Abstract
Aim:
We performed a systematic review and meta-analysis to identify the prevalence of small intestinal bacterial overgrowth (SIBO) in patients with gastroparesis.
Background:
Several studies have suggested an association between SIBO and gastroparesis, which is characterized by delayed gastric emptying in the absence of mechanical obstruction.
Methods:
A comprehensive search was performed using MEDLINE, EMBASE, Scopus and Cochrane Central Register of Controlled Trials (CENTRAL) through January, 2022 for randomized controlled trials and observational studies reporting the prevalence of SIBO in gastroparesis. Pooled prevalence was estimated using a random effects model. Heterogeneity was assessed by using the inconsistency index (I2).
Results:
Among the 976 articles identified, 43 studies were selected for full text review. Six studies, with 385 patients, were deemed eligible for inclusion, with a perfect agreement between investigators (kappa=1.0). Overall, 379 patients were diagnosed with gastroparesis by gastric emptying scintigraphy and six were diagnosed with a wireless motility capsule. The pooled prevalence of SIBO was 41% (95% confidence interval 0.23-0.58). SIBO was diagnosed using jejunal aspirate cultures (N=15, 8.4%), lactulose breath test (N=80, 44.7%), glucose breath test (N=30, 16.8%), D-xylose breath test (N=52, 29.1%), and hydrogen breath test (N=2, 1.1%). Heterogeneity was significant and noted to be high at 91%. Only one study reported SIBO diagnosis in controls, therefore no pooled odds ratio was calculated.
Conclusion:
SIBO was present in almost half of the patients with gastroparesis. Future studies should examine and identify the association between SIBO and gastroparesis.
Key Words: Gastroparesis, SIBO, Motility, Gut-brain axis
Introduction
Gastroparesis is characterized by episodes of postprandial nausea, early satiety, postprandial fullness, dyspepsia, and bloating (1) in the absence of mechanical obstruction (2) Gastroparesis most commonly occurs due to diabetes, postsurgical, neuropathic, and myopathic causes (3). However, almost 40% of cases have an unknown cause, which is considered an "idiopathic gastroparesis" and is currently being studied (4). Recently, an investigation estimated the prevalence of gastroparesis as 0.16% in the U.S. population (5). Furthermore, the prevalence among women was shown to be approximately 38 per 100,000 individuals and 10 per 100,000 individuals in men (6).
Symptoms attributable to gastroparesis, such as bloating, malnutrition, and weight loss, may overlap with other conditions such as small intestinal bacterial overgrowth (SIBO) (7). SIBO encompasses a spectrum of clinical symptoms and signs such as bloating, flatulence and/or diarrhea along with laboratory findings of increased numbers of bacteria in the small intestine (1, 8–14). The clinical profile of a patient diagnosed with SIBO is difficult to define and can usually be confused with other conditions (14, 15).
SIBO has been associated with disorders of protective antibacterial mechanisms, anatomical abnormalities, and motility disorders (such as scleroderma, autonomic neuropathy in diabetes mellitus, post-radiation enteropathy and small intestinal pseudo-obstruction) (7). Delays in bowel transit time may predispose to bacterial overgrowth, however it is not clear if the same pathophysiology occurs with altered stomach motility seen in gastroparesis (1, 13, 14). In addition, the discomfort of SIBO may overlap with the clinical course of gastroparesis, which may lead to further gastrointestinal motility issues and a worsening of the clinical course (14). The prevalence of SIBO in patients with gastroparesis has been scarcely reported worldwide with percentages ranging from 9.5 to 60%, and there is scarce literature reporting an association between SIBO and gastroparesis (1, 10–14). To determine the association between SIBO and gastroparesis and further characterize the risk factors, we performed a comprehensive systematic review and meta-analysis to identify the prevalence of SIBO in patients with gastroparesis.
Methods
Design and eligibility criteria
This systematic review was performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement (16). In this meta-analysis we included all randomized controlled trials (RCT) and observational studies reporting the prevalence of SIBO in patients diagnosed with gastroparesis. Gastroparesis and SIBO could be diagnosed using any definition provided by the study. Studies such as commentaries, conference abstracts, case reports, experimental in vitro studies and letters to the editor were excluded. There were no language or data limitations to this study. The protocol for this study was conceived during June 2021 but was not registered in a database; however, a preliminary abstract was accepted and presented at the American College of Gastroenterology annual meeting in October 2021.
