Abstract
Background
Previous research has reported the association between social isolation and cognitive impairment. However, biological mechanisms underlying this association are understudied. It is also unclear whether there are sex differences in these biological mechanisms.
Objectives
To examine whether chronic inflammation biomarkers are potential mediators of the association between social isolation and cognitive functioning among older men and women.
Methods
Data were the National Health and Nutrition Examination Survey 1999–2002. A total of 2,535 older adults aged 60 and older were included. Chronic inflammation was measured by C-reactive protein (CRP), plasma fibrinogen, and serum albumin. Cognitive functioning was assessed by the Digit Symbol Substitution Test (DSST). Social isolation was defined using a 4-point composite index of items pertaining to the strength of social network and support. Linear regression models and formal mediation analysis were applied.
Results
Social isolation was associated with lower DSST scores [β (SE) = −2.445 (1.180), p < 0.01 for men; β (SE) = −5.478 (1.167), p < 0.001 for women]. For older men, social isolation was associated with higher levels of CRP (β [SE] = 0.226 (0.110), p < 0.05) and fibrinogen (β [SE] = 0.058 (0.026), p < 0.05). In mediation analyses, among older men, CRP mediated 6.1% and fibrinogen mediated 12.0% of the association of social isolation with DSST.
Conclusion
Social isolation was associated with poorer cognitive functioning partially via heightened inflammatory responses in older men. Defining these associations’ mechanisms in sex-specific contexts could inform preventive and therapeutic strategies for cognitive impairment in older adults.
Keywords: Loneliness, Gender differences, Dementia, Cognitive testing, Mild Cognitive Impairment (MCI)
1. Introduction
Cognitive functioning is one of the major determinants of well-being in later life. Prevention of cognitive decline could prevent cognitive impairment, a major risk factor that contributes to disability and social care needs among the older population globally. In 2021, over 6.2 million older adults in the United States (US) were living with Alzheimer’s disease and related dementia (ADRD), and the cost for all individuals with ADRD is $355 billion (Alzheimer’s Association, 2021). As poorer cognitive functioning is one of the main manifestations of cognitive impairment and/or ADRD (Plassman et al., 2011), exploring risk factors and pathways underlying poorer cognitive functioning will inform interventions to prevent and/or manage cognitive impairment and/or ADRD.
Being socially connected is a fundamental drive and core human need (Cacioppo et al., 2014). Social isolation, defined as having few social relationships or infrequent social contact with others, has been associated with higher risks for cognitive impairment and ADRD (Biddle et al., 2019; Joyce et al., 2022; Shen et al., 2022; Yu et al., 2020). Behavioral and psychological processes that linking social isolation with ADRD have been proposed. Social isolation may promote unhealthy behaviors such as smoking and physical inactivity (Shankar et al., 2011), which can increase the risks of dementia (Livingston et al., 2020). Moreover, depression is implicated in the association between perceived social isolation and poor cognitive health (Donovan et al., 2017), which can also be extended to dementia (Livingston et al., 2020). Several theories have been proposed to explain the association of social isolation and diminished cognitive functioning. The “use it or lose it” theory (Hultsch et al., 1999), which argues that engaging in social and physical activities stimulates the brain. The decreased engagement in social activities may result in the lack of use of mental faculties, thus, leading to cognitive decline. Another is the stress-buffering theory (Ditzen & Heinrichs, 2014), which proposes that maintaining social connection is beneficial when coping with stressful situations. Social connection may prevent or modulate responses to stressful events that are damaging to cognitive health.
