21.1% benzodiazepines, and 11.8% Z-drugs), compared with those receiving gabapentin (48.6% antidepressants, 16.3% benzodiazepines, and 8.2% Z-drugs) (Table 2).
Discussion
To our knowledge, this is the first study reporting UK trends in gabapentinoid prescribing during the period immediately before and after their reclassification as controlled drugs in April 2019. We hypothesised that reclassification and the associated publicity regarding the risks of gabapentinoids during the preceding consultation period would reverse the rising trend in UK gabapentinoid prescribing. However, whilst our findings suggest that the dramatic rise in incident gabapentinoid prescribing may have peaked, we observed different temporal trends for gabapentin and pregabalin. Incident gabapentin prescribing peaked first in 2016–17. Subsequently, a steady downward trend in incident gabapentin prescribing is evident, which started prior to reclassification and continued at a similar rate immediately afterwards. Consequently, by April 2019, incident gabapentin prescribing had already fallen by around 22%. However, incident pregabalin prescribing continued to rise for longer, before plateauing from 2017 to 18, and no significant change to a downward trend in annual incident pregabalin prescribing was identified. Monthly prescribing data (October 2017–September 2019) suggest the start of a downward trend in monthly incident pregabalin prescribing from January 2019. However, our data is limited to the first 6 months following reclassification in April 2019 and the gabapentinoid prescribing trends beyond this are uncertain. There was no significant change to a downward trend in annual prevalent prescribing for either gabapentin or pregabalin.
The rising trend in incident gabapentinoid prescribing to 2016–17 is consistent with previous UK studies.4,6 The timing of the subsequent downward trend in incident gabapentin prescribing is consistent with our hypothesis, in so far as it coincides with the publicity and consultation period regarding proposals to reclassify gabapentinoids as controlled drugs. However, the downward trend was well established by the time of reclassification itself. It is noteworthy that, although pregabalin's higher misuse potential received more adverse publicity during the period before and around the time of reclassification (2016–2019),7,9 gabapentin prescribing fell first, whilst pregabalin prescribing did not fall significantly prior to 2019. One possible contributing factor is that the expiry of UK pregabalin patents in 2014 (epilepsy and anxiety) and 2017 (neuropathic pain) resulted in cheaper generic pregabalin becoming available, which may have made clinicians more likely to prescribe pregabalin first line for neuropathic pain rather than gabapentin. This occurred in Canada and Australia following subsidies that reduced the cost of pregabalin.2,3 Another possible factor is that, unlike gabapentin, pregabalin has a UK licence for anxiety, a common condition among patients with chronic pain.
Consistent with publicly available prescriptions data,19 we demonstrated limited immediate impact of reclassification on prevalent gabapentinoid prescribing, suggesting that prescribers were not stopping gabapentinoid prescribing for existing users to any great extent.19 In contrast, prevalent tramadol prescribing fell immediately and substantially following a similar UK reclassification in 2014.20 However, patients on tramadol (an opioid) may have been switched to an alternative opioid or to another class of pain medicines, such as gabapentinoids, as happened following changes to US opioid regulations.21 By comparison, there is a lack of effective pharmacological alternatives for patients currently using gabapentinoids for chronic pain.
Over 50% of the patients we identified with an incident gabapentinoid prescription did not have a recorded diagnosis of a licensed indication for these drugs and almost one-fifth had a recorded diagnosis of a potential unlicensed indication, mainly chronic back pain. This is lower than a previous study in UK primary care, which identified potential prescribing indications in 60% of patients newly prescribed gabapentinoids and reported that, in 2017, over 50% of these were for unlicensed indications.4 This is likely to reflect their use of a longer, more sensitive 1-year time window prior to first prescription for attribution to a diagnostic code.4 Studies in Canada, Australia, and the US also reported widespread off-label pregabalin prescribing for chronic pain,2,3,22 most commonly for musculoskeletal conditions such as low back pain. Off-label prescribing for non-neuropathic pain is also suggested by our finding that, during the 12 months prior to starting gabapentinoids, prescribing of analgesic medicines typically used in non-neuropathic pain was common, with around two-thirds of patients prescribed opioids and nearly half prescribed NSAIDs.
