Abstract
Background
Integrated molecular testing could be an opportunity to detect and provide care for both tuberculosis and COVID-19. Many high tuberculosis burden countries, such as Peru, have existing GeneXpert systems for tuberculosis testing with GeneXpert Xpert MTB/RIF Ultra (Xpert Ultra), and a GeneXpert SARS-CoV-2 assay, GeneXpert Xpert Xpress SARS-CoV-2 (Xpert Xpress), is also available. We aimed to assess the feasibility of integrating tuberculosis and COVID-19 testing using one sputum specimen with Xpert Ultra and Xpert Xpress in Lima, Peru.
Methods
In this cross-sectional, diagnostic accuracy study, we recruited adults presenting with clinical symptoms or suggestive history of tuberculosis or COVID-19, or both. Participants were recruited from a total of 35 primary health facilities in Lima, Peru. Participants provided one nasopharyngeal swab and one sputum sample. For COVID-19, we tested nasopharyngeal swabs and sputum using Xpert Xpress; for tuberculosis, we tested sputum using culture and Xpert Ultra. We compared diagnostic accuracy of sputum testing using Xpert Xpress with nasopharyngeal swab testing using Xpert Xpress. Individuals with positive Xpert Xpress nasopharyngeal swab results were considered COVID-19 positive, and a positive culture indicated tuberculosis. To assess testing integration, the proportion of cases identified in sputum by Xpert Xpress was compared with Xpert Xpress on nasopharyngeal swabs, and sputum by Xpert Ultra was compared with culture.
Findings
Between Jan 11, 2021, and April 26, 2022, we recruited 600 participants (312 [52%] women and 288 [48%] men). In-study prevalence of tuberculosis was 13% (80 participants, 95% CI 11–16) and of SARS-CoV-2 was 35% (212 participants, 32–39). Among tuberculosis cases, 13 (2·2%, 1·2–3·7) participants were concurrently positive for SARS-CoV-2. Regarding the diagnostic yield of integrated testing, Xpert Ultra detected 96% (89–99) of culture-confirmed tuberculosis cases (n=77), and Xpert Xpress-sputum detected 67% (60–73) of COVID-19 cases (n=134). All five study staff reported that integrated molecular testing was easy and acceptable.
Interpretation
The diagnostic yield of Xpert Xpress on sputum was moderate, but integrated testing for tuberculosis and COVID-19 with GeneXpert was feasible. However, systematic testing for both diseases might not be the ideal approach for everyone presenting with presumptive tuberculosis or COVID-19, as concurrent positive cases were rare during the study period. Further research might help to identify when integrated testing is most worthwhile and its optimal implementation.
Funding
Canadian Institutes of Health Research and International Development Research Centre.
Translation
For the Spanish translation of the abstract see Supplementary Materials section.
Introduction
Tuberculosis was the leading cause of death by an infectious disease, until the emergence of COVID-19. WHO estimated that there were 10·6 million tuberculosis cases and 1·6 million tuberculosis-related deaths in 2021.1 Since the emergence of COVID-19, there have been over 676 million cases and nearly 6·88 million deaths attributable to COVID-19 as of March 22, 2023, according to the COVID-19 dashboard by the Center for Systems Science and Engineering at Johns Hopkins University. Some of the vast pandemic-related resource mobilisation2 has come at the expense of control programmes of other diseases, with existing personnel, facilities, and supplies reallocated to the COVID-19 response; additionally, routine health services have been disrupted.3, 4 An estimated 4·2 million people who developed tuberculosis in 2021 were not diagnosed or notified1 and, consequently, did not receive proper care; thus, strategies and catch-up efforts to mitigate the detrimental effects of COVID-19 are urgently needed.
In response, in 2021, the Stop TB Partnership and US Agency for International Development recommended simultaneous, integrated (on a multiplex platform) testing approaches for tuberculosis and COVID-19 in countries with a high burden of tuberculosis (as reported by WHO) for individuals presumed to have either disease.5 In late 2021, The Global Fund to Fight AIDS, Tuberculosis and Malaria released a briefing note recommending that individuals whose clinical signs and symptoms meet the case definitions for tuberculosis and COVID-19 undergo testing for both diseases, using sputum for tuberculosis and nasopharyngeal swabs for COVID-19.6 WHO has not issued a formal policy recommendation on integrated testing.
Research in context.
Evidence before this study
We searched PubMed from database inception to July 13, 2022, to identify articles of any language that studied integrated molecular testing approaches for tuberculosis and COVID-19. A search including terms for the concepts of tuberculosis and COVID-19—ie, (“tuberculosis”[Title/Abstract] OR “TB”[Title/Abstract]) AND (“sars-cov-2”[Title/Abstract] OR “covid-19”[Title/Abstract])—with the human filter applied and with no other restrictions yielded 882 citations. These results included 28 systematic reviews, although none focused on integrated testing or screening approaches for tuberculosis and COVID-19. One systematic review and meta-analysis estimated that, on the basis of findings from 43 studies, the pooled prevalence of concurrent tuberculosis among patients with COVID-19 was 1·1% (95% CI 0·81–1·36) with values ranging from 0·2% to 14·4%. Additionally, we searched PubMed using the same search criteria and dates for evaluations of Xpert Xpress SARS-CoV-2, the index test of interest, using sputum samples. This search yielded three publications: a case report describing concurrent presence of Mycobacterium tuberculosis and SARS-CoV-2 from a sputum sample; a methodological paper describing the development of a protocol for sputum testing with results from less than 30 individuals; and one study evaluating Xpert Xpress SARS-CoV-2 with alternative specimens that presented diagnostic accuracy estimates of all lower respiratory specimens in aggregate.
