Abstract
Background
Acupuncture is increasingly used in people with epilepsy. It remains unclear whether existing evidence is rigorous enough to support its use. This is an update of a Cochrane review first published in 2008.
Objectives
To determine the effectiveness and safety of acupuncture in people with epilepsy.
Search methods
We searched the Cochrane Epilepsy Group Specialised Register (June 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2013, Issue 5), MEDLINE, EMBASE, CINAHL, AMED and other databases (from inception to June 2013). We reviewed reference lists from relevant trials. We did not impose any language restrictions.
Selection criteria
Randomised controlled trials (RCTs) comparing acupuncture with placebo or sham treatment, antiepileptic drugs or no treatment; or comparing acupuncture plus other treatments with the same other treatments, involving people of any age with any type of epilepsy.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration.
Main results
We included 17 RCTs with 1538 participants that had a wide age range and were suffering mainly from generalized epilepsy. The duration of treatment varied from 7.5 weeks to 1 year. All included trials had a high risk of bias with short follow‐up. Compared with Chinese herbs, needle acupuncture plus Chinese herbs was not effective in achieving at least 50% reduction in seizure frequency (80% in control group versus 90% in intervention group, RR 1.13, 95% CI 0.97 to 1.31, 2 trials; assumed risk 500 per 1000, corresponding risk 485 to 655 per 1000). Compared with valproate, needle acupuncture plus valproate was not effective in achieving freedom from seizures (44% in control group versus 42.7% in intervention group, RR 0.97, 95% CI 0.72 to 1.30, 2 trials; assumed risk 136 per 1000, corresponding risk 97 to 177 per 1000) or at least 50% reduction in seizure frequency (69.3% in control group versus 81.3% in intervention group, RR 1.34, 95% CI 0.52 to 3.48, 2 trials; assumed risk 556 per 1000, corresponding risk 289 to 1000 per 1000) but may have achieved better quality of life (QOL) after treatment (QOLIE‐31 score (higher score indicated better QOL) mean 170.22 points in the control group versus 180.32 points in the intervention group, MD 10.10 points, 95% CI 2.51 to 17.69 points, 1 trial). Compared with phenytoin, needle acupuncture was not effective in achieving at least 50% reduction in seizure frequency (70% in control group versus 94.4% in intervention group, RR 1.43, 95% CI 0.46 to 4.44, 2 trials; assumed risk 700 per 1000, corresponding risk 322 to 1000 per 1000). Compared with valproate, needle acupuncture was not effective in achieving seizure freedom (14.1% in control group versus 25.2% in intervention group, RR 1.75, 95% CI 0.93 to 3.27, 2 trials; assumed risk 136 per 1000, corresponding risk 126 to 445 per 1000) but may be effective in achieving at least 50% reduction in seizure frequency (55.3% in control group versus 73.7% in intervention group, RR 1.32, 95% CI 1.05 to 1.66, 2 trials; assumed risk 556 per 1000, corresponding risk 583 to 923 per 1000) and better QOL after treatment (QOLIE‐31 score mean 172.6 points in the control group versus 184.64 points in the intervention group, MD 12.04 points, 95% CI 4.05 to 20.03 points, 1 trial). Compared with antiepileptic drugs, catgut implantation at acupoints plus antiepileptic drugs was not effective in achieving seizure freedom (13% in control group versus 19.6% in intervention group, RR 1.51, 95% CI 0.93 to 2.43, 4 trials; assumed risk 127 per 1000, corresponding risk 118 to 309 per 1000) but may be effective in achieving at least 50% reduction in seizure frequency (63.1% in control group versus 82% in intervention group, RR 1.42, 95% CI 1.07 to 1.89, 5 trials; assumed risk 444 per 1000, corresponding risk 475 to 840 per 1000) and better QOL after treatment (QOLIE‐31 score (higher score indicated worse quality of life) mean 53.21 points in the control group versus 45.67 points in the intervention group, MD ‐7.54 points, 95% CI ‐14.47 to ‐0.61 points, 1 trial). Compared with valproate, catgut implantation may be effective in achieving seizure freedom (8% in control group versus 19.7% in intervention group, RR 2.82, 95% CI 1.61 to 4.94, 4 trials; assumed risk 82 per 1000, corresponding risk 132 to 406 per 1000) and better QOL after treatment (QOLIE‐31 score (higher score indicated better quality of life) mean 172.6 points in the control group versus 191.33 points in the intervention group, MD 18.73 points, 95% CI 11.10 to 26.36 points, 1 trial) but not at least 50% reduction in seizure frequency (65.6% in control group versus 91.7% in intervention group, RR 1.31, 95% CI 0.94 to 1.84, 4 trials; assumed risk 721 per 1000, corresponding risk 677 to 1000 per 1000). Acupuncture did not have excess adverse events compared to control treatment in the included trials.
Authors' conclusions
Available RCTs are small, heterogeneous and have high risk of bias. The current evidence does not support acupuncture for treating epilepsy.
Plain language summary
Acupuncture for epilepsy
People with epilepsy are currently treated with antiepileptic drugs but a significant number of people continue to have seizures and many experience adverse effects to the drugs. As a result, there is increasing interest in alternative therapies and acupuncture is one of those. Seventeen randomised controlled trials with 1538 participants were included in the current systematic review (literature search conducted on 3rd June 2013).
Compared with Chinese herbs, needle acupuncture plus Chinese herbs was not effective in achieving satisfactory seizure control (at least 50% reduction in seizure frequency). If we assumed that 500 out of 1000 patients treated with Chinese herbs alone normally achieved satisfactory seizure control, we estimated that 485 to 655 out of 1000 patients treated with needle acupuncture plus Chinese herbs would achieve satisfactory seizure control. Compared with valproate, needle acupuncture plus valproate was not effective in achieving freedom from seizures or satisfactory seizure control. If we assumed that 136 out of 1000 patients treated with valproate alone normally achieved seizure freedom, we estimated that about 97 to 177 out of 1000 patients treated with acupuncture plus valproate would achieve seizure freedom; if we assumed that 556 out of 1000 patients treated with valproate alone normally achieved satisfactory seizure control, we estimated that about 289 to 1000 out of 1000 patients treated with acupuncture plus valproate would achieve satisfactory seizure control. Compared with phenytoin, needle acupuncture was not effective in achieving satisfactory seizure control. If we assumed that 700 out of 1000 patients treated with phenytoin alone normally achieved satisfactory seizure control, we estimated that about 322 to 1000 out of 1000 patients treated with acupuncture alone would achieve satisfactory seizure control. Compared with valproate, needle acupuncture was not effective in achieving seizure freedom but it may have been better in achieving satisfactory seizure control. If we assumed that 136 out of 1000 patients treated with valproate alone normally achieved seizure freedom, we estimated that about 126 to 445 out of 1000 patients treated with acupuncture alone would achieve seizure freedom; if we assumed that 556 out of 1000 patients treated with valproate alone normally achieved satisfactory seizure control, we estimated that about 583 to 923 out of 1000 patients treated with acupuncture alone would achieve satisfactory seizure control. Compared with antiepileptic drugs, catgut implantation at acupoints plus antiepileptic drugs was not effective in achieving seizure freedom, but it may have been better in achieving satisfactory seizure control. If we assumed that 127 out of 1000 patients treated with antiepileptic drugs alone normally achieved seizure freedom, we estimated that about 118 to 309 out of 1000 patients treated with catgut implantation at acupoints plus antiepileptic drugs would achieve seizure freedom; If we assumed that 444 out of 1000 patients treated with antiepileptic drugs alone normally achieved satisfactory seizure control, we estimated that about 475 to 840 out of 1000 patients treated with catgut implantation at acupoints plus antiepileptic drugs would achieve satisfactory seizure control. Compared with valproate, catgut implantation may have been better in achieving seizure freedom but not satisfactory seizure control. If we assumed that 82 out of 1000 patients treated with valproate alone normally achieved seizure freedom, we estimated that about 132 to 406 out of 1000 patients treated with catgut implantation at acupoints alone would achieve seizure freedom; if we assumed that 721 out of 1000 patients treated with valproate alone normally achieved satisfactory seizure control, we estimated that about 677 to 1000 out of 1000 patients treated with catgut implantation at acupoints alone would achieve satisfactory seizure control.
Acupuncture did not have excess adverse events compared to control treatment in the included trials. However, the included trials were small, heterogeneous and had a high risk of bias. It remains uncertain whether acupuncture is effective and safe for treating people with epilepsy.
Summary of findings
Summary of findings for the main comparison. Summary of findings: needle acupuncture plus Chinese herbs versus Chinese herbs alone.
| Needle acupuncture plus Chinese herbs compared with Chinese herbs for epilepsy | ||||||
|
Patient or population: children (0‐18 years) with generalised epilepsy Settings: hospital inpatients and outpatients Intervention: Needle acupuncture plus Chinese herbs Comparison: Chinese herbs alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Chinese herbs alone | Needle acupuncture plus Chinese herbs | |||||
|
50% or greater reduction in seizure frequency (follow‐up: 6 months) |
500 per 1000 | 565 per 1000 (485 to 655) | RR 1.13 (0.97 to 1.31) | 120 (2) | ⊕⊕⊕⊝ moderatea | |
|
Adverse effects (follow‐up: 6 months) |
See comment | See comment | Not estimable | 120 (2) |
See comment | None of included studies reported adverse effects. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. Evidence from RCT downgraded by one level because of high risk of bias in study design.
Summary of findings 2. Summary of findings: needle acupuncture plus valproate versus valproate alone.
| Needle acupuncture plus valproate compared with valproate alone for epilepsy | ||||||
|
Patient or population: participants with generalised epilepsy Settings: hospital outpatients (one included study recruited outpatients only, the other included study did not specify the patient settings) Intervention: Needle acupuncture plus valproate Comparison: Valproate alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| valproate alone | Needle acupuncture plus valproate | |||||
|
Seizure freedom (follow‐up: 3‐6 months) |
136 per 1000 | 132 per 1000 (97 to 177) | RR 0.97 (0.72 to 1.30) | 150 (2) | ⊕⊕⊕⊝ moderatea | |
|
50% or greater reduction in seizure frequency (follow‐up: 3‐6 months) |
556 per 1000 | 745 per 1000 (289 to 1000) | RR 1.34 (0.52 to 3.48) | 150 (2) | ⊕⊕⊝⊝ lowb | |
|
Post‐treatment quality of life (QOLIE‐31 score, which has a range of 0‐200, with higher score indicates better quality of life) (follow‐up: 6 months) |
The mean post‐treatment quality of life across control groups ranged from 170.22 to 172.6 points. | The mean post‐treatment quality of life in the intervention group was 10.1 points higher (2.51 points higher to 17.69 points higher). | 90 (1) |
⊕⊕⊝⊝ lowb | ||
|
Frequency of adverse effects ‐ dizziness (follow‐up: 6 months) |
160 per 1000 | 107 per 1000 (19 to 608) | RR 0.67 (0.12 to 3.80) | 90 (1) | ⊕⊕⊝⊝ lowb | |
|
Frequency of adverse effects ‐ malaise (follow‐up: 6 months) |
233 per 1000 | 193 per 1000 (62 to 592) | RR 0.83 (0.27 to 2.54) | 90 (1) | ⊕⊕⊝⊝ lowb | |
|
Frequency of adverse effects ‐ nausea (follow‐up: 6 months) |
140 per 1000 | 96 per 1000 (21 to 331) | RR 0.60 (0.15 to 2.36) | 90 (1) | ⊕⊕⊝⊝ lowb | |
|
Frequency of adverse effects ‐ sleepiness (follow‐up: 6 months) |
119 per 1000 | 84 per 1000 (28 to 248) | RR 0.71 (0.24 to 2.08) | 90 (1) | ⊕⊕⊝⊝ lowb | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. Evidence from RCT downgraded by one level because of high risk of bias in study design.
b. Evidence from RCT downgraded by two levels because of high risk of bias in study design and imprecise result.
Summary of findings 3. Summary of findings: needle acupuncture versus sham acupuncture.
| Needle acupuncture compared with sham acupuncture for epilepsy | ||||||
|
Patient or population: adults with intractable epilepsy Settings: hospital outpatients Intervention: Needle acupuncture Comparison: Sham acupuncture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| sham acupuncture | needle acupuncture | |||||
|
Percentage reduction in seizure frequency (follow‐up: 12 weeks) |
The median reduction in seizure frequency was 20%. | The median reduction in seizure frequency was 45%. | 34 (1) |
⊕⊕⊝⊝ lowa | No standard deviation or confidence interval or P value was provided to estimate the confidence interval. | |
|
Improvement in quality of life (QOLIE‐89 score, which has a range of 0‐100, with higher score indicates better quality of life) (follow‐up: 12 weeks) |
The mean improvement in quality of life was 1.7 points. | The mean improvement in quality of life in the intervention group was 3.4 points lower (14.45 points lower to 7.65 points higher). | 22 (1) |
⊕⊕⊝⊝ lowa | ||
|
Withdrawal due to lack of efficacy (follow‐up: 12 weeks) |
125 per 1000 | 166 per 1000 (31 to 875) | RR 1.33 (0.25 to 7.00) | 34 (1) | ⊕⊕⊝⊝ lowa | |
|
Adverse effects (follow‐up: 12 weeks) |
See comment | See comment | Not estimable | 120 (2) |
See comment | The included study did not report adverse effects. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. Evidence from RCT downgraded by two levels because of high risk of bias in study design and imprecise result.
Summary of findings 4. Summary of findings: needle acupuncture versus phenytoin.
| Needle acupuncture compared with phenytoin for epilepsy | ||||||
|
Patient or population: participants with epilepsy Settings: hospital outpatients (one included study recruited outpatients only, the other included study did not specify the patient settings) Intervention: needle acupuncture Comparison: phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| phenytoin | needle acupuncture | |||||
|
50% or greater reduction in seizure frequency (follow‐up: 6 months) |
700 per 1000 | 1000 per 1000 (322 to 1000) | RR 1.43 (0.46 to 4.44) | 150 (2) | ⊕⊕⊝⊝ lowa | |
|
Adverse effects (follow‐up: 6 months) |
See comment | See comment | Not estimable | 120 (2) |
See comment | The included study did not report adverse effects. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. Evidence from RCT downgraded by two levels because of high risk of bias in study design and imprecise result.
Summary of findings 5. Summary of findings: needle acupuncture versus valproate.
| Needle acupuncture compared with valproate for epilepsy | ||||||
|
Patient or population: participants with epilepsy (one included study only recruited children with absence epilepsy while another included study recruited both children and adults with generalised epilepsy) Settings: hospital inpatients and outpatients Intervention: needle acupuncture Comparison: valproate | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| valproate | needle acupuncture | |||||
|
Seizure freedom (follow‐up: 3 months to 1 year) |
136 per 1000 | 238 per 1000 (126 to 445) | RR 1.75 (0.93 to 3.27) | 180 (2) | ⊕⊕⊝⊝ lowa | |
|
50% or greater reduction in seizure frequency (follow‐up: 3 months to 1 year) |
556 per 1000 | 734 per 1000 (583 to 923) | RR 1.32 (1.05 to 1.66) | 180 (2) | ⊕⊕⊝⊝ lowa | |
|
Post‐treatment quality of life (QOLIE‐31 score, which has a range of 0‐200, with higher score indicates better quality of life) (follow‐up: 3 months) |
The mean post‐treatment quality of life across control groups ranged from 170.22 to 172.6 points. | The mean post‐treatment quality of life in the intervention group was 12.04 points higher (4.05 points higher to 20.03 points higher). | 100 (1) |
⊕⊕⊝⊝ lowa | ||
|
Frequency of adverse effects ‐ dizziness (follow‐up: 3 months) |
160 per 1000 | 181 per 1000 (75 to 429) | RR 1.13 (0.47 to 2.68) | 100 (1) |
⊕⊕⊝⊝ lowa | |
|
Frequency of adverse effects ‐ malaise (follow‐up: 3 months) |
233 per 1000 | 161 per 1000 (76 to 343) | RR 0.69 (0.33 to 1.47) | 100 (1) |
⊕⊕⊝⊝ lowa | |
|
Frequency of adverse effects ‐ nausea (follow‐up: 3 months) |
140 per 1000 | 20 per 1000 (2 to 157) | RR 0.14 (0.02 to 1.12) | 100 (1) |
⊕⊕⊝⊝ lowa | |
|
Frequency of adverse effects ‐ sleepiness (follow‐up: 3 months) |
119 per 1000 | 71 per 1000 (17 to 284) | RR 0.60 (0.15 to 2.38) | 100 (1) |
⊕⊕⊝⊝ lowa | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. Evidence from RCT downgraded by two levels because of high risk of bias in study design and imprecise result.
Summary of findings 6. Summary of findings: catgut implantation at acupoints plus antiepileptic drugs versus antiepileptic drugs alone.
| Catgut implantation at acupoints plus antiepileptic drugs compared with antiepileptic drugs alone for epilepsy | ||||||
|
Patient or population: participants with epilepsy Settings: hospital inpatients and outpatients Intervention: catgut implantation at acupoints plus antiepileptic drugs Comparison: antiepileptic drugs alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| antiepileptic drugs alone | catgut implantation at acupoints plus antiepileptic drugs | |||||
|
Seizure freedom (follow‐up: 2 months to 1 year) |
127 per 1000 | 192 per 1000 (118 to 309) | RR 1.51 (0.93 to 2.43) | 361 (4) | ⊕⊕⊝⊝ lowa | |
|
50% or greater reduction in seizure frequency (follow‐up: 2 months to 1 year) |
444 per 1000 | 630 per 1000 (475 to 840) | RR 1.42 (1.07 to 1.89) | 401 (5) | ⊕⊕⊝⊝ lowa | |
|
Post‐treatment quality of life (QOLIE‐31 score, which has a range of 0‐100, with higher score indicates worse quality of life) (follow‐up: 3 months) |
The mean post‐treatment quality of life was 53.21 points. | The mean post‐treatment quality of life in the intervention group was 7.54 points lower (14.47 points lower to 0.61 points lower). | 120 (1) | ⊕⊕⊝⊝ lowa | ||
|
Frequency of adverse effects ‐ dizziness (follow‐up: 3 months) |
160 per 1000 | 53 per 1000 (20 to 138) | RR 0.33 (0.13 to 0.86) | 120 (1) | ⊕⊕⊝⊝ lowa | |
|
Frequency of adverse effects ‐ malaise (follow‐up: 3 months) |
233 per 1000 | 117 per 1000 (51 to 268) | RR 0.50 (0.22 to 1.15) | 120 (1) | ⊕⊕⊝⊝ lowa | |
|
Frequency of adverse effects ‐ nausea (follow‐up: 3 months) |
140 per 1000 | 46 per 1000 (12 to 164) | RR 0.33 (0.09 to 1.17) | 120 (1) | ⊕⊕⊝⊝ lowa | |
|
Frequency of adverse effects ‐ anorexia (follow‐up: 3 months) |
180 per 1000 | 45 per 1000 (10 to 204) | RR 0.25 (0.06 to 1.13) | 120 (1) | ⊕⊕⊝⊝ lowa | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. Evidence from RCT downgraded by two levels because of high risk of bias in study design and imprecise result.
Summary of findings 7. Summary of findings: catgut implantation at acupoints versus valproate.
| Catgut implantation at acupoints compared with valproate for epilepsy | ||||||
|
Patient or population: participants with generalised epilepsy Settings: hospital inpatients and outpatients Intervention: catgut implantation at acupoints Comparison: valproate | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| valproate | catgut implantation at acupoints | |||||
|
Seizure freedom (follow‐up: 3 months to 1 year) |
82 per 1000 | 231 per 1000 (132 to 406) | RR 2.82 (1.61 to 4.94) | 381 (4) |
⊕⊕⊝⊝ lowa | |
|
50% or greater reduction in seizure frequency (follow‐up: 3 months to 1 year) |
721 per 1000 | 945 per 1000 (677 to 1000) | RR 1.31 (0.94 to 1.84) | 381 (4) |
⊕⊕⊝⊝ lowa | |
|
Post‐treatment quality of life (QOLIE‐31 score, which has a range of 0‐200, with higher score indicates better quality of life) (follow‐up: 3 months) |
The mean post‐treatment quality of life across control groups ranged from 170.22 to 172.6 points. | The mean post‐treatment quality of life in the intervention group was 18.73 points higher (11.10 points higher to 26.36 points higher). | 100 (1) |
⊕⊕⊝⊝ lowa | ||
|
Frequency of adverse effects ‐ dizziness (follow‐up: 3 months) |
160 per 1000 | 101 per 1000 (35 to 285) | RR 0.63 (0.22 to 1.78) | 100 (1) |
⊕⊕⊝⊝ lowa | |
|
Frequency of adverse effects ‐ malaise (follow‐up: 3 months) |
233 per 1000 | 214 per 1000 (109 to 425) | RR 0.92 (0.47 to 1.82) | 100 (1) |
⊕⊕⊝⊝ lowa | |
|
Frequency of adverse effects ‐ nausea (follow‐up: 3 months) |
140 per 1000 | 20 per 1000 (2 to 157) | RR 0.14 (0.02 to 1.12) | 100 (1) |
⊕⊕⊝⊝ lowa | |
|
Frequency of adverse effects ‐ anorexia (follow‐up: 3 months) |
180 per 1000 | 40 per 1000 (9 to 177) | RR 0.22 (0.05 to 0.98) | 100 (1) |
⊕⊕⊝⊝ lowa | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. Evidence from RCT downgraded by two levels because of high risk of bias in study design and imprecise result.
