Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), has spread around the world, spurring the biomedical community to find and create antiviral therapies. The agent remdesivir, which has undergone a protracted and tortuous developmental path, is one potential therapeutic strategy now being assessed in several clinical trials. A broad-spectrum antiviral drug called remdesivir has already shown antiviral effects against filoviruses. Remdesivir was suggested as an exploratory medicine early in the pandemic because in vitro tests showed it to have antiviral effectiveness against SARS-CoV-2.
Methods
We conducted a retrospective cohort study that examined patient data captured through an electronic medical system at the Abu Arish General Hospital between 2021 and 2022. Data analysis was performed with SPSS version 25.0 (Armonk, NY: IBM Corp.).
Results
A total of 88 patients were included in this study. With the usage of remdesivir, our risk model is able to forecast adverse events and the case fatality rate. In contrast to D-dimer and c-reactive proteins, we showed that alanine transaminase (ALT), aspartate aminotransferase (AST), serum creatinine, and hemoglobin are relevant variables.
Conclusion
Our risk model can predict the adverse reactions and case fatality rate with the use of remdesivir. We demonstrated ALT, AST, serum creatinine, and hemoglobin as important variables rather than D-dimer and c-reactive proteins.
Keywords: hemoglobin, serum creatinine, ast, alt, covid-19, remdesivir
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease 2019 (COVID-19), has high morbidity and mortality rates [1-3]. Risk factors for the development of acute respiratory distress syndrome and death include advanced age, male sex, neutrophilia, organ dysfunction, coagulopathy, and high D-dimer levels [4]. Due to the COVID-19 pandemic’s rapid evolution during the first wave, health authorities concentrated on repurposing already-approved medications to create timely and affordable therapeutic approaches [5,6]. The mainstay of medical care for hospitalized COVID-19 patients continues to be supportive care, which includes providing oxygen and administering dexamethasone to patients who have invasive mechanical ventilation [7]. Various medications were subject to expanded access programs (EAP) and emergency use authorization (EUA). EAP has enabled drug repositioning for COVID-19, allowing for the use of drugs, such as remdesivir, baricitinib, tocilizumab, nirmatrelvir-ritonavir, and molnupiravir, as well as early access to COVID-19 convalescent plasma, casirivimab-imdevimab, bamlanivimab/etesevimab, and sotrovimab [1,7-9]. Broad-spectrum action against members of various viral families, such as filoviruses, paramyxoviruses, and coronaviruses, has been demonstrated, and the drug has been shown to have both preventive and therapeutic effects against these coronaviruses in nonclinical animals [10-12]. Accordingly, remdesivir was advertised as a candidate medication for COVID-19 therapy [13-16].
The European Medicines Agency has prepared paperwork for compassionate use that summarizes the pharmacokinetics of remdesivir (EMA, 2020). Remdesivir is injected intravenously (IV) and is given in two doses: a loading dose on day 1 (200 mg in adults; pediatric patients receive a dose that is tailored for body weight) and a daily maintenance dose (100 mg in adults) for a maximum of 10 days. Remdesivir has been shown to have a short plasma half-life in nonhuman primates (t1/2 = 0.39 h), but intracellular levels of the triphosphate form are sustained after daily dosing of 10 mg/kg [17,18]. Remdesivir’s effectiveness against SARS-CoV-2 and related coronaviruses was supported by in vitro and preclinical in vivo animal models. Among these is a current in vitro investigation of remdesivir’s antiviral activity against SARS-CoV-2 (formerly known as 2019-nCov, strain nCoV-2019BetaCoV/Wuhan/WIV04/2019) that measured the viral copy number in infected Vero E6 cells using qRT-PCR. In this work, an IC50 of 770 nM and an IC90 of 1,760 nM (at cytotoxic concentrations > 100 mM) were found [19,20]. Remdesivir’s effectiveness in vivo in inhibiting viral replication and reducing viral-associated pathologies against related coronaviruses was further established in research by Sheahan et al. and de Wit et al. [11,21].
As mentioned above, remdesivir (GS-5734) is a phosphoramidate prodrug of a monophosphate nucleoside analog (GS-441524) that inhibits the replication of viral genomes by RNA-dependent RNA polymerase (RdRp) [22,23]. Nucleoside analogs are not thought to pass easily through the cell wall. After entering the host cell, they must undergo phosphorylation to form nucleoside triphosphate (NTP), which is similar to adenosine triphosphate (ATP) and can be utilized for genome replication by RdRp enzymes or complexes [24-26]. Remdesivir is metabolized by host cells into its pharmacologically active analog adenosine triphosphate (GS-443902), which then competes with ATP for integration into the nascent RNA strand by the RdRp complex. This results in the termination of RNA synthesis, which restricts viral replication after a few more nucleotides have been incorporated [23,24]. In primary human airway epithelial cultures and human lung cells, remdesivir showed strong antiviral efficacy against SARS-CoV-2. With a half-maximal effective concentration, remdesivir also has a dose-dependent inhibitory effect on SARS-CoV-2 replication (EC50) [27,28]. Remdesivir is a substrate for the cytochrome P450 (CYP450) enzymes CYP2C8, CYP2D6, and CYP3A4, as well as the organic anion-transporting polypeptide OATP1B1, OATP1B3, and P-glycoprotein (P-gp) transporters. Remdesivir and its metabolites are thought to be CYP inhibitors in vitro, but there is no proof that they induce CYP. The possibility of clinically significant drug-drug interactions (DDIs) may be constrained by the drug’s mode of administration and quick clearance. However, more clinical research is necessary to assess how remdesivir interacts with the cytochrome P450 system and to identify any potential drug-drug interactions [29,30].
Finding the incidence of remdesivir use in the Intensive Care Unit (ICU) at Abu Arish General Hospital was one of the study’s key goals. Other goals of this study included determining the baseline traits of patients at the beginning of remdesivir treatment and in patients following remdesivir therapy. This study also evaluated the clinical results after using remdesivir.
