Abstract
Background and Objectives:
Klebsiella pneumoniae causes challenging nosocomial fatal infections including neonatal sepsis. Our study aims at clarifying the contribution of integrons in the observed reduced susceptibility of multidrug-resistant (MDR) K. pneumoniae isolated from septicemic neonates to the clinically used antimicrobial agents and biocides.
Materials and Methods:
Eighty-six K. pneumoniae isolates were collected from Mansoura University Children’s Hospital from septicemic neonates. Isolates were subjected to antibiotic and biocide susceptibility using disk diffusion and the agar dilution method, respectively. The distribution of different classes of integrons was screened in the isolates by PCR. Detected inegron I was sequenced in selected isolates.
Results:
Fifty-seven isolates (66.27%) were MDR. In the MDR isolates, class I integron was detected in 23 (40.3%), integron III was detected in 20 (35%), whereas integron II could not be detected. Sequencing results of integron I from MDR K. pneumoniae isolates revealed that only aminoglycoside and folate synthesis inhibitors gene cassettes were detected, while the rest of the resistance genes were not associated with integron I.
Conclusion:
The presence of integron I in MDR K. pneumoniae tested isolates may contribute only to some biocide resistance, however, it does not seem to be the only contributor in multiple drug resistance.
Keywords: Klebsiella pneumoniae, Integrons, Disinfectants, Drug resistance, Newborn
INTRODUCTION
In underdeveloped and developing nations, Gram-negative bacteria are the most common cause of newborn sepsis (1). Klebsiella pneumoniae represents an important pathogen that causes nosocomial infections and subsequently neonatal sepsis (2). Neonatal sepsis represents a major cause of mortality among neonates. Neonatal sepsis cause one million death per year according to WHO statistics (3, 4). K. pneumoniae is responsible for the majority of neonatal sepsis, especially in developing countries (5).
The challenge of K. pneumoniae stems from its ability to acquire resistance against many antimicrobial agents including biocides (6). There are many mechanisms through which the pathogen can acquire resistance against antimicrobial agents. Mobile genetic elements including integrons are considered the major factor in the dissemination of multidrug resistance (MDR) (7). As a result of acquiring resistance to at least one agent in three or more antimicrobial groups, MDR was coined (8). According to their genomic context, integrons can be found as mobile integrons when they are related to mobile DNA elements such as insertion sequences, transposons, conjugative plasmids, and as chromosome integrons when they are found in bacterial chromosomes (9). The risk of multiple resistance of Klebsiella infection is exaggerated, which leads to an increase in hospital stay time and also poses difficulty for the medical team to choose the best treatment (9, 10). There is a possible linkage between biocides resistance and antibiotic resistance established on the presence of mobile genetic elements (11). Integrons are recombination and expression mechanisms that can grab exogenous gene cassettes and recombine them (9). Furthermore, integrons may play an important role in the pathogenicity of microorganisms, as well as enable the transfer of virulence factors among different bacteria. According to the reports available, integrons have a wide distribution among clinically isolated bacteria; also, their mobility has become a major problem in antibiotic resistance in clinical specimens (2). Class I, II, and III integrons represent the most predominate classes that confer multiple resistance in Gram-negative bacteria including Enterobacteriaceae (12). Controlling the nosocomial infections in health care institutes depends mainly on using different biocides either disinfectants for non-living surfaces or antiseptics for living surfaces (11). Glutaraldehyde is a disinfectant and a sterilant aldehyde. The bactericidal action of glutaraldehyde might be attributed to the denaturation of proteins and nucleic acids through alkylation; the reaction is irreversible at the level of nucleic acids (13). Chloroxylenol (Dettol) is a potent chlorinated phenolic disinfectant, antiseptic and bactericide. Chloroxylenol acts as a bactericide by distrusting the cell membrane of bacteria (14). Povidone-iodine is a pharmaceutical preparation made up of elemental iodine, hydrogen iodide, and povidone (15). It kills bacteria by releasing iodine, which causes lipids to be iodinated and cytoplasmic and membrane components to be oxidized (15). The extensive misuse of biocides in hospitals led to the emergence of resistance among many bacteria, especially those that have the ability to acquire resistance (6).
The current study aimed at determining the resistance profile of K. pneumoniae isolated from neonates in Mansoura University Children’s Hospital against both antimicrobial agents and biocides. Furthermore, to layout the possible correlation between the presence of integrons among MDR K. pneumoniae, being resistant to three or more classes of antimicrobial agents and the reduced susceptibility of these isolates to different clinically used antimicrobial agents.
MATERIALS AND METHODS
Bacterial isolates.
Eighty-six K. pneumoniae isolates were collected from neonatal sepsis through blood samples from Mansoura University Children’s Hospital, Egypt. The isolates were identified through culturing on nutrient agar media and MacConkey’s agar (Oxoid, UK.). The characteristic formed colonies of K. pneumoniae were subjected to typical biochemical tests including TSI (Triple Sugar Iron Agar), indole, methyl Red (MR), Voges–Proskauer (VP), citrate (C), and motility test.
Antibiotic susceptibility testing.
