Highlights
-
•
Two thirds of patients with sterile pyuria were exposed to antibiotics treatment.
-
•
Neisseria gonorrhoeae and Trichomonas vaginalis were major cause of pyuria.
-
•
Moderate leukocytes in urine predict the presence of urogenital pathogen.
-
•
Females patients had significantly more urogenital pathogens than males patients.
Keywords: Sexual transmitted infection, Pyuria, UTI culture negative, leukocyte esterase
Abstract
Background
Urogenital pathogens such as Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium and Trichomonas vaginalis have been reported to cause pyuria, however they are not routinely cultured from urine samples of patients clinically diagnosed to have urinary tract infections (UTI). In this study, pathogen specific PCR was done to identify the urogenital pathogens in the urine samples among clinically diagnosed UTI patients with negative routine urine culture.
Methods
A cross-sectional study was conducted involving 227 archived urine samples from clinically diagnosed UTI patients with positive leucocyte esterase but negative urine culture results. The urogenital pathogens were detected using pathogen specific singleplex PCR. Data were cleaned and analyzed using STATA version 15.
Results
The median age of patients was 31[IQR 23 – 51] years and the majority (174, 76.7%) were females. Two thirds of patients had history of antibiotic use two weeks prior to recruitment (154, 67.8%). A total of 62(27.3%) urine samples were positive for at least one urogenital pathogen. Of 62 positive samples, 9 had two urogenital pathogens and 1 had three urogenital pathogens. The most predominant urogenital pathogen detected was Neisseria gonorrhoeae 25(34.2%) and Trichomonas vaginalis 24(32.9%). Being female (aOR 2.4; 95% CI: 1.04 – 5.49; p-value 0.039) and having history of using antibiotics in the past two weeks (aOR 1.9; 95%CI: 1.04 – 3.60; p-value 0.036) was independently associated with the presence of urogenital pathogens.
Conclusion
More than a quarter of female patients with clinical symptoms of UTI and routine urine culture negative results were infected with urogenital pathogens mainly Neisseria gonorrhoeae and Trichomonas vaginalis. Further research with a larger sample set in a range of settings is required to understand the implications of these finding generally.
Introduction
Background
Sterile pyuria is a presence of greater than ten white blood cells (pus cells) per high power field in culture negative urine samples [1,2]. About 13.9% of women and 2.6% of men globally are diagnosed to have sterile pyuria [1]. Sterile pyuria has been linked with a number of causes including urogenital pathogens (like viruses, fungi, and atypical or fastidious bacteria causing sexual transmitted infections (STIs)), antibiotic use, genitourinary tuberculosis, interstitial cystitis, bladder cancer and cystitis [1]. The burden of sterile pyuria varies in the population due to a number of reasons including sex, causative agents, use of antibiotics and the frequency of laboratory testing for urogenital pathogens [3]. In the general population, sterile pyuria is positively associated with pregnancy, menopause, untreated urinary tract infections (UTI), and vaginal or penile discharge [2].
Although urogenital pathogens like C. trachomatis, N. gonorrhoeae, M. genitalium and T. vaginalis have been documented to cause pus cells in urine [4], they are not easily captured with routine urine culture methods [4,5]. This has led to misdiagnosis of these patients and hence delayed appropriate treatment for the underlying conditions predisposing patients to various associated complications [2,6,7]. Additionally, in resource limited settings, UTI has been the common working diagnosis for patients presenting with pyuria in community health facilities [8]. In higher health facilities where quantitative urine culture is conducted, patients with leucocyte positive and culture negative are denied from treatment due to lack of treatment guidelines to cover for this group [9]. Furthermore, treatment guidelines are silent on pyuria of unknown origin, which further delays the management of these patients, subjecting them to the consequences of self-medications, both of which may lead to a worsening of the underlying condition [2].
The pattern of urogenital pathogens among patients with pyuria but urine culture negative results is not clearly established. This study was done to establish the pattern of urogenital pathogens and factors associated with PCR positive urogenital pathogens in urine samples of patient with sterile pyuria.