Background
Description of the condition
Epilepsy is a common neurological disorder with an estimated annual incidence of 50 per 100,000 and a prevalence of 5 to 10 per 1000 in developed countries (Sander 1996). About 2% to 3% of the general population will be given a diagnosis of epilepsy at some time in their lives (Hauser 1993), the majority of whom will go into remission. About 70% of patients with epilepsy become seizure free but up to 30% continue to have seizures despite treatment with adequate doses of antiepileptic drugs (AEDs), that is they become drug resistant. Hence there is a constant search for newer modes of treatment. Furthermore, the commonly used AEDs can have adverse effects such as causing gingival hyperplasia; gastrointestinal disturbances (nausea, vomiting); osteoporosis, osteomalacia, and bone marrow toxicity; hepatotoxicity; nephrotoxicity; neurological symptoms (ataxia, dizziness, diplopia, somnolence); cognitive, mood and behavioural disturbances; endocrine dysfunction; and teratogenicity; as well as allergic reactions including skin rashes, toxic epidermal necrolysis or Steven Johnson syndrome (Holland 2001). Other treatments for epilepsy such as a ketogenic diet, use of a vagal nerve stimulator, and epilepsy surgery have their own limitations and complications. As a result, many people are turning to alternative complementary therapy for treatment of their condition and acupuncture is one of the popular options.
Description of the intervention
Acupuncture is a procedure in which specific body areas, the meridian points, are pierced with fine needles for therapeutic purposes. It is one of the major modalities of treatment in traditional Chinese medicine. In China its use can be traced back more than 2000 years (Wu 1996). Apart from the traditional needle acupuncture various forms of acupuncture have been developed, including electroacupuncture, laser acupuncture, acupressure, and catgut implantation at acupoints. Being a relatively simple, inexpensive and safe treatment, acupuncture has been well accepted by inhabitants in China and people worldwide who are of Chinese origin. Acupuncture is widely used by many Chinese practitioners in various neurological disorders as an alternative treatment approach (Johansson 1993). It is also increasingly practiced in some Western countries (NIH 1998).
How the intervention might work
Acupuncture involves complex theories of regulation of the five elements (fire, earth, metal, water, and wood), yin and yang, Qi, and blood and body fluids. By stimulating various meridian points disharmony and dysregulation of organ systems is corrected to relieve symptoms and restore natural internal homeostasis (Maciocia 1989). Many studies in animals and humans have demonstrated that acupuncture can cause multiple biological responses (Wang 2001). These responses can occur both locally or close to the site of application (Jansen 1989) and at a distance, mediated mainly by the sensory neurons to many structures that are within the central nervous system (Magnusson 1994). The result is activation of pathways affecting various physiological systems in the brain as well as in the periphery (Liu 2004; Middlekauff 2004; Sun 2001).
There are both anecdotal reports and animal studies that suggest acupuncture may inhibit seizures. In an experiment of penicillin‐induced epilepsy in rats electroacupuncture was found to inhibit seizures, possibly through decreasing neuronal and inducible nitric oxide synthase transcription in the hippocampus (Huang 1999; Yang 2000). Antagonism of gamma‐aminobutyric acid type A (GABA‐A) receptors was found to attenuate the antiepileptic effect of electroacupuncture, whilst electroacupuncture acted synergistically with the antagonists of non‐N‐methyl‐D‐aspartate (non‐NMDA) receptors (Liu 1997). Electroacupuncture may theoretically have an effect on epilepsy by increasing the release of inhibitory neurotransmitters (Liu 1995; Wu 1992) such as serotonin, GABA, or opioid peptides. Beneficial effects on human epilepsy have been reported in uncontrolled studies (Shi 1987; Yang 1990).
Why it is important to do this review
Reports on the effects of acupuncture on electroencephalographic recordings have been conflicting (Chen 1983; Kloster 1999) and it is unclear whether the existing evidence is scientifically rigorous enough to recommend acupuncture for routine use in people with epilepsy. We examined the efficacy and safety of acupuncture therapy in epilepsy in a systematic review of randomised controlled trials.
This is an update of a Cochrane review first published in 2006.
Objectives
To determine the effectiveness and safety of acupuncture in people with epilepsy.
We investigated the following hypotheses.
Acupuncture can increase the probability of becoming seizure free.
Acupuncture can reduce the frequency and duration of seizures.
Acupuncture can improve quality of life.
Acupuncture is associated with adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials using truly random or quasi‐random allocation of treatment in the review.
We included studies comparing acupuncture with at least one control group that used no treatment, placebo treatment, sham treatment or AED.
Studies were single or double blind or unblinded.
We included parallel group or cross‐over designs.
Types of participants
People with an epilepsy syndrome of any type (Commission 1989) who were of any age and of either gender.
Types of interventions
We planned to include trials evaluating all forms of acupuncture therapy including acupressure, laser acupuncture, electroacupuncture or catgut implantation at acupoints in the review regardless of times of treatment and length of the treatment period. We included both traditional acupuncture in classical meridian points and contemporary acupuncture in non‐meridian or trigger points regardless of the source or methods of stimulation (for example, hand, needle, laser, electrical stimulation, or catgut implantation). Acupuncture could be given alone or as an add‐on to AEDs.
The control interventions that were considered included placebo acupuncture, sham acupuncture or AEDs. Placebo acupuncture refers to a needle attached to the skin surface (not penetrating the skin but at the same acupoint) (Furlan 1999). Sham acupuncture refers to a needle placed in an area close to, but not in, the acupuncture point (Furlan 1999) or subliminal skin electrostimulation via electrodes attached to the skin (SCSSS 1999).
We investigated the following treatment comparisons.
Acupuncture alone compared with no treatment.
Acupuncture alone compared with placebo or sham treatment or antiepileptic medication.
Acupuncture in addition to baseline AED compared with baseline AED alone.
Acupuncture in addition to baseline AED compared with placebo or sham treatment in addition to baseline AED.
Trials that only compared different forms of acupuncture were excluded since we did not intend to investigate whether one type of acupuncture was more effective than another. Trials that compared acupuncture in addition to herbal medicines or other alternative therapies with AED treatment were also excluded since such trial cannot resolve which component of the combination, that is acupuncture or herbs, is more effective than control treatment.
Types of outcome measures
Primary outcomes
Seizure freedom.
Satisfactory seizure control: 50% or greater reduction in seizure frequency.
Absolute or percentage reduction in seizure frequency and duration.
Improved quality of life if assessed by validated, reliable scales.
Secondary outcomes
-
Incidence of adverse or harmful effects:
sedation;
cognitive side effects;
allergic reactions ‐ skin rashes, Steven Johnson syndrome.
Withdrawals due to side effects or lack of efficacy.
Search methods for identification of studies
Electronic searches
We searched the following databases.
Cochrane Epilepsy Group Specialised Register (3 June 2013).
Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2013, Issue 5) searched on 3 June 2013 using the search strategy set out in Appendix 1.
MEDLINE (Ovid) (1946 to 30 May 2013) using the search strategy set out in Appendix 2.
EMBASE (Ovid) (1947 to 4 June 2013) using the search strategy set out in Appendix 3
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (EBSCOhost) (1937 to 3 June 2013) using the search strategy set out in Appendix 4.
AMED (Allied and Complementary Medicine Database) (EBSCOhost) (1985 to 3 June 2013) using the search strategy set out in Appendix 5.
World Health Organization International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/ searched on 3 June 2013) using the search terms "epilep* AND acupuncture", and "seizure* AND acupuncture".
Chinese literature databases, including China Journals Full‐text Database, China Master thesis Full‐text Database, and China Doctor Dissertations Full‐text Database (1999 to 4 June 2013) using the search strategy set out in Appendix 6.
Searching other resources
We searched the reference lists of all relevant papers for further studies. In addition, we contacted colleagues and experts in the field to ascertain any unpublished or ongoing studies. There were no language restrictions either in the search or inclusion of studies. We considered multiple publications reporting the same groups of patients or subsets as the same trial.
Data collection and analysis
Selection of studies
Two review authors (Daniel Ka Leung Cheuk and Virginia Wong) independently assessed trials for inclusion. We resolved any disagreements between the two review authors by mutual discussion.
Data extraction and management
We extracted the following data, if available.
-
Study methods:
design (e.g. parallel or cross‐over design);
randomisation method (including list generation);
method of allocation concealment;
blinding method;
stratification factors.
-
Participants:
inclusion and exclusion criteria;
number (total and per group);
age and sex distribution;
seizure type and epilepsy syndrome;
duration of epilepsy;
etiology of epilepsy;
seizure frequency;
presence of neurological signs;
number and types of AEDs taken.
-
Intervention and control:
type of acupuncture;
details of treatment regimen including duration of treatment;
type of control;
details of control treatment including drug dosage;
washout period if cross‐over design.
-
Follow‐up data:
duration of follow‐up;
dates of treatment withdrawal and reasons for treatment withdrawal;
withdrawal rates.
-
Outcome data:
as described above.
-
Analysis data:
methods of analysis (intention‐to‐treat and per protocol analysis);
comparability of groups at baseline (yes or no);
statistical techniques.
Assessment of risk of bias in included studies
Two review authors (Daniel Ka Leung Cheuk and Virginia Wong) independently assessed the risk of bias in each included study according to the Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011, section 8.5.1). We resolved any disagreements between the two review authors by mutual discussion.
For each study we assessed the following items to see whether:
the allocation sequence was adequately generated ('sequence generation');
the allocation was adequately concealed ('allocation concealment');
knowledge of the allocated interventions was adequately prevented during the study ('blinding'), for participants, personnel and outcome assessors;
incomplete outcome data were adequately addressed;
reports of the study were free of suggestion of selective outcome reporting;
the study was apparently free of other problems that could put it at high risk of bias.
We allocated each domain one of three possible categories: 'Yes' for low risk of bias, 'No' for high risk of bias, and 'Unclear' where the risk of bias was uncertain or unknown.
Measures of treatment effect
We used risk ratios (RR) with 95% confidence intervals (CI) for binary outcomes (seizure freedom, 50% or greater reduction in seizure frequency, frequency of adverse effects, and withdrawal due to adverse effects), and mean differences (MD) with 95% CI for continuous outcomes (quality of life, absolute or percentage reduction in seizure frequency and duration). To account for multiple statistical testing we used 99% confidence intervals for the frequency of adverse effects if there were several adverse effects reported.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the distribution of important participant factors between trials (age, gender, seizure type, duration of epilepsy, number of AEDs taken at time of randomisation) and trial factors (randomisation concealment, blinding, losses to follow‐up). Statistical heterogeneity was assessed by the I2 statistic (Higgins 2011, section 9.5.2), where a value greater than 50% was considered to indicate substantial heterogeneity.
Assessment of reporting biases
We planned to use funnel plots (effect size against standard error) if sufficient studies (more than five) were found. Asymmetry could be due to publication bias but could also be due to a relationship between trial size and effect size. In the event that a relationship was found, we planned to examine the clinical diversity of the studies (Higgins 2011, section 10.4). However, there were no more than five studies reporting the same outcome and therefore we did not draw a funnel plot.
Data synthesis
Where the interventions were the same or similar enough, and if there was no important clinical heterogeneity, we synthesised the results in a meta‐analysis. If no significant statistical heterogeneity was present we synthesised the data using a fixed‐effect model, otherwise we used a random‐effects model for analysis. The analyses included all participants in the treatment groups to which they were allocated (that is intention‐to‐treat analyses) as far as possible.
Subgroup analysis and investigation of heterogeneity
We planned to assess the impact of important patient characteristics including seizure type, duration and aetiology of epilepsy and presence of neurological signs upon the outcome, although insufficient data were available to do so.
Sensitivity analysis
We also planned to undertake sensitivity analyses including: (i) all studies; (ii) only those not having a high risk of bias. However, since all included studies had a high risk of bias, we did not perform a sensitivity analysis.
Results
Description of studies
Results of the search
We obtained a total of 387 results from the electronic search of the databases. We did not identify any additional articles from references of relevant articles. After we removed duplicates, 361 articles remained. We screened the titles and abstracts of these against the inclusion and exclusion criteria for study selection and excluded 329 references based on titles or abstracts alone. We obtained the full texts of the remaining 32 articles and assessed these for eligibility. We excluded 11 of these studies, with the reasons stated in the table Characteristics of excluded studies. One of the remaining studies is ongoing and is described in the table Characteristics of ongoing studies. The remaining 17 studies described by 20 papers (three studies were published in two papers each) fulfilled the inclusion criteria and we included these in the review (described in the table Characteristics of included studies). The flow of records is summarised in Figure 1. This included one new study in the current updated review (June 2013).
1.
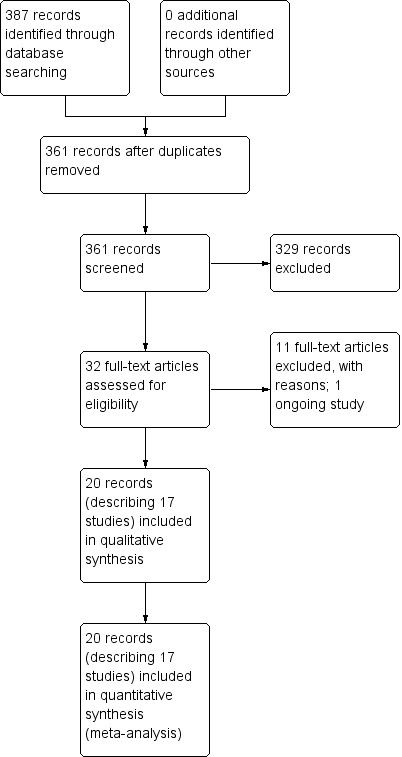
Study flow diagram.
Included studies
Seventeen randomised controlled trials met the inclusion criteria (Deng 2001a; Han 2008; Kloster 1999; Leng 2000; Li 2007; Ma 2001; Mao 2011; Peng 2003; Shi 2001; Xiong 2003; Yi 2009; Yu 1999; Zhang 2006a; Zhang 2006b; Zhou 2000; Zhuang 2004; Zhuang 2006). Details of the included studies are summarised below.
Three of the included trials were published in two records (Kloster 1999; Ma 2001; Peng 2003). One trial was performed in Norway and was published in English (Kloster 1999) while the remaining 16 trials were performed in China and published in Chinese (Deng 2001a; Han 2008; Leng 2000; Li 2007; Ma 2001; Mao 2011; Peng 2003; Shi 2001; Xiong 2003; Yi 2009; Yu 1999; Zhang 2006a; Zhang 2006b; Zhou 2000; Zhuang 2004; Zhuang 2006); they were translated for this review. The 17 included trials recruited a total of 1538 people with epilepsy. All trials recruited participants in the hospital setting. Five trials recruited hospital inpatients and outpatients (Li 2007; Ma 2001; Shi 2001; Zhang 2006b; Zhuang 2006), while four trials recruited outpatients only (Kloster 1999; Mao 2011; Yi 2009; Yu 1999) and eight trials did not specify the patient setting (Deng 2001a; Han 2008; Leng 2000; Peng 2003; Xiong 2003; Zhang 2006a; Zhou 2000; Zhuang 2004). One trial included only adults (Kloster 1999), four trials included only children (Ma 2001; Peng 2003; Shi 2001; Xiong 2003), and the remaining 12 trials included both adults and children (Deng 2001a; Han 2008; Leng 2000; Li 2007; Mao 2011; Yi 2009; Yu 1999; Zhang 2006a; Zhang 2006b; Zhou 2000; Zhuang 2004; Zhuang 2006). Four trials included patients with both partial and generalized epilepsy (Kloster 1999; Yu 1999; Zhou 2000; Zhuang 2004), while 12 trials included only patients with generalized epilepsy (Deng 2001a; Han 2008; Leng 2000; Li 2007; Ma 2001; Mao 2011; Peng 2003; Xiong 2003; Yi 2009; Zhang 2006a; Zhang 2006b; Zhuang 2006), and one trial included only patients with childhood absence epilepsy (Shi 2001). The duration of epilepsy was highly variable in the included trials, ranging from three days to over 30 years. The baseline seizure frequency varied from once every four to six months to many times a day. Aetiologies of epilepsy in the patients were reported in only four trials, which encompassed a wide variety in three of the trials (Ma 2001; Xiong 2003; Zhuang 2004) and only childhood absence in one trial (Shi 2001). Neurological signs were not mentioned in any of the trials. One trial reported that patients were taking a mean of two AEDs before entering the trial (Kloster 1999) and one trial excluded patients who were currently using an AED (Peng 2003). One trial summarised what kinds of drugs patients were taking without mentioning the average number of AEDs that patients required before entering the trial (Xiong 2003). Two trials simply mentioned that some patients were taking AEDs without giving details (Ma 2001; Yu 1999). The remaining 12 trials did not mention anything about the drug history of the participants (Deng 2001a; Han 2008; Leng 2000; Li 2007; Mao 2011; Shi 2001; Yi 2009; Zhou 2000; Zhang 2006a; Zhang 2006b; Zhuang 2004; Zhuang 2006).
The type of acupuncture used in the included trials varied. Traditional needle acupuncture was used in six trials (Ma 2001; Shi 2001; Xiong 2003; Yi 2009; Yu 1999; Zhou 2000) and catgut implantation into acupoints was used in eight trials (Deng 2001a; Han 2008; Leng 2000; Li 2007; Mao 2011; Peng 2003; Zhuang 2004; Zhuang 2006). Two trials included two treatment groups, catgut implantation in one group and needle acupuncture in the other (Zhang 2006a; Zhang 2006b). One trial combined traditional needle acupuncture and electroacupuncture in the treatment group (Kloster 1999). The acupoints chosen were highly variable in the included trials . While the chosen acupoints were fixed and universally applied to all patients in eight trials (Leng 2000; Peng 2003; Shi 2001; Xiong 2003; Yu 1999; Zhang 2006a; Zhuang 2004; Zhuang 2006), the remaining nine trials (Deng 2001a; Han 2008; Kloster 1999; Li 2007; Ma 2001; Mao 2011; Yi 2009; Zhang 2006b; Zhou 2000) allowed some flexibility in the use of additional acupoints on top of the protocol acupoints set for all patients.
All 17 included trials used a parallel group, randomised controlled design. Two trials had two intervention groups (catgut implantation in acupoints with or without valproate, and needle acupuncture with or without valproate) and one control group (valproate alone) (Zhang 2006a; Zhang 2006b). One trial (Xiong 2003) employed two control groups (carbamazepine in one control group and Chinese herbs in the other), while the other 16 trials used only one control group. The controls chosen were sham acupuncture in one trial (Kloster 1999), Chinese herbal tablet in one trial (Ma 2001), phenytoin in two trials (Yu 1999; Zhou 2000), phenobarbital plus phenytoin in one trial (Zhuang 2004), and valproate in 12 trials (Deng 2001a; Han 2008; Leng 2000; Li 2007; Mao 2011; Peng 2003; Shi 2001; Yi 2009; Zhang 2006a; Zhang 2006b; Zhuang 2006). The duration of treatment ranged from 7.5 weeks to 12 months and the duration of follow‐up ranged from 12 weeks to 12 months.
Ten trials used freedom from seizures as an outcome (Deng 2001a; Leng 2000; Li 2007; Mao 2011; Peng 2003; Shi 2001; Yi 2009; Zhang 2006a; Zhang 2006b; Zhuang 2006). Fifteen trials reported the number of patients with good (75% or over), moderate (50% to 74%), or mild (25% to 49%) reductions in seizure frequency as outcomes (Deng 2001a; Leng 2000; Li 2007; Ma 2001; Mao 2011; Peng 2003; Shi 2001; Xiong 2003; Yi 2009; Yu 1999; Zhang 2006a; Zhang 2006b; Zhou 2000; Zhuang 2004; Zhuang 2006). Two trials reported post‐treatment seizure frequency as an outcome (Mao 2011; Zhou 2000). Two trials reported good (75% or over) or moderate (50% to 74%) reductions in seizure duration as outcomes (Ma 2001; Xiong 2003). Seven trials reported epilepsy score (Han 2008; Li 2007; Yi 2009; Zhang 2006a; Zhuang 2006; Zhang 2006b; Zhuang 2006) and four trials reported different degrees of epilepsy score improvement (Han 2008; Li 2007; Yi 2009; Zhang 2006b) as outcomes, but the epilepsy score was not clearly defined in any of the trials. One trial (Kloster 1999) used the percentage reduction in seizure frequency; percentage increase in seizure‐free weeks; and the numbers of patients who had their seizure frequency improved, remain static, or worsened as outcomes. This trial also reported withdrawal due to lack of efficacy (Kloster 1999). Four trials reported the quality of life (QOL) of the patients as an outcome (Kloster 1999; Li 2007; Yi 2009; Zhang 2006b). One trial also reported Global Clinical Improvement scores (Li 2007). Seven trials reported post‐treatment electroencephalogram (EEG) abnormality (Deng 2001a; Li 2007; Ma 2001; Xiong 2003; Yi 2009; Zhang 2006b) or degrees of EEG improvement (Kloster 1999; Ma 2001) as outcomes. Four trials reported adverse effects of treatment (Kloster 1999; Li 2007; Yi 2009; Zhang 2006b).
Excluded studies
The reasons for exclusion of the 11 studies were: non‐epilepsy population (Luo 2004; Yi 2005), comparisons of different acupuncture methods (Li 2004; Lin 2001; Xu 2003), acupuncture plus other treatment against acupuncture (Kuang 1996), acupuncture plus other treatment against AEDs or a different treatment (Chui 2006; Deng 2001b; Han 2005; Wu 2008; Xu 2004).
Risk of bias in included studies
All 17 iIncluded studies were of poor methodological quality with at least one area at high risk of bias. These findings are summarised in the table Characteristics of included studies, Figure 2 and Figure 3, and described below.
2.