Added value of this study
We describe the clinical symptoms and symptom duration of 600 adults with presumptive tuberculosis or COVID-19. Our study is one of the first to evaluate simultaneous, integrated molecular testing for tuberculosis and COVID-19 among this group. Within our study population, we observed a relatively low concurrent positivity rate. We showed that using samples collected in one clinical encounter and running them on a multi-disease PCR-based platform was feasible for diagnosing both tuberculosis and COVID-19. Of note, our study also showed that detecting M tuberculosis and SARS-CoV-2 from a single sputum sample was possible, a finding that, until now, has only been documented in a case report. Additionally, our study is the largest to describe the diagnostic accuracy of Xpert Xpress SARS-CoV-2 using sputum samples, a sample type that has largely been ignored in COVID-19 testing.
Implications of all the available evidence
Despite policy briefs from major global health stakeholders calling for integrated testing for tuberculosis and COVID-19, evidence to support these policies has remained scarce. Our findings suggest that integrated testing for both diseases is likely to be feasible, particularly in settings that already use multidisease testing PCR platforms with experienced laboratory and clinical staff. However, sputum might not be the ideal sample for integrated testing, as the proportion of COVID-19 cases identified through sputum testing was only a moderate proportion of total cases identified with nasopharyngeal swabs. Also, molecular testing is relatively expensive and resource-intensive; the relatively low rate of COVID-19 diagnoses among people with culture-confirmed tuberculosis suggests that integrated testing approaches should be applied in a context-specific manner as opposed to universally. More research will be needed to better understand when exactly and in which populations these approaches should be adopted.
One platform that might be used for this purpose is GeneXpert (Cepheid, Sunnyvale, CA, USA), which runs cartridge-based, automated PCR tests for various diseases, including tuberculosis and COVID-19. GeneXpert Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) and the newer generation GeneXpert Xpert MTB/RIF Ultra (hereafter referred to as Xpert Ultra; Cepheid, Sunnyvale, CA, USA) are WHO-endorsed molecular tuberculosis tests that use sputum samples.7 The GeneXpert Xpert Xpress SARS-CoV-2 (hereafter referred to as Xpert Xpress; Cepheid, Sunnyvale, CA, USA) test has received US Food and Drug Administration Emergency Use Authorisation for SARS-CoV-2 detection with nasopharyngeal swab samples.8 Both Xpert Ultra and Xpert Xpress cartridges are available to national tuberculosis programmes at concessional prices via the Global Drug Facility of Stop TB Partnership.9 Therefore, GeneXpert-based testing provides an opportunity for presumably affected patients to receive care for tuberculosis and COVID-19 in one clinical encounter, because existing equipment, staff, and expertise can be leveraged.
This type of integrated intervention could be particularly applicable in countries such as Peru, a country with a high burden of tuberculosis that has been particularly affected by the COVID-19 pandemic and has one of the world's highest COVID-19 cumulative mortality rates, according to the COVID-19 dashboard. Peru has also had a pandemic-associated drop in tuberculosis case notifications: there were 8093 fewer tuberculosis case notifications from March to October, 2020, than in that same period in 2019, a drop of about 20%.10 Since early 2020, there have been approximately 4·2 million notified cases of COVID-19 and 217 000 COVID-19-related deaths in Peru, according to the COVID-19 dashboard (appendix 2 p 2). Other countries in the region have also reported substantial case notification declines.11 Due to high endemic levels of tuberculosis and multidrug resistant tuberculosis, there is an existing network of GeneXpert platforms across seven of the 24 regions of the country, and use of Xpert MTB/RIF or Xpert Ultra is recommended for tuberculosis diagnosis among key populations, such as children and people living with HIV.12
Therefore, we investigated integrated tuberculosis and COVID-19 molecular testing using GeneXpert (ie, simultaneously testing for both diseases in the same clinical encounter, including with a single sputum specimen). To do so, we first estimated the accuracy of Xpert Xpress on sputum samples versus nasopharyngeal swabs for COVID-19 diagnosis. We also compared the proportion of tuberculosis cases identified using sputum on Xpert Ultra and COVID-19 cases identified using sputum on Xpert Xpress when integrated testing is in place versus standard-of-care methods. We also comment on the feasibility of integrated testing.
Methods
Study design and population
We did a cross-sectional, diagnostic accuracy study of adult (aged ≥18 years) outpatients in Lima, Peru, with presumptive COVID-19 or tuberculosis, or both (ie, symptoms including but not limited to coughs, fever, difficulty breathing, and sore throat of any duration) or epidemiologic history suggestive of COVID-19 or tuberculosis. Participants were excluded if they had a history of COVID-19 in the previous 3 months or anti-tuberculosis therapy in the previous 6 months. Participants had to be able to spontaneously produce a sputum sample.
Weekly COVID-19 incidence in Lima during the study period is provided in appendix 2 (p 2). Before July 2021, study participants were recruited from three sites across Lima: the COVID-19 clinic at Huaycán Hospital (a secondary referral hospital in the Ate-Vitarte district); the tuberculosis clinic at Huascar XV Health Centre (a primary health facility in the San Juan de Lurigancho district); and the tuberculosis clinic at Max Arias Health Centre (a primary health facility in the La Victoria district). After July 2021, participants were also recruited from tuberculosis clinics of 32 other primary health facilities in the San Juan de Lurigancho district. We expanded recruitment from the three initial sites in an attempt to increase the rate of participant enrolment.
All participants provided written informed consent. This study received ethical approval from the Comité Institucional de Ética en Investigación at Universidad Peruana Cayetano Heredia (SIDISI 202931) and the McGill University Health Centre Research Ethics Board (2021-6866). This study was registered in the PRISA repository at Instituto Nacional de Salud in Peru (number EI00000001484).
