Abstract
Dermatomyositis (DM) is an idiopathic inflammatory myopathy that commonly manifests with proximal muscle weakness and is associated with extramuscular pathology including characteristic skin lesions such as Gottron’s papules and heliotrope rash, as well as lung, gastrointestinal, joint, and cardiac involvement. Systemic corticosteroids are a cornerstone of therapy, and more recently intravenous immunoglobulin (IVIG; OCTAGAM®) has been approved by the US Food and Drug Administration for the treatment of adults with DM. Both steroids and IVIG represent nonspecific anti-inflammatory therapy, and more targeted approaches are lacking. Transcriptomics has identified upregulation of interferon (IFN)–regulated genes as key features of both adult DM and juvenile DM (JDM). Accordingly, blocking IFN signaling through inhibition of the Janus kinase (JAK) pathway represents a potential treatment option for DM. Placebo-controlled trial data assessing the use of JAK inhibitors for the treatment of DM are limited; as such, a systematic literature review was undertaken to assess the evidence of JAK inhibitors in the treatment of patients with DM. Terms related to DM and JAK inhibitors were searched using PubMed, Embase, Web of Science, Scopus, and Dimensions to identify peer-reviewed publications reporting patients with DM who were treated with a JAK inhibitor. Baseline demographics, clinical characteristics, and treatment outcome data were extracted. A total of 48 publications reporting 145 unique patients (adult DM, n=84; JDM, n=61) were identified. Among cases of adult DM, 61 of 84 (73%) had refractory skin disease at baseline, and all (61 of 61) reported improvement in cutaneous symptoms. Of patients with adult DM, 16 of 84 (19%) had refractory muscle disease at baseline, and all (16 of 16) reported improvement in muscle symptoms. In patients with adult DM complicated by interstitial lung disease (ILD; n=33), 31 (94%) patients improved with JAK inhibitor treatment. Among cases of JDM with refractory skin disease at baseline (60 of 61), most patients (57 of 60; 95%) showed improvements in skin symptoms after JAK inhibitor treatment. Of patients with JDM with refractory muscle disease at baseline (36 of 61), most (30 of 36; 83%) reported improvement in muscle symptoms. Four patients with JDM and ILD experienced improvement in lung disease activity following treatment with a JAK inhibitor. Among both DM and JDM cases, all patients (17 with DM and 16 with JDM) who had elevated serum IFN and/or IFN-stimulated gene expression at baseline showed reduction in IFN or IFN gene expression. Although the conclusions that can be drawn from this analysis are limited because of the differences in assessments used across publications, overall treatment of patients with DM or JDM with a JAK inhibitor was associated with significant improvement of a wide range of DM manifestations, including skin lesions, muscle weakness, and ILD. Our systematic literature review suggests that JAK inhibitors may be a viable treatment option for DM/JDM, and randomized controlled trials are necessary to confirm these findings.
Keywords: Baricitinib, Brepocitinib, Dermatomyositis, Idiopathic Inflammatory Myopathies, JAK, Janus Kinase Inhibitor, Juvenile Dermatomyositis, Myositis, Ruxolitinib, Tofacitinib
Introduction
Dermatomyositis (DM) is a rare idiopathic autoimmune disease associated with muscle and skin inflammation that can lead to significant morbidity and mortality (1). Patients with DM are frequently treated off-label with immunosuppressive agents, and only in the past year has the US Food and Drug Administration approved intravenous immunoglobulin (IVIG; OCTAGAM®) to treat DM in adults. There is an urgent, unmet need to develop additional disease-modifying treatments for DM.
In both adult DM and juvenile DM (JDM), transcriptomic analyses demonstrate an upregulation of interferon (IFN)–regulated genes (2-4). In particular, in myocytes of patients with DM, robust expression of both type I IFN– and type II IFN–inducible genes correlates with expression of genes associated with inflammation and regeneration (5). Given the substantial evidence demonstrating the importance of IFN-regulated genes in DM and the obligate role of Janus kinases (JAKs) in IFN signal transduction (6), JAK inhibitors have been used therapeutically. The various approved and investigational JAK inhibitors have distinct pharmacologic activity at the four human JAK isoforms (JAK1–3, tyrosine kinase 2 [TYK2]), and several are known to potently inhibit JAK1 and/or TYK2 and accordingly inhibit types I and II IFN signaling (7).
The first report of DM responsive to a JAK inhibitor, ruxolitinib, was in 2014, of a 72-year-old woman with recalcitrant DM and myelofibrosis (8). Although there was controversy about whether the treatment of her underlying myelofibrosis contributed to the remission of her DM (9,10), a subsequent case report and a case series demonstrated the efficacy of a JAK inhibitor in treating refractory skin disease (11,12). More recently, a proof-of-concept study of tofacitinib in refractory DM also showed safety and efficacy as measured by the validated American College of Rheumatology and European League Against Rheumatism (ACR/EULAR) Myositis Response Criteria (13). This study required washout of other immunosuppressive agents, thereby highlighting the therapeutic potential of JAK inhibitors as monotherapy in refractory skin-predominant disease. Beyond the treatment of skin-predominant disease, JAK inhibitors have also been reported to be efficacious in myositis-associated interstitial lung disease (ILD), in particular melanoma differentiation-associated 5 (MDA5)–associated ILD (14).
Given the promising therapeutic potential of JAK inhibitors in DM, the purpose of this systematic literature review is to examine the evidence available for JAK inhibitor use in this disease. Although adult-onset DM and JDM have clinical similarities, there are also notable distinctions, including markedly diminished malignancy risk and increased calcinosis in JDM compared with adult-onset DM (15); thus, we report findings for the two diseases separately.
Methods
Search Strategy
The systematic literature review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A comprehensive electronic search strategy of databases—including PubMed, Embase, Web of Science, Scopus, and Dimensions—was performed August 18–20, 2021, with the terms ((“dermatomyositis” OR “myositis” OR “inflammatory myopathy” OR “inflammatory myopathies”) AND (“JAK” OR “janus kinase” OR “tofacitinib” OR “baricitinib” OR “ruxolitinib” OR “upadacitinib” OR “filgotinib”)) queried for the title, abstract, or keywords. The same terms and delimiters were also queried in published abstracts between 2012 and 2021 from the following congress proceedings: ACR, EULAR, Paediatric Rheumatology European Society, Asia-Pacific League of Associations for Rheumatology, and Pan-American League of Rheumatology Associations. For each identified publication, citations both within and of that paper were reviewed.
Articles and conference abstracts were eligible for inclusion if they were primary publications of patients with DM or JDM who were treated with JAK inhibitors. All study designs (ie, case reports, case series, retrospective studies, observational studies, randomized controlled trials) were eligible for inclusion. Publications were excluded if they did not document patient clinical characteristics, prior and/or concomitant therapies, or outcomes following treatment with JAK inhibitors. Review articles and nonprimary case reports were also excluded. Publications were included even if individual patients were subsequently included in another primary publication (eg, case reports that were also included in a retrospective study), to compile all relevant data for each patient. Patients documented in multiple publications were only counted once as unique patients. We identified unique reports (ie, individual peer-reviewed article or congress proceeding), unique analyses (ie, all reports that possibly or likely presented the same patient in multiple publications such as patients included in a study that were also included in a case report), and unique patients (ie, individual patients, counted from only one report), and for clarity we present results as unique patients.