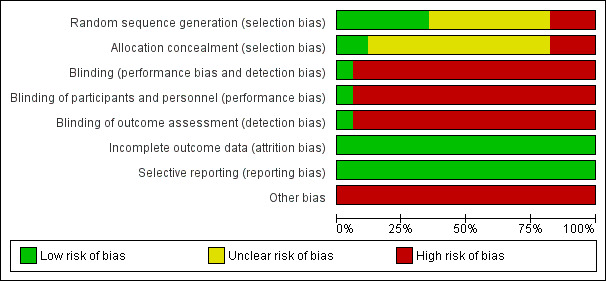
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
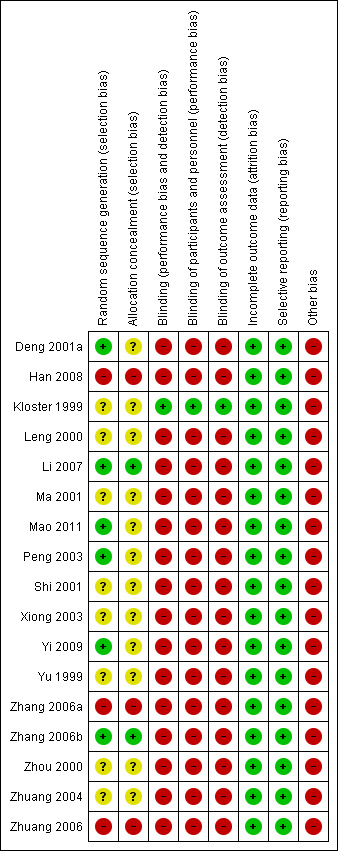
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Although all trials mentioned that the patients were randomly allocated to the intervention and control groups, the method of randomisation was not described in eight trials (Kloster 1999; Leng 2000; Ma 2001; Shi 2001; Xiong 2003; Yu 1999; Zhou 2000; Zhuang 2004) and sequence generation was considered to be inadequate in three trials (Han 2008; Zhang 2006a; Zhuang 2006) since they allocated participants according to sequence of attendance. Allocation concealment was not reported in 12 trials (Deng 2001a; Kloster 1999; Leng 2000; Ma 2001; Mao 2011; Peng 2003; Shi 2001; Xiong 2003; Yi 2009; Yu 1999; Zhou 2000; Zhuang 2004) and was considered inadequate in three trials (Han 2008; Zhang 2006a; Zhuang 2006).
Blinding
Sixteen of the included trials did not blind the participants, the personnel, or the outcome assessors (Deng 2001a; Han 2008; Leng 2000; Li 2007; Ma 2001; Mao 2011; Peng 2003; Shi 2001; Xiong 2003; Yi 2009; Yu 1999; Zhang 2006a; Zhang 2006b; Zhou 2000; Zhuang 2004; Zhuang 2006). Only one trial blinded all parties (Kloster 1999).
Incomplete outcome data
There were no dropouts in 15 trials (Deng 2001a; Han 2008; Leng 2000; Li 2007; Ma 2001; Mao 2011; Peng 2003; Shi 2001; Xiong 2003; Yu 1999; Zhang 2006a; Zhang 2006b; Zhou 2000; Zhuang 2004; Zhuang 2006) and dropouts were accounted for in one trial (Kloster 1999) as the dropouts were mentioned to be due to lack of efficacy requiring change of AED. Dropouts were not explained in one trial (Yi 2009). Among those trials which reported dropouts, the number was small and we considered it unlikely to result in significant bias.
Selective reporting
In all included trials, all predefined or expected outcomes were reported and selective reporting was not evident.
Other potential sources of bias
Twelve trials (Deng 2001a; Han 2008; Leng 2000; Peng 2003; Shi 2001; Xiong 2003; Yi 2009; Yu 1999; Zhang 2006a; Zhang 2006b; Zhuang 2004; Zhuang 2006) did not provide data on important baseline characteristics of the intervention and control groups to judge the comparability of the two groups. In three trials (Kloster 1999; Ma 2001; Zhou 2000) the authors claimed that the two groups were comparable at baseline but the data they provided suggested otherwise since one group seemed to have more frequent seizures than the other. Furthermore, the acupuncture treatment in 12 trials was not standardised (Deng 2001a; Han 2008; Kloster 1999; Li 2007; Ma 2001; Mao 2011; Yi 2009; Yu 1999; Zhang 2006a; Zhang 2006b; Zhou 2000; Zhuang 2006). Eleven trials relied on the discretion of the clinician in choosing acupoints (Deng 2001a; Han 2008; Kloster 1999; Li 2007; Ma 2001; Mao 2011; Yi 2009; Zhang 2006a; Zhang 2006b; Zhou 2000; Zhuang 2006) and in two trials the number of courses of acupuncture or the interval between courses was variable (Deng 2001a; Yu 1999). The control treatment was also not standardised for two trials. In one trial, whether valproate or carbamazepine was used in the control patients depended on the clinician's judgement and preference (Deng 2001a). In another trial, the dose of phenytoin used was variable (Zhou 2000). In 16 studies (Deng 2001a; Han 2008; Leng 2000; Li 2007; Ma 2001; Mao 2011; Peng 2003; Shi 2001; Xiong 2003; Yi 2009; Yu 1999; Zhang 2006a; Zhang 2006b; Zhou 2000; Zhuang 2004; Zhuang 2006) no sham or placebo control was used and the placebo effect might have caused bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
All effect sizes were calculated with the fixed‐effect model unless otherwise specified.
Needle acupuncture plus Chinese herbs compared with Chinese herbs alone
Two trials compared needle acupuncture plus Chinese herbs with Chinese herbs alone (Ma 2001; Xiong 2003). Although the Chinese herbs used were different in the two studies, they were the same in the treatment and control groups in each study. Therefore, unless significant interaction between the effects of acupuncture and the particular type of Chinese herbs was present, it was expected that the comparison of outcomes between the treatment and control groups represented the net effect of acupuncture. Where the described outcomes were comparable, we combined the results in a meta‐analysis. We found that apart from using different acupoints and different control herbs, there was no significant clinical heterogeneity between the two trials. Both trials included only paediatric patients with generalized epilepsy of widely differing durations. There was also no significant statistical heterogeneity in the various outcomes reported and hence the results were combined in meta‐analyses using the fixed‐effect model. Results on adverse effects and the available primary outcomes are summarised in Table 1.
Primary outcomes
Seizure freedom
Neither of the trials (Ma 2001; Xiong 2003) reported our predetermined primary outcome of seizure freedom.
A 50% or greater reduction in seizure frequency
Both trials (Ma 2001; Xiong 2003) showed a mild positive effect of acupuncture in achieving 50% or greater reduction in seizure frequency (pooled RR 1.13, 95% CI 0.97 to 1.31, 2 studies, 120 participants) but this did not reach statistical significance (P = 0.13) (Analysis 1.1; Figure 4).
1.1. Analysis.
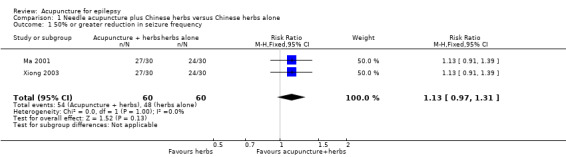
Comparison 1 Needle acupuncture plus Chinese herbs versus Chinese herbs alone, Outcome 1 50% or greater reduction in seizure frequency.
4.
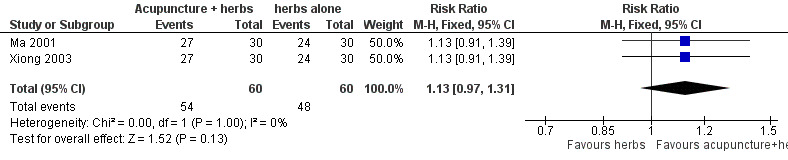
Forest plot of comparison: 1 Needle acupuncture plus Chinese herbs versus Chinese herbs alone, outcome: 1.1 50% or greater reduction in seizure frequency.
Absolute or percentage reduction in seizure frequency and duration
The two trials (Ma 2001; Xiong 2003) did not report absolute or percentage reduction in seizure frequency or duration.
Quality of life
The two trials (Ma 2001; Xiong 2003) did not report quality of life changes.
Secondary outcomes
The two trials (Ma 2001; Xiong 2003) did not report adverse effects or withdrawal due to adverse effects or lack of efficacy.
Additional outcomes
A 75% or greater reduction in seizure frequency
We found a statistically significant difference (P = 0.007) between the treatment and control groups in the number of patients with 75% or greater reduction in seizure frequency (pooled RR 1.52, 95% CI 1.12 to 2.05, 2 studies, 120 participants) favouring acupuncture treatment (Analysis 1.2).
1.2. Analysis.
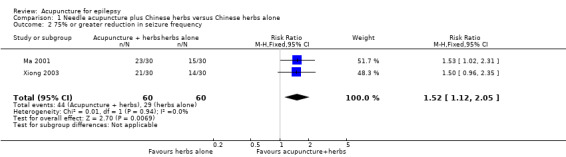
Comparison 1 Needle acupuncture plus Chinese herbs versus Chinese herbs alone, Outcome 2 75% or greater reduction in seizure frequency.
A 25% or greater reduction in seizure frequency
There was no significant difference (P = 0.7) between the treatment and the control groups in the number of patients with 25% or greater reduction in seizure frequency (pooled RR 1.02, 95% CI 0.93 to 1.11, 2 studies, 120 participants) (Analysis 1.3).
1.3. Analysis.
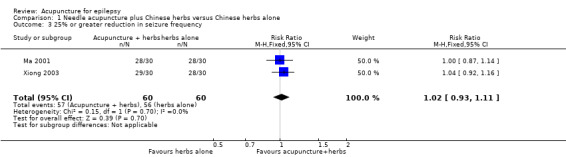
Comparison 1 Needle acupuncture plus Chinese herbs versus Chinese herbs alone, Outcome 3 25% or greater reduction in seizure frequency.
A 75% or greater reduction in seizure duration
There was no significant difference (P = 0.06) between the treatment and the control groups in the number of patients with 25% or greater reduction in seizure duration (pooled RR 1.90, 95% CI 0.97 to 3.74, 2 studies, 120 participants) (Analysis 1.4).
1.4. Analysis.
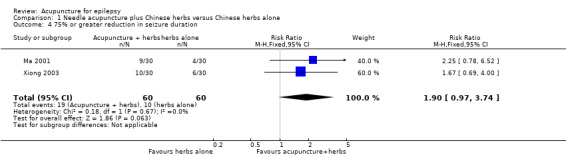
Comparison 1 Needle acupuncture plus Chinese herbs versus Chinese herbs alone, Outcome 4 75% or greater reduction in seizure duration.
A 50% or greater reduction in seizure duration
The treatment group was significantly more likely (P = 0.03) to have 50% or greater reduction in seizure duration compared with the control group (pooled RR 1.29, 95% CI 1.03 to 1.62, 2 studies, 120 participants) (Analysis 1.5).
1.5. Analysis.
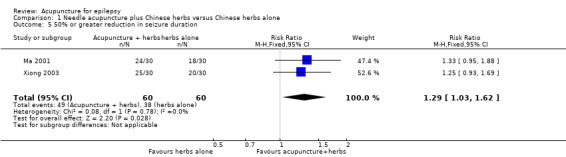
Comparison 1 Needle acupuncture plus Chinese herbs versus Chinese herbs alone, Outcome 5 50% or greater reduction in seizure duration.
Post‐treatment EEG abnormality
One study (Ma 2001) graded EEG abnormalities using a scoring system, with a higher score representing more severe abnormality. There was no significant difference between the treatment and the control groups in the proportion of patients with 4 points or greater improvement in EEG abnormality (RR 1.67, 95% CI 0.89 to 3.11, 1 study, 52 participants, P = 0.11) (Analysis 1.6), or 2 points or greater improvement in EEG abnormality (RR 1.04, 95% CI 0.87 to 1.25, 1 study, 52 participants, P = 0.64) (Analysis 1.7). Two studies (Ma 2001; Xiong 2003) classified the EEG abnormalities into different grades (borderline, mild, moderate, and severe abnormality). There was no statistically significant difference between the treatment and the control groups in the number of patients with different degrees of post‐treatment EEG abnormality (any abnormality: pooled RR 0.79, 95% CI 0.59 to 1.04, 2 studies, 120 participants, P = 0.09; severe abnormality: RR 0.33, 95% CI 0.01 to 7.87, 1 study, 60 participants, P = 0.5; moderate abnormality: RR 0.67, 95% CI 0.21 to 2.13, 1 study, 60 participants, P = 0.49; mild abnormality: RR 0.82, 95% CI 0.40 to 1.68, 1 study, 60 participants, P = 0.59; and borderline abnormality: RR 1.33, 95% CI 0.33 to 5.45, 1 study, 60 participants, P = 0.69) (Analysis 1.8).
1.6. Analysis.
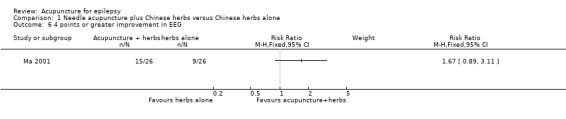
Comparison 1 Needle acupuncture plus Chinese herbs versus Chinese herbs alone, Outcome 6 4 points or greater improvement in EEG.
1.7. Analysis.
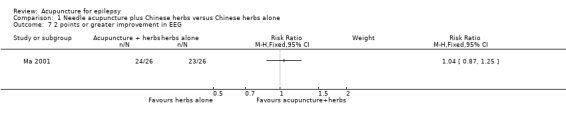
Comparison 1 Needle acupuncture plus Chinese herbs versus Chinese herbs alone, Outcome 7 2 points or greater improvement in EEG.
1.8. Analysis.
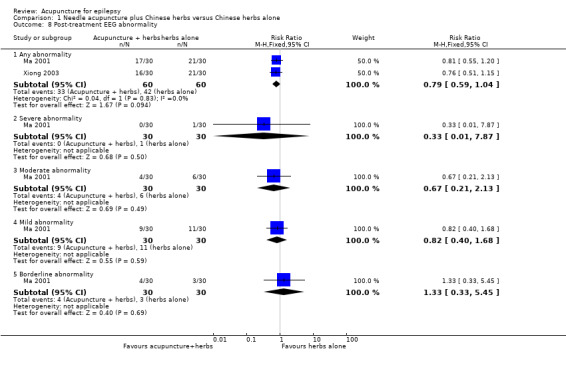
Comparison 1 Needle acupuncture plus Chinese herbs versus Chinese herbs alone, Outcome 8 Post‐treatment EEG abnormality.
Needle acupuncture plus valproate compared with valproate alone
Two trials compared needle acupuncture plus valproate with valproate alone (Yi 2009; Zhang 2006a). Apart from using different acupoints there was no significant clinical heterogeneity between the two trials. Both trials recruited patients with generalized epilepsy. The participants in the trial by Zhang 2006a appeared to have a longer duration of epilepsy than those in Yi 2009. Results on adverse effects and the available primary outcomes are summarised in Table 2.
Primary outcomes
Seizure freedom
The pooled result of the two trials (Yi 2009; Zhang 2006a) showed no significant difference (P = 0.84) in seizure freedom between the treatment and the control groups (pooled RR 0.97, 95% CI 0.72 to 1.30, 2 studies, 150 participants) (Analysis 2.1).
2.1. Analysis.
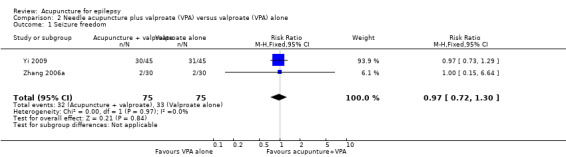
Comparison 2 Needle acupuncture plus valproate (VPA) versus valproate (VPA) alone, Outcome 1 Seizure freedom.
A 50% or greater reduction in seizure frequency
There was also no significant difference (P = 0.55) in the number of patients achieving 50% or greater reduction in seizure frequency (pooled RR 1.34, 95% CI 0.52 to 3.48, random‐effects model, 2 studies, 150 participants) (Analysis 2.2; Figure 5). However, there was a high degree of statistical heterogeneity between the trials (I2 = 91%). The included trials differed in the age of the patients, duration of epilepsy, and acupuncture methods.
2.2. Analysis.
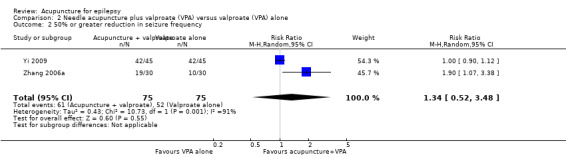
Comparison 2 Needle acupuncture plus valproate (VPA) versus valproate (VPA) alone, Outcome 2 50% or greater reduction in seizure frequency.
5.
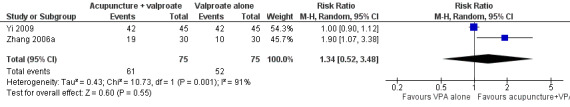
Forest plot of comparison: 2 Needle acupuncture plus valproate (VPA) versus valproate (VPA) alone, outcome: 2.2 50% or greater reduction in seizure frequency.
Absolute or percentage reduction in seizure frequency and duration
The two trials (Yi 2009; Zhang 2006a) did not report absolute or percentage reduction in seizure frequency or duration.
Quality of life
One trial (Yi 2009) reported that the treatment group had a significantly better (P = 0.009) quality of life after treatment compared with the control group by the QOLIE‐31 score (MD 10.10, 95% CI 2.51 to 17.69, 1 study, 90 participants) (Analysis 2.3). Higher score indicated better quality of life; a positive MD indicated benefit of the treatment compared with control.
2.3. Analysis.
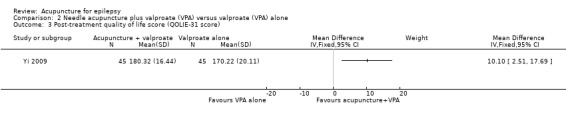
Comparison 2 Needle acupuncture plus valproate (VPA) versus valproate (VPA) alone, Outcome 3 Post‐treatment quality of life score (QOLIE‐31 score).
Secondary outcomes
Frequency of adverse effects
One trial (Yi 2009) with 90 participants reported the frequencies of several adverse effects and found no statistically significant differences between the treatment and the control groups (dizziness: RR 0.67, 99% CI 0.07 to 6.51, P = 0.65; malaise: RR 0.83, 99% CI 0.19 to 3.60, P = 0.75; nausea: RR 0.60, 99% CI 0.10 to 3.63, P = 0.47; anorexia: RR 0.94, 99% CI 0.48 to 1.87, P = 0.83; impaired concentration: RR 0.65, 99% CI 0.34 to 1.26, P = 0.10; and sleepiness: RR 0.71, 99% CI 0.17 to 2.92, P = 0.54) (Analysis 2.4).
2.4. Analysis.
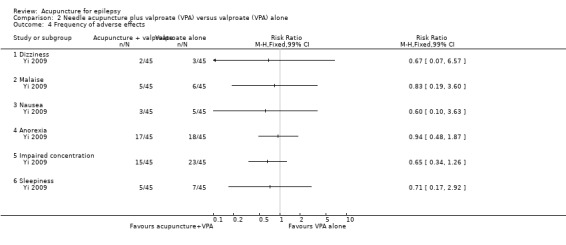
Comparison 2 Needle acupuncture plus valproate (VPA) versus valproate (VPA) alone, Outcome 4 Frequency of adverse effects.
Withdrawals due to adverse effects or lack of efficacy
The two trials did not report any withdrawals due to adverse effects or lack of efficacy (Yi 2009; Zhang 2006a).
Additional outcomes
A 75% or greater reduction in seizure frequency
We found no significant difference (P = 0.57) between the treatment and control groups in the number of patients with 75% or greater reduction in seizure frequency when the results of the two trials (Yi 2009; Zhang 2006a) were combined (pooled RR 1.23, 95% CI 0.61 to 2.50, random‐effects model, 2 studies, 150 participants) (Analysis 2.5). However, there was moderate statistical heterogeneity between the trials (I2 = 52%). The included trials differed in the age of the patients, duration of epilepsy, and acupuncture methods.
2.5. Analysis.
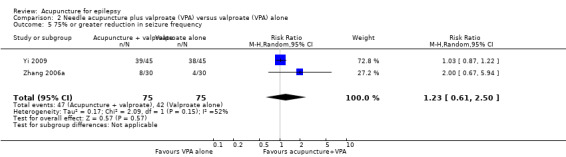
Comparison 2 Needle acupuncture plus valproate (VPA) versus valproate (VPA) alone, Outcome 5 75% or greater reduction in seizure frequency.
A 25% or greater reduction in seizure frequency
There was also no significant difference (P = 0.78) between the treatment and control groups in the number of patients with 25% or greater reduction in seizure frequency (pooled RR 1.03, 95% CI 0.83 to 1.28, random‐effects model, 2 studies, 150 participants) (Analysis 2.6). There was moderate statistical heterogeneity between the trials (I2 = 68%). The included trials differed in the age of the patients, duration of epilepsy, and acupuncture methods.
2.6. Analysis.
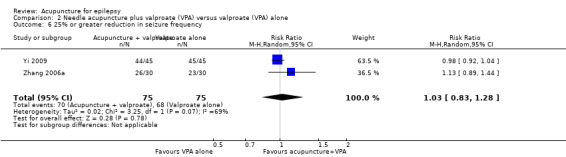
Comparison 2 Needle acupuncture plus valproate (VPA) versus valproate (VPA) alone, Outcome 6 25% or greater reduction in seizure frequency.
Post‐treatment epilepsy score
Both trials (Yi 2009; Zhang 2006a) reported the post‐treatment epilepsy score (a higher score indicated more severe epilepsy), which was not significantly different (P = 0.29) between the treatment and the control group when the results of the trials (Yi 2009; Zhang 2006a) were combined (pooled MD ‐1.10, 95% CI ‐3.11 to 0.13, random‐effects model, 2 studies, 150 participants) (Analysis 2.7). A negative MD indicated benefit of treatment compared with control. However, there was moderate statistical heterogeneity between the trials (I2 = 51%). The included trials differed in the age of the patients, duration of epilepsy, and acupuncture methods.
2.7. Analysis.
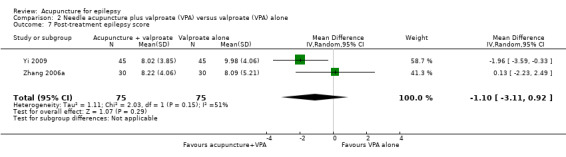
Comparison 2 Needle acupuncture plus valproate (VPA) versus valproate (VPA) alone, Outcome 7 Post‐treatment epilepsy score.
A 70% or greater reduction in epilepsy score
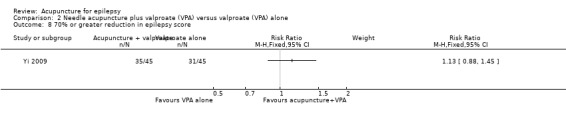
One trial (Yi 2009) reported no significant difference (P = 0.34) between the treatment and the control groups in the number of patients with 70% or greater reduction in epilepsy score (RR 1.13, 95% CI 0.88 to 1.45, 1 study, 90 participants) (Analysis 2.8).