Procedures
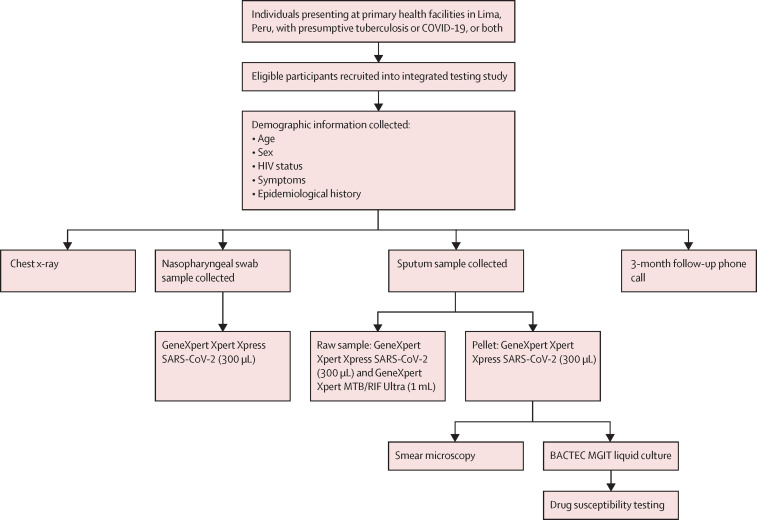
Eligible individuals presenting at recruitment sites were invited to participate in our study. Participants provided self-reported demographic and symptom data on questionnaires upon enrolment. Due to logistical considerations, COVID-19-related clinic closures, and irregular staff availability, consecutive sampling was not feasible and so convenience sampling was used. Nasopharyngeal swabs and sputum samples were stored and transported at 2–8°C and processed within the same day. Procedures and details are in figure 1 and appendix 2 (p 2).
Figure 1.
Study procedures
Each participant provided one nasopharyngeal swab and one sputum sample. During the phone call, we asked whether the participant was living at time of follow-up, was hospitalised since enrolling in the study, had received a diagnosis of tuberculosis or COVID-19 after enrolling in our study, or had any current symptoms, and how their overall physical and mental health were compared with usual health.
Briefly, each nasopharyngeal swab was transported in approximately 3 mL of transport media. 300 μL was used for testing on Xpert Xpress as per manufacturer's protocol. Approximately 5 mL of expectorated sputum was collected per participant. Glass beads were used to homogenise the sample for easier separation. For tuberculosis testing, 1 mL was used for Xpert Ultra; for COVID-19 testing, 300 μL was run on Xpert Xpress. The remaining sputum was decontaminated and used for smear microscopy and one bacteriological culture (BD BACTEC MGIT, BD, Franklin Lakes, NJ, USA).
The laboratory staff that did all the assays were masked to participants' clinical details. Laboratory procedures were done at the Humberto Guerra Alisson laboratory, a reference-level laboratory at the Instituto de Medicina Tropical Alexander von Humboldt at Universidad Peruana Cayetano Heredia (Lima, Peru). Study data were collected and managed with Research Electronic Data Capture (REDCap) tools hosted at the Research Institute of the McGill University Health Centre.13 REDCap is a secure, web-based application designed to support data capture for research studies.
Information on testing integration feasibility was collected through semi-structured interviews with study staff, which took place at study completion—ie, when staff could reflect on their experience of the study (appendix 2 pp 2–3).
Clinical staff at recruitment sites were already experienced at collecting sputum samples and nasopharyngeal swabs and had existing protocols regarding personal protective equipment and biosafety for these procedures, so study-specific additional safety measures were not required. Sputum specimen and nasopharyngeal swabs were collected outdoors or in well-ventilated areas to reduce biosafety concerns. With respect to the laboratory aspects of integrated testing, splitting sputum for use in multiple assays was anticipated to be challenging, particularly for samples with a thick consistency, laboratory staff used glass beads to homogenise the sample before allocating the appropriate volume to each cartridge.
Outcomes
The study aimed to identify, among people with presumed tuberculosis or COVID-19, the diagnostic accuracy of Xpert Xpress using sputum, compared with the performance on manufacturer-recommended nasopharyngeal swabs. The other main outcome was the proportion of COVID-19 cases and tuberculosis cases detected using a single sputum sample, compared with cases identified by reference standards. Additionally, the prevalence of concurrent tuberculosis and COVID-19, and the feasibility of integrated testing, were examined.
Statistical analysis
The Xpert Xpress test on nasopharyngeal swabs, the manufacturer-recommended sample, can detect SARS-CoV-2 with very high accuracy14 owing to its low limit of detection.15 Due to its resultant high sensitivity, Xpert Xpress on nasopharyngeal swabs was used as the reference test and individuals with a positive Xpert Xpress result with nasopharyngeal swabs were classified as COVID-19 positive. The diagnostic accuracy of Xpert Xpress on sputum, our primary index test of interest, compared with Xpert Xpress on nasopharyngeal swabs was identified via a contingency table, with the 95% CI calculated by exact method. Tests that produced errors, no result, or inconclusive results were repeated with repeated results included in the analysis. After repeat testing, test results that were errors, no result, or inconclusive were excluded from the analysis. A sensitivity analysis was done that included only individuals who had complete test results for all assays (known as complete cases).
The index test for tuberculosis was Xpert Ultra in our study. Tuberculosis has no perfect reference standard, but microbiological confirmation of Mycobacterium tuberculosis by liquid culture is acknowledged to be the most accurate option and positivity served as reference standard for confirmed tuberculosis. The index test for COVID-19 is Xpert Xpress on sputum and secondarily Xpert Xpress on nasopharyngeal swabs. We compared the proportion of cases identified by Xpert Xpress on sputum with those identified by Xpert Xpress on nasopharyngeal swabs. Tests that produced errors, no result, or inconclusive results were repeated with repeated results included in the analysis. After repeat testing, test results that were errors, no result, or inconclusive were excluded from the analysis. All analyses were done with the epiR package (version 2.0.48) in RStudio.16 Overlap in test results was visualised with VennDiagram (version 1.7.1) in Rstudio.17
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between Jan 11, 2021, and April 26, 2022, 607 eligible individuals were enrolled in our study, with seven excluded due to inadequate sputum specimen volume. Thus, 600 participants were included in our analyses. Demographic characteristics are shown in table 1 . 171 (29%) of 600 of our study population had previously been tested for SARS-CoV-2. The study population (median age 40 years, range 18–87) was balanced between men (48%) and women (52%; table 1). Almost all participants self-reported that they were mildly ill (533 [89%] of 600). The most reported symptoms at study enrolment were cough (94%, 68% less than 2 weeks in duration, 26% longer than 2 weeks), headache (86%), and general malaise (85%). As producing a sputum sample was an eligibility criterion, there was most likely a selection bias against the inclusion of asymptomatic individuals who had difficulty expectorating.