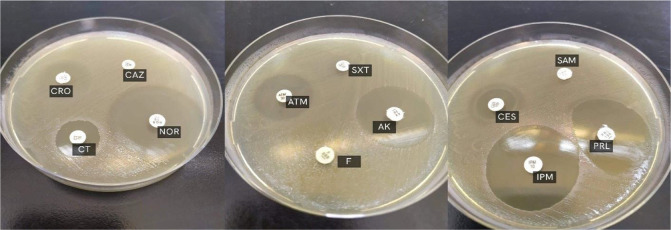
The antibiogram of K. pneumoniae isolates was determined using different clinically available antimicrobial agents according to the Modified Kirby-Bauer method (16). The antibacterial agents used were; cefoperazone/sulbactam (CES, 75/30 μg), ceftazidime (CAZ, 30 μg), cefepime (FEP, 30 μg), imipenem (IPM, 10 μg), colistin (CT, 10 μg), ofloxacin (OFX, 5 μg), gatifloxacin (GAT, 5 μg), norfloxacin (NOR, 10 μg), amikacin (AK, 30 μg), sulfamethoxazole/trimethoprim (SXT, 1.25/23.75 μg), aztreonam (ATM, 30 μg), ampicillin/sulbactam (SAM, 20 μg), piperacillin (PRL, 100μg), ceftriaxone (CRO, 30 μg), and nitrofurantoin (F, 300 μg). All of the used antimicrobial disks were purchased from Oxoid (Hampshire, England). As suggested by CLSI, the inoculum was optimized to 0.5 McFarland turbidity standard and inoculated on Mueller Hinton Agar (Oxoid, Hampshire, England). After adding the antimicrobial discs, the plates were incubated at 37°C for 18 hours. The inhibitory zones were measured, and the results were interpreted using CLSI criteria (2019) (17).
Biocide susceptibility test.
Three different biocides were tested to determine the minimum inhibitory concentrations (MIC) by the agar dilution method (18). The following biocides were tested: glutaraldehyde (El-Nasr Pharmaceutical Company, Cairo, Egypt), betadine (Iodine-povidone complex 10%) (El-Nile Pharmaceutical Company, Cairo, Egypt), and Dettol (4.8% chloroxylenol) (Royal cosmetic Co., Egypt), starting with the concentrations previously recommended. All biocides were added to the sterilized Mueller–Hinton agar medium at a temperature between 50–55°C (11) to obtain concentration ranges: 500–4000 mg L−1 (5–40 mmol) for glutaraldehyde, 100–800 mg L−1 (0.35–2.8 mmol) for betadine, and 10–80 mg L−1 (0.02–0.16 mmol) for Dettol.
Colony PCR for detection of class I, II, and III integrons.
DNA was extracted by colony PCR method for each MDR K. pneumoniae isolate (19). A pure colony of K. pneumoniae strains was suspended in 200 μL of sterile deionized water and incubated at 100°C for 10 min. After centrifugation at 12,000 rpm for 2 min, the supernatant was used as a template DNA and stored at −20°C until use.
Each PCR reaction consisted of 12.5 μL of MyTaq Red Mix (Bioline Co., UK), 1 μL of each primer (10 μM each, see Table 1), 2.5 μL DNA template, and nuclease-free water to the final volume (25 μL). PCR reaction was performed under the following thermal cycling conditions, denaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing temperature as indicated in Table 1 for 30 s, extension at 72°C for 1 min and final extension at 72°C for 7 min.
Table 1.
Primers used for integrons detections.
| Target Sequence | Primer | Sequence 5′-3′ | Annealing Temp. | Product size | Ref. |
|---|---|---|---|---|---|
| Integron I | hep58F-18 | TCATGGCTTGTTATGACTGT | 55°C | Variable | (20) |
| Hep59R-18 | GTAGGGCTTATTATGCACGC | ||||
| Integron II | intI2L F-18 | CACGGATATGCGACAAAAAGGT | 57°C | Variable | (21) |
| intI2RR-18 | GTAGCAAACGAGTGACGAAATG | ||||
| Integron III | intI3L F-18 | GCCTCCGGCAGCGACTTTCAG | 58°C | Variable | |
| intI3RR-18 | ACGGATCTGCCAAACCTGACT |
Sequencing of Integron-I positive isolates.
Six integron I positive amplicons representing various MDR K. pneumoniae isolates were chosen, purified, and sequenced to evaluate the relationship between integron I prevalence and antibiotic/biocide resistance. QIAquick PCR Purification (Qiagen, Hilden, Germany) was used to clean up PCR amplicons according to the manufacturer's instructions. The concentration and purity of eluted DNA was determined using NanoDropTM 2000/2000c Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Purified PCR amplicons were sent for sequencing from both directions using the forward and reverse primers intended to amplify integron I (Colors Medical Labs, Cairo, Egypt). Sequenced products were analyzed using the BLAST database (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistical analysis.
The susceptibility pattern of tested K. pneumoniae isolates against different antimicrobials agents in association to presence or absence of Integron I and Integron III were analyzed using Fisher's exact test. Statistical analysis was carried out using GraphPad Prism (version 9.3.1). Significance was accepted at (P < 0.05).
RESULTS
Identification of K. pneumoniae isolates.
K. pneumoniae isolates were confirmed among 86 isolates out of 257 clinical specimens. They were identified through the characteristic morphology of pink mucoid colonies on MacConkey agar. All K. pneumoniae isolates were confirmed as, lactose fermenter, A/A with TSI, indole negative, MR negative, VP positive, citrate positive, and motility negative Gram-negative bacilli.
Antimicrobial susceptibility pattern of K. pneumoniae.