Potentially interacting medicines with CNS depressant effects, including opioids, antidepressants, benzodiazepines and Z-drug hypnotics, were frequently co-prescribed with gabapentinoids. Previously published estimates of gabapentinoid-opioid co-prescribing rates vary depending on the patient cohort, healthcare setting and co-prescribing definition used. Our finding that around 60% of patients are co-prescribed gabapentinoids with opioids is consistent with a Scottish study, which reported that around 50% of patients prescribed gabapentinoids were co-prescribed opioids and almost 60% were co-prescribed opioids, benzodiazepines or both.23 Another UK study using CRPD4,12 reported that, between 2007 and 2017, rates of gabapentinoid co-prescribing with an opioid and/or benzodiazepine, increased over time in proportion to the rate of incident gabapentinoid prescriptions, with 21.8% and 24.1%, of those newly prescribed gabapentin and pregabalin respectively, receiving a same day prescription, primarily for opioids, in 2017. The lower proportion identified in this study is likely to reflect their less sensitive definition of same-day co-prescribing rather than overlapping use. Opioid-gabapentinoid co-prescribing rates of 32%, 38%, and 50% have been reported in the US, Australia, and Canada respectively.2,3,22 Higher opioid-gabapentinoid co-prescribing rates over 70% have been reported in patients newly diagnosed with osteoarthritis24 and patients with a diagnosis of chronic pain.3 Our finding that around one fifth of patients are co-prescribed benzodiazepines with gabapentinoids is similar to the rates reported in previous studies from the US, Australia and Canada.2,3,22
Frequent co-prescribing of gabapentinoids with opioids, benzodiazepines, antidepressants and Z-drugs is likely to reflect high levels of distress and sleep disturbance among people with chronic pain and the limitations of current pharmacotherapy, leading to widespread use of polypharmacy for chronic pain.25 However, there is potential for pharmacokinetic and pharmacodynamic interactions. Co-administration of gabapentinoids with opioids may potentiate the respiratory depressant effect of opioids, increasing the risk of overdose, respiratory complications, and medication-related hospitalisation.26, 27, 28 Older patients and those with respiratory or neurological disease and renal impairment are particularly at risk.28 Oversedation due to concurrent use of gabapentinoids with CNS depressants may also potentially increase the risk of falls and related injuries, particularly in the elderly.29 This is particularly concerning given that, consistent with previous studies,1,6,22,23 we found higher gabapentinoid prescribing rates in older age groups. It also highlights the dilemma facing clinicians treating older patients who often have comorbidities and polypharmacy which, alongside the pharmacokinetic and pharmacodynamic changes associated with ageing, increase the risk of gabapentinoid side-effects and adverse events.30
A key strength of our study arises from the large size of the combined GOLD and AURUM CPRD datasets, which allowed the analysis of gabapentinoid prescribing trends in a large cohort of primary care patients representing the whole of the UK. Gabapentinoids are only available on prescription in the UK and, as all prescribing in primary care is electronically recorded at the point of medication issue, missing data are unlikely. Our study has some limitations. First, the data are for primary care and do not capture gabapentinoid prescriptions issued by specialists in secondary care. However, in the UK prescriptions recommended by specialists will usually still be prescribed by primary care and, whilst first prescriptions may occasionally be initiated by specialists, continuation will be by general practice prescribing and therefore the findings reported are still likely to accurately reflect UK gabapentinoid prescribing trends. In keeping with all database studies, our study is based on prescriptions issued and cannot account for patients not taking their prescribed medication as directed. Whilst multiple prescription events per person suggest patients are taking their medication, this is not certain. In addition, as diagnoses in the CPRD are not directly linked with issued prescriptions, it is not possible to definitively attribute prescribing to indications, these can only be inferred from the medical record and we did not identify recorded diagnoses representing potential licensed or unlicensed prescribing indications in around one-third of patients. It is possible that we could have identified more diagnoses for unlicensed indications by expanding the time window beyond −30 days to +90 days but previous CPRD studies allowing up to 1 year before the prescription dates were still unable to attribute prescribing to a diagnosis in at least 40% of cases.4,6 Furthermore, our analysis may overestimate the proportion attributable to an unlicensed indication because licensed and unlicensed measures were not mutually exclusive, and a 10% overlap was observed. It is also possible that our definition of co-prescribing (overlapping prescribing periods) may overestimate the proportion co-prescribed potentially interacting medicines by capturing patients who are switching from one drug to another. Finally, our analyses include six months of data after the date gabapentinoids were reclassified as controlled drugs and the impact of reclassification beyond that remains unknown.
In conclusion, whilst UK gabapentinoid prescribing may have peaked, the immediate impact of their reclassification as controlled drugs on the prevalence of gabapentinoid prescribing has been limited. This is concerning, given the lack of evidence for their effectiveness outside clearly defined licensed indications and their potential to cause harm, particularly when co-prescribed with other CNS depressant medication. It may reflect the lack of effective pharmaceutical alternatives and limited access to non-pharmacological therapies for chronic pain, making it particularly challenging for clinicians to support patients to stop gabapentinoids in UK primary care. The next step should be to investigate the risk of gabapentinoid-related adverse events and the factors associated with poor outcome, to inform interventions aimed at improving the appropriateness and safety of gabapentinoid prescribing.
