Abstract
Direct revascularization of critical ischemia of the limb is often unsuccessful due to the anatomic extent and distribution of arterial occlusive disease, and no pharmacologic treatment has proved effective in treating this condition. Patients with ischemic limb may eventually require amputation and may develop serious morbidity and mortality. The goal of limb salvage in these patients has stimulated research into alternative treatment methods, including angiogenesis.
Attempts have been made to apply growth factors directly or to encode DNA for such factors, but it is unknown whether these factors remain at the target site long enough to be effective. We report our strategy of using vascular endothelial growth factor in a fibrin network, which enables the sustained release of biologic material at the target site.
Key words: Endothelial growth factors/therapeutic use; ischemia/therapy; leg/blood supply; neovas-cularization, physiologic/drug effects
Epidemiologic studies report that critical ischemia of the limb develops in approximately 500 to 1000 people per million per year. 1 Direct revascularization may be unsuccessful in most of these cases due to the anatomic extent and distribution of arterial occlusive disease. 2,3 No pharmacologic treatment has been shown to alter the natural history of critical ischemia of the leg. 4 Patients with this condition may eventually require amputation and may develop serious morbidity and mortality. 5,6 The goal of limb salvage in this group of patients has stimulated research into alternative treatment methods, including angiogenesis. 7–9
In angiogenesis, vascular cells proliferate and form new capillary structures. 10 Ischemic tissue injury causes the release of endogenous biochemical agents, which include growth factors that stimulate angiogenesis and growth of the collateral vessels. 11–13 It is also known that under pathologic conditions in which blood flow is limited by stenosis in the vascular system, the heart or any other organ is capable of growing new arteries, provided that the vascular narrowing or occlusion does not proceed too quickly. However, this physiologic response to pathophysiologic processes is often inadequate to prevent clinical manifestations of ischemia.
Attempts have been made to apply growth factors directly or to encode DNA for such factors, 14,15 but it is unknown whether these factors remain at the target site long enough to be effective.
A fibrin network is critical for effective wound healing, and it is biodegradable through routine tissue fibrinolysis. Since fibrin sealant is lysed slowly, it can serve as a vehicle to deliver various agents that may act to help heal wounds, 16–24 to promote new vessel growth, 17,25 or to store and slowly release antibiotics and other therapeutic agents. 19 Following balloon injury in an experimental animal, we demonstrated the possibility of reconstructing the arterial wall using fibrin sealant to deliver endothelial cells. 26
In this case report, we present an angiogenic strategy that uses vascular endothelial growth factor (VEGF) in a fibrin network, which enables the sustained release of biologic material at the target site.
Case History
In July 1998, a 66-year-old man was referred to us because he was experiencing pain in both legs on minimal exertion (walking 15 feet) and sometimes at rest. Treatment with analgesics and pentoxifylline provided incomplete relief. He was also being treated for hypertension and coronary artery disease. He had no history of diabetes, smoking, or cerebrovascular disease.
Physical examination revealed no carotid bruit, and cardiovascular examination was normal. Femoral pulses were palpable, but the popliteal pulse was feeble, and the dorsalis pedis pulses were absent. Noninvasive testing showed moderate reduction (0.60) of the ankle-brachial index (ABI) on the right leg at rest, moderate digital ischemia of the right 1st, 2nd, 3rd, and 4th toes, and severe ischemia of the right 5th toe. Peripheral angiography revealed bilateral occlusion of the superficial femoral artery (SFA) and diffuse distal disease of the arteries of the right lower extremity (Fig. 1A). Under the compassionate-use protocol of the Human Research Review Board of the Medical College of Wisconsin, the patient was deemed appropriate for angiogenic treatment.