Although behavioral and psychological processes linking social isolation to cognitive impairment and ADRD have been studied (Donovan et al., 2017; Shankar et al., 2011), biophysiological mechanisms underlying these links are not well elucidated. Social isolation is proposed to have a biological impact because it acts as a social stressor and activates the stress response (Cacioppo et al., 2015). Studies have shown that activation of the stress response (e.g., hypothalamic-pituitary-adrenocortical [HPA] axis) has a direct effect on the heightened inflammatory response (Ayoub, 2010; Eisenberger et al., 2017). In addition, an evolutionary explanation for the activation of the inflammatory system in response to social isolation has been proposed (Cacioppo et al., 2014, 2015; Eisenberger et al., 2017). This is because individuals would have been more likely to be attacked or injured if they were socially isolated than if they were around people who could defend them. Thus, the activation of the inflammatory response when someone is socially isolated could confer an evolutionary advantage as he/she would be prepared to respond biologically to the increased stress. This explanation has also been supported by prior research which found that social isolation is linked to higher systemic, low-grade, chronic inflammation markers, such as C-reactive protein (CRP) (Cudjoe et al., 2021; Das, 2013; Yang et al., 2013), interleukin-6 (IL-6) (Cudjoe et al., 2021; Glei et al., 2012), and high fibrinogen levels (Shankar et al., 2011; Yang et al., 2013)
Inflammation is a biological response to infection or injury in which the body produces more of a number of chemicals that aid in the defense against infection (Ayoub, 2010). An elevation in inflammatory markers is independently associated with smaller regional brain volumes and larger ventricular volume (Walker et al., 2017), and chronic inflammation has been implicated in the neuropathological cascade culminating in ADRD (Gorelick, 2010). Studies suggested that inflammatory markers, such as high-sensitive CRP, IL-6, high fibrinogen levels, and low albumin levels, are predictive of global markers of brain atrophy (Satizabal et al., 2012), an accelerated cognitive decline (Kipinoinen et al., 2022), and the risk of dementia (Lewis & Knight, 2021). However, whether and how inflammatory responses mediate the association between social isolation and cognitive functioning is largely unknown.
Prior research suggests that older men with a higher level of social disconnection are more vulnerable to dementia and mortality than older women (Yang et al., 2013; Zhou et al., 2018). For example, a nationwide cohort study found that older Chinese men who felt lonely were more likely to suffer from dementia than older Chinese women (Zhou et al., 2018). Another 18-year longitudinal study identified notable sex differences in which social isolation has greater mortality effects for men (Yang et al., 2013). Potential explanations for this finding have emerged from studies showing sex differences in the biological processes influenced by social isolation. Reviews of studies on social isolation in relation to health outcomes suggest sex differences in physiological responses to social isolation. For instance, one study found that the association between social isolation and elevated CRP is stronger for older men compared with older women (Loucks et al., 2006). Another study suggests sex differences in the associations of social isolation with inflammation (Yang et al., 2013). However, it remains unclear whether there are significant sex differences in the moderating role of chronic inflammation on the associations between social isolation and cognitive functioning.
Using a nationally representative population-based study, we extend previous research to test the hypothesis that social isolation is associated with poorer cognitive functioning via elevated inflammation. We address additional gaps in the literature by hypothesizing that these associations will be more prominent among older men.
2. Methods
2.1. Study design and population
The National Health and Nutrition Examination Survey (NHANES) is a consecutive cross-sectional survey performed in two-year cycles among non-institutionalized US civilians. The NHANES used a stratified, multistage, clustered probability sampling approach to represent the overall population in the US; it is administered by the US National Center for Health Statistics (NCHS) that collects sociodemographic, dietary, and health information through in-home interviews and clinical examinations in mobile examination centers. The survey oversampled the racial/ethnic minorities and adults aged 60 and above to improve statistical power in analyses. Detailed information on the survey design and data collection can be found elsewhere (Curtin et al., 2012). All data collection procedures for the survey were approved by the NCHS research ethics review board.