Table 1.
Demographic characteristics of study population
| Participants (N=600) | ||
|---|---|---|
| Sex | ||
| Female | 313 (52%) | |
| Male | 287 (48%) | |
| Age, years | 40 (29–54) | |
| Known exposure to COVID-19 case 2 weeks before symptom onset | 174 (29%) | |
| Disease severity at study entry* | ||
| Not ill | 0 | |
| Mildly ill | 533 (89%) | |
| Moderately ill | 67 (11%) | |
| Gravely ill | 0 | |
| Smoking status | ||
| Current | 32 (5%) | |
| Former | 178 (30%) | |
| Never routine | 390 (65%) | |
| Chest radiography results | ||
| No abnormalities | 63 (11%) | |
| Abnormalities present | 302 (50%) | |
| Data not available | 235 (39%) | |
| Comorbidities | ||
| Cardiovascular disease | 37 (6%) | |
| Asthma | 26 (4%) | |
| Other chronic respiratory condition | 5 (1%) | |
| Diabetes | 23 (4%) | |
| Known HIV infection | 6 (1%) | |
| Chronic kidney disease | 2 (<1%) | |
| Current cancer | 2 (<1%) | |
| Symptom positivity | ||
| Breathing difficulties | 230 (38%) | |
| Cough | 565 (94%) | |
| Cough for less than 2 weeks | 407 (68%) | |
| Cough for 2 weeks or longer | 158 (26%) | |
| Fatigue | 448 (75%) | |
| Fever | 343 (57%) | |
| General malaise | 509 (85%) | |
| Headache | 518 (86%) | |
| Loss of smell | 106 (18%) | |
| Loss of taste | 100 (17%) | |
| Muscle pain | 481 (80%) | |
| Sore throat | 523 (87%) | |
| Thoracic pain | 427 (71%) | |
Data are n (%) and median (IQR).
Disease severity definitions are as follows: not ill refers to healthy and strong impression throughout examination; mildly ill refers to ability to carry out routine activities but symptomatic (eg, fatigue or cough) upon careful inspection; moderately ill refers to some impairment of activities (ie, visibly ill to a lay person, still ambulatory and mostly self-sufficient, but clearly symptomatic); and gravely ill refers to inability to carry out usual activities, visibly distressed, and requires hospitalisation.
In our study population, 80 of 600 (13%, 95% CI 11–16) participants had culture-confirmed tuberculosis, four of whom had multidrug-resistant tuberculosis. 212 participants (35%, 32–39) were deemed positive for COVID-19 on the basis of detection of SARS-CoV-2 using Xpert Xpress on nasopharyngeal swabs. 13 participants (2·2%, 1·2–3·7) were concurrently positive on tuberculosis culture and Xpert Xpress on nasopharyngeal swabs or sputum.
Among the 80 participants with culture-confirmed tuberculosis, those who also tested positive for SARS-CoV-2 varied by specimen: 11 on nasopharyngeal swabs (14%, 95% CI 7·1–23) and three on sputum (3·8%, 1·0–11). Test result patterns among culture-confirmed tuberculosis cases (appendix 2 p 4) showed that the observed concurrent tuberculosis and COVID-19 rate partly depends on the specimen used for SARS-CoV-2 detection. Alternatively, of the 212 participants with a positive COVID-19 result with Xpert Xpress on nasopharyngeal swabs, 11 (5%) had culture-confirmed tuberculosis.
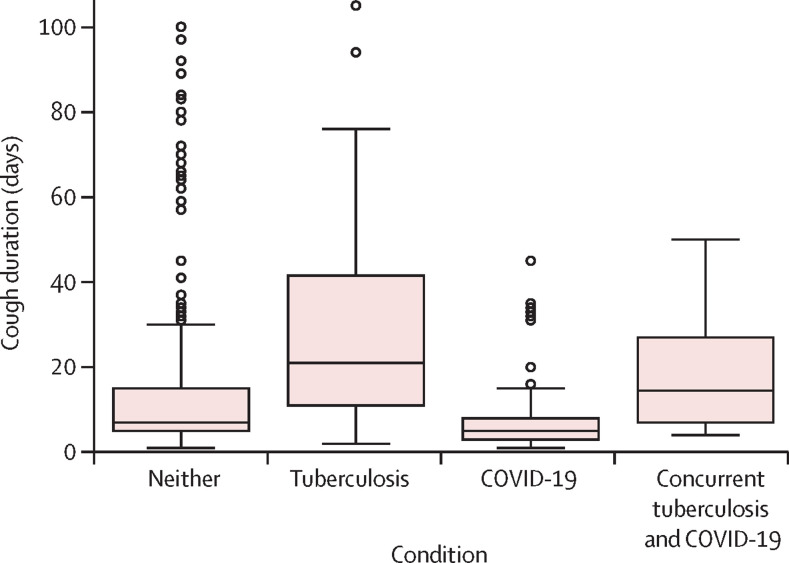
Symptoms are stratified by diagnosis (table 2 ). Participants with COVID-19 had higher rates of specific symptoms than participants with tuberculosis, including fatigue, headache, and loss of smell or taste. However, participants with tuberculosis typically reported having a particular symptom longer than those with COVID-19. For example, breathing difficulties were reported in 36 (45%) of 80 participants with tuberculosis and 91 (43%) of 212 participants with COVID-19; however, the median duration of having breathing difficulties for participants with tuberculosis was 10 days (IQR 4–15) compared with 3 days (IQR 2–4) for participants with COVID-19. Cough lasting longer than 2 weeks was uncommon in participants with COVID-19 (23 [11%] of 212) but was observed in 52 (65%) of 80 of participants with tuberculosis (figure 2 ). Yet, cough for less than 2 weeks could not rule out either disease, as 176 (83%) of 212 participants with COVID-19 and 24 (30%) 80 of participants with tuberculosis reported short-term coughs. Symptoms are also presented for the 13 participants with tuberculosis and a positive Xpert Xpress on nasopharyngeal swabs or sputum (table 2), which is a small proportion of the study population (13 [2%] of 600), and therefore strong inferences should not be drawn.