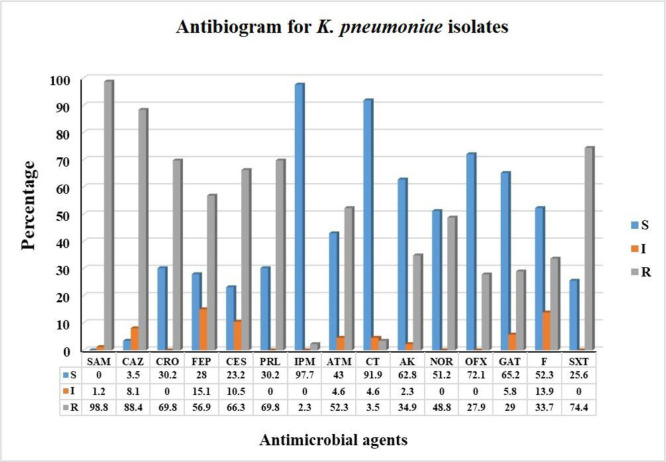
Susceptibility patterns to the tested antimicrobial agents are shown in Figs. 1 and 2. The antimicrobial susceptibility data revealed that 57 (66.27%) isolates were MDR. High resistance percentage was detected among cephalosporins with resistance rates of 56.9%, 66.3%, 69.8%, and 88.4% for cefepime, cefoperazone/sulbactam, ceftriaxone, and ceftazidime, respectively. The highest resistance was detected for ampicillin/sulbactam (98.8%). Moderate resistance was detected for antimicrobial agents of the fluoroquinolones and aminoglycosides with resistance rates of 27.9%, 29%, 34.9%, and 48.8% for ofloxacin, gatifloxacin, amikacin, and norfloxacin, respectively. The lowest resistance was detected for colistin and imipenem with resistance prevalence of 2.3 and 3.5%, respectively.
Fig. 1.
The antimicrobial susceptibility of clinical K. pneumoniae isolates to different antibiotics by disk diffusion method.
Fig. 2.
Antibiogram pattern of K. pneumoniae isolates against antimicrobial agents.
* R: resistant – I: Intermediate resistant – S: sensitive.
Biocide sensitivity.
The MICs of three different biocides were determined for all K. pneumoniae isolates. In case of MDR isolates, 0.8 mg/ml of glutaraldehyde was inhibitory to all MDR isolates (the MIC50 was 0.55). For betadine, 4 mg / ml inhibited the growth of all MDR isolates (MIC50 was 1.4), while in case of Dettol, 1.6 mg / ml inhibited the growth of all MDR isolates (MIC50 was 1.3).
Integron prevalence.
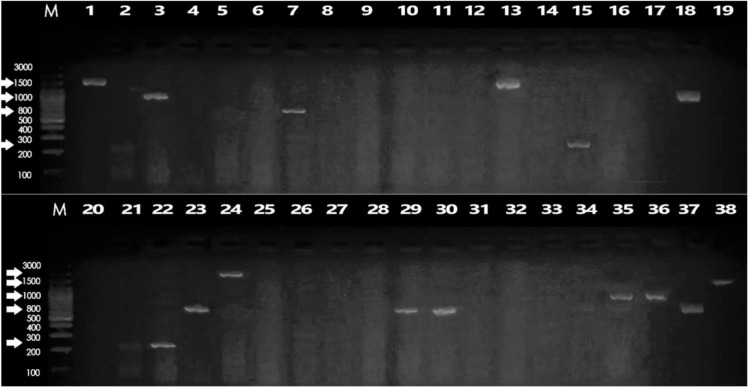
PCR amplification of the integron I variable region revealed that only 23 (40.3%) of the MDR isolates were integron I positive. For integron II, no MDR isolates were integron II positive. In case of integron III, only 20 of the MDR isolates (35%) were integron III positive as shown in Fig. 3.
Fig. 3.
Electrophoretic graph of conventional PCR products on 1.5% agarose gel stained with ethidium bromide of some representative K. pneumoniae isolates for the detection of integron I. Lanes M: represent 100 bp DNA ladder, Lane 18: positive control (reagent control mixture with DNA of the standard strain), Lane 19: negative control (reagent control mixture without DNA), Lanes 1–17 and 20–38: clinical K. pneumoniae isolates.
The presence of different classes of integrons, resistance genes, as well as reduced susceptibility to biocides and antibiotic resistance profiles are shown in Table 2. The data showed that almost all of the integron I-positive isolates had reduced susceptibility to the tested biocides.
Table 2.
Incidence of integrons, resistance genes, and reduced susceptibility to biocides in MDR K. pneumoniae isolates
| Isolate No. | Integron type (bp) | Disinfectant resistance profile | Antibiotic resistance profile |
|---|---|---|---|
| 1 | I (1500) | Dettol, GLU | PRL, SAM, CAZ, CRO, FEP, CES, IPM, ATM, NOR, AK, SXT, F |
| 2 | III (300) | Dettol, GLU | SAM, CAZ, FEP, NOR, OFX, GAT, AK, SXT, F |
| 5 | III (300) | Dettol, GLU | PRL, SAM, CAZ, FEP, CES, OFX, GAT, SXT |
| 6 | -- | Dettol, GLU | PRL, SAM, CAZ, CRO, CES, ATM, NOR, AK, SXT, F |
| 7 | I (800) | PVP-I, Dettol | PRL, SAM, CAZ, CRO, FEP, CES, ATM, NOR, OFX, GAT, SXT |
| 10 | -- | GLU | PRL, SAM, CAZ, CRO, FEP, CES, ATM, AK, SXT |
| 13 | I (1500) & III (300) | Dettol | PRL, SAM, CAZ, CRO, FEP, CES, ATM, AK, NOR, SXT, F |