Contributors
J.A., S.M., T.H., S.A.H., R.W. and C.D.M. conceptualised, designed and obtained funding for the study. S.M., R.B., R.W., and J.B. accessed, processed and verified the underlying data. S.M., R.W. and R.B. take responsibility for the integrity of the data and the accuracy of the analyses. R.W. and R.B. analysed the data with supervision from S.M. J.A., R.B., S.M., T.H., S.A.H. and C.D.M. interpreted the data. J.A. drafted the manuscript. R.B. and J.A. prepared the figures and tables. All authors critically revised the manuscript, had access to the data reported in the study, and approved the decision to submit for publication.
Data sharing statement
Data may be obtained from a third party and are not publicly available. The data were obtained from the Clinical Practice Research Datalink (CPRD). CPRD data governance does not allow us to distribute patient data to other parties. Researchers may apply for data access at http://www.CPRD.com/.
Ethical approval
The CPRD Group holds research ethics approval from the National Research Ethics Service Committee for all purely observational research, using anonymised CPRD data, and not requiring any direct patient involvement (Reference 05/MRE04/87). The Independent Scientific Advisory Committee (ISAC) provided permission for data access and related analysis (ISAC protocol 19_214A).
Declaration of interests
The authors have no conflicts of interest to declare.
Acknowledgements
This study is based in part on data from the Clinical Practice Research Datalink (CPRD) obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone.
Funding: This project was funded by the National Institute for Health and Care Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Reference PB-PG-1217-20011). Christian Mallen Sara Muller, and Ram Bajpai are supported by the NIHR Applied Research Collaboration West Midlands and Christian Mallen also by the NIHR School for Primary Care Research. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2022.100579.
Appendix A. Supplementary data
References
- 1.Johansen M.E. Gabapentinoid use in the United States 2002 through 2015. JAMA Intern Med. 2018;178(2):292–294. doi: 10.1001/jamainternmed.2017.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwok H., Khuu W., Fernandes K., et al. Impact of unrestricted access to pregabalin on the use of opioids and other CNS-active medications: a cross-sectional time series analysis. Pain Med. 2017;18(6):1019–1026. doi: 10.1093/pm/pnw351. [DOI] [PubMed] [Google Scholar]
- 3.Schaffer A.L., Busingye D., Chidwick K., Brett J., Blogg S. Pregabalin prescribing patterns in Australian general practice, 2012-2018: a cross-sectional study. BJGP Open. 2021;5(1) doi: 10.3399/bjgpopen20X101120. bjgpopen20X101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montastruc F., Loo S.Y., Renoux C. Trends in first gabapentin and pregabalin prescriptions in primary care in the United Kingdom, 1993-2017. JAMA. 2018;320(20):2149–2151. doi: 10.1001/jama.2018.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman C.W., Brett A.S. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern Med. 2019;179(5):695–701. doi: 10.1001/jamainternmed.2019.0086. [DOI] [PubMed] [Google Scholar]
- 6.Appleyard T., Ashworth J., Bedson J., Yu D., Peat G. Trends in gabapentinoid prescribing in patients with osteoarthritis: a United Kingdom national cohort study in primary care. Osteoarthritis Cartilage. 2019;27(10):1437–1444. doi: 10.1016/j.joca.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Evoy K.E., Sadrameli S., Contreras J., Covvey J.R., Peckham A.M., Morrison M.D. Abuse and misuse of pregabalin and gabapentin: a systematic review update. Drugs. 2021;81(1):125–156. doi: 10.1007/s40265-020-01432-7. [DOI] [PubMed] [Google Scholar]
- 8.Chiappini S., Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency's 'Suspected Adverse Drug Reactions' Database. CNS Drugs. 2016;30(7):647–654. doi: 10.1007/s40263-016-0359-y. [DOI] [PubMed] [Google Scholar]
- 9.Hakkinen M., Vuori E., Kalso E., Gergov M., Ojanpera I. Profiles of pregabalin and gabapentin abuse by postmortem toxicology. Forensic Sci Int. 2014;241:1–6. doi: 10.1016/j.forsciint.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Office for National Statistics Deaths related to drug poisoning in England and Wales: 2016 registrations 2016. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsrelatedtodrugpoisoninginenglandandwales/2016registrations