Data Extraction and Assessments
One researcher (GL) reviewed search results and extracted data from each identified publication, and another researcher (AG) reviewed search results and extracted data from a random 10% of all identified publications to ensure consistency, as done in a similarly performed analysis (16). Discrepancies were resolved by consensus. From included publications, the following information was extracted: study type and follow-up time, JAK inhibitor used, number of patients, patient baseline demographics and clinical characteristics, symptoms, treatment history, concomitant medications, and treatment outcomes, IFN signature, muscle enzyme (creatine kinase [CK], aldolase) levels, and myositis-specific and myositis-associated autoantibodies (MSAs, MAAs). Efficacy outcomes included Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) activity score (scale 0–100; higher score indicating more severe disease), total improvement score (TIS), and individual core set measures (CSMs) of the TIS such as manual muscle testing (MMT-8; scale 0–150, respectively; higher scores indicating greater strength). Data were also extracted on serum IFN or IFN-regulated gene expression. For publications reporting JDM cases, data were also extracted for the Childhood Myositis Activity Score (CMAS; scale 0–52; higher scores indicating greater muscle strength) and Global Disease Activity Score (DAS; total 0–20 consisting of skin DAS [0–9] and muscle DAS [0–11]). In patients with ILD, data were extracted for diffusing capacity for carbon monoxide (DLCO%) and forced vital capacity (FVC%). The above parameters were extracted from text, tables, figures, and/or supplementary materials depending on the information reported in each publication. If relevant data with discrete numeric values (eg, CDASI scores, individual CSMs, TIS) were only provided in chart form, WebPlotDigitizer was used to extract numeric values from the images (17). Extraction of safety-related data was beyond the scope of this review.
Risk of Bias Assessment
Risk of bias was assessed using the National Institutes of Health (NIH) quality assessment tool for case series studies (18). One researcher (GL) rated each study as low, high, or unclear risk of bias, and a second researcher (AG) assessed 10% of the identified publications to ensure consistency. Discrepancies were resolved by consensus.
Results
Publications Search Overview
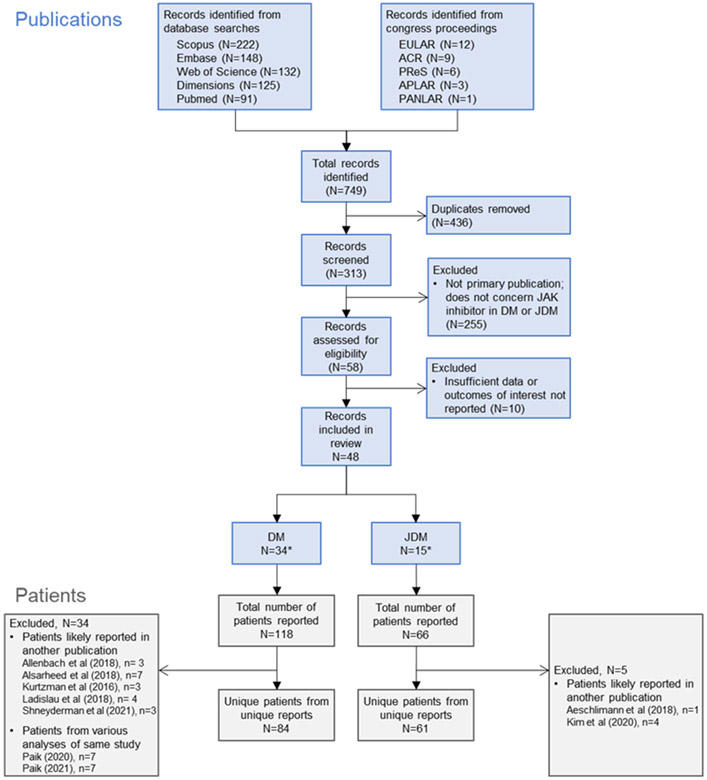
The literature search yielded a total of 749 records (Figure 1), 313 of which were unique records screened for eligibility. Of these, 265 did not meet inclusion criteria. This resulted in 48 records published between December 2014 and August 2021. From these publications, individual patients were reported in unique reports (n = 39; clinical trials, retrospective studies, case series, or case reports), included in multiple publications of a study (eg, STIR primary study and long-term analysis), or reported in a case series and likely included in a larger study (eg, a retrospective study that includes patients previously described in a case study). Data were extracted from reports of 145 individual patients with adult DM or JDM. Of these, 34 publications reported DM in 84 unique adult patients, and 15 reported JDM in 61 unique pediatric patients (one report included patients with adult DM and JDM (19)). In 16 publications (12 adult DM, 4 JDM), 33 patients with DM and 10 patients with JDM were primarily treated for DM-ILD. The characteristics of included studies are presented in Supplemental Table 1 for publications on adult DM and Supplemental Table 2 for publications on JDM.
Figure 1.
Systematic literature review search strategy and article attrition. ACR, American College of Rheumatology; APLAR, Asia-Pacific League of Associations for Rheumatology; DM, dermatomyositis; EULAR, European League Against Rheumatism; JAK, Janus kinase; JDM, juvenile dermatomyositis; PANLAR, Pan-American League of Rheumatology Associations; PReS, Paediatric Rheumatology European Society. *1 report included patients with DM and JDM (19).
Of patients with adult DM, 64 patients (76%) were female. Of patients with JDM, 34 (56%) were female; sex was not reported for one patient. Of patients with DM, 67 were treated with tofacitinib, eight with baricitinib, and nine with ruxolitinib; of patients with JDM, 19 were treated with tofacitinib, eight with baricitinib, 27 with ruxolitinib, and seven with baricitinib or ruxolitinib (specific JAK inhibitor for each patient not reported in one study). Most patients (92% DM, 100% JDM) received concomitant therapies while initiating JAK inhibitor treatment. These were typically the standard-of-care agents, including corticosteroids, immunosuppressants (eg, methotrexate, azathioprine), and IVIG. All patients with DM had documented prior therapy, and 66/84 were initiated on JAK inhibitor treatment owing to refractory cutaneous or muscle disease, ILD, and/or other symptoms. Prior therapy for most patients (83/84) included corticosteroids (Supplemental Table 3). Of patients with refractory DM who were receiving corticosteroids when JAK inhibitor therapy was initiated, 90% (43/48) were able to taper or discontinue corticosteroid therapy. Among patients with JDM, 60/61 presented with refractory disease (as assessed by the investigator), and specific prior therapy was reported for 27 of 61 patients. The most common prior therapy was corticosteroids (27/27; Supplemental Table 3). Of patients with JDM, concomitant steroid therapy was reported for 47 patients. Of these patients, 23 tapered or discontinued corticosteroid therapy; the remaining studies reporting concomitant corticosteroid use did not report changes to corticosteroid therapy during JAK inhibitor therapy.
Overall, treatment with a JAK inhibitor significantly improved or resolved symptoms of disease for patients with DM and JDM with cutaneous or muscle disease or with ILD (Supplemental Table 4).
JAK Inhibition in Adult Dermatomyositis
Cutaneous Disease
A total of 28 publications included 61 patients with DM who had refractory cutaneous disease (Table 1). All patients (61/61) improved with JAK inhibitor treatment. In the 24 unique patients for whom individual pre- and post-treatment CDASI scores (scale 0–100) were reported, all 24 patients showed improvements (lowering of scores). Among these patients, baseline CDASI scores ranged from 12–57 (8,11,19-24). In studies that reported score changes after 4–12 weeks of JAK inhibitor use, improvements ranged from 2–41 points from baseline, with posttreatment CDASI scores ranging from 0–15 (8,19-23). Patients continued to experience improvement in CDASI scores in studies with long-term follow-up of JAK inhibitor treatment (20–96 weeks) (21-23).
