Abstract
Introduction:
Abnormally invasive placenta (AIP, aka placenta accreta spectrum; PAS) is an increasingly common pregnancy pathology, which, despite significant morbidity risk to the mother, is often undiagnosed prior to delivery. We tested several potential biomarkers in plasma from PAS mothers to determine whether any were sufficiently robust for a formal, diagnostic accuracy study.
Methods:
We examined hyperglycosylated hCG (h-hCG), decorin and IL-8, based on biological plausibility and literature indications that they might be altered in PAS. These analytes were assayed by ELISA in maternal plasma from five groups, comprising (1) normal term controls, (2) placenta previa controls, and cases of (3) placenta increta/percreta without placenta previa, (4) placenta previa increta/percreta and (5) placenta previa accreta.
Results:
There were no differences in h-hCG, ß-hCG or the h-hCG/ß-hCG ratio between the groups. Mean decorin levels were increased in previa controls (Group 2) compared to the other groups, but there was substantial overlap between the individual values. While an initial multiplex assay showed a greater value for IL-8 in the placenta previa increta/percreta group (Group 4) compared to placenta previa controls (Group 2), the subsequent validation ELISA for IL-8 showed no differences between the groups.
Discussion:
We conclude that the absence of differences and the extent of overlap between cases and controls does not justify further assessment of these biomarkers.
Keywords: Placenta accreta spectrum, Cytokine, Hyperglycosylated human chorionic, gonadotropin, Decorin, IL-8
1. Introduction
Abnormally invasive placenta (AIP, aka placenta accreta spectrum; PAS), is a placental over-invasion pathology which potentially has severe clinical consequences. It has become one of the leading causes of postpartum hemorrhage in the U.S. and is a significant contributor to maternal morbidity and mortality. Although the risk factors of placenta previa and prior uterine damage (primarily Caesarean section) are of major epidemiological importance in identifying possible cases, there are nevertheless many cases where the presence of both risk factors is not associated with PAS. We here refer to PAS because all cases in our series are histopathologically confirmed as Grade 1 (accreta, absence of decidua and direct interaction of villi with myometrium), 2 (increta) and 3 (percreta) by histologic criteria on the recent FIGO Clinical Classification System [1]. Both ultrasound and MRI have been used effectively to diagnose cases of PAS however the subjectivity involved in assessing qualitative ultrasound markers has restricted them to expert use. The result is that many cases of PAS are still undiagnosed or misdiagnosed, leading to poor maternal outcomes. In view of these circumstances, having a biomarker for PAS would be of considerable benefit, enabling physicians to add another diagnostic tool, early enough to prepare for the complex delivery often necessitated in these cases. We here assessed three potential PAS biomarkers, hyperglycosylated hCG, decorin and IL-8. This was a pilot study to determine whether these factors should be subject to a full diagnostic accuracy study.
Hyperglycosylated hCG (h-hCG) has been shown to be released by human extravillous trophoblast cells (EVT) and to stimulate EVT invasion [2,3]. It has been suggested as a biomarker for gestational trophoblastic diseases such as choriocarcinoma. In normal pregnancies h-hCG concentrations in maternal serum decrease over gestation, whereas maternal circulating concentrations of ß-hCG show an increase from 9 to 11 weeks of gestation followed by a decline thereafter [3]. It has been speculated that h-hCG reflects the activity of the invasive EVT and we thus hypothesized that the over-invasion characteristic of PAS would lead to increased secretion of h-hCG into the maternal circulation in these cases compared to controls.
Decorin is a TGFß-binding proteoglycan, secreted by decidual cells in the term placenta, which has been shown to suppress EVT proliferation and invasiveness [4]. Decorin is up-regulated in the preeclamptic placenta and it is hypothesized that it may play a role in the etiology of this pathology by limiting EVT invasion [5,6]. Another report suggests that while decorin is synthesized and secreted by normal, term decidua, it is neither synthesized nor secreted by decidual cells in PAS [7]. This data suggests that a reduction or loss of secreted decorin might serve as a biomarker for PAS.
IL-8 has been identified at high levels in cancer cells undergoing the epithelial to mesenchymal transition (EMT), which is the molecular program that underpins conversion of the parent cytotrphoblast cells to the invasive, more phenotypically mesenchymal EVT. The EMT promotes angiogenesis and maintenance of an invasive cellular phenotype [8], characteristics shared with PAS [9,10]. IL-8 has also been shown to promote the migration and invasion of placental extravillous trophoblast cells during pregnancy [11]. These data suggested that IL-8 might be capable of acting as a biomarker of PAS.
