Abstract
Aim:
The aim of the present study was to conduct a meta-analysis of the frequency of isoniazid-induced liver injury (INH-ILI) in patients receiving isoniazid (INH) preventative therapy (IPT).
Background:
The frequency of hepatotoxicity (drug-induced liver injury: DILI) of antituberculosis drugs has been studied, especially when INH, rifampin, and pyrazinamide are co-administered. However, little is known about the frequency of DILI in patients with latent tuberculosis infection (LTBI), where IPT is indicated.
Methods:
We searched PubMed, Google Scholar, and the Cochrane Database of Systematic Reviews for studies reporting the frequency of INH-ILI in patients with IPT using one or more diagnostic indicators included in the criteria of the DILI Expert Working Group.
Results:
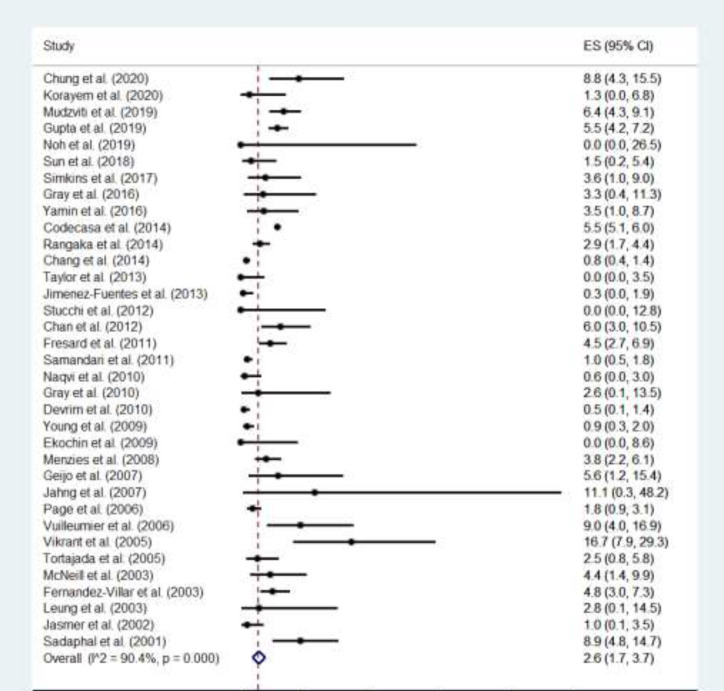
Thirty-five studies comprising a total of 22,193 participants were included. The overall average frequency of INH-ILI was 2.6% (95% CI, 1.7-3.7%). The mortality associated with INH-DILI was 0.02% (4/22193). Subgroup analysis revealed no significant differences in the frequency of INH-ILI in patients older or younger than 50 years, children, patients with HIV, candidates for liver, kidney, or lung transplant, or according to the type of study design.
Conclusion:
The frequency of INH-ILI in patients receiving IPT is low. Studies on INH-ILI are needed where the current DILI criteria are used.
Key Words: Isoniazid, Latent tuberculosis, Drug induced liver injury, Adverse drug reaction, Liver injury
Introduction
Isoniazid (isonicotinic acid hydrazide: INH) was discovered 110 years ago and has been used for the prophylaxis and treatment of tuberculosis (TB) for 70 years (1). Isoniazid preventative therapy (IPT) is indicated in patients with latent tuberculosis infection (LTBI), which is defined as a state of persistent immune response to stimulation by M. tuberculosis antigens with no evidence of clinically manifest active TB (2). IPT is indicated in household contacts of pulmonary TB or multidrug-resistant TB, people who are initiating anti-TNF treatment, receiving dialysis, preparing for an organ or hematological transplant, or living with silicosis (2). Additionally, at-risk LTBI populations where IPT may be indicated include prisoners, healthcare workers, immigrants from countries with a high TB burden, homeless people, people living with HIV, and people who use intravenous drugs (2).
The two most important adverse reactions of INH are hepatotoxicity (drug-induced liver injury: DILI) and neuropathy, the latter being potentially preventable with the administration of pyridoxine. The exact frequency of INH-induced liver injury (INH-ILI) is difficult to determine for three reasons. Firstly, most reports refer to hepatotoxicity induced by combinations of antituberculosis drugs (i.e., when INH is co-administered with other drugs such as rifampin and pyrazinamide, which are also potentially hepatotoxic) (3). One strategy for determining the specific frequency of INH-ILI is the administration of INH alone, that is, as IPT for 6 to 9 months (4). Secondly, various criteria have been used over time to determine the frequency of hepatotoxicity of antituberculosis drugs; for example, in a narrative review, up to 9 different criteria were reported (3). Thirdly, INH can transiently elevate transaminases, and depending on the DILI criteria used and time allowed for remission of the transient transaminase elevation (“transaminitis”), the frequency rates of INH-ILI can vary (5).