Table 1.
Publications of Adult DM That Report Efficacy of JAK Inhibitors on Skin Disease
| Publication | N | Time to Outcome |
Baseline CDASI Assessment |
Posttreatment or Change in CDASI Assessment |
Other Clinical Improvements |
|---|---|---|---|---|---|
| Hornung et al (2014) (8) | 1 | 2 months | 12 | 0 | – |
| Paik and Christopher-Stine (2017) (12) | 1 | 2–6 months | NR | NR | Improvement in cutaneous symptoms within 2 months; prednisone tapered without worsening of skin lesions |
| Hornig et al (2018) (33) | 1 | 2 months | NR | NR | Improved skin condition at 2 months |
| Kurasawa et al (2018) (14) | 5 | 12 months | NR | NR | Skin rash improved within 30 days for all 3 surviving patients |
| Landon-Cardinal et al (2019) (27) | 12 | 3, 11.6 ± 6.8 months | Mean, 31 | Mean, 16 (3 months); 8 (11.6 months) | At 3 months, 11/12 patients had >5-point improvement in CDASI score |
| Allenbach et al (2018) (63)* | 3 | 3 months | NR | NR | Improvement in skin lesions |
| Ladislau et al (2018) (20)* | 4 | 3 months | Mean, 34.3 | Mean, 12.8 | All patients had improved facial rash |
| Moghadam-Kia et al (2019) (37) | 4 | 3–6 months | NR | NR | All patients had improvement in skin lesions or rash |
| Wendel et al (2019) (23) | 2 | 12–28 weeks | 25† | 10 at 12 weeks, 3 at 28 weeks† | Rapid improvement in skin lesions |
| Delvino et al (2020) (30) | 1 | 3–12 months | NR | NR | Rapid and significant improvement in cutaneous lesions by 3 months |
| Fetter et al (2020) (31) | 1 | 4 months | NR | NR | Noticeable improvement in skin condition after 4 months; hair regrowth on scalp and eyebrows |
| Fischer et al (2020) (32) | 1 | 8 months | NR | NR | Improvement of skin lesions |
| Ishikawa et al (2020) (34) | 1 | 6–12 months | NR | NR | Gradual improvement of skin lesions |
| Jalles et al (2020) (35) | 1 | 4 months | NR | NR | Clinical remission of skin disease after 4 months |
| Navarro-Navarro et al (2020) (21) | 2 | 4–20 weeks | Mean, 19.5 | Mean, 4 | Both patients had cutaneous response within 4 weeks |
| Riggle et al (2020) (22) | 4 | 6 months | Mean, 16.5 | Mean, 5.3 | All patients had significant improvement of cutaneous disease activity and pruritus |
| Shinjo and de Souza (2020) (39) | 1 | 2 months | NR | NR | Skin lesions significantly improved within 3–4 weeks |
| Williams and McKinney (2020) (40) | 1 | 6 months | NR | NR | Substantial improvement in rash |
| Crespo Cruz et al (2021) (29) | 1 | Up to 7 months | NR | NR | All skin lesions significantly improved and complete resolution of pruritus |
| Jasmine et al (2021) (36) | 1 | 2 months | NR | NR | No recurrence of rash after 2 months |
| Min et al (2021) (19) | 9 | 1–2 months | Mean, 27.2 | Mean, 9.2 | All patients had clinically significant improvement in pruritus if present; all patients had CDASI improvement of ≥11 points |
| Alsarheed et al (2018) (28)‡ | 7 | 4–20 months | – | Mean change, 13 | 6/7 patients transitioned from moderate or severe cutaneous disease to mild disease as determined by CDASI scores; all patients had improvement in pruritis |
| Kurtzman et al (2016) (11)‡ | 3 | 4 weeks | Mean 28.3 | Mean, 16.3 | All patients had improvement in pruritus |
| Ohmura et al (2021) (38) | 1 | 7.5 months | NR | NR | Significant improvement in skin lesions after 80 days; symptom-free after 7.5 months |
| Paik et al (2021a) (13) | 10 | 12 weeks | Mean, 28 | Mean, 9.5 | Skin improvement evident as early as 4 weeks |
| Shneyderman et al (2021) (24)§ | 3 | 12 weeks | Mean 31.3 | Mean, 8 | – |
| Paik et al (2020) (25)§ | 7 | 42, 68 weeks | Mean, 25.4 | Mean, 3.9 (at week 42); 5.4 (at week 68) | – |
| Paik et al (2021b) (26)§ | 7 | 96 weeks | Mean, 25.4 | Mean, 4.7 | – |
CDASI, Cutaneous Dermatomyositis Disease Area and Severity Index; DM, dermatomyositis; NR, not reported.
Some or all patients likely to have been reported in Landon-Cardinal et al (2019) (27).
Reported for one patient.
Some or all patients likely to have been reported in Min et al (2021) (19).
Patients reported in Paik et al (2021a) (13).
Three analyses in 5 publications reported mean scores, accounting for 29 patients (some with individual scores reported in separate analyses as described above). In the open-label STIR trial of tofacitinib in 10 adult patients with refractory DM, mean CDASI score at baseline was 28 and at week 12 was 9.5 (13,25). At week 96 of the STIR long-term extension trial, the mean score reported for seven patients was 4.71 (26). In a case series of 12 patients treated with either baricitinib or ruxolitinib, the mean baseline CDASI score was 31 and by week 12 was 16; 11 of 12 patients showed clinically significant improvement with JAK inhibitor treatment, defined as a >5-point improvement in CDASI score (27). Mean CDASI was further reduced to a score of 8 after long-term (~50 weeks) follow-up of these 12 patients. Another case series reported 7 patients treated with tofacitinib, with mean improvement in CDASI score of 13 points (28).
Among the 14 publications that indicated an outcome related to refractory cutaneous symptoms but did not report pre- and posttreatment CDASI scores, 21 of 21 patients improved after treatment with a JAK inhibitor (12,29-40). In one study of five patients with cutaneous disease in addition to rapidly progressive ILD, skin symptoms of heliotrope rash, Gottron’s papules, and erythema improved with JAK inhibitor treatment, although two patients later died (see ILD section) (14).
Muscle Disease
A total of 14 publications included 16 patients presenting with refractory muscle disease (Table 2). Patients treated with a JAK inhibitor displayed significant improvements in muscle strength. Of the 16 adult patients, 15 (93.8%) had patient- or clinician-reported improvement, decreased edema on magnetic resonance imaging, and/or improvement in muscle strength measurements (ie, MMT-8, Medical Research Council Muscle Scale [MRC]); one study did not report outcomes specific to muscle disease. The STIR open-label trial reported one patient with adult DM involving active, refractory muscle disease (13). This patient had a baseline MMT-8 score (scale 0–150) of 127 that improved to 136 at week 12 of treatment with a JAK inhibitor. In another study, one patient demonstrated improved arm abductor strength measured by handheld dynamometry; scores improved from 4-/5 to 5-/5 (12). MRC scoring (scale 0–5) was used in two patients with muscle disease; scores improved from a baseline of 3/5 to 4/5 in both patients (20).
Table 2.