Materials and methods
Retrospective data analysis was performed on all patients treated with remdesivir at Abu Arish General Hospital between 2021 and 2022. The ethics committee’s approval was required before the study could be carried out. Information was gathered from the individual hospitals’ medical records departments. This study was initiated after approval from the Jazan Health Ethics Committee (reference number: 22110), which granted a waiver of written informed consent.
All patients aged 18 years and over with critical illnesses whose doctors recommended remdesivir for COVID-19 between 2021 and 2022 were included. Critically ill was defined as patients with an oxygen saturation of 94% or needing additional oxygen or mechanical breathing. Patients under the age of 18 years were excluded, as were cases where remdesivir was prescribed for a condition other than COVID-19. Those who were not diagnosed with COVID-19 or not seriously unwell were also excluded.
The data was collected from the electronic medical system of the hospital in Jazan. The collected data comprised patient demographic data. Prior to the completion of the remdesivir course, baseline data on clinical features, such as a history of hypertension, dyslipidemia, hypothyroidism, kidney dysfunction, heart failure (HF), diabetes mellitus, and the levels of serum creatinine (SrCr) (mmol/L), hemoglobin, glucose, alanine transaminase (ALT), and aspartate aminotransferase (AST), were determined. Data were reported as median and ranges, means, and standard deviations (SDs) for continuous variables. A chi-square test for association and analysis of variance (ANOVA) for mean differences were applied accordingly. All analyses were done at 5% significance using SPSS version 25.0 (Armonk, NY: IBM Corp.).
Results
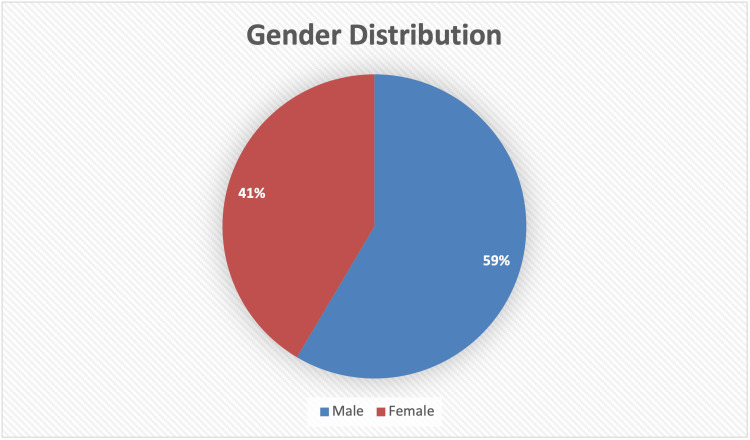
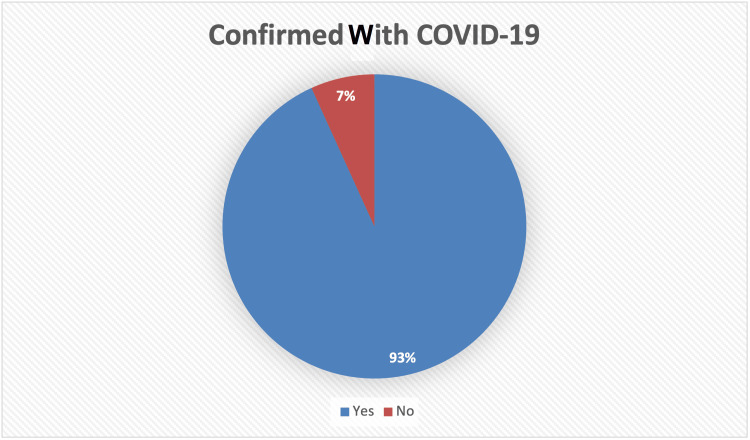
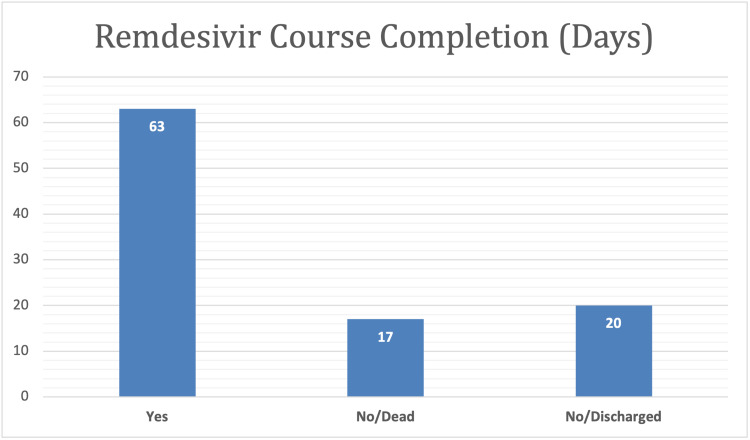
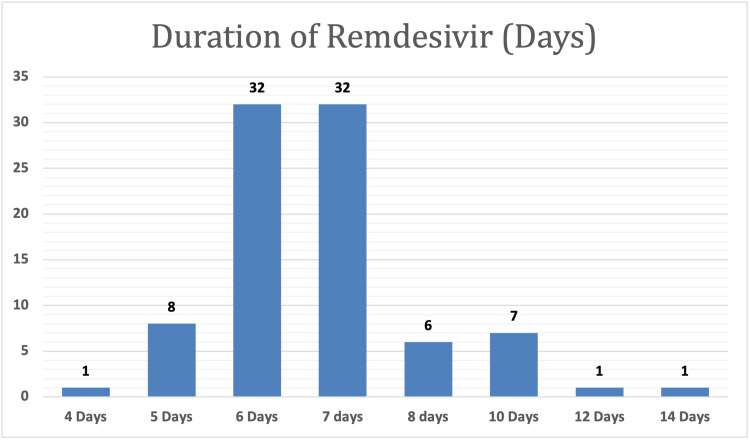
To explore the demographic and clinical characteristics of the study population, data were collected from a sample of 88 patients who had been administered “remdesivir” medicine, and the majority (93.2%) of them were confirmed to have COVID-19. Of these patients, 63% completed the course, 17% died, and 20% were discharged from the hospital. Furthermore, the results indicated that the majority of the patients were male (59.1%) and had used remdesivir for six to seven days (72.4%) (Table 1 and Figures 1-6).