The present systematic study aimed to determine the frequency of INH-ILI in patients with LTBI who received IPT using indicators included in the criteria of the DILI Expert Working Group (6).
Methods
This study followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (7) (Supplementary Table S1).
Search strategy
Two independent investigators (JL and DIJ) performed a systematic search in PubMed, Google Scholar, and the Cochrane Database of Systematic Reviews for studies published from 1991 (when a similar study was published) (8) to August 2022. In addition, a secondary search was conducted based on the reference lists of retrieved articles. In the search strategy in PubMed, MeSH (Medical Subject Headings) terms such as “Isoniazid,” “Antitubercular Agents,” “Drug-Induced Liver Injury,” “Drug-Induced Hepatitis,” and “Latent Tuberculosis” were used.
Eligibility criteria
We searched for randomized controlled trials (RCTs) and observational studies reporting data on the frequency of INH-ILI in patients with IPT. We considered studies in English or other languages that included participants of any age and met the following criteria: (i) studies directly reported the frequency of hepatotoxicity in patients with IPT; (ii) when the prevalence with its confidence interval was not directly reported, studies reported enough data to calculate a percentage of hospital admissions with their corresponding 95% confidence interval (CI) (i.e., number of patients with INH-ILI as numerator and total number of patients with INH during the study period as denominator); iii) use of diagnostic criteria similar to the criteria of the DILI Expert Working Group and the Council for International Organizations of Medical Sciences/Roussel Uclaf Causality Assessment Method (9), which consists of the presence of one of the following: levels of alanine aminotransferase (ALT) equal to or increased 5 times above the upper limit of normal (ULN), alkaline phosphatase (AP) equal to or greater than 2 times above the ULN (especially if accompanied by elevation in the concentration of 5′-nucleotidase or gamma glutamyl transpeptidase, GGT), in the absence of bone pathology known to increase alkaline phosphatase; or elevation greater than or equal to 3 times the ULN of ALT concentration and simultaneous elevation of bilirubin concentration exceeding 2 × ULN.
Quality assessment
The quality of observational studies (cohort, case-control, and cross-sectional studies) and randomized controlled trials (RCTs) was appraised according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) (10) and Consolidated Standards of Reporting Trials (CONSORT) (11), respectively. Two investigators independently evaluated the quality of the studies.
Data extraction
Using a common data extraction template, all relevant information was independently abstracted from the selected studies by both reviewers. Information was collated on (i) study characteristics—names of the authors, institutions, geographical location, year of publication, duration, type of hospital and care setting, design, sample size, and method for DILI identification (interview, clinical records, others); and (ii) diagnostic criteria for INH-ILI.
Statistical analyses
This study focused on the population in whom INH had been indicated as the only drug for TB prophylaxis. The frequency of INH-ILI was calculated in each study, taking into account the total number of exposed patients meeting DILI criteria divided by the total number of patients exposed to INH. The overall estimates in the pooled analysis were obtained using Stata 13 software (Stata Corp LP, College Station, TX) and the Meta XL (www.epigear.com) add-in for Microsoft Excel (12). A pooled prevalence was calculated with 95% CI by combining estimates from selected studies based on a random-effects model (13); this is a variant of the inverse of the variance method, and it incorporates intra- and inter-study variability. Heterogeneity between estimates was assessed using the I2 statistic, which describes the percentage of variation not due to sampling error across studies. Subgroup analyses were performed by age group (above and below 50 years), children, patients living with HIV, INH treatment duration, study provenance, candidates for kidney and lung transplantation, and type of study.
Results
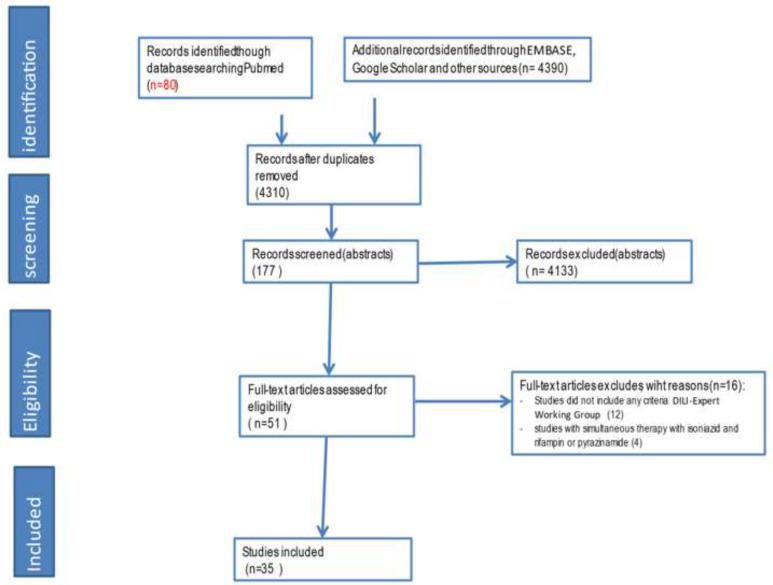
Thirty-five studies comprising a total of 22,193 participants were included (clinical trial: 13; cohort: 14, cross-sectional: 8). Mean participant age was 37.2 (SD 13.9) years, and 46.9% of participants were female (Table 1 and Figure 1). The mean STROBE score of observational studies was 64.5% (SD 14.1), and the mean CONSORT score for clinical trials was 68.6% (SD 10.1). Studies from 16 countries were included: USA (14, 15, 24, 16–23) (11), Spain (25–28) (4), South Africa (29, 30) (2), Botswana (31, 32) (2), Brazil (33) (1), Hong Kong (34) (1), India (35) (1), Italy (36) (1), Korea (37, 38) (2), Pakistan (39) (1), Saudi Arabia (40) (1), Switzerland (41, 42) (2), Taiwan (43, 44) (2), Turkey (45) (1), Australia (46) (1), Zimbabwe (47) (1), and one study included patients of 3 countries (Canada, Brazil, and Saudi Arabia) (48).