Table 2.
Number of study participants reporting symptoms and corresponding symptom durations
|
People with tuberculosis (N=80) |
People with COVID-19 (N=212) |
People with tuberculosis with concurrent positive Xpert Xpress test (N=13) |
||||
|---|---|---|---|---|---|---|
| Participants reporting symptoms | Symptom duration, days | Participants reporting symptoms | Symptom duration, days | Participants reporting symptoms | Symptom duration, days | |
| Breathing difficulties | 36 (45%) | 10 (4–15) | 91 (43%) | 3 (2–4) | 7 (54%) | 7 (6–15) |
| Cough | 76 (95%) | 20 (10–37) | 199 (94%) | 5 (3–8) | 12 (92%) | 15 (7–24) |
| Cough less than 2 weeks | 24 (30%) | 7 (5–9) | 176 (83%) | 5 (3–7) | 5 (39%) | 7 (4–7) |
| Cough 2 weeks or longer | 52 (65%) | 32 (20–52) | 23 (11%) | 20 (15–33) | 7 (54%) | 20 (17–42) |
| Fatigue | 56 (70%) | 14 (7–19) | 162 (76%) | 4 (3–7) | 9 (69%) | 7 (5–15) |
| Fever | 55 (69%) | 5 (3–14) | 141 (67%) | 2 (2–4) | 7 (54%) | 3 (2–10) |
| General malaise | 65 (81%) | 12 (6–18) | 187 (88%) | 4 (3–7) | 11 (85%) | 7 (5–15) |
| Headache | 58 (73%) | 10 (5–15) | 188 (89%) | 4 (3–6) | 9 (69%) | 13 (6–15) |
| Loss of smell | 7 (9%) | 7 (6–11) | 66 (31%) | 3 (2–5) | 2 (15%) | 5 (4–6) |
| Loss of taste | 8 (10%) | 6 (5–9) | 56 (26%) | 3 (2–5) | 1 (8%) | 3 (3–3) |
| Muscle pain | 59 (74%) | 10 (7–15) | 169 (80%) | 4 (3–7) | 8 (62%) | 7 (5–15) |
| Sore throat | 72 (90%) | 15 (7–33) | 184 (87%) | 4 (3–7) | 12 (92%) | 7 (6–9) |
| Thoracic pain | 60 (75%) | 14 (7–25) | 135 (64%) | 4 (3–6) | 11 (85%) | 7 (7–16) |
Data are n (%) or median (IQR). Data stratified by disease diagnosis in 80 people with tuberculosis, as defined by culture-positivity; in 212 people with COVID-19, as defined by a positive result on Xpert Xpress with nasopharyngeal swab; and in 13 people with culture-positive tuberculosis and a concurrently positive Xpert Xpress test (nasopharyngeal swab or sputum).
Figure 2.
Boxplots of cough duration stratified by disease condition
Tuberculosis was defined by culture positivity (n=80). COVID-19 was defined by a positive test result on Xpert Xpress with nasopharyngeal swabs (n=212). Concurrent tuberculosis and COVID-19 was defined as concurrent tuberculosis culture and a positive result on Xpert Xpress with nasopharyngeal swabs or sputum (n=13).
The diagnostic accuracy of Xpert Xpress on sputum is not well characterised. With sputum, Xpert Xpress sensitivity was 67% (95% CI 60–73) and specificity was 97% (94–98) compared with Xpert Xpress using the manufacturer-recommended sample, which is nasopharyngeal swabs (table 3 ). A sensitivity analysis including complete cases only (table 3) yielded similar results: Xpert Xpress on sputum had sensitivity of 67% (60–73) and specificity of 97% (94–98).
Table 3.
Two-by-two contingency table of Xpert Xpress results for SARS-CoV-2
| Positive on nasopharyngeal swab | Negative on nasopharyngeal swab | |
|---|---|---|
| All available results (n=586) | ||
| Positive on sputum | 134 | 13 |
| Negative on sputum | 67 | 372 |
| Complete cases only (n=584) | ||
| Positive on sputum | 134 | 13 |
| Negative on sputum | 66 | 371 |
Xpert Xpress results on sputum for SARS-CoV-2 detection (index test) compared with Xpert Xpress results on nasopharyngeal swabs (reference test) for all participants with available data (n=586), and for participants without any missing test results (n=584).