Table 1. Demographic and clinical characteristics of the study population (N = 88).
N is the total number of participants.
| Variable | Level | N | % |
| Gender | Male | 52 | 59.1 |
| Female | 36 | 40.9 | |
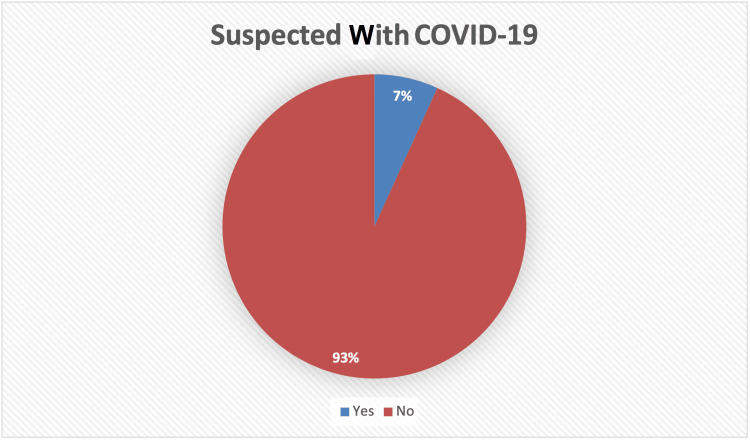
| Suspected with COVID-19 | Yes | 6 | 6.8 |
| No | 82 | 93.2 | |
| Confirmed with COVID-19 | Yes | 82 | 93.2 |
| No | 6 | 6.8 | |
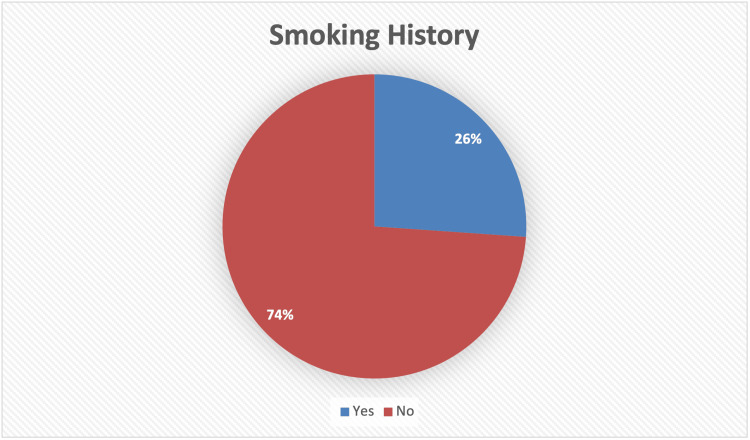
| Smoking history | Yes | 23 | 26.1 |
| No | 65 | 73.9 | |
| Remdesivir course completion | Yes | 55 | 63.0 |
| No/dead | 15 | 17.0 | |
| No/discharged | 18 | 20.0 | |
| Duration of remdesivir (days) | 4 | 1 | 1.1 |
| 5 | 8 | 9.1 | |
| 6 | 32 | 36.4 | |
| 7 | 32 | 36.4 | |
| 8 | 6 | 6.8 | |
| 10 | 7 | 8.0 | |
| 12 | 1 | 1.1 | |
| 14 | 1 | 1.1 |
Figure 1. Graphical representation of the gender distribution of study population.
Figure 2. Graphical representation of the smoking history of the study population.
Figure 3. Graphical representation of the suspected participants with COVID-19 distribution.
COVID-19: coronavirus disease 2019
Figure 4. Graphical representation of the confirmed participants with COVID-19 distribution.
COVID-19: coronavirus disease 2019
Figure 5. Graphical representation of the remdesivir course completion distribution in days.
Figure 6. Graphical representation of the duration of remdesivir in days.
Descriptive statistics, such as mean, standard deviation, minimum, and maximum, were calculated for the baseline data before the remdesivir course completion. Clinical characteristics included a history of hypertension, dyslipidemia, hypothyroidism, kidney dysfunction, HF, and diabetes mellitus; and levels of SrCr (mmol/L), hemoglobin, glucose, ALT, and AST (Table 2).
Table 2. Demographic and clinical characteristics before remdesivir course completion.
BMI: body mass index; ALT: alanine transaminase; AST: aspartate aminotransferase; SrCr: serum creatinine; CrCl: creatinine clearance; SD: standard deviation
| Variable | Mean | SD | Minimum | Maximum |
| Age | 69.23 | 17.02 | 16.00 | 108.00 |
| Weight | 72.05 | 15.94 | 23.00 | 120.00 |
| Height | 164.94 | 5.90 | 146.00 | 188.00 |
| BMI | 26.41 | 5.48 | 10.79 | 44.92 |
| ALT | 47.18 | 88.48 | 6.20 | 663.00 |
| AST | 94.65 | 265.36 | 10.20 | 2378.00 |
| Albumin | 30.78 | 6.07 | 17.40 | 44.48 |
| Bilirubin | 15.73 | 18.82 | 2.90 | 124.30 |
| Glucose | 8.69 | 4.76 | 2.63 | 31.70 |
| Hemoglobin | 12.02 | 2.30 | 5.73 | 16.00 |
| SrCr mmol/L | 103.75 | 49.25 | 24.00 | 291.00 |
| CrCl mL/min | 66.16 | 37.63 | 20.00 | 256.00 |
The results regarding the remdesivir loading dose of 200 mg/day and the maintenance dose of 100 mg/day indicated all patients received the loading dose. However, 97.27% of the patients received the maintenance dose (Table 3).