Table 1.
Characteristics of studies on isoniazid-induced liver injury (INH-ILI) in patients with latent tuberculosis infection (LTBI) receiving Isoniazid Preventive Therapy (IPT).
| Author (year) | Country | Study | Type of patients | Total | n (INH-ILI) | Mean age (years) | Female (%) | DILI criteria | Quality assessment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| design | sample | STROBE (%) | CONSORT (%) | ||||||||||||||||||
| Chung et al (2020) | Korea | cohort | Healthcare workers | 114 | 10 | 42 | 69.3 | A | 83 | ||||||||||||
| Korayem et al. (2020) | Saudi Arabia | cohort | Lung transplant recipients | 80 | 1 | 38 | 35 | A | 68 | ||||||||||||
| Mudzviti et al (2019) | Zimbabwe | cohort | HIV-infected paediatric and adolescent patients on antiretroviral therapy (ART). | 438 | 28 | 10 | 46.1 | A | 77 | ||||||||||||
| Gupta et al. (2019) | USA | clinical trial | Pregnant women with HIV infection and ART | 956 | 53 | 29 | 100 | A, B | 67 | ||||||||||||
| Noh et al. (2019) | Korea | cross sect | Older patients | 12 | 0 | 69 | 70 | A, B | 77 | ||||||||||||
| Sun et al. (2018) | Taiwan | clinical trial | Patients aged ≥12 in a general hospital | 132 | 2 | 31.7 | 38.6 | A | 79 | ||||||||||||
| Simkins et al. (2017) | USA | cross sect | Renal transplant candidates (94%) | 110 | 4 | 59.81 | 34 | A | 60 | ||||||||||||
| Gray et al. (2016) | Australia | cross sect | Patients with LTBI in a general hospital | 61 | 2 | 50 | 55 | A | 73 | ||||||||||||
| Yamin et al. (2016) | USA | cohort | HIV positive (44%) | 115 | 4 | 38.3 | 29 | A | 80 | ||||||||||||
| Codecasa et al (2014) | Italy | cross sect | Patients of the reference centre for anti-tuberculosis treatment | 11963 | 662 | 29.9 | 45.2 | A | 63 | ||||||||||||
| Rangaka et al (2014) | South Africa | clinical trial | HIV patients | 662 | 19 | 34 | 76 | A | 71 | ||||||||||||
| Chang et al (2014) | USA | cross sect | Patients of paediatric clinic | 1582 | 13 | NR | 50 | A, B | 77 | ||||||||||||
| Taylor et. al. (2013) | Botswana | cohort | Pregnant HIV-infected women | 103 | 0 | 28 | 100 | A | 77 | ||||||||||||
| Jimenez-Fuentes et al. (2013) | Spain | clinical trial | Immigrants with LTBI | 294 | 1 | 26.5 | 60 | A | 63 | ||||||||||||
| Stucchi et al. (2012) | Brazil | cross sect | Liver transplantation candidates | 27 | 0 | 50.5 | 21 | A | 53 | ||||||||||||
| Chan et al (2012) | Taiwan | clinical trial | Male prison inmates | 183 | 11 | 0 | A, B | 78 | |||||||||||||
| Fresard et al (2011) | Switzerland | cohort | Patients with LTBI in the outpatient clinic | 426 | 19 | 32 | 49.3 | A | 67 | ||||||||||||
| Samandari et al. (2011) | Botswana | clinical trial | Adults with HIV infection | 1006 | 10 | 34 | 71 | A | 71 | ||||||||||||
| Naqvi et. al. (2010) | Pakistan | clinical trial | Renal transplant recipients | 181 | 1 | 32.84 | 22.1 | B | 47 | ||||||||||||
| Gray et. al. (2010) | South Africa | cohort | HIV-infected children on antiretroviral therapy | 39 | 1 | 1.8 | 36 | A | 63 | ||||||||||||
| Devrim et al (2010) | Turkey | cohort | Children | 617 | 3 | NR | NR | A | 27 | ||||||||||||
| Young et. al. (2009) | USA | cross sect | Patients >18 years of public health clinic | 639 | 6 | NR | NR | A | 67 | ||||||||||||
| Ekochin et al. (2009) | USA | cohort | Patients on methotrexate treatment | 41 | 0 | 49 | NR | A | 37 | ||||||||||||
| Continued | |||||||||||||||||||||