2. Methods and materials
2.1. Selection of subjects
This study was approved by the Institutional Review Board for Hackensack University Medical Center. All subjects gave written, informed consent for use of the blood samples. Placenta percreta and uncomplicated placenta previa cases (controls) were identified by antenatal ultrasound and MRI assessment. The prenatal diagnoses were later confirmed by histopathological determination after delivery of the placenta following elective Caesarean section (normal, placenta previa) or after Caesarean hysterectomy (placenta accreta, increta/percreta or placenta previa accreta/increta/percreta). Samples were divided into five groups; maternal blood from pregnancies defined as 1) normal, term without previa or PAS, 2) placenta previa, 3) placenta increta/percreta without placenta previa, 4) placenta previa increta/percreta and 5) placenta previa accreta. In the case of groups 3 and 4, each group contained only one case of placenta increta; the remainder were placenta percreta. Gestational age was calculated from the last menstrual period and confirmed by first trimester ultrasound. Birthweight centile was calculated using the Fenton 2013 Pre-term Growth Calculator for neonates <37 weeks gestational age (https://peditools.org/fenton2013/).
2.2. Blood sampling and plasma preparation
Maternal blood was taken into K-EDTA tubes, mixed and stored on ice prior to plasma preparation. Preparation of plasma took place within 1 h of sample collection. Samples were centrifuged at 2500 g for 10 min then the plasma layer was removed and stored in 0.5 mL aliquots at −80°C.
2.3. Assays and statistics
A multiplex cytokine assay was performed on a Luminex 200 unit (Luminex Corp., Austin, TX) using the Human High Sensitivity Cytokine Premixed Magnetic Luminex Performance Assay kit from R & D Systems (Minneapolis, MN), according to the manufacturer’s instructions. Quantification of h-hCG, ß-hCG, decorin and IL-8 by ELISA was carried out using kits specific for these analytes and according to the manufacturers’ instructions. The h-hCG kit was obtained from MyBiosource (San Diego, CA) and the ß-hCG kit from Genway Biotech Inc. (San Diego, CA). The decorin ELISA kit was obtained from Thermo Scientific (Fair Lawn, NJ). The IL-8 assay kit was obtained from Abcam (Cambridge, MA). Measurements were made using a BioTek Synergy HT plate reader (Winooski, VT). Samples were assayed in batch to minimize interassay variability. Each sample was assayed in duplicate and the mean of the two values was taken as representative of the specific variable for that individual. Following normality testing (Shapiro-Wilk test), groups were compared using one-way ANOVA (Tukey multiple comparison post-test) or the Kruskal-Wallis test (Dunn’s multiple comparison post-test) using Graphpad Prism software (v8.2, San Diego, CA). The family-wise significance level was set as 0.05. Data is presented as mean ± SEM or median ± IR.
3. Results
3.1. Subject demographics
Baseline characteristics for all subjects are given in Table 1A (maternal) and 1B (pregnancy). There were no differences in maternal age and pre-pregnancy BMI. What is apparent is the greater numbers with parity >2 and prior Caesarean sections >2 in Group 4 (increta/percreta-previa) compared to all the other groups. Not surprisingly, Group 1 (normal, term) pregnancies delivered significantly later in gestation than the other groups. Birthweight obviously differed between the groups due to gestational age differences, but when fetal sex and gestational age at delivery were taken into account, the birthweight centiles did not differ between the groups.
Table 1A.
Maternal demographics.
| Group | Diagnosis (n) | Maternal Age (years) | Pre-pregnancy BMI | Category | Parity (n) | Prior C/s (n) |
|---|---|---|---|---|---|---|
| 1 | Normal (14) | 36.0 ± 0.9 | 25.2 ± 1.3 | 0 | 1 | 1 |
| ≤ 2 | 11 | 11 | ||||
| >2 | 2 | 2 | ||||
| 2 | Previa (18) | 33.3 ± 1.6 | 25.9 ± 1.3 | 0 | 6 | 11 |
| ≤ 2 | 11 | 7 | ||||
| >2 | 1 | 0 | ||||
| 3 | Increta/Percreta (7) | 38.3 ± 1.5 | 23.1 ± 1.8 | 0 | 0 | 1 |
| ≤ 2 | 5 | 6 | ||||
| >2 | 2 | 0 | ||||
| 4 | Increta/Percreta-Previa (42) | 33.8 ± 0.6 | 28.2 ± 1.0 | 0 | 2 | 2 |
| ≤ 2 | 16 | 16 | ||||
| >2 | 24 | 24 | ||||
| 5 | Accreta-Previa (7) | 34.4 ± 2.0 | 28.4 ± 3.3 | 0 | 2 | 1 |
| ≤ 2 | 2 | 4 | ||||
| >2 | 3 | 2 |
3.2. Assay parameters
Different subsets of these subjects were used for the various assays, depending on time of assay and sample availability. The range of ages at the time of blood sampling is given in Table 1B. Although we were able to obtain blood samples prior to the third trimester from a number of subjects, we did not have sufficient to perform a cross-sectional comparison on values prior to late second trimester (24 weeks). We therefore compared the values for all samples taken after 24 weeks of gestation (≥25 weeks); the mean gestational age at the time of blood sampling for the samples taken at ≥ 25 weeks is also given in Table 1B. We did however separately examine the gestational age dependence of each analyte within the defined groups, including samples covering the second trimester.
