Abstract
Background
In high‐income countries, over the last three decades, the length of hospital stays for people with serious mental illness has reduced drastically although considerable variation remains. In lower‐income countries this variation may be greater. Some argue that reduction in hospital stay leads to 'revolving door admissions' and worsening mental health outcomes despite apparent cost savings, whilst others suggest longer stays may be more harmful by institutionalising people to hospital care.
Objectives
To evaluate the effect of short stay/brief admission hospital care with long stay/standard in‐patient care in people with serious mental illness.
Search methods
We searched the Cochrane Schizophrenia Group's register of trials, July 2007 and updated this search in May 2012.
Selection criteria
We included all randomised controlled trials comparing planned short/brief with long/standard hospital stays for people with serious mental illnesses.
Data collection and analysis
We extracted data independently. For dichotomous data we calculated risk ratios (RR) and their 95% confidence intervals (CI) on an intention‐to‐treat basis based using a fixed‐effect model. For continuous data, had we identified such data, we planned to calculate fixed‐effect mean differences (MD). We assessed risk of bias for included studies and rated quality of evidence using GRADE.
Main results
We included six relevant trials undertaken between 1969 and 1980. We found no significant difference in death (n = 175, 1 RCT, RR in the longer term 0.42, CI 0.10 to 1.83, very low quality evidence). In the long term, there was no difference in improvement of mental state (n = 61, 1 RCT, RR 3.39, CI 0.76 to 15.02, very low quality evidence). There was no difference in readmission to hospital (n = 651, 4 RCTs, RR by the long term 1.26, CI 1.00 to 1.57, low quality evidence). Data for leaving the study prematurely by the longer term showed no difference (n = 229, 2 RCTs, (RR 0.77, CI 0.34 to 1.77, low quality evidence). There was a significant difference favouring short stay (P = 0.01) in numbers of participants with delayed discharge from hospital exceeding the time planned in study (n = 404, 3 RCTs, RR in the longer term 0.54, CI 0.33 to 0.88, low quality evidence). There was no difference in numbers of participants lost to follow‐up (n = 404, 3 RCTs, RR by the longer term 1.07, CI 0.70 to 1.62, low quality evidence). Finally, there was a significant difference favouring short‐stay hospitalisation for social functioning, including unemployment, unable to housekeep, or unknown employment status (n = 330, 2 RCTs, RR by longer term 0.61, CI 0.50 to 0.76, very low quality evidence).
Authors' conclusions
The effects of hospital care and the length of stay is important for mental health policy. We found limited low and very low quality data which were all over 30 years old. Outcomes from these studies do suggest that a planned short‐stay policy does not encourage a 'revolving door' pattern of admission and disjointed care for people with serious mental illness. More large, well‐designed and reported trials are justified especially where a short‐stay policy is not routine care.
Keywords: Humans, Length of Stay, Hospitalization, Hospitalization/statistics & numerical data, Institutionalization, Mental Disorders, Mental Disorders/rehabilitation, Patient Readmission, Patient Readmission/statistics & numerical data, Randomized Controlled Trials as Topic
Plain language summary
Length of stay in hospital for people with severe mental illness
Since the 1960s, in North America and most of Europe, large psychiatric hospitals have been closed and small local hospital units established. Medical opinion as to whether people with mental illness should stay in hospital for months and years or just a few weeks has changed. Care in the community has been helped by the advent of medication for people with mental illness. Consequently, in the developed world, hospital stays are now relatively short and large psychiatric hospitals or asylums have almost disappeared. However, there is still some doubt about whether short admissions are good because the person does not get institutionalised, or harmful because the causes and symptoms of the illness are not completely addressed. This is further complicated because there are patients who have short but frequent admissions (‘revolving door patients’) in contrast to others who despite treatment stay in hospital for a long time (‘new long stay patients’).
The review aims to determine what length of stay in hospital is the most helpful and is now based on a 2012 search. Six randomised trials are included that compare short stay in hospital with either long stay in hospital or standard care. No differences were found between groups in readmission to hospital, mental state, leaving the study early, risk of death and people lost to follow‐up. There was a significant difference favouring short‐stay hospitalisation for social functioning. There was limited information that suggested that short‐stay hospitalisation does not encourage a ‘revolving door’ pattern of admission to hospital and disjointed or poor care.
This should reassure people with mental illness coming into hospital that a short stay (of less than 28 days) means they are no more likely to be readmitted, to leave hospital abruptly, or to lose contact with services after leaving hospital than if they received long‐stay care. Short‐stay patients are also more likely to leave hospital on their planned discharge date and possibly have a greater chance of finding employment. For psychiatrists, policy makers and health professionals it is important to know that short‐stay hospitalisation does not lead to a ‘revolving door’ pattern of admission to hospital and poor or fragmented care.
However, all evidence in this review was rated by the review authors to be low quality. More large, well‐designed and well‐reported trials are justified that focus on important outcomes such as death, self‐harm, harm to others, employment, criminal behaviour, mental state, satisfaction with treatment and services, homelessness, social or family relationships and costs.
This plain language summary has been written by a consumer Benjamin Gray, Service User and Service User Expert, Rethink Mental Illness.
Summary of findings
Summary of findings for the main comparison. SHORT versus LONG HOSPITAL STAY for people with severe mental illness.
| SHORT versus LONG HOSPITAL STAY for people with severe mental illness | ||||||
| Patient or population: patients with people with severe mental illness Settings: Intervention: SHORT versus LONG HOSPITAL STAY | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | SHORT versus LONG HOSPITAL STAY | |||||
| Death ‐ 2 years follow‐up Number of deaths Follow‐up: mean 2 years | Study population | RR 0.42 (0.1 to 1.83) | 175 (1 study) | ⊕⊝⊝⊝ very low2,3,4,5 | ||
| 63 per 10001 | 27 per 1000 (6 to 116) | |||||
| Moderate | ||||||
| 64 per 10001 | 27 per 1000 (6 to 117) | |||||
| No clinically important changes in specific symptoms ‐ positive and negative symptoms of schizophrenia (long term) Mental state: Not improved ‐ by 2 years ‐ as measured by PEF scale PEF scale Follow‐up: mean 2 years | Study population | RR 3.39 (0.76 to 15.02) | 61 (1 study) | ⊕⊝⊝⊝ very low2,3,4,5 | ||
| 67 per 10001 | 226 per 1000 (51 to 1000) | |||||
| Moderate | ||||||
| 67 per 10001 | 227 per 1000 (51 to 1000) | |||||
| Service outcomes: 1a. Readmission to hospital (homogeneous studies) long term Number of participants readmitted to hospital Follow‐up: mean 12 months | Study population6 | RR 1.26 (1 to 1.57) | 651 (4 studies) | ⊕⊕⊝⊝ low7 | ||
| 246 per 1000 | 310 per 1000 (246 to 387) | |||||
| Moderate6 | ||||||
| 264 per 1000 | 333 per 1000 (264 to 414) | |||||
| Service outcomes: 3. Leaving hospital prematurely ‐ by 2 years Participants leaving the study prematurely Follow‐up: mean 2 years | Study population6 | RR 0.77 (0.34 to 1.77) | 229 (2 studies) | ⊕⊕⊝⊝ low4,7 | ||
| 103 per 1000 | 79 per 1000 (35 to 182) | |||||
| Moderate6 | ||||||
| 104 per 1000 | 80 per 1000 (35 to 184) | |||||
| Service outcomes: 4. Discharge delayed beyond the time planned in study ‐ 2‐year data Number of participants kept beyond projected time of discharge Follow‐up: mean 2 years | Study population6 | RR 0.54 (0.33 to 0.88) | 404 (3 studies) | ⊕⊕⊝⊝ low7,8 | ||
| 178 per 1000 | 96 per 1000 (59 to 156) | |||||
| Moderate6 | ||||||
| 125 per 1000 | 68 per 1000 (41 to 110) | |||||
| Leaving the study early (long term) Participants leaving the study prematurely Follow‐up: mean 2 years | Study population6 | RR 1.07 (0.70 to 1.62) | 404 (3 studies) | ⊕⊕⊝⊝ low7,8 | ||
| 144 per 1000 | 155 per 1000 (101 to 234) | |||||
| Moderate6 | ||||||
| 81 per 1000 | 87 per 1000 (57 to 131) | |||||
| General functioning: Unemployed, unable to housekeep or unknown employment status ‐ by 2 years Number of participants with poor occupational functioning in 2 years Follow‐up: mean 2 years | Study population6 | RR 0.61 (0.5 to 0.76) | 330 (2 studies) | ⊕⊝⊝⊝ very low4,7,9 | ||
| 545 per 1000 | 333 per 1000 (273 to 415) | |||||
| Moderate6 | ||||||
| 580 per 1000 | 354 per 1000 (290 to 441) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 As there was only one study found, the control risk is calculated as the mean baseline risk. 2 Risk of bias rated serious as there was only one study. 3 Single study available so no analysis of inconsistency. 4 Imprecision rated serious as the total population size was less than 400. 5 Publication bias rated strongly suspected as there was only one study found. 6 Median control group risk calculated as there was little variation in baseline control group risk in the studies included. 7 Inconsistency rated serious as there were large differences in effect between the studies. 8 Risk of bias rated serious as one of the three studies, (Herz,1975) has a high risk of random sequence generation, allocation concealment and blinding. 9 Risk of bias rated serious as one of the 2 studies, (Herz,1975) has a high risk of random sequence generation, allocation concealment and blinding.
Background
Description of the condition
In Europe, hospital provision for mentally ill people dates back over eight hundred years. These were frequently overcrowded and included provision for the 'poor'. The distinction between 'pauper' and 'lunatic' only began to be recognised by the eighteenth century. At the turn of the nineteenth century growing public concern about mental illness led to greater provision of asylums and restrictive custodial care. This was off‐set, however, by a liberal movement called 'Moral Treatment', pioneered in France (Pinel 1806) and England (Tuke 1813). This approach favoured releasing people who were physically restrained into bigger asylums (Connolly 1856) and introducing social forms of treatment based on human respect. By the end of the nineteenth century, this policy was reversed in favour of restrictive custodial approaches due to staff shortage, over‐crowding and concern about safety. In the 1930's the pendulum swung again with the introduction of electroconvulsive therapy (ECT) and insulin coma therapy. Optimism in the effectiveness of new treatments paralleled a move to more liberal care policies. Social attitudes continued to change after World War II, particularly with the introduction of anti‐psychotic medication in 1952 (chlorpromazine and later drugs) making rehabilitation feasible even for those with serious mental illnesses. At the same time, the large asylums were criticised for being 'total institutions' (Goffman 1961), inhumane and repressive (Wing 1970).
Description of the intervention
Since the 1960s, in North America and Europe, large hospitals have been closed and small local general hospital units established. This has been a gradual process apart from in Italy where public mental hospitals were rapidly closed and admissions to asylums prohibited (Jones 1985). At the same time, many types of approaches to community care have evolved. For example, case management, a widely used community care regimen, which is now a statutory obligation in several countries. Current mental health policies and guidance in the UK encourage short hospital stay with follow‐up by community programmes such as case management and the Care Programme Approach (CPA) (DOH 1999). Community care, however, has come under criticism for its failure to provide adequate care (DoH 1994), particularly for mentally ill 'revolving door patients' (people who have repeated, frequent admissions (Glick 1975), and 'new long stay patients' (such as people with both mental illness and behaviour problems (Todd 1976)).
How the intervention might work
In many 'Western' countries, whilst bed numbers have declined, admission rates have risen (Anonymous 1996), perhaps as a direct result of community care policy (Marshall 1999). Frequent short admissions are a common pattern of hospital care for people with serious mental illness. Other countries have had quite different experiences. One of the performance indicators in the National Service Framework for Mental Health in the UK is the reduction in the psychiatric emergency readmission rate (DOH 1999).