| Menzies et al. (2008) | Canada, Brazil, and Saudi Arabia | clinical trial | Tuberculosis clinics located in university hospitals in 3 countries | 422 | 16 | NR | 47 | A | 84 | ||||||||||||
| Geijo et al. (2007) | Spain | clinical trial | Outpatient clinic of the general hospital | 54 | 3 | 44.16 | 51 | A | 59 | ||||||||||||
| Jahng et al. (2007) | USA | cohort | Cirrhosis patients during transplant | 9 | 1 | 49.1 | 33 | A | 57 | ||||||||||||
| Page et al. (2006) | USA | cross sect | HIV positive (18.8%) | 670 | 12 | 30.1 | 58.8 | A | 77 | ||||||||||||
| Vuilleumier et al. (2006) | Switzerland | cohort | Pharmacogenetics study (NAT2 and CYP2E1) | 89 | 8 | 31 | 45 | A | 53 | ||||||||||||
| Vikrant et al. (2005) | India | clinical trial | Patients with end-stage renal disease (ESRD) on renal replacement therapy | 54 | 9 | 30.9 | 17.5 | A | 71 | ||||||||||||
| Tortajada et al. (2005) | Spain | clinical trial | Outpatient clinics of public health care facilities | 199 | 5 | 52 | A | 78 | |||||||||||||
| McNeill et al. (2003) | USA | cohort | Patients in a community setting | 114 | 5 | 34.7 | 39 | A | 53 | ||||||||||||
| Fernandez-Villar et al. (2003) | Spain | cohort | Drug users | 415 | 20 | A | 60 | ||||||||||||||
| Leung et al. (2003) | Hong Kong | clinical trial | Patients living with silicosis | 36 | 1 | 57.6 | 3 | A | 63 | ||||||||||||
| Jasmer et al. (2002) | USA | clinical trial | Three urban public health tuberculosis clinics | 204 | 2 | 35 | 45 | A | 79 | ||||||||||||
| Sadaphal et al (2001) | USA | cohort | Injection drug users. HIV (25%). | 146 | 13 | 42 | 14 | A | 70 | ||||||||||||
STROBE: Strengthening the Reporting of Observational studies in Epidemiology. CONSORT: Consolidated Standards of Reporting Trials. LTBI: latent tuberculosis infection. IPT: Isoniazid Preventative Therapy. INH-ILI: isoniazid-induced liver injury. A: alanine aminotransferase (ALT) > 5 times above the upper limit of normal (ULN). B: ALT> 3 ULN if bilirubin >2 ULN. NR: not reported.
Figure 1.
Study screening Flowchart
The overall average frequency of INH-ILI was 2.6% (95% CI: 1.7-3.7%) (Figure 2). In 34/35 studies, the DILI criterion of more than or equal to a fivefold elevation above the ULN for ALT was used; one study used the criterion of more than or equal to a threefold elevation in ALT concentration and simultaneous elevation of bilirubin concentration exceeding 2 times the ULN; and one study used the two criteria outlined above. No study used the criteria of more than or equal to a twofold elevation above the ULN for alkaline phosphatase (ALP) (particularly with accompanying elevations in concentrations of 5′-nucleotidase or γ-glutamyl transpeptidase in the absence of known bone pathology driving the rise). No study reported specific clinical characteristics of INH-ILI (e.g., clinical symptoms of hepatitis). No asymmetry was observed in relation to the management of publication bias, (see the funnel plot in Table S2 in the supplemental file).
Figure 2.
Forest plot of frequencies of isoniazid-induced liver injury (INH-ILI) in patients with latent tuberculosis infection (LTBI) receiving Isoniazid Preventive Therapy (IPT)
Mortality associated with INH-ILI was 0.02% (4/22193). Subgroup analysis (Table 2) revealed no significant differences in the frequency of INH-ILI in patients older or younger than 50 years, treatment duration (6, 9, or 12 months), children, patients living with HIV, candidates for liver, kidney, or lung transplant, type of study design, or study provenance.
Table 2.