Table 1B.
Pregnancy demographics.
| Group | Diagnosis (n) | GA sampling range (weeks) | GA@ sampling ≥25 weeks (n) | GA@ delivery (weeks) | Birthweight (grams) | Birthweight centile |
|---|---|---|---|---|---|---|
| 1 | Normal (14) | 16–39 | 32.1 ± 1.4 (9) | 37.6 ± 0.3* | 3170 ± 98 | 62 ± 5 |
| 2 | Previa (18) | 25–38 | 33.5 ± 0.8 (18) | 35.8 ± 0.5 | 2750 ± 129 | 59 ± 6 |
| 3 | Increta/Percreta (7) | 28–35 | 32.8 ± 0.9 (7) | 34.2 ± 0.3 | 2221 ± 112 | 49 ± 10 |
| 4 | Increta/Percreta-Previa (42) | 22–35 | 31.5 ± 0.4 (39) | 33.5 ± 02 | 2285 ± 65 | 62 ± 3 |
| 5 | Accreta-Previa (6) | 16–37 | 34.0 ± 0.8 (6) | 33.7 ± 0.8 | 2089 ± 208 | 48 ± 7 |
> 3, 4, 5; p < 0.05 (Kruskal-Wallis).
3.3. h-hCG
The results of the assay for h-hCG are shown in Fig. 1A. There were no differences between any of the groups (range 25–36 weeks) and overlap of the individual values between the groups. We also assayed ß-hCG in the same samples and the results are shown in Fig. 1B, again demonstrating no differences between the groups. Finally, as the profiles of h-hCG and ß-hCG have been shown to differ and might magnify group differences, we calculated the ratio of h-hCG to ß-hCG in individual samples and analyzed the ratio by groups (Fig. 1C). There were no differences in the ratio values between the groups. We also plotted the gestational age dependence of the values for h-hCG, ß-hCG and the h-hCG/ß-hCG ratio, including those samples obtained prior to 25 weeks of gestation. The graphs are shown in Fig. 2A–C. For h-hCG, both group 1 (normal) and group 5 (accreta-previa) showed a significant deviation from a zero slope, but the slopes were not significantly different from each other.
Fig. 1. Maternal plasma concentrations of h-hCG, β-hCG and the h-hCG/β-hCG ratio:
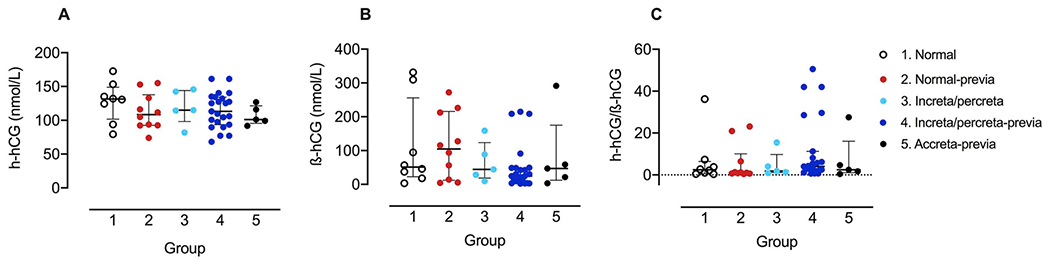
Concentration (nmol/L) of h-hCG (A) and ß-hCG (B) in maternal serum and the ratio of h-hCG to ß-hCG (C) in (1) normal pregnancies (open circles, n = 8) and pregnancies with (2) placenta previa (red, n = 10), (3) increta/percreta without placenta previa (light blue, n = 5), (4) increta/percreta with placenta previa (dark blue, n = 23) or (5) accreta with placenta previa (black, n = 5). Data plotted as mean ± interquartile range (IR).