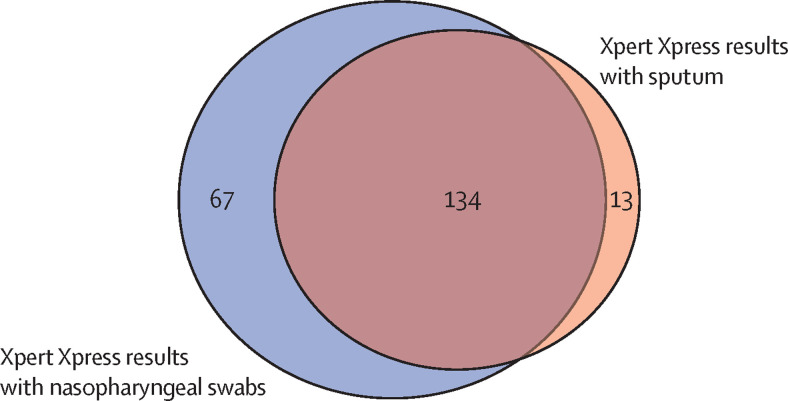
We investigated the diagnostic yield of a single sputum sample for diagnosing tuberculosis or COVID-19, on the basis of the proportion of cases identified compared with the Universidad Peruana Cayetano Heredia standard-of-care tests. Compared with culture-positivity, Xpert Ultra detected 77 of 80 (96%, 95% CI 89–99) tuberculosis cases, including all four cases with rifampicin-resistance. Xpert Xpress on sputum identified 134 of 201 (67%, 60–73) COVID-19 cases diagnosed by Xpert Xpress with nasopharyngeal swabs (figure 3 ). Counts and explanations for unavailable test results are reported in appendix 2 (p 4). As 13 (2%) of 600 participants had culture-positive tuberculosis and a positive Xpert Xpress result on either nasopharyngeal swabs or sputum, at least 46 people with presumptive tuberculosis or COVID-19, or both, would have to be tested to find one person with both diseases.
Figure 3.
Venn diagram of Xpert Xpress results for SARS-CoV-2 detection with nasopharyngeal swabs and sputum
Numbers in the figure correspond to the number of individuals with positive test results on either nasopharyngeal swab (n=67), sputum (n=13), or both (n=134). Here, data from individuals with any missing test results were excluded from the figure.
According to semi-structured interviews with two study nurses and three laboratory staff, integrated tuberculosis and COVID-19 testing using sputum was considered very feasible. Collecting additional nasopharyngeal swabs was not deemed difficult and was considered simpler than sputum, as some participants needed coaching to produce sputum. One study nurse suggested that integrated testing might be preferentially introduced at tuberculosis clinics. Given the epidemiological situation in Peru, individuals presenting for tuberculosis testing were comfortable with also providing different samples for concurrent COVID-19 testing. Conversely, some individuals presenting for COVID-19 testing were more sceptical of undergoing tuberculosis testing: “When they were told that also they can leave a sample to rule-out tuberculosis, some did not really want to because they were more concerned about what COVID was, but not what tuberculosis was, no, because that was what ‘was on trend’, as they say. There are other patients who had a history or relatives with TB and they did leave their samples [for TB]… But some did not want to because in truth for time and because sometimes they were unaware of the disease [TB] and were more worried about COVID.” One laboratory staff member noted that as all GeneXpert cartridges look very similar, it could be possible to use the incorrect sample or sample volume in a particular assay during busy periods, and that this happened a few times during the study. Otherwise, as Universidad Peruana Cayetano Heredia already use Xpert Ultra in their laboratory workflow, the addition of Xpert Xpress was straightforward, regardless of specimen. Additional laboratory biosafety protocols were not required. The minimal manipulation and hands-on time needed to run Xpert Ultra and Xpert Xpress tests aided feasibility of integrated testing.
Discussion
We investigated the integration of COVID-19 and tuberculosis testing by testing a single sputum sample on the GeneXpert platform. Compared with the standards-of-care in our setting (Lima, Peru), this approach identified 96% of tuberculosis cases and 67% of COVID-19 cases. In a hypothetical population of 1000 people diagnosed with COVID-19 by Xpert Xpress with nasopharyngeal swabs, Xpert Xpress on sputum would miss 333 individuals. Using a single sputum specimen on GeneXpert to universally test for both diseases was a feasible but suboptimal integrated testing approach, since Xpert Xpress had only moderate sensitivity on sputum.
Recommendations from The Global Fund include testing for COVID-19 and tuberculosis when clinical signs and symptoms meet the case definitions for both diseases.6 Our concurrent positivity rate was low despite multiple COVID-19 surges during the recruitment period (appendix 2 p 2). Study activity was paused in January, 2022, as most staff were isolating due to SARS-CoV-2 exposure, so some concurrently positive cases were likely to be missed. Our positivity rates are consistent with a 2021 systematic review and meta-analysis, which pooled 43 studies to estimate the prevalence of tuberculosis among people with COVID-19; prevalence estimates from included publication ranged from 0·18% to 14%, with pooled prevalence of 1·1% (95% CI 0·81–1·4).18 These findings and our findings suggest that testing everyone with either presumptive tuberculosis or COVID-19 for both diseases might not be a worthwhile approach, and universal implementation of integrated testing probably should not be adopted. However, when COVID-19 prevalence surges, integrated testing might gain value and yield more cases, highlighting the importance of strong disease surveillance systems that indicate when interventions, such as integrated testing, might be appropriate. Further work will be required to understand how integrated testing approaches can be optimised.19, 20
Symptom duration alone could not reliably differentiate participants diagnosed with tuberculosis from people diagnosed with COVID-19. Our study population, who were predominately mildly ill, were almost all symptomatic. Many but not all symptoms were reported at similar rates by both groups. For some symptoms, such as fever or general malaise, the IQRs in people with tuberculosis and those with COVID-19 overlapped, presenting a challenge to using clinical picture as a rule-out tool. In individuals with culture-positive tuberculosis and a positive Xpert Xpress test, the moderately longer reported symptom durations perhaps indicates that these individuals had incidental SARS-CoV-2 infections. Altogether, 14% of people with culture-confirmed tuberculosis also tested positive for SARS-CoV-2 on Xpert Xpress with nasopharyngeal swabs; therefore, integrated disease testing might be more pertinent in people with presumptive tuberculosis in the context of increased local COVID-19 outbreaks.