Why it is important to do this review
Japan has increased bed provision in the last 30 years (Hafner 1987) and many 'developing' countries provide either non‐specialist 'standard' medical care or continue to use institutions unchanged for decades (Appleby 1991). Most countries, however, are under pressure to change their bed provision and the effect of hospitalisation remains important to all. A degree of hospital care is likely to remain an integral part of any mental health service. Community care policies may change the pattern of hospital care for people with severe mental illness, favouring shorter admissions. It is therefore important to determine the effects of different lengths of hospital stay. This will help inform policy‐makers in developing safe, appropriate, efficient and effective mental health services in the future.
Objectives
To evaluate the effect of 'short stay'/'brief admission' hospital care with 'long stay'/'standard' in‐patient care in people with serious mental illness.
In addition, we also aimed to test the following hypotheses via sensitivity analyses:
those given a short stay in hospital defined as less than 28 days (an arbitrary compulsory length of stay as defined by the Mental Health Act 1983 of England and Wales) have differential response to those in hospital greater than 28 days; and that:
those over 65 years old have a differential response to length of hospital stay than younger people;
findings from trials conducted in the presence of community care programmes (however defined) have a differential response to studies conducted in the absence of community care programmes.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised trials. Quasi‐randomised trials (those that have employed alternating allocation, allocation by letter of the alphabet or day of the week) were also identified but excluded from the main analysis. We used data from these in a sensitivity analysis in order to see if the exclusions were justified.
Types of participants
We included trials of people with schizophrenia, related disorders or 'severe/chronic mental disorders/illnesses', however defined.
Types of interventions
1. Planned short stay/brief admission ‐ however defined within the studies
2. Long stay or standard care ‐ however defined within the studies
A hypothesis relating to these definitions will, however, be tested (please see Objectives).
Types of outcome measures
We grouped outcomes into short term (less than three months); medium term (three to six months); long term (six months to one year); longer term (one to two years or more from admission date).
Primary outcomes
1. Global state
1.1 No clinically important change in global state (as defined by individual studies)
Secondary outcomes
1. Death (suicides and all‐causes)
2. Global state
2.1 Relapse (as defined by the individual studies)
3. Mental state
3.1 No clinically important change in general mental state score 3.2 Average endpoint general mental state score 3.3 Average change in general mental state score 3.4 No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia) 3.5 Average endpoint specific symptom score 3.6 Average change in specific symptom score
4. Leaving the studies early (any reason, adverse events, inefficacy of treatment)
5. Service outcomes
5.1 Readmission to hospital 5.2 Leaving hospital prematurely 5.3 Discharge delayed beyond the time planned 5.4 Community care
6. Behaviour
6.1 Violent incidents (self, others, property) 6.2 Social functioning
7. User satisfaction
8. Quality of life
8.1 No clinically important change in general quality of life 8.2 Average endpoint general quality of life score 8.3 Average change in general quality of life score
9. Self esteem/psychological well‐being
10. Family burden
11. General functioning
11.1 Imprisonment 11.2 Employment status 11.3 Independent living
12. Economic outcomes
12.1 Total cost of care 12.2 Total health cost
13. 'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2008) and used the GRADEPRO profiler to import data from Review Manager (RevMan) to create a 'Summary of findings' table. The Table 1 provides outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient‐care and decision making. We selected the following main outcomes for inclusion in the 'Summary of findings' table.
Death (suicides and all‐causes) ‐ long term
No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia) ‐ long term
Service outcomes: readmission to hospital ‐ long term
Leaving hospital prematurely ‐ long term
Service outcomes: discharge delayed beyond the time planned in study ‐ long term
Leaving the study early ‐ long term
General functioning: unemployed, unable to housekeep or unknown employment ‐ long term
Search methods for identification of studies
Electronic searches
1. Cochrane Schizophrenia Group Trial Register (May 2012)
We searched the Cochrane Schizophrenia Group's register (May 2012), which is based on regular searches of MEDLINE, EMBASE, CINAHL and PsycINFO. We constructed the following search phrase to assist identification using the following search strategy:
[((short* or brief* or length*) in same field as (admission* or hospital*) in REFERENCE and (*hospitali*) in intervention of STUDY]
This register is compiled by systematic searches of major databases, handsearches and conference proceedings (see Group Module; see also Appendix 1 for previous searches).
Searching other resources
1. Reference searching
We inspected references of all identified studies for further relevant studies.
2. Personal contact
We contacted the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Methods used in data collection and analysis for this update are below; for methods used in previous versions please see Appendix 2.
Selection of studies
For this update BO and VG independently inspected citations from the new electronic search and identified relevant abstracts. BO and VG also inspected full articles of the abstracts meeting inclusion criteria. SS carried out the reliability check of all citations from the new electronic search.
Data extraction and management
1. Extraction
For this update, BO and VG extracted data from included studies. We extracted data presented only in graphs and figures whenever possible. When further information was necessary, we contacted authors of studies in order to obtain missing data or for clarification. If studies were multi‐centre, where possible, we extracted data relevant to each component centre separately.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
Had we encountered any continuous data, we would have included such data from rating scales only if: a. the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); and b. the measuring instrument has not been written or modified by one of the trialists for that particular trial.
For future updates of this review where continuous data may be identified; ideally the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist).
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult to measure conditions such as schizophrenia. Had we encountered such data, we would have combined endpoint and change data in the analysis and used mean differences (MD) rather than standardised mean differences (SMD) throughout (Higgins 2011, Chapter 9.4.5.2).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we would have applied the following standards to all data before inclusion: a) standard deviations (SDs) and means are reported in the paper or obtainable from the authors; b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution (Altman 1996)); c) if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS, Kay 1986), which can have values from 30 to 210), we modified the calculation described above to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐S min), where S is the mean score and S min is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied. We would have entered skewed endpoint data from studies of fewer than 200 participants as other data within the data and analysis section tables rather than into an analysis. Skewed data pose less of a problem when looking at mean if the sample size is large; we would have entered skewed endpoint data from large trials into syntheses. We found skewed data in three included studies (Glick 1975; Herz 1975; Kennedy 1980), which were presented in 'other data' tables.
2.5 Common measure
To facilitate comparison between trials, we converted variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the PANSS (Kay 1986), this could be considered as a clinically significant response (Leucht 2005; Leucht 2005a). This method will be useful in future updates of this review, where continuous data may be identified.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for planned short‐stay/brief admission. Where keeping to this made it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'Not improved'), we reported data where the left of the line indicates an unfavourable outcome. This was noted in the relevant graphs.
Assessment of risk of bias in included studies
For this update, BO and VG worked independently using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess trial quality. This new set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain additional information.
We have noted the level of risk of bias in both the text of the review and in the Table 1.
Measures of treatment effect
1. Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). For statistically significant results, we used 'Summary of findings' tables to calculate the number needed to treat to provide benefit (NNTB) /to induce harm (NNTH) statistic and its 95% CI.
2. Continuous data
We did not identify any useable non‐skew continuous data in this review; had we identified such data, we had planned to estimate the mean difference (MD) between groups. We would prefer not to calculate effect size measures (standardised mean difference (SMD)). However, if scales of very considerable similarity had been used, we would have presumed there was a small difference in measurement, and we would have calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data poses problems. Authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
For future updates of this review where cluster‐randomised studies are identified and where clustering is not accounted for in primary studies, we will present data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC is not reported it will be assumed to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would be possible using the generic inverse variance technique. Again, no such data were identified for this current version of the review.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, had we identified such trials, we would only have used data of the first phase of cross‐over studies; this will be the case for future updated versions of the review.
3. Studies with multiple treatment groups
Where a study involves more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary we simply added these and combined within the two‐by‐two table. If data had been continuous, we would have combined data following the formula in section 7.7.3.8 (Combining groups) of the Handbook (Higgins 2011). Where the additional treatment arms were not relevant, we did not reproduce these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we did not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we marked such data with (*) to indicate that such a result may well be prone to bias; this was the case with Hirsch 1979*.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, the rate of those who stayed in the study ‐ in that particular arm of the trial ‐ was used for those who did not. We had planned to undertake a sensitivity analysis to test how prone the primary outcomes were to change when data only from people who complete the study to that point were compared to the intention‐to‐treat analysis using the above assumptions; however, no study reported outcome data for our primary outcome of clinically important change in global state.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50%, and data only from people who completed the study to that point were reported, we planned to present and use these data.
3.2 Standard deviations
If standard deviations (SDs) were not reported, we first tried to obtain the missing values from the authors. If not available, where there are missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals (CIs) available for group means, and either a P value or T value available for differences in mean, we can calculate them according to the rules described in the Handbook (Higgins 2011): When only the SE is reported, SDs are calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Handbook (Higgins 2011) present detailed formulae for estimating SDs from P values, T or F values, CIs, ranges or other statistics. If these formulae do not apply, we would calculate the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Last observation carried forward
We anticipated that in some studies the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, where LOCF data have been used in the trial, if less than 50% of the data have been assumed, we reproduced these data and indicated that they are the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise. When such situations or participant groups arose, we fully discussed these.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise. When such methodological outliers arose, we fully discussed these.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 method alongside the Chi2 P value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. P value from Chi2 test, or a confidence interval for I2). An I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic was interpreted as evidence of substantial levels of heterogeneity (Higgins 2011). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10 of the Handbook (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We planned not to use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model: it puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose the fixed‐effect model for all analyses. The reader is, however, able to choose to inspect the data using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses ‐ only primary outcomes
1.1 Clinical state, stage or problem
We proposed to undertake this review and provide an overview of the effects of length of hospitalisation for people with schizophrenia, related disorders or 'severe/chronic mental disorders/illnesses', however defined. In addition, however, we tried to report data on subgroups of people in the same clinical state, stage and with similar problems but data were not available.
2. Investigation of heterogeneity
If inconsistency was high, we have reported this. First, we investigated whether data had been entered correctly. Second, if data were correct, we visually inspected the graph and successively removed outlying studies to see if homogeneity was restored. For this review we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present data. If not, then we would not pool data and discussed issues. We know of no supporting research for this 10% cut‐off, but we used prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity is obvious, we simply stated hypotheses regarding these for future reviews or versions of this review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
We planned to apply all sensitivity analyses to the primary outcomes of this review. This included: (i) removing studies that implied randomisation from synthesis, leaving other studies with a better description of randomisation remaining; (ii) testing how prone results were to change when 'completer' data only were compared to any imputed data; (iii) analysing the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation; and (iv) synthesising data using a random‐effects model. However, no study reported outcome data for our primary outcome of clinically important change in global state.
Results
Description of studies
For detailed description, see Characteristics of included studies and Characteristics of excluded studies.
Results of the search
From the initial electronic search we identified 206 citations. Thirty‐four were ordered and assessed against the inclusion criteria. Of these, five randomised controlled trials were included in the main analysis. For the 2005 update we found 306 citations, and seven were assessed against inclusion criteria with one study included (Kennedy 1980). Kennedy 1980 included participants from unselected acute psychiatric admissions and we therefore analysed this trial in a separate sensitivity analysis. For the 2007 update we did not find any additional studies to include. For the 2012 search update, 477 reference from 256 studies were identified; four studies were obtained as full articles but excluded as they compared day hospital care with inpatient stay and/or focused on economic evaluation (see Figure 1). Therefore, the number of included studies remains six.
1.

Study flow diagram (for 2012 update search).
Included studies
All included studies, published between the 1969‐1980, were stated to be randomised controlled trials where patients were allocated 'at random'. Although the Glick 1975 study used randomisation, important differences were found between the groups on important variables (education, socio‐economic status, pre‐morbid adjustment, mean dosage of chlorpromazine equivalent) that favoured the long‐stay group. These differences may well have occurred by chance but it is difficult to assess the degree of confounding that this introduces. Kennedy 1980 allocated participants randomly, although blinding was not reported. Kennedy 1980, included participants from unselected acute psychiatric admissions and we therefore analysed this trial in a separate sensitivity analysis.One study, Burhan 1969, met the inclusion criteria but used unusual methods in both its design and conduct. The results of this study, where 100 patients from a hospital cohort of over 1000 were 'randomly selected' for a short‐stay package, added heterogeneity to the pooled results. We therefore presented the data from this trial and discussed the data separately. For both the 2007 and 2012 updates, we did not find any additional studies that we could include.