| 14 | -- | PVP-I, Dettol, GLU | PRL, SAM, CAZ, CRO, FEP, CES, ATM, NOR, OFX, GAT, SXT, F |
| 15 | I (250) & III (400) | Dettol | SAM, CAZ, CRO, CES, SXT, F |
| 16 | -- | Dettol | PRL, SAM, CAZ, CRO, CES, ATM, NOR, SXT, F |
| 18 | I (1000) | -- | PRL, SAM, CAZ, CRO, FEP, CES, ATM, NOR, OFX, GAT, AK, SXT, F |
| 19 | -- | Dettol, GLU | SAM, CAZ, CRO, FEP, CES, NOR, AK, SXT, F |
| 20 | -- | PVP-I, Dettol, GLU PRL, | SAM, CAZ, CRO, FEP, CES, ATM, CT, NOR, OFX, GAT, AK, SXT, F |
| 21 | -- | -- | PRL, SAM, CAZ, CRO, FEP, CES, ATM, AK, SXT |
| 22 | I (250) & III (500) | Dettol | PRL, SAM, CAZ, CRO, FEP, CES, ATM, NOR, OFX, GAT, AK, SXT, F |
| 23 | I (800) | -- | PRL, SAM, CAZ, CRO, FEP, CES, ATM, CT, SXT |
| 24 | I (2000) & III (400) | PVP-I, Dettol, GLU | PRL, SAM, CAZ, CRO, FEP, CES, ATM, CT, NOR, AK, SXT, F |
| 25 | III (300) | PVP-I, GLU | PRL, SAM, CAZ, FEP, CES, NOR, F |
| 26 | -- | GLU | PRL, SAM, CAZ, CRO, FEP, CES, ATM, CT, SXT, F |
| 28 | -- | -- | PRL, SAM, CAZ, CRO, FEP, CES, ATM, NOR, OFX, GAT, AK, SXT, F |
| 29 | I (800) | -- | PRL, SAM, CAZ, CRO, CES, ATM, NOR, SXT |
| 30 | I (800) & III (150) | Dettol | PRL, SAM, CAZ, CRO, FEP, CES, ATM, CT, NOR, OFX, GAT, SXT, F |
| 31 | -- | Dettol | PRL, SAM, CAZ, CRO, FEP, CES, ATM, CT, NOR, OFX, GAT, SXT, F |
| 32 | -- | -- | PRL, SAM, CAZ, CRO, CES, ATM, NOR, AK, SXT |
| 33 | -- | Dettol | SAM, CAZ, NOR, AK, SXT, F |
| 34 | -- | Dettol | PRL, SAM, CAZ, CRO, FEP, CES, ATM, NOR, OFX, GAT, AK, SXT, F |
| 35 | I (1000) & III (150) | PVP-I, Dettol, GLU PRL, | SAM, CAZ, CRO, FEP, CES, ATM, CT, NOR, OFX, GAT, AK, SXT, F |
| 37 | I (800) & III (300) | PVP-I, Dettol, GLU | PRL, SAM, CAZ, FEP, CES, OFX, GAT, SXT |
| 38 | I (1500) | Dettol, GLU | PRL, SAM, CAZ, CRO, CES, ATM, NOR, AK, SXT, F |
| 39 | I (800) | PVP-I | PRL, SAM, CAZ, CRO, FEP, CES, ATM, NOR, OFX, GAT, SXT, F |
| 41 | -- | PVP-I | PRL, SAM, CAZ, CRO, FEP, CES, ATM, AK, SXT |
| 42 | III (300) | Dettol, GLU | PRL, SAM, CAZ, FEP, CES, OFX, GAT, SXT |
| 44 | I (250) & III (400) | PVP-I | SAM, CAZ, CRO, CES, SXT, F |
| 45 | -- | Dettol | PRL, SAM, CAZ, CRO, CES, ATM, NOR, SXT, F |
| 46 | -- | PVP-I | PRL, SAM, CAZ, CRO, FEP, CES, ATM, AK, SXT |
| 48 | I (1500) & III (300) | -- | PRL, SAM, CAZ, CRO, FEP, CES, ATM, NOR, SXT, F |
| 50 | -- | Dettol | PRL, SAM, CAZ, CRO, CES, ATM, NOR, AK, SXT |
| 51 | -- | Dettol | SAM, CAZ, NOR, AK, SXT, F |
| 54 | I (1500) & III (300) | -- | PRL, SAM, CAZ, CRO, FEP, CES, ATM, NOR, SXT, F |
| 55 | -- | PVP-I, GLU | PRL, SAM, CAZ, CRO, FEP, CES, ATM, NOR, OFX, GAT, AK, SXT, F |
| 56 | I (800) | -- | PRL, SAM, CAZ, CRO, CES, ATM, NOR, SXT |
| 60 | III (300) | -- | PRL, SAM, CAZ, FEP, CES, NOR, F |
| 62 | I (250) & III (400) | Dettol | SAM, CAZ, CRO, CES, SXT, F |
| 64 | -- | -- | PRL, SAM, CAZ, CRO, CES, ATM, NOR, SXT |
| 66 | I (1500) | Dettol, GLU | PRL, SAM, CAZ, CRO, FEP, CES, IPM, ATM, NOR, AK, SXT, F |
| 67 | III (300) | Dettol, GLU | SAM, CAZ, FEP, NOR, OFX, GAT, AK, SXT, F |
| 68 | -- | Dettol | PRL, SAM, CAZ, CRO, FEP, CES, ATM, NOR, OFX, GAT, AK, SXT, F |
| 69 | I (250) & III (400) | PVP-I, Dettol | SAM, CAZ, CRO, CES, SXT, F |
| 71 | -- | Dettol | SAM, CAZ, NOR, AK, SXT, F |
| 74 | I (800) | -- | PRL, SAM, CAZ, CRO, CES, ATM, NOR, SXT |
| 75 | -- | -- | PRL, SAM, CAZ, CRO, FEP, CES, ATM, AK, SXT |
| 76 | III (300) | GLU | PRL, SAM, CAZ, FEP, CES, NOR, F |