2.8. Analysis.
Comparison 2 Needle acupuncture plus valproate (VPA) versus valproate (VPA) alone, Outcome 8 70% or greater reduction in epilepsy score.
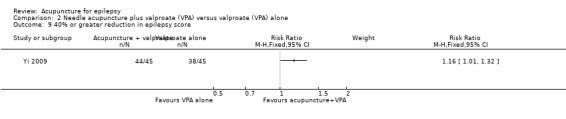
A 40% or greater reduction in epilepsy score
The same trial (Yi 2009) reported that the treatment group was significantly more likely (P = 0.03) to have 40% or greater improvement in epilepsy score compared with the control group (RR 1.16, 95% CI 1.01 to 1.32, 1 study, 90 participants) (Analysis 2.9).
2.9. Analysis.
Comparison 2 Needle acupuncture plus valproate (VPA) versus valproate (VPA) alone, Outcome 9 40% or greater reduction in epilepsy score.
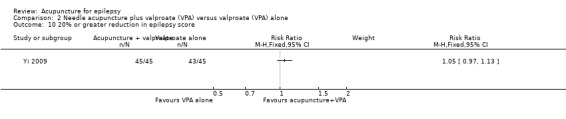
A 20% or greater reduction in epilepsy score
The same trial (Yi 2009) reported no significant difference (P = 0.24) between the treatment and the control groups in the number of patients with 20% or greater reduction in epilepsy score (pooled RR 1.05, 95% CI 0.97 to 1.13, 1 study, 90 participants) (Analysis 2.10).
2.10. Analysis.
Comparison 2 Needle acupuncture plus valproate (VPA) versus valproate (VPA) alone, Outcome 10 20% or greater reduction in epilepsy score.
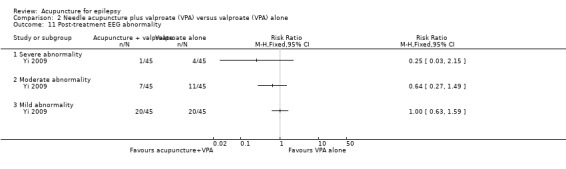
Post‐treatment EEG abnormality
This trial (Yi 2009) with 90 participants also reported post‐treatment EEG abnormalities and found no significant difference between the treatment and the control groups (severe abnormality: RR 0.25, 95% CI 0.03 to 2.15, P = 0.21; moderate abnormality: RR 0.64, 95% CI 0.27 to 1.49, P = 0.3; mild abnormality: RR 1.00, 95% CI 0.63 to 1.59, P = 1.0) (Analysis 2.11).
2.11. Analysis.
Comparison 2 Needle acupuncture plus valproate (VPA) versus valproate (VPA) alone, Outcome 11 Post‐treatment EEG abnormality.
Needle acupuncture compared with sham acupuncture
Only one trial used sham acupuncture as control treatment (Kloster 1999). Results on adverse effects and the available primary outcomes are summarised in Table 3.
Primary outcomes
Seizure freedom
This trial (Kloster 1999) did not report seizure freedom.
A 50% or greater reduction in seizure frequency
This trial (Kloster 1999) also did not report the number of patients with 50% or greater reduction in seizure frequency.
Percentage reduction in seizure frequency
The percentage reduction in seizure frequency was reported to be higher in the treatment group (median 45%, 18 participants) compared with the control group (median 20%, 16 participants) but this difference did not reach statistical significance (P = 0.38) (Analysis 3.1). Since no mean or standard deviation (SD) was reported, we could not perform a re‐analysis.
3.1. Analysis.
Comparison 3 Needle acupuncture versus sham acupuncture, Outcome 1 Percentage reduction in seizure frequency.
| Percentage reduction in seizure frequency | ||
|---|---|---|
| Study | Treatment | Control |
| Kloster 1999 | median 45% (n=18) | median 20% (n=16) |
Quality of life
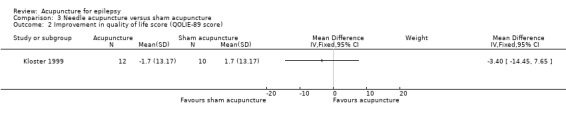
This trial (Kloster 1999) reported no significant difference (P = 0.55) in quality of life improvement between the treatment and the control groups (MD ‐3.4, 95% CI ‐14.45 to 7.65, 1 study, 22 participants) (Analysis 3.2). The authors also did not find any significant differences between the treatment and the control groups in any of the 17 subscores of the various items in the questionnaire.
3.2. Analysis.
Comparison 3 Needle acupuncture versus sham acupuncture, Outcome 2 Improvement in quality of life score (QOLIE‐89 score).
Secondary outcomes
Frequency of adverse effects
The included trial (Kloster 1999) reported that a number of participants in the trial experienced adverse effects of treatment, such as changes in the sense of well‐being, sleep, bowel movements, micturition, menstruation, nausea and vomiting. However, the frequencies of their occurrence and the treatment groups these patients were allocated to were not reported.
Withdrawals due to lack of efficacy
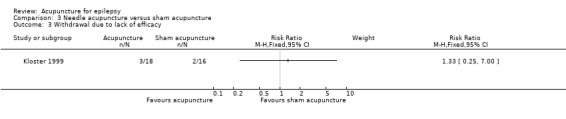
This trial (Kloster 1999) reported dropouts due to lack of efficacy and there was no significant difference (P = 0.73) between the treatment and the control groups (RR 1.33, 95% CI 0.25 to 7.00, 1 study, 34 participants) (Analysis 3.3).
3.3. Analysis.
Comparison 3 Needle acupuncture versus sham acupuncture, Outcome 3 Withdrawal due to lack of efficacy.
Additional outcomes
Reduction in seizure frequency
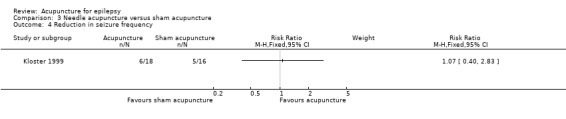
Six out of 18 patients in the treatment group compared with five out of 16 in the control group had fewer seizures on follow‐up, which was not significantly different (RR 1.07, 95% CI 0.40 to 2.83, 1 study, 34 participants, P = 0.9) (Analysis 3.4).
3.4. Analysis.
Comparison 3 Needle acupuncture versus sham acupuncture, Outcome 4 Reduction in seizure frequency.
No increase in seizure frequency
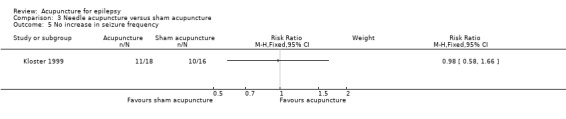
Similarly, the numbers of patients without an increase in seizures on follow‐up were not significantly different (P = 0.93) between the treatment and the control groups (RR 0.98, 95% CI 0.58 to 1.66, 1 study, 34 participants) (Analysis 3.5).
3.5. Analysis.
Comparison 3 Needle acupuncture versus sham acupuncture, Outcome 5 No increase in seizure frequency.
Percentage increase in seizure‐free weeks
The percentage increase in seizure‐free weeks was reported to be lower in the treatment group (median 50%, 18 participants) compared with the control group (median 100%, 16 participants) (Analysis 3.6). However, no statistical test was performed and therefore we were uncertain whether the difference was statistically significant, or not. Since no mean or SD was reported we did not perform a re‐analysis.
3.6. Analysis.
Comparison 3 Needle acupuncture versus sham acupuncture, Outcome 6 Percentage increase in seizure‐free weeks.
| Percentage increase in seizure‐free weeks | ||
|---|---|---|
| Study | Treatment | Control |
| Kloster 1999 | median 50% (n=18) | median 100% (n=16) |
Improvement in EEG abnormality
This trial (Kloster 1999) reported that there was no difference between the two groups in changes in EEG abnormality. However, no further data were provided.
Needle acupuncture compared with phenytoin
Two trials compared needle acupuncture with phenytoin (Yu 1999; Zhou 2000). The acupuncture regimen and acupoints chosen were different in these two trials. Otherwise the trials appeared similar. Results on adverse effects and the available primary outcomes are summarised in Table 4.
Primary outcomes
Seizure freedom
Neither of the trials (Yu 1999; Zhou 2000) reported our predetermined primary outcome of seizure freedom.
A 50% or greater reduction in seizure frequency
The combined results of the two trials (Yu 1999; Zhou 2000) showed no significant difference (P = 0.54) in the number of participants with 50% or greater reduction in seizure frequency (pooled RR 1.43, 95% CI 0.46 to 4.44, random‐effects model, 2 studies, 150 participants) (Analysis 4.1). However, the two trials exhibited very high statistical heterogeneity (I2 = 97%). The included trials differed in acupuncture methods.
4.1. Analysis.
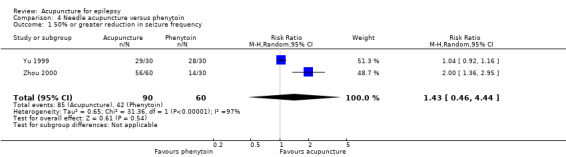
Comparison 4 Needle acupuncture versus phenytoin, Outcome 1 50% or greater reduction in seizure frequency.
Absolute or percentage reduction in seizure frequency and duration
The two trials (Yu 1999; Zhou 2000) did not report absolute or percentage reduction in seizure frequency or duration.
Quality of life
The two trials (Yu 1999; Zhou 2000) also did not report quality of life changes.
Secondary outcomes
The two trials (Yu 1999; Zhou 2000) did not report adverse effects or dropouts due to adverse effects or lack of efficacy.
Additional outcomes
A 75% or greater reduction in seizure frequency
There were significantly more patients in the treatment group who achieved 75% or greater reduction in seizure frequency (pooled RR 2.14, 95% CI 1.47 to 3.1, 2 studies, 150 participants, P < 0.0001) (Analysis 4.2).
4.2. Analysis.
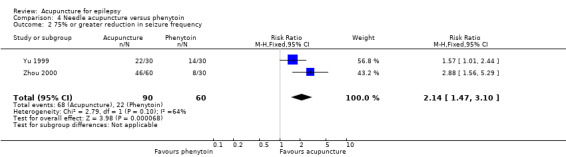
Comparison 4 Needle acupuncture versus phenytoin, Outcome 2 75% or greater reduction in seizure frequency.
A 25% or greater reduction in seizure frequency
One study (Zhou 2000) also reported significantly more patients in the treatment group who achieved 25% or greater reduction in seizure frequency (RR 1.61, 95% CI 1.2 to 2.17, 1 study, 90 participants, P = 0.0002) (Analysis 4.3).
4.3. Analysis.
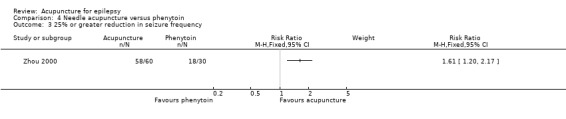
Comparison 4 Needle acupuncture versus phenytoin, Outcome 3 25% or greater reduction in seizure frequency.
Post‐treatment seizure frequency
The same trial (Zhou 2000) reported a significantly lower seizure frequency after treatment (MD ‐25.1 per year, 95% CI ‐35.96 to ‐14.24 per year, 1 study, 90 participants, P < 0.00001) (Analysis 4.4).
4.4. Analysis.
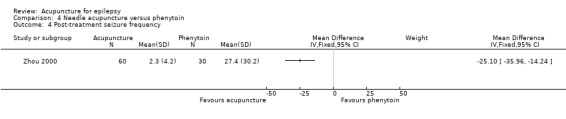
Comparison 4 Needle acupuncture versus phenytoin, Outcome 4 Post‐treatment seizure frequency.
Needle acupuncture compared with valproate
Two trials compared needle acupuncture with valproate (Shi 2001; Zhang 2006b). One trial recruited patients with childhood absence epilepsy (Shi 2001) while the other trial recruited patients aged 15 to 60 years with generalized tonic‐clonic epilepsy (Zhang 2006b). Their acupuncture protocols were different. Results on adverse effects and the available primary outcomes are summarised in Table 5.
Primary outcomes
Seizure freedom
The pooled result of the two trials (Shi 2001; Zhang 2006b) showed no significant difference (P = 0.08) in seizure freedom between the treatment and the control groups (RR 1.75, 95% CI 0.93 to 3.27, 2 studies, 180 participants) (Analysis 5.1).
5.1. Analysis.
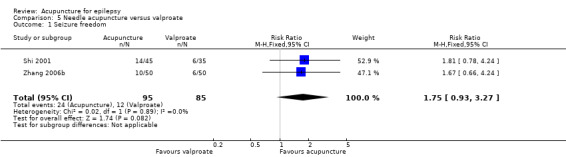
Comparison 5 Needle acupuncture versus valproate, Outcome 1 Seizure freedom.
A 50% or greater reduction in seizure frequency
There were significantly more patients in the treatment group who achieved 50% or greater reduction in seizure frequency (pooled RR 1.32, 95% CI 1.05 to 1.66, 2 studies, 180 participants, P = 0.02) (Analysis 5.2).
5.2. Analysis.
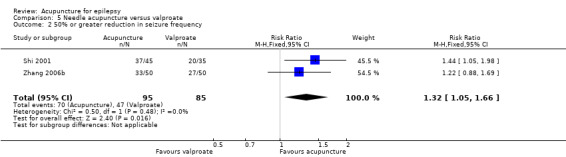
Comparison 5 Needle acupuncture versus valproate, Outcome 2 50% or greater reduction in seizure frequency.
Absolute or percentage reduction in seizure frequency and duration
The two trials (Shi 2001; Zhang 2006b) did not report absolute or percentage reduction in seizure frequency or duration.
Quality of life
One trial (Zhang 2006b) reported that the treatment group had significantly better quality of life after treatment compared with the control group by the QOLIE‐31 score (MD 12.04, 95% CI 4.05 to 20.03, 1 study, 100 participants, P = 0.003) (Analysis 5.3).
5.3. Analysis.
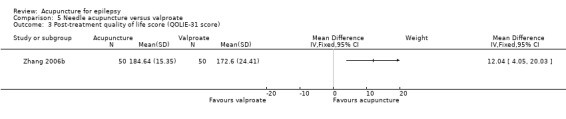
Comparison 5 Needle acupuncture versus valproate, Outcome 3 Post‐treatment quality of life score (QOLIE‐31 score).
Secondary outcomes
Frequency of adverse effects
One trial (Zhang 2006b) with 100 participants reported the frequencies of several adverse effects and found that the treatment group had significantly fewer participants with impaired concentration compared with the control group (RR 0.36, 99% CI 0.16 to 0.79, P = 0.0009), but no significant difference in dizziness (RR 1.13, 99% CI 0.36 to 3.52, P = 0.79); malaise (RR 0.69, 99% CI 0.26 to 1.87, P = 0.34); nausea (RR 0.14, 99% CI 0.01 to 2.14, P = 0.06); anorexia (RR 0.33, 99% CI 0.06 to 1.72, P = 0.08); and sleepiness (RR 0.60, 99% CI 0.10 to 3.66, P = 0.47) (Analysis 5.4).
5.4. Analysis.
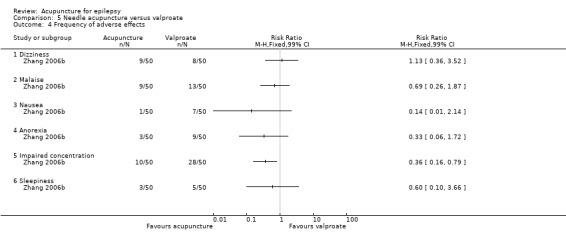
Comparison 5 Needle acupuncture versus valproate, Outcome 4 Frequency of adverse effects.
Withdrawals due to adverse effects or lack of efficacy
Withdrawals due to adverse effects or lack of efficacy were not reported in either trial (Shi 2001; Zhang 2006b).
Additional outcomes
A 75% or greater reduction in seizure frequency
One trial (Zhang 2006b) reported that significantly more patients in the treatment group experienced 75% or greater reduction in seizure frequency (RR 1.80, 95% CI 1.10 to 2.95, 1 study, 100 participants, P = 0.02) (Analysis 5.5).
5.5. Analysis.
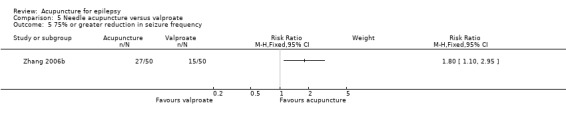
Comparison 5 Needle acupuncture versus valproate, Outcome 5 75% or greater reduction in seizure frequency.
A 25% or greater reduction in seizure frequency
There was no significant difference (P = 0.34) between the treatment and control groups in the number of patients with 25% or greater reduction in seizure frequency (RR 1.07, 95% CI 0.93 to 1.23, 1 study, 100 participants) (Analysis 5.6).
5.6. Analysis.
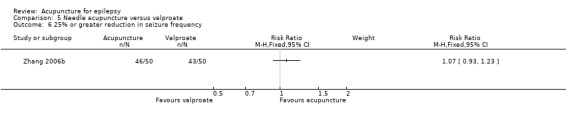
Comparison 5 Needle acupuncture versus valproate, Outcome 6 25% or greater reduction in seizure frequency.
Post‐treatment epilepsy score
One trial (Zhang 2006b) reported the post‐treatment epilepsy score, which was not significantly different (P = 0.84) between the treatment group and the control group (MD 0.19, 95% CI ‐1.64 to 2.02, 1 study, 100 participants) (Analysis 5.7).
5.7. Analysis.
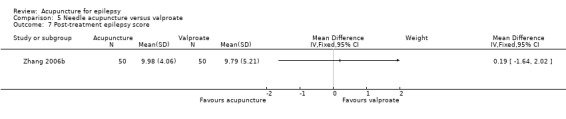
Comparison 5 Needle acupuncture versus valproate, Outcome 7 Post‐treatment epilepsy score.
A 70% or greater reduction in epilepsy score
This trial (Zhang 2006b) reported that significantly more patients in the treatment group achieved 70% or greater reduction in epilepsy score compared with the control group (RR 1.73, 95% CI 1.05 to 2.86, 1 study, 100 participants, P = 0.03) (Analysis 5.8).
5.8. Analysis.
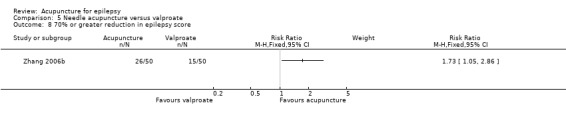
Comparison 5 Needle acupuncture versus valproate, Outcome 8 70% or greater reduction in epilepsy score.
A 40% or greater reduction in epilepsy score
There was no difference (P = 0.11) between the treatment and the control groups in the number of participants who experienced 40% or greater improvement in epilepsy score (RR 1.31, 95% CI 0.94 to 1.81, 1 study, 100 participants) (Analysis 5.9).
5.9. Analysis.
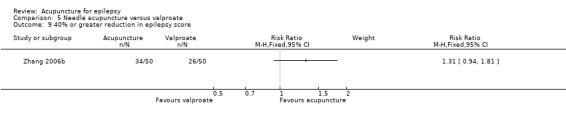
Comparison 5 Needle acupuncture versus valproate, Outcome 9 40% or greater reduction in epilepsy score.
A 20% or greater reduction in epilepsy score
There was no significant difference (P = 0.14) between the treatment and the control groups in the number of participants who experienced 20% or greater improvement in epilepsy score (RR 1.12, 95% CI 0.96 to 1.31, 1 study, 100 participants) (Analysis 5.10).
5.10. Analysis.

Comparison 5 Needle acupuncture versus valproate, Outcome 10 20% or greater reduction in epilepsy score.
Post‐treatment EEG abnormality
The same trial (Zhang 2006b) with 100 participants also reported post‐treatment EEG abnormalities and found no significant difference between the treatment and the control groups (severe abnormality: RR 0.74, 95% CI 0.45 to 1.21, P = 0.23; moderate abnormality: RR 1.06, 95% CI 0.61 to 1.86, P = 0.83; mild abnormality: RR 1.38, 95% CI 0.60 to 3.13, P = 0.45) (Analysis 5.11).
5.11. Analysis.
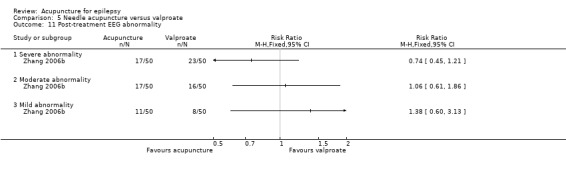
Comparison 5 Needle acupuncture versus valproate, Outcome 11 Post‐treatment EEG abnormality.
Catgut implantation at acupoints plus antiepileptic drugs (AEDs) compared with AEDs alone
Five trials compared catgut implantation at acupoints plus AEDs with AEDs alone (Deng 2001a; Li 2007; Mao 2011; Zhang 2006a; Zhuang 2004). Three trials used valproate (Li 2007; Mao 2011; Zhang 2006a), one trial used phenobarbital plus phenytoin (Zhuang 2004), and one trial used either valproate or carbamazepine (Deng 2001a); which drug was used in the treatment group depended on the discretion of the clinician and the dose of AED used in the treatment group was just half that used in the control group in this trial. All five trials recruited patients with generalized epilepsy and their age and duration of epilepsy were similar. The acupoints chosen were the same for two of the five trials (Li 2007; Zhang 2006a). Results on adverse effects and the available primary outcomes are summarised in Table 6.
Primary outcomes
Seizure freedom
The pooled result of four trials (Deng 2001a; Li 2007; Mao 2011; Zhang 2006a) showed no significant difference (P = 0.09) in seizure freedom between the treatment and the control groups (pooled RR 1.51, 95% CI 0.93 to 2.43, 4 studies, 361 participants) (Analysis 6.1).
6.1. Analysis.

Comparison 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, Outcome 1 Seizure freedom.
A 50% or greater reduction in seizure frequency
There were significantly more patients in the treatment group who achieved 50% or greater reduction in seizure frequency when the results of five trials (Deng 2001a; Li 2007; Mao 2011; Zhang 2006a; Zhuang 2004) were combined (pooled RR 1.42, 95% CI 1.07 to 1.89, random‐effects model, 5 studies, 401 participants, P = 0.02) (Analysis 6.2; Figure 6). However, there was a high degree of statistical heterogeneity (I2 = 78%). The included trials were heterogeneous in the age of the patients, duration of epilepsy, and acupuncture methods.