Frequency of isoniazid-induced liver injury (INH-ILI) in patients with latent tuberculosis infection (LTBI) receiving Isoniazid Preventive Therapy (IPT). Subgroup analyses
| Subgroup analysis | Studies (n) | Proportion (%) (95% CI) | P |
|---|---|---|---|
| Mean age (years) | 0.896 | ||
| < 50 | 26 | 2.8 (1.7 – 4.0) | |
| ≥ 50 | 5 | 2.0 (0.3 – 4.7) | |
| Children (<18y) vs adults | 0.416 | ||
| Children (<18y) | 4 | 1.8 (0.1 – 5.1) | |
| adults (>=18y) | 31 | 2.8 (1.8 – 3.9) | |
| HIV (human immunodeficiency virus) infection | 0.937 | ||
| HIV-positive persons | 9 | 3.1 (1.5 – 5.1) | |
| HIV-negative persons | 26 | 2.5 (1.4 – 3.9) | |
| Duration of INH treatment | 0.115 | ||
| 6 months | 17 | 3.7 (2.4 – 5.1) | |
| ≥ 9 months | 17 | 1.8 (0.8 – 3.0) | |
| Continent where the study was conducted | 0.842 | ||
| Asia | 8 | 3.5 (0.9 – 7.4) | |
| America | 13 | 2.2 (0.9 – 3.8) | |
| Africa | 5 | 2.0 (0.4 – 4.7) | |
| Europe | 8 | 3.3 (1.4 – 5.7) | |
| Oceania (Australia, New Zealand) | 1 | 3.3 (0.4 – 11.3) | |
| Transplant recipients or candidates for kidney, lung or liver transplant | 0.661 | ||
| Yes | 6 | 2.8 (0.0 – 8.2) | |
| No | 29 | 2.7 (1.8 – 3.9) | |
| Type of study | 0.735 | ||
| Clinical trial | 13 | 2.7 (1.4 – 4.3) | |
| Observational (cohort cross-sectional study) | 22 | 2.6 (1.4 – 4.2) |
Discussion
In the present study, the frequency of INH-ILI in patients with IPT was found to be 2.6%. No significant differences were found in the frequency of INH-ILI in patients older or younger than 50 years, children, patients living with HIV, candidates for liver, kidney, or lung transplant, treatment duration (6, 9, or 12 months), type of study design, or study provenance.
In 1991, Steele et al. published a systematic review of 34 studies (published between 1966 and 1989) including a total of 38,257 patients in which they found an overall frequency of INH hepatotoxicity of 0.6% in adults, and 0.2% in children; however, unlike our study, the criterion used was clinical manifestations of hepatitis in conjunction with aspartate aminotransferase (AST) levels exceeding 100 units/dl (8), which is not currently in the DILI criteria. In a meta-analysis published in 2013, Sharma et al. included 5 clinical trials and found an INH-ILI frequency of 4.6% in HIV-negative persons (49), where the criterion used was ALT > 5.0 times the ULN (5, 50).
In 2009 Holty et al. performed a systematic study of IPT in 139 liver/kidney/lung transplant candidates, finding an INH-ILI frequency of 6% as cause of treatment suspension (51). The present study found no statistically significant difference in the frequency of INH-ILI in patients with IPT in relation to an administration time of 6 (4%), 9 (3%), or 12 (2%) months, suggesting that the frequency of INH-ILI may not be related to these administration durations. The INH-ILI-associated mortality in patients with IPT was 0.02% (4/22193). In 1993, Salpeter reviewed the mortality associated with INH-ILI in patients with IPT and found a frequency of 0.001% (2 of 202,497) using criteria of the 1983 American Thoracic Society guidelines (52). The present study found no significant difference between the frequency of INH-ILI in patients older or younger than 50 years. In a previous meta-analysis, Hosford et al. found a higher frequency of DILI among those older than 60 years with LTBI; however, the DILI criteria differed from those used in the present study (elevation in liver blood tests > 2–5 times the upper reference level, equivalent elevated liver enzymes, and/or clinical symptoms of hepatitis) (53).
The main limitation of the present study is that none of the included studies had complete and updated DILI criteria (9). In 34/35 studies, the criterion used for DILI was ALT > 5 times above the ULN, and a single study reported bilirubin > 2 times and ALT > 3 times above the ULN. None of the included studies reported alkaline phosphatase levels; therefore, it was not possible to characterize the hepatotoxicity pattern (cholestatic, hepatocellular, or mixed). Studies that did not consider any of the indicators present in updated DILI criteria were excluded, for example, those with the only criterion being AST > 3 times above the ULN (54). In the included studies, no analysis of causality with an updated instrument recommended for the diagnosis of DILI was reported (9). It is necessary to conduct studies with updated DILI criteria in studies with antituberculosis drugs, especially with INH.
Conclusion
In conclusion, with the criteria used (ALT > 5 times above the ULN), the frequency of INH-ILI is low; however, it will be important to perform studies with updated and complete DILI criteria so as to know the patterns and real frequency of INH-ILI.