1. Length of studies
The study duration of Hirsch 1979* and Kennedy 1980 was one year. Burhan 1969, Glick 1975 and Herz 1975 evaluated participants for two years, and Glick 1976 was the longest study lasting just over two years (26 months).
2. Setting
Four trials were undertaken in the US (Burhan 1969; Glick 1975; Glick 1976; Herz 1975) and two in the UK (Hirsch 1979*, Kennedy 1980).
3. Participants
All trial participants were 'seriously mentally ill' with psychiatric disorders such as schizophrenia, affective disorders and severe personality disorders. All trials focused on adults, excluding children, adolescents, the elderly, and those with learning disabilities, organic brain disease, drug and alcohol abuse.
4. Study size
Burhan 1969 was the largest study with 1169 participants randomised and Glick 1976 was the smallest study with 74 participants. The other studies ranged in size between 141 and 247 participants.
5. Interventions
In three studies, short stay varied from one week (Herz 1975) to 21 to 28 days (Glick 1975; Glick 1976). Those allocated to short stay received other treatments such as discharge planning and crisis resolution training (Glick 1975; Glick 1976). Kennedy 1980 reported allocating participants to either short stay or control without defining the length of short stay. No specific 'community‐based' interventions were reported except for Hirsch 1979* (day care). The effects of the presence or absence of these programmes are discussed.
Two trials clearly reported the minimum and maximum duration of long stay before the trial (Glick 1975; Glick 1976). Otherwise, professional carers determined the length of stay. Two trials specified a cut‐off for long stays (at 45 days, Hirsch 1979*; 60 days, Herz 1975). No specific intervention was reported for those allocated to the long‐stay group after discharge. Antipsychotic drugs were the main treatment for participants and most trials reported similar use in both long‐ and short‐stay participants.
6. Outcomes
Data relating to readmissions (not relapse), loss to follow‐up, premature discharge, delayed discharge, and employment were possible to extract. Deaths (suicides and all‐causes) were noted in three trials but attributed to experimental or control groups in only one (Herz 1975). Parasuicide episodes and readmission to hospital due to parasuicide were reported in one study (Kennedy 1980). No data were reported on relapse, criminal behaviour or imprisonment. Trialists used many different scales but all were reported without any reference to standard deviations, and therefore could not be summated. Only one paper presented data from a continuous measure that could be extracted in dichotomous (percentage of people improved (Glick 1975).
6.1. Mental state
6.1.1 Health‐Sickness rating scale ‐ HSRS (Luborsky 1962) This is a global rating of psychiatric functioning. Ratings are on a scale of zero, (severely disabled) to 100 (very effective functioning). Glick 1975 reported data from this scale.
6.1.2 Psychiatric Evaluation Form ‐ PEF (Endicott 1972) A clinician‐rated scale used to assess psychological functioning during the week prior to interview. This consists of 24 individual and eight summary scales. Scoring on each scale ranges from one to five with higher scores indicating greater impairment. Glick 1975 reported data from this scale.
7. Awaiting assessment
No studies await assessment.
8. Ongoing studies
We are not aware of any ongoing studies.
Excluded studies
Sixteen studies were identified of which we excluded nine because they did not meet the inclusion criteria (see Characteristics of excluded studies).
Risk of bias in included studies
For a graphical overview, see Figure 2 and Figure 3.
2.
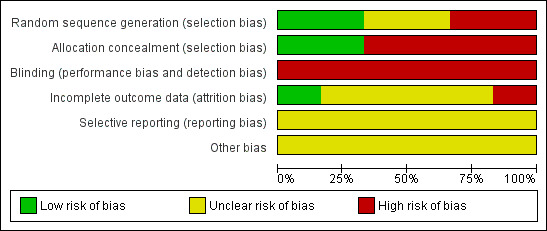
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
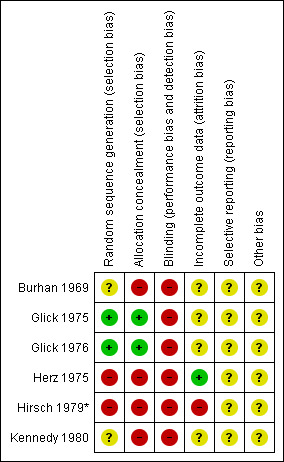
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
No trials made fully explicit the means by which randomisation took place (Higgins 2006). All studies, however, reported random allocation or, in the case of Burhan 1969, random selection from a large sample. Although this latter technique is unusual, it did not invalidate the study.
Blinding
No trial mentioned blinding of observers. Glick 1975 and Glick 1976 specifically mention that participants and investigators both "had knowledge" of the group to which they were assigned to.
Incomplete outcome data
One trial, Burhan 1969, included all randomised people, that is, undertook an intention‐to‐treat analysis. The remaining trials reported exclusions in their analyses. Glick 1975 reported 4.5% exclusion at two years, Glick 1976 11% at two years, Herz 1975 30% at two years and Hirsch 1979* 53% at one year. In this latter study, only data from two outcomes were used (readmissions and lost to follow‐up and one year) as intention‐to‐treat numbers could not be calculated. The limited reporting of randomisation, lack of blindness for these outcomes and unclear reasons for loss to follow‐up would suggest that all estimates of effect are prone to bias (Moher 1998).
Selective reporting
Review authors did not detect any selective reporting biases.
Other potential sources of bias
Review authors did not detect any other potential sources of bias.
Effects of interventions
See: Table 1
1. COMPARISON: SHORT versus LONG HOSPITAL STAY
1.1 Death
We found no significant difference in the number of reported deaths at 2 years follow‐up (3/112 deaths in the short‐stay group, and 4/63 in the long‐stay group) (n = 175, 1 RCT, risk ratio (RR) 0.42, confidence interval (CI) 0.10 to 1.83, Analysis 1.1).
1.1. Analysis.
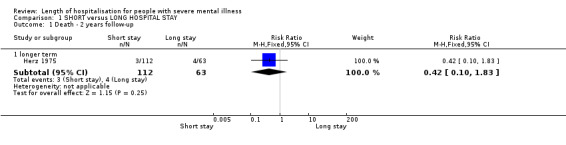
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 1 Death ‐ 2 years follow‐up.
1.2 Mental state
Only one trial (Glick 1975) reported percentages of people 'not improved'. We found no significant difference between short‐ and long‐stay groups as measured by the Psychiatric Evaluation Form (PEF) scale (n = 61, 1 RCT, RR 3.39 CI 0.76 to 15.02) or the Health‐Sickness rating scale (HSRS) (n = 61, 1 RCT, RR 0.97, CI 0.31 to 3.01, Analysis 1.2). These outcomes were presented only in the preliminary report of the study, and are a subset of a larger trial.
1.2. Analysis.
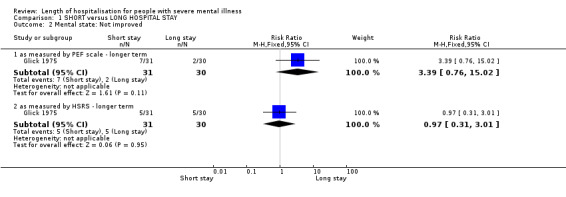
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 2 Mental state: Not improved.
1.3 Service outcomes
1.3.1 Readmissions
All trials reported readmission data. We found no significant difference between short‐ and long‐stay groups by long term (one year) (n = 651, 4 RCTs, RR 1.26 CI 1.00 to 1.57), and by longer term (two years) (n = 229, 2 RCTs, RR 1.03 CI 0.78 to 1.36, Analysis 1.3). Adding Burhan 1969, which reported very significantly fewer readmissions for those in the short‐stay group throughout the two‐year period, introduced heterogeneity (I2 = 71.7% at one year and 92.7% at 2 years). As this study had always been unusual in its methods and interventions, we removed these results from the others and presented and discuss them separately (n = 1169, RR 0.22 CI 0.07 to 0.67 at one year, and n = 1169, RR 0.21 CI 0.11 to 0.41 at two years, Analysis 1.4).
1.3. Analysis.
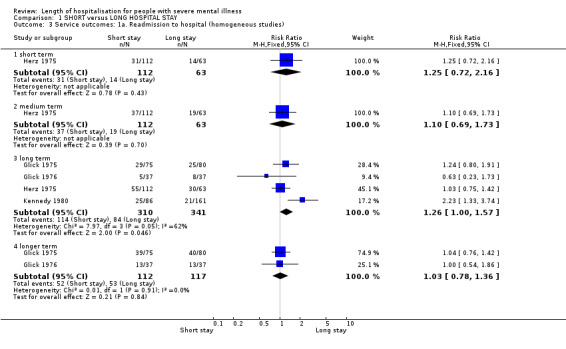
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 3 Service outcomes: 1a. Readmission to hospital (homogeneous studies).
1.4. Analysis.
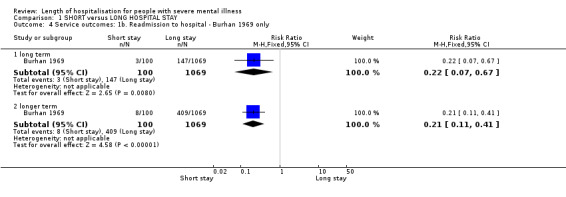
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 4 Service outcomes: 1b. Readmission to hospital ‐ Burhan 1969 only.
Adding Kennedy 1980, which is a trial that randomised all (unselected) acute psychiatric admissions, introduced heterogeneity (I2 = 62.4% at one year), and produced an opposite trend to Burhan 1969. The short‐stay group had a higher number of readmissions compared to the standard stay group. The results have not been summated; we have presented these separately (RR 2.23 CI 1.3 to 3.7 at one year). The differences were statistically significant between the two groups. Although the readmission rates in the experimental wards were twice as high as the control wards; the average duration of a readmission to the experimental wards were only a third as long as the average readmission to the control wards. We found the average duration of stay in short‐stay wards were shorter for both first readmissions and all admissions by one year.
No significant differences were found between the two groups in the number of admissions to hospital because of a parasuicidal act by one year (Kennedy 1980, n = 246, RR 1.05 CI 0.36 to 3.04, Analysis 1.5).
1.5. Analysis.
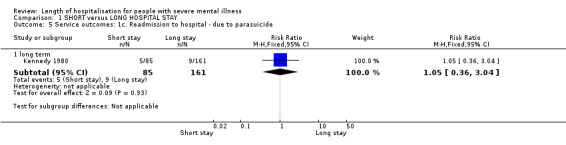
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 5 Service outcomes: 1c. Readmission to hospital ‐ due to parasuicide.
1.3.2 Length of stay
Apart from Kennedy 1980, there were no standard deviations reported for average length of stay and we were unable to summate the data. For those allocated to short stays in hospital, the average length of stay ranged from 10.8 days (Herz 1975; Kennedy 1980) to 25.0 days (Glick 1975) and the long stay averages ranged from 24 days (Kennedy 1980) to 94 days (Glick 1975). The standard deviation in Kennedy 1980 is greater than the mean so these data are skewed and presented separately (Analysis 1.6).
1.6. Analysis.
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 6 Service outcomes: 2. Average length of stay (days).
| Service outcomes: 2. Average length of stay (days) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| long term | ||||
| Kennedy 1980 | Short stay | 10.8 | 15.65 | 86 |
| Kennedy 1980 | Long stay | 24.1 | 26.09 | 161 |
| longer term | ||||
| Glick 1975 | Short stay | 25 | No SD | 75 |
| Glick 1975 | Long stay | 94 | No SD | 80 |
| Herz 1975 | Short stay | 10.8 | No SD | 112 |
| Herz 1975 | Long stay | 50 | No SD | 63 |
1.3.3 Premature discharge from hospital
Two trials reported abrupt, premature, discharge, against medical advice (Glick 1975; Glick 1976), and we found no significant difference between groups (n = 229, RR 0.77 CI 0.34 to 1.77, Analysis 1.7).
1.7. Analysis.
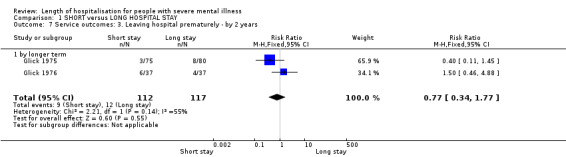
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 7 Service outcomes: 3. Leaving hospital prematurely ‐ by 2 years.