Methods
Study design settings and participants
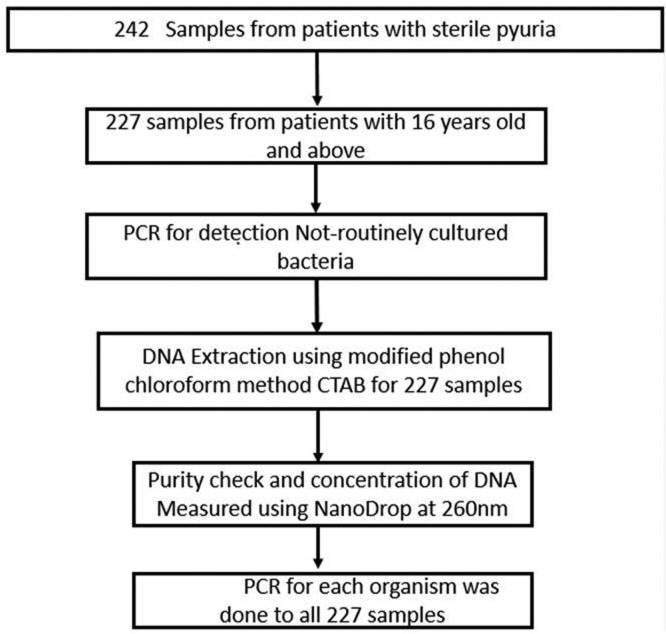
A laboratory based cross-sectional study was conducted from May to July 2021 at the Catholic University of Health and Allied Sciences (CUHAS) - Molecular biology research laboratory in Mwanza and National Institute for Medical Research (NIMR) - molecular laboratory Mwanza Centre. The study used clean catch midstream urine samples which was self-collected by the patients with clinical diagnosis of UTI from 10 different health facilities in Mwanza, Kilimanjaro and Mbeya during the Holistic Approach to Unravel Antibacterial Resistance in East Africa (HATUA) project from [10]. In HATUA project urine samples were stored under -80°c within two hours of collection. The minimum sample size of 138 urine samples was obtained using the Kish Leslie formula (1965) with 10% prevalence of urogenital pathogens obtained among adult patients presenting with UTI symptoms [3]. However, the current study enrolled 227 archived urine samples from patients with a sign and symptoms of UTI like fever, lower abdominal pain, flank/back pain, painful urination, dysuria, hematuria, increased frequency of urination but urine culture negative results with >125 leucocyte/μl, as shown in Fig. 1. Urine leucocyte esterase was measured by urine chemistry dipstick (Siemens Healthineers AG, Pennsylvania, United States).
Fig. 1.
Flow chart diagram for sample retrieval and processing.
DNA extraction
DNA extraction was done using cetlytrimethyl ammonium bromide (CTAB) modified phenol – chloroform extraction method as described previously [11,12]. Where 10 mL of urine sample was centrifuged for 10 minutes at 2000 × g and supernatant was discarded. A total of 100μl urine deposit was suspended into 200μl of CTAB and incubated at 65οC for 30 minutes. Then 200μl of chloroform was added to each tube, capped tightly, inverted 10 times to mix and centrifuged at 13,000rpm for 5 minutes at 4οC to separate the phases.
PCR Amplification and detection
Using specific primers, samples were tested for the presence of urogenital pathogens (C. trachomatis, M. genitalium, N. gonorrhoeae and T. vaginalis) as previously described [2], detailed in Table 1. PCR amplification was performed in a 25μl PCR reaction volume master mix containing 10μl of DNA template for C. trachomatis, M. genitalium, T. vaginalis and 5μl of DNA template for N. gonorrhoeae, 0.5μl deoxynucleotide triphosphate (dNTP) mix, 2.5μl of 10X standard Taq reaction buffer, 0.5μl of forward and reverse primers, 1μl of Taq polymerase (New England BioLabs) and the remaining volume of 10μl and 15μl Nuclease free water were added up to 25μl reaction. All reactions were performed in a T-Gradient Thermoblock PCR system (Biometra, Goettingen, Germany). The cycling conditions was set at initial denaturation at 95οC for 30 seconds, denaturation at 95οC for 30 seconds, annealing for each primer set targeting 16SrRNA as indicated in Table 1 and elongation at 68οC for 1 minute, and final extension at 72οC for 5 minutes for 30 cycles of PCR [13].
Table 1.
Details of the primer sets and expected amplicon size.
| Microorganism | Primer name | Primer Sequence 5’- 3’ | Amplicon size (bp) | Ta |
|---|---|---|---|---|
| C. trachomatis | 16SrRNA-S | CGAGTCGGCATCTAATACTAT | 402 | 44°C |
| 16SrRNA-AS | AAAACGACATTTCTGCCGC | |||
| N. gonorrhoeae | 16SrRNA-S | TAGCCAAGCCGCGAGGC | 694 | 49.3°C |
| 16SrRNA-AS | GGCGCAGACGGTTACTTAAGCAGGA | |||
| M. genitalium | 16SrRNA-S | GTAATACATAGGTCGCAAGCGTTATC | 771 | 51.4°C |
| 16SrRNA-AS | CACCACCTGTCACTCGGTTAACCTC | |||
| T. vaginalis | β-tubulin gen-S | ATCGTAAAGAGCTTCGTTATCAATG | 89 | 42°C |
| β-tubulingen-AS | GCATGTTGT GCC GGA CAT AAC CAT |
Ta is annealing temperature and bp is the base pair.