Table 3. Descriptive statistics of remdesivir loading and maintenance dose per day.
N: total number; SD: standard deviation; COVID-19: coronavirus disease 2019
| Remdesivir | COVID-19 confirmed | N | Mean | SD | Minimum | Maximum |
| Loading dose 200 mg/day | Yes | 82 | 198.78 | 11.04 | 100.00 | 200.00 |
| No | 6 | 200.00 | 0.00 | 200.00 | 200.00 | |
| Maintenance dose 100 mg/day | Yes | 80 | 100.00 | 0.00 | 100.00 | 100.00 |
| No | 6 | 100.00 | 0.00 | 100.00 | 100.00 |
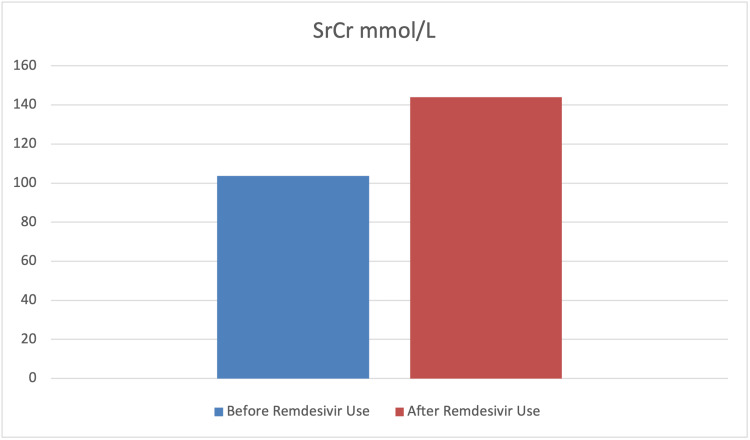
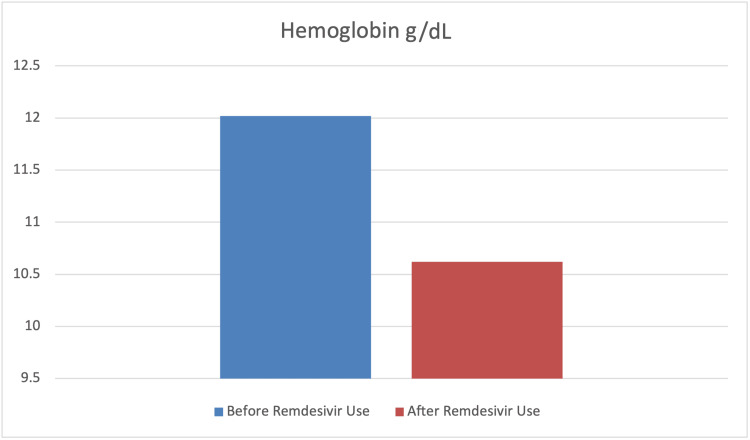
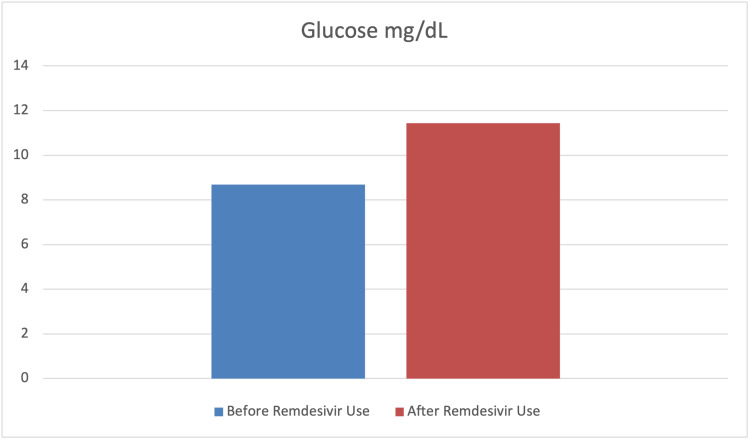
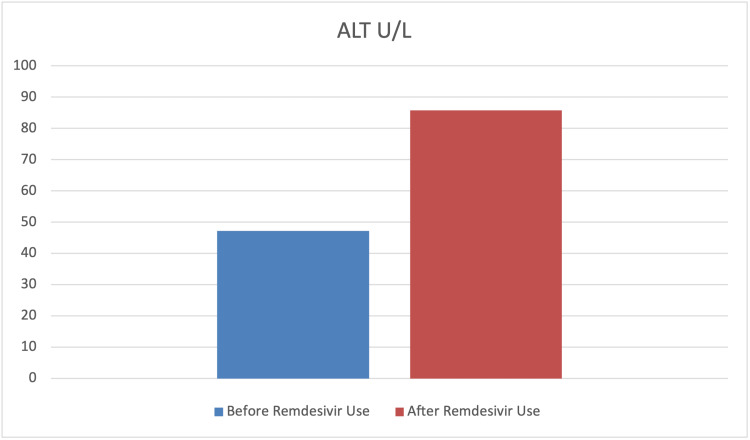
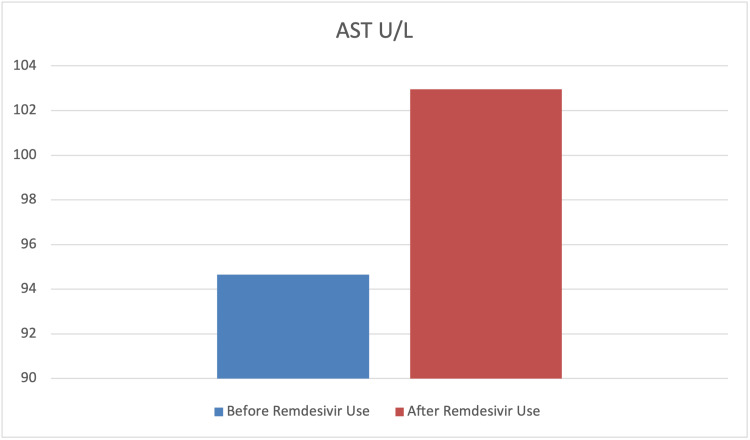
To assess the effect of remdesivir use on the levels of SrCr, hemoglobin, glucose, ALT, and AST paired sample t-tests were carried out. The findings indicated a significant mean difference before and after remdesivir use in terms of SrCr, hemoglobin, glucose, and ALT levels (p<0.001). However, the mean difference before and after remdesivir use in terms of AST was not statistically significant (p>0.05). Furthermore, the results indicated that the levels of SrCr, glucose, and ALT had significantly increased after the use of remdesivir. In contrast, the level of hemoglobin significantly decreased after its use. The findings also indicated that although the level of AST increased after the use of remdesivir, this difference was not statistically significant (p>0.05) (Table 4 and Figures 7-11).