Search strategy and study selection
A comprehensive search was performed by a medical librarian (MR) using the following databases: MEDLINE (via PubMed), EMBASE, Scopus and Cochrane Central Register of Controlled Trials (CENTRAL). The search was performed from inception to January 2022. The full search strategy is presented in Supplementary Appendix 1. Two researchers (RB and ARM) independently reviewed the titles and abstracts to identify potentially relevant articles for a full-text review. Both reviewers reviewed the full-text of the articles in detail. When required, disagreements were resolved by consensus or by a third party (GC). References of the selected studies were examined to identify any additional relevant studies. Additionally, authors from selected manuscripts were contacted if additional information was required.
Data extraction
The data were extracted independently (RB and ARM). The disparities were resolved by a third party (GC). Data extracted consisted of (i) author, (ii) year of publication, (iii) study design, (iv) location of the study, (v) duration of follow-up, (vi) total number of patients included, (vii) number of patients diagnosed with gastroparesis and SIBO and their respective diagnostic tests (viii) diagnostic test used for gastroparesis and cut off values, (ix) number of patients with SIBO, (x) diagnostic test used for SIBO and cut off values and, (xi) type and amount of substrate used for diagnosis of SIBO and (xii) percentage of SIBO in patients with gastroparesis.
Study quality and certainty of the evidence
Two reviewers (EM and FSPM) assessed each study and determined its quality as poor, fair, or good using the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional (17). The tool includes 14 questions that evaluated the internal validity of each study. A good overall quality rating was registered if all the domains were favorably assessed.
Statistical analysis
The outcome was to estimate the prevalence of SIBO in patients with gastroparesis across all the included studies. The data are expressed as proportions or percentages with the corresponding confidence interval (CI). Statistical significance was set at p-value less than 0.05. Heterogeneity was evaluated using the inconsistency index (I2), and a score < 40% did not represent significant heterogeneity. We considered it appropriate to use a random effects model due to the heterogeneity in the population and study design. Publication bias was analyzed and represented using a funnel plot graph. Finally, the Egger test was used in order to assess asymmetry (18).
Results
Study Selection
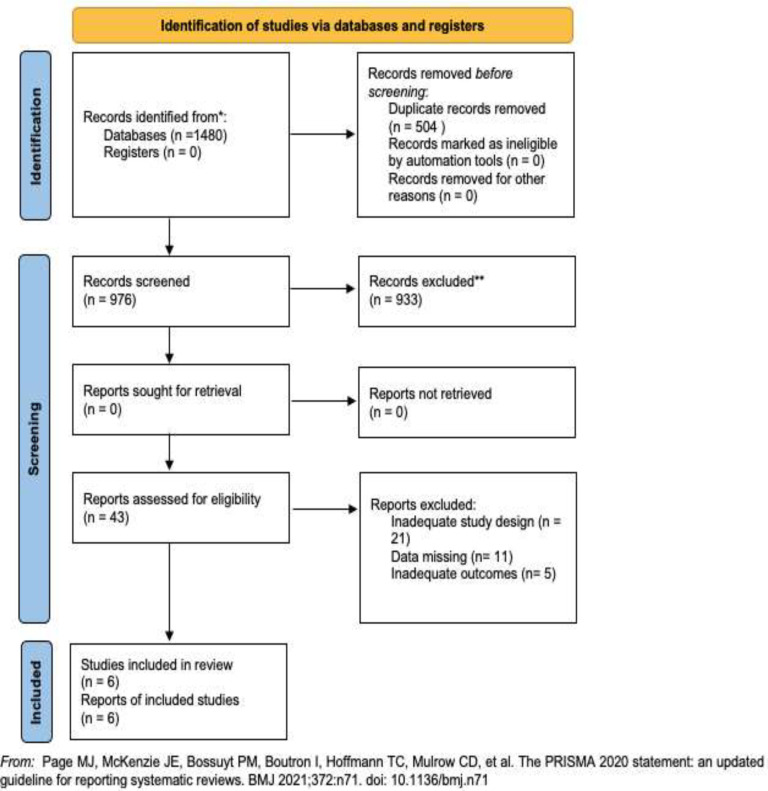
A total of 1480 articles were retrieved and screened. Ultimately, 43 articles were selected for full-text review of which six full-text articles, including 385 patients, were ultimately included in the review. Overall, only observational studies were included. Four were retrospective studies, and two of them were cross-sectional studies (1, 10–14). The flowchart of the study is shown in Figure 1.