Publications of Adult DM That Report Efficacy of JAK Inhibitors in Muscle Disease Activity
| Publication | N* | Time to Outcome |
Baseline Muscle Strength Assessment |
Posttreatment Muscle Strength Assessment |
Other Clinical Improvements |
|---|---|---|---|---|---|
| Hornung et al (2014) (8) | 1 | 2 months | NR | NR | Patient regained muscle strength |
| Paik and Christopher-Stine (2017) (12) | 1 | 2–6 months | Handheld dynamometry, 4/5 | Handheld dynamometry, 5/5 | Muscle strength improved within 2 months; prednisone tapered at 2 months without worsening of muscle weakness |
| Allenbach et al (2018) (63)† | 1 | 3 months | Muscle strength improved | ||
| Ladislau et al (2018) (20)† | 2 | 3 months | Mean MRC, 3 | Mean MRC, 5 | Muscle strength improved |
| Moghadam-Kia et al (2019) (37) | 1 | 6 months | NR | NR | Improved MMT at 6 months in patient with active muscle disease |
| Wendel et al (2019) (23) | 1 | 12, 28 weeks | NR | NR | Muscle strength improved after 12 weeks |
| Delvino et al (2020) (30) | 1 | 3–12 months | NR | NR | Subjective improvement in strength within 3 months, which was sustained at 12 months |
| Fetter et al (2020) (31) | 1 | 4 months | NR | NR | Noticeable improvement in muscle strength |
| Jalles et al (2020) (35) | 1 | 4 months | NR | NR | Patient regained muscle strength; clinical remission |
| Navarro-Navarro et al (2020) (21) | 1 | 20 weeks | NR | NR | Subjective improvement in strength and fatigue; muscle enzymes returned to normal range |
| Williams and McKinney (2020) (40) | 1 | 6 months | NR | NR | Patient experienced regained muscle strength |
| Min et al (2021) (19) | 2 active, 2 mild | 1–2 months | NR | NR | Resolution of muscle disease activity in patients with refractory muscle weakness; subjective improvement in strength and fatigue in patients with mild muscle weakness |
| Kurtzman et al (2016) (11)‡ | 2 mild | 4 weeks | NR | NR | Subjective improvement in strength and fatigue |
| Paik et al (2021a) (13) | 1 | 12 weeks | MMT-8, 127 | MMT-8, 136 | Evidence of edema on baseline muscle MRI, which improved by week 12 |
DM, dermatomyositis; MMT, manual muscle testing; MRC, Medical Research Council MMT Scale; MRI, magnetic resonance imaging; NR, not reported.
Number of patients with active muscle disease; publication may have included additional patients without active muscle disease.
Some or all patients likely to have been reported in another publication.
Some or all patients likely to have been reported in Min et al (2021) (19).
Interstitial Lung Disease
In 12 publications, 33 unique adult patients had DM-ILD, 32 of whom were seropositive for anti-MDA5 antibodies, including many with poor prognostic factors (eg, hyperferritinemia). Most patients (32/33) were treated with tofacitinib, and one was treated with ruxolitinib. Overall, 31 patients (94%) improved with JAK inhibitor treatment. In an open-label trial of tofacitinib in 18 patients with DM-ILD who were anti-MDA5-Ab positive, a 100% 6-month survival rate was reported vs 78% of historical controls (41). In a patient who was negative for anti-MDA5 antibodies but positive for anti-Jo1 and antinuclear antibodies, treatment with tofacitinib was also effective (42). In a case series of patients with DM-ILD and poor prognostic factors (pertaining to serum ferritin levels and lung opacity unresponsive to triple therapy) who received triple therapy (glucocorticoid pulse therapy followed by prednisolone, cyclophosphamide, and cyclosporine A) and tofacitinib (n=5), three patients recovered and two patients died within 2 months of combination therapy (due to respiratory failure [one patient] and liver failure subsequent to bacterial infection, respiratory failure, and shock [one patient]). In comparison, six patients receiving only triple therapy (historical controls) died within 2 months (14). Baseline FVC% or DLCO% measurements were reported for 23 patients with DM-ILD, and improvements, although not explicitly quantified in all cases, were noted in all 23 patients treated with JAK inhibitors (23,33,38,41-43).
Calcinosis and Arthralgia
There were six cases of adults with calcinosis reported in four publications, all of which improved after treatment with a JAK inhibitor (13,22,23). In a case series of three patients with calcinosis from the STIR open-label study, improvement in calcinosis was noted on imaging after 3 months of treatment (24). Two of these patients were positive for antinuclear matrix protein 2 (NXP2) antibodies, and the third was positive for anti-transcription intermediary factor 1 γ (TIF1-γ) antibodies.
Arthralgia improved with JAK inhibitor treatment in all cases for which outcomes (either subjective or objective) for arthralgia were reported (12,23,30,32,34,36,37,40).
IFN Gene Signature
In the five studies reporting data for 17 unique adult patients in which serum IFN levels and/or IFN-stimulated gene expression were measured, all 17 patients showed reduction in IFN or IFN gene signature with JAK inhibitor treatment (13,20,32,33,35).
Laboratory Parameters
Although not all patients were surveyed for autoantibodies, antibody testing was reported in 69 patients with DM. Of these patients, 63 (91%) were seropositive for at least one MSA or MAA. Of MSAs, 20 patients were positive for anti–TIF1-γ, 33 for anti-MDA5, four for anti-NXP2, three for anti-Mi2, four for anti-small ubiquitin like modifier activating enzyme heterodimer (SAE), and one for anti-Jo1 antibodies. Of MAAs, 12 patients were positive for anti-Ro (−52 or −60) antibodies. Six patients were MSA/MAA negative. Other antibodies that were reported included antinuclear antibody (ANA; for which six patients were positive), rheumatoid factor (two patients), and anti–cyclic citrullinated peptide (two patients).
In four studies, CK levels were reported for four unique patients. Baseline CK (range, 354–4112 U/L) improved with JAK inhibitor therapy (range, 32–308 U/L) (20,21,37). One case (baseline CK, 535 U/L) reported levels as normal after therapy (44).
Total Improvement Score
In the STIR trial, all 10 patients achieved the ACR/EULAR criteria for at least minimal improvement (TIS ≥20) after 12 weeks of tofacitinib treatment, and five of the 10 patients achieved at least moderate improvement (TIS ≥40) (13,25,26). In the long-term extension study of up to 96 weeks, six of seven patients demonstrated at least minimal improvement on the TIS (26).
Juvenile Dermatomyositis
Cutaneous Disease
Of the 61 unique patients with JDM, 60 had active cutaneous disease (Table 3). Of these patients, 57 had significant improvement in skin symptoms after JAK inhibitor treatment. One patient (1.6%) had initial improvement but experienced relapse of skin rash after 8 weeks of treatment with a JAK inhibitor.
Table 3.
Publications of JDM That Report Efficacy of JAK Inhibitors on Skin Disease
| Publication | N | Time to Outcome |
Baseline CDASI or Skin DAS Assessment |
Posttreatment or Change in CDASI or Skin DAS Assessment |
Other Clinical Improvements |
|---|---|---|---|---|---|
| Papadopoulou et al (2019) (48) | 1 | 6 months | Modified skin DAS, 5 | Modified skin DAS, 1 | – |
| Sabbagh et al (2019) (46) | 2 | 6, 12 months | Mean CDASI, 21 | Mean CDASI, 9.5 | – |
| El-Lateef (2020) (56) | 1 | 6 months | NR | NR | Remission of skin lesions |
| Heinen et al (2020) (51) | 1 | 7 months | NR | NR | Reduction in sternal rash |
| Kim et al (2020a) (45) | 4 | 24, 72 weeks | CDASI mean, 42.5 | CDASI mean, 26.3 (24 weeks); 17.8 (72 weeks) | Significant improvement in CDASI by week 4 |
| Yu et al (2020) (50) | 3 | 3, 6 months | Skin DAS mean, 4.7 | Skin DAS mean, 1.7 (3 months); 0 (6 months) | Significant improvement in skin disease activity by 3 months; near complete resolution of Gottron’s papules and heliotrope rash in one patient |
| Ding et al (2021) (55) | 25* | 3–18 months | NR | NR | All patients showed improvement in rashes within 1–2 weeks; no clinically observable rash present after 12 weeks; one patient experienced relapse of rash |
| Kostic et al (2021) (64) | 2 | 5, 7 months | NR | NR | Partial or complete response within 5 or 7 months, respectively, by physician assessment |
| Le Voyer et al (2021) (49) | 10 | 3–6 months | Skin DAS mean, 6.0 | Skin DAS mean, 1 (6 months) † | – |
| Aeschliman et al (2018) (47)‡ | 1 | 2 months | Skin DAS, 4 | Skin DAS, 0 | |
| Zhou et al (2021) (53) | 1 | 6, 17 months | NR | NR | Skin lesions fully resolved within 6 months (physician assessment) |
CDASI, Cutaneous Dermatomyositis Disease Area and Severity Index; DAS, disease activity score; JDM, juvenile dermatomyositis; NR, not reported.