Table 4. Mean comparison before and after remdesivir use in SrCr, hemoglobin, glucose, ALT, and AST levels.
ALT: alanine transaminase; AST: aspartate aminotransferase; SrCr: serum creatinine; SD: standard deviation; t: total number; SD: standard deviation; COVID-19: coronavirus disease 2019
| Variable | Before remdesivir use | After remdesivir use | t (87) | p-Value | Cohen’s syndrome | ||
| Mean | SD | Mean | SD | ||||
| SrCr mmol/L | 103.75 | 49.25 | 143.91 | 103.39 | -4.22 | <0.001 | 0.45 |
| Hemoglobin | 12.02 | 2.30 | 10.62 | 2.07 | 9.92 | <0.001 | 1.06 |
| Glucose | 8.69 | 4.76 | 11.43 | 6.18 | -5.84 | <0.001 | 0.62 |
| ALT | 47.18 | 88.48 | 85.77 | 143.76 | -3.05 | 0.003 | 0.33 |
| AST | 94.65 | 265.36 | 102.95 | 140.21 | -0.31 | 0.378 | 0.03 |
Figure 7. Graphical representation of the level of SrCr mmol/L before and after remdesivir use.
SrCr: serum creatinine
Figure 8. Graphical representation of the level of hemoglobin g/dL before and after remdesivir use.
Figure 9. Graphical representation of the level of glucose mg/dL before and after remdesivir use.
Figure 10. Graphical representation of the level of ALT U/L before and after remdesivir use.
ALT: alanine transaminase
Figure 11. Graphical representation of the level of AST U/L before and after remdesivir use.
AST: aspartate aminotransferase
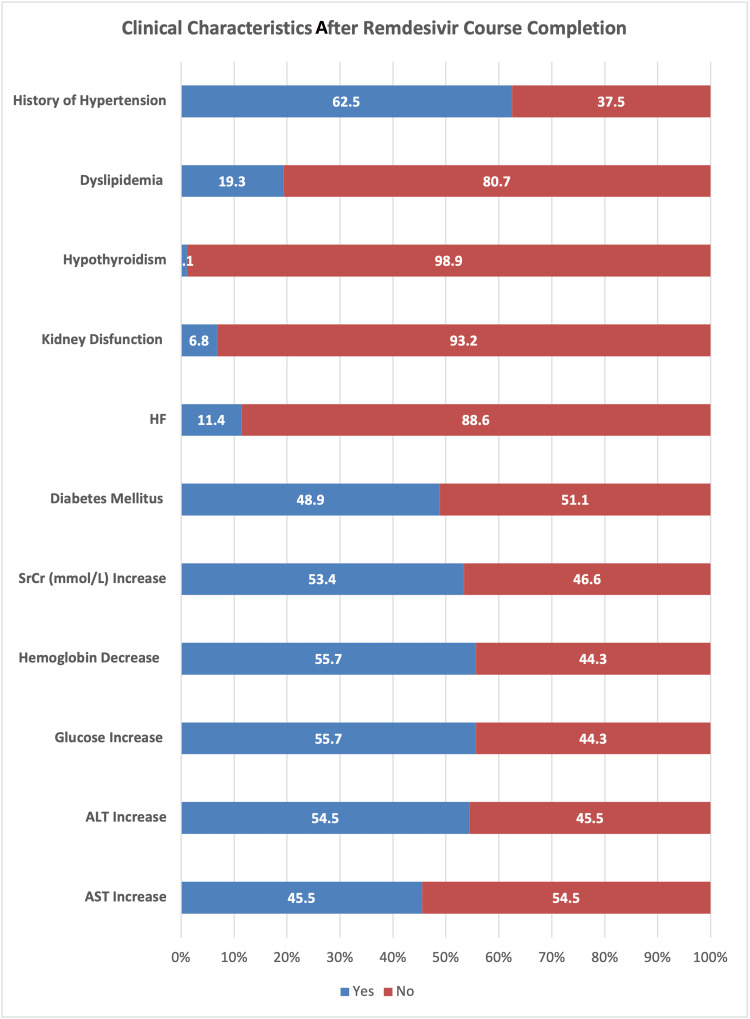
When examining the clinical characteristics after administering remdesivir, the results indicated that a history of hypertension was found in more than 62% of the patients. However, the histories of dyslipidemia (19.3%), hypothyroidism (1.1%), kidney dysfunction (6.8%), and HF (11.4%) were found to be very low. Furthermore, the findings indicated that after administering remdesivir, increases in the levels of SrCr (mmol/L), glucose, ALT, and AST were noted in 53.4%, 55.7%, 54.5%, and 45.5% of the patients, respectively. However, the hemoglobin level decreased in more than 55% of the patients (Table 5 and Figure 12).