Figure 1.
Flowchart of the systematic review search process
Study characteristics
The characteristics of the included studies are summarized in Table 1. All the included studies were published between 1991 and 2021. Five of the studies (1, 11–14) were conducted in the United States, whereas one was conducted in United Kingdom (10). Of the 385 patients with gastroparesis included in the review, 379 (98.4%) were diagnosed with gastroparesis by gastric emptying scintigraphy (GES) and six (1.6%) were diagnosed by wireless motility capsule. The meal of the six patients had 260 kcal, 2% fat and 2 g fiber. SIBO diagnosis was made using jejunal aspirate cultures (N=15, 8.4%), lactulose breath test (N=80, 44.7%), glucose breath test (N=30, 16.8%), D-xylose breath test (N=52, 29.1%), and hydrogen breath test (N=2, 1.1%). In regards the amount of substrate for diagnosis of SIBO, they used 25 g lactulose in 250 ml water (N=79, 44.1%), 50 g Glucose in 150 ml water (N=30, 16.7%), 10 g Lactulose (N=1, 0.5%) or nonabsorbable dietary saccharides (200 g mashed potato, 100 g baked beans and 6.25 g glucose) in 25 ml water (N=2, 1.1%). Only three studies included controls (N= 325) which prevented the calculation of the pooled OR or RR. Patients with non-delayed gastric emptying (N=40, 12.3%), normal GES undergoing lactulose breath test (LBT, N=270, 83%) and healthy volunteers (N=15, 4.6%) were considered as controls (Table 1).
Table 1.
Characteristics of included studies: SIBO in patients with gastroparesis
| Study, year, reference | Study design | Country | Female (%) | Inclusion criteria for patients | Method for Gastroparesis diagnosis | Cut off for Gastroparesis | Etiology of Gastroparesis | Gastroparesis (n) |
Method for SIBO diagnosis | Cut off for SIBO | SIBO (n) |
Controls | Prevalence: n SIBO/tot of patients with Gastroparesis (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Waldron 1991 |
Cross Sectional | UK | 36 (72) | Patients being evaluated for non-ulcerative dyspepsia. Excluded: post-surgical, GERD, IBS, cancer, gallstones |
Gastric emptying scintigraphy (GES) |
Tc labeled scrambled eggs time to 50% of emptying, views were obtained at 5 min interval for 1 hour and then regular intervals until passage > 50% | Idiopathic with dyspepsia symptoms | 21 | Hydrogen/Non absorbable saccharides Breath Test | The presence of a dual peak of breath hydrogen: (the first one >10 ppm) | 2 | Healthy volunteers | 2/21 (9.5%) |
| Reddymasu 2010 |
Retrospective Cohort | USA | 25 (83) | Patients with Gastroparesis on GES with persistent abdominal pain, flatulence and bloating. Excluded: none |
Gastric emptying scintigraphy (GES) |
>10% of radionuclide meal with a standardized caloric content at the end of 4 hours | Diabetes, Idiopathic and Postsurgical | 50 | Glucose Breath Test | 1) Baseline Hydrogen or Methaneconcentration of >15 ppm 2) A rise that exceeded 20 ppm if baseline Hydrogenor Methane <10 ppm 3) A doubling of thebaseline methane | 30 | NA | 30/50 (60%) |
| George 2014 |
Retrospective Cohort | USA | 588 (79.5) | Patient undergoing Lactulose Breath Test (LBT) for evaluation of SIBO and had delayed gastric emptying on GES Excluded: none |
Gastric emptying scintigraphy (GES) |