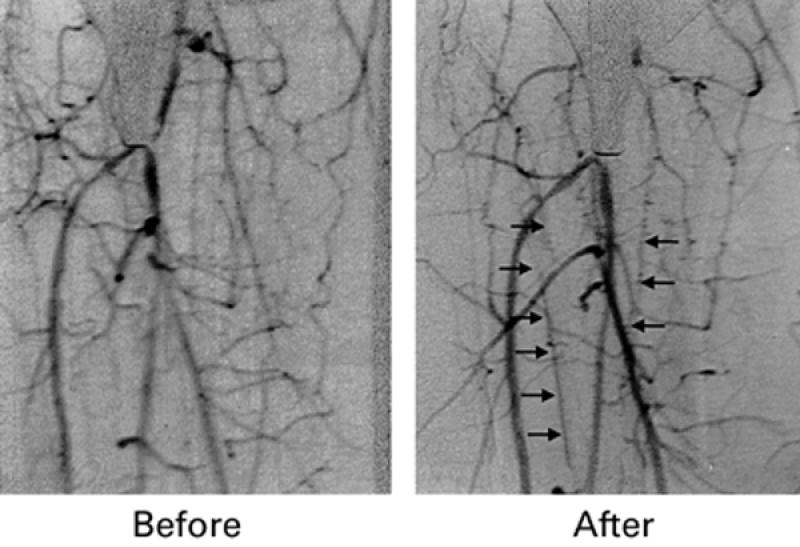
Fig. 1 Angiogram of a patient with critical limb ischemia before treatment (A) and 3 months following treatment (B). Note vessel growth and better visualization of peripheral arteries.
The procedure was performed on an outpatient basis. Intramuscular application of VEGF and modified fibrin glue was carried out with a transdermal needle attached to a FibriJet™ dual syringe system (Micro-medics Inc.; Eagan, Minn). The patient was given diazepam, and 5 mL of lidocaine was administered topically. We then administered the following fibrin glue composition: 5,000 U of thrombin (Parke Davis; Morris Plains, NJ); 10 mL of cryoprecipitate (prepared at the Blood Center of Southeastern Wisconsin, Milwaukee, Wis); 100 μg of deferoxamine (Ciba; Summit, NJ); and 100 μg of VEGF (Intergen; New York, NY). This cocktail was injected into the right popliteal region, close to the popliteal artery.
Two weeks after the procedure, the patient was feeling better and could walk approximately 500 feet without experiencing significant pain, a more than thirty-fold increase of walking distance. Clinical examination remained unchanged, and the patient's need for analgesics decreased at this time. The patient was followed up once every 4 weeks thereafter. His ABI at 4 weeks was 0.69, a slight increase over the prior examination; he had residual arterial ischemia of the right 1st digit but not of the other 4.
Twelve weeks later, the patient no longer required any analgesics for his claudication pain, and his exercise tolerance had improved remarkably. Four months after treatment, angiography revealed substantial growth of new vessels in the infra-popliteal segment at the site where growth factor and fibrin had been injected (Fig. 1B). Noninvasive ABI measurements performed 20 weeks after the procedure showed significant increase (0.75) in comparison with the preprocedural ABI.
Discussion
Several studies have shown that angiogenic growth factors are effective in experimental models of myo-cardial ischemia 27 and hind-limb ischemia. 28,29 Moreover, VEGF was used in clinic to treat critical limb ischemia 30,31 and advanced coronary artery disease. Providing further support for the concept, Isner and colleagues 30 reported improvement of peripheral arterial ischemia after they used a hydrogel-coated balloon catheter to transfect the peripheral arteries with cDNA encoding VEGF. Subsequently, the same group initiated intramuscular injection of VEGF165-encoding naked human plasmid cDNA in 9 patients (10 ischemic limbs). 31 In this initial series of intramuscular (rather than intra-arterial) injections of an angiogenic gene, blood flow improved in 8 of 10 limbs, as demonstrated by magnetic resonance angiography, and there was an overall increase of 30% in ABI, a key measure of improved limb blood supply. Patients were excluded if they had conditions that might be associated with untoward sequelae of “inappropriate angiogenesis,” such as cancer or diabetic retinopathy.
Isner's group 32 also injected naked plasmid DNA directly into the myocardium through a minimally invasive incision in the chest wall. They concluded that the procedure is safe and may lead to reduced symptoms and improved myocardial perfusion in selected patients with chronic myocardial ischemia.