Conflict of interests
The authors certify that they have no conflict of interests.
Supplementary Materials
References
- 1.Unissa AN, Subbian S, Hanna LE, Selvakumar N. Overview on mechanisms of isoniazid action and resistance in Mycobacterium tuberculosis. Infect Genet Evol. 2016;45:474–492. doi: 10.1016/j.meegid.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Licence: CC BY-NC-SA 3.0 IGO. n.d, authors. WHO consolidated guidelines on tuberculosis. Module 1: prevention – tuberculosis preventive treatment. Geneva: World Health Organization; 2020. [PubMed] [Google Scholar]
- 3.Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WCM, van der Ven AJAM, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: Concise up-to-date review. J Gastroenterol Hepatol. 2008;23:192–202. doi: 10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 4.Mølhave M, Wejse C. Historical review of studies on the effect of treating latent tuberculosis. Int J Infect Dis. 2020;92:31–36. doi: 10.1016/j.ijid.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 6.Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806–815. doi: 10.1038/clpt.2011.58. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 [PMC free article] [PubMed] [Google Scholar]
- 8.Steele MA, Burk RF, DesPrez RM. Toxic hepatitis with isoniazid and rifampin. Chest. 1991;99:465–471. doi: 10.1378/chest.99.2.465. [DOI] [PubMed] [Google Scholar]
- 9.Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806–815. doi: 10.1038/clpt.2011.58. [DOI] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansfield KE, Sim J, Jordan JL, Jordan KP. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain. 2016;157:55–64. doi: 10.1097/j.pain.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Montepiedra G, Aaron L, Theron G, McCarthy K, Bradford S, et al. Isoniazid preventive therapy in HIV-infected pregnant and postpartum women. N Engl J Med. 2019;381:1333–1346. doi: 10.1056/NEJMoa1813060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simkins J, Abbo LM, Camargo JF, Rosa R, Morris MI. Twelve-week rifapentine plus isoniazid versus 9-month isoniazid for the treatment of latent tuberculosis in renal transplant candidates. Transplantation. 2017;101:1468–1472. doi: 10.1097/TP.0000000000001329. [DOI] [PubMed] [Google Scholar]
- 16.Yamin A, Bornstein E, Hensel R, Mohamed O, Kempker RR. Predictors of latent tuberculosis infection treatment after introduction of a new regimen: a retrospective cohort study at an inner city clinic. Open Forum Infect Dis. 2016:3. doi: 10.1093/ofid/ofw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young H, Wessolossky M, Ellis J, Kaminski M, Daly JS. A retrospective evaluation of completion rates, total cost, and adverse effects for treatment of latent tuberculosis infection in a public health clinic in central Massachusetts. Clin Infect Dis. 2009;49:424–427. doi: 10.1086/600394. [DOI] [PubMed] [Google Scholar]
- 18.Ekochin LH, Manadan AM, Aggarwal R, Sequeira W, Block JA. Absence of transaminase elevation during concomitant methotrexate and isoniazid therapy. J Rheumatol. 2009;36:2127. doi: 10.3899/jrheum.090149. [DOI] [PubMed] [Google Scholar]
- 19.Jahng AW, Tran T, Bui L, Joyner JL. Safety of Treatment of Latent Tuberculosis Infection in Compensated Cirrhotic Patients During Transplant Candidacy Period. Transplantation. 2007;83:1557–1562. doi: 10.1097/01.tp.0000266578.45634.4f. [DOI] [PubMed] [Google Scholar]
- 20.Page KR, Sifakis F, Montes de Oca R, Cronin WA, Doherty MC, Federline L, et al. Improved adherence and less toxicity with rifampin vs isoniazid for treatment of latent tuberculosis. Arch Intern Med. 2006;166:1863. doi: 10.1001/archinte.166.17.1863. [DOI] [PubMed] [Google Scholar]
- 21.McNeill L, Allen M, Estrada C, Cook P. Pyrazinamide and rifampin vs isoniazid for the treatment of latent tuberculosis. Chest. 2003;123:102–106. doi: 10.1378/chest.123.1.102. [DOI] [PubMed] [Google Scholar]
- 22.Jasmer RM, Saukkonen JJ, Blumberg HM, Daley CL, Bernardo J, Vittinghoff E, et al. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann Intern Med. 2002;137:640. doi: 10.7326/0003-4819-137-8-200210150-00007. [DOI] [PubMed] [Google Scholar]