Gel electrophoresis
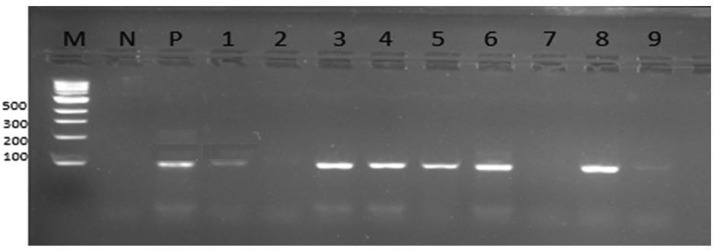
To enhance interpretation of the band size 8μl of 100bp ladder was included in the electrophoresis of 1.2% gel. Gel electrophoresis was carried at 100V for 120 minutes (IntrogenTM, Carlsbad, CA) and visualization was done by UV-light image reader (Alpha Imager, Alpha Innotech, U.S.A) as indicated in Fig. 2 for T. vaginalis.
Fig. 2.
Representative Images for PCR products electrophoresed on a 1.2% (wt/vol) agarose gel, stained with red safe and photographed under UV light: M=Marker 100bp, P-positive control, N- Negative control, lane number 1,3,4,5,6,8 and 9 are positive for T. vaginalis with 89bp.
Quality control
Quality control was performed using in house-typed isolate from cervical swab sample for C. trachomatis, T. vaginalis, N. gonorrhoeae and M. genitalium.
Data analysis
Data were analyzed using STATA version 15.0. Categorical variables were summarized as proportions while continuous variables were summarized as median (interquartile range). Univariate and multivariate logistic regression models were fitted to determine the associated factors of urogenital infection. Variables with a p-value of less than 0.2 on univariate analysis were fitted into the multivariate logistic regression model and their odds ratios and 95% confidence intervals were reported. Variables with the p-value of less than 0.05 at multivariate logistic regression model were considered statistically significant.
Results
Descriptive data of study participants
In this study, the majority of samples, 174 (76.7%), were from female patients with a median age of 31 [IQR 23 – 51] years. There were 162 (71.4%) samples from married patients, 127 (55.9%) were from patients who had primary level of education and one third of samples, 73 (32.2%) were from patients who had a history of antibiotic use in the past two weeks (Table 2).
Table 2.
Social demographic and clinical characteristics of 227 studied patient.
| Variables | Frequency (n) | Percent (%) |
|---|---|---|
| Resident | ||
| Urban health facilities | 118 | 52 |
| Rural health facilities | 109 | 48 |
| Sex | ||
| Female | 174 | 76.7 |
| Male | 53 | 23.3 |
| Marital status | ||
| Married | 161 | 70.9 |
| Not married | 66 | 29.1 |
| Antibiotics use in a past 2 weeks | ||
| Yes | 73 | 32.2 |
| No | 154 | 67.8 |
| Urine characteristics | ||
| Clear | 67 | 29.5 |
| Turbid | 158 | 69.6 |
| Blood | 2 | 0.9 |
| Urine pH | ||
| >7.0 | 162 | 71.4 |
| <7.0 | 65 | 28.6 |
| Leucocyte | ||
| Small | 30 | 13.2 |
| Moderate | 118 | 52.0 |
| Large | 79 | 34.8 |
Small, moderate and large is when leukocyte is below 75, 120 and above 500 respectively.
PCR detection of urogenital pathogens (main results)
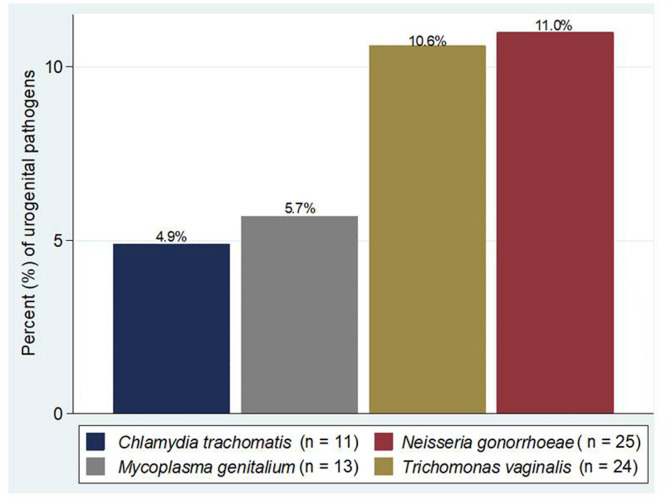
Of the 227 samples examined, 62 (27.3%) were positive for at least one urogenital pathogen. Of the 62 positive samples, nine had two urogenital pathogens and one had three, making a total of 73 urogenital pathogens detected. The most frequent urogenital pathogens detected were Neisseria gonorrhoeae (n=25, 11%) and Trichomonas vaginalis (n=24, 10.6), Fig. 3.