1 patient did not have active cutaneous disease.
Reported in patients with outcome data at 6 months.
Patient likely to have been reported in Le Voyer et al (2021) (49).
CDASI scores (pre- and post-JAK inhibitor treatment) were reported for eight patients with JDM (19,45,46). At baseline, CDASI scores ranged from 20–53. After treatment with a JAK inhibitor, improvements ranged from 7–27 points from baseline after 4–12 weeks, 9–34 points from baseline after 52 weeks, and 14–36 points from baseline after 72 weeks. Skin DAS (scale 0–9) or modified skin DAS (scale 0–5) scores were reported for 14 patients (47-50). Baseline skin DAS scores ranged from 2–8, and posttreatment initiation scores ranged from 0–8. Eight patients had complete resolution (0 on skin DAS; 57%), and two had scores that did not improve (14%) (47,49,50). One patient with a baseline modified skin DAS score of 5 improved to a score of 1 (48).
Muscle Disease
A total of 36 patients with JDM from 10 analyses (11 publications) had active, refractory muscle disease (Table 4). Overall, improvement with JAK inhibitor treatment was reported in 30 patients (83%). Pre- and posttreatment muscle activity scores (MMT-8, Muscle DAS, or CMAS) were available for 25 patients with muscle disease (45-48,50-54). Of these 25 patients, seven (28%) did not show objective improvements in muscle disease with JAK inhibitor therapy. Three of the seven patients were reported in a retrospective study of patients treated with ruxolitinib or tofacitinib (55) in which 7/10 patients had muscle improvement as measured by the CMAS (mean CMAS scores: baseline, 24.9; posttreatment, 38.2). Of the three patients from this study with CMAS scores that were unchanged from baseline after treatment, two reported qualitative improvements in fatigue and activity tolerance; the third patient was not evaluated using CMAS before treatment owing to joint involvement (55). In a retrospective study in which nine patients had JDM with muscle involvement and were treated with ruxolitinib or baricitinib, four achieved complete responses (by MMT-8 and CMAS) (49). However, three patients experienced muscle relapse after partially responding, one patient with partial response discontinued owing to insufficient efficacy, and one patient was considered a nonresponder (49).
Table 4.
Muscle Disease Activity Results Among Publications of JDM That Report Muscle Symptom Outcomes
| Publication | N* | Time to Outcome |
Baseline Muscle Strength Assessment |
Posttreatment Muscle Strength Assessment |
Other Clinical Improvements |
|---|---|---|---|---|---|
| Papadopoulou et al (2019) (48) | 1 | 6 months | MMT, 59 CMAS, 46 |
MMT, 70 CMAS, 50 |
Clinical improvement in muscle symptoms |
| Sabbagh et al (2019) (46) | 2 | 6, 12 months | Mean MMT-8, 136 CMAS, 21† |
MMT-8 (6 months), 148† MMT-8 (12 months), 149† CMAS, 49 (12 months)† |
– |
| Heinen et al (2020) (51) | 1 | 7 months | CMAS, 18 | CMAS, 40 | Increased muscle strength within 3 months; absence of inflammation in quadriceps on MRI |
| Kim et al (2020a) (45) | 2 | 24, 72 weeks | Mean MMT-8, 112 | Mean MMT-8, 138.5 (24 weeks); 146 (72 weeks) | Muscle improvement seen as early as week 4 with clinical improvement (per MMT-8) by week 8 and confirmed by MRI |
| Sozeri et al (2020) (52) | 2 | 3 months | Mean CMAS, 30 | Mean CMAS, 51 | – |
| Yu et al (2020) (50) | 3 | 3, 6 months | Mean MMT, 48.3 Mean CMAS, 30 Mean Muscle DAS, 7.3 |
Mean MMT, 69 (3 months); 79.3 (6 months) Mean CMAS, 45 (3 months); 50.3 (6 months) Mean Muscle DAS, 3 (3 months); 0.3 (6 months) |
Significant improvement in muscle weakness by 3 months |
| Ding et al (2021) (55) | 10 | 3–18 months | Mean CMAS, 24.9§ | Mean CMAS, 38.2§ | 2 of 9 patients with CMAS assessment had no improvement but reported subjective improvement in fatigue and activity tolerance |
| Le Voyer et al (2021) (49) | 9 active, 1 mild | 3–6 months | Mean MMT, 53.5 Mean CMAS, 26.6 |
Mean MMT, 69 Mean CMAS, 40.4 |
4 of 9 patients had complete response; 5 of 9 had muscle relapse, were non-responders, or had insufficient efficacy |
| Aeschliman et al (2018) (47)‡ | 1 | 2 months | Mean MMT, 57 Mean CMAS, 47 |
Mean MMT, 79 Mean CMAS, 52 |
Resolution of muscle disease within 2 months |
| Wang et al (2021) (54) | 5 | 12–31 months | Mean CMAS, 1.6 | Mean CMAS, 33 | – |
| Zhou et al (2021) (53) | 1 | 6, 17 months | CMAS, 36 | CMAS, 40 (6 months); 48 (17 months) | – |
CMAS, Childhood Myositis Activity Score; DAS, disease activity score; JAK, Janus kinase; JDM, juvenile dermatomyositis; MMT, manual muscle testing; MRC, Medical Research Council MMT Scale; MRI, magnetic resonance imaging; NR, not reported.
Number of patients with active muscle disease; publication may have included additional patients without active muscle disease.
Score reported for one patient.
Patient likely to have been reported in Le Voyer et al (2021) (49).
1 patient with baseline muscle weakness did not undergo CMAS assessment before initiating JAK inhibitor.
Of the patients with muscle disease who had objective muscle activity responses to JAK inhibitor treatment, baseline MMT-8 ranged from 108–142 (45,46), and baseline CMAS ranged from 0–46 (47-52,54). MMT-8 scores improved by 25–26 points from baseline after 12 weeks of treatment with a JAK inhibitor (45); in patients with longer-term use, scores improved by 7–39 points (45,46). With long-term JAK inhibitor use (12–31 months), CMAS scores improved by 18–41 points from baseline (54).
Interstitial Lung Disease
In four publications, 10 patients had JDM-ILD, and four were anti-MDA5 positive (46,50,54,55). Of the 10 patients with ILD, all experienced improvement in lung disease activity with JAK inhibitor treatment (46,50). Four patients had individual DLCO% reported (all baseline DLCO% ≤60%); one patient improved from 55% to 96%, whereas three patients experienced smaller improvements (posttreatment DLCO%, 60%–75%). No outcomes specific to ILD were reported in the remaining two studies (six patients) (54,55).