Fig. 2. Gestational age dependence of h-hCG, β-hCG and the h-hCG/β-hCG ratio:
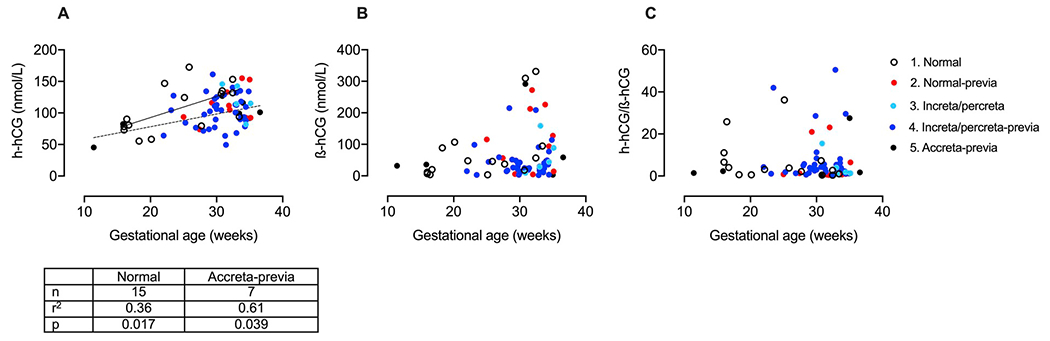
Concentration (nmol/L) of h-hCG (A) and ß-hCG (B) in maternal serum and the ratio of h-hCG to ß-hCG (C) plotted against gestational age in (1) normal pregnancies (open circles, n = 15) and pregnancies with (2) placenta previa (red, n = 10), (3) increta/percreta without placenta previa (light blue, n = 5), (4) increta/percreta with placenta previa (dark blue, n = 36) or (5) accreta with placenta previa (black, n = 7). Linear regression lines are plotted for normal pregnancies (Group 1; dashed line) and for accreta with placenta previa (Group 5; solid line).
3.4. Decorin
We assayed samples for decorin using a specific ELISA. Fig. 3A shows the plasma decorin concentration for groups 1–4. There was an increased mean concentration in the previa group (Group 2) vs. the other groups. It is notable however that examination of the individual values in Fig. 3A shows substantial overlap between Group 2 and the values in Groups 1, 3 and 4. We also analyzed the groups by gestational age (at sampling) and the results are shown in Fig. 3B. The negative slope for Group 3 (increta/percreta) was significantly different from zero however, there were only 4 data points in this group; all the other groups, including the increta/percreta-previa group which contained 20 data points, had slopes which did not differ from zero, indicating no change with gestational age.
Fig. 3. Maternal plasma decorin concentration and gestational age dependence:
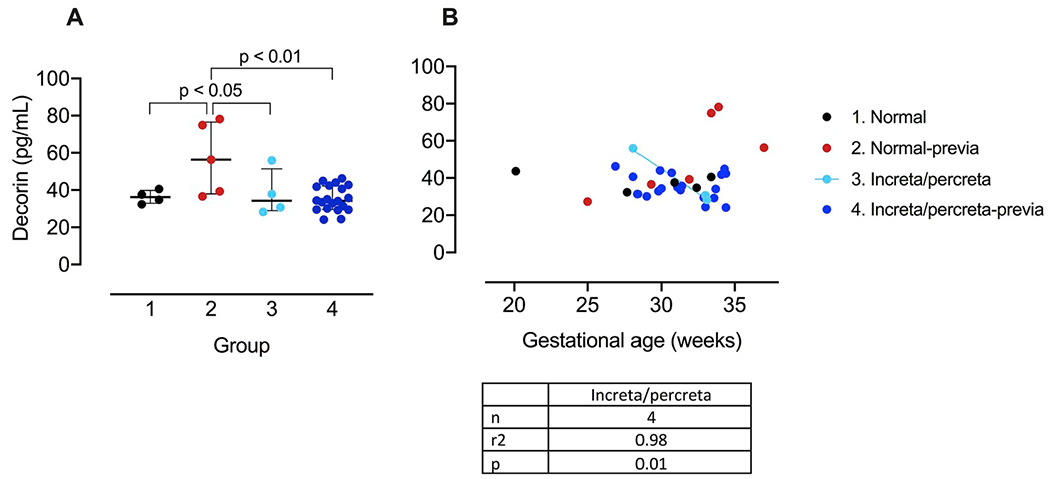
A. Decorin concentration (pg/mL) for (1) normal pregnancies (open circles, n = 4) and pregnancies with (2) placenta previa (red, n = 5), (3) increta/percreta without placenta previa (light blue, n = 4), (4) increta/percreta with placenta previa (dark blue, n = 20). Data plotted as mean ± IR. B. Gestational age dependence of decorin concentration for (1) normal pregnancies (open circles, n = 5) and pregnancies with (2) placenta previa (red, n = 6), (3) increta/percreta without placenta previa (light blue, n = 4), (4) increta/percreta with placenta previa (dark blue, n = 20). Linear regression line is plotted for pregnancies with increta/percreta but without placenta previa (Group 3; light blue line).