- 23.Sadaphal P, Astemborski J, Graham NMH, Sheely L, Bonds M, Madison A, et al. Isoniazid preventive therapy, hepatitis C virus infection, and hepatotoxicity among injection drug users infected with mycobacterium tuberculosis. Clin Infect Dis. 2001;33:1687–1691. doi: 10.1086/323896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang S-H, Nahid P, Eitzman SR. Hepatotoxicity in children receiving isoniazid therapy for latent tuberculosis infection. J Pediatric Infect Dis Soc. 2014;3:221–227. doi: 10.1093/jpids/pit089. [DOI] [PubMed] [Google Scholar]
- 25.Jiménez-Fuentes MA, de Souza-Galvao ML, Mila Augé C, Solsona Peiró J, Altet-Gómez MN. Rifampicin plus isoniazid for the prevention of tuberculosis in an immigrant population. Int J Tuberc Lung Dis. 2013;17:326–332. doi: 10.5588/ijtld.12.0510. [DOI] [PubMed] [Google Scholar]
- 26.Geijo MP, Herranz CR, Vaño D, García ÁJ, García M, Dimas JF. Pauta corta de isoniazida y rifampicina comparada con isoniazida para la infección latente de tuberculosis Ensayo clínico aleatorizado. Enferm Infecc Microbiol Clin. 2007;25:300–304. doi: 10.1157/13102264. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez‐Villar A, Sopena B, Vazquez R, Ulloa F, Fluiters E, Mosteiro M, et al. Isoniazid Hepatotoxicity among Drug Users: The Role of Hepatitis C. Clin Infect Dis. 2003;36:293–298. doi: 10.1086/345906. [DOI] [PubMed] [Google Scholar]
- 28.Tortajada C, Martínez-Lacasa J, Sánchez F, Jiménez-Fuentes A, De Souza ML, García JF, et al. Is the combination of pyrazinamide plus rifampicin safe for treating latent tuberculosis infection in persons not infected by the human immunodeficiency virus? Int J Tuberc Lung Dis. 2005;9:276–281. [PubMed] [Google Scholar]
- 29.Rangaka MX, Wilkinson RJ, Boulle A, Glynn JR, Fielding K, van Cutsem G, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet. 2014;384:682–690. doi: 10.1016/S0140-6736(14)60162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray D, Nuttall J, Lombard C, Davies M -a, Workman L, Apolles P, et al. Low rates of hepatotoxicity in HIV-infected children on anti-retroviral therapy with and without isoniazid prophylaxis. J Trop Pediatr. 2010;56:159–165. doi: 10.1093/tropej/fmp079. [DOI] [PubMed] [Google Scholar]
- 31.Taylor AW, Mosimaneotsile B, Mathebula U, Mathoma A, Moathlodi R, Theebetsile I, et al. Pregnancy outcomes in HIV-infected women receiving long-term isoniazid prophylaxis for tuberculosis and antiretroviral therapy. Infect Dis Obstet Gynecol. 2013;2013:1–5. doi: 10.1155/2013/195637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samandari T, Agizew TB, Nyirenda S, Tedla Z, Sibanda T, Shang N, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 33.Stucchi RSB, Boin IFSF, Angerami RN, Zanaga L, Ataide EC, Udo EY. Is isoniazid safe for liver transplant candidates with latent tuberculosis? Transplant Proc. 2012;44:2406–2410. doi: 10.1016/j.transproceed.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 34.Leung CC, Law WS, Chang KC, Tam CM, Yew WW, Chan CK, et al. Initial experience on rifampin and pyrazinamide vs isoniazid in the treatment of latent tuberculosis infection among patients with silicosis in Hong Kong. Chest. 2003;124:2112–2118. doi: 10.1378/chest.124.6.2112. [DOI] [PubMed] [Google Scholar]
- 35.Vikrant S, Agarwal SK, Gupta S, Bhowmik D, Tiwari SC, Dash SC, et al. Prospective randomized control trial of isoniazid chemoprophylaxis during renal replacement therapy. Transpl Infect Dis. 2005;7:99–108. doi: 10.1111/j.1399-3062.2005.00103.x. [DOI] [PubMed] [Google Scholar]
- 36.Codecasa LR, Murgia N, Ferrarese M, Delmastro M, Repossi AC, Casali L, et al. Isoniazid preventive treatment: predictors of adverse events and treatment completion. Int J Tuberc Lung Dis. 2013;17:903–908. doi: 10.5588/ijtld.12.0677. [DOI] [PubMed] [Google Scholar]
- 37.Chung SJ, Lee H, Koo GW, Min J-H, Yeo Y, Park DW, et al. Adherence to nine-month isoniazid for latent tuberculosis infection in healthcare workers: a prospective study in a tertiary hospital. Sci Rep. 2020;10:6462. doi: 10.1038/s41598-020-63156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noh CS, Kim H Il, Choi H, Kim Y, Kim C-H, Choi J-H, et al. Completion rate of latent tuberculosis infection treatment in patients aged 65 years and older. Respir Med. 2019;157:52–58. doi: 10.1016/j.rmed.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Naqvi R, Naqvi A, Akhtar S, Ahmed E, Noor H, Saeed T, et al. Use of isoniazid chemoprophylaxis in renal transplant recipients. Nephrol Dial Transplant. 2010;25:634–637. doi: 10.1093/ndt/gfp489. [DOI] [PubMed] [Google Scholar]