6.2. Analysis.
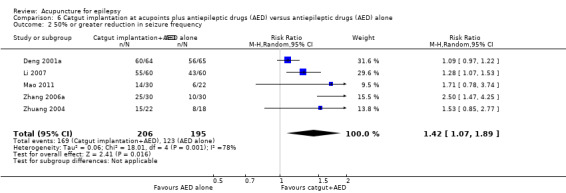
Comparison 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, Outcome 2 50% or greater reduction in seizure frequency.
6.
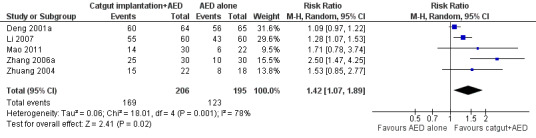
Forest plot of comparison: 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, outcome: 6.2 50% or greater reduction in seizure frequency.
Absolute or percentage reduction in seizure frequency and duration
None of the trials reported absolute or percentage reduction in seizure frequency or duration.
Quality of life
One trial (Li 2007) reported that the treatment group had a significantly better quality of life after treatment compared with the control group by the QOLIE‐31 score (MD ‐7.54, 95% CI ‐14.47 to ‐0.61, 1 study, 120 participants, P = 0.03); a higher score indicated worse quality of life, a negative MD indicated benefit of treatment compared with control (Analysis 6.3).
6.3. Analysis.
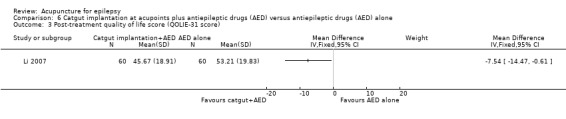
Comparison 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, Outcome 3 Post‐treatment quality of life score (QOLIE‐31 score).
Secondary outcomes
Frequency of adverse effects
One trial (Li 2007) with 120 participants reported the frequencies of several adverse effects and found that the treatment and the control groups were not significantly different for malaise (RR 0.50, 99% CI 0.17 to 1.50, P = 0.1), nausea (RR 0.33, 99% CI 0.06 to 1.74, P = 0.09), and anorexia (RR 0.25, 99% CI 0.03 to 1.81, P = 0.07); dizziness (RR 0.33, 99% CI 0.10 to 1.60, P = 0.02) and impaired concentration (RR 0.43, 99% CI 0.17 to 1.07, P = 0.02) were significantly reduced (Analysis 6.4).
6.4. Analysis.
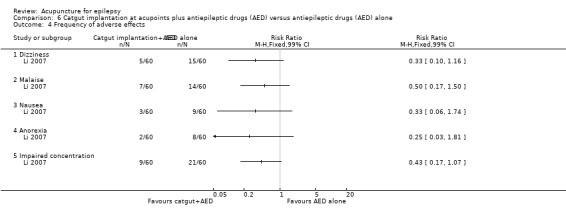
Comparison 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, Outcome 4 Frequency of adverse effects.
Withdrawals due to adverse effects or lack of efficacy
Withdrawals due to adverse effects or lack of efficacy were not reported in any of the five trials (Deng 2001a; Li 2007; Mao 2011; Zhang 2006a; Zhuang 2004).
Additional outcomes
A 75% or greater reduction in seizure frequency
The combined results of five trials (Deng 2001a; Li 2007; Mao 2011; Zhang 2006a; Zhuang 2004) revealed that significantly more patients in the treatment group experienced 75% or greater reduction in seizure frequency (pooled RR 1.48, 95% CI 1.22 to 1.79, 5 studies, 401 participants, P < 0.0001) (Analysis 6.5).
6.5. Analysis.
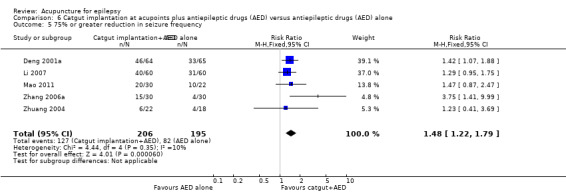
Comparison 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, Outcome 5 75% or greater reduction in seizure frequency.
A 25% or greater reduction in seizure frequency
There were also significantly more patients in the treatment group who experienced 25% or greater reduction in seizure frequency (pooled RR 1.09, 95% CI 1.03 to 1.16, 5 studies, 401 participants, P = 0.005) (Analysis 6.6).
6.6. Analysis.
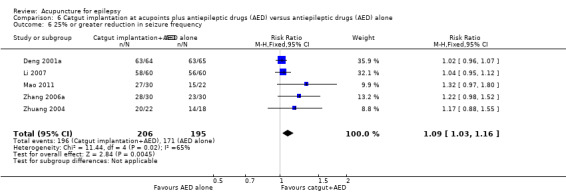
Comparison 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, Outcome 6 25% or greater reduction in seizure frequency.
Post‐treatment seizure frequency
One trial (Mao 2011) reported a significantly lower seizure frequency after treatment (MD ‐0.90 per year, 95% CI ‐1.58 to ‐0.22 per year, 1 study, 52 participants, P = 0.009) (Analysis 6.7).
6.7. Analysis.

Comparison 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, Outcome 7 Post‐treatment seizure frequency.
Post‐treatment epilepsy score
Two trials (Li 2007; Zhang 2006a) reported the post‐treatment epilepsy score, which was not significantly different (P = 0.15) between the treatment and the control groups when the results were combined (MD ‐1.56 per year, 95% CI ‐3.59 to 0.57, random‐effects model, 2 studies, 180 participants) (Analysis 6.8). However, there was moderate statistical heterogeneity between the trials (I2 = 58%). The included trials differed in acupuncture methods.
6.8. Analysis.
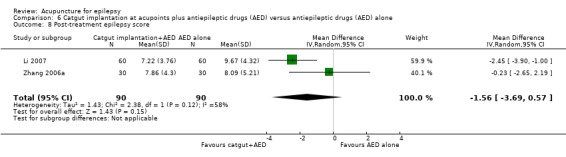
Comparison 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, Outcome 8 Post‐treatment epilepsy score.
A 70% or greater reduction in epilepsy score
One trial (Li 2007) reported that significantly more patients in the treatment group achieved 70% or greater reduction in epilepsy score compared with the control group (RR 2.06, 95% CI 1.28 to 3.33, 1 study, 120 participants, P = 0.003) (Analysis 6.9).
6.9. Analysis.
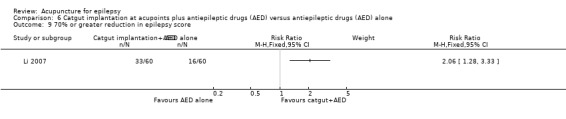
Comparison 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, Outcome 9 70% or greater reduction in epilepsy score.
A 40% or greater reduction in epilepsy score
There were also significantly more patients in the treatment group who achieved 40% or greater reduction in epilepsy score compared with the control group (RR 1.36, 95% CI 1.10 to 1.67, 1 study, 120 participants, P = 0.004) (Analysis 6.10).
6.10. Analysis.
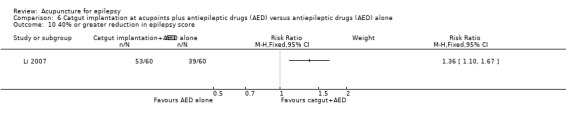
Comparison 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, Outcome 10 40% or greater reduction in epilepsy score.
A 20% or greater reduction in epilepsy score
There was no significant difference (P = 0.12) between the treatment and the control groups in the number of participants who experienced 20% or greater improvement in epilepsy score (RR 1.10, 95% CI 0.98 to 1.23, 1 study, 120 participants) (Analysis 6.11).
6.11. Analysis.
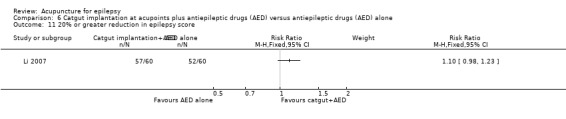
Comparison 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, Outcome 11 20% or greater reduction in epilepsy score.
Post‐treatment Global Clinical Impression (CGI) score
One trial (Li 2007) with 120 participants reported this outcome and found that the treatment group had a significantly higher efficacy index (MD 2.01, 95% CI 1.85 to 2.17, P < 0.00001) but not severity index (MD 0.06, 95% CI ‐0.29 to 0.41, P = 0.73) or global improvement index (MD 0.17, 95% CI ‐0.23 to 0.57, P = 0.41) (Analysis 6.12).
6.12. Analysis.
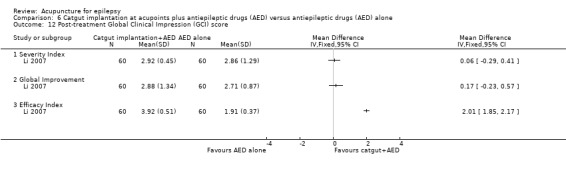
Comparison 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, Outcome 12 Post‐treatment Global Clinical Impression (GCI) score.
Post‐treatment EEG abnormality
Two trials (Deng 2001a; Li 2007) with 249 participants reported post‐treatment EEG abnormalities and found no significant difference between the treatment and the control groups (severe abnormality: pooled RR 0.83, 95% CI 0.43 to 1.60, P = 0.57; moderate abnormality: pooled RR 0.75, 95% CI 0.53 to 1.05, P = 0.1; mild abnormality: pooled RR 1.29, 95% CI 0.91 to 1.84, P = 0.16; borderline abnormality: pooled RR 0.80, 95% CI 0.23 to 2.83, P = 0.73) (Analysis 6.13).
6.13. Analysis.
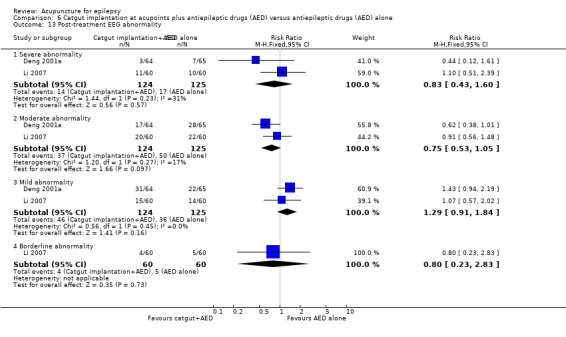
Comparison 6 Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone, Outcome 13 Post‐treatment EEG abnormality.
Catgut implantation at acupoints compared with valproate
Five trials compared catgut implantation at acupoints with valproate (Han 2008; Leng 2000; Peng 2003; Zhang 2006b; Zhuang 2006). One trial (Peng 2003) only included paediatric patients while the other four trials (Han 2008; Leng 2000; Zhang 2006b; Zhuang 2006) included patients from all age groups. The acupuncture regimens were the same in three trials (Han 2008; Zhang 2006b; Zhuang 2006), while the remaining two trials (Leng 2000; Peng 2003) used different regimens. The trials had different but overlapping sets of outcomes. Results on adverse effects and the available primary outcomes are summarised in Table 7.
Primary outcomes
Seizure freedom
The pooled result of four trials (Leng 2000; Peng 2003; Zhang 2006b; Zhuang 2006) showed that significantly more patients in the treatment group achieved seizure freedom compared with the control group (pooled RR 2.82, 95% CI 1.61 to 4.94, 4 studies, 381 participants, P = 0.0003) (Analysis 7.1).
7.1. Analysis.
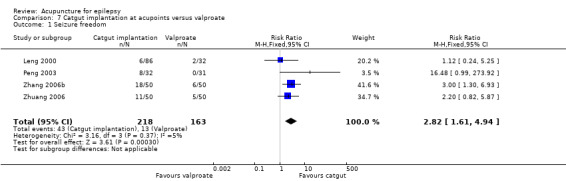
Comparison 7 Catgut implantation at acupoints versus valproate, Outcome 1 Seizure freedom.
A 50% or greater reduction in seizure frequency
The pooled result of the four trials (Leng 2000; Peng 2003; Zhang 2006b; Zhuang 2006) showed no significant difference (P = 0.11) between the treatment and the control groups in the number of participants who achieved 50% or greater reduction in seizure frequency (pooled RR 1.31, 95% CI 0.94 to 1.84, random‐effects model, 4 studies, 381 participants) (Analysis 7.2). However, there was a high degree of statistical heterogeneity (I2 = 92%). The included trials were heterogeneous in the age of the patients, duration of epilepsy, and acupuncture methods.
7.2. Analysis.
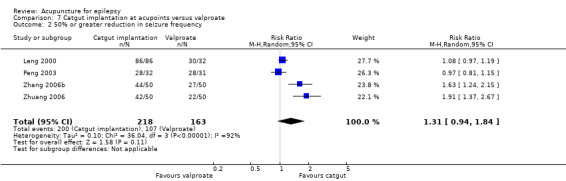
Comparison 7 Catgut implantation at acupoints versus valproate, Outcome 2 50% or greater reduction in seizure frequency.
Absolute or percentage reduction in seizure frequency and duration
None of the trials reported absolute or percentage reduction in seizure frequency or duration.
Quality of life
One trial (Zhang 2006b) reported that the treatment group had significantly better quality of life after treatment compared with the control group by the QOLIE‐31 score (MD 18.73, 95% CI 11.10 to 26.36, 1 study, 180 participants, P < 0.00001) (Analysis 7.3).
7.3. Analysis.
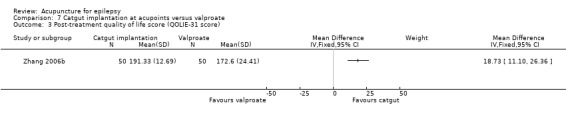
Comparison 7 Catgut implantation at acupoints versus valproate, Outcome 3 Post‐treatment quality of life score (QOLIE‐31 score).
Secondary outcomes
Frequency of adverse effects
One trial (Zhang 2006b) with 100 participants reported the frequencies of several adverse effects and found that the treatment group and the control group had no significant difference in dizziness (RR 0.63, 99% CI 0.16 to 2.47, P = 0.38), malaise (RR 0.92, 99% CI 0.38 to 2.26, P = 0.82), nausea (RR 0.14, 99% CI 0.01 to 2.14, P = 0.06), impaired concentration (RR 0.68, 99% CI 0.39 to 1.20, P = 0.08), and sleepiness (RR 0.40, 99% CI 0.05 to 3.24, P = 0.26); anorexia (RR 0.22, 99% CI 0.03 to 1.56, P = 0.05) was just significantly reduced (Analysis 7.4).
7.4. Analysis.
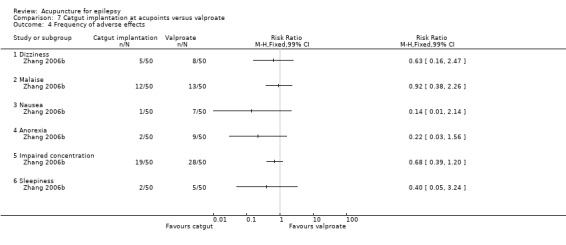
Comparison 7 Catgut implantation at acupoints versus valproate, Outcome 4 Frequency of adverse effects.
Withdrawals due to adverse effects or lack of efficacy
Withdrawals due to adverse effects or lack of efficacy were not reported in these trials (Han 2008; Leng 2000; Peng 2003; Zhang 2006b; Zhuang 2006).
Additional outcomes
A 75% or greater reduction in seizure frequency
The combined result of three trials (Peng 2003; Zhang 2006b; Zhuang 2006) revealed that significantly more patients in the treatment group experienced 75% or greater reduction in seizure frequency (pooled RR 2.12, 95% CI 1.15 to 3.91, random‐effects model, 3 studies, 263 participants, P = 0.02) (Analysis 7.5). However, there was moderate statistical heterogeneity between the trials (I2 = 79%). The included trials differed in the age of the patients, duration of epilepsy, and acupuncture methods.
7.5. Analysis.
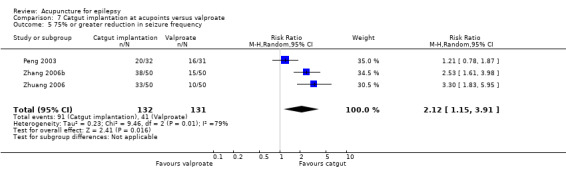
Comparison 7 Catgut implantation at acupoints versus valproate, Outcome 5 75% or greater reduction in seizure frequency.
A 25% or greater reduction in seizure frequency
The combined result of two trials (Zhang 2006b; Zhuang 2006) showed significantly more patients in the treatment group experienced 25% or greater reduction in seizure frequency (pooled RR 1.12, 95% CI 1.01 to 1.24, 2 studies, 200 participants, P = 0.03) (Analysis 7.6).
7.6. Analysis.
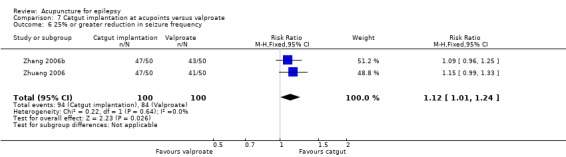
Comparison 7 Catgut implantation at acupoints versus valproate, Outcome 6 25% or greater reduction in seizure frequency.
Post‐treatment epilepsy score
Three trials (Han 2008; Zhang 2006b; Zhuang 2006) reported the post‐treatment epilepsy score, which was not significantly different (P = 0.83) between the treatment and the control groups when the results were combined (pooled MD 0.09, 95% CI ‐0.69 to 0.87, 3 studies, 370 participants) (Analysis 7.7).
7.7. Analysis.
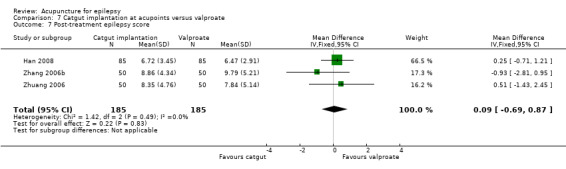
Comparison 7 Catgut implantation at acupoints versus valproate, Outcome 7 Post‐treatment epilepsy score.
A 70% or greater reduction in epilepsy score
One trial (Zhang 2006b) reported that significantly more patients in the treatment group achieved 70% or greater reduction in epilepsy score compared with the control group (RR 2.53, 95% CI 1.61 to 3.98, 1 study, 100 participants, P <0.0001) (Analysis 7.8).
7.8. Analysis.
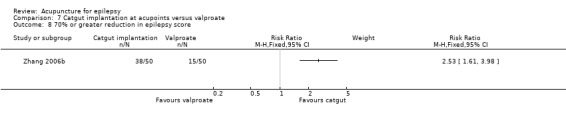
Comparison 7 Catgut implantation at acupoints versus valproate, Outcome 8 70% or greater reduction in epilepsy score.
A 40% or greater reduction in epilepsy score
There were also significantly more patients in the treatment group who achieved 40% or greater reduction in epilepsy score compared with the control group (RR 1.73, 95% CI 1.31 to 2.29, 1 study, 100 participants, P = 0.0001) (Analysis 7.9).
7.9. Analysis.
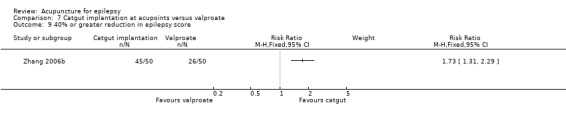
Comparison 7 Catgut implantation at acupoints versus valproate, Outcome 9 40% or greater reduction in epilepsy score.
A 30% or greater reduction in epilepsy score
Another trial (Han 2008) reported no significant difference (P = 0.17) between the treatment and the control groups in the number of participants who experienced 30% or greater improvement in epilepsy score (RR 0.90, 95% CI 0.78 to 1.04, 1 study, 170 participants) (Analysis 7.10).
7.10. Analysis.
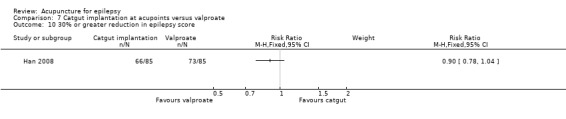
Comparison 7 Catgut implantation at acupoints versus valproate, Outcome 10 30% or greater reduction in epilepsy score.
A 20% or greater reduction in epilepsy score
One trial (Zhang 2006b) reported that significantly more patients in the treatment group achieved 20% or greater reduction in epilepsy score compared with the control group (RR 1.17, 95% CI 1.02 to 1.35, 1 study, 100 participants, P = 0.03) (Analysis 7.11).
7.11. Analysis.
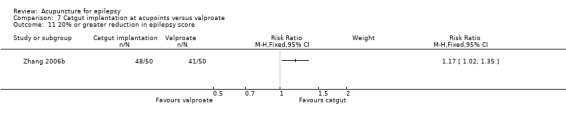
Comparison 7 Catgut implantation at acupoints versus valproate, Outcome 11 20% or greater reduction in epilepsy score.
Post‐treatment EEG abnormality
The same trial (Zhang 2006b) with 100 participants reported post‐treatment EEG abnormalities and found no significant difference between the treatment and the control groups (severe abnormality: RR 0.87, 95% CI 0.55 to 1.37, P = 0.55; moderate abnormality: RR 0.94, 95% CI 0.52 to 1.68, P = 0.83; mild abnormality: RR 1.50, 95% CI 0.67 to 3.35, P = 0.32) (Analysis 7.12).
7.12. Analysis.
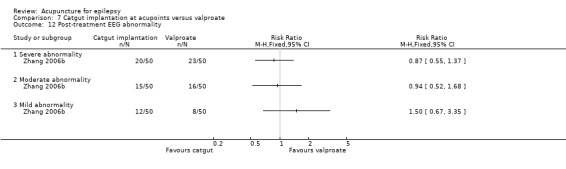
Comparison 7 Catgut implantation at acupoints versus valproate, Outcome 12 Post‐treatment EEG abnormality.
Discussion
Summary of main results
In the current review, we included a total of 17 randomised controlled trials with 1538 participants suffering mainly from generalized epilepsy. The participants in the different trials had a wide age range. The included trials were small, heterogeneous, and had a high risk of bias and short follow‐up.