Fig. 3.
Pattern of the detected urogenital pathogens.
Of the 10 samples with multiple urogenital pathogens, 9/10(90%) were from female patients, 7/10 (70%) had moderate number of leucocytes (250 leucocyte/μl), Table 3.
Table 3.
The description of the patients with more than one urogenital pathogens.
| SN | Age (years) | Sex | Macroscopic appearance | Leukocyte | Antibiotic use | Detected pathogen(s) |
|---|---|---|---|---|---|---|
| 1. | 15-35 | F | Turbid | Moderate | Yes | T. vaginalis and M. genitalium |
| 3. | 15-35 | F | Turbid | Moderate | Yes | T. vaginalis and M. genitalium |
| 4. | 15-35 | F | Turbid | Moderate | Yes | T. vaginalis and M. genitalium |
| 2. | 36-55 | M | Turbid | Moderate | Yes | T. vaginalis and C. trachomatis |
| 6. | Above 55 | F | Turbid | Moderate | Yes | T. vaginalis and N. gonorrhoeae |
| 7. | 36-55 | F | Turbid | Moderate | No | T. vaginalis and N. gonorrhoeae |
| 5. | 15-35 | F | Turbid | Large | Yes | C. trachomatis and M. genitalium |
| 8. | 36-45 | F | Turbid | Large | Yes | C. trachomatis and N. gonorrhoeae |
| 9. | 15-35 | F | Turbid | large | Yes | C. trachomatis and N. gonorrhoeae |
| 10. | 15-35 | F | Turbid | Moderate | Yes | C. trachomatis, T. vaginalis and M. genitalium |
Factors associated with PCR positive urogenital pathogens in urine samples of patient with sterile pyuria
Using univariate logistic regression analysis, being female (OR 2.5; 95% CI: 1.1 – 5.7; p-value = 0.026) and having a history of using antibiotics in the past two weeks (OR 2.0; 95% CI: 1.1 – 3.7; p-value = 0.026) were factors associated with PCR positive urogenital pathogens (4). On stepwise multivariate logistic regression analysis being female (OR 2.4; 95%CI 1.0-5.8; P=0.039) and have history of using antibiotic two weeks prior to recruitment to the study (OR 2.0; 95% CI 1.0-3.6; P=0.036) indecently predicted the PCR positive urogenital pathogens (Table 4).
Table 4.
Factors associated with PCR positive urogenital pathogen.
| Variable | PCR positive for urogenital infection N (%) | Univariate OR [95%CI] | P- value | Multivariate OR [95%CI] | P- value |
|---|---|---|---|---|---|
| Age (years) | 31 [23 - 51] | 1.0 [0.9 – 1.0] | 0.156 | 1.0 [0.9 – 1.0] | 0.961 |
| Sex | |||||
| Male (53) | 8 (15.1 | 1.0 | |||
| Female (174 | 54 (31.0 | 2.5 [1.1 – 5.7] | 0.026 | 2.4 [1.0 – 5.8] | 0.039 |
| Residence | |||||
| Rural (109) | 27 (22.7) | 1.0 | |||
| Urban (118) | 35 (32.4) | 1.6 [0.9 – 2.9] | 0.102 | 2.0 [0.9 – 3.1] | 0.086 |
| Marital status | |||||
| Not married (41) | 17 (26.2) | 1.0 | |||
| Married (113) | 45 (27.8) | 0.9 [0.5 – 1.8] | 0.804 | - | - |
| Antibiotic use in a past 2 weeks | |||||
| No (154) | 35 (22.7) | 1.0 | |||
| Yes (73) | 27 (37.0) | 2.0 [1.1 – 3.7] | 0.026 | 2.0 [1.0 – 3.6] | 0.036 |
| Urine characteristics | |||||
| Clear (67) | 13 (19.4) | 1.0 | |||
| Turbid (158) | 48 (30.4) | 2.0 [0.9 – 3.6] | 0.093 | - | * |
| Blood (2) | 1 (50.0) | 4.2 [0.2 – 70.9] | 0.325 | - | - |
| Urine pH | |||||
| ≥7.0 (162) | 17 (26.2) | 1.0 | |||
| < 7.0 (65) | 45 (27.8) | 0.9 [0.5 – 1.8] | 0.804 | - | - |
| Leucocyte | |||||
| Small (30) | 8 (26.7) | 1.0 | |||
| Moderate (118) | 31 (26.3) | 1.1 [0.4 – 2.9] | 0.800 | - | - |
| Large (79) | 23 (29.1) | 1.0 [0.4 – 2.4] | 0.965 | - | - |
Urine characteristics had a p-value of <0.2 (0.093) but was not included in multivariate analysis due to multicollinearity with sex.