| 78 | -- | PVP-I GLU | PRL, SAM, CAZ, CRO, FEP, CES, ATM, NOR, OFX, GAT, AK, SXT, F |
| 79 | -- | -- | PRL, SAM, CAZ, CRO, FEP, CES, ATM, AK, SXT |
| 81 | I (250) & III (500) | Dettol | PRL, SAM, CAZ, CRO, FEP, CES, ATM, NOR, OFX, GAT, AK, SXT, F |
| 82 | -- | Dettol | SAM, CAZ, NOR, AK, SXT, F |
| 85 | -- | Dettol, GLU | SAM, CAZ, CRO, FEP, CES, NOR, AK, SXT, F |
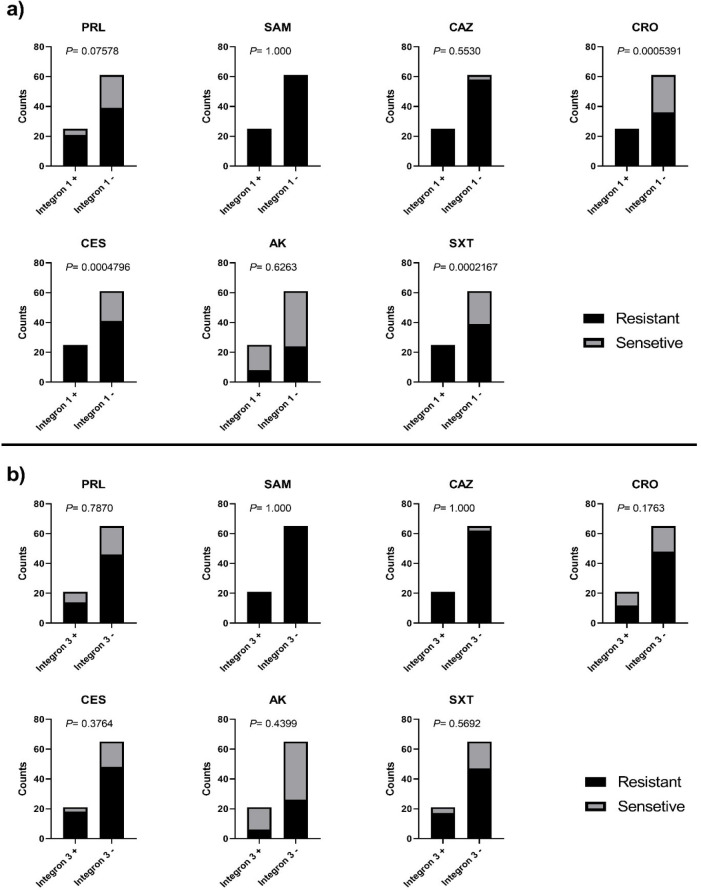
The correlation between the presence or absence of integron I/integron III and the susceptibility of the tested K. pneumoniae isolates to different antimicrobial agents was determined. Except for CRO, CES and SXT and Integron I, our results showed that no correlation was found between the presence of either integron I or integron III and the increase in resistance against most of the tested antimicrobial agents as shown in Table 3 and Fig. 4.
Table 3.
Incidence of integrons and resistance percentage of K. pneumoniae isolates against tested antimicrobial agents.
| Intergron I | ||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||
| + | - | |||||||||||||||||||||||||||||||||||||
| PRL | SAM | CAZ | CRO | CES | AK | SXT | PRL | SAM | CAZ | CRO | CES | AK | SXT | |||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||||
| R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | |||||||||||
| Number | 21 | 4 | 25 | - | 25 | 24 | 1 | 25 | - | 8 | 17 | 25 | - | 39 | 22 | 61 | 58 | 3 | 36 | 25 | 41 | 20 | 24 | 37 | 39 | 22 | ||||||||||||
| % | 84 | 16 | 100 | - | 100 | - | 96 | 4 | 100 | - | 32 | 68 | 100 | - | 64 | 36 | 100 | - | 95 | 5 | 59 | 41 | 67 | 33 | 39 | 61 | 64 | 36 | ||||||||||
|
| ||||||||||||||||||||||||||||||||||||||
| Intergron III | ||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||
| + | - | |||||||||||||||||||||||||||||||||||||
| PRL | SAM | CAZ | CRO | CES | AK | SXT | PRL | SAM | CAZ | CRO | CES | AK | SXT | |||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||||
| R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | |||||||||||
| Number | 14 | 7 | 21 | - | 21 | - | 12 | 9 | 18 | 3 | 6 | 15 | 17 | 4 | 46 | 19 | 65 | - | 62 | 3 | 48 | 17 | 48 | 17 | 26 | 39 | 47 | 18 | ||||||||||
| % | 67 | 33 | 100 | - | 100 | - | 57 | 43 | 86 | 14 | 29 | 71 | 81 | 19 | 71 | 29 | 100 | - | 95 | 5 | 74 | 26 | 74 | 26 | 40 | 60 | 72 | 28 | ||||||||||
R: mean resistant, S: mean sensitive, +: mean present, −: mean absent
Fig. 4.
Correlation between the susceptibility of the tested K. pneumoniae isolates to different antimicrobials agents and the presence or absence of: (a) integron I and (b) integron III
Integron sequence analysis.
The variable and three conserved regions of the integrase gene were sequenced following PCR amplification of integron I. Multiple sequence alignment revealed that the 1.5 kb integron I have sequence similarity to aadA gene variants (aadA1) that encodes resistance genes against streptomycin and other aminoglycosides members (Table 4). The sequence alignment of the 2 kb integron I (isolate 24) showed the presence of N-acetyltransferase AAC (6')-Il that is associated with aminoglycoside resistance. Moreover, this integron was found to harbor an additional gene of OXA β-lactamases that confers resistance to different types of penicillins. On the other hand, sequence alignment for integron I of 1 kb or smaller was found to encode resistance genes for trimethoprim as shown in Table 4.