3.5. IL-8
We initially performed a multiplex cytokine assay, the results of which are shown in Table 2, where we compared placenta previa controls (n = 9) with a placenta previa accreta/percreta group (n = 4/14). The value of IL-8 for the PAS-previa group was higher than for the control previa group. The accreta values were not significantly different from the percreta values. To validate this result, we performed measurement of IL-8 by ELISA. This assay revealed no differences between the experimental groups (Fig. 4A). When the groups were analyzed against gestational age at the time of sampling, both Groups 1 and 4 had non-zero slopes (Fig. 4B). In the case of Group 1 (normal, term), there were only 4 data points. For Group 4 (increta/percreta-previa), the 18 data points indicate an increasing IL-8 concentration over gestation (p < 0.042). Nevertheless, the 95% prediction bands for the best-fit linear regression lines show complete overlap between the normal and increta/percreta-previa groups, suggesting that while the slopes of these curves may be different, differentiation between these groups based on gestational age changes would not be possible.
Table 2.
Multiplex cytokine assay.
| Group | IL-1ß | IL-2 | IL4 | IL-5 | IL-6 | IL-8 | IL-10 | IFNγ | TNFα |
|---|---|---|---|---|---|---|---|---|---|
| Previa | 0.87 ± 0.15 | 1.36 ± 0.10 | 6.57 ± 0.39 | 0.69 ± 0.15 | 16.7 ± 8.7 | 14.7 ± 4.1 | 3.96 ± 0.35 | 27.6 ± 0.9 | 12.8 ± 0.8 |
| AIP-Previa | 1.03 ± 0.19 | 1.44 ± 0.10 | 7.18 ± 0.41 | 1.18 ± 0.23 | 15.7 ± 5.6 | 33.2 ± 2.7* | 3.98 ± 0.33 | 28.2 ± 0.8 | 16.0 ± 0.9 |
Cytokines results in pg/mL. Data presented as mean ± SD.
AIP-Previa > Previa, p < 0.01.
Fig. 4. Maternal plasma IL-8 concentration and gestational age dependence:
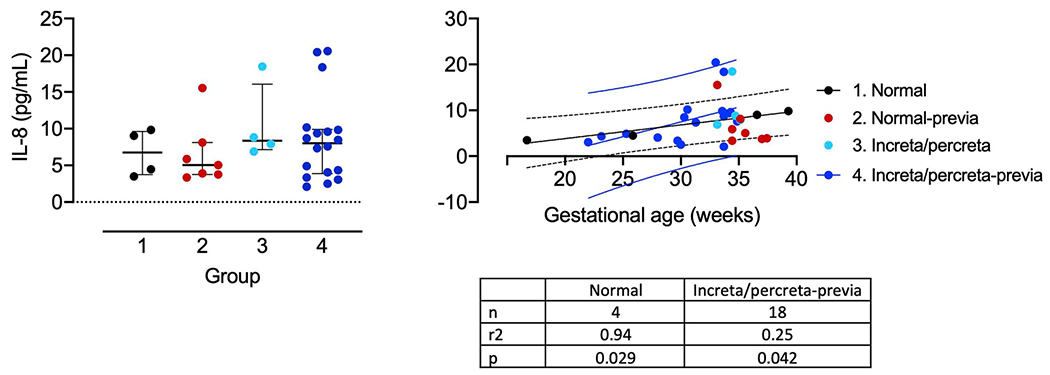
A. IL-8 concentration (pg/mL) for (1) normal pregnancies (open circles, n = 4) and pregnancies with (2) placenta previa (red, n = 7), (3) increta/percreta without placenta previa (light blue, n = 4), (4) increta/percreta with placenta previa (dark blue, n = 18). Data plotted as mean ± IR. B. Gestational age dependence of IL-8 concentration for the same groups. Linear regression lines are plotted for normal pregnancies (Group 1; black line) and with increta/percreta with placenta previa (Group 4; dark blue line). The dashed black and dark blue lines show the 95% prediction bands for the best-fit line for Group1 and Group 4 data respectively.
4. Discussion
This report examined maternal plasma for three potential biomarkers of PAS. Samples from normal term pregnancies, cases of uncomplicated placenta previa and PAS were assayed for h- and ß-hCG, for decorin and for IL-8. There was only one situation where a difference was observed. The mean decorin concentration in Group 2 (previa controls) was significantly higher than that for the Group 1 (normal controls), 3 (increta/percreta) and 4 (increta/percreta-previa), consistent with the prediction of a reduced decorin in PAS, as a result of the restriction of invasion caused by decorin. The relationship between biomarkers and gestational age was only of significance for h-hCG, where both groups 1 (normal) and 5 (accreta-previa) showed an increasing serum concentration with gestational age. For all the biomarkers there was substantial overlap in the range of individual values between cases and controls. The results show that there is no definitive separation between PAS and controls for any of the analytes, making them unsuitable as biomarkers.