Discussion
Sterile pyuria in patients with sign and symptoms of UTI are of public health importance as it represents a hidden group with STI. Poor knowledge of the patients on differences of UTI and STI and stigma within the community act as barriers to treatment of STI considering that UTI is socially accepted and the treatment options is available [14,15]. Previous study conducted in Arusha Tanzania reported the seroprevalence of STI causing pathogens like C. trachomatis, N. gonorrhoeae and Treponema pallidum to be above 50% [16]. Testing for urogenital pathogens such as C. trachomatis, N. gonorrhoeae, M. genitalium and T. vaginalis in urine samples of patients with pyuria and negative urine culture, is not routinely done due to lack of treatment guidelines and advanced diagnostic tools e.g., molecular techniques to detect these urogenital pathogens [4]. The current study is among the first studies to investigate the pattern of urogenital pathogens and associated factors from urine samples of patients with pyuria but negative results of urine culture for UTI diagnosis in the study setting. In this report we have documented the importance of the four urogenital pathogens (C. trachomatis, N. gonorrhoeae, M. genitalium and T. vaginalis) as causes of apparently sterile pyuria as detected by molecular techniques.
In the current study, 27.3% of urine samples were positive for at least one of the four urogenital pathogens tested. The prevalence in this study is relatively higher than the 19.5% reported among adult's patients presenting with urinary tract infection symptoms in primary health care clinic in Zimbabwe [3] and 20.8% reported from Kilifi, Kenya among pregnant women [17]. The variations in findings could be explained by the fact that the current study used urine samples from patients with clinical diagnosis of UTI and positive urine leucocytes (pyuria) while the previous study in Kilifi urine samples from pregnant women attending antenatal clinic not necessarily having pyuria [17]. The prevalence in this study is similar to the previous studies which reported a prevalence of 23.9% among adult's women in Metro Health Medical Center- USA presenting with sign of UTI [8,18].
In this study the most common urogenital pathogen was N. gonorrhoeae with a prevalence of 11.0%. The prevalence of N. gonorrhoeae is similar to the Zimbabwe study which reported a prevalence of 8.7% among adult patients presenting with urinary tract infection symptoms in primary health care clinic [3]. Moreover, the prevalence in this study is higher than that reported (6.7%) among pregnant adolescent girls in the city of Mwanza [19] and the 3.3% reported for reproductive aged women in sub-Saharan Africa [20]. Sampling differences may partly explain the variation in prevalence of urogenital pathogens observed. In this current study, we enrolled patients with pyuria while in the previous studies in Mwanza and sub-Saharan Africa they enrolled participants without pyuria [19,20].
The prevalence of T. vaginalis in this study was 10.6%. The finding is comparable to 12.4%, 8.0% and 7.4% reported among pregnant adolescent girls in Mwanza Tanzania [19], adults presenting with UTI like symptoms in Harare, Zimbabwe [3] and pregnant women in Kilifi Kenya [17], respectively. However, the prevalence in the current study is slightly lower than the previous study which reported the prevalence of 16.5% among adult's women presenting at emergency department [8]. This variation in prevalence could be attributed to the difference in the study participant characteristics where previous study enrolled adult women with majority of them harbored Trichomonas vaginalis. This has also been proven in the current study where Trichomonas vaginalis was among the most common detected pathogen.
In the current study M. genitalium had prevalence of 5.7% similar to the previous study in Kenyan pregnant women aged between 18 and 44 years, which reported the prevalence of 6.3% [21]. Also comparable to the previous study which reported the prevalence of 3.2% among HIV patients in Kilimanjaro [22]. This can be due to the fact that M. genitalium is rarely associated with urogenital infections compared to the other pathogens like N. gonorrhoeae and C. trachomatis.
The prevalence of C. trachomatis in this study was 4.9% lower compared to the previous studies which reported the prevalence of 11.4% among pregnant adolescent girls and 36.2% among infertile women in the same setting, Mwanza [19,23]. The higher prevalence of C. trachomatis among infertile women may be attributable by the type of sample collected, endo-cervical swabs, whereby in the current study we collected mid-stream urine (MSU) samples. Studies have reported that endo-cervical swabs have high yields of C. trachomatis than MSU samples [24], [25], [26]. Although endo-cervical swab is not a convenient sample to the majority of the patients. Therefore, introduction of a convenient sample such as MSU is required.