Tc–sulfur colloid radiolabeled egg meal, delayed ifsolid meal present after either 2 h(60 % retention) or 4 h (10 % retention) | Diabetes, Idiopathic and Postsurgical | 201 | Lactulose Breath Test (LBT) | 1) Breath hydrogen level increase (>20 ppm above baseline)2) Dual breath hydrogen peaks 3) Breath methane increase (>20 ppm over baseline) | 79 | Normal GES undergoing LBT | 79/201 (39.3%) |
| Schatz 2015 |
Cross Sectional | USA | 728 (78.1) | Patients with bloating, chronic abdominal pain, abdominal distension or diarrhea Excluded: none |
Gastric emptying scintigraphy (GES) |
Radiolabeled egg meal, based on national consensus recommendations for GES 2008: imaging, at 0, 1, 2, and 4 h after radiolabeled meal ingestion | Diabetes, Idiopathic and secondary to medications | 74 | D-Xylose Breath Test | A greater than 2 standard deviations rise in CO2 (14C) above normal range at any one or more of the following: 30 min, 60 min or 180 min | 52 | NA | 52/74 (70.3%) |
| Triadafilopoulos 2016 |
Retrospective Cohort | USA | 41 (78.8) | Patients undergoing evaluation for dysphagia, heartburn, acid regurgitation, chest pain and/or belching Excluded: scleroderma, achalasia, history of surgery or endoscopic treatment |
Wireless motility capsule | Gastric emptying time defined as time from capsule ingestion to entry into the alkaline duodenal environment: if > 5 hours was categorized as Gastroparesis | Idiopathic with bloating symptoms | 6 | Lactulose Breath Test | An increase in hydrogen of 20 ppm within 60–90 min and two distinct peaks | 1 | NA | 1/6 (16.7%) |
| Continued | |||||||||||||
| Calderon 2021 |
Retrospective Cohort | USA | 68 (93) | Patients who presented for small bowel enteroscopy for diagnostic evaluation of SIBO. Excluded: previous intake of prebiotics, probiotics or antibiotics. |
Gastric emptying scintigraphy (GES) |
Ingestion of a low-fat solid test meal. Delayed gastric emptying defined as greater than 60% retention at 2 hours or greater than 10% retention at 4 hours | Diabetes, Idiopathic and Postsurgical | 33 | Jejunal Aspirate Culture | Presence of either colonic-type or upper aerodigestive tract (UAT) SIBO | 15 | Non-delayed gastric emptying patients | 15/33 (45.4%) |
Prevalence of SIBO in Gastroparesis
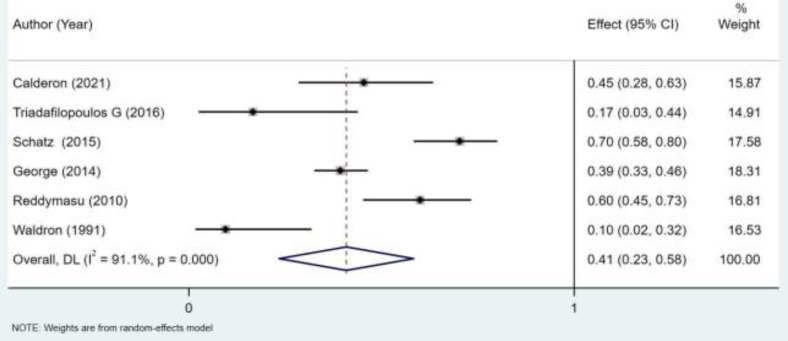
The prevalence of SIBO among patients with gastroparesis ranged from 16.7% to 70.3% among in all studies (1, 10–14). These values varied according to the total number of patients with gastroparesis per study. Regarding the main outcome, the pooled prevalence of SIBO was 41% (95% CI 0.23-0.58) (Figure 2). The two studies that had larger effects on the results were: George (2014) and Schatz (2015) with 18.31% and 17.58% of weight respectively.
Figure 2.
Forest plot of the prevalence of small intestinal bacterial overgrowth (SIBO) in adults with gastroparesis
Quality assessment
An independent researcher used the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies to assess the risk of bias in each study. The tool included 14 questions that evaluated the internal validity of each study. Table 2 shows the results of the quality assessment of the included studies. Three studies were reported as poor quality, due to limitations in adjusting for confounders, implementing the dependent variable in all study participants, having an exact timeframe to evaluate an association. Meanwhile, others were reported to be of fair quality.
Table 1.