It should be noted that, given the limited number of samples obtained prior to 25 weeks gestational age, interpretation of the gestational age dependence data should be treated with caution. Nevertheless, the data from pre-25 week samples is consistent with the data from the post-24 week samples and provides some information on the likely concentration of potential biomarkers in the earlier gestation period.
We separated increta/percreta without placenta previa (Group 3) from cases of increta/percreta with placenta previa (Group 4) as we were not sure whether the two groups were necessarily the same pathology. In the event, both groups showed similar expression values across the three potential biomarkers. Combining these two groups did not change any of the statistical analyses and we therefore present them as separate groups to demonstrate this lack of difference.
4.1. h-hCG
The hCG results presented here show no discrimination between the groups, whether for h-hCG, ß-hCG or for the h-hCG/ß-hCG ratio. The values we obtained are similar to those of Einerson et al. [12] and show a similar degree of scatter, however our study separately examined placenta previa controls in addition to the normal term pregnancies used as controls by Einerson et al. The mean value for the previa controls (group 2, 112 ± 9 pmol/L) was, if anything, closer to the PAS groups (group 3, 107 ± 16; group 4, 104 ± 2; group 5, 107 ± 6 pmol/mL) than that for the normal controls (128 ± 11 pmol/mL). The limited nature of the results did not justify further analysis.
4.2. Decorin
The decorin results showed that the mean for Group 2 (previa controls; 57 ± 9 pg/mL) was greater than that for Group 1 (normal controls; 36 ± 2 pg/mL), Group 3 (increta/percreta; 38 ± 6 pg/mL) and Group 4 (increta/percreta-previa; 35 ± 2 pg/mL). The previa group was composed of only five samples which overlapped with the range of values for the other groups, precluding definitive separation between the groups. Moreover, while the higher value for the previa controls compared to the PAS samples is consistent with an invasion-restricting role for decorin, the lower values of the normal controls is not. Combining the normal samples with the previa controls brings the mean value for the combined samples closer to those for the PAS samples and any difference is lost. There was a non-zero slope for the group 3 (increta/percreta) samples as a function of gestational age, however there were only four samples in this group, too few to rely on for a definitive assessment. When combined with group 4 (increta/percreta-previa) the slope was not significantly different from zero. Although loss of decorin in the PAS samples is consistent with trophoblast over-invasion, the overlap between the previa and PAS samples precludes the use of decorin as a biomarker.
4.3. IL-8
The only significant results for IL-8 were the non-zero slopes for the normal and increta/percreta-previa groups (groups 1 and 4) as a function of gestational age. However, there are only four samples in group 1 and thus this analysis cannot be taken as reliable indicator. The prediction bands for the two slopes show substantial overlap down to the mid-2nd trimester (Fig. 4B), suggesting that despite the differences in slope between the previa controls and the increta/percreta-previa cases, this data cannot be used to distinguish between them.
4.4. Other biomarker studies
One area of recurrent research in PAS has been the search for biomarkers which can predict PAS and/or various aspects of PAS (see Bartels et al. for a review; [13]). Several requirements are necessary of any PAS biomarker study. These include the histopathologic confirmation of case diagnosis and differentiation between PAS grades; use of the FIGO Clinical Classification System is recommended to allow for multi-center standardization. In addition, appropriate controls (placenta previa), sufficient samples for assessment and absence of overlap between control and case values are also necessary.
Several reports have measured cell-free RNA in the maternal circulation, finding increased levels in PAS [14–18], however they all suffer from low numbers of PAS cases and, in addition, are deficient in one or other of the requirements listed above. Multiple retrospective studies have taken advantage of testing of screening proteins such as alpha-fetoprotein, ß-hCG and PAPP-A to assess whether PAS is detectable in early gestation [19–24]. Not only are some of the results contradictory, there is overlap between values for cases and controls, there are low numbers of cases and in many, an absence of histopathologic confirmation or gradation into type of PAS. The question of timing is also important in these samples; while the samples are taken early enough that case-control discrimination would allow for therapeutic measures or early preparation, in many cases they may be earlier than disease begins to manifest in many cases. Finally, there are specific marker proteins which have been tested for differences in PAS. Angiogenic proteins such as VEGF and sFlt have shown contradictory results [25,26], as has the cardiac marker creatine kinase (CK [27,28]). Assessment of soluble TRAIL-R2, one of the receptors for TRAIL (TNFalpha-related apoptosis inducing ligand) in maternal serum in control, previa and PAS pregnancies and showed a decreased level of TRAIL-R2 in the PAS samples [29] however this was solely for placenta accreta, which may have included clinically rather than histopathologically confirmed cases. Moreover, the absence of individual values precludes assessment of overlap. Even those biomarker studies reported in the literature which have demonstrated a difference between case and control have not been evaluated earlier in pregnancy and/or have shown too much overlap between individual case and control values to be useful clinically.