- 40.Korayem GB, Alissa DA, AlSuhaibani NI, AlSwailem GS, AlShammari MA, Yaqoob I, et al. Empiric vs screening‐based use of isoniazid for tuberculosis prophylaxis: Safety and effectiveness in lung transplant recipients in Saudi Arabia. Transpl Infect Dis. 2021:23. doi: 10.1111/tid.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frésard I, Bridevaux P, Rochat T, Janssens J. Adverse effects and adherence to treatment of rifampicine 4 months vs isoniazid 6 months for latent tuberculosis. doi: 10.4414/smw.2011.13240. Swiss Med Wkly 2011. [DOI] [PubMed] [Google Scholar]
- 42.Vuilleumier N, Rossier MF, Chiappe A, Degoumois F, Dayer P, Mermillod B, et al. CYP2E1 genotype and isoniazid-induced hepatotoxicity in patients treated for latent tuberculosis. Eur J Clin Pharmacol. 2006;62:423–429. doi: 10.1007/s00228-006-0111-5. [DOI] [PubMed] [Google Scholar]
- 43.Sun H-Y, Huang Y-W, Huang W-C, Chang L-Y, Chan P-C, Chuang Y-C, et al. Twelve-dose weekly rifapentine plus isoniazid for latent tuberculosis infection: a multicentre randomised controlled trial in Taiwan. Tuberculosis. 2018;111:121–126. doi: 10.1016/j.tube.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 44.Chan P-C, Yang C-H, Chang L-Y, Wang K-F, Lu B-Y, Lu C-Y, et al. Latent tuberculosis infection treatment for prison inmates: a randomised controlled trial. Int J Tuberc Lung Dis. 2012;16:633–638. doi: 10.5588/ijtld.11.0504. [DOI] [PubMed] [Google Scholar]
- 45.Devrim İ, Olukman Ö, Can D, Dizdarer C. Risk Factors for isoniazid hepatotoxicity in children with latent TB and TB: difference from adults. Chest. 2010;137:737–738. doi: 10.1378/chest.09-2120. [DOI] [PubMed] [Google Scholar]
- 46.Gray EL, Goldberg HF. Baseline abnormal liver function tests are more important than age in the development of isoniazid-induced hepatoxicity for patients receiving preventive therapy for latent tuberculosis infection. Intern Med J. 2016;46:281–287. doi: 10.1111/imj.12979. [DOI] [PubMed] [Google Scholar]
- 47.Mudzviti T, Shamu T, Chimbetete C, Munengerwa T, Bote S, Pascoe M. Tolerability of isoniazid preventive therapy in an HIV-infected cohort of paediatric and adolescent patients on antiretroviral therapy from a resource-limited setting: a retrospective cohort study. Drugs - Real World Outcomes. 2019;6:37–42. doi: 10.1007/s40801-019-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menzies D, Long R, Trajman A, Dion M-J, Yang J, Al Jahdali H, et al. Adverse events with 4 months of rifampin therapy or 9 months of isoniazid therapy for latent tuberculosis infection. Ann Intern Med. 2008;149:689. doi: 10.7326/0003-4819-149-10-200811180-00003. [DOI] [PubMed] [Google Scholar]
- 49.Sharma SK, Sharma A, Kadhiravan T, Tharyan P. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD007545.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Cancer Institute. Common terminology criteria for adverse events, version 2 0. Bethesda, MD, USA: 2003. [Google Scholar]
- 51.Holty J-EC, Gould MK, Meinke L, Keeffe EB, Ruoss SJ. Tuberculosis in liver transplant recipients: A systematic review and meta-analysis of individual patient data. Liver Transplant. 2009;15:894–906. doi: 10.1002/lt.21709. [DOI] [PubMed] [Google Scholar]
- 52.Salpeter SR. Fatal isoniazid-induced hepatitis Its risk during chemoprophylaxis. West J Med. 1993;159:560–564. [PMC free article] [PubMed] [Google Scholar]
- 53.Hosford JD, von Fricken ME, Lauzardo M, Chang M, Dai Y, Lyon JA, et al. Hepatotoxicity from antituberculous therapy in the elderly: A systematic review. Tuberculosis. 2015;95:112–122. doi: 10.1016/j.tube.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bliven-Sizemore EE, Sterling TR, Shang N, Benator D, Schwartzman K, Reves R, et al. Three months of weekly rifapentine plus isoniazid is less hepatotoxic than nine months of daily isoniazid for LTBI. Int J Tuberc Lung Dis. 2015;19:1039–1044. doi: 10.5588/ijtld.14.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.