Quality assessment of included studies
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Quality rating (good, fair or poor) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Waldron et al | Yes | Yes | NR | Yes | No | No | No | NR | Yes | No | Yes | NA | NR | No | Poor |
| Reddymasu | Yes | Yes | NR | Yes | No | Yes | Yes | NR | Yes | No | NR | NA | Yes | Yes | Fair |
| George | Yes | Yes | NR | Yes | No | Yes | Yes | NR | Yes | No | NR | NA | NR | No | Poor |
| Schatz et al | Yes | Yes | NR | Yes | NR | Yes | Yes | NR | Yes | No | Yes | NR | NR | Yes | Fair |
| Triadafilopoulos et al | Yes | Yes | NR | Yes | No | NR | Yes | NR | NR | No | NR | No | NR | NR | Poor |
| Calderon et al | Yes | Yes | NR | Yes | NR | Yes | Yes | NR | Yes | No | Yes | NR | NR | Yes | Fair |
Q1. Was the research question or objective in this paper clearly stated?
Q2. Was the study population clearly specified and defined?
Q3. Was the participation rate of eligible persons at least 50%?
Q4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants?
Q5. Was a sample size justification, power description, or variance and effect estimates provided?
Q6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured?
Q7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed?
Q8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (eg, categories of exposure, or exposure measured as continuous variable)?
Q9. Were the exposure measures (independent variables) clearly defined, valid, reliable and implemented consistently across all study participants?
Q10. Was the exposure(s) assessed more than once over time?
Q11. Were the outcome measures (dependent variables) clearly defined, valid, reliable and implemented consistently across all study participants?
Q12. Were the outcome assessors blinded to the exposure status of participants?
Q13. Was loss to follow-up after baseline 20% or less?
Q14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)?
NA, not applicable; NR, not reported.
Heterogeneity
Heterogeneity was significant and noted to be high at 91% (Figure 2).
Publication bias
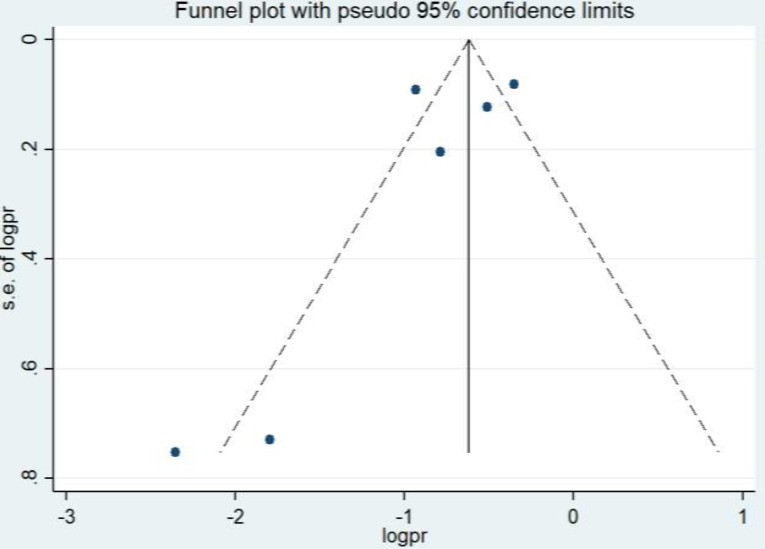
Publication bias analysis was performed and analyzed using a funnel plot (Figure 3). There is an asymmetry distribution of the studies revealing that publication bias was present. In addition, Egger’s test did not show statistical significance (-1.24, p=0.282), which contradicts the funnel plot interpretation; however, a limitation of this test is that it usually has low power as the number of studies are small.
Figure 3.
Publication bias: Funnel plot of the prevalence of small intestinal bacterial overgrowth (SIBO) in patients with gastroparesis
Discussion
The present study is the first systematic review and meta-analysis to assess the prevalence of SIBO among patients with gastroparesis. This study has shown that the pooled prevalence of SIBO in patients diagnosed with gastroparesis is 41%. This implies that almost half the patients with gastroparesis had SIBO.
Previous studies have concluded SIBO prevalence in gastroparesis to range from 40-60%. Schatz et al. reported a SIBO prevalence of 67.5% using xylose breath tests in patients with gastroparesis (12). This prevalence was similar to that reported in a previous study conducted by Reddymasu et al. which reported 60% (30/50) of patients with gastroparesis had SIBO using a glucose breath test (1). The prevalence was lower in a cohort performed by George et al. where 39% of patients with gastroparesis tested positive for SIBO on the lactulose breath test (13). In 2021, a retrospective study performed by Calderon et al. showed a prevalence of 45.5% of SIBO among patients with gastroparesis (14). To the best of our knowledge, this is the first systematic review and meta-analysis evaluating the prevalence of SIBO in patients with gastroparesis.