4.5. Summary
Biomarker studies thus far are inconclusive. None of those tested here have satisfied the necessary criteria for PAS predictive markers. The >40 individual cases of increta/percreta-previa utilized in this study is the largest sample sizes of histopathologically confirmed PAS (grade 2 or 3 on the FIGO Clinical Classification System) that have been tested to date. The relative dearth of accreta (Grade 1) cases is due to the requirement for histopathological confirmation, as uterine conservation can be achieved in most of these cases. Most prior studies have not had the numbers necessary to confirm biomarker status. It is likely that a more sophisticated biomarker discovery process using multi-center sampling will be necessary to identify markers of value.
References
- [1].Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, Fox KA, Collins S, Diagnosis FPA, Management Expert Consensus P, FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders, Int. J. Gynaecol. Obstet 146 (1) (2019) 20–24. [DOI] [PubMed] [Google Scholar]
- [2].Handschuh K, Guibourdenche J, Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, Fournier T, Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell invasion and is down-regulated by peroxisome proliferator-activated receptor-gamma, Endocrinology 148 (10) (2007) 5011–5019. [DOI] [PubMed] [Google Scholar]
- [3].Guibourdenche J, Handschuh K, Tsatsaris V, Gerbaud P, Leguy MC, Muller F, Brion DE, Fournier T, Hyperglycosylated hCG is a marker of early human trophoblast invasion, J. Clin. Endocrinol. Metabol 95 (10) (2010) E240–244. [DOI] [PubMed] [Google Scholar]
- [4].Zou Y, Yu X, Lu J, Jiang Z, Zuo Q, Fan M, Huang S, Sun L, Decorin-mediated inhibition of human trophoblast cells proliferation, migration, and invasion and promotion of apoptosis in vitro, BioMed. Res. Int 2015 (2015) 201629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nandi P, Siddiqui MF, Lala PK, Restraint of trophoblast invasion of the uterus by decorin: role in pre-edampsia, Am. J. Reprod. Immunol 75 (3) (2016) 351–360. [DOI] [PubMed] [Google Scholar]
- [6].Siddiqui MF, Nandi P, Girish GV, Nygard K, Eastabrook G, de Vrijer B, Han VK, Lala PK, Decorin over-expression by decidual cells in preeclampsia: a potential blood biomarker, Am. J. Obstet. Gynecol 215 (3) (2016), 361 e361–361 e315. [DOI] [PubMed] [Google Scholar]
- [7].Borbely AU, Daher S, Ishigai MM, Mattar R, Sun SY, Knofler M, Bevilacqua E, Oliveira SF, Decorin and biglycan immunolocalization in non-villous structures of healthy and pathological human placentas, Histopathology 64 (5) (2014) 616–625. [DOI] [PubMed] [Google Scholar]
- [8].Waugh DJ, Wilson C, The interleukin-8 pathway in cancer, Clin. Cane. Res 14 (21)(2008) 6735–6741. [DOI] [PubMed] [Google Scholar]
- [9].DaSilva-Arnold S, James JL, Al-Khan A, Zamudio S, Illsley NP, Differentiation of first trimester cytotrophoblast to extravillous trophoblast involves an epithelial-mesenchymal transition, Placenta 36 (12) (2015) 1412–1418. [DOI] [PubMed] [Google Scholar]
- [10].DaSilva-Arnold SC, Zamudio S, Al-Khan A, Alvarez-Perez J, Mannion C, Koenig C, Luke D, Perez AM, Petroff M, Alvarez M, Illsley NP, Human trophoblast epithelial-mesenchymal transition in abnormally invasive placenta, Biol. Reprod 99 (2) (2018) 409–421. [DOI] [PubMed] [Google Scholar]
- [11].Jovanovic M, Stefanoska I, Radojcic L, Vicovac L, Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1, Reproduction 139 (4) (2010) 789–798. [DOI] [PubMed] [Google Scholar]
- [12].Einerson BD, Straubhar A, Soisson S, Szczotka K, Dodson MK, Silver RM, Soisson AP, Hyperglycosylated hCG and placenta accreta spectrum, Am. J. Perinatol 36 (1) (2019) 22–26. [DOI] [PubMed] [Google Scholar]
- [13].Bartels HC, Postle JD, Downey P, Brennan DJ, Placenta accreta spectrum: a review of pathology, molecular biology, and biomarkers, Dis. Markers 2018 (2018) 1507674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Miura K, Miura S, Yamasaki K, Yoshida A, Yoshiura K, Nakayama D, Niikawa N, Masuzaki H, Increased level of cell-free placental mRNA in a subgroup of placenta previa that needs hysterectomy, Prenat. Diagn 28 (9) (2008) 805–809. [DOI] [PubMed] [Google Scholar]