Calcinosis
Calcinosis was reported in eight patients with JDM, seven of whom experienced improvement in calcinosis following JAK inhibitor treatment (46,48,49,52,53,56).
IFN Gene Signature
In six analyses, 18 patients had elevated serum IFN or IFN-stimulated gene expression; in all patients for whom posttreatment measurements were taken (n=16), IFN levels or gene signatures were decreased following JAK inhibitor treatment (45-49,51).
Laboratory Parameters
Antibody testing was reported for 56 patients, 11 of whom were MSA-/MAA-negative. Of MSAs, six patients were positive for anti–TIF1-γ antibodies, six for anti-MDA5, 24 for anti-NXP2, one for anti-Mi2, one for anti-Jo1, and one for anti-PL7. Of MAAs, one patient was positive for anti-Ku antibodies, one for anti–U1-RNP, and 11 for anti-Ro (−60 and/or −52). A total of 14 patients also tested positive for ANA.
CK and aldolase levels were reported for four and two patients, respectively. Two patients with baseline CK levels of 403 and 1797 U/L decreased to 61 and 70 to 109 U/L, respectively, after JAK inhibitor treatment (48,50); two patients with baseline CK levels of 640 and 1440 U/L were reported as normal after treatment (52). Aldolase levels of two patients decreased from 9.6 to 7 U/L and 10.1 to 4.9 U/L (46).
Risk of Bias
Risk of bias, assessed using an NIH quality assessment tool for case series studies, was found to be low for all eight open-label trials included. Of the 40 remaining case series, case reports, and observational or retrospective studies identified, risk of bias was assessed to be low for 31 reports; 9 reports had unclear risk of bias.
Discussion
JAK inhibitors add to the growing armamentarium of potential therapeutic options for adult DM and JDM and have emerged as a potential treatment for refractory DM following the demonstrated disease-modifying effects of JAK inhibition in rheumatic diseases (57,58). The patients reported in the publications in this systematic literature review displayed persistent refractory symptoms and did not see improvement with several first- and second-line treatments (eg, methotrexate, mycophenolate, azathioprine), including corticosteroids and other immunomodulatory agents such as IVIG. Outcome assessments varied; however, our review demonstrates that treatment with a JAK inhibitor was associated with a wide range of significantly improved or resolved DM manifestations, including skin lesions, muscle weakness, ILD, and calcinosis. Clinical efficacy in patients with DM was seen with similar daily JAK inhibitor doses used in other autoimmune diseases (Supplemental Tables 1 and 2; (59-61).
Our findings highlight that clinical improvement was most striking in adult patients with pronounced skin symptoms. When included as an outcome measure, CDASI scores improved by a mean of 19 points overall across studies of patients with DM and JDM (8,13,19-23,26,27,45,46). Improvements after treatment with a JAK inhibitor were reported for most cases of muscle disease in adults with DM, and most were described as an increase in muscle strength. Patients with JDM had a mean improvement in MMT-8 scores of 24.9 points, highlighting improvement in muscle strength in children. Overall, the composite assessment of improvement in JDM as measured by the CMAS also demonstrated a clinically significant improvement of 16 points (a 1.5- to 3-point change in CMAS can be considered clinically meaningful for patients with JDM (62)).
Patients with DM have dysregulation of the type I IFN pathway (3), which is mediated through JAK1 and TYK2 activation (6). Among the studies that reported IFN levels or IFN-regulated gene expression after JAK inhibitor initiation, all showed that the clinical response corresponded with the downregulation of IFN activity, providing further evidence that JAK inhibitors may similarly benefit patients with DM by decreasing type I IFN signaling.
Although most patients were on concomitant immunosuppression, an open-label proof-of-concept study demonstrated that JAK inhibitor monotherapy was efficacious (13). Furthermore, most patients with DM were able to taper or discontinue concomitant corticosteroid therapy while on JAK inhibitor therapy, further supporting the therapeutic potential of JAK inhibitors in DM.
No JAK inhibitor is currently indicated for DM/JDM, limiting their clinical use. JAK inhibitors currently approved for autoimmune diseases are baricitinib (JAK1/2 inhibitor), upadacitinib (JAK1 inhibitor), and tofacitinib (JAK1/2/3 inhibitor) (Supplemental Table 5). In addition to the approved drugs that target JAK1 and/or JAK2, several drugs that target other members of the JAK family are in development. TYK2 inhibitors, such as deucravacitinib (TYK2) and brepocitinib (JAK1/TYK2), may be especially of interest for mediating IFN signaling. Randomized controlled trials are needed to further elucidate the therapeutic utility of JAK inhibition in DM and JDM.
Although this study is the most comprehensive systematic review on JAK inhibitor therapy and DM to date, it is not without limitations. The studies included are heterogeneous, often with differing outcome measures; thus, direct comparison of results is difficult. If a publication included patients that were likely included in another publication (ie, from a larger analysis or from an ongoing trial), the patients from the publication in question were not included in the count of unique patients, and therefore the number of unique patients may be underrepresented. Evaluation of safety-related data was beyond the scope of this review.
Our systematic review demonstrated that treatment with a JAK inhibitor was associated with reduced IFN markers and improved or resolved symptoms of DM and JDM, including skin, muscle, and lung disease. There is a need for carefully designed randomized controlled trials to confirm the role of JAK inhibition in these encouraging findings.
Supplementary Material
Acknowledgments
Medical writing and editorial support was provided by Tania R. Iqbal, PhD, CMPP, and Krystina Neuman, PhD, CMPP, of The Curry Rockefeller Group, LLC (Tarrytown, NY), and was funded by Priovant Therapeutics, Inc. (New York, NY). Funding for this study was provided by Priovant Therapeutics.
Conflicts of Interest
JJP has received research support from Corbus Pharmaceuticals, Kezar Life Sciences, and Pfizer Inc.; has received consulting fees and/or honoraria from Alexion, EMD Serono, Guidepoint consulting, Pfizer Inc., and Roivant Sciences; and is supported by grant number K23AR073927 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
GL, AG, PNM, and MPP are employees of Priovant Therapeutics.
LCS has received consulting fees and/or honoraria from Allogene, ArgenX, Boehringer-Ingelheim, the Dysimmune Disease Foundation, Janssen, Mallinckrodt, Octapharma, Roivant Sciences, and EMD Serono and has received research support from Corbus Pharmaceuticals, Kezar Life Sciences, and Pfizer Inc.