SIBO is also associated with other comorbidities. In a recent meta-analysis published in 2021, the prevalence of SIBO in patients with diabetes mellitus was found to be at 29% (19). Additionally, the prevalence of SIBO in other gastrointestinal disorders, such as inflammatory bowel disease has been studied in a recent metanalysis from 11 studies (20). The overall prevalence was 22.3%, with a higher prevalence in patients with Crohn´s disease with 25.4% compared to a lower prevalence in those with ulcerative colitis (14.3%) (21). Irritable bowel syndrome has been associated with SIBO (35.5% vs. 29.7% in the controls group); however, the investigators considered the overall quality of the data and evidence as low (21). The risk of and prevalence of SIBO in patients with obesity, nonalcoholic fatty liver disease, and using of proton pump inhibitors, among others, have been also described in recent systematic reviews and with high prevalences among these individuals (22–24). It is important to highlight as well, that SIBO was identified in all the studies with reliable diagnostic tools such as jejunal aspirate culture (which is the gold standard) and using breath tests which have been found in previous studies to have a sensitivity of 62.5% and specificity of 82% with Glucose Breath Test and 52% and 86% with Lactulose Breath Test (25, 26).
Several hypotheses have attempted to explain the association between SIBO and gastroparesis. One of the most widely accepted currently posits is that the delayed small bowel motility transit that allows bacteria to grow and colonize easily in these affected areas, following a pathophysiological pathway similar to that evidenced in the etiologies of patients with gastroparesis, such as diabetes and scleroderma, where SIBO was also found. However, there are no known mechanisms regarding gastric motility itself (1). Another potential mechanism highlights the importance of the phase 3 migratory motor complex (27). These are considered protective between meals and their transit, and their decreased function or absence is associated with the presence of SIBO (28). In addition, previous studies have shown impaired intestinal motility in patients with gastroparesis, regardless of etiology (29). Also, some of the risk factors are common for SIBO and Gastroparesis. In the studies considered for this study, only two of them excluded patients with other GI comorbidities and with previous abdominal surgeries or endoscopic procedures (10, 11). Most of the studies included patients with Gastroparesis caused by type 2 Diabetes which is associated to other metabolic conditions that put you are risk of SIBO. Besides, none of the studies analyzed excluded patient taking medications such as opiates or anticholinergics which can affect motility and also cause delayed gastric emptying (2). This may represent another possible explanation for the co-presence of both etiologies.
Currently, Gastric emptying scintigraphy (GES) is considered the “gold standard” for the diagnosis of gastroparesis given its accuracy and therefore has been recommended by different guidelines (2, 30). This diagnostic study consists of ingesting a technetium-99 labeled meal (low-fat, solid-phase meal) followed by serial gamma camera scans to evaluate the transit of the meal through the upper gastrointestinal tract (31, 32). To differentiate an adequate gastric emptying, imaging is usually performed up to 4 hours after meal consumption (31, 32). Only one study utilized a wireless motility capsule study to distinguish patients with gastroparesis (11). This modality is based on the usage of a wireless ingestible capsule that can measure the pH, pressure and temperature. The timing of the gastric emptying is demonstrated by an abrupt change in pH into the alkaline range after passage into the duodenum (typically a rise in pH from the gastric baseline to >4.0 in the duodenum) (2). The total cutoff used for this testing in order to define delayed gastric emptying is 5 h (31–33). Other less widely used diagnostic modalities such as stable isotope breath testing, electrogastrography, and gastroduodenal manometry were not used in the reviewed studies (32). Recently, manometry has gained more attention because it can also reveal tonic and phasic pressure activity of the pylorus and may provide some information about pyloric dysfunction and lead to pyloric interventions (2, 34).