Compared with Chinese herbs, needle acupuncture plus Chinese herbs was not effective in achieving at least 50% reduction in seizure frequency (two trials). Compared with valproate, needle acupuncture plus valproate was not effective in achieving freedom from seizures (two trials) or at least a 50% reduction in seizure frequency (two trials) but may have achieved a better quality of life (QOL) after treatment (one trial). Compared with phenytoin, needle acupuncture was not effective in achieving at least 50% reduction in seizure frequency (two trials). Compared with valproate, needle acupuncture was not effective in achieving seizure freedom (two trials) but may have been effective in achieving at least a 50% reduction in seizure frequency (two trials) and better QOL after treatment (one trial). Compared with antiepileptic drugs, catgut implantation at acupoints plus antiepileptic drugs (AEDs) was not effective in achieving seizure freedom (four trials) but may have been effective in achieving at least 50% reduction in seizure frequency (five trials) and better QOL after treatment (one trial). Compared with valproate, catgut implantation may be effective in achieving seizure freedom (four trials) and better QOL after treatment (one trial), but not at least a 50% reduction in seizure frequency (four trials). Acupuncture did not have excess adverse events compared to the control treatment in the included trials.
Although needle acupuncture and catgut implantation at acupoints appeared better than control treatment in some outcome measures they were not effective in many other outcome measures. Moreover, some outcome measures were reported by single trials only and no meta‐analysis was possible. On the other hand, the single trial with a sham control (Kloster 1999) concluded that acupuncture was not effective in reducing seizure frequency, increasing the number of seizure‐free weeks or improving QOL in any aspect. However, its small sample size might have limited the statistical power to detect a small difference. Since this trial focused on adults only, whether acupuncture has different effects on different age groups warrants further investigation.
Based on the current systematic review, no firm conclusions can be drawn regarding the effect of acupuncture on epilepsy. Although we identified 17 randomised controlled trials, they were heterogeneous with respect to the treatment and comparison groups used, and the number of patients recruited in each trial was small. The included trials were also heterogeneous with respect to the age of the patients, underlying epilepsy types, acupuncture and control regimens, and outcomes chosen, which limits the reliability of the pooled results.
Overall completeness and applicability of evidence
The available evidence on acupuncture for treatment of epilepsy is far from complete. The included trials were all quite small, recruiting only 34 to 170 patients in each trial (18 to 85 patients in the treatment groups and 16 to 85 patients in the control groups), thus limiting the statistical power and precision of the effect estimates. In addition, no trial reported a sample size calculation, which is essential for ensuring adequate sample size and statistical power. Because of the many different comparisons, which further limited the number of patients in each comparison, the generalizability of the findings is questionable. The follow‐up period used in the trials was also quite short, just one year at most. Whether acupuncture is effective in the long term certainly needs further investigation.
Some of the included trials compared acupuncture with AEDs only, without a concurrent placebo or sham control group. We cannot tell whether acupuncture is effective per se in these trials. The drugs used in the control group might not be appropriate for all patients, as the patients might have been resistant to those treatments already. The dosage of the AED used in the control group might also be inadequate. Sometimes the AED used might aggravate seizures in some patients, resulting in an apparent effectiveness of acupuncture when in fact it was not effective. Furthermore, we cannot reliably determine which AED acupuncture might be superior to as the control treatment was not standardised in some trials. Similarly, which acupuncture regimen is effective and in which patients cannot be reliably determined as the treatment regimen was individualised in some of the trials. As the current review only included trials on needle acupuncture (with manual or electrical stimulation) and catgut implantation at acupoints, whether other forms of acupuncture such as laser acupuncture or acupressure are effective for treating epilepsy is uncertain.
Although no excessive adverse effects were found in the trials that were included, we cannot assure the safety of acupuncture in epilepsy patients since the small sample sizes might have limited the power of detecting rare adverse effects. Although the existing literature on acupuncture in epilepsy patients and patients with a variety of other conditions has supported that it is a relatively safe treatment modality, which can obviate the side effects of AEDs, acupuncture is not without risks. Infections and inappropriate needle placement causing inadvertent damage do occur occasionally. Acupuncture should, therefore, be performed by a well‐trained therapist who is experienced, understands the theories behind it, and takes the necessary precautions.
Quality of the evidence
The included trials all carried a high risk of bias, as evidenced by the lack of descriptions of the randomisation methods, concealment of randomisation, or attempts at blinding in most trials. The reliability of randomisation was questionable in most trials since the treatment and the control groups might not have been comparable at baseline. The treatment and control groups had at least one potentially important baseline difference in three trials (Kloster 1999; Ma 2001; Zhou 2000) and important baseline characteristics were not mentioned in most of the other trials. Furthermore, most trials did not include a placebo or sham control group and therefore a placebo effect could not be excluded, and these trials might bias the results in favour of acupuncture. On the other hand, the acupuncture methods used were not standardised within the intervention or the control groups in some trials, nor were different methods stratified within a treatment group, making it difficult to draw conclusions about the effectiveness of a particular acupuncture protocol.
Potential biases in the review process
We searched extensively in the international and Chinese literature. Some studies that were not published in English or Chinese and not reached by our search strategies might have been missed. Publication bias was also possible although the small number of trials precluded further investigation of this by constructing a funnel plot.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this is the only systematic review on the effects of acupuncture for epilepsy. All relevant studies have been included in the current review.
Authors' conclusions
Implications for practice.
Although numerous observational human studies and experimental animal studies have suggested potential benefits of acupuncture for treating epilepsy, there is a paucity of high quality clinical evidence. The current evidence does not support the use of acupuncture as a treatment for epilepsy.
Implications for research.
There are not many randomised controlled trials assessing acupuncture for epilepsy, especially using control groups that allow estimation of the net effect of acupuncture. The existing trials are of small size with a high risk of bias. Further high quality studies with larger sample sizes and with appropriate standardised treatment and control groups are needed to assess the effectiveness of acupuncture in treating epilepsy. The randomisation method used should be rigorous and concealed. Although blinding of the therapist applying acupuncture is difficult, blinding of the patients, the other care providers, and outcome assessors should be attempted in order to minimise performance and detection biases. Since epilepsy is a highly heterogeneous disease with different aetiologies and severities, acupuncture is likely to have different effects, if any, on different subgroups of patients. Future clinical trials should, therefore, be focused on a particular subgroup or include a very large sample size to delineate the effect of acupuncture on different types of patients. The effectiveness of different forms of acupuncture at different acupoints using different regimens should also be systematically investigated, in a standardised way. Since epilepsy in children and adults have many important differences, both paediatric and adult clinical trials are needed before the results can be safely generalized to all age groups. Since epilepsy is a chronic disease which is well known to wax and wane, with or without treatment, a longer follow‐up period with serial measurements of outcomes is also highly recommended to determine the genuine effectiveness of acupuncture and its long‐term effects.
Feedback
Query regarding excluded studies
Summary
The following query was made on 27 June 2006. Why were trials which compared acupuncture alone with other treatments (pragmatic studies) excluded from the review? How many such trials were there? I think these are a valid form of evaluation and would like to know what data, if any, these studies yielded.
Reply
The objective of this review is to evaluate whether acupuncture is effective for treatment of epilepsy. Therefore only studies that yielded the net treatment effect of acupuncture were included. Net treatment effect of acupuncture can be determined by comparing acupuncture with placebo or sham or no treatment, or comparing acupuncture plus other treatments with the same other treatments.
Studies comparing acupuncture with another treatment alone cannot help us to determine whether acupuncture itself is effective or not, because even though these studies show that acupuncture is more effective than another treatment for example antiepileptic drug or herbs, it does not necessarily mean that acupuncture per se is effective as the drug may have a negative impact on the outcomes such that even no treatment or an ineffective treatment is better than the comparator drug. Therefore these studies comparing acupuncture with another treatment alone were excluded.
Studies comparing acupuncture with another treatment alone that we identified through a systematic search of the literature are included in the table 'Characteristics of excluded studies'. The detailed findings of these studies are not shown but interested readers may refer to the references listed.
NB: 17 July 2008. Please note that this review has now been updated (Issue 4, 2008) to include the excluded studies i.e. studies comparing acupuncture with antiepileptic drugs.
Contributors
Comment made by Dr Catherine Zollman. Daniel Cheuk replied to the comment on behalf of the review authors.
What's new
| Date | Event | Description |
|---|---|---|
| 3 June 2013 | New citation required but conclusions have not changed | One new study has been included; conclusions remain unchanged. |
| 3 June 2013 | New search has been performed | Searches updated 3 June 2013. |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 17 July 2008 | New citation required but conclusions have not changed | The review now includes eight new studies which previously appeared in the 'excluded studies' section. There are now a total of 914 participants. |
| 1 March 2008 | New search has been performed | The searches have been updated (1st March 2008). |
| 17 August 2006 | Feedback has been incorporated | Feedback incorporated along with contact author's response. |
Acknowledgements
We are grateful to the staff of the Medical Library of the University of Hong Kong for their help in finding the articles relevant to this review. We also thank Miss Alison Beamond and Mr Graham Chan for their assistance in the search of relevant trials.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Acupuncture explode all trees
#2 (acupuncture)
#3 (acupressure)
#4 (electroacupuncture)
#5 (meridian*)
#6 (acupoint*)
#7 MeSH descriptor Acupressure explode all trees
#8 MeSH descriptor Acupuncture Therapy explode all trees
#9 MeSH descriptor Acupuncture Points explode all trees
#10 MeSH descriptor Electroacupuncture explode all trees
#11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10)
#12 MeSH descriptor Epilepsy explode all trees
#13 MeSH descriptor Seizures explode all trees
#14 (epilep*) or (seizure*) or (convulsi*)
#15 (#12 OR #13 OR #14)
#16 (#11 AND #15)
Appendix 2. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomised trials published in Lefebvre 2009.
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. clinical trials as topic.sh.
6. randomly.ab.
7. trial.ti.
8. 7 or 5 or 2 or 6 or 1 or 4 or 3
9. exp animals/ not humans.sh.
10. 8 not 9
11. exp epilepsy/
12. epilep$.tw.
13. exp seizures/
14. seizure$.tw.
15. convulsi$.tw.
16. or/11‐15
17. exp Acupuncture Therapy/
18. exp Acupuncture/
19. exp Acupuncture Points/
20. (acupuncture or acupressure or electroacupuncture).tw.
21. (meridian$ or acupoint$).tw.
22. exp Electroacupuncture/
23. exp Acupressure/
24. or/17‐23
25. 10 and 16 and 24
Appendix 3. EMBASE search strategy
1. randomised controlled trial/
2. controlled clinical trial/
3. randomized.ab.
4. placebo.ab.
5. clinical trials/
6. randomly.ab.
7. trial.ti.
8. or/1‐7
9. exp animals/ not humans.sh.
10. 8 not 9
11. exp epilepsy/
12. epilep$.tw.
13. exp seizures/
14. seizure$.tw.
15. convulsi$.tw.
16. or/11‐15
17. exp Acupuncture Therapy/
18. exp Acupuncture/
19. exp Acupuncture Points/
20. (acupuncture or acupressure or electroacupuncture).tw.
21. (meridian$ or acupoints$).tw.
22. exp Electroacupuncture/
23. exp Acupressure/
24. or/17‐23
25. 10 and 16 and 24
Appendix 4. CINAHL search strategy
| S8 | S1 AND S7 |
| S7 | S2 OR S3 OR S4 OR S5 OR S6 |
| S6 | acupressure or acupuncture or electroacupuncture |
| S5 | acupoint* or meridian* |
| S4 | (MM "Acupressure+") |
| S3 | (MM "Electroacupuncture") |
| S2 | (MM "Acupuncture+") OR (MM "Acupuncture Points") |
| S1 | epilep* or seizure* or convulsi* |
Appendix 5. AMED search strategy
| S5 | S1 and S4 |
| S4 | S2 or S3 |
| S3 | meridian* or acupoint* |
| S2 | acupuncture or acupressure or electroacupuncture |
| S1 | epilep* or seizure* or convulsi* |
Appendix 6. Search strategy for China Journals Full‐text Database, China Master thesis Full‐text Database, and China Doctor Dissertations Full‐text Database
1. "Dianxian" (epilepsy)
2. "ZhenJiu" (Acupuncture) or "ZhenCi" (Acupuncture) or "DianZhen" (Electro‐acupuncture) or "ZhenYa" (Acupressure) or "XueWei" (acupoints)
3. 1 and 2
Data and analyses
Comparison 1. Needle acupuncture plus Chinese herbs versus Chinese herbs alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 50% or greater reduction in seizure frequency | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.97, 1.31] |
| 2 75% or greater reduction in seizure frequency | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.12, 2.05] |
| 3 25% or greater reduction in seizure frequency | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.11] |
| 4 75% or greater reduction in seizure duration | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.9 [0.97, 3.74] |
| 5 50% or greater reduction in seizure duration | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.03, 1.62] |
| 6 4 points or greater improvement in EEG | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 2 points or greater improvement in EEG | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8 Post‐treatment EEG abnormality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Any abnormality | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.59, 1.04] |
| 8.2 Severe abnormality | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.87] |
| 8.3 Moderate abnormality | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.21, 2.13] |
| 8.4 Mild abnormality | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.40, 1.68] |
| 8.5 Borderline abnormality | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.33, 5.45] |
Comparison 2. Needle acupuncture plus valproate (VPA) versus valproate (VPA) alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure freedom | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.72, 1.30] |
| 2 50% or greater reduction in seizure frequency | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.52, 3.48] |
| 3 Post‐treatment quality of life score (QOLIE‐31 score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Frequency of adverse effects | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| 4.1 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Malaise | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Nausea | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Anorexia | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Impaired concentration | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Sleepiness | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 5 75% or greater reduction in seizure frequency | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.61, 2.50] |
| 6 25% or greater reduction in seizure frequency | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.83, 1.28] |
| 7 Post‐treatment epilepsy score | 2 | 150 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐3.11, 0.92] |
| 8 70% or greater reduction in epilepsy score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9 40% or greater reduction in epilepsy score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 20% or greater reduction in epilepsy score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11 Post‐treatment EEG abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 Severe abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Moderate abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Mild abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Needle acupuncture versus sham acupuncture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percentage reduction in seizure frequency | Other data | No numeric data | ||
| 2 Improvement in quality of life score (QOLIE‐89 score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Withdrawal due to lack of efficacy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Reduction in seizure frequency | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5 No increase in seizure frequency | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Percentage increase in seizure‐free weeks | Other data | No numeric data |
Comparison 4. Needle acupuncture versus phenytoin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 50% or greater reduction in seizure frequency | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.46, 4.44] |
| 2 75% or greater reduction in seizure frequency | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.47, 3.10] |
| 3 25% or greater reduction in seizure frequency | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Post‐treatment seizure frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 5. Needle acupuncture versus valproate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure freedom | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.93, 3.27] |
| 2 50% or greater reduction in seizure frequency | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.05, 1.66] |
| 3 Post‐treatment quality of life score (QOLIE‐31 score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Frequency of adverse effects | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| 4.1 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Malaise | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Nausea | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Anorexia | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Impaired concentration | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Sleepiness | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 5 75% or greater reduction in seizure frequency | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 25% or greater reduction in seizure frequency | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Post‐treatment epilepsy score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 70% or greater reduction in epilepsy score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9 40% or greater reduction in epilepsy score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 20% or greater reduction in epilepsy score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11 Post‐treatment EEG abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 Severe abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Moderate abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Mild abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Catgut implantation at acupoints plus antiepileptic drugs (AED) versus antiepileptic drugs (AED) alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure freedom | 4 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.93, 2.43] |
| 2 50% or greater reduction in seizure frequency | 5 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.07, 1.89] |
| 3 Post‐treatment quality of life score (QOLIE‐31 score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Frequency of adverse effects | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| 4.1 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Malaise | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Nausea | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Anorexia | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Impaired concentration | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 5 75% or greater reduction in seizure frequency | 5 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.22, 1.79] |
| 6 25% or greater reduction in seizure frequency | 5 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.03, 1.16] |
| 7 Post‐treatment seizure frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Post‐treatment epilepsy score | 2 | 180 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐3.69, 0.57] |
| 9 70% or greater reduction in epilepsy score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 40% or greater reduction in epilepsy score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11 20% or greater reduction in epilepsy score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12 Post‐treatment Global Clinical Impression (GCI) score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Severity Index | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Global Improvement | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Efficacy Index | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Post‐treatment EEG abnormality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Severe abnormality | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.43, 1.60] |
| 13.2 Moderate abnormality | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.53, 1.05] |
| 13.3 Mild abnormality | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.91, 1.84] |
| 13.4 Borderline abnormality | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.83] |
Comparison 7. Catgut implantation at acupoints versus valproate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure freedom | 4 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [1.61, 4.94] |
| 2 50% or greater reduction in seizure frequency | 4 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.94, 1.84] |
| 3 Post‐treatment quality of life score (QOLIE‐31 score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Frequency of adverse effects | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| 4.1 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Malaise | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Nausea | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Anorexia | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Impaired concentration | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Sleepiness | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 5 75% or greater reduction in seizure frequency | 3 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [1.15, 3.91] |
| 6 25% or greater reduction in seizure frequency | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.01, 1.24] |
| 7 Post‐treatment epilepsy score | 3 | 370 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.69, 0.87] |
| 8 70% or greater reduction in epilepsy score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9 40% or greater reduction in epilepsy score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 30% or greater reduction in epilepsy score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11 20% or greater reduction in epilepsy score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12 Post‐treatment EEG abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.1 Severe abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Moderate abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Mild abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Deng 2001a.
| Methods | Randomised controlled trial, parallel groups | |
| Participants | Setting: hospital patients Treatment group (males): 64 (30) Control group (males): 65 (28) Age: Treatment group: mean 21.75 (SD 12.03) years; Control group: mean 22.43 (SD 13.25) years Inclusion: generalized tonic‐clonic epilepsy Exclusion: none Seizure type: generalized tonic‐clonic epilepsy Duration of epilepsy: Treatment group: mean 7.36 ± 7.03 years; Control group: mean 7.85 ± 8.02 years Aetiology of epilepsy: not available Baseline seizure frequency: not available Number of AEDs taken: not available |
|
| Interventions | Treatment group: catgut implantation at 3 to 4 of 7 acupoints, every 25 to 30 days, for 4 to 5 times, plus carbamazepine or valproate at half doses + aminobutyric acid 500 mg 2 to 3 times/day + vitamin B6 20 to 30 mg 2 to 3 times/day + cinnarizine 25 to 50 mg 2 to 3 times/day Control group: carbamazepine 100 to 200 mg 2 to 3 times/day or valproate 200 mg 2 to 3 times/day +aminobutyric acid 500 mg 2 to 3 times/day + Vitamin B6 20 to 30 mg 2 to 3 times/day + cinnarizine 25‐50 mg 2 to 3 times/day Duration of treatment: 1 year | |
| Outcomes | Seizure freedom: Treatment group 75% or greater reduction in seizure frequency 50% or greater reduction in seizure frequency 25% or greater reduction in seizure frequency Post‐treatment EEG abnormality: severe, moderate, mild |
|
| Notes | Duration of follow‐up: 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by computer‐generated random number |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | Comparability of the groups at baseline was uncertain since there were no data on aetiology of epilepsy, current AED treatments, and frequency of seizures at baseline. There was no sham or placebo control and hence there might be placebo effect which causes bias. Treatment was variable within the treatment group and might introduce bias |
Han 2008.
| Methods | Randomised controlled trial, parallel groups | |
| Participants | Setting: hospital patients Treatment group (males): 85 (45) Control group (males): 85 (53) Age: Treatment group: mean 33.4 (SD 20.15) years; Control group: mean 34.9 (SD 15.57) years Inclusion: generalized epilepsy Exclusion: none Seizure type: generalized epilepsy Duration of epilepsy: not available Aetiology of epilepsy: not available Baseline seizure frequency: not available Number of AEDs taken: not available |
|
| Interventions | Treatment group: catgut implantation at 9 acupoints (9 points were divided into 3 groups, each group was used in alternate cycle) plus 1 additional acupoints according to Traditional Chinese Medicine diagnosis, applied every 15 days Control group: sodium valproate 200 mg tds Duration of treatment: 90 days | |
| Outcomes | 30% or greater reduction in epilepsy score Post‐treatment epilepsy score |
|
| Notes | Duration of follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation to treatment groups according to sequence of attendance (quasi‐random) |
| Allocation concealment (selection bias) | High risk | Allocation to treatment groups was done according to sequence of attendance and hence allocation was considered not concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | Comparability of the groups at baseline was uncertain since there were no data on aetiology and duration of epilepsy, current AED treatments, and frequency of seizures at baseline. There was no sham or placebo control and hence there might be placebo effect which causes bias. Treatment was variable within the treatment group and might introduce bias |
Kloster 1999.