References
- 1.MARIE I: Morbidity and mortality in adult polymyositis and dermatomyositis. Curr Rheumatol Rep 2012. 14(3): 275–85. [DOI] [PubMed] [Google Scholar]
- 2.BAECHLER EC, BAUER JW, SLATTERY CA et al. : An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol Med 2007. 13(1-2): 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GREENBERG SA, PINKUS JL, PINKUS GS et al. : Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol 2005. 57(5): 664–78. [DOI] [PubMed] [Google Scholar]
- 4.TEZAK Z, HOFFMAN EP, LUTZ JL et al. : Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. J Immunol 2002. 168(8): 4154–63. [DOI] [PubMed] [Google Scholar]
- 5.PINAL-FERNANDEZ I, CASAL-DOMINGUEZ M, DERFOUL A et al. : Identification of distinctive interferon gene signatures in different types of myositis. Neurology 2019. 93(12): e1193–e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CASAL-DOMINGUEZ M, PINAL-FERNANDEZ I, MAMMEN AL: Inhibiting interferon pathways in dermatomyositis: rationale and preliminary evidence. Current Treatment Options in Rheumatology 2021. 7: 258–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DOWTY ME, LIN TH, JESSON MI et al. : Janus kinase inhibitors for the treatment of rheumatoid arthritis demonstrate similar profiles of in vitro cytokine receptor inhibition. Pharmacol Res Perspect 2019. 7(6): e00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.HORNUNG T, JANZEN V, HEIDGEN FJ, WOLF D, BIEBER T, WENZEL J: Remission of recalcitrant dermatomyositis treated with ruxolitinib. N Engl J Med 2014. 371(26): 2537–8. [DOI] [PubMed] [Google Scholar]
- 9.HORNUNG T, WOLF D, WENZEL J: More on remission of recalcitrant dermatomyositis treated with ruxolitinib. N Engl J Med 2015. 372(13): 1274. [DOI] [PubMed] [Google Scholar]
- 10.SELVA-O'CALLAGHAN A, TRALLERO-ARAGUAS E, LABRADOR-HORRILLO M: More on remission of recalcitrant dermatomyositis treated with ruxolitinib. N Engl J Med 2015. 372(13): 1273–4. [DOI] [PubMed] [Google Scholar]
- 11.KURTZMAN DJ, WRIGHT NA, LIN J et al. : Tofacitinib citrate for refractory cutaneous dermatomyositis: an alternative treatment. JAMA Dermatol 2016. 152(8): 944–5. [DOI] [PubMed] [Google Scholar]
- 12.PAIK JJ, CHRISTOPHER-STINE L: A case of refractory dermatomyositis responsive to tofacitinib. Semin Arthritis Rheum 2017. 46(4): e19. [DOI] [PubMed] [Google Scholar]
- 13.PAIK JJ, CASCIOLA-ROSEN L, SHIN JY et al. : Study of tofacitinib in refractory dermatomyositis: an open-label pilot study of ten patients. Arthritis Rheumatol 2021. 73(5): 858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.KURASAWA K, ARAI S, NAMIKI Y et al. : Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology (Oxford) 2018. 57(12): 2114–9. [DOI] [PubMed] [Google Scholar]
- 15.TANSLEY SL, MCHUGH NJ, WEDDERBURN LR: Adult and juvenile dermatomyositis: are the distinct clinical features explained by our current understanding of serological subgroups and pathogenic mechanisms? Arthritis Res Ther 2013. 15(2): 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EMERY P, POPE JE, KRUGER K et al. : Efficacy of monotherapy with biologics and JAK inhibitors for the treatment of rheumatoid arthritis: a systematic review. Adv Ther 2018. 35(10): 1535–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ROHATGI A: WebPlotDigitizer, Version 4.5. Available at: https://automeris.io/WebPlotDigitizer. [Google Scholar]
- 18.NATIONAL HEART, LUNG, AND BLOOD INSTITUTE: Study quality assessment tools. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed September 15, 2021.
- 19.MIN MS, ALSARHEED A, KASSAMALI B et al. : Tofacitinib as treatment for refractory dermatomyositis: a retrospective study from two academic medical centers. J Am Acad Dermatol 2021: doi: 10.1016/j.jaad.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 20.LADISLAU L, SUAREZ-CALVET X, TOQUET S et al. : JAK inhibitor improves type I interferon induced damage: proof of concept in dermatomyositis. Brain 2018. 141(6): 1609–21. [DOI] [PubMed] [Google Scholar]
- 21.NAVARRO-NAVARRO I, JIMENEZ-GALLO D, RODRIGUEZ-MATEOS ME, RODRIGUEZ-HERNANDEZ C, LINARES-BARRIOS M: Treatment of refractory anti-NXP2 and anti-TIF1gamma dermatomyositis with tofacitinib. J Dtsch Dermatol Ges 2021. 19(3): 443–7. [DOI] [PubMed] [Google Scholar]
- 22.RIGGLE K, PATEL A, AGGARWAL R: Ab0599 Treatment of refractory dermatomyositis with tofacitinib. Ann Rheum Dis 2020. 79(suppl 1): 1596. [Google Scholar]
- 23.WENDEL S, VENHOFF N, FRYE BC et al. : Successful treatment of extensive calcifications and acute pulmonary involvement in dermatomyositis with the Janus-Kinase inhibitor tofacitinib - a report of two cases. J Autoimmun 2019. 100: 131–6. [DOI] [PubMed] [Google Scholar]
- 24.SHNEYDERMAN M, AHLAWAT S, CHRISTOPHER-STINE L, PAIK JJ: Calcinosis in refractory dermatomyositis improves with tofacitinib monotherapy: a case series. Rheumatology (Oxford) 2021: doi: 10.1093/rheumatology/keab421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.PAIK J, ALBAYDA J, TINIAKOU E, PURWIN G, KOENIG A, CHRISTOPHER-STINE L: Long term open label extension of study of tofacitinib in refractory dermatomyositis [abstract #1094]. Arthritis Rheumatol 2020. 72(suppl 10). [Google Scholar]
- 26.PAIK JJ, SHNEYDERMAN M, GUTIERREZ-ALAMILLO L et al.: Long term extension study of tofacitinib in refractory dermatomyositis. Arthritis Rheumatol 2021: doi: 10.1002/art.41944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LANDON-CARDINAL O, GUILLAUME-JUGNOT P, BOLKO L et al. : JAK inhibitors: a promising molecular-targeted therapy in dermatomyositis [abstract #1280]. Arthritis Rheumatol 2019. 71(suppl 10). [Google Scholar]
- 28.ALSARHEED A, PATEL M, VLEUGELS R: 503 Tofacitinib for recalcitrant cutaneous dermatomyositis. J Invest Dermatol 2018. 138(5): S85. [Google Scholar]
- 29.CRESPO CRUZ A, DEL BOZ J, ROMERO GÓMEZ C: Good response to tofacitinib in refractory amyopathic dermatomyositis. Actas Dermo-Sifiliográficas (English Edition) 2021. 112(4): 374–6. [DOI] [PubMed] [Google Scholar]
- 30.DELVINO P, BARTOLETTI A, MONTI S et al. : Successful treatment with baricitinib in a patient with refractory cutaneous dermatomyositis. Rheumatology (Oxford) 2020. 59(12): e125–e7. [DOI] [PubMed] [Google Scholar]
- 31.FETTER T, RIOS GC, NIEBEL D, BIEBER T, WENZEL J: Unexpected hair regrowth in a patient with longstanding alopecia universalis during treatment of recalcitrant dermatomyositis with the janus kinase inhibitor ruxolitinib. Acta Derm Venereol 2020. 100(10): adv00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FISCHER K, ARINGER M, BEISSERT S, GÜNTHER C: Klinisches ansprechen der dermatomyositis auf therapie mit dem JAK inhibitor baricitinib [abstract DK01/04]. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 2020. 18(S1): 2–43.32030878 [Google Scholar]