Table 5. Clinical characteristics after remdesivir course completion (N = 88).
HF: heart failure; ALT: alanine transaminase; AST: aspartate aminotransferase; SrCr: serum creatinine; N: total participants
| Variable | Level | N | % |
| History of hypertension | Yes | 55 | 62.5 |
| No | 33 | 37.5 | |
| Dyslipidemia | Yes | 17 | 19.3 |
| No | 71 | 80.7 | |
| Hypothyroidism | Yes | 1 | 1.1 |
| No | 87 | 98.9 | |
| Kidney dysfunction | Yes | 6 | 6.8 |
| No | 82 | 93.2 | |
| HF | Yes | 10 | 11.4 |
| No | 78 | 88.6 | |
| Diabetes mellitus | Yes | 43 | 48.9 |
| No | 45 | 51.1 | |
| SrCr (mmol/L) increase | Yes | 47 | 53.4 |
| No | 41 | 46.6 | |
| Hemoglobin decrease | Yes | 49 | 55.7 |
| No | 39 | 44.3 | |
| Glucose increase | Yes | 49 | 55.7 |
| No | 39 | 44.3 | |
| ALT increase | Yes | 48 | 54.5 |
| No | 40 | 45.5 | |
| AST increase | Yes | 40 | 45.5 |
| No | 48 | 54.5 |
Figure 12. Graphical representation of the clinical characteristics after remdesivir course completion.
HF: heart failure; ALT: alanine transaminase; AST: aspartate aminotransferase; SrCr: serum creatinine
Discussion
At present, no specific antiviral drug is available for SARS-CoV-2 infection. Remdesivir, an adenosine analog developed to work specifically against the Ebola virus in 2014, is used on a compassionate basis in moderate-to-severely infected COVID-19 patients due to the lack of any other proven antiviral. Remdesivir is normally given for 5-10 days, which may be extended in severely ill patients, along with steroids. In our study, 63% of the patients received a complete course of remdesivir, 17% died, and 20% were discharged during the treatment course.
SARS-CoV-2 infection predominately involves the host’s respiratory system; however, SARS-CoV-2-induced renal and hepatic damage has also been reported [31,32]. Major pathophysiological features of liver injury in SARS-CoV-2 infection include hepatic lobular and portal inflammation, steatosis, congestion, focal necrosis with neutrophil infiltration, microthrombosis, and mild-to-moderate increases in liver aminotransferase (five times the upper limit of normal {ULN}) [33]. Remdesivir use in SARS-CoV-2 patients is associated with mild-to-moderate self-limiting hepatic injury, with no signs of jaundice [34]. Our study reported a statistically significant increase (p=0.003) in ALT in 54.5% of patients, while there were non-significant increases in AST seen in 45.5% of COVID-19-recovered patients after using remdesivir. Our results were almost similar to earlier studies, which have also reported mild-to-moderately elevated aminotransferase levels in 10-60% of COVID-19 patients after parenteral use of remdesivir with yet unknown mechanisms [1,35-37]. In our study, ALT was more increased than AST, which is contrary to some of the results published [38] but similar to others [39]. This self-limiting slight increase could be associated with the virus itself (as higher ACE2 expression on the hepatocyte’s surface), vascular endothelial injury and hypoxia [40], systemic inflammation [41], and drug-induced liver injury (DILI) [42]. Possible mechanisms of DILI include insulin resistance, lipid dystrophy, oxidative stress, and the inhibition of mitochondrial RNA polymerase. Remdesivir is metabolized in the liver by OATP1B and CYP3A4, which indicates the susceptibility of hepatocytes to drug-induced liver damage [43]. More than a 10 ULN increase in liver enzymes is alarming and should be addressed with great caution.
Hospitalized COVID-19 patients requiring intensive care may develop hyperglycemia (an increase in blood glucose {BG}), mainly due to increased glucose production, virus-induced immune response, and insulin resistance [44]. Moreover, direct SARS-CoV-2 damage to ACE3 BG receptors in pancreatic islets can cause hyperglycemia, even in non-diabetic COVID-19 patients. Acute-phase hyperglycemia could increase ACE2 expression, resulting in more viral particles entering host cells and delaying the recovery phase. Due to transient damage to beta cells, hyperglycemia may persist for three years after recovery from COVID-19 and is generally associated with poor outcomes of the disease [45,46]. As remdesivir is usually administered in combination with steroids, hyperglycemia is considered an indirect adverse drug reaction [47]. Our study reported increased BG levels in 55.7% of patients, which were statistically significant (p<0.001). These findings are similar to those reported earlier by Beigel et al., Awad et al., Hajjar et al., and Shrestha et al. [48-51].
Remdesivir is associated with acute kidney injury (AKI), an important complication in COVID-19 patients associated with a poor prognosis of disease, and AKI itself is associated with a 1.5-fold increase in serum creatinine levels from baseline [52]. Our study showed a statistically significant (p<0.001) increase in serum creatine levels in 53.4% of patients, which is similar to the findings reported by Kuno et al. and Sedighi et al., but contrary to those of Wong et al. [53-55].
Our study reported a significant decrease (p<0.001) in blood hemoglobin (Hb) levels in 55.7% of patients, which is similar to other findings in the literature [56,57]. The use of remdesivir is associated with a 2.8-fold risk of AKI [58]. Another study reported that SARS-CoV-2 can bind to the beta chain of hemoglobin through its surface glycoproteins, and their inhibition causes blood complications [59]. Elsewhere, SARS-CoV-2's ability to capture porphyrin has been examined, which in turn can inhibit heme metabolism. The interaction of SARS-CoV-2 with hemoglobin through ACE2, clusters of differentiation (CD)26, and CD147 has already been confirmed [60]. Decreased blood hemoglobin levels may result in sideroblastic anemia-like possibilities, along with myelodysplastic features and the need to replace worn-out erythrocytes. Red cell distribution width (RDW) is a marker of myelodysplasias, and COVID-19 data shows increased RDW in severely ill patients.