1.3.4 Delayed discharge from hospital
There were significantly fewer delayed discharges in the short‐stay group compared with those in long stay (n = 404, 3 RCTs, RR 0.54 CI 0.33 to 0.88, Analysis 1.8). Including data from quasi‐randomised trials reduced this to no effect, and introduced significant heterogeneity (Chi‐square 27.45, df 4, P < 0.001).
1.8. Analysis.
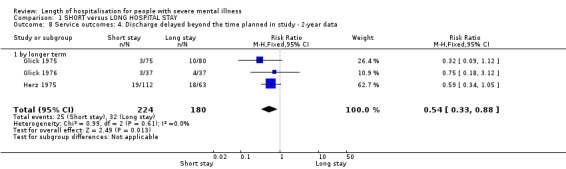
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 8 Service outcomes: 4. Discharge delayed beyond the time planned in study ‐ 2‐year data.
1.3.5 Day care
We found significantly more post‐discharge daycare given to participants in the short‐stay group than those in the standard‐stay group (Kennedy 1980, n = 247, RR 4.52 CI 2.74 to 7.45, NNTH 3 CI 2 to 6, Analysis 1.9).
1.9. Analysis.
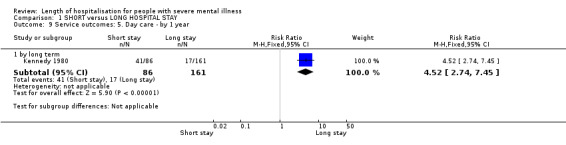
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 9 Service outcomes: 5. Day care ‐ by 1 year.
1.4 Behaviour
1.4.1 Violent acts to self (parasuicide episodes)
Parasuicide episodes were reported in the medium in one study (Kennedy 1980); however, results demonstrate no difference between groups (n = 247, 1 RCT, RR 0.17 CI 0.02 to 1.30, Analysis 1.10).
1.10. Analysis.
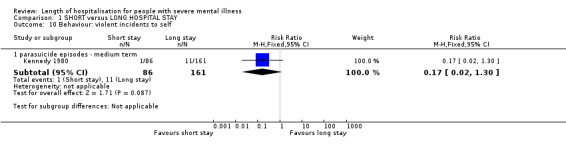
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 10 Behaviour: violent incidents to self.
1.5 Leaving the study early
We found no significant difference in loss to follow‐up between short‐ or long‐stay groups at one year (n = 453, 3 RCTs, RR 0.87 CI 0.68 to 1.11) and two years (n = 404, 3 RCTs, RR 1.07 CI 0.70 to 1.62, Analysis 1.11). At one year, just over 5% of people in both groups were lost to follow‐up, rising to 14% by two years. Six‐month data by Herz 1975 were equivocal.
1.11. Analysis.
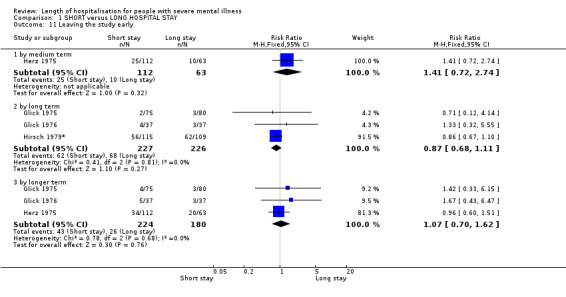
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 11 Leaving the study early.
1.6 General functioning
1.6.1 Unemployed, unable to housekeep or unknown employment status ‐ by two years
People from the short‐stay groups were more likely to be employed at two years than those allocated to long stays (n = 330, 2 RCTs, RR 0.61 CI 0.50 to 0.76, NNTB 5, CI 4 to 8, Analysis 1.12).
1.12. Analysis.
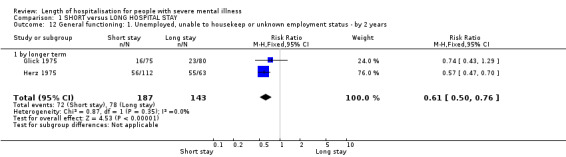
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 12 General functioning: 1. Unemployed, unable to housekeep or unknown employment status ‐ by 2 years.
1.6.2 Work attendance
One study reported work attendance during the week before each assessment at three weeks (short term) and four months (medium term); these data demonstrate no difference between groups at either short term (n = 247, 1 RCT, RR 1.50 CI 0.61 to 3.65) or medium term (n = 247, 1 RCT, RR 1.70 CI 0.75 to 3.85, Analysis 1.13).
1.13. Analysis.
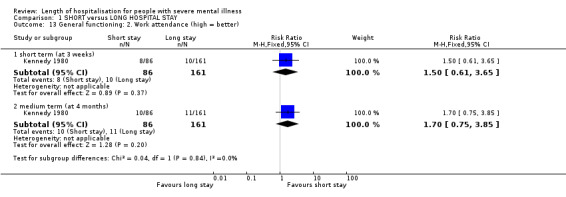
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 13 General functioning: 2. Work attendance (high = better).
1.7 Cost of care
Only one study (Glick 1975) reported costs for outpatient services, but data are skewed (wide confidence intervals), although the data suggested that short‐stay care is slightly more expensive overall (Analysis 1.14).
1.14. Analysis.
Comparison 1 SHORT versus LONG HOSPITAL STAY, Outcome 14 Economic outcomes: Total costs of care.
| Economic outcomes: Total costs of care | ||||
|---|---|---|---|---|
| Study | Intervention | Mean cost (US$) | SD | Notes |
| by longer term | ||||
| Glick 1975 | Short term admission (21‐28 days) | 2498 | No SD | Post hospital costs only (1973 costings). |
| Glick 1975 | Long term admission (90‐120 days) | 2255 | No SD | Post hospital costs only (1973 costings). |
2. Sensitivity analysis
Combining the results from Kennedy 1980 for readmissions at one year increased the relative risk to 1.26 (95% CI 1.0 to 1.6). This introduced heterogeneity (I2 = 62.4%). We had planned to apply all sensitivity analyses to the primary outcomes of this review. This included (i) removing studies that implied randomisation from synthesis and other studies with a better description of randomisation; (ii) testing how prone results were to change when 'completer' data only were compared to any imputed data; (iii) analysing the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation; and (iv) synthesising data using a random‐effects model. However, no study reported outcome data for our primary outcome of clinically important change in global state.
Discussion
Summary of main results
1. COMPARISON: SHORT versus LONG HOSPITAL STAY
1.1 Death
Deaths were very poorly recorded with the exception of Herz 1975. Further information on death rates was sought from trialists, but we were unable to obtain additional information. No trial mentioned violence, criminal offence or imprisonment as an outcome.
1.2 Mental state
All continuous data on mental outcomes could not be synthesised as standard deviations were not reported. One small trial, Glick 1975, did record relevant, but equivocal data. This came from a subset (n = 61) of the full study (n = 141). Data reported in their final report of the study could not be extracted. It is, therefore, inadvisable to draw firm conclusions from so little data but currently there is no evidence that shorter lengths of stay are harmful to a person's mental state.
1.3 Service outcomes
1.3.1 Readmissions to hospital
There were no significant differences for readmission rates between the short‐ and long‐stay groups at one and two years with the homogeneous studies (Glick 1975; Glick 1976; Herz 1975). There is no evidence to support the theory that short‐stay policies, in themselves, promote a 'revolving door' pattern of admissions for those with serious mental illnesses. The unusual Burhan 1969 study showed how a remarkable degree of support for those allocated to a short‐stay policy may well substantially decrease the numbers readmitted. It is thought that this support, involving the author providing sole aftercare including daily visits, counselling and 24 hours personal access via a telephone was the reason for the introduction of heterogeneity. Lessons should be drawn for practice and research from this unusual trial, providing remarkable planning and supervision for short‐stay admissions. The other studies did not offer this degree of support. Only Kennedy 1980 reported data for the outcome of readmission because of a parasuicidal act and being allocated to an initial short stay did not increase the likelihood of being admitted to hospital for a suicide attempt. Again, this is reassuring.
1.3.2 Delayed discharge/Leaving hospital prematurely
Hospitals were significantly more successful in achieving discharge on time for those allocated to short stay. However, those in the short‐stay policy groups were no more likely to have a premature/abrupt discharge than the long‐stay group. The theories of institutionalisation (Goffman 1961) could explain successful discharge of short‐stay patients, suggesting that longer hospitalisation leads to difficulties for patients to re‐enter the real world. Planning discharge and aftercare planning may also be more likely in the short‐stay group; the impetus to institute these processes might not be present when there are no restrictions to the length of the admission. Also, from the consumer perspective, knowing that the admission will be short may improve engagement in discharge planning.
1.3.3 Day care
We found those people in the short‐stay group were prescribed significantly more day care in the Kennedy 1980 study, although no data were available from this study for readmission rates. It is not clear from other studies that this is a common outcome of a short‐stay policy. This may partly explain the findings in Glick 1975 where the post‐discharge cost of the short stay policy is really no different, and may be more, than the standard approach to hospitalisation.
1.4 Behaviour
Only one study reported parasuicide episodes in the medium term (Kennedy 1980) and it showed no demonstrable difference between the groups. No study reported violent incidents to others or to property.
1.5 Leaving the study early
The results suggest that, over two years, short‐stay patients are not significantly at greater risk to being lost to follow‐up than those allocated to long stays.
1.6 General functioning
Available studies did not specifically highlight participants' social functioning although general functioning was addressed in the context of employment and work attendance.
Although data do suggest higher rates of employment/independent living for those allocated to short‐stay policies, these should be interpreted with caution. The trial that provided greater aftercare for the short‐stay group (Herz 1975) showed greatest effect on employment status. This important finding should be replicated.
Only one study provided data on work attendance and no difference was shown between groups both in the short and medium terms.
1.6 Economic outcomes: Costs of care
Economic data were very poor and difficult to interpret. No study reported indirect costs (that is travel, family costs, etc.) and intangible costs (such as inconvenience). Once a person is discharged, Glick 1975 suggests that there is really not much difference between the two groups, and if anything the short‐stay group cost more once they had left hospital. This could, perhaps be explained if it was common to use more day hospital for these people compared with those whose hospital stay was longer as was seen in Kennedy 1980. However, should the mean length of stay be used as a measure of resources consumed, the long stay average costs would be much more than the short stay.
2. Sensitivity analysis
Adding Kennedy 1980 introduced moderate heterogeneity to the outcome of readmission rate at one year. Including this study increased the relative risk of being readmitted to hospital if in the short‐stay group at one year to 1.26 (95% CI 1.0 to 11.6). This study is considerably different from the other included studies in its type of participants. It randomised all acute, unselected psychiatric admissions including people with organic brain disorders and alcohol problems.
Overall completeness and applicability of evidence
1. Completeness
All trials failed to report continuous data in a way that was useful to the review authors. These included outcomes for mental state, social functioning and family burden. Fourteen different scales, some of unknown validity were used. No trial reported user satisfaction, perhaps because the measurement of the consumers' views were not considered important in the 1960s and 1970s. Questions regarding the effect of a short‐stay policy on these important variables remain unanswered.
2. Applicability
The review utilised data from six trials reported from 1969 to 1980. Four trials came from the US and two from the UK. It is difficult to know whether the results would be equally applicable to the psychiatry of the low‐ and middle‐income countries. In addition, the American definition of schizophrenia has been shown to be broader than other countries during this period (WHO 1973; Cooper 1972) and psychiatric services differ between the US and the UK. This may have yet further implications for generalisability of the results of this review. However, the fact that the findings of trials within these two very different care cultures are not substantially different suggests that the results of this review can, at least, be considered for those who work within diverse settings.
The fact that all these trials were mainly published in the 1970s may be the result of a window of opportunity to conduct studies of this sort before the large institutions closed in these countries. These trials, therefore, offer unique information about health policy that would be difficult to repeat today in high‐income countries where large institutions have closed. If questions remain unanswered as regards the effect of a short‐stay versus a longer‐stay policy, it may be for countries other than the US and the UK to help supply the answers.