Table 4.
Sequencing analysis for the selected K. pneumoniae isolates
| Isolates No | Sequence ID | CDS | Function |
|---|---|---|---|
| 1 | AM937245.2 | streptomycin / spectinomycin adenyltransferase | streptomycin / spectinomycin resistance |
| CP048298.1 | ANT(3'')-Ia family aminoglycoside nucleotidyltransferase AadA1 | aminoglycoside resistance protein; | |
| 3 | FJ001873.1 | dihydrofolate reductase type VII | confers resistance to trimethoprim |
| MK816931.1 | dihydrofolate reductase DfrA7 | resistance to trimethoprim | |
| 7 | HQ880274.1 | dihydrofolate reductase | confers resistance to trimethoprim |
| EU523055.1 | dihydrofolate reductase | confers resistance to trimethoprim | |
| 13 | CP049307.1 | ANT(3'')-Ia family aminoglycoside | |
| nucleotidyltransferase AadA1 | aminoglycoside resistance protein | ||
| CP038443.1 | ANT(3'')-Ia family aminoglycoside | aminoglycoside resistance | |
| nucleotidyltransferase AadA1 | |||
| 15 | MN543585.1 | Aminoglycoside 3''-nucleotidyltransferase | aminoglycoside resistance |
| 24 | CP034436.1 | aminoglycoside N-acetyltransferase AAC(6')-Il | aminoglycoside resistance |
| CP058133.1 | oxacillin-hydrolyzing class D beta-lactamase OXA-10 | Penicillin-binding protein transpeptidase domain |
DISCUSSION
Klebsiella pneumoniae is a key pathogen implicated in community-acquired and nosocomial neonatal infections, with case fatality rates ranging from 18 to 68% (22, 23). In the recent years, the importance of the K. pneumoniae has grown due to the severe consequences of having limited antibiotic options, increased hospital costs, and poor neonatal outcomes (24).
Among the 86 K. pneumoniae isolates from neonates in this study, 66.27% were MDR. This detected resistance rate is higher than that reported before by Albasha et al. in Sudan (25), where the prevalence of MDR in K. pneumoniae isolates was only 35%. Thankfully, our clinical isolates are still highly sensitive to imipenem (97.7%) and colistin (91.9%) as reported previously by Albasha et al. in Sudan (25), and Jahanbin et al. in Iran (26). We found that the prevalence of resistance to β-lactams was the highest among the tested antibacterial agents, where resistance rates to ampicillin/sulbactam, ceftazidime, and cefoperazone/sulbactam were 98.8%, 88.4%, and 66.3%, respectively. These high β-lactam resistance rates were reported also in previous studies in Iran by Firoozeh et al. (2), and in Egypt by Hassuna et al. (27), which necessitates the future use of other alternatives. In the current study, resistance rates to ceftazidime, nitrofurantoin (the most important antimicrobial agents against K. pneumoniae), piperacillin (the most effective against Enterobacteriaceae and Pseudomonas aeruginosa) were 88.4%, 33.7%, and 69.8%, respectively. Similarly, resistance rates reported in previous studies in Sudan by Albasha et al. (25) and in Iran by Jahanbin et al. (26), and Heidary et al. (28), were 70%, 22.4%, and 62%, respectively. On the other hand, we found that the susceptibility rate of K. pneumoniae isolates toward the folate synthesis inhibitor Sulfamethoxazole/trimethoprim was less than that reported by Firoozeh et al. (2). Moreover, our study found a high resistance to aztreonam, which is in line with another study carried out by Heidary et al. (28) in Iran, who revealed a resistance rate of 64% among K. pneumoniae isolates. Similar to another study in Iran (28), we found that amikacin resistance rate of K. pneumoniae isolates was 34.9%.
Unfortunately, uncontrolled use of these antibacterial agents among people worldwide is highly common, which enhances the selective pressure on bacteria that leads to emergence of resistance. Our data revealed that imipenem, followed by colistin, and then ofloxacin were the best therapeutic options for treating K. pneumoniae infections.
Integrons have been found to be a major source of resistance genes and are thought to act as antimicrobial resistance gene reservoirs in microbial communities including K. pneumoniae (29) that can be distributed among bacteria through horizontal gene transfer. The spread of integron-positive isolates in hospitals led to the spread of multi-drug resistant isolates (2). The current study reported the presence of class I integron in 40.3% of the K. pneumoniae isolates under investigation, which is relatively higher than that detected in previous studies carried out where the prevalence of integron I was 25.8% and 12.2% (20, 30). On the other hand, another study carried out in China by Xu et al. (31) showed a relatively higher percentage of class I integron (60.1%) among K. pneumoniae isolated from Chinese tertiary hospitals.
Integron III was found only in 20 of the MDR isolates (35%). This is higher than a previous study carried out in the Netherlands, which discovered that only 10.97% of the tested isolates had integron III (32). On the other hand, studies conducted in Iran by Firoozeh et al. (2) and Jahanbin et al. (26), reported that none of the studied MDR K. pneumoniae isolates had class III integrons. This observed increased prevalence of integron III among our MDR K. pneumoniae isolates represents an additional player that might contribute significantly in the spread of bacterial resistance.
Our study reports the absence of class II integron among the tested K. pneumoniae isolates which was similar to findings obtained by Xu et al. in China (31), Zeighami et al. (33), and Mobarak-Qamsari in Iran (34).