Strengths and limitations
Our study had some limitations. First, several diagnostic tools were used to determine the presence of SIBO in various studies while only two instruments were used to diagnose gastroparesis. The most commonly used SIBO diagnostic test was the LBT. George et al. (13) and Triadafilopoulos (11) described the sampling process. The two most important gases analyzed were methane and hydrogen. However, the amount of lactulose administered and the cut-off point to determine a positive LBT were different in the two studies. In the first study, the three different criteria were required for a positive LBT. These criteria were as follows: 1) an increase in breath hydrogen level of >20 ppm above baseline by 90 min, 2) an increase in dual breath hydrogen peaks >10 ppm increase over baseline with a decrease of >5 ppm before the second peak of >20 ppm above baseline and 3) an increase in breath methane >20 ppm over baseline by 90 min. In comparison, the second study focused only on the measurement of hydrogen through an elevated breath hydrogen concentration within 90 min, with two distinct peaks and an increase of >20 ppm. Finally, heterogeneity remained high which could be due to the variations in sample size, study design and sampling among the studies. On the other hand, Gastroparesis was diagnosed using the gold standard test which is the GES in five of the six studies included and also using a standardized cut off of 4 hours readings in most of these studies.
Conclusion
In summary, our study is the first systematic review and meta-analysis to report the prevalence of SIBO among patients with gastroparesis. Approximately 41% of patients with gastroparesis may have underlying SIBO; therefore, SIBO should be tested in patients with adequately treated gastroparesis and persistent symptoms. Future studies should further examine and identify the association between SIBO and gastroparesis.
Conflict of interests
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest in the subject matter or materials discussed in this manuscript.
Supplementary Materials
References
- 1.Reddymasu SC, McCallum RW. Small intestinal bacterial overgrowth in gastroparesis: are there any predictors? J Clin Gastroenterol. 2010;44:8–13. doi: 10.1097/MCG.0b013e3181aec746. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Sanders KM. Gastroparesis. Gastroenterology. 2022:162:68–87. doi: 10.1053/j.gastro.2021.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkman HP, Yates K, Hasler WL, Nguyen L, Pasricha PJ, Snape WJ, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101–115. doi: 10.1053/j.gastro.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.So Ykan I, Ri S, Siek S, Kiernan B, Mccallum RW. Demography, clinical characteristics, psychological and abuse Pro® les, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–2404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 5.Syed AR, Wolfe MM, Calles-Escandon J. Epidemiology and diagnosis of gastroparesis in the United States: a population-based study. J Clin Gastroenterol. 2020;54:50–54. doi: 10.1097/MCG.0000000000001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moshiree B, Potter M, Talley NJ. Epidemiology and pathophysiology of gastroparesis. Gastrointest Endosc Clin North Am. 2019;29:1–14. doi: 10.1016/j.giec.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978–2990. doi: 10.3748/wjg.v16.i24.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quigley EMM, Murray JA, Pimentel M. AGA clinical practice update on small intestinal bacterial overgrowth: expert review. Gastroenterology. 2020;159:1526–1532. doi: 10.1053/j.gastro.2020.06.090. [DOI] [PubMed] [Google Scholar]
- 9.Quigley EMM. The spectrum of small intestinal bacterial overgrowth (SIBO) Curr Gastroenterol Rep. 2019;21:3. doi: 10.1007/s11894-019-0671-z. [DOI] [PubMed] [Google Scholar]
- 10.Waldron B, Cullen PT, Kumar R, Smith D, Jankowski J, Hopwood D, et al. Evidence for hypomotility in non-ulcer dyspepsia: a prospective multifactorial study. Gut. 1991;32:246–251. doi: 10.1136/gut.32.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Triadafilopoulos G. Utility of wireless motility capsule and lactulose breath testing in the evaluation of patients with chronic functional bloating. BMJ Open Gastroenterol. 2016;3:000110. doi: 10.1136/bmjgast-2016-000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schatz RA, Zhang Q, Lodhia N, Shuster J, Toskes PP, Moshiree B. Predisposing factors for positive D-Xylose breath test for evaluation of small intestinal bacterial overgrowth: a retrospective study of 932 patients. World J Gastroenterol. 2015;21:4574–4582. doi: 10.3748/wjg.v21.i15.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George NS, Sankineni A, Parkman HP. Small intestinal bacterial overgrowth in gastroparesis. Dig Dis Sci. 2014;59:645–652. doi: 10.1007/s10620-012-2426-7. [DOI] [PubMed] [Google Scholar]
- 14.Calderon G, Siwiec RM, Bohm ME, Nowak T v, Wo JM, Gupta A, et al. Delayed gastric emptying is not associated with a microbiological diagnosis of small intestinal bacterial overgrowth. Dig Dis Sci. 2021;66:160–166. doi: 10.1007/s10620-020-06153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohm M, Siwiec RM, Wo JM. Diagnosis and management of small intestinal bacterial overgrowth. Nutr Clin Pract. 2013;28:289–99. doi: 10.1177/0884533613485882. [DOI] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies-NHLBI, NIH . Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort.