- [15].El Behery MM, Rasha LE, El Alfy Y, Cell-free placental mRNA in maternal plasma to predict placental invasion in patients with placenta accreta, Int. J. Gynaecol. Obstet 109 (1) (2010) 30–33. [DOI] [PubMed] [Google Scholar]
- [16].Kawashima A, Sekizawa A, Ventura W, Koide K, Hori K, Okai T, Masashi Y, Furuya K, Mizumoto Y, Increased levels of cell-free human placental lactogen mRNA at 28-32 gestational weeks in plasma of pregnant women with placenta previa and invasive placenta, Reprod. Sci 21 (2) (2014) 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou J, Li J, Yan P, Ye YH, Peng W, Wang S, Wang XT, Maternal plasma levels of cell-free beta-HCG mRNA as a prenatal diagnostic indicator of placenta accreta, Placenta 35 (9) (2014) 691–695. [DOI] [PubMed] [Google Scholar]
- [18].Naghshineh E, Azadehrah M, Rouholamin S, Portal vein and superior mesenteric vein thrombosis after cesarean hysterectomy, J. Res. Med. Sci 19 (2) (2014) 197. [PMC free article] [PubMed] [Google Scholar]
- [19].Zelop C, Nadel A, Frigoletto FD Jr., Pauker S, MacMillan M, Benacerraf BR, Placenta accreta/percreta/increta: a cause of elevated maternal serum alpha-fetoprotein, Obstet. Gynecol 80 (4) (1992) 693–694. [PubMed] [Google Scholar]
- [20].Kupferminc MJ, Tamura RK, Wigton TR, Glassenberg R, Socol ML, Placenta accreta is associated with elevated maternal serum alpha-fetoprotein, Obstet. Gynecol 82 (2) (1993) 266–269. [PubMed] [Google Scholar]
- [21].Desai N, Krantz D, Roman A, Fleischer A, Boulis S, Rochelson B, Elevated first trimester PAPP-A is associated with increased risk of placenta accreta, Prenat. Diagn 34 (2) (2014) 159–162. [DOI] [PubMed] [Google Scholar]
- [22].Dreux S, Salomon LJ, Muller F, Goffinet F, Oury JF, A.B.A.S. Group, Sentilhes L, Second-trimester maternal serum markers and placenta accreta, Prenat. Diagn 32 (10) (2012) 1010–1012. [DOI] [PubMed] [Google Scholar]
- [23].Thompson O, Otigbah C, Nnochiri A, Sumithran E, Spencer K, First trimester maternal serum biochemical markers of aneuploidy in pregnancies with abnormally invasive placentation, BJOG 122 (10) (2015) 1370–1376. [DOI] [PubMed] [Google Scholar]
- [24].Butler EL, Dashe JS, Ramus RM, Association between maternal serum alpha-fetoprotein and adverse outcomes in pregnancies with placenta previa, Obstet. Gynecol 97 (1) (2001) 35–38. [DOI] [PubMed] [Google Scholar]
- [25].Wehrum MJ, Buhimschi IA, Salafia C, Thung S, Bahtiyar MO, Werner EF, Campbell KH, Laky C, Sfakianaki AK, Zhao G, Funai EF, Buhimschi CS, Accreta complicating complete placenta previa is characterized by reduced systemic levels of vascular endothelial growth factor and by epithelial-to-mesenchymal transition of the invasive trophoblast, Am. J. Obstet. Gynecol 204 (5) (2011), 411.e411–411.e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Biberoglu E, Kirbas A, Daglar K, Biberoglu K, Timur H, Demirtas C, Karabulut E, Danisman N, Serum angiogenic profile in abnormal placentation, J. Matern. Fetal Neonatal Med 29 (19) (2016) 3193–3197. [DOI] [PubMed] [Google Scholar]
- [27].Ophir E, Tendler R, Odeh M, Khouri S, Oettinger M, Creatine kinase as a biochemical marker in diagnosis of placenta increta and percreta, Am. J. Obstet. Gynecol 180 (4) (1999) 1039–1040. [DOI] [PubMed] [Google Scholar]
- [28].Ersoy AO, Oztas E, Ozler S, Ersoy E, Erkenekli K, Uygur D, Caglar AT, Danisman N, Can venous ProBNP levels predict placenta accreta? J. Matern. Fetal Neonatal Med 29 (24) (2016) 4020–4024. [DOI] [PubMed] [Google Scholar]
- [29].Oztas E, Ozler S, Ersoy AO, Ersoy E, Caglar AT, Uygur D, Yucel A, Ergin M, Danisman N, Decreased placental and maternal serum TRAIL-R2 levels are associated with placenta accreta, Placenta 39 (2016) 1–6. [DOI] [PubMed] [Google Scholar]