Therefore, from our study population, it appears that integrated tuberculosis and COVID-19 molecular testing might be most warranted when individuals are presenting with symptoms lasting less than 1 week, whereas for those with longer-lasting symptoms, seemingly only tuberculosis testing is needed. This observation might be particularly relevant as the incubation periods of newer SARS-CoV-2 variants continue to shorten.21, 22 In particular, to ensure proper care, it would be important to understand whether a patient who requires steroids as part of COVID-19 treatment also has tuberculosis. More research will help identify whether this trend is maintained in other high tuberculosis burden settings and as SARS-CoV-2 variants continue to arise.
Using already widespread molecular testing platforms to concurrently investigate tuberculosis and COVID-19 could be one way to reach the millions of people with tuberculosis who have not received care since the pandemic's onset. However, as rapid antigen tests for SARS-CoV-2 detection are now widely available, the role of molecular testing has reduced, which has probably diminished the opportunity to use COVID-19 testing as an opportunity to identify people with undiagnosed tuberculosis. Even so, finding these missing cases remains important because people with previously diagnosed or current tuberculosis are at a high risk of complications and death if infected by SARS-CoV-2. A multicountry cohort investigating the effect of COVID-19 on 767 people with current or previous tuberculosis reported that 61·7% were hospitalised for COVID-19 and more than one in ten died (85 [11%] of 767).23 Data from Peru suggest that mortality risk in people with concurrent tuberculosis and COVID-19 is 7·3%, compared with 4·6% in people with tuberculosis alone.10 Thus, asking presumptive tuberculosis patients about their COVID-19 history in tuberculosis-endemic settings might be appropriate as a prognostic factor.18
As resources have been reallocated towards the pandemic response and away from existing health services, available tools must be deployed in a maximally efficient manner. Sample collection supply shortages have driven the investigation into nasopharyngeal swab alternatives for molecular testing. For example, using saliva for molecular testing had a similar accuracy to nasopharyngeal swabs24 and would be less resource-intensive in terms of personnel and cost.25 Swabbed saliva transported in sterilising buffer also had good accuracy,26 an approach that might have some merit for sputum.
In planning for future pandemics, and when considering the breadth of infectious diseases endemic to many settings with high burden of tuberculosis, integrated testing using multidisease platforms seems an obvious intervention. Performing multiple tests simultaneously requires substantial resources, so designing appropriate testing algorithms is crucial. GeneXpert is already available in many high tuberculosis burden countries,27 but other well established platforms (eg, Abbott m2000 RealTime System [Abbott, Abbott Park, IL, USA]), or more novel options (eg, Truelab [Molbio, Verna, Goa, India]), exist for which tuberculosis tests are also WHO-endorsed. It is particularly relevant for lower-resource settings to invest in platforms that can be quickly updated to incorporate assays for emerging pathogens, as budget constraints might preclude procurement of multiple stand-alone systems. Diagnostic companies must ensure these tools are accessible.
Our study is one of the first to investigate integrated molecular tuberculosis and COVID-19 testing, and the diagnostic accuracy of Xpert Xpress on sputum. Integrated testing feasibility findings are likely to be generalisable to other urban settings with high burdens of tuberculosis and COVID-19. Additionally, we have shown that using a single sputum sample to test for both M tuberculosis and SARS-CoV-2 in a single clinical encounter was feasible. We contribute evidence that sputum might be a usable sample type.
There were limitations in this study. We faced many logistical issues related to the COVID-19 pandemic. Most crucially, consecutive participant sampling was not feasible due to limited personnel and availability. At multiple points throughout the study, clinical or laboratory staff members had to isolate due to infection or contact with SARS-CoV-2, which limited or halted recruitment. Additionally, although we repeated tests when an initial assay did not produce a definite result, results for Xpert Xpress on sputum were unavailable for several participants. As participants needed to provide a sputum sample, our results are likely to be most generalisable to settings testing mostly symptomatic individuals (because asymptomatic people typically cannot produce sputum), and are not universally applicable.
The diagnostic yield of Xpert Xpress on sputum was moderate, but integrated testing for tuberculosis and COVID-19 using GeneXpert was feasible. However, systematic testing for both diseases might not be a worthwhile approach in all people presenting with presumptive tuberculosis or COVID-19, as concurrently positive cases were rare in our study population. More research is needed to identify the epidemiological situations wherein integrated testing would be most valuable and appropriate.
Data sharing
Deidentified participant data that underlie the results reported in this Article, with an accompanying data dictionary, will be made available to investigators whose proposed use of the data has been approved by an independent review committee.
Declaration of interests
MP serves as an advisor to the following non-profit agencies in global health: Bill & Melinda Gates Foundation, Foundation for Innovative New Diagnostics, WHO, and Stop TB Partnership. CU-G reports receiving funding from the Foundation of Innovative New Diagnostics, US National Institutes of Health, Abbott, and National Research Council Canada for grants in tuberculosis or COVID research, or both; receiving payment or honoraria for lectures, presentations, or educational events from Abbott and Molbio; and participating on an advisory board for point-of-care testing in infectious diseases.
Acknowledgments
Acknowledgments
This project was funded by the Canadian Institutes of Health Research and International Development Research Centre (project number 109554). We are grateful to the Global Drug Facility for providing Xpert Xpress SARS-CoV-2 cartridges. EL-HM was supported by a doctoral fellowship from the Fonds de Recherce de Quebec – Sante. MP holds a Canada Research Chair award from the Canadian Institutes of Health Research. We are also grateful to Roberto Bazzani for his close support during the project.
Contributors
EL-HM, GS, MK, MP, and CU-G conceptualised the study. EL-HM, LV-C, and PE-L curated the data. EL-HM did the formal analysis. EL-HM and MP acquired the funding. LV-C, PE-L, TC, CU-G did the investigation. EL-HM, GS, MK, MP, and CU-G did the methodology. MP and CU-G supervised the study. EL-HM wrote the original draft of the report. EL-HM, LV-C, PE-L, TC, GS, MK, MP, and CU-G reviewed and edited the draft. EL-HM and LV-C accessed and verified all the data reported in the study. All authors agreed with the decision to submit for publication.