Despite this very encouraging early experience, it is not known whether new collaterals will have hemodynamic significance. Recent trials using VEGF, recombinant fibroblast growth factor-2 (FGF-2), and basic fibroblast growth factor (bFGF) demonstrate minimal or no improvement of myocardial perfusion following angiogenic procedures. 33–36
Recently, Terai and colleagues 37 presented data suggesting that VEGF may play a role in the pathogenesis of acute Kawasaki syndrome. Indeed, the 1st peak of VEGF levels is observed in the early stage of the illness, when skin rash, edema of the extremities, or both, are present. Studies of skin histopathology in acute Kawasaki syndrome demonstrate vascular dilatation with endothelial gap formation and sub-endothelial edema. 38,39 Pathologic examination has also demonstrated that coronary artery vasculitis is present in all patients with Kawasaki syndrome. 38 Other studies have demonstrated that there is endothelial injury and adventitial edema in the coronary arteries of patients with Kawasaki syndrome. 39 Moreover, recent studies have documented abnormal endothelial responses in patients who have Kawasaki syndrome and normal coronary anatomy. 40
In view of current clinical studies involving the use of VEGF and related growth factors in the treatment of diffuse coronary artery disease, these data raise questions regarding the safety of the therapeutic angiogenesis procedure: could VEGF therapy possibly induce further damage to coronary circulation? Additional studies are needed to investigate the safety of introducing VEGF into ischemic tissue. It is possible that concomitant anti-inflammatory therapy or use of delivery vehicles that localize the effect of VEGF 18,41,42 could make this therapy safer. Last, the possibility that VEGF accelerates atherosclerosis should be investigated. 43
We believe that fibrin glue enhances the effect of VEGF. Even without the addition of growth factors, fibrin glue applied subcutaneously has been shown to stimulate angiogenesis. 18,44 Experiments demonstrate that the glue becomes vascularized (Fig. 2), indicating that plasma proteins alone can perform some of the functions of the extracellular matrix involved in anchoring endothelial cells to the vascular wall. As described above in regard to ischemic tissue, the fibrin glue serves as a temporary matrix for the gradual development of granulation tissue that is characterized by a high degree of vascularity. Intravascular administration of growth factors without fibrin glue can stimulate angiogenesis in some animal models, 15,45–47 but not in others, 48 which further supports the value of adding the glue.