Our risk model allowed us to predict the adverse reactions and case fatality rates with the use of remdesivir. We demonstrated ALT, AST, serum creatinine, and hemoglobin as important variables rather than D-dimer and c-reactive proteins. The small sample size and retrospective nature of the analysis are the main limitations of this study.
Conclusions
The scientific community has rallied to sufficiently discover and assess new medicines and vaccinations as the COVID-19 pandemic continues to spread across the globe, and this community includes academia, government laboratories, small biotechnology firms, and global pharmaceutical corporations. The quickest therapeutic approach to stopping the pandemic’s spread involves repurposing or repositioning an efficient small-molecule drug. Remdesivir, one of these prospective treatments, has proven effective against coronaviruses in both in vitro and in vivo settings.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Jazan Health Ethics Committee issued approval #22110
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Huang C, Wang Y, Li X, et al. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.From SARS to MERS, thrusting coronaviruses into the spotlight. Song Z, Xu Y, Bao L, et al. Viruses. 2019;11 doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Geller C, Varbanov M, Duval RE. Viruses. 2012;4:3044–3068. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Zhou F, Yu T, Du R, et al. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Expanded access programs, compassionate drug use, and emergency use authorizations during the COVID-19 pandemic. Rizk JG, Forthal DN, Kalantar-Zadeh K, et al. Drug Discov Today. 2021;26:593–603. doi: 10.1016/j.drudis.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fields BN, Knipe DM, Howley PM. Philadelphia, PA: Lippincott Williams and Wilkins; 2007. Fields' virology. [Google Scholar]
- 7.Effect of dexamethasone in hospitalized patients with COVID-19 - preliminary report. Horby P, Lim WS, Emberson J, et al. MedRxiv. 2020 [Google Scholar]
- 8.A pneumonia outbreak associated with a new coronavirus of probable bat origin. Zhou P, Yang XL, Wang XG, et al. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Chen N, Zhou M, Dong X, et al. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GS-5734 and its parent nucleoside analog inhibit filo-, pneumo-, and paramyxoviruses. Lo MK, Jordan R, Arvey A, et al. Sci Rep. 2017;7 doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Sheahan TP, Sims AC, Leist SR, et al. Nat Commun. 2020;11 doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sheahan TP, Sims AC, Graham RL, et al. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compassionate use of remdesivir for patients with severe COVID-19. Grein J, Ohmagari N, Shin D, et al. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. Wu Z, McGoogan JM. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 15.Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. Li Q, Guan X, Wu P, et al. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cell-based assays to identify inhibitors of viral disease. Green N, Ott RD, Isaacs RJ, Fang H. Expert Opin Drug Discov. 2008;3:671–676. doi: 10.1517/17460441.3.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jumping species-a mechanism for coronavirus persistence and survival. Menachery VD, Graham RL, Baric RS. Curr Opin Virol. 2017;23:1–7. doi: 10.1016/j.coviro.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Uzunova K, Filipova E, Pavlova V, Vekov T. Biomed Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middle East respiratory syndrome coronavirus (MERS-CoV): animal to human interaction. Omrani AS, Al-Tawfiq JA, Memish ZA. Pathog Glob Health. 2015;109:354–362. doi: 10.1080/20477724.2015.1122852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. de Wit E, Feldmann F, Cronin J, et al. Proc Natl Acad Sci USA. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of ebola and emerging viruses. Siegel D, Hui HC, Doerffler E, et al. J Med Chem. 2017;60:1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 23.Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. Amirian ES, Levy JK. One Health. 2020;9 doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M. J Biol Chem. 2020;295:4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. Agostini ML, Andres EL, Sims AC, et al. mBio. 2018;9 doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, Hall MD. ACS Cent Sci. 2020;6:672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Wang M, Cao R, Zhang L, et al. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Pruijssers AJ, George AS, Schäfer A, et al. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pharmacokinetic, pharmacodynamic, and drug-interaction profile of remdesivir, a SARS-CoV-2 replication inhibitor. Humeniuk R, Mathias A, Kirby BJ, et al. Clin Pharmacokinet. 2021;60:569–583. doi: 10.1007/s40262-021-00984-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.What do we know about remdesivir drug interactions? Yang K. Clin Transl Sci. 2020;13:842–844. doi: 10.1111/cts.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.COVID-19 and liver injury: role of inflammatory endotheliopathy, platelet dysfunction, and thrombosis. McConnell MJ, Kondo R, Kawaguchi N, Iwakiri Y. Hepatol Commun. 2022;6:255–269. doi: 10.1002/hep4.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long-term kidney function recovery and mortality after COVID-19-associated acute kidney injury: an international multi-centre observational cohort study. Tan BW, Tan BW, Tan AL, et al. EClinicalMedicine. 2023;55 doi: 10.1016/j.eclinm.2022.101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liver injury in COVID-19: clinical features, potential mechanisms, risk factors and clinical treatments. Zhao SW, Li YM, Li YL, Su C. World J Gastroenterol. 2023;29:241–256. doi: 10.3748/wjg.v29.i2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theranostics for COVID-19 antiviral drugs: prospects and challenges for worldwide precision/personalized medicine. Manóchio C, Torres-Loureiro S, Scudeler MM, Miwa B, Souza-Santos FC, Rodrigues-Soares F. OMICS. 2023;27:6–14. doi: 10.1089/omi.2022.0151. [DOI] [PubMed] [Google Scholar]