The findings from this study shows about 1 in every 4 patients with apparent sterile pyuria is infected with urogenital pathogens not detected by the urine culture media used routinely in microbiology laboratory, which is likely to lead to report a false negative result. Failure to treat these patients will lead to return to the health care center and this has an extra burden/cost to the healthcare centres and the patients. Untreated STIs can be further transmitted to others and may lead to further complications in other individuals and may promote patients seeking over the counter medication. The use of over-the-counter medications is highly associated with antimicrobial resistance development in individual patients and community at large due to inappropriate use of antibiotics [27]. The findings cemented on the need of considering urogenital pathogens especially when leucocyte is positive while urine culture results is negative.
In this study 16.2% of the samples had coinfection of two or three urogenital pathogens. The findings are comparable to the previous study among adolescence pregnant girls in Mwanza city which reported 15% [19]. The prevalence of coinfection in the current study is relatively higher than 2.6% reported among adults presenting with UTI like symptoms [3]. In the current study, 70% of those with multiple infections were positive for T. vaginalis and 50% were positive for M. genitalium this may be due to the close symbiotic relationship among these pathogens [28].
The present study shows that female patients are more likely to be infected with urogenital pathogen, similar to the previous study which showed women are relatively highly infected with urogenital pathogens like N. gonorrhoeae, T. vaginalis, M. genitalium and C. trachomatis [29,30]. T. vaginalis infection among women has been associated with the shortness of urethra and its anatomical position under labia which create warm and moist environment for its survival [31]. Furthermore, the observation can partly be explained by the fact that women delay treatment due to late onset of symptoms and dependence on male partner for financial coverage of health care costs [32,33]. Moreover, the stratified squamous epithelium lining of the female urogenital tract is resistant to urogenital pathogens which subject them to asymptomatic infection leading to delayed treatment [34].
In the current study, use of antibiotics past two weeks was found to be associated with infection of the urogenital pathogens. This has also been observed previously in the study among adult patients presenting with UTI symptoms in primary health care clinics in Zimbabwe [3]. This could be due to collision in clinical signs and symptoms with UTI which lead to lower suspicious index among clinicians for STI pathogens [3]. When UTI is suspected in most cases urine culture becomes negative leading to denial for treatment. Consequently, this subjects patients to seek for self-medication with antimicrobials which does not clear the urogenital pathogens and predisposing to chronic infection and emergence of antimicrobial resistance. The findings from this study also underline the need for accurate, rapid tests for routine screening of urogenital pathogens in our setting and development of treatment guidelines as previously documented [3,30] especially for patients with pyuria but urine culture for UTI diagnosis negative.
Study limitation
In this study frozen mid-stream urine samples were used instead of first fresh voided urine which is recommended for detection of urogenital pathogens, this might lead to underestimation of the prevalence of N. gonorrhoeae, T. vaginalis, M. genitalium and C. trachomatis. Furthermore, the study did not document the non-infectious causes of sterile pyuria such as interstitial nephritis, nephrolithiasis, uroepithelial tumor, autoimmune diseases such as SLE, contamination of urine sample with vaginal leukocytes which might dilute the reported findings.
Conclusion
Our data demonstrate the importance of non-cultivated urinary pathogens Neisseria gonorrhoeae, Trichomonas vaginalis, Mycoplasma genitalium and Chlamydia trachomatis as a cause of sterile pyuria. Clinician should consider screening for these pathogens among patients at high risk. Further research with a larger sample set in a range of settings is required to understand the implications of these finding generally.
Declarations
Ethics approval and consent to participate
The protocol to conduct this study was approved by the joint CUHAS/BMC research ethics with certificate number CREC/4712021. The ethic committee is formed by members of the two sister institutions that's Catholic University of Health and Allied Science and Bugando Medical Centre all located in Mwanza, Tanzania. The study use data generated from HATUA study that receive all ethical permission [10]. All research participants provided written informed consent before recruitment to the project.
All data were treated as confidential.
Consent to publish
None applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interest
None declared.
Funding
This study is part of the Holistic Approach to Unravel Antibacterial Resistance in East Africa (HATUA) project funded by the National Institute for Health Research, Medical Research Council and the Department of Health and Social Care, Award (MR/S004785/1).
Author contribution
BM, MFM, BK, BO and SEM designed the work. BM, MFM, DNM, VS and MMM performed laboratory investigations and results interpretations. MFM, ETK, JRM, SG and SEM analyzed and interpreted the data. BM and MFM wrote the first draft of the manuscript which was critically reviewed by KK, WS, SG, AMB, AS and MH. All authors read and approved the final version of the manuscript.
Acknowledgements
Authors would like to acknowledge the support provided by the HATUA project and Department of Microbiology and immunology of the Catholic University of Health and Allied Sciences, Mwanza, Tanzania.