| Methods | Randomised controlled trial, parallel groups Block randomisation | |
| Participants | Setting: hospital outpatient Treatment group (males): 18 (9) Control group (males): 16 (7) Age: Treatment group: mean 37.7 (SD 12.8) years; Control group: mean 37.4 (SD 15) years. Inclusion: chronic intractable epilepsy, adult > 18 years, duration > 2 years, verified diagnosis of epilepsy, partial or generalized, ≥ 1 seizures/week Exclusion: non‐epileptic seizures ± epilepsy, inability to co‐operate, progressive cerebral illness; seizure type: partial or generalised ‐ primary generalized (1 treatment, 5 controls), partial with generalization (17 treatment, 11 controls) Duration of epilepsy: Treatment group: mean 27.6 ± 14.3 years; Control group: mean 26.4 ± 12.1 years Aetiology of epilepsy: not available Baseline seizure frequency: Treatment group: 3.5/week; Control: 2/week Number of AEDs taken: Treatment: mean 2 ± 0.8; Control: mean 2.3 ± 0.8 |
|
| Interventions | Treatment group: needle acupuncture, at LR3, L14, GV20, plus ≥1 acupoints chosen according to Traditional Chinese Medicine diagnosis, Suzhou Hwato acupuncture needles with diameter 0.3 mm, length 25 to 55 mm; stimulation given until patient felt needle sensation; needles inserted to varying depths and angles, stimulated by manual rotation or electrically, 3 Hz, 3‐20 mA depending on patient's endurance, using standard TENS apparatus Control group: sham acupuncture, with bilateral needling of 3 points: S1 (2.5cun lateral to umbilicus), S2 (3cun above midpoint of patella), S3 (1cun distal to midpoint between LI15 and TE14), sterilised Suzhou Hwato acupuncture needles diameter 0.25 mm, length 13 mm, to a depth < 5mm; minimal manual stimulation, no electrical stimulation Both: 30min/session, 3 sessions/week for 7.5 weeks with 4 days break in the middle | |
| Outcomes | Percentage reduction of seizure frequency
Reduction in seizure frequency. No increase in seizure frequency Percentage increase in number of seizure‐free weeks Improvement in quality of life score (QOLIE‐89 score) Improvement in EEG abnormality Withdrawal due to lack of efficacy Adverse effects |
|
| Notes | Duration of follow‐up: 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The participants and personnel and outcome assessors were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants and personnel and outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts due to lack of efficacy, requiring changes of AED: Treatment group: 3 participants; Control group: 2 participants. Dropouts accounted for less than 20% of the participants with reasons provided. They were similar in both groups and considered unlikely to affect the conclusion |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | The treatment and control groups were not comparable at baseline as they had different baseline seizure frequency and percentage of patients with primary generalised epilepsy and might introduce bias. Treatment was variable within the treatment group and might introduce bias |
Leng 2000.
| Methods | Randomised controlled trial, parallel groups | |
| Participants | Setting: hospital patients Treatment group (males): 86 (67) Control group (males): 32 (21) Age: Treatment group: mean 29.7 (range 3 to 43) years; Control group: mean 28 (range 17 to 45) years Inclusion: generalized epilepsy Exclusion: none Seizure type: generalized epilepsy Duration of epilepsy: Treatment group: 3 days to 4 years; Control group: 7 days to 3 years Aetiology of epilepsy: not available Baseline seizure frequency: not available Number of AEDs taken: not available |
|
| Interventions | Treatment group: catgut implantation at 9 acupoints monthlyControl group: Na valproate 100 mg bd Duration of treatment: 6 months | |
| Outcomes | Seizure freedom 50% or greater reduction in seizure frequency | |
| Notes | Duration of follow‐up: 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | The comparability of the groups at baseline was questionable since there were no data on aetiology of epilepsy, current AED treatments, and frequency of seizures at baseline. There was no sham or placebo control and hence there might be placebo effect which causes bias |
Li 2007.
| Methods | Randomised controlled trial, parallel groups | |
| Participants | Setting: hospital inpatients and outpatients Treatment group (males): 60 (35) Control group (males):60 (38) Age: Treatment group: 15 to 30 years (32), 30 to 45 years (22), 45 to 60 years (6); Control group: 15 to 30 years (34), 30 to 45 years (19), 45 to 60 years (7) Inclusion: aged 15 to 60 years, generalized tonic‐clonic epilepsy, no medication within 2 weeks, informed consent signed Exclusion: no seizure within past 6 months, received treatment that may affect current study, concomitant diseases of heart, liver, spleen, lung, or kidney, or brain tumour or psychiatric diseases, or bleeding disorders, cannot persevere to study completion Seizure type: generalized tonic‐clonic epilepsy Duration of epilepsy: Treatment group: < 5 years (37), 5 to 10 years (18), > 10 years (5); Control group: < 5 years (34), 5 to 10 years (22), > 10 years (4) Aetiology of epilepsy: not available Baseline seizure frequency: Treatment group: > = once daily (2), 1 to 6 times per week (11), 1 to 3 times per month (17), 1 to 3 per 1 to 2 months (19), 1 to 3 times per 3 months (10), 1 to 3 times per 4 to 5 months (1); Control group: ≥ once daily (3), 1 to 6 times per week (8), 1 to 3 times per month (16), 1 to 3 per 1 to 2 months (24), 1 to 3 times per 3 months (8), 1 to 3 times per 4 to 5 months (1) Number of AEDs taken: not available |
|
| Interventions | Treatment group: catgut implantation at 9 acupoints plus 1 additional acupoints according to Traditional Chinese Medicine diagnosis, applied every 15 days, sodium valproate 200mg tds Control group: sodium valproate 200mg tds Duration of treatment: 90 days | |
| Outcomes | Seizure freedom
75% or greater reduction in seizure frequency
50% or greater reduction in seizure frequency
25% or greater reduction in seizure frequency 70% or greater reduction in epilepsy score 40% or greater reduction in epilepsy score 20% or greater reduction in epilepsy score Post‐treatment epilepsy score Post‐treatment quality of life (QOLIE‐31) score Post‐treatment Global Clinical Impression score): global improvement, efficacy index, severity index Post‐treatment EEG abnormality: severe, moderate, mild, borderline Adverse effects: dizziness, malaise, nausea, anorexia, impaired concentration |
|
| Notes | Duration of follow‐up: 90 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by computer‐generated random number |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | The treatment and the control groups were comparable at baseline. However, there was no sham or placebo control and hence there might be placebo effect which causes bias. Treatment was variable within the treatment group and might introduce bias |
Ma 2001.
| Methods | Randomised controlled trial, parallel groups | |
| Participants | Setting: hospital inpatients and outpatients Treatment group: (boys): 30 (23) Control group (boys): 30 (18) Age: Treatment group: < 5 years (4), 5 to 10 years (6), 10 to 16 years (20); Control group: < 5 years (2), 5 to 10 years (9), 10 to 16 years (19) Inclusion: generalized epilepsy, < 16 years Seizure type: generalized epilepsy Duration of epilepsy: Treatment group: < 1 year (10), 1 to 5 years (12), 5 to 10 years (5), 10 to 15 years (3); Control group: < 1 year (13), 1 to 5 (10), 5 to 10 years (5), 10 to 15 years (2) Aetiology of epilepsy: Treatment group: antenatal problems (2), caesarian section (5), forceps delivery (2), birth asphyxia (4), history of febrile convulsion (8), history of trauma (6), history of brain disorder (4), mental retardation (2), history of intoxication (2), history of phobia (2), family history of epilepsy (1); Control group: antenatal problems (5), caesarian section (5), forceps delivery (2), birth asphyxia (5), history of febrile convulsion (7), history of trauma (5), history of brain disorder (5), mental retardation (1), history of intoxication (6), history of phobia (2), family history of epilepsy (1), prematurity (1), history of hypocalcaemic seizure (1) Baseline seizure frequency: Treatment group: > 1/day (12), 1 to 6/week (7), 1 to 3/month (6), 1/1 to 2 months (2), 1/2 to 4 months (1), 1/4 to 6 months (2); Control group: > 1/day (5), 1 to 6/week (3), 1 to 3/month (9), 1/1 to 2 months (4), 1/2 to 4 months (6), 1/4 to 6 months (3) Number of AEDs taken: not available |
|
| Interventions | Treatment group: acupuncture + mixed Chinese herbal capsule (Xi Feng capsule). Acupuncture at 6 points for 30 minutes, daily for 8 days then rest for 2 days then begin another course. Additional acupuncture at 1or 2 acupoints depending on Traditional Chinese Medicine diagnosis. Xi Feng capsule to be taken 3 times at age‐dependent dosage: < 1 year (1 tab), 1 to 3 years (2 tabs), 4 to 16 years (age‐1 tabs, max 8 tabs) Control group: Xi Feng capsule alone Duration of treatment: 6 months | |
| Outcomes | 75% or greater reduction in seizure frequency
50% or greater reduction in seizure frequency
25% or greater reduction in seizure frequency 75% or greater reduction in seizure duration 50% or greater reduction in seizure duration 4 points or greater improvement in EEG 2 points or greater improvement in EEG Post‐treatment EEG abnormality: severe, moderate, mild, borderline |
|
| Notes | Duration of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | The treatment and the control groups were not comparable at baseline since the treatment group had more frequent seizures. There was no sham or placebo control and hence there might be placebo effect which causes bias. Treatment was variable within the treatment group and might introduce bias |
Mao 2011.
| Methods | Randomised controlled trial, parallel groups Study period: March 2004 to March 2009 |
|
| Participants | Setting: hospital outpatients Treatment group (males): 30 (16) Control group (males): 22 (12) Age: Treatment group : mean 29.93.56 (SD 14.31) years; Control group: mean 33.62 (SD 16.17) years Inclusion: generalized tonic‐clonic epilepsy Exclusion: partial epilepsy, pseudoseizure, syncope, migraine, transient ischemic attack, hyperventilation, hepatic or renal dysfunction Seizure type: generalized tonic‐clonic epilepsy Duration of epilepsy: Treatment group: mean 16.45 (SD 11.05) years; Control group: mean 15.89 (SD 9.62) years Aetiology of epilepsy: not available Baseline seizure frequency: Treatment group: mean 5.17 (SD 1.91) times/year; Control group: mean 5.27 (SD 1.96) times/year Number of AEDs taken: not available |
|
| Interventions | Treatment group: catgut implantation in 4 acupoints, plus 1‐3 additional acupoints according to Traditional Chinese Medicine diagnosis. Implantation once every month for 6 months. Valproate 2 g/day divided into 2 doses for adults, 50mg/kg/day divided into 3 doses for children, for 1 year Control group: Valproate 2 g/day divided into 2 doses for adults, 50mg/kg/day divided into 3 doses for children, for 1 year Duration of treatment: 12 months | |
| Outcomes | Seizure freedom 75% or greater reduction in seizure frequency 50% or greater reduction in seizure frequency 25% or greater reduction in seizure frequency Post‐treatment seizure frequency (times/year) | |
| Notes | Duration of follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to treatment groups according to random number |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | Comparability of groups at baseline was uncertain since there were no data on aetiology of epilepsy and current AED treatments. There was no sham or placebo control and hence there might be placebo effect which causes bias. Treatment was variable within the treatment group and might introduce bias |
Peng 2003.
| Methods | Randomised controlled trial, parallel groups | |
| Participants | Setting: hospital patients Treatment group (males): 32 (20) Control group (males): 31 (17) Age: Treatment group: mean 11.25 (range 6 to 16) years; Control group: mean 12.13 (range 6 to 15) years Inclusion: patients with primary epilepsy aged 6 to 16 years not being treated with Western medicines Exclusion: patients with secondary epilepsy and patients being treated with antiepileptics were excluded Seizure type: generalized epilepsy Duration of epilepsy: Treatment group: mean 6.53 (range 4 to 13) years; Control group: mean 5.69 (range 3 to 11) years Aetiology of epilepsy: not available Baseline seizure frequency: not available Number of AEDs taken: 0 |
|
| Interventions | Treatment group: catgut implantation in 6 acupoints (GV1, CV15, bilateral BL15 and bilateral ST36) every 20 days for 3 times Control group: Na valproate 5 to 1010 mg/kg/day divided into 3 doses Duration of treatment: 60 days | |
| Outcomes | Seizure freedom 75% or greater reduction in seizure frequency 50% or greater reduction in seizure frequency | |
| Notes | Duration of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by computer‐generated random number |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | The comparability of the groups at baseline was questionable since there were no data on aetiology and frequency of seizures at baseline. There was no sham or placebo control and hence there might be placebo effect which causes bias |
Shi 2001.
| Methods | Randomised controlled trial, parallel groups | |
| Participants | Setting: hospital inpatients and outpatients Treatment group (males): 45 (20) Control group (males): 35 (16) Age: Treatment group: mean 9 (range 5 to 14) years; Control group: mean 10 (range 6 to 13) years Inclusion: childhood absence epilepsy Exclusion: none Seizure type: childhood absence epilepsy Duration of epilepsy: Treatment group: 10 months to 4 years; Control group: 6 months to 5 years Aetiology of epilepsy: not available Baseline seizure frequency: not available Number of AEDs taken: not available |
|
| Interventions | Treament group: acupuncture at 6 acupoints (including injection of diazepam to 2 acupoints) alternate day Control group: Na valproate 200 mg tds + piracetam 800 mg tds Duration of treatment: 80 days | |
| Outcomes | Seizure freedom 50% or greater reduction in seizure frequency | |
| Notes | Duration of follow‐up: 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | Comparability of groups at baseline was uncertain since there were no data on aetiology of epilepsy, current AED treatments, and frequency of seizures at baseline. There was no sham or placebo control and hence there might be placebo effect which causes bias |
Xiong 2003.
| Methods | Randomised controlled trial, parallel groups (3 groups) | |
| Participants | Setting: hospital patients Treatment group: 30 Control group 1: 30 Control group 2: 30 Overall 64 boys Age: 1 to 4 years (14), 4 to 10 years (52), 10 to 17 years (24) Inclusion: generalized epilepsy, < 18 years Exclusion: none Seizure type: generalized epilepsy Duration of epilepsy: < 1 year (18), 1 to 3 years (45), 3 to 6 years (10), 6 to 10 years (7) Aetiology of epilepsy: history of febrile convulsion (28), trauma (19), intoxication (13), forceps delivery (3), positive family history (7), perinatal asphyxia (2), brain disease (1) Baseline seizure frequency: not available AEDs used: carbamazepine (19), valproate (22), Chinese herbs (25), other drugs (5), drug naive (19) |
|
| Interventions | Treatment group: acupuncture + Chinese herb mixtures. Acupuncture at 10 points for 30 minutes, daily for 10 days then rest for 2 days then begin another course. Chinese herb mixture to be taken twice daily at age‐dependent dosage: < 3 years (50 to 100 ml), 3 to 9 years (100 to 200 ml), 9 to 17 years (200 to 500 ml)
Control group 1: Chinese herb mixture alone
Control group 2: carbamazepine alone, dosage according to age Duration of treatment: 6 months |
|
| Outcomes | 75% or greater reduction in seizure frequency 50% or greater reduction in seizure frequency 25% or greater reduction in seizure frequency 75% or greater reduction in seizure duration 50% or greater reduction in seizure duration Post‐treatment EEG abnormality |
|
| Notes | Duration of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | The comparability of the groups at baseline was questionable since there were no data on aetiology and duration of epilepsy, current AED treatments, and frequency of seizures at baseline. There was no sham or placebo control and hence there might be placebo effect which causes bias |
Yi 2009.
| Methods | Randomised controlled trial, parallel groups Study period: December 2007 to June 2009 |
|
| Participants | Setting: hospital outpatients Treatment group (males): 46 Control group: 46 Age: Treatment group: mean 22.23 (SD 2.5) years; Control group: mean 21.56 (SD 2.66) years Inclusion: age 15 to 60 years, generalised epilepsy, informed consent signed Exclusion: received other treatment concurrently or cannot cooperate with study treatment, concomitant diseases of heart, liver, spleen, lung, or kidney, or brain tumour or psychiatric diseases Seizure type: generalized epilepsy Duration of epilepsy: Treatment group: mean 3.46 (SD 1.67) years; Control group: 3.55 (SD 1.21) years Aetiology of epilepsy: not available Baseline seizure frequency: not available Number of AEDs taken: not available |
|
| Interventions | Treatment group: Acupuncture at 1 common point, plus 2 to 3 acupoints depending on Traditional Chinese Medicine diagnosis. Needles left for 30 minutes, with 20 minutes of electrical stimulation. Treatment applied once on alternate day for 10 times then rest for 2 days (1 course). A total of 4 courses given. Sodium valproate 200 mg tds Control group: sodium valproate 200 mg tds Duration of treatment: 3 months | |
| Outcomes | Seizure freedom 75% or greater reduction in seizure frequency 50% or greater reduction in seizure frequency 25% or greater reduction in seizure frequency 70% or greater reduction in epilepsy score 40% or greater reduction in epilepsy score 20% or greater reduction in epilepsy score Post‐treatment epilepsy score Post‐treatment quality of life score (QOLIE‐31 score) Post‐treatment EEG abnormality: severe, moderate, mild Adverse effects: dizziness, malaise, nausea, anorexia, impaired concentration, sleepiness |
|
| Notes | Duration of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by computer‐generated random number |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 dropout in each group, reasons not reported. Dropouts constituted small proportion of participants and were considered unlikely to affect outcome |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | The comparability of the groups at baseline was questionable since there were no data on aetiology and frequency of seizures at baseline. There was no sham or placebo control and hence there might be placebo effect which causes bias. Treatment was variable within the treatment group and might introduce bias |
Yu 1999.
| Methods | Randomised controlled trial, parallel groups. Study period: August 1995 to December 1998 |
|
| Participants | Setting: hospital outpatients Treatment group (males): 30 (18) Control group: 30 (not available) Age Treatment group: 6 to 43 years; Control group: not available Inclusion: Epilepsy Exclusion: space occupying lesions on CT scan Seizure type: not available Duration of epilepsy Treatment group: 3 months to 10 years; Control group: not available Aetiology of epilepsy: not available Baseline seizure frequency: not available Number of AEDs taken: not available |
|
| Interventions | Treatment group: acupuncture at 7 acupoints every 3 to 5 days for 20 to 30 times Control group: phenytoin 100 mg tds ± oryzanol Duration of treatment: 60 to 150 days | |
| Outcomes | 75% or greater reduction in seizure frequency 50% or greater reduction in seizure frequency | |
| Notes | Duration of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | The comparability of the groups at baseline was questionable since there were no data on aetiology and duration of epilepsy, current AED treatments, and frequency of seizures at baseline. There was no sham or placebo control and hence there might be placebo effect which causes bias |
Zhang 2006a.
| Methods | Randomised controlled trial, parallel groups (3 groups) | |
| Participants | Setting: hospital patients Treatment group 1 (catgut implantation at acupoints + valproate) (males): 30 (14) Treatment group 2 (needle acupuncture + valproate) (males): 30 (12) Control group (males): 30 (12) Age Treatment group 1: mean 33.56 (SD 12.75) years; Treatment roup 2: mean 35.02 (SD 12.05) years; Control group: mean 31.79 (SD 11.77) years Inclusion: generalized tonic‐clonic epilepsy Exclusion: none Seizure type: generalized tonic‐clonic epilepsy Duration of epilepsy Treatment group 1: mean 7.3 (SD 7.03) years; Treatment group 2: mean 7.96 (SD 7.28) years; Control group: mean 7.68 (SD 6.94) years Aetiology of epilepsy: not available Baseline seizure frequency: not available Number of AEDs taken: not available |
|
| Interventions | Treatment group 1: catgut implantation in 9 acupoints (9 points were divided into 3 groups, each group was used in alternate cycle), plus 1 additional acupoints according to Traditional Chinese Medicine diagnosis. Implantation once every 15 days. Valproate 200 mg tds Treatment group 2: needle acupuncture at same acupoints alternate days + valproate 200 mg tds Control group: Valproate 200 mg tds Duration of treatment: 3 months | |
| Outcomes | Seizure freedom 75% or greater reduction in seizure frequency 50% or greater reduction in seizure frequency 25% or greater reduction in seizure frequency Post‐treatment epilepsy score | |
| Notes | Duration of follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation to treatment groups according to sequence of attendance (quasi‐random) |
| Allocation concealment (selection bias) | High risk | Allocation to treatment groups was done according to sequence of attendance and hence allocation was considered not concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | The comparability of the groups at baseline was questionable since there were no data on aetiology of epilepsy, current AED treatments, and frequency of seizures at baseline. There was no sham or placebo control and hence there might be placebo effect which causes bias |
Zhang 2006b.
| Methods | Randomised controlled trial, parallel groups | |
| Participants | Setting: hospital inpatients and outpatients Treatment group 1 (catgut implantation at acupoints) (males): 50 (27) Treatment group 2 (needle acupuncture) (males): 50 (25) Control group (males): 50 (24) Age: Treatment group 1: 15 to 30 years (28), 30 to 45 years (19), 45 to 60 years (3); Treatment roup 2: 15 to 30 years (30), 30 to 45 years (13), 45 to 60 years (7); Control group: 15 to 30 years (26), 30 to 45 years (20), 45 to 60 years (4) Inclusion: aged 15 to 60 years, generalized tonic‐clonic epilepsy, informed consent signed Exclusion: no seizure within past 6 months, received treatment that may affect current study, concomitant diseases of heart, liver, spleen, lung, or kidney, or brain tumour or psychiatric diseases, or bleeding disorders, cannot comply with study treatment Seizure type: generalized tonic‐clonic epilepsy Duration of epilepsy Treatment group 1: < 5years (24), 5 to 10 years (20), > 10 years (6); Treatment group 2: < 5years (28), 5 to 10 years (13), > 10 years (9) Control group: < 5years (25), 5 to 10 years (19), > 10 years (6) Aetiology of epilepsy: not available Baseline seizure frequency: not available Number of AEDs taken: not available |
|
| Interventions | Treatment group 1: catgut implantation in 9 acupoints (9 points were divided into 3 groups, each group was used in alternate cycle), plus 1 additional acupoints according to Traditional Chinese Medicine diagnosis. Implantation once every 15 days Treatment group 2: needle acupuncture at same acupoints, needle left in place for 20 minutes. Treatment applied once daily on alternate days Control group: Sodium valproate 200 mg tds Duration of treatment: 3 months | |
| Outcomes | Seizure freedom
75% or greater reduction in seizure frequency
50% or greater reduction in seizure frequency
25% or greater reduction in seizure frequency 70% or greater reduction in epilepsy score 40% or greater reduction in epilepsy score 20% or greater reduction in epilepsy score Post‐treatment epilepsy score Post‐treatment quality of life score (QOLIE‐31 score) Post‐treatment EEG abnormality: severe, moderate, mild Frequency of adverse effects: dizziness, malaise, nausea, anorexia, impaired concentration, sleepiness |
|
| Notes | Duration of follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done by computer‐generated random number |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | The comparability of the groups at baseline was questionable since there were no data on aetiology of epilepsy, current AED treatments, and frequency of seizures at baseline. There was no sham or placebo control and hence there might be placebo effect which causes bias. Treatment was variable within the treatment group and might introduce bias |
Zhou 2000.