- 35.Remdesivir and favipiravir changes hepato-renal profile in COVID-19 patients: a cross sectional observation in Bangladesh. Perveen R, Nasir M, Murshed M, Nazneen R, Ahmad S. Int J Med Sci Clin Invent. 2021;8:5196–5201. [Google Scholar]
- 36.Pharmacogenetic variants and risk of remdesivir-associated liver enzyme elevations in Million Veteran Program participants hospitalized with COVID-19. Tuteja S, Yu Z, Wilson O, et al. Clin Transl Sci. 2022;15:1880–1886. doi: 10.1111/cts.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Overview on remdesivir. Shambharkar SS. Int J Sci Res. 2021;10:1344–1349. [Google Scholar]
- 38.Liver and kidney function in patients with COVID-19 treated with remdesivir. van Laar SA, de Boer MG, Gombert-Handoko KB, Guchelaar HJ, Zwaveling J. Br J Clin Pharmacol. 2021;87:4450–4454. doi: 10.1111/bcp.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adverse events following remdesivir administration in moderately ill COVID-19 patients - a retrospective analysis. Gandham R, Eerike M, Raj GM, Bisoi D, Priyadarshini R, Agarwal N. J Family Med Prim Care. 2022;11:3693–3698. doi: 10.4103/jfmpc.jfmpc_2468_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prevalence and characteristics of hypoxic hepatitis in COVID-19 patients in the intensive care unit: a first retrospective study. Huang H, Li H, Chen S, et al. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.607206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Surviving the storm: cytokine biosignature in SARS-CoV-2 severity prediction. Ahmad R, Haque M. Vaccines (Basel) 2022;10 doi: 10.3390/vaccines10040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mechanism of SARS-CoV-2 invasion into the liver and hepatic injury in patients with COVID-19. Zhang X, Yu Y, Zhang C, et al. Mediterr J Hematol Infect Dis. 2022;14 doi: 10.4084/MJHID.2022.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoofnagle JH. Drug-Induced Liver Disease. Third Edition. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2013. LiverTox: a website on drug-induced liver injury; pp. 725–732. [Google Scholar]
- 44.Impact of COVID-19 therapy on hyperglycemia. Parise R, Deruiter J, Ren J, et al. Diab Vasc Dis Res. 2022;19 doi: 10.1177/14791641221095091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Singh AK, Singh R. Diabetes Res Clin Pract. 2020;167 doi: 10.1016/j.diabres.2020.108382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Yang JK, Lin SS, Ji XJ, Guo LM. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The effects of increased glucose level and glycolysis on SARS CoV-2 infection. Ozlem Zurnaci F, Guzel M. Mini Rev Med Chem. 2022;22:2344–2349. doi: 10.2174/1389557522666220318115350. [DOI] [PubMed] [Google Scholar]
- 48.Remdesivir for the Treatment of COVID-19 - final report. Beigel JH, Tomashek KM, Dodd LE, et al. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Risk of mortality due to sudden hyperglycemia in COVID-19 patients. Awad W, Kadhim HM, Hassan DM, Al-Yassiry KA. https://ijwph.ir/article-1-992-en.html Iran J Public Health. 2021;13:199–202. [Google Scholar]
- 50.Intensive care management of patients with COVID-19: a practical approach. Hajjar LA, Costa IB, Rizk SI, et al. Ann Intensive Care. 2021;11 doi: 10.1186/s13613-021-00820-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.New-onset diabetes in COVID-19 and clinical outcomes: a systematic review and meta-analysis. Shrestha DB, Budhathoki P, Raut S, et al. World J Virol. 2021;10:275–287. doi: 10.5501/wjv.v10.i5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acute kidney injury in hospitalized children with sickle cell anemia. Batte A, Menon S, Ssenkusu J, et al. BMC Nephrol. 2022;23 doi: 10.1186/s12882-022-02731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prediction of in-hospital mortality with machine learning for COVID-19 patients treated with steroid and remdesivir. Kuno T, Sahashi Y, Kawahito S, Takahashi M, Iwagami M, Egorova NN. J Med Virol. 2022;94:958–964. doi: 10.1002/jmv.27393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linear mixed model analysis to evaluate correlations between remdesivir adverse effects with age and gender of patients with mild Covid-19 pneumonia. Sedighi M, Amanollahi A, Moradi Moghaddam O, Basir Ghafouri H, Hoseini SE, Tavakoli N. J Med Virol. 2022;94:3783–3790. doi: 10.1002/jmv.27800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Remdesivir use and risks of acute kidney injury and acute liver injury among patients hospitalised with COVID-19: a self-controlled case series study. Wong CK, Au IC, Cheng WY, et al. Aliment Pharmacol Ther. 2022;56:121–130. doi: 10.1111/apt.16894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clinical course and outcomes of COVID-19 in kidney transplant recipients. Bajpai D, Deb S, Bose S, et al. Indian J Nephrol. 2022;32:467–475. doi: 10.4103/ijn.IJN_509_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Effectiveness of remdesivir with corticosteroids for COVID-19 patients in intensive care unit: a hospital-based observational study. Hanafusa M, Nawa N, Goto Y, et al. J Med Virol. 2023;95 doi: 10.1002/jmv.28168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Acute kidney injury associated with remdesivir: a comprehensive pharmacovigilance analysis of COVID-19 reports in FAERS. Wu B, Luo M, Wu F, He Z, Li Y, Xu T. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.692828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.COVID-19 disease: ORF8 and surface glycoprotein inhibit heme metabolism by binding to porphyrin. Liu W, Li H. https://chemrxiv.org/engage/chemrxiv/article-details/60c74e1dbb8c1ab62f3db6cb ChemRxiv. 2020 [Google Scholar]
- 60.COVID- 19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. Liu W, Hualan L. https://chemrxiv.org/engage/chemrxiv/article-details/60c74fa50f50db305139743d ChemRxiv. 2020 [Google Scholar]