Sequencing of class I integron revealed that aminoglycosides and folate synthesis inhibitors gene cassettes were predominant. Likewise, Liao et al. (35) and Xu et al. (31) reported the abundance of trimethoprim (dfr) and aminoglycosides (aac, aad) in class I integrons. On the other hand, other resistance genes may be found, however, at lower abundance. We detected the presence of oxacillin-hydrolyzing class D beta-lactamase OXA-10 in only one of the sequenced integrons. Poirel et al. showed that the acquired class D β-lactamase genes are mostly linked to class I integron or to insertion sequences (36). Correlation studies for the association between the presence of integrons and the observed antimicrobial resistance revealed that resistance to CRO, CEF and SXT, where the only statistically associated with integron I presence. Therefore, integron I in MDR K. pneumoniae seems to contribute only to limited antibacterial and biocide resistance.
On the other hand, our observations from susceptibility of integron positive and integron negative isolates of K. pneumoniae to different biocides and the absence of detected genes associated with biocide resistance in the sequenced integrons from isolates resistant to different biocides, suggest that there is no current linkage between the presence of integrons and resistance to the tested biocides. Moreover, our results indicate that there is no possible linkage between antibacterial resistance and biocide resistance mediated via integrons as this linkage has been a point of concern for several decades (37).
CONCLUSION
The presence of integron I in MDR K. pneumoniae isolates from neonatal sepsis may contribute only to limited antibacterial and biocide resistance, however, it does not seem to be the major contributor. Thankfully, imipenem, colistin and ofloxacin are still valid options for treatment, however, to preserve their activity implementation of antimicrobial stewardship and antibiotic policies becomes a must.
ACKNOWLEDGEMENTS
A particular appreciation to Dr. Amir Mohamed Abdelhamid, Department of Pharmacology, Faculty of Pharmacy, Delta University for Science and Technology, Gamasa, Egypt, for his cooperation with the statistical analysis for this work.
REFERENCES
- 1.Saleem AF, Qamar FN, Shahzad H, Qadir M, Zaidi AK. Trends in antibiotic susceptibility and incidence of late-onset Klebsiella pneumoniae neonatal sepsis over a six-year period in a neonatal intensive care unit in karachi, pakistan. Int J Infect Dis 2013; 17(11): e961–5. [DOI] [PubMed] [Google Scholar]
- 2.Firoozeh F, Mahluji Z, Khorshidi A, Zibaei M. Molecular characterization of class 1, 2 and 3 integrons in clinical multi-drug resistant Klebsiella pneumoniae isolates. Antimicrob Resist Infect Control 2019; 8: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team . 4 million neonatal deaths: When? Where? Why? Lancet 2005; 365: 891–900. [DOI] [PubMed] [Google Scholar]
- 4.Sands K, Carvalho MJ, Portal E, Thomson K, Dyer C, Akpulu C, et al. Characterization of antimicrobial-resistant gram-negative bacteria that cause neonatal sepsis in seven low-and middle-income countries. Nat Microbiol 2021; 6: 512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajul SV, Mohite ST, Mangalgi SS, Wavare SM, Kakade SV. Klebsiella pneumoniae in septicemic neonates with special reference to extended spectrum β-lactamase, ampc, metallo β-lactamase production and multiple drug resistance in tertiary care hospital. J Lab Physicians 2015; 7: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijayakumar R, Sandle T, Al-Aboody MS, AlFonaisan MK, Alturaiki W, Mickymaray S., et al. Distribution of biocide resistant genes and biocides susceptibility in multidrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii—a first report from the kingdom of saudi arabia. J Infect Public Health 2018; 11: 812–816. [DOI] [PubMed] [Google Scholar]
- 7.Odumosu BT, Adeniyi BA, Chandra R. Analysis of integrons and associated gene cassettes in clinical isolates of multidrug resistant Pseudomonas aeruginosa from southwest nigeria. Ann Clin Microbiol Antimicrob 2013; 12: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18: 268–281. [DOI] [PubMed] [Google Scholar]
- 9.Mazel D. Integrons: Agents of bacterial evolution. Nat Rev Microbiol 2006; 4: 608–620. [DOI] [PubMed] [Google Scholar]
- 10.Roy Chowdhury P, Ingold A, Vanegas N, Martínez E, Merlino J, Merkier AK, et al. Dissemination of multiple drug resistance genes by class 1 integrons in Klebsiella pneumoniae isolates from four countries: A comparative study. Antimicrob Agents Chemother 2011; 55: 3140–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadry AA, Serry FM, El-Ganiny AM, El-Baz AM. Integron occurrence is linked to reduced biocide susceptibility in multidrug resistant Pseudomonas aeruginosa. Br J Biomed Sci 2017; 74: 78–84. [DOI] [PubMed] [Google Scholar]