None of the included trials had participants aged 65 years and over, therefore, we were unable to test the hypothesis that this age group may have a differential response to younger age groups in this review.
Quality of the evidence
Of the six included studies, all are pre‐CONSORT (Moher 2001) and the methodological quality of these studies was judged to be poor; rated either low or very low quality.To a large extent, these studies have not mentioned that they followed a specific method of randomisation and much of the data was unusable. Poor methodological standards have been associated with an overestimate of treatment effect, which should be kept in mind when viewing the results (Schulz 1995).
Potential biases in the review process
For the 2012 update search, review authors OB and VG independently screened results for eligible inclusion in the review, this was cross‐checked by SS. No new studies were included, and therefore there is little chance that any biases arose in this updating process. There may remain potential publication bias, where we may have overlooked unpublished studies. Every effort was made, however, to extensively search references from our search results, in order to identify any other completed or ongoing studies. 'Summary of findings' outcomes were selected by BO and VG; their decision to use the selected outcomes was not influenced by the data already present in the previously published review.
Agreements and disagreements with other studies or reviews
As no new studies were added to the previous version of this systematic review, the findings and conclusions drawn remain in agreement, with the addition of providing a visual summary of the quality of the evidence, and a more extensive investigation into the risk of bias associated with each study.
Authors' conclusions
Implications for practice.
1. For people with serious mental illness and their carers
Data from this review can partially reassure those coming into a hospital that has a short‐stay policy (of less than 28 days) that they are no more likely to be readmitted, to leave hospital abruptly, or to lose contact with services after leaving hospital than had they received long stay care. They are also more likely to leave hospital on their planned discharge date and possibly have a greater chance of finding employment.
2. For clinicians
For clinicians concerned about the uncertainty and safety of a short‐stay policy, there is some reassurance that it does not promote a 'revolving door' pattern of admissions and possible fragmentation of care.
3. For policy makers and commissioners of care
Length‐of‐stay policies have a direct relationship to the size and provision of in‐patient facilities. These, in turn, have a major impact on how resources are used. Traditionally, planners assess levels of in‐patient provision based on national and international comparisons, rather than on the effectiveness of short‐ versus long‐stay policies. This review attempts to address this and, based on limited data so far, commissioning short‐stay policies appears to be an appropriate use of resource. It also indirectly supports the commissioning of services where discharge and aftercare planning is a priority.
Implications for research.
1. General
The studies we identified were pioneering and important. If their findings had been more clearly reported, as is now recommended by the CONSORT statements (Begg 1996;, Moher 2001), this review would have had more findings to report. Continuous data should be presented with standard deviations. With ALLTRIALS perhaps, some day, more data will become available even from the old studies included in this review.
2. Specific
Further trials are needed in order to fill important gaps in knowledge, strengthen existing evidence, and allow greater generalisability to other care cultures. Trials should be large, simple, and clearly reported. These trials should address questions regarding the processes of discharge and aftercare planning. Criteria for entry could be broad, not focusing on 'perfectly' defined single disease categories, but well described, so that readers could extrapolate results for their own circumstances. This also applies to the interventions. These too can be pragmatic but well described. Simple outcomes should also be reported. For example, death, self‐harm, harm to others, criminal behaviour, employment, and homelessness are not difficult to record. Mental, social and family outcomes, user satisfaction, and costs may be more problematic, but can often be recorded clearly in order to inform a wide readership (Table 2).
1. Suggested design of study.
| Type of study | Allocation: the randomisation process should be clearly described. Double‐blind evaluation of the outcomes of a lifestyle intervention is extremely difficult, and probably impossible. Trialists should, take every precaution to minimise the effect of biases by using blinded or independent raters. Intention‐to‐treat analysis is preferable. Trialists should describe from which groups withdrawals came, why they occurred and what was their outcome. Duration: Two‐year follow‐up at minimum. Setting: in a situation where hospitalisation of people from schizophrenia tends to extend well beyond 28 days ‐ perhaps in a low‐middle‐income country setting. |
| Participants | Diagnosis: people with schizophrenia or schizophrenia‐like illnesses. Age: all ages . Sex: men & women. N = 300.* History: people needing admission. |
| Interventions | 1. Short‐stay policy: discharge planning for before day 28. N = 150. 2. Standard stay: discharge planning as before. N = 150. |
| Outcomes | Service outcomes: readmission, use of day hospital. Loss to follow‐up. Functioning: including employment. Serious events: any, list. Satisfaction. Quality of life. Economic outcomes. |
| Notes | * Size of study with sufficient power to highlight ˜10% difference between groups for primary outcome. |
Feedback
General
Summary
1. Category: Discussion The authors state that brief admissions to a psychiatric hospital "do not encourage a 'revolving door' pattern of care for people with serious mental illness and may be more effective than standard care." Such a conclusion would be erroneous and, in an era of aggressive cost containment, dangerous. This review merely presents a meta‐analysis of four old and very different studies, each comparing 'long' with 'short' hospital stays. All the studies were performed more than 20 years ago, before the adoption of current diagnostic criteria (Diagnostic and Statistical Manual of Mental Disorders, fourth edition, and International Classification of Diseases, ninth revision) and modern treatment methods, such as use of selective serotonin reuptake inhibitors and atypical antipsychotic drugs. Further, the prominent decrease in psychiatric facilities (notably the American state hospital system) has meant that many of the patients with chronic mental illness who were institutionalised two decades ago are now subject to repeated acute care admissions to general hospitals. In the two included studies by Glick et al, a short admission was defined as 21‐28 days and a long admission as 90‐120 days. Clearly, the adjectives short and long have since come to have very different meanings: a four‐week hospital stay today would generally be considered to be long. The other two studies were of a mixed sample of patients with either exclusively schizophrenic or 'functional psychiatric' disorders that conceivably could encompass all personality, mood and psychotic conditions. Can one draw an informed conclusion from pooling such outdated and heterogeneous data? At best this meta‐analysis presents a historical snapshot of distant relevance to today's world of inpatient psychiatry. Nevertheless, profit‐driven managed care companies may interpret this paper as justifying a solution to mental health cost control through the imposition of inappropriate limits on inpatient care.
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
1. Category: Discussion The commentator is right that only old trials that met inclusion criteria for our systematic review on short versus long stays were identified, but he is wrong when he says that our findings are of distant relevance to today's psychiatry. We started the review with an important question. Over the past 40 years the lengths of patients' stays in hospital have been reduced so that mental institutions can be closed and to contain costs in many countries. As a result, there is serious public concern about the alternative community care after many deaths and repeated acute care admissions of seriously mentally ill patients (Todd 1976, DoH 1994). Some governments are now suggesting increasing hospital‐based care as part of their modernisation programmes (DOH 1999). With all these policy changes, we simply asked: which is more effective from the patient's point of view, longer or shorter stays? The question is important to today's mental health service, and so the low level of research is both a disappointment and a challenge. We also share the concern that policy is driven by little research evidence, whether made by managed care companies in the United States or by the NHS in the United Kingdom (Knapp 1990). Yet most resources are spent on wards, staff, and buildings. There have been important advances in the treatment of serious mental illnesses, so why is there no recent robust and pragmatic research on how hospital care is organised and delivered?
References 1. Department of Health. Report of the inquiry into the care and treatment of Christopher Clunis. London: HMSO , 1994. 2. Todd NA, Bennie EH, Carlisle JM. Some features of new long‐stay male schizophrenics. British Journal of Psychiatry 1976; 129: 424‐427 3. Department of Health. Government increases number of secure beds for mental health patients. London: Department of Health, 1999 (Press release 1999/0439.). 4. Knapp M, Beecham J. Costing mental health services. Psychological Medicine 1990; 20: 893‐908.
Contributors
Comment from Spencer Eth, New York, November 1999. Reply from Paul Johnstone, Middlesbrough & Gabriella Zolese, London, November 1999.
What's new
| Date | Event | Description |
|---|---|---|
| 16 December 2013 | New citation required but conclusions have not changed | Results of update have not changed overall conclusions of the review. |
| 5 April 2013 | New search has been performed | Cochrane Schizophrenia Group Trial Register Searched (May 2012). No new studies found. 'Summary of findings' table added. 'Risk of bias' tables extended. |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 4, 1999
| Date | Event | Description |
|---|---|---|
| 6 October 2010 | Amended | Contact details updated. |
| 22 July 2009 | Amended | Plain language summary added. |
| 30 October 2008 | Amended | Converted to new review format. |
| 1 June 2007 | New search has been performed | Cochrane Schizophrenia Group Trials Register searched |
Acknowledgements
We would like to thank Clive Adams and Tessa Grant for general support, advice and editing, Robert Pugh and Dinesh Sethi for their helpful comments, Judith Wright for her assistance in the electronic literature search, and John Rathbone for his help in updating previous versions of this review.
We would like to acknowledge the contributions of a previous author, Gabrielle Zolese, who was involved in protocol writing, searching, trial selection, data extraction and completion of earlier reports. We have been unable to contact her during the completion of the latest report.
We would also like to thank Fuwen Yu, Xiaochun Qiu and Qinghua Shang for peer reviewing the version based on 2012 search. Their comments were much appreciated.
Appendices
Appendix 1. Previous searches
1 Update search We searched the Cochrane Schizophrenia Group Trials Register (July 2007) using the phrase:
[((short* or brief* or length*) in same field as (admission* or hospital*) in REFERENCE and (*hospitali*) in STUDY]
This register is compiled by systematic searches of major databases, hand searches and conference proceedings (see Group Module).
1.2 Previous electronic searches
1.2.1 We searched The Cochrane Schizophrenia Group's Trials Register (June 2005) using the phrase:
[((short* or brief* or length*) in same field as (admission* or hospital*) in REFERENCE and (*hospitali*) in STUDY]
1.2.2 We searched Cochrane Schizophrenia Group's Register (December 1998) using the phrase:
[and ((short or brief) near (admission* or hospitali$ation*) or #42 = 114 or 327)]
#42 is the 'intervention' field of this register and '114 or 327' is the code for length of hospital stay.
1.2.3 We searched Biological Abstracts (January 1982 to May 1995) using the Cochrane Schizophrenia Group's phrase for both randomised controlled trials and schizophrenia combined with the phrase:
[and ((short or brief) near (admission* or hospitali$ation*))]
1.2.4 We searched EMBASE (January 1980 to May 1998) using the Cochrane Schizophrenia Group's phrase for both randomised controlled trials and schizophrenia combined with the phrase:
[and ((short or brief) near (admission* or hospitali$ation*))]
1.2.5 We searched MEDLINE (January 1966 to May 1998) using the Cochrane Schizophrenia Group's phrase for both randomised controlled trials and schizophrenia combined with the phrase:
[and ((short or brief) near (admission* or hospitali$ation*))]
1.2.6 We searched PsycLIT (January 1974 to May 1995) using the Cochrane Schizophrenia Group's phrase for both randomised controlled trials and schizophrenia combined with the phrase:
[and ((short or brief) near (admission* or hospitali$ation*))]
1. Reference searching We inspected the references of all identified studies for more studies.
2. SCISEARCH We sought each of the included studies as a citation on the SCISEARCH (May 1998) database. Reports of articles that had cited these studies were inspected in order to identify further trials.
3. Personal contact We sought the results from unpublished trials from authors of key studies. We contacted authors of published studies to request original data if appropriate or to seek clarifications.
Appendix 2. Previous method and data collection
[For definitions of terms used in this, and other sections, please refer to the Glossary]
1. Selection of trials PJ and GZ undertook the original search for trials, and independently inspected all reports. Any disagreements were resolved by discussion, and where doubt remained, we acquired the full article for further inspection. Once the full articles were obtained, we independently decided whether the studies met the review criteria. If disagreement could not be resolved by discussion, again we sought further information and added these trials to the list of those awaiting assessment. For the updated version of the review, material downloaded from electronic resources included details of author, institution or journal of publication. NA inspected and selected all reports which were then reinspected by PJ to ensure reliable selection. We obtained full articles of the selected abstracts and independently decided whether the studies met the review criteria. If disagreement could not be resolved by discussion, we sought further information and added these trials to the list of those awaiting assessment.