| Methods | Randomised controlled trial, parallel groups | |
| Participants | Setting: hospital patients Treatment group (males): 60 (32) Control group: 30 (17) Age Treatment group: 0.5 to 65 years; Control group: 1 to 59 years. Inclusion: epilepsy Exclusion: nil Seizure type: Treatment group: generalized epilepsy (46), partial epilepsy (14); Control group: generalized epilepsy (20), partial epilepsy (10) Duration of epilepsy: not available Aetiology of epilepsy: not available Baseline seizure frequency: not available Number of AEDs taken: not available |
|
| Interventions | Treatment group: acupuncture at 12 standard acupoints, plus 1‐2 additional acupoints according to Traditional Chinese Medicine diagnosis, daily for 30 days then weekly for 5 months Control: phenytoin Duration of treatment: 6 months | |
| Outcomes | 75% or greater reduction in seizure frequency 50% or greater reduction in seizure frequency 25% or greater reduction in seizure frequency Post‐treatment seizure frequency (times/year) |
|
| Notes | Duration of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | The treatment and the control groups were not comparable at baseline because the treatment group had lower baseline seizure frequency. There was no sham or placebo control and hence there might be placebo effect which causes bias. Treatment was variable within the treatment group and might introduce bias |
Zhuang 2004.
| Methods | Randomised controlled trial, parallel groups Study period: April 2002 to March 2003 |
|
| Participants | Setting: hospital patients Treatment group: 22 Control group: 18 Age: 4.5 to 50 years Inclusion: epilepsy Exclusion: none Seizure type: not available Duration of epilepsy: not available Aetiology of epilepsy: Treatment group: idiopathic epilepsy (12), symptomatic epilepsy (10); Control group: idiopathic epilepsy (10), symptomatic epilepsy (8) Baseline seizure frequency: not available Number of AEDs taken: not available |
|
| Interventions | Treatment group: catgut implantation in 4 acupoints (4 points were divided into 2 groups, each group was used in alternate cycle). Implantation once every 14 days Control group: phenobarbital 30 mg tds and phenytoin 100 mg tds Duration of treatment: 2 months | |
| Outcomes | 75% or greater reduction in seizure frequency 50% or greater reduction in seizure frequency 25% or greater reduction in seizure frequency | |
| Notes | Duration of follow‐up: 2 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | The comparability of the groups at baseline was questionable since there were no data on aetiology of epilepsy, current AED treatments, and frequency of seizures at baseline. There was no sham or placebo control and hence there might be placebo effect which causes bias |
Zhuang 2006.
| Methods | Randomised controlled trial, parallel groups Study period; January 2003 to December 2005 |
|
| Participants | Setting: hospital inpatients and outpatients Treatment group (males): 50 (22) Control group (males): 50 (27) Age Treatment group: mean 30.05 (SD 12.03) years; Control group: mean 33.2 (SD 11.65) years Inclusion: generalised epilepsy Exclusion: none Seizure type: generalized epilepsy Duration of epilepsy: not available Aetiology of epilepsy: not available Baseline seizure frequency: not available Number of AEDs taken: not available |
|
| Interventions | Treatment group: catgut implantation in 9 acupoints (9 points were divided into 3 groups, each group was used in alternate cycle), plus 1 additional acupoints according to Traditional Chinese Medicine diagnosis. Implantation once every 15 days Control group: valproate 200 mg tds Duration of treatment: 3 months | |
| Outcomes | Seizure freedom 75% or greater reduction in seizure frequency 50% or greater reduction in seizure frequency 25% or greater reduction in seizure frequency Post‐treatment epilepsy score | |
| Notes | Duration of follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation to treatment groups according to sequence of attendance (quasi‐random) |
| Allocation concealment (selection bias) | High risk | Allocation to treatment groups was done according to sequence of attendance and hence allocation was considered not concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | The comparability of the groups at baseline was questionable since there were no data on aetiology and duration of epilepsy, current AED treatments, and frequency of seizures at baseline. There was no sham or placebo control and hence there might be placebo effect which causes bias |
AEDs: antiepileptic drugs bd: twice daily CT: computed tomography EEG: electroencephalogram GCI: Global Clinical Improvement SD: standard deviation tds: three times daily
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chui 2006 | Acupuncture combined with Chinese herbs compared with antiepileptic drugs. No placebo or sham or no‐treatment control |
| Deng 2001b | Acupuncture combined with Chinese herbs compared with antiepileptic drugs. No placebo or sham or no‐treatment control |
| Han 2005 | Acupuncture combined with Chinese herbs compared with anti‐epileptic drugs. No placebo or sham or no‐treatment control |
| Kuang 1996 | Acupuncture combined with Chinese herbs compared with acupuncture only. No placebo or sham or no‐treatment control |
| Li 2004 | Comparison of different acupuncture methods. No placebo or sham or no‐treatment control |
| Lin 2001 | Comparison of different acupuncture methods. No placebo or sham or no‐treatment control |
| Luo 2004 | Trial of acupuncture for treatment of febrile convulsion, not epilepsy |
| Wu 2008 | Acupuncture combined with tuina (another form of alternative complementary therapy) compared with rehabilitation. No placebo or sham or no‐treatment control |
| Xu 2003 | Comparison of different acupuncture methods. No placebo or sham or no‐treatment control |
| Xu 2004 | Acupuncture combined with Chinese herbs compared with sodium valproate. No placebo or sham or no‐treatment control |
| Yi 2005 | Trial of blood‐letting puncture for treatment of apoplexy, not epilepsy |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐TRC‐10001023.
| Trial name or title | Ear‐acupuncture therapy for epilepsy |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: 1. Male or female patients aged 12 to 65 years. 2. Patients meeting the diagnosis of partial epilepsy according to Classification of epilepsy issued by International league against epilepsy, 1981, who get no remission after following routine antiepileptics treatment for 1 year or more. 3. The seizure attacked over 4 times per month within 8 weeks before recruitment. 4. Patients are receiving at least one type of antiepileptics, and have no change in medication within 8 weeks before recruitment. 5. No brain tumour, progressive brain disease or neural degeneration confirmed by EEG within 6 months or by previous MRI or CT scan. 6. No other somatic disease and can count the frequency of seizure attack. 7. Women at reproductive age have take effective contraceptive interventions. Exclusion criteria: 1. Patients with CNS disease. 2. Non‐epileptic seizures or pseudoepilepsy. 3. Patients with history of status Epilepticus or cluster seizures. 4. Patients with severe mental retardation or unstable mental state. 5. Patients with severe heart, liver, kidney disease or haematologic disease. 6. Women undergoing pregnancy or lactation. 7. Patients have enrolled in other antiepileptics trial within 8 weeks before recruitment. 8. Patients have implanted stimulator of the Cervical Vagus Nerve. 9. Patients with poor compliance. |
| Interventions | Treatment group: acupuncture on auricular non‐acupoints with 1mA electric needle for 30 min, three times a day. Treatment lasts for 4 weeks. Control group: acupuncture on auricular non‐acupoints with 1mA electric needle for 30 min, three times a day. Treatment lasts for 4 weeks. Then patients will be transferred to treatment group if no effect is observed. |
| Outcomes | Frequency of epilepsy attack |
| Starting date | 1 Jan 2009 |
| Contact information | Dr Yuxue Zhao, Institute of Acupuncture, China Academy of Traditional Chinese Medicine, 16 Nanxiaojie of Dongzhimen, Beijing, China +86 10 64014411 2772 claricezhao@yahoo.com.cn |
| Notes | Still recruiting participants |
CNS: central nervous system CT: computed tomography MRI: magnetic resonance imaging
Differences between protocol and review
The assessment of risk of bias was updated to adhere to the recommendation by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). As a result, the Jadad score was abandoned. The plan for the sensitivity analysis was also changed accordingly to exclude studies which carried a high risk of bias. We updated the analysis to use 99% confidence intervals (instead of 95% confidence intervals) for frequency of adverse effects if there were several adverse effects reported in order to account for multiple statistical testing.
Contributions of authors
Both review authors contributed to the design, development, and editing of the protocol and undertook all parts of the review.
Sources of support
Internal sources
The University of Hong Kong, Hong Kong.
External sources
No sources of support supplied
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Deng 2001a {published data only}
- Deng Y, Wang J, Lin Y, Liu W, Wang L. Clinical observation on treatment of epilepsy general tonic‐clonic attack with catgut implantation at acupoint plus antiepileptic Western medicine of small dose. Chinese Acupuncture and Moxibustion 2001;21(5):271‐3. [Google Scholar]
Han 2008 {published data only}
- Han D, Sun J, Zhuang L. Catgut embedding for treatment of 85 cases of generalized epilepsy. Journal of Acupuncture and Tuina Science 2008;24(6):35‐6. [Google Scholar]
Kloster 1999 {published data only}
- Kloster R, Lasson PG, Lossius R, Nakken KO, Dahl R, Zui‐Ling X, et al. The effect of acupuncture in chronic intractable epilepsy. Seizure 1999;8(3):170‐4. [DOI] [PubMed] [Google Scholar]
- Stavem K, Kloster R, Rossberg E, Larsson PLG, Dahl R, Kinge E, et al. Acupuncture in intractable epilepsy: lack of effect on health‐related quality of life. Seizure 2000;9(6):422‐6. [DOI] [PubMed] [Google Scholar]
Leng 2000 {published data only}
- Leng Y, Pan Q, Chen X, Yang T. Epilepsy treated by point thread embedding. Report of 86 cases. Journal of External Therapy of Traditional Chinese Medicine 2000;9(4):16. [Google Scholar]
Li 2007 {published data only}
- Li Y. Clinical research of treating generalized tonic‐clonic seizure with catgut embedding and medicine. Thesis (MPhil). Guangzhou University of Traditional Chinese Medicine 2007.
Ma 2001 {published data only}
- Ma R, Zhang X, Liu Y, Li X, Yang C, Xiong J. Clinical observation on treatment of tonoclonic attack of infantile epilepsy with acupuncture plus Xi Feng capsule. Journal of Traditional Chinese Medicine 2001;42(5):276‐8. [Google Scholar]
- Zhang X. Clinical and experimental study on acupuncture combined with Xi Feng Jiao Nang for treatment of epilepsy of childhood. Thesis (MPhil). Tianjin University of Traditional Chinese Medicine 2000.
Mao 2011 {published data only}
- Mao ZN, Gao ZG, Zhang GW, Wang SB. Observation on therapeutic effect of acupoint catgut‐embedding combined western medicine for epilepsy of generalized seizures type. Zhongguo Zhen Jiu 2011;31(6):509‐12. [PubMed] [Google Scholar]
Peng 2003 {published data only}
- Peng Y. Clinical study on treatment of infantile primary epilepsy with acupoint catgut‐implantation therapy. World Journal of Acupuncture‐Moxibustion 2003;13(1):38‐41. [Google Scholar]
- Peng Y. Studies of the clinic and experiment with embedding catgut in points to treat childhood primary epilepsy. Thesis (PhD). Guangzhou University of Traditional Chinese Medicine 2000.
Shi 2001 {published data only}
- Shi J. Absence seizures of epilepsy treated by scalp acupuncture combined with hydro‐acupuncture at renying point. Report of 45 cases. Shaanxi Journal of Chinese Traditional Medicine 2001;22(1):43‐4. [Google Scholar]
Xiong 2003 {published data only}
- Xiong X, Zhang G, Huang W, Sun J. Clinical observation of acupuncture and Chinese medicine for treatment of epilepsy in children. Chinese Journal of Information on Traditional Chinese Medicine 2003;10(7):62‐3. [Google Scholar]
Yi 2009 {published data only}
- Yi H. Observation of electro‐acupuncture treatment of vasoconstriction district for grand mal seizures. Thesis (MPhil). Guangzhou University of Traditional Chinese Medicine 2009.
Yu 1999 {published data only}
- Yu D, Ping X, Zhao X. Epilepsy treated by acupuncture with growing fluid and extinguishing wind. Report of 30 cases. Jiangsu Journal of Traditional Chinese Medicine 1999;20(11):37. [Google Scholar]
Zhang 2006a {published data only}
- Zhang J, Li Y, Zhuang L. Clinical observation on catgut implantation at acupoint for treatment of generalised tonic‐clonic epilepsy. Report of 90 cases . Journal of Clinical Acupuncture and Moxibustion 2006;22(6):8‐10. [PubMed] [Google Scholar]
Zhang 2006b {published data only}
- Zhang J. Study of curative effect of generalized tonic‐clonic seizure (GTCS) treated by catgut implantation at acupoint. Thesis (M. Phil.). Guangzhou University of Traditional Chinese Medicine 2006.
Zhou 2000 {published data only}
- Zhou Y. Epilepsy treated by acupuncture with complementation with Yin and Yang. Report of 60 cases. Journal of Traditional Chinese Medicine 2000;41(7):441. [Google Scholar]
Zhuang 2004 {published data only}
- Zhuang L, Ding X, Chen M. Catgut embedding for treatment of epilepsy: observation of 22 cases. New Chinese Medicine 2004;36(1):46‐7. [Google Scholar]
Zhuang 2006 {published data only}
- Zhuang Lx, Zhang J, Li YZ. Clinical observation on catgut implantation at acupoint for treatment of general paroxysmal epilepsy. Chinese Acupuncture and Moxibustion 2006;26(9):611‐3. [PubMed] [Google Scholar]
References to studies excluded from this review
Chui 2006 {published data only}
- Chui H, Pin J, Cao R. Catgut embedding and oral herbal medicines for treatment of 68 cases of epilepsy. Traditional Chinese Medicine Research 2006;19(9):61‐2. [Google Scholar]
Deng 2001b {published data only}
- Deng Y, Huang Z, Lin Y, Liu J, Huang L. Clinical observation on 88 cases of epilepsy with general tonicoclonic attack treated with medicated thread implantation at acupoint. Journal of Traditional Chinese Medicine 2001;42(7):406‐8. [Google Scholar]
Han 2005 {published data only}
- Han G, Han R. Catgut embedding for treatment of 800 cases of epilepsy. China's Naturopathy 2005;13(10):23‐4. [Google Scholar]
Kuang 1996 {published data only}
- Kuang K, Zhou D, Yu X. Clinical observation on 40 cases of epilepsy treated with acupuncture and Chinese herbs. Jiangxi Journal of Traditional Chinese Medicine 1996;27(6):47. [Google Scholar]
Li 2004 {published data only}
- Li H, Zhang J. Catgut embedding in scalp acupoint for treatment of 112 cases of epilepsy. Journal of Clinical Acupuncture and Moxibustion 2004;20(6):46‐7. [Google Scholar]
Lin 2001 {published data only}
- Lin J, Deng Q, Zhang J. Observation on therapeutic effect of 160 cases of epilepsy treated with acupoint catgut embedding therapy. Chinese Acupuncture and Moxibustion 2001;21(11):653‐4. [Google Scholar]
Luo 2004 {published data only}
- Luo WP, Miao YN. Observations on the curative effect of combined acupuncture and medication on infantile hyperpyretic convulsion. Shanghai Journal of Acupuncture and Moxibustion 2004;23(7):12. [Google Scholar]
Wu 2008 {published data only}
- Wu Y, Zou LP, Han TL, Zheng H, Caspi O, Wong V, et al. Randomized controlled trial of traditional Chinese medicine (acupuncture and tuina) in cerebral palsy: part 1‐‐any increase in seizure in integrated acupuncture and rehabilitation group versus rehabilitation group?. Journal of Alternative and Complementary Medicine 2008;14(8):1005‐9. [DOI] [PubMed] [Google Scholar]
Xu 2003 {published data only}
- Xu Y, Zhang J, Deng Q. Acupoint catgut embedding therapy and its application in the treatment of epilepsy. Information on Tcm 2003;20(1):35. [Google Scholar]
Xu 2004 {published data only}
- Xu J, Lie X, Lie D. Acupuncture combined with herbal seizure tablet for treatment of epilepsy in children. Report of 64 cases. Journal of Traditional Chinese Medicine 2004;45(5):349. [Google Scholar]
Yi 2005 {published data only}
- Yi G, Xiuyun W, Tangping X, Zhihua D, Yunchen L. Effect of blood‐letting puncture at twelve well‐points of hand on consciousness and heart rate in patients with apoplexy. Journal of Traditional Chinese Medicine = Chung i Tsa Chih Ying Wen Pan / Sponsored by All‐China Association of Traditional Chinese Medicine, Academy of Traditional Chinese Medicine 2005;25(2):85‐9. [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR‐TRC‐10001023 {published data only}
- Zhao Y, Rong P. Ear‐acupuncture therapy for epilepsy. WHO International Clinical Trials Registry Platform: http://apps.who.int/trialsearch/Trial.aspx?TrialID=ChiCTR‐TRC‐10001023 2010. [http://apps.who.int/trialsearch/Trial.aspx?TrialID=ChiCTR‐TRC‐10001023]
Additional references
Chen 1983
- Chen K, Chen G, Feng X. Observation of immediate effect of acupuncture on electroencephalograms in epileptic patients. Journal of Traditional Chinese Medicine 1983;3:121‐4. [PubMed] [Google Scholar]
Commission 1989
- Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30:389‐99. [DOI] [PubMed] [Google Scholar]
Furlan 1999
- Furlan AD, Tulder MW, Cherkin DC, Tsukayama H, Lao L, Koes BW, et al. Acupuncture and dry‐needling for low back pain. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD001351] [DOI] [PubMed] [Google Scholar]
Hauser 1993
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935‐1984. Epilepsia 1993;34:453‐68. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Holland 2001
- Holland KD. Efficacy, pharmacology, and adverse effects of antiepileptic drugs. Neurologic Clinics 2001;19(2):313‐45. [DOI] [PubMed] [Google Scholar]
Huang 1999
- Huang ZN, Yang R, Chen G, Cheng JS. Effect of electroacupuncture and 7‐NI on penicillin‐induced epilepsy and their relation with intrahippocampal NO changes. Sheng Li Xue Bao 1999;51(5):508‐14. [PubMed] [Google Scholar]
Jansen 1989
- Jansen G, Lundeberg T, Kjartansson S, Samuelson UE. Acupuncture and sensory neuropeptides increase cutaneous blood flow in rats. Neuroscience Letters 1989;97:305‐9. [DOI] [PubMed] [Google Scholar]
Johansson 1993
- Johansson K, Lindgren I, Widner H, Wiklund I, Johansson BB. Can sensory stimulation improve the functional outcome in stroke patients?. Neurology 1993;43:2189‐92. [DOI] [PubMed] [Google Scholar]
Lefebvre 2009
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Liu 1995
- Liu J, Cheng J. Changes of amino acids release in rat's hippocampus during kainic acid induced epilepsy and acupuncture. Zhen Ci Yan Jiu 1995;20(3):50‐4. [PubMed] [Google Scholar]
Liu 1997
- Liu J, Cheng JS. Hippocampal non‐NMDA and GABA‐A receptors in benzylpenicillin‐induced epilepsy and electro‐acupuncture antiepilepsy. Zhongguo Yao Li Xue Bao 1997;18(2):189‐91. [PubMed] [Google Scholar]
Liu 2004
- Liu JH, Yan J, Yi SX, Chang XR, Lin YP, Hu JM. Effects of electroacupuncture on gastric myoelectric activity and substance P in the dorsal vagal complex of rats. Neuroscience Letters 2004;356(2):99‐102. [DOI] [PubMed] [Google Scholar]
Maciocia 1989
- Maciocia G. Foundations of Chinese Medicine: A comprehensive Text for Acupuncturists and Herbalists. Edinburgh: Churchill Livingstone, 1989. [Google Scholar]
Magnusson 1994
- Magnusson M, Johansson K, Johansson BB. Sensory stimulation promotes normalization of postural control after stroke. Stroke 1994;25:1176‐80. [DOI] [PubMed] [Google Scholar]
Middlekauff 2004
- Middlekauff HR, Shah JB, Yu JL, Hui K. Acupuncture effects on autonomic responses to cold pressor and handgrip exercise in healthy humans. Clinical Autonomic Research: official journal of the Clinical Autonomic Research Society 2004;14(2):113‐8. [DOI] [PubMed] [Google Scholar]
NIH 1998
- NIH Consensus Conference. Acupuncture. JAMA 1998;280(17):1518‐24. [PubMed] [Google Scholar]
Sander 1996
- Sander JWAS, Shorvon SD. Epidemiology of the epilepsies. Journal of Neurology, Neurosurgery, and Psychiatry 1996;61(5):433‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
SCSSS 1999
- Swedish Collaboration on Sensory Stimulation in Stroke. Sensory stimulation after stroke: a randomized controlled trial. Cerebrovascular Diseases 1999;9 Suppl 1:1‐28. [Google Scholar]
Shi 1987
- Shi Z, Gong B, Jia Y, Hua Z. The efficacy of electroacupuncture on 98 cases of epilepsy. Journal of Traditional Chinese Medicine 1987;7:21‐2. [PubMed] [Google Scholar]
Sun 2001
- Sun HL, Li XM. Clinical study on treatment of cerebral apoplexy with penetration needling of scalp acupoints [tou xue tou ci zhi liao nao zu zhong lin chuang yan jiu]. Chinese Acupuncture and Moxibustion 2001;21(5):275‐8. [Google Scholar]
Wang 2001
- Wang G, Jiang N, He Z. Effects of scalp acupuncture on plasma ET‐1, MDA and NO contents in the patient of cerebral infarction. Chinese Acupuncture and Moxibustion 2001;21(4):241‐2. [Google Scholar]
Wu 1992
- Wu D. Mechanism of acupuncture in suppressing epileptic seizures. Journal of Traditional Chinese Medicine 1992;12:187‐92. [PubMed] [Google Scholar]
Wu 1996
- Wu JN. A short history of acupuncture. Journal of Alternative and Complementary Medicine 1996;2(1):19‐21. [DOI] [PubMed] [Google Scholar]
Yang 1990
- Yang J. Treatment of status epilepticus with acupuncture. Journal of Traditional Chinese Medicine 1990;10:101‐2. [PubMed] [Google Scholar]
Yang 2000
- Yang R, Huang ZN, Cheng JS. Anticonvulsion effect of acupuncture might be related to the decrease of neuronal and inducible nitric oxide synthases. Acupuncture Electrotherapy Research 2000;25(3‐4):137‐43. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cheuk 2008
- Cheuk DKL, Wong V. Acupuncture for epilepsy. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD005062.pub3] [DOI] [PubMed] [Google Scholar]