- 12.Malek MM, Amer FA, Allam AA, El-Sokkary RH, Gheith T, Arafa M. Occurrence of classes i and ii integrons in Enterobacteriaceae collected from zagazig university hospitals, egypt. Front Microbiol 2015; 6: 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sehmi SK, Allan E, MacRobert AJ, Parkin I. The bactericidal activity of glutaraldehyde-impregnated polyurethane. Microbiologyopen 2016; 5: 891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukabwe H, Kajabwangu R, Mugisha D, Mayengo H, Munyanderu B, Baluku A, et al. Effectiveness of preoperative bath using chloroxylenol antiseptic soap on the incidence of post emergency cesarean section surgical site infection at Mbarara Regional Referral hospital, Uganda: a randomized controlled trial. Pan Afr Med J 2022; 41: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durani P, Leaper D. Povidone-iodine: Use in hand disinfection, skin preparation and antiseptic irrigation. Int Wound J 2008; 5: 376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao H, Liu J, Jiang X, Chen F, Lu X, Zhang J. Analysis of the clinical effect of combined drug susceptibility to guide medication for carbapenem-resistant Klebsiella pneumoniae patients based on the Kirby-Bauer disk diffusion method. Infect Drug Resist 2021; 14: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(CLSI) , Performance standards for antimicrobial susceptibility Tests. Clinical and laboratory standards institute, Wayne, PA: 2019, In 29th edition document M100. [Google Scholar]
- 18.Kawamura-Sato K, Wachino J-I, Kondo T, Ito H, Arakawa Y. Correlation between reduced susceptibility to disinfectants and multidrug resistance among clinical isolates of Acinetobacter species. J Antimicrob Chemother 2010; 65: 1975–1983. [DOI] [PubMed] [Google Scholar]
- 19.Marinho SA, Teixeira AB, Santos OS, Cazanova RF, Ferreira CAS, Cherubini K, et al. Identification of Candida spp. By phenotypic tests and PCR. Braz J Microbiol 2010; 41: 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White PA, McIver CJ, Deng Y, Rawlinson WD. Characterisation of two new gene cassettes, aada5 and dfra17. FEMS Microbiol Lett 2000; 182: 265–269. [DOI] [PubMed] [Google Scholar]
- 21.Ploy M-C, Denis F, Courvalin P, Lambert T. Molecular characterization of integrons in Acinetobacter baumannii: Description of a hybrid class 2 integron. Antimicrob Agents Chemother 2000; 44: 2684–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du Q, Pan F, Wang C, Yu F, Shi Y, Liu W, et al. Nosocomial dissemination of hypervirulent Klebsiella pneumoniae with high-risk clones among children in shanghai. Front Cell Infect Microbiol 2022; 12: 984180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Lv Y, Yang W, Zhao P, Yin C. Epidemiology and clinical characteristics of infection/colonization due to carbapenemase-producing enterobacterales in neonatal patients. BMC Microbiol 2022; 22: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballot DE, Bandini R, Nana T, Bosman N, Thomas T, Davies VA, et al. A review of -multidrug-resistant Enterobacteriaceae in a neonatal unit in johannesburg, south africa. BMC Pediatr 2019; 19: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albasha AM, Osman EH, Abd-Alhalim S, Alshaib EF, Al-Hassan L, Altayb HN. Detection of several carbapenems resistant and virulence genes in classical and hyper-virulent strains of Klebsiella pneumoniae isolated from hospitalized neonates and adults in khartoum. BMC Res Notes 2020; 13: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jahanbin F, Marashifard M, Jamshidi S, Zamanzadeh M, Dehshiri M, Malek Hosseini SAA, et al. Investigation of integron-associated resistance gene cassettes in urinary isolates of Klebsiella pneumoniae in yasuj, southwestern iran during 2015–16. Avicenna J Med Biotechnol 2020; 12: 124–131. [PMC free article] [PubMed] [Google Scholar]
- 27.Hassuna NA, AbdelAziz RA, Zakaria A, Abdelhakeem M. Extensively-drug resistant Klebsiella pneumoniae recovered from neonatal sepsis cases from a major nicu in egypt. Front Microbiol 2020; 11: 1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heidary M, Goudarzi H, Hashemi A, Eslami G, Goudarzi M, Salimi Chirani A, et al. Prevalence of quinolone resistance genes in Klebsiella pneumoniae strains isolated from hospitalized patients during 2013–2014. Arch Pediatr Infect Dis 2016; 5(4): e38343. [Google Scholar]
- 29.Su J, Shi L, Yang L, Xiao Z, Li X, Yamasaki S. Analysis of integrons in clinical isolates of Escherichia coli in china during the last six years. FEMS Microbiol Lett 2006; 254: 75–80. [DOI] [PubMed] [Google Scholar]
- 30.Derakhshan S, Najar Peerayeh S, Fallah F, Bakhshi B, Rahbar M, Ashrafi A. Detection of class 1, 2, and 3 integrons among Klebsiella pneumoniae isolated from children in tehran hospitals. Arch Pediatr Infect Dis 2014; 2: 164–168. [Google Scholar]
- 31.Xu X, Li X, Luo M, Liu P, Su K, Qing Y, et al. Molecular characterisations of integrons in clinical isolates of Klebsiella pneumoniae in a chinese tertiary hospital. Microb Pathog 2017; 104: 164–170. [DOI] [PubMed] [Google Scholar]
- 32.Fluit AC, Schmitz F-J. Resistance integrons and super-integrons. Clin Microbiol Infect 2004; 10: 272–288. [DOI] [PubMed] [Google Scholar]
- 33.Zeighami H, Haghi F, Hajiahmadi F. Molecular characterization of integrons in clinical isolates of beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in iran. J Chemother 2015; 27: 145–151. [DOI] [PubMed] [Google Scholar]
- 34.Mobarak-Qamsari M, Ashayeri-Panah M, Eftekhar F, Feizabadi MM. Integron mediated multidrug resistance in extended spectrum beta-lactamase producing clinical isolates of Klebsiella pneumoniae. Braz J Microbiol 2014; 44: 849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao W, Li D, Liu F, Du F-L, Long D, Zhang W, et al. Distribution of integrons and phylogenetic groups among highly virulent serotypes of Klebsiella pneumoniae in a chinese tertiary hospital. J Glob Antimicrob Resist 2020; 21: 278–284. [DOI] [PubMed] [Google Scholar]
- 36.Poirel L, Naas T, Nordmann P. Diversity, epidemiology, and genetics of class d beta-lactamases. Antimicrob Agents Chemother 2010; 54: 24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pal C, Bengtsson-Palme J, Kristiansson E, Larsson DGJ. Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genomics 2015; 16: 964. [DOI] [PMC free article] [PubMed] [Google Scholar]