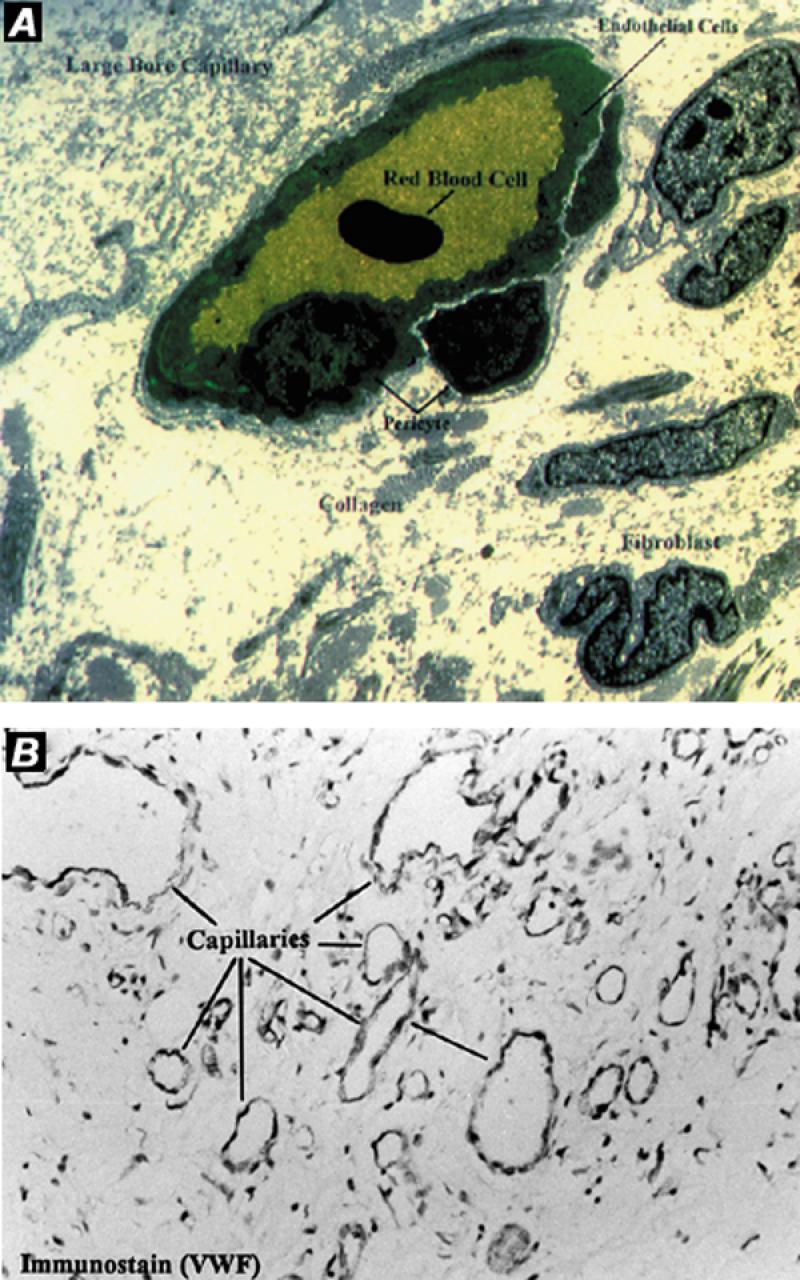
Fig. 2 Angiogenesis in an experimental model of dynamic cardiomyoplasty in a sheep. Fibrin glue was used to form a provisional reservoir between the myocardial wall and transplanted skeletal muscle. A) Transmission electron micrograph. Note neocapillaries and arterioles formed in a fibrin network. B) Immunohistochemical micrographs of latissimus dorsi muscle show evidence of neovascularization.
Fasol and colleagues 17 demonstrated that implantation of modified fibrin glue containing the angiogenic growth factor bFGF induced significant site-directed formation of new blood vessel structures in a rat model.
Laube and colleagues* applied human recombinant basic fibroblast growth factor in a fibrin matrix directly to the epicardium during conventional bypass grafting in 8 patients with diffuse coronary artery disease. Follow-up angiography and thallium scans demonstrated induction of a collateral network of capillaries.
Thrombin and fibrinogen obtained from animal sources can lead to antibody production. 49–51 This problem would be eliminated by the use of human fibrinogen. Although use of human fibrinogen carries a theoretical risk of disease transmission, a summary of several European studies has shown no evidence of human immunodeficiency virus or hep-atitis transmission in over 1,000,000 patients. 52 Alternatively, use of autologous cryoprecipitate 53 or recombinant DNA 54 would provide safe sources of fibrin meshwork.
We have previously demonstrated that VEGF and fibrin accelerated the wound healing process in a patient who had bilateral ischemic gangrene of the feet. Unfortunately, the patient still required bilateral below-knee amputation; however, this was less extensive and severe than anticipated before the protocol. 42
In the case reported here, it is clear that there was substantial improvement in symptoms and, consequently, in the patient's quality of life. We have objectively demonstrated by angiography the growth of new blood vessels after treatment with VEGF and fibrin. The fibrin glue aids in the slow release of the growth factor and thus prolongs its availability, sustaining angiogenesis and improving oxygen supply. We propose that this strategy can be used as a possible therapeutic intervention in the management of limb ischemia by inducing growth of new blood vessels. Further clinical study appears warranted.
Footnotes
*Personal communication to senior author (NK).
Address for reprints: Nicholas Kipshidze, MD, PhD, Lennox Hill Hospital, Interventional Cardiology, 9th Floor, 130 77th Street, New York, NY 10021
These studies were supported in part by a research grant from A. Ward Ford Memorial Institute.