References
- 1.Goonewardene S, Persad R. Sterile pyuria: a forgotten entity. Ther Adv Urol. 2015;7:295–298. doi: 10.1177/1756287215592570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wise GJ, Schlegel PN. Sterile pyuria. N Engl J Med. 2015;372:1048–1054. doi: 10.1056/NEJMra1410052. [DOI] [PubMed] [Google Scholar]
- 3.Olaru ID, Chisenga M, Yeung S, Mabey D, Marks M, Chonzi P, et al. Sexually transmitted infections and prior antibiotic use as important causes for negative urine cultures among adults presenting with urinary tract infection symptoms to primary care clinics in Zimbabwe: a cross-sectional study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nassar FA, Abu-Elamreen FH, Shubair ME, Sharif FA. Detection of Chlamydia trachomatis and Mycoplasma hominis, genitalium and Ureaplasma urealyticum by polymerase chain reaction in patients with sterile pyuria. Adv Med Sci. 2008;53:80–86. doi: 10.2478/v10039-008-0020-1. [DOI] [PubMed] [Google Scholar]
- 5.Grad AI, Vica ML, Matei HV, Grad DL, Coman I, Tataru DA. Polymerase chain reaction as a diagnostic tool for six sexually transmitted infections - preliminary results. Clujul Med. 2015;88:33–37. doi: 10.15386/cjmed-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colgan R, Williams M, Johnson JR. Diagnosis and treatment of acute pyelonephritis in women. Am Fam Physician. 2011;84:519–526. [PubMed] [Google Scholar]
- 7.Anger J, Lee U, Ackerman AL, Chou R, Chughtai B, Clemens JQ, et al. Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J Urol. 2019;202:282–289. doi: 10.1097/JU.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 8.Tomas ME, Getman D, Donskey CJ, Hecker MT. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol. 2015;53:2686–2692. doi: 10.1128/JCM.00670-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabe M, Bartoletti R, Bjerklund-Johansen T, Cai T, Cek M, Köves B, et al. European Association of Urology; The Netherlands: 2015. Guidelines on urological infections. [Google Scholar]
- 10.Asiimwe BB, Kiiru J, Mshana SE, Neema S, Keenan K, Kesby M, et al. Protocol for an interdisciplinary cross-sectional study investigating the social, biological and community-level drivers of antimicrobial resistance (AMR): Holistic Approach to Unravel Antibacterial Resistance in East Africa (HATUA) BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Meyer T, Mampaey E, Vlemmix M, Denil S, Trooskens G, Renard JP, et al. Quality evaluation of methyl binding domain based kits for enrichment DNA-methylation sequencing. PLoS One. 2013;8:e59068. doi: 10.1371/journal.pone.0059068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasman H, Saputra D, Sicheritz-Ponten T, Lund O, Svendsen CA, Frimodt-Møller N, et al. Rapid whole-genome sequencing for detection and characterization of microorganisms directly from clinical samples. J Clin Microbiol. 2014;52:139–146. doi: 10.1128/JCM.02452-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguilera-Arreola MG, González-Cardel AM, Tenorio AM, Curiel-Quesada E, Castro-Escarpulli G. Highly specific and efficient primers for in-house multiplex PCR detection of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma hominis and Ureaplasma urealyticum. BMC Res Notes. 2014;7:433. doi: 10.1186/1756-0500-7-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wight D, Plummer M, Ross D. The need to promote behaviour change at the cultural level: one factor explaining the limited impact of the MEMA kwa Vijana adolescent sexual health intervention in rural Tanzania. A process evaluation. BMC Public Health. 2012;12:788. doi: 10.1186/1471-2458-12-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patra S. Socio-cultural correlates and risky sexual behaviour influencing prevalence of HIV/AIDS and STIs in Uganda: a gender perspective. Cogent Soc Sci. 2016;2 doi: 10.1080/23311886.2016.1166472. [DOI] [Google Scholar]
- 16.Nkya WM, Gillespie SH, Howlett W, Elford J, Nyamuryekunge C, Assenga C, et al. Sexually transmitted diseases in prostitutes in Moshi and Arusha, Northern Tanzania. Int J STD AIDS. 1991;2:432–435. doi: 10.1177/095646249100200608. [DOI] [PubMed] [Google Scholar]
- 17.Masha SC, Wahome E, Vaneechoutte M, Cools P, Crucitti T, Sanders EJ. High prevalence of curable sexually transmitted infections among pregnant women in a rural county hospital in Kilifi, Kenya. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottesman T, Yossepowitch O, Samra Z, Rosenberg S, Dan M. Prevalence of Mycoplasma genitalium in men with urethritis and in high risk asymptomatic males in Tel Aviv: a prospective study. Int J STD AIDS. 2017;28:127–132. doi: 10.1177/0956462416630675. [DOI] [PubMed] [Google Scholar]