2. Quality assessment We assessed the methodological quality of included studies using the criteria described in the Cochrane Handbook (Higgins 2006), which is based on the degree of allocation concealment. Poor concealment has been associated with overestimation of treatment effect (Schulz 1995). Category A includes studies in which allocation has been randomised and concealment is explicit. Category B studies are those which have randomised allocation but in which concealment is not explicit. Category C studies are those in which allocation has neither been randomised nor concealed. We only included trials that were stated to be randomised (categories A or B of the handbook) in this review. The categories are defined below:
A. Low risk of bias (adequate allocation concealment) B. Moderate risk of bias (some doubt about the results) C. High risk of bias (inadequate allocation concealment).
3. Data extraction We independently extracted data from selected trials. When disputes arose we attempted to resolve these by discussion. When this was not possible and further information was necessary to resolve the dilemma, we did not enter data and added the trial to the list of those awaiting assessment.
4. Data management 4.1 Loss to follow up (intention‐to‐treat /ITT analysis) We excluded data from studies where more than 50% of participants in any group were lost to follow up (this did not include the outcome of 'leaving the study early'). In studies with less than 50% dropout rate, we assumed people leaving early had a negative outcome, except for the event of death. We analysed the impact of including studies with high attrition rates (25‐50%) in a sensitivity analysis. If inclusion of data from this latter group resulted in a substantive change in the estimate of effect, we did not add their data to trials with less attrition, but presented them separately.
4.2 Dichotomous data For binary outcomes we calculated the relative risk (RR) and its 95% confidence interval (CI) based on the fixed effects model. Relative Risk is more intuitive (Boissel 1999) than odds ratios and odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation leads to an overestimate of the impression of the effect. When the overall results were significant and homogeneous we calculated the number needed to treat/harm (NNT/H).
4.3 Continuous data 4.3.1 Normal distribution Continuous data on outcomes in trials relevant to mental health issues are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data we applied the following standards to continuous final value endpoint data before inclusion: (a) standard deviations and means were reported in the paper or were obtainable from the authors; (b) when a scale started from zero, the standard deviation, when multiplied by two, should be less than the mean (otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution ‐ Altman 1996); In cases with data that are greater than the mean they were entered into 'Other data' table as skewed data. If a scale starts from a positive value (such as PANSS, which can have values from 30 to 210) the calculation described above in (b) should be modified to take the scale starting point into account. In these cases skewness is present if 2SD>(S‐Smin), where S is the mean score and Smin is the minimum score. We reported non‐normally distributed data (skewed) in the 'other data types' tables.
For change data (mean change from baseline on a rating scale) it is impossible to tell whether data are non‐normally distributed (skewed) or not, unless individual patient data are available. After consulting the ALLSTAT electronic statistics mailing list, we entered change data in RevMan analyses and reported the finding in the text to summarise available information. In doing this, we assumed either that data were not skewed or that the analysis could cope with the unknown degree of skew.
4.3.2 Final endpoint value versus change data Where both final endpoint data and change data were available for the same outcome category, we only presented final endpoint data. We acknowledge that by doing this much of the published change data may be excluded, but argue that endpoint data is more clinically relevant and that if change data were to be presented along with endpoint data, it would be given undeserved equal prominence. Where studies reported only change data we contacted authors for endpoint figures.
4.3.3 Data synthesis For continuous outcomes we estimated a weighted mean difference (WMD) between groups based on a fixed effects model.
4.4 Scale derived data A wide range of scales/instruments are available to measure outcomes in psychiatric care. These instruments vary greatly in quality. Instruments should be reliable (have a known degree of stability when a measurement is repeated under identical conditions) and valid (really measure what it actually purports to measure (Rust 1989). In this review we only used outcomes measured by instruments which have been published in peer reviewed journals. In addition, data from rating scales were only used if it was (i) self‐reported or completed by an independent rater or relative; and (ii) more than 50% complete.
4.5 Cluster trials Studies increasingly employ cluster randomisation (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a unit‐of‐analysis error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes Type I errors (Bland 1997; Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra‐class correlation co‐efficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a design effect. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation co‐efficient (ICC) [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999). If cluster studies had been appropriately analysed taking into account intra‐class correlation coefficients and relevant data documented in the report, we synthesised these with other studies using the generic inverse variance technique.
5. Investigation for heterogeneity Firstly, we considered all the included studies within any comparison to judge for clinical heterogeneity. Then we visually inspected graphs to investigate the possibility of statistical heterogeneity. This was supplemented using, primarily, the I‐squared statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I‐squared estimate was greater than or equal to 75%, this was interpreted as indicating the presence of high levels of heterogeneity, and when it was between 50‐75% it was interpreted as indicating moderate levels of heterogeneity (Higgins 2003). If inconsistency were thought to be high, data were not summated and presented as part of the main results, but were presented separately and a sensitivity analysis performed. Reasons for heterogeneity were investigated whenever possible.
6. Sensitivity analysis We excluded data from quasi‐randomised trials and from trials including unselected psychiatric admissions from the main analysis and these were entered into a sensitivity analysis to see if these exclusions were justified.
7. General issues Where possible, we entered data into RevMan in such a way that the area to the left of the 'line of no effect' indicated a 'favourable' outcome for the treatment group. Where this was not possible, we labelled the graphs in RevMan analyses accordingly so that the direction of any effects was clear.
Appendix 3. Previous Plain Language Summary
Over the last hundred years medical opinion as to whether people with a severe mental illness should stay in hospital for months and years versus a few weeks has changed. This has been helped by the advent of medication for some of these illnesses. Consequently, in the developed world hospital stays are now relatively short. However even in these countries there is still some doubt as to whether really short admissions are helpful because the person does not get institutionalised, or harmful because the symptoms and possible causes of the illness are not completely addressed. There are a group of patients who have short but frequent admissions (‘revolving door patients’) and others who despite a variety of treatments stay in hospital for a long time (‘new long stay patients’).
To identify what length of stay in hospital is most helpful; this review looks at trials comparing short hospital stays ranged from one week to 21‐28 days with long hospital stays, which was only clearly reported in two studies (90 to 120 days), otherwise professional carers determined length of stay. Six studies were found containing a total of 2030 people, four in the USA and two in the UK. However, differences in the design of the trials made them difficult to compare. In addition all of the trials were done before 1980 when there was less choice of medication and greater differences in the diagnoses between the US and the UK. One study showed a statistically significant drop in the number of people readmitted to hospital in the short stay group, but these people had almost daily input from a clinician. The remaining studies showed no significant difference between the two groups. In two trials, the short‐stay group were more likely to be employed after two years. In three trials the long‐stay group were more likely to stay in hospital after the date they were supposed to be discharged. Although the data from these trials is not extensive there is a suggestion that short stays in hospital in themselves do not cause people to become ‘revolving door patients’. A new trial that is large, simple and clearly recorded, would help to confirm these results.
(Plain language summary prepared for this review by Janey Antoniou of RETHINK, UK www.rethink.org).
Data and analyses
Comparison 1. SHORT versus LONG HOSPITAL STAY.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death ‐ 2 years follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 longer term | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.10, 1.83] |
| 2 Mental state: Not improved | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 as measured by PEF scale ‐ longer term | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.39 [0.76, 15.02] |
| 2.2 as measured by HSRS ‐ longer term | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.31, 3.01] |
| 3 Service outcomes: 1a. Readmission to hospital (homogeneous studies) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 short term | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.72, 2.16] |
| 3.2 medium term | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.69, 1.73] |
| 3.3 long term | 4 | 651 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.00, 1.57] |
| 3.4 longer term | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.78, 1.36] |
| 4 Service outcomes: 1b. Readmission to hospital ‐ Burhan 1969 only | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 long term | 1 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.07, 0.67] |
| 4.2 longer term | 1 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.11, 0.41] |
| 5 Service outcomes: 1c. Readmission to hospital ‐ due to parasuicide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 long term | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.36, 3.04] |
| 6 Service outcomes: 2. Average length of stay (days) | Other data | No numeric data | ||
| 6.1 long term | Other data | No numeric data | ||
| 6.2 longer term | Other data | No numeric data | ||
| 7 Service outcomes: 3. Leaving hospital prematurely ‐ by 2 years | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.34, 1.77] |
| 7.1 by longer term | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.34, 1.77] |
| 8 Service outcomes: 4. Discharge delayed beyond the time planned in study ‐ 2‐year data | 3 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.33, 0.88] |
| 8.1 by longer term | 3 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.33, 0.88] |
| 9 Service outcomes: 5. Day care ‐ by 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 by long term | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.52 [2.74, 7.45] |
| 10 Behaviour: violent incidents to self | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 parasuicide episodes ‐ medium term | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.30] |
| 11 Leaving the study early | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 by medium term | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.72, 2.74] |
| 11.2 by long term | 3 | 453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.11] |
| 11.3 by longer term | 3 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.70, 1.62] |
| 12 General functioning: 1. Unemployed, unable to housekeep or unknown employment status ‐ by 2 years | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.50, 0.76] |
| 12.1 by longer term | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.50, 0.76] |
| 13 General functioning: 2. Work attendance (high = better) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 short term (at 3 weeks) | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.61, 3.65] |
| 13.2 medium term (at 4 months) | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.75, 3.85] |
| 14 Economic outcomes: Total costs of care | Other data | No numeric data | ||
| 14.1 by longer term | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Burhan 1969.
| Methods | Allocation: 'randomly selected from total hospital intake by admissions office' ‐ no further details. Blinding: none. Duration: 2 years. Setting: Ohio USA. | |
| Participants | Diagnosis: 'mixture of psychotic and psychoneurotic'. N = 1169. Age: adults, no further details. Sex: unknown. Excluded: 'geriatric' and 'mentally retarded' people. | |
| Interventions | 1. Short stay (2‐3 weeks): informed re short stay on admission, daily psychiatrist visit (5 days), counselling both recipient & family at discharge, telephone access to psychiatrist thereafter. N = 100. 2. Long stay: standard care. N = 1069. All participants had medication, and out patient care as required. |
|
| Outcomes | Service outcomes: readmission to hospital. Unable to use ‐ Mental state: (Hoffer‐Osmond diagnostic test, MMPI (no SD). Service outcomes: length of stay (no SD). General functioning: employment (no data). Economic data (no SD). |
|
| Notes | This study adds heterogeneity and the results are presented separately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...100 were randomly assigned by the admissions office to the study cohort"‐ no further details provided. |
| Allocation concealment (selection bias) | High risk | Quote: "The patients in the cohort were assigned to me as the investigator, and I became their therapist". Comment: probably not done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "I interviewed each patient in the study cohort as soon as possible after he was admitted. I told him and his family that he would leave hospital in two or three weeks, sooner if possible.." Comment: probably not done. All patients and investigators had the knowledge to which group the patients were assigned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details. |
| Selective reporting (reporting bias) | Unclear risk | No details. |
| Other bias | Unclear risk | None detected. |
Glick 1975.
| Methods | Allocation: 'random allocation', no further details. Blinding: none. Duration: 2 years. Setting: San Francisco, USA. | |
| Participants | Diagnosis: schizophrenia (57% paranoid) (Mosher criteria). N = 141. Age: mean 23, range 15‐38 years. Sex: 50% women. Marital status: 84% single. Race: 10% black. Social class: 85% III ‐V. History: 'short‐term' 31; 'long‐term' 29. | |
| Interventions | 1. Short‐term admission (21‐28 days): early discharge plan, rapid assessment, "crisis resolution" management ethos, phenothiazine medication; long‐range recommendations for further post‐discharge treatment or rehabilitation. N = 71. 2. Long‐term admission (90‐120 days): assessment (2‐3 weeks), psychotherapy, phenothiazine medication and/or "major rehabilitative measures" (change of home/job or workshop placement). N = 70. Similar fixed drug regimes across groups. |
|
| Outcomes | Mental state. HSRS, PEF.