References
- 1.Second European Consensus Document on chronic critical leg ischemia. Circulation 1991;84(4 Suppl):IV1–26. [PubMed]
- 2.Dormandy J, Mahir M, Ascady G, Balsano F, De Leeuw P, Blombery P, et al. Fate of the patient with chronic legischaemia. A review article. J Cardiovasc Surg (Torino) 1989;30:50–7. [PubMed]
- 3.Isner JM, Rosenfield K. Redefining the treatment of peripheral artery disease. Circulation 1993;88:1534–57. [DOI] [PubMed]
- 4.Isner JM, Rosenfield K. Redefining the treatment of peripheral artery disease. Role of percutaneous revascularization. Circulation 1993;88(4 Pt 1):1534–57. [DOI] [PubMed]
- 5.Gregg RO. Bypass or amputation? Concomitant review of bypass arterial grafting and major amputations. Am J Surg 1985;149:397–402. [DOI] [PubMed]
- 6.Ouriel K, Fiore WM, Geary JE. Limb-threatening ischemia in the medically compromised patient: amputation or revascularization? Surgery 1988;104:667–72. [PubMed]
- 7.Graham AM, Schniederman A, Jothy S, Homan J, Symes JF. Staged reversal of venous flow for revascularization of the severely ischemic limb. J Surg Res 1983;35:11–20. [DOI] [PubMed]
- 8.Goldsmith HS, Griffith AL, Catsimpoolas N. Increased vascular perfusion after administration of an omental lipid fraction. Surg Gynecol Obstet 1986;162:579–83. [PubMed]
- 9.Pevec WC, Hendricks D, Rosenthal MS, Shestak KC, Steed DL, Webster MW. Revascularization of an ischemic limb by use of a muscle pedicle flap: a rabbit model. J Vasc Surg 1991;13:385–90. [DOI] [PubMed]
- 10.D'Amore PA, Thompson RW. Mechanisms of angiogenesis. Annu Rev Physiol 1987;49:453–64. [DOI] [PubMed]
- 11.Engler DA. Use of vascular endothelial growth factor for therapeutic angiogenesis. Circulation 1996,94:1496–8. [DOI] [PubMed]
- 12.Shou M, Thirumurti V, Rajanayagam S, Lazarous DF, Hodge E, Stiber JA, et al. Effect of basic fibroblast growth factor on myocardial angiogenesis in dogs with mature collateral vessels. J Am Coll Cardiol 1997;29:1102–6. [DOI] [PubMed]
- 13.Thompson JA, Anderson KD, DiPietro JM, Zwiebel JA, Zametta M, Anderson WF, et al. Site-directed neovessel formation in vivo. Science 1988;241:1349–52. [DOI] [PubMed]
- 14.Bauters C, Asahara T, Zheng LP, Takeshita S, Bunting S, Ferrara N, et al. Site-specific therapeutic angiogenesis after systemic administration of vascular endothelial growth factor. J Vasc Surg 1995;21:314–25. [DOI] [PubMed]
- 15.Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow ST, Passeri J, et al. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation 1996;94:3281–90. [DOI] [PubMed]
- 16.Kipshidze N, Ferguson J, Macris MP, Clubb F, Cloy M, Horn J, et al. Percutaneous application of fibrin sealant to achieve hemostasis following arterial catheterization. J Invas Cardiol 1998;10:133–41. [PubMed]
- 17.Fasol R, Schumacher B, Schlaudraff K, Hauenstein KH, Seitelberger R. Experimental use of a modified fibrin glue to induce site-directed angiogenesis from the aorta to the heart. J Thorac Cardiovasc Surg 1994;107:1432–9. [PubMed]
- 18.Chekanov V, Nikolaychik V, Tchekanov G. The use of biologic glue for better adhesions between the skeletal muscle flap and the myocardium and for increasing capillary ingrowth. J Thorac Cardiovasc Surg 1996;111:678–80. [DOI] [PubMed]
- 19.Boyce ST, Holder IA, Supp AP, Warden GD, Greenhalgh DG. Delivery and activity of antimicrobial drugs released from human fibrin sealant. J Burn Care Rehabil 1994;15: 251–5. [DOI] [PubMed]