- 18.Rothstein Hannah, Sutton AJ, Borenstein Michael. Publication bias in meta-analysis : prevention, assessment and adjustments. Wiley; 2005. p. 356 . [Google Scholar]
- 19.975 AGING [Internet] 2022. Available from: www.aging-us.com.
- 20.Shah A, Morrison M, Burger D, et al. Systematic review with meta-analysis: the prevalence of small intestinal bacterial overgrowth in inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49:624–635. doi: 10.1111/apt.15133. [DOI] [PubMed] [Google Scholar]
- 21.Shah A, Talley NJ, Jones M, Kendall BJ, Koloski N, Walker MM, et al. Small intestinal bacterial overgrowth in irritable bowel syndrome: a systematic review and meta-analysis of case-control studies. Am J Gastroenterol. 2020;115:190–201. doi: 10.14309/ajg.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 22.Wijarnpreecha K, Werlang ME, Watthanasuntorn K, Panjawatanan P, Cheungpasitporn W, Gomez V, et al. Obesity and risk of small intestine bacterial overgrowth: a systematic review and meta-analysis. Dig Dis Sci. 2020;65:1414–1422. doi: 10.1007/s10620-019-05887-x. [DOI] [PubMed] [Google Scholar]
- 23.Wijarnpreecha K, Lou S, Watthanasuntorn K, Kroner PT, Cheungpasitporn W, Lukens FJ, et al. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2020;32:601–608. doi: 10.1097/MEG.0000000000001541. [DOI] [PubMed] [Google Scholar]
- 24.Su T, Lai S, Lee A, He X, Chen S. Meta-analysis: proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. J Gastroenterol. 2018;53:27–36. doi: 10.1007/s00535-017-1371-9. [DOI] [PubMed] [Google Scholar]
- 25.Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol . 2010;16:2978–2990. doi: 10.3748/wjg.v16.i24.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanjeevi R, Jamwal KD, Dhar Chowdhury S, et al. Assessment of small intestinal bacterial overgrowth in chronic pancreatitis patients using jejunal aspirate culture and glucose hydrogen breath test. Scand J Gastroenterol. 2021;56:588–593. doi: 10.1080/00365521.2021.1900383. [DOI] [PubMed] [Google Scholar]
- 27.Szurszewski JH. A migrating electric complex of the canine small intestine. Am J Physiol. 1969;217:1757–1763. doi: 10.1152/ajplegacy.1969.217.6.1757. [DOI] [PubMed] [Google Scholar]
- 28.Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977;59:1158–1166. doi: 10.1172/JCI108740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camilleri M, Malagelada JR. Abnormal intestinal motility in diabetics with the gastroparesis syndrome. Eur J Clin Invest. 1984;14:420–427. doi: 10.1111/j.1365-2362.1984.tb01206.x. [DOI] [PubMed] [Google Scholar]
- 30.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camilleri M, Chedid V, Ford AC, Haruma K, Horowitz M, Jones KL, et al. Gastroparesis. Nat Rev Dis Primers. 2018;4:41. doi: 10.1038/s41572-018-0038-z. [DOI] [PubMed] [Google Scholar]
- 32.Usai-Satta P, Bellini M, Morelli O, Geri F, Lai M, Bassotti G. Gastroparesis: new insights into an old disease. World J Gastroenterol. 2020;26:2333–2348. doi: 10.3748/wjg.v26.i19.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee AA, Rao S, Nguyen LA, Moshiree B, Sarosiek I, Schulman MI, et al. Validation of diagnostic and performance characteristics of the wireless motility capsule in patients with suspected gastroparesis. Clin Gastroenterol Hepatol. 2019;17:1770–1779. doi: 10.1016/j.cgh.2018.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986;90:1919–1925. doi: 10.1016/0016-5085(86)90262-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.