Service outcomes: readmission, average length of hospital stay, delay in discharge, leaving hospital prematurely.
Leaving the study early.
General functioning: unemployed. Unable to use ‐ Social function: KAS, Behavior Inventory (no SD). User satisfaction: PSECS (no SD). Family burden: PEF (no SD). Employment status: BFR, PEF, PSECS , FMECS (no SD). |
|
| Notes | Mosher criteria ‐ see Mosher 1971. Differences reported between groups (long stay ‐ more education, higher socio‐economic status, better pre‐morbid adjustment & higher mean dose antipsychotics ‐ 644 mg CPZ equivalents versus 328 mg). First report on subset of 61 people (Glick 1974) presented only usable data from PEF & HSRS (percentage not improved). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'All patients admitted to the ward during a twenty‐six month period....were randomly assigned to either short‐term or long‐term hospitalisation...' 'Patients remained in the long or short term group regardless of final diagnosis, to avoid any incentive to bias diagnostic judgments...' 'Envelopes with the assignment to short ‐ or long ‐ term treatment enclosed, were prepared by the research staff and opened by the ward clerk one at a time after each patient was admitted... |
| Allocation concealment (selection bias) | Low risk | 'Envelopes with the assignment to short ‐ or long ‐ term treatment enclosed, were prepared by the research staff and opened by the ward clerk one at a time after each patient was admitted... All patients were told their treatment assignment within an hour or so of their admission. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Both patients and investigators had knowledge of the group to which they were assigned. Patients in treatment group were informed of the duration of their stay and investigators were not blinded either. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 255 patients admitted during the 26‐month intake period of the study, 20 were excluded. The 235 patients left, remained in the study until completion. Of these, 141 were diagnosed as having schizophrenia and 74 as 'non‐schizophrenics' with diagnoses of affective disorders, neuroses and severe personality disorders. |
| Selective reporting (reporting bias) | Unclear risk | No details. |
| Other bias | Unclear risk | None detected. |
Glick 1976.
| Methods | Allocation: 'randomly assigned' ‐ no further details. Blindness: not reported. Duration: 26 months. Setting: USA. | |
| Participants | Diagnosis: 'non‐schizophrenics' (DSM‐II) included affective disorders, neuroses and severe personality disorders. N = 74. Age: not stated. Excluded: alcohol and drug dependencies, organic disease. | |
| Interventions | 1. Short term (21‐28 days): emphasis on 'crisis resolution' & discharge planning. N = 37. 2. Long term (90‐120 days): emphasis on diagnosis, treatment, psycho‐social intervention, rehabilitation, & referral to post hospital care. N = 37. Both groups received individual, family & group psycho‐therapy. |
|
| Outcomes | Service outcomes: readmission, leaving hospital prematurely, delayed discharge from hospital.
Leaving the study early. Unable to use ‐ Behaviour: KAS, Behavior Inventory (no SD). User satisfaction: PSECS (no SD). Family burden: PEF (no SD). General functioning: employment status: BFR, PEF, PSECS, FMECS (no SD). |
|
| Notes | No difference between diagnostic categories between groups. No specific community programme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'All patients admitted to the ward during a twenty‐six month period....were randomly assigned to either short‐term or long‐term hospitalisation...' 'Patients remained in the long or short term group regardless of final diagnosis, to avoid any incentive to bias diagnostic judgments...' 'Envelopes with the assignment to short ‐ or long ‐ term treatment enclosed, were prepared by the research staff and opened by the ward clerk one at a time after each patient was admitted... |
| Allocation concealment (selection bias) | Low risk | 'Envelopes with the assignment to short ‐ or long ‐ term treatment enclosed, were prepared by the research staff and opened by the ward clerk one at a time after each patient was admitted... All patients were told their treatment assignment within an hour or so of their admission...' |
| Blinding (performance bias and detection bias) All outcomes | High risk | '...none of the ward staff knew which treatment assignment the next patient would receive but they were also told of the assignment when the envelope was opened ...' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 255 patients admitted during the 26‐month intake period of the study, 20 were excluded. The 235 patients left, remained in the study until completion. Of these, 141 were diagnosed as having schizophrenia and 74 as 'non‐schizophrenics' with diagnoses of affective disorders, neuroses and severe personality disorders. |
| Selective reporting (reporting bias) | Unclear risk | No details. |
| Other bias | Unclear risk | None detected. |
Herz 1975.
| Methods | Allocation: 'randomly assigned' ‐ no further details. Blinding: none. Duration: 2 years. Setting: New York, USA. | |
| Participants | Diagnosis: ˜60% schizophrenia. N = 175. Race: 'ethnically diverse'. Social class: 'low income'. Excluded: those < 16 years; not living with responsible adult; with concurrent serious medical illness; substance misuse; organic brain syndrome; anti‐social behaviour, and/or adolescent behaviour disorder. | |
| Interventions | 1. Brief‐day: planned discharge by 7 days, then day care & OPD when necessary. N = 61. 2. Brief‐out: planned discharge & OPD when necessary. N = 51. 3. Standard treatment: length of stay determined by carers, OPD when necessary. Maximum length of stay to first significant release was 60 days. N = 63. |
|
| Outcomes | Death.
Service outcomes: readmission, average length of hospital stay, delayed discharge.
General functioning: unemployed. Leaving the study early. Unable to use ‐ Global functioning: GAS (no SD). Mental state: MSER, PSS (no SD). Family burden: FEF (no SD). |
|
| Notes | Both brief groups received less psychotropic medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The study randomly assigned 175 newly admitted inpatients to 3 treatment groups. It gave no details as to how this was done, particularly whether there was any process of random sequence generation. |
| Allocation concealment (selection bias) | High risk | Other than broadly stating (as above) that the participants were randomised to the study groups, the paper made no reference to allocation concealment during the process of randomisation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The methodology makes no reference to either single or double blinding. The therapeutic teams as well as participants were apparently aware of what treatment group had been assigned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study made no reference to attrition or drop outs. All 175 participants apparently completed the study. |
| Selective reporting (reporting bias) | Unclear risk | No details. |
| Other bias | Unclear risk | None detected. |
Hirsch 1979*.
| Methods | Allocation: 'at random' ‐ no further details. Blinding: none. Duration: 1 year. Setting: central London, UK. | |
| Participants | Diagnosis: 'functional psychiatric disorder'. N = 224. History: just admitted. Age: >16 years. Sex: brief group = 47% males, standard group = 40% males. Excluded: outside catchment, < 16 years, organic brain syndrome. | |
| Interventions | 1. Brief admission: discharge planned in < 8 days + community day care. N = 115. 2. Standard admission: discharged at carers discretion. N = 109. |
|
| Outcomes | Leaving the study early. Unable to use ‐ Service outcomes: readmission. (> 50% loss to follow‐up). Mental state: PSE (no SD). Economic outcomes. (no data). Behaviour: PBAS (details not published). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "immediately on admission, patients were allocated at random... allocation was done the moment the patients were admitted to avoid any possibility of bias...". These were the only references to randomisation in the methodology. There is no reference to any process of random sequence generation. |
| Allocation concealment (selection bias) | High risk | There was no process of allocation concealment described in the methodology. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study did not utilise single or double blinding during allocation or treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15% of eligible participants were missed or excluded because of failure to obtain or complete an interview. 20% of eligible participants assigned to one or the other group were excluded because they left hospital in less than four days. |
| Selective reporting (reporting bias) | Unclear risk | No details. |
| Other bias | Unclear risk | None detected. |
Kennedy 1980.
| Methods | Allocation: randomised. Blinding: none. Duration: 1 year. Setting: Edinburgh, Scotland, UK. | |
| Participants | Diagnosis: 'any psychiatric disorder: organic, schizophrenia, affective, alcoholic, neurotic and other' N = 247. Age: any. Sex: males = 49%. Excluded: 1 in 4 from randomisation ' in interest of continuity of care' | |
| Interventions | 1. Admission to short‐stay ward. N = 86. 2. Admission to one of two control wards. N = 161. |
|
| Outcomes | Service outcomes: readmission rates, day care, parasuicide admissions, length of stay. Work. Reported parasuicide episodes. Unable to use ‐ Psychiatric Status Schedule (no means or SDs reported). FEF (no means or SDs reported). GP form (no data reported for individual groups). ECT treatment (no data reported). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote 1: "... 73 per cent of patients admitted to the experimental ward were randomised and 78 per cent of patients admitted to the control wards" (page 206). Quote 2: "Once a patient had been allotted, any readmissions were to the same ward. Patients could be excluded from randomisation in the interests of continuity of care, and about 1 in 4 were so managed" (page 205‐206). Comment: probably done; however randomisation process was not explicitly defined. Allocation was randomised at the start however this was then breached for 25% of readmitted patients to ensure the continuity of care. |
| Allocation concealment (selection bias) | High risk | Quote: "Both patient and informant were then advised that discharge to out‐patient care would be arranged as soon as possible, probably within a week". (page 206) Comment: Both clinicians and patiens had knowledge of the allocation and patients allocated to the short‐stay group were informed of the discharge plan at the time of assignment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Probably not done. Comment: No mention of blinding of observers. The authors and clinical team had knowledge of allocated intervention after assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided on incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | It is not clear whether the outcome of previously determined three measures (PSS, FEF, GP form) were reported for both experimental and control groups. |
| Other bias | Unclear risk | None detected. |
General CPZ ‐ chlorpromazine ECT ‐ electroconvulsive treatment SD ‐ standard deviation
Diagnostic tools DSM‐II ‐ Diagnostic Statistical Manual version 2
Scales Global functioning GAS ‐ Global Assessment Score
Mental state measures MMPI ‐ Minnesota Multiphasic Personality Inventory MSER ‐ Mental State Examination Record HSRS ‐ Health‐Sickness rating scale PSS ‐ Psychiatric Status Schedule PSE ‐ Present State Examination
Social function/behaviour scales KAS ‐ Katz Adjustment Score PBAS ‐ Patient Behaviour Assessment Scale
Satisfaction scales FEF ‐ Family Evaluation Form. PSECS ‐ Patient self evaluation of current status
Employment status BFR ‐ Unknown PEF ‐ Psychiatric Evaluation Form FMECS (work) ‐ Family Evaluation of Current Status
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Appleby 1993 | Allocation: not randomised ‐ retrospective study. |
| Caffey 1968 | Allocation: quasi‐randomised 'assigned in rotation to one of three groups'. |
| Hafner 1986 | Allocation: not randomised ‐ cohort study. |
| Lehrman 1961 | Allocation: not randomised ‐ cohort study. |
| May 1968 | Allocation: randomised. Participants: people with schizophrenia. Intervention: individual psychotherapy versus ataraxic drugs versus individual psychotherapy plus ataraxic drugs versus ECT versus no extra treatment. |
| Mendel 1966 | Allocation: not randomised ‐ cohort study. |
| Olfson 1990 | Allocation: not randomised, from emergency room by nurse and psychiatrist 'according to need and bed availability'. |
| Rosen 1976 | Allocation: assigned on admission on a 'first come first served basis, by date of application and availability of beds' ‐ quasi‐randomised. |
| Singer 1975 | Allocation: not randomised ‐ retrospective cohort. |
ECT ‐ electroconvulsive treatment
Differences between protocol and review
See Table 4 for differences between the original protocol and review, and the updated review.
2. Differences between review and 2013 update.
| Original review | Update (2013) | Change to results/ conclusion |
| Change in wording of outcome timeframe: "We grouped outcomes into (less than three months), (up to six months), (up to one year), and (two years or more from admission date)" |
Changed to: "We grouped outcomes into short term (less than three months); medium term (three to six months); long term (six months to one year); longer term (one to two years or more from admission date)" |
No |
Contributions of authors
Nisreen Alwan ‐ updating the review: searching, trial selection, data extraction from newly included studies, analysis, completion of report.
Paul Johnstone ‐ protocol writing, searching, trial selection, data extraction, completion of report, updating.
Babalola Olufemi ‐ screening results for 2012 update search, completion of 2012 update report.
Vahdet Gormez ‐ screening results for 2012 update search, completion of 2012 update report.
Stephanie Sampson ‐ assistance in completion of 2012 update report.