- 20.Deyerling W, Haverich A, Potel J, Hetzer R. A suspension of fibrin glue and antibiotic for local treatment of mycotic aneurysms in endocarditis—an experimental study. Thorac Cardiovasc Surg 1984;32:369–72. [DOI] [PubMed]
- 21.Zilch H, Lambiris E. The sustained release of cefotaxim from a fibrin-cefotaxim compound in treatment of osteitis. Pharmacokinetic study and clinical results. Arch Orthop Trauma Surg 1986;106:36–41. [DOI] [PubMed]
- 22.Haverich A, Hirt S, Karck M, Siclari F, Wahlig H. Prevention of graft infection by bonding of gentamycin to Dacron prostheses. J Vasc Surg 1992;15:187–93. [DOI] [PubMed]
- 23.Kabuto M, Kubota T, Kobayashi H, Nakagawa T, Arai Y, Kitai R. Experimental study of intraoperative local chemo-therapy with fibrin glue containing nitrosourea for malignant gliomas. Surg Neurol 1995;44:151–7. [DOI] [PubMed]
- 24.Lasa C Jr, Hollinger J, Drohan W, MacPhee M. Delivery of demineralized bone powder by fibrin sealant. Plast Reconstr Surg 1995;96:1409–18. [DOI] [PubMed]
- 25.Kang SS, Gosselin C, Ren D, Greisler HP. Selective stimulation of endothelial cell proliferation with inhibition of smooth muscle cell proliferation by fibroblast growth factor-1 plus heparin delivered from fibrin glue suspensions. Surgery 1995;118:280–7. [DOI] [PubMed]
- 26.Baker JE, Nikolaychik V, Sahota H, Keelan MH, Komarowski R, Keane SD, et al. Reconstruction of balloon-injured artery with fibrin glue/endothelial cell matrix. Circulation 1994;90;I–492.
- 27.Yanagisawa-Miwa A, Uchida Y, Nakamura F, Tomaru T, Kido H, Kamijo T, et al. Salvage of infarcted myocardium by angiogenic action of basic fibroblast growth factor. Science 1992;257:1401–3. [DOI] [PubMed]
- 28.Baffour R, Berman J, Garb JL, Rhee SW, Kaufman J, Friedmann P. Enhanced angiogenesis and growth of collaterals by in vivo administration of recombinant basic fibroblast growth factor in a rabbit model of acute lower limb ischemia: dose-response effect of basic fibroblast growth factor. J Vasc Surg 1992;16:181–91. [PubMed]
- 29.Pu LQ, Sniderman AD, Arekat Z, Graham AM, Brassard R, Symes JF. Angiogenic growth factor and revascularization of this ischemic limb: evaluation in a rabbit model. J Surg Res 1993;54:575–83. [DOI] [PubMed]
- 30.Isner JM, Walsh K, Symes J, Pieczek A, Takeshita S, Lowry J, et al. Arterial gene therapy for therapeutic angiogenesis in patients with peripheral artery disease. Circulation 1995; 91:2687–92. [DOI] [PubMed]
- 31.Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation 1998;97:1114–23 [DOI] [PubMed]
- 32.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation 1998:98;2800–4. [DOI] [PubMed]
- 33.Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordono FJ, Simons M, et al. Results of Intracoronary Recombinant Human Vascular Endothelial Growth Factor (rhVEGF) Administration Trial [abstract]. J Am Coll Cardiol 1998;31:65A.
- 34.Henry TD, Annex BH, Azrin MA, McKendall GR, Willerson JT, Hendel RC, et al. Double blind, placebo controlled trial of recombinant human vascular endothelial growth factor–The VIVA Trial [abstract]. J Am Coll Cardiol 1999; 33:384A.
- 35.Laham RJ, Leimbach M, Chronos NA, Vansant JP, Pearlman JD, Pettigrew R, et al. Intracoronary administration of Recombinant Fibroblast Growth Factor-2 (rFGF-2) in patients with severe coronary artery disease: Results of Phase I [abstract]. J Am Coll Cardiol 1999;33:383A.