- 19.Hokororo A, Kihunrwa A, Hoekstra P, Kalluvya SE, Changalucha JM, Fitzgerald DW, et al. High prevalence of sexually transmitted infections in pregnant adolescent girls in Tanzania: a multi-community cross-sectional study. Sex Transm Infect. 2015;91:473–478. doi: 10.1136/sextrans-2014-051952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassa ZY, Hussen S, Hadra N, Moges Y, Bonja F. Prevalence of Neisseria gonorrhoeae infection among women of reproductive age in sub-Saharan Africa: a systematic review and meta-analysis. Eur J Contracept Reprod Health Care. 2020;25:365–371. doi: 10.1080/13625187.2020.1779688. [DOI] [PubMed] [Google Scholar]
- 21.Masha SC, Cools P, Descheemaeker P, Reynders M, Sanders EJ, Vaneechoutte M. Urogenital pathogens, associated with Trichomonas vaginalis, among pregnant women in Kilifi, Kenya: a nested case-control study. BMC Infect Dis. 2018;18:549. doi: 10.1186/s12879-018-3455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapiga SH, Sam NE, Mlay J, Aboud S, Ballard RC, Shao JF, et al. The epidemiology of HIV-1 infection in northern Tanzania: results from a community-based study. AIDS Care. 2006;18:379–387. doi: 10.1080/09540120500465012. [DOI] [PubMed] [Google Scholar]
- 23.Ramadhani MY, Mirambo MM, Mbena H, Kihunrwa A, Mshana SE. High prevalence of Chlamydia trachomatis infection among infertile women in Mwanza city, Tanzania: a need to introduce screening and treatment programme. Sex Transm Infect. 2017;93:111. doi: 10.1136/sextrans-2016-052795. [DOI] [PubMed] [Google Scholar]
- 24.Van Der Pol B, Ferrero DV, Buck-Barrington L, Hook E, III, Lenderman C, Quinn T, et al. Multicenter evaluation of the BDProbeTec ET system for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J Clin Microbiol. 2001;39:1008–1016. doi: 10.1128/JCM.39.3.1008-1016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schachter J, McCormack WM, Chernesky MA, Martin DH, Van Der Pol B, Rice PA, et al. Vaginal swabs are appropriate specimens for diagnosis of genital tract infection with Chlamydia trachomatis. J Clin Microbiol. 2003;41:3784–3789. doi: 10.1128/JCM.41.8.3784-3789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shafer MA, Moncada J, Boyer CB, Betsinger K, Flinn SD, Schachter J. Comparing first-void urine specimens, self-collected vaginal swabs, and endocervical specimens to detect Chlamydia trachomatis and Neisseria gonorrhoeae by a nucleic acid amplification test. J Clin Microbiol. 2003;41:4395–4399. doi: 10.1128/JCM.41.9.4395-4399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pokharel S, Adhikari B. Antimicrobial resistance and over the counter use of drugs in Nepal. J Glob Health. 2020;10 doi: 10.7189/jogh.10.010360. 010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiesenfeld HC, Manhart LE. Mycoplasma genitalium in women: current knowledge and research priorities for this recently emerged pathogen. J Infect Dis. 2017;216:S389–S395. doi: 10.1093/infdis/jix198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juliana NCA, Deb S, Ouburg S, Chauhan A, Pleijster J, Ali SM, et al. The prevalence of chlamydia trachomatis and three other non-viral sexually transmitted infections among pregnant women in Pemba Island Tanzania. Pathogens. 2020;9:625. doi: 10.3390/pathogens9080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zenebe MH, Mekonnen Z, Loha E, Padalko E. Prevalence, risk factors and association with delivery outcome of curable sexually transmitted infections among pregnant women in southern Ethiopia. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sumati AH, Saritha NK. Association of urinary tract infection in women with bacterial vaginosis. J Glob Infect Dis. 2009;1:151–152. doi: 10.4103/0974-777X.56254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutasingwa LV, Mbirigenda SK. MEASURE Evaluation; Dar es Salaam: 2017. Investigating risky sexual behaviours among youth in the context of the HIV epidemic in Mbeya Region, Tanzania. [Google Scholar]
- 33.Scheidell JD, Beau De Rochars VMB, Séraphin MN, Hobbs MM, Morris JG, Jr, Célestin JP, et al. Socioeconomic vulnerability and sexually transmitted infection among pregnant Haitian women. Sex Transm Dis. 2018;45:626–631. doi: 10.1097/OLQ.0000000000000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blaskewicz CD, Pudney J, Anderson DJ. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod. 2011;85:97–104. doi: 10.1095/biolreprod.110.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.