- 33.HORNIG J, WEINHAGE T, SCHMIDT LH et al. : [Response of dermatomyositis with lung involvement to Janus kinase inhibitor treatment]. Z Rheumatol 2018. 77(10): 952–7. [DOI] [PubMed] [Google Scholar]
- 34.ISHIKAWA Y, KASUYA T, FUJIWARA M, KITA Y: Tofacitinib for recurrence of antimelanoma differentiation-associated gene 5 antibody-positive clinically amyopathic dermatomyositis after remission: a case report. Medicine (Baltimore) 2020. 99(37): e21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.JALLES C, DEROUX A, TARDIEU M et al. : [Severe MDA5 dermatomyositis associated with cancer and controlled by JAK inhibitor]. Rev Med Interne 2020. 41(6): 421–4. [DOI] [PubMed] [Google Scholar]
- 36.JASMINE SW, SEN D, JONES HA: AB019. Response to tofacitinib in a case of refractory TIF-1 positive amyopathic dermatomyositis with arthritis. Ann Transl Med 2021. 9(5): AB019–AB. [Google Scholar]
- 37.MOGHADAM-KIA S, CHARLTON D, AGGARWAL R, ODDIS CV: Management of refractory cutaneous dermatomyositis: potential role of Janus kinase inhibition with tofacitinib. Rheumatology (Oxford) 2019. 58(6): 1011–5. [DOI] [PubMed] [Google Scholar]
- 38.OHMURA SI, YAMABE T, NANIWA T: Successful dose escalation of tofacitinib for refractory dermatomyositis and interstitial lung disease with anti-melanoma differentiation-associated gene 5 antibodies. Mod Rheumatol Case Rep 2021. 5(1): 76–81. [DOI] [PubMed] [Google Scholar]
- 39.SHINJO SK, DE SOUZA FHC: Tofacitinib as a treatment for refractory dermatomyositis: a case report. Open J Rheumatol Autoimmune Dis 2020. 10(01): 39–43. [Google Scholar]
- 40.WILLIAMS P, MCKINNEY B: Refractory dermatomyositis-systemic lupus erythematosus overlap syndrome and response to tofacitinib. Proc Bayl Univ Med Cent 2020. 34(1): 116–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CHEN Z, WANG X, YE S: Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease. N Engl J Med 2019. 381(3): 291–3. [DOI] [PubMed] [Google Scholar]
- 42.CONCA W, WEHEBA I, ABOUZIED ME et al. : Iacta alea est: the inexorable advance of tofacitinib in the treatment of dermatomyositis-associated rapidly progressive interstitial lung disease. a case report. Front Pharmacol 2020. 11: 585761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.TAKATANI A, KOGA T, FUJITA Y et al. : Efficacy of tofacitinib for slowly progressive interstitial lung disease in a patient with anti-MDA5 antibody-positive dermatomyositis. Clin Immunol 2020. 215: 108451. [DOI] [PubMed] [Google Scholar]
- 44.MACHIYAMA T, SHIRAI T, FUJITA Y et al. : Successful concomitant therapy with tofacitinib for anti- melanoma differentiation associated gene 5 antibody-positive rapidly progressive interstitial lung disease with poor prognostic factors. Med Case Rep Study Protoc 2021. 2(1): doi: 10.1097/MD9.0000000000000026. [DOI] [Google Scholar]
- 45.KIM H, DILL S, O'BRIEN M et al. : Janus kinase (JAK) inhibition with baricitinib in refractory juvenile dermatomyositis. Ann Rheum Dis 2020: [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.SABBAGH S, ALMEIDA DE JESUS A, HWANG S et al. : Treatment of anti-MDA5 autoantibody-positive juvenile dermatomyositis using tofacitinib. Brain 2019. 142(11): e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.AESCHLIMANN FA, FREMOND ML, DUFFY D et al. : A child with severe juvenile dermatomyositis treated with ruxolitinib. Brain 2018. 141(11): e80. [DOI] [PubMed] [Google Scholar]
- 48.PAPADOPOULOU C, HONG Y, OMOYINMI E, BROGAN PA, ELEFTHERIOU D: Janus kinase 1/2 inhibition with baricitinib in the treatment of juvenile dermatomyositis. Brain 2019. 142(3): e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LE VOYER T, GITIAUX C, AUTHIER FJ et al.: JAK inhibitors are effective in a subset of patients with juvenile dermatomyositis: a monocentric retrospective study. Rheumatology (Oxford) 2021: keab116. [DOI] [PubMed] [Google Scholar]
- 50.YU Z, WANG L, QUAN M, ZHANG T, SONG H: Successful management with Janus kinase inhibitor tofacitinib in refractory juvenile dermatomyositis: a pilot study and literature review. Rheumatology (Oxford) 2021. 60(4): 1700–7. [DOI] [PubMed] [Google Scholar]
- 51.HEINEN A, SCHNABEL A, BRUCK N et al. : Interferon signature guiding therapeutic decision making: ruxolitinib as first-line therapy for severe juvenile dermatomyositis? Rheumatology (Oxford) 2021. 60(4): e136–e8. [DOI] [PubMed] [Google Scholar]
- 52.SOZERI B, DEMIR F: A striking treatment option for recalcitrant calcinosis in juvenile dermatomyositis: tofacitinib citrate. Rheumatology (Oxford) 2020. 59(12): e140–e1. [DOI] [PubMed] [Google Scholar]
- 53.ZHOU Q, WENG R, XIA Y: Refractory juvenile dermatomyositis: response to tofacitinib. Med Clin (Barc) 2021: doi: 10.1016/j.medcli.2021.03.022. [DOI] [PubMed] [Google Scholar]
- 54.WANG X, DING Y, ZHOU Z, HOU J, XU Y, LI J: Clinical characteristics and poor predictors of anti-NXP2 antibody-associated Chinese JDM children. Pediatr Rheumatol Online J 2021. 19(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DING Y, HUANG B, WANG Y et al. : Janus kinase inhibitor significantly improved rash and muscle strength in juvenile dermatomyositis. Ann Rheum Dis 2020: doi: 10.1136/annrheumdis-2020-218582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.EL-LATEEF HA: Ruxolitinib therapeutic trial in refractory cutaneous juvenile dermatomyositis (case report) [abstract #AB023]. Pediatric Rheumatology 2020. 18(suppl 2): 82. [Google Scholar]
- 57.HARRINGTON R, AL NOKHATHA SA, CONWAY R: JAK inhibitors in rheumatoid arthritis: an evidence-based review on the emerging clinical data. J Inflamm Res 2020. 13: 519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.WANG F, SUN L, WANG S et al. : Efficacy and safety of tofacitinib, baricitinib, and upadacitinib for rheumatoid arthritis: a systematic review and meta-analysis. Mayo Clin Proc 2020. 95(7): 1404–19. [DOI] [PubMed] [Google Scholar]
- 59.OLUMIANT® (Baricitinib). Full Prescribing Information. Indianapolis, IN USA, Eli Lilly and Company; 2018. [Google Scholar]
- 60.XELJANZ® (Tofacitinib). Full Prescribing Information. New York, NY USA, Pfizer Labs; 2012. [Google Scholar]
- 61.JAKAFI® (Ruxolitinib). Full Prescribing Information. Wilmington, DE USA, Incyte Corporation; 2011. [Google Scholar]
- 62.HUBER AM, FELDMAN BM, RENNEBOHM RM et al. : Validation and clinical significance of the Childhood Myositis Assessment Scale for assessment of muscle function in the juvenile idiopathic inflammatory myopathies. Arthritis Rheum 2004. 50(5): 1595–603. [DOI] [PubMed] [Google Scholar]
- 63.ALLENBACH Y, TOQUET S, LANDON-CARDINAL O, BENVENISTE O: Reply: A child with severe juvenile dermatomyositis treated with ruxolitinib. Brain 2018. 141(11): e81. [DOI] [PubMed] [Google Scholar]
- 64.KOSTIK M, RAUPOV R, SUSPITSIN E et al. : Pos0080 Tofacitinib treatment in children with rheumatic diseases: single-center experience. Ann Rheum Dis 2021. 80(suppl 1): 247.1–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.