- 11.Gov U.K. Pregabalin and gabapentin to be controlled as class C drugs Online: UK Home Office. 2018. https://www.gov.uk/government/news/pregabalin-and-gabapentin-to-be-controlled-as-class-c-drugs Available from:
- 12.Rahman A., Kane J., Montastruc F., Renoux C. Trends in new prescription of gabapentinoids and of coprescription with opioids in the 4 nations of the UK, 1993-2017. Br J Clin Pharmacol. 2021;87(8):3349–3353. doi: 10.1111/bcp.14727. [DOI] [PubMed] [Google Scholar]
- 13.Wolf A., Dedman D., Campbell J., et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. Int J Epidemiol. 2019;48(6) doi: 10.1093/ije/dyz034. 1740–1740g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedson J., Chen Y., Ashworth J., et al. Trends in long-term opioid prescribing in primary care patients with musculoskeletal conditions: an observational database study. Pain. 2016;157(7):1525–1531. doi: 10.1097/j.pain.0000000000000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute for Health and Clinical Excellence Neuropathic pain in adults: pharmacological management in non-specialist settings 2017. https://www.nice.org.uk/guidance/cg173 [PubMed]
- 16.Molero Y., Larsson H., D'Onofrio B.M., Sharp D.J., Fazel S. Associations between gabapentinoids and suicidal behaviour, unintentional overdoses, injuries, road traffic incidents, and violent crime: population based cohort study in Sweden. BMJ. 2019;365:l2147. doi: 10.1136/bmj.l2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riley R.D., Lambert P.C., Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 18.Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Mahase E. Gabapentinoids: has reclassification really solved the problem? BMJ. 2020;368:m114. doi: 10.1136/bmj.m114. [DOI] [PubMed] [Google Scholar]
- 20.Chen T.C., Chen L.C., Knaggs R.D. A 15-year overview of increasing tramadol utilisation and associated mortality and the impact of tramadol classification in the United Kingdom. Pharmacoepidemiol Drug Saf. 2018;27(5):487–494. doi: 10.1002/pds.4320. [DOI] [PubMed] [Google Scholar]
- 21.Shen Y., Hincapie-Castillo J.M., Vouri S.M., Dewar M.A., Sumfest J.M., Goodin A.J. Prescription patterns of adjuvant pain medications following an opioid supply restriction law: an interrupted time series analysis. Med Care. 2022;60(6):432–436. doi: 10.1097/MLR.0000000000001719. [DOI] [PubMed] [Google Scholar]
- 22.Zhou L., Bhattacharjee S., Kwoh C.K., et al. Trends, patient and prescriber characteristics in gabapentinoid use in a sample of United States ambulatory care visits from 2003 to 2016. J Clin Med. 2019;9(1):83. doi: 10.3390/jcm9010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torrance N., Veluchamy A., Zhou Y., et al. Trends in gabapentinoid prescribing, co-prescribing of opioids and benzodiazepines, and associated deaths in Scotland. Br J Anaesth. 2020;125(2):159–167. doi: 10.1016/j.bja.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Yu D., Appleyard T., Cottrell E., Peat G. Co-prescription of gabapentinoids and opioids among adults with and without osteoarthritis in the United Kingdom between 1995 and 2017. Rheumatology (Oxford) 2021;60(4):1942–1950. doi: 10.1093/rheumatology/keaa586. [DOI] [PubMed] [Google Scholar]
- 25.Gilron I., Jensen T.S., Dickenson A.H. Combination pharmacotherapy for management of chronic pain: from bench to bedside. Lancet Neurol. 2013;12(11):1084–1095. doi: 10.1016/S1474-4422(13)70193-5. [DOI] [PubMed] [Google Scholar]
- 26.Gomes T., Juurlink D.N., Antoniou T., Mamdani M.M., Paterson J.M., van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10) doi: 10.1371/journal.pmed.1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes T., Greaves S., van den Brink W., et al. Pregabalin and the risk for opioid-related death: a nested case-control study. Ann Intern Med. 2018;169(10):732–734. doi: 10.7326/M18-1136. [DOI] [PubMed] [Google Scholar]
- 28.US Federal Drugs Agency (FDA) https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-warns-about-serious-breathing-problems-seizure-and-nerve-pain-medicines-gabapentin-neurontin
- 29.Chen T.C., Knaggs R.D., Chen L.C. Association between opioid-related deaths and persistent opioid prescribing in primary care in England: a nested case-control study. Br J Clin Pharmacol. 2022;88(2):798–809. doi: 10.1111/bcp.15028. [DOI] [PubMed] [Google Scholar]
- 30.Pickering G. Antiepileptics for post-herpetic neuralgia in the elderly: current and future prospects. Drugs Aging. 2014;31(9):653–660. doi: 10.1007/s40266-014-0202-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.