Supplementary Materials
References
- 1.WHO . World Health Organization; Geneva: 2022. Global tuberculosis report 2022. [Google Scholar]
- 2.Global Preparedness Monitoring Board . World Health Organization; Geneva: 2020. A world in disorder: Global Preparedness Monitoring Board annual report 2020. [Google Scholar]
- 3.Klinton JS, Heitkamp P, Rashid A, et al. One year of COVID-19 and its impact on private provider engagement for TB: a rapid assessment of intermediary NGOs in seven high TB burden countries. J Clin Tuberc Other Mycobact Dis. 2021;25 doi: 10.1016/j.jctube.2021.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Migliori GB, Thong PM, Akkerman O, et al. Worldwide effects of coronavirus disease pandemic on tuberculosis services, January–April 2020. Emerg Infect Dis. 2020;26:2709–2712. doi: 10.3201/eid2611.203163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Agency for International Development. Stop TB Partnership . Stop TB Partnership; Geneva: 2021. Simultaneous, integrated diagnostic testing approach to detect COVID-19 and TB in high TB burden countries. [Google Scholar]
- 6.The Global Fund . The Global Fund; Geneva: 2021. Briefing note: testing for both tuberculosis and SARS-CoV-2. [Google Scholar]
- 7.WHO . World Health Organization; Geneva: 2020. Molecular assays intended as initial tests for the diagnosis of pulmonary and extrapulmonary TB and rifampicin resistance in adults and children: rapid communication. Policy update. [Google Scholar]
- 8.Cepheid Cepheid receives emergency use authorization from FDA for rapid SARS-CoV-2 test. March 21, 2020. http://cepheid.mediaroom.com/2020-03-21-Cepheid-Receives-Emergency-Use-Authorization-from-FDA-for-Rapid-SARS-CoV-2-Test
- 9.Stop TB Partnership . Stop TB Partnership; Geneva: 2021. Global drug facility: diagnostics, medical devices & other health products catalog, November 2021. [Google Scholar]
- 10.Ugarte-Gil C, Curisinche M, Figueroa CJ, Gotuzzo E, Rios R. COVID-19 among tuberculosis patients in Peru: an operational report from the national registry. 52nd Union World Conference on Lung Health; Oct 19–22, 2021 (abstr LB-1879-20).
- 11.Souza MDR, da Paz WS, Sales VBDS, et al. Impact of the COVID-19 pandemic on the diagnosis of tuberculosis in Brazil: is the WHO End TB Strategy at risk? Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.891711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ministerio de Salud, Perú . Ministerio de Salud; Lima: 2018. Norma téchnica de salud para la prevencion y control de la coninfeccion tuberculosis y virus de la immunodeficiencia humane en el Peru. [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinnes J, Deeks JJ, Berhane S, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;3 doi: 10.1002/14651858.CD013705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeffelholz MJ, Alland D, Butler-Wu SM, et al. Multicenter evaluation of the Cepheid Xpert Xpress SARS-CoV-2 test. J Clin Microbiol. 2020;58:e00926–e00930. doi: 10.1128/JCM.00926-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevenson M. Tools for the analysis of epidemiological data: package ‘epiR’. July 30, 2021. https://mran.microsoft.com/snapshot/2021-09-01/web/packages/epiR/epiR.pdf
- 17.Chen H. University of California Los Angeles; Los Angeles, CA: 2021. VennDiagram: generate high-resolution Venn and Euler plots. [Google Scholar]
- 18.Aggarwal AN, Agarwal R, Dhooria S, Prasad KT, Sehgal IS, Muthu V. Active pulmonary tuberculosis and coronavirus disease 2019: a systematic review and meta-analysis. PloS one. 2021;16 doi: 10.1371/journal.pone.0259006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLean EL, Villa-Castillo L, Ruhwald M, Ugarte-Gil C, Pai M. Integrated testing for TB and COVID-19. Med (N Y) 2022;3:162–166. doi: 10.1016/j.medj.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruhwald M, Hannay E, Sarin S, Kao K, Sen R, Chadha S. Considerations for simultaneous testing of COVID-19 and tuberculosis in high-burden countries. Lancet Glob Health. 2022;10:e465–e466. doi: 10.1016/S2214-109X(22)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xin H, Wong JY, Murphy C, et al. The incubation period distribution of coronavirus disease 2019: a systematic review and meta-analysis. Clin Infect Dis. 2021;73:2344–2352. doi: 10.1093/cid/ciab501. [DOI] [PubMed] [Google Scholar]
- 22.Del Águila-Mejía J, Wallmann R, Calvo-Montes J, Rodríguez-Lozano J, Valle-Madrazo T, Aginagalde-Llorente A. Secondary attack rate, transmission and incubation periods, and serial interval of SARS-CoV-2 omicron variant, Spain. Emerg Infect Dis. 2022;28:1224–1228. doi: 10.3201/eid2806.220158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.TB/COVID-19 Global Study Group Tuberculosis and COVID-19 co-infection: description of the global cohort. Eur Respir J. 2021;59 doi: 10.1183/13993003.02538-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181:353–360. doi: 10.1001/jamainternmed.2020.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell JR, Uppal A, Oxlade O, et al. Active testing of groups at increased risk of acquiring SARS-CoV-2 in Canada: costs and human resource needs. CMAJ. 2020;192:E1146–E1155. doi: 10.1503/cmaj.201128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banada P, Elson D, Daivaa N, et al. Sample collection and transport strategies to enhance yield, accessibility, and biosafety of COVID-19 RT-PCR testing. J Med Microbiol. 2021;70 doi: 10.1099/jmm.0.001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Médecins Sans Frontières. Stop TB Partnership . Medicins Sans Frontieres; Geneva: 2020. Step up for TB 2020: tuberculosis Policies in 37 countries. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified participant data that underlie the results reported in this Article, with an accompanying data dictionary, will be made available to investigators whose proposed use of the data has been approved by an independent review committee.