- 36.Laham RJ, Sellke FW, Edelman ER, Pearlman JD, Ware JA, Brown DL, Gold JP, Simons M. Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary bypass surgery: results of a phase I randomized, double-blind, placebo-controlled trial. Circulation 1999;100(18):1865–71. [DOI] [PubMed]
- 37.Terai M, Yasukawa K, Narumoto S, Tateno S, Oana S, Kohno Y. Vascular endothelial growth factor in acute Kawasaki disease. Am J Cardiol 1999;83:337–9. [DOI] [PubMed]
- 38.Hirose S, Hamashima Y. Morphological observations on the vasculitis in the mucocutaneous lymph node syndrome. A skin biopsy study of 27 patients. Eur J Pediatr 1978;129: 17–27. [DOI] [PubMed]
- 39.Fujiwara H, Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics 1978;61:100–7. [PubMed]
- 40.Yamakawa R, Ishii M, Sugimura T, Akagi T, Eto G, Iemura M, et al. Coronary endothelial dysfunction after Kawasaki disease: evaluation by intracoronary injection of acetylcholine. J Am Coll Cardiol 1998;31:1074–1080. [DOI] [PubMed]
- 41.Mack CA, Patel SR, Schwarz EA, Zanzonico P, Hahn RT, Ilercil A, et al. Biologic bypass with the use of adenovirus-mediated gene transfer of the complementary deoxyribonucleic acid for vascular endothelial growth factor 121 improves myocardial perfusion and function in the ischemic porcine heart. J Thorac Cardiovasc Surg 1998;115: 168–77. [DOI] [PMC free article] [PubMed]
- 42.Kipshidze N, Chekanov V, Kappes S, Abdurahman A, Hammen D, Chawla PS, et al. Therapeutic angiogenesis for critical limb ischemia to limit or avoid amputation. J Invas Cardiol 1999;11:25–8. [PubMed]
- 43.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation 1999;99: 1726–32. [DOI] [PubMed]
- 44.Chekanov VS, Tchekanov GV, Rieder MA, Eisenstein R, Wankowski DM, Schmidt DH, et al. Biologic glue increases capillary growth after cardiomyoplasty in an ischemic cardiomyopathy model. ASAIO J 1996;42:M480–7. [DOI] [PubMed]
- 45.Banai S, Jaklitsch MT, Casscells W, Shou M, Shrivastav S, Correa R, et al. Effects of acidic fibroblast growth factor on normal and ischemic myocardium. Circ Res 1991;69: 76–85. [DOI] [PubMed]
- 46.Baffour R, Berman J, Garb JL, Rhee SW, Kaufman J, Friedmann P. Enhanced angiogenesis and growth of collaterals by in vivo administration of recombinant basic fibroblast growth factor in a rabbit model of acute lower limb ischemia: dose-response effect of basic fibroblast growth factor. J Vasc Surg 1992;16:181–91. [PubMed]
- 47.Rivard A, Isner M. Angiogenesis and vasculogenesis in treatment of cardiovascular disease. Mol Med 1998;4:429–40. [PMC free article] [PubMed]
- 48.Folkman J, Shing Y. Angiogenesis. J Biol Chem 1992;267: 10931–4. [PubMed]
- 49.Nichols WL, Daniels TM, Fisher PK, Owen WG, Pineda AA, Mann KG. Antibodies to bovine thrombin and coagulation factor V associated with surgical use of topical bovine thrombin or fibrin “glue”: A frequent finding [abstract]. Blood 1993;82(Suppl 1):59a.
- 50.Israels SJ, Israels ED. Development of antibodies to bovine and human factor V in two children after exposure to topical bovine thrombin. Am J Pediatr Hematol Oncol 1994; 16:249–54. [DOI] [PubMed]
- 51.Costa JM, Fiessinger JN, Capron L, Aiach M. Partial characterization of an autoantibody recognizing the secondary binding site(s) of thrombin in a patient with recurrent spontaneous arterial thrombosis. Thromb Haemost 1992; 67:193–9. [PubMed]
- 52.Gibble JW, Ness PM. Fibrin glue: the perfect operative sealant? Transfusion 1990;30:741–7. [DOI] [PubMed]
- 53.Oz MC, Jeevanandam V, Smith CR, Williams MR, Kaynar AM, Frank RA, et al. Autologous fibrin glue from intraoperatively collected platelet-rich plasma. Ann Thorac Surg 1992;53:530–1. [DOI] [PubMed]
- 54.Prunkard D, Cottingham I, Garner I, Bruce S, Dalrymple M, Lasser G, et al. High-level expression of recombinant human fibrinogen in the milk of transgenic mice. Nat Biotechnol 1996;14:867–71. [DOI] [PubMed]