Sources of support
Internal sources
Berkshire Health Authority, UK.
Pathfinder Mental Health Services NHS Trust, UK.
Anglia and Oxford Regional Health Authority, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Burhan 1969 {published data only}
- Burhan AS. Short term hospital treatment: a study. Hospital and Community Psychiatry 1969;20:369‐70. [DOI] [PubMed] [Google Scholar]
Glick 1975 {published data only}
- Glick ID, Hargreaves WA, Drues JMA, Showstack JA. Short versus long hospitalization: a prospective controlled study. IV. One‐year follow‐up results for schizophrenic patients. American Journal of Psychiatry 1976;133:509‐14. [DOI] [PubMed] [Google Scholar]
- Glick ID, Hargreaves WA, Goldfield MD. Short versus long hospitalization. Archives of General Psychiatry 1974;30:363‐9. [DOI] [PubMed] [Google Scholar]
- Glick ID, Hargreaves WA, Raskin M, Kutner SJ. Short versus long hospitalization. II. Results for schizophrenia in‐patients. American Journal of Psychiatry 1975;123:385‐90. [DOI] [PubMed] [Google Scholar]
- Hargreaves WA, Glick ID, Drues J, Showstack JA, Feigenbaum E. Short versus long hospitalization: a prospective controlled study. VI. Two‐year follow‐up results for schizophrenics. Archives of General Psychiatry 1977;34:305‐11. [DOI] [PubMed] [Google Scholar]
- Young RC, Gould E, Glick ID. Personality inventory correlates of outcome in a follow‐up study of psychiatric hospitalization. Psychological Reports 1980;46:903‐6. [DOI] [PubMed] [Google Scholar]
Glick 1976 {published data only}
- Glick ID, Hargreaves WA, Drues JA, Showstack JA, Katzow JJ. Short versus long hospitalization: a prospective controlled study. VII. Two‐year follow‐up results for non‐schizophrenics. Archives of General Psychiatry 1977;34:314‐7. [DOI] [PubMed] [Google Scholar]
- Glick ID, Hargreaves WA, Drues JMA, Showstack JA. Short versus long hospitalization: a controlled study. III. Inpatient results for nonschizophrenics. Archives of General Psychiatry 1976;33:78‐83. [DOI] [PubMed] [Google Scholar]
- Glick ID, Hargreaves WA, Drues JMA, Showstack JA. Short versus long hospitalization: a prospective controlled study. V. One‐year follow up for non‐schizophrenics. American Journal of Psychiatry 1976;133:515‐7. [DOI] [PubMed] [Google Scholar]
Herz 1975 {published data only}
- Endicott J, Cohen J, Nee J, Fleiss JL, Herz MI. Brief vs standard hospitalization. For whom?. Archives of General Psychiatry 1979;36:706‐12. [DOI] [PubMed] [Google Scholar]
- Herz MI, Endicott J, Gibbon M. Brief hospitalization: two‐year follow‐up. Archives of General Psychiatry 1979;36:701‐5. [DOI] [PubMed] [Google Scholar]
- Herz MI, Endicott J, Spitzer RL. Brief hospitalization of patients with families: initial results. American Journal of Psychiatry 1975;132:413‐8. [DOI] [PubMed] [Google Scholar]
- Herz MI, Endicott J, Spitzer RL. Brief hospitalization: a two‐year follow‐up. American Journal of Psychiatry 1977;134:502‐7. [DOI] [PubMed] [Google Scholar]
- Herz ML, Endicott J, Spitzer RL. Brief versus standard hospitalization: the families. American Journal of Psychiatry 1976;133:795‐801. [DOI] [PubMed] [Google Scholar]
- Rebel S, Herz MI. Limitations of brief hospital treatment. American Journal of Psychiatry 1976;133:518‐21. [DOI] [PubMed] [Google Scholar]
Hirsch 1979* {published data only}
- Hirsch SR, Platt S, Knights A, Weyman A. Shortening hospital stay for psychiatric care: effect on patients and their families. British Medical Journal 1979;1:442‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knights A, Hirsch SR, Platt SD. Clinical change as a function of brief admission to hospital in a controlled study using the present state examination. British Journal of Psychiatry 1980;137:170‐80. [DOI] [PubMed] [Google Scholar]
- Platt S, Hirsch S. The effects of brief hospitalization upon the psychiatric patient's household. Acta Psychiatrica Scandinavica 1981;64:199‐216. [DOI] [PubMed] [Google Scholar]
- Platt SD, Hirsch SR, Knights AC. Effects of brief hospitalization on psychiatric patients' behaviour and social functioning. Acta Psychiatrica Scandinavica 1981;63:117‐28. [DOI] [PubMed] [Google Scholar]
Kennedy 1980 {published data only}
- Kennedy P, Hird F. Description and evaluation of a short‐stay admission ward. British Journal of Psychiatry 1980;136:205‐15. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Appleby 1993 {published data only}
- Appleby L, Prakash ND, Luchins DJ, Gibbons RD, Hedeker DR. Length of stay and recidivism in schizophrenia: a study of public psychiatric hospital patients. American Journal of Psychiatry 1993;150:72‐6. [DOI] [PubMed] [Google Scholar]
Caffey 1968 {published data only}
- Caffey EM, Galbrecht CR, Klett CJ. Brief hospitalization and aftercare in the treatment of schizophrenia. Archives of General Psychiatry 1971;24:81‐6. [DOI] [PubMed] [Google Scholar]
- Caffey EM, Jones RD, Diamond LS, Burton E, Bowen WT. Brief hospital treatment of schizophrenia: early results of a multiple hospital study. Hospital and Community Psychiatry 1968;19:282‐7. [PubMed] [Google Scholar]
Hafner 1986 {published data only}
- Hafner H, an der Heiden W, Buchholz W, Bardens R, Klug J, Krumm B. Organisation, Wirksamkeit und Wirkschaftlichkeit komplementarer Versorgung schizophrener. Nervenartz 1986;57:214‐26. [PubMed] [Google Scholar]
Lehrman 1961 {published data only}
- Lehrman NS. Follow‐up of brief versus prolonged psychiatric hospitalization. Comprehensive Psychiatry 1961;2:227‐40. [DOI] [PubMed] [Google Scholar]
May 1968 {published data only}
- May PRA. Treatment of Schizophrenia: a Comparative Study of Five Treatment Methods. New York: Science House, 1968. [Google Scholar]
- May PRA, Tuma AH, Yale C, Potepan P, Dixon WJ. Schizophrenia ‐ a follow‐up study of results of treatment. II. Hospital stay over two to five years. Archives of General Psychiatry 1976;33:481‐6. [DOI] [PubMed] [Google Scholar]
Mendel 1966 {published data only}
- Mendel WM. Effect of length of hospitalization on rate and quality of remission from acute psychotic episodes. Journal of Nervous and Mental Disease 1966;143:226‐33. [DOI] [PubMed] [Google Scholar]
Olfson 1990 {published data only}
- Olfson M, Plotke GS, Harding CM, Jones J, Mayfield VW. A controlled evaluation of inpatient crisis treatment for acute schizophrenia episodes. Psychiatric Quarterly 1990;61:143‐54. [DOI] [PubMed] [Google Scholar]
Rosen 1976 {published data only}
- Mattes JA, Klein DF, Millan D, Rosen B. Comparison of the clinical effectiveness of 'short' versus 'long' stay psychiatric hospitalization. Journal of Nervous and Mental Disease 1979;167:175‐81. [DOI] [PubMed] [Google Scholar]
- Mattes JA, Rosen B, Klein DF. Comparison of the clinical effectiveness of short versus long stay intensive psychiatric hospitalization. II. Results of a 3‐year post‐hospital follow‐up. Journal of Nervous and Mental Diseases 1977;165:387‐94. [DOI] [PubMed] [Google Scholar]
- Rosen B, Katzoff A, Carillo C, Klein DF. Clinical effectiveness of short versus long psychiatric hospitalisation. I. Inpatient results. Archives of General Psychiatry 1976;33:1316‐22. [DOI] [PubMed] [Google Scholar]
Singer 1975 {published data only}
- Singer JE, Grob MC. Short term versus long term hospitalization in a private psychiatric facility. A follow‐up study. Hospital and Community Psychiatry 1975;26:745‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [LH020600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 1996
- Anonymous. British Medical Journal Headlines. BMJ 1996;313:8. [Google Scholar]
Appleby 1991
- Appleby L, Araya R (eds). Mental Health and the Global Village. Royal College of Psychiatrists. London: Gaskell, 1991. [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Mower D, Alkin I, et al. Improving quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637‐9. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Connolly 1856
- Connolly J. The Treatment of the Insane without Mechanical Restraints. London: Elger & Company, 1856. [Google Scholar]
Cooper 1972
- Cooper JE, Kendell RE, Gurland BJ, Sharpe L, Copeland JRM, Simon R. Psychiatric Diagnosis in New York and London. Vol. 20, Oxford: Oxford University Press, 1972. [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Abstracts of 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. 2000.
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
DoH 1994
- Department of Health, England, Wales. Report of the Inquiry into the Care and Treatment of Christopher Clunis. London: HMSO, 1994. [Google Scholar]
DOH 1999
- Department of Health, England, Wales. National Service Framework for Mental Health. London: HMSO, 1999. [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Endicott 1972
- Endicott J, Spitzer RL. What! Another rating scale? The Psychiatric Evaluation Form. Journal of Nervous and Mental Disease 1972;154(2):88‐104. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. [DOI] [PubMed] [Google Scholar]
Glick 1974
- Glick ID, Hargreaves WA, Goldfield MD. Short versus long hospitalization. Archives of General Psychiatry 1974;30:363‐9. [DOI] [PubMed] [Google Scholar]
Goffman 1961
- Goffman E. Asylums: Essays on the Social Situation of Mental Patients and Other Inmates. New York: Doubleday, 1961. [Google Scholar]
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Hafner 1987
- Hafner H. Do we still need beds for psychiatric patients? An analysis of changing patterns of mental health care. Acta Psychiatrica Scandinavica 1987;75:113‐26. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. In: Higgins JPT, Green S editor(s). The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd., 2006. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org..
Jones 1985
- Jones K, Poletti A. Understanding the Italian experience. British Journal of Psychiatry 1985;146:341. [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Leucht 2005
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF?. Schizophrenia Bulletin 2007;33(1):183‐91. [PUBMED: 16905632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luborsky 1962
- Luborsky L. Clinicians' judgments of mental health: a proposed scale. Archives of General Psychiatry 1962;7(6):407‐17. [DOI] [PubMed] [Google Scholar]
Marshall 1999
- Marshall M, Gray A, Lockwood A, Green R. Case management for people with severe mental disorders. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD000050.pub2] [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D, CONSORT Group. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐91. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Pinel 1806
- Pinel P (translated Davis DD). A Treatise on Insanity. Sheffield: Caddell & Davils, 1806. [Google Scholar]
Rust 1989
- Rust J, Golombok S. Modern Psychometrics. London: Routledge, 1989. [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83. [Google Scholar]
Todd 1976
- Todd NA, Bennie EH, Carlisle JM. Some features of new long‐stay male schizophrenic. British Journal of Psychiatry 1976;129:424‐7. [DOI] [PubMed] [Google Scholar]
Tuke 1813
- Tuke S. A Description of the Retreat. York: Alexander & Webb, 1813. [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. [PubMed] [Google Scholar]
WHO 1973
- World Health Organization. Report of the International Pilot Study of Schizophrenia. Vol. 1, Geneva: World Health Organization, 1973. [Google Scholar]
Wing 1970
- Wing JK, Brown GW. Institutionalism and Schizophrenia: A Comparative Study of Three Mental Hospitals, 1960‐1968. Cambridge: Cambridge University Press, 1970. [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
References to other published versions of this review
Alwan 2008
- Alwan N, Johnstone P, Zolese G. Length of hospitalisation for people with severe mental illness. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000384.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnstone 1999
- Johnstone P, Zolese G. Length of hospitalisation for people with severe mental illness. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD000384] [DOI] [PubMed] [Google Scholar]