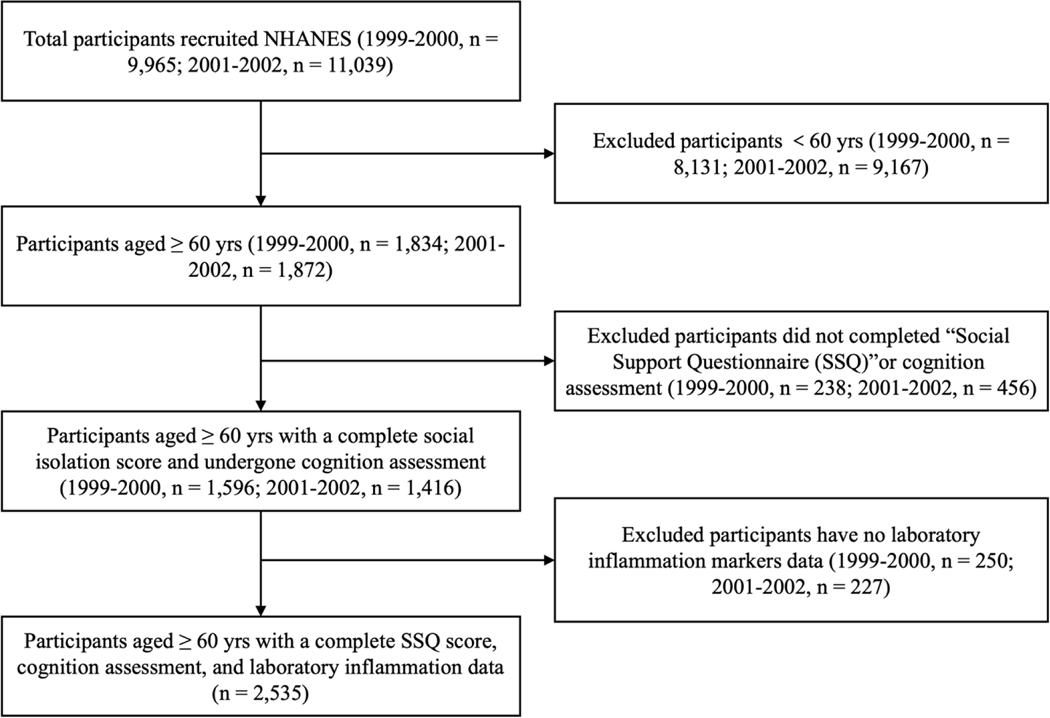
The NHANES 1999–2000 and 2001–2002 cycles were selected for analysis because they are the only cycles for which key variables to test our hypotheses were measured. The NHANES 1999–2002 has an overall sample of 3,706 older adults aged 60 and older. We applied the following exclusions in the study: 1) did not complete the “Social Support Questionnaire” (287 excluded), 2) cognitive measurement data were missing (407 excluded), and 3) had no laboratory inflammation markers data (477 excluded). A total of 2,535 eligible participants were included in the analyses. The flowchart of sample selection is present in Figure 1.
Figure 1. Flow chart of participants in the National Health and Nutrition Examination Survey Study 1999–2002.
2.2. Measurements
2.2.1. Assessment of social isolation
Social isolation in this study is derived from the Yale Health and Aging Study (Seeman & Berkman, 1988) and the social network index developed by Berkman and Syme (Berkman & Syme, 1979), which summarizes the strength of the social network and support across four domains: marital status, number of close friends or confidants, emotional support, and financial support. The first question asked whether the participant was married or living with a partner (0 = married or living with a partner; 1 = widowed, divorced, separated, or never married). The second question asked, “How many close friends or confidants do you have?” (0 = at least one close friend or confidant; 1 = no close friends or confidants). A third question characterized the availability and adequacy of emotional support. Participants were asked if they had no one to provide emotional support (e.g., talking over problems or helping to make a difficult decision) in the past 12 months. Participants who were “at risk” for inadequate emotional support if they answered “yes” (inadequate emotional support = 1). The fourth question asked about the availability of financial support, “If you need some extra financial help (e.g., paying any bills, housing costs, hospital visits, or providing you with food or clothes), could you count on anyone to help?” Those who answered “no” were coded as “1”.
The four indicators of social isolation were summed up to create a social isolation score (SIS). The SIS ranged from 0 to 4, with higher scores indicating greater isolation. The Cronbach’s alpha for the SIS is 0.703, demonstrating good internal consistency (Tavakol & Dennick, 2011). This valid measure has been used to evaluate social isolation of US adults in previous studies (Coyle et al., 2017; Mick et al., 2014). Theoretically, it would follow that those with a higher SIS can be considered socially isolated (Shankar et al., 2011). Following the methods of previous studies using NHANES (Coyle et al., 2017; Mick et al., 2014), participants were coded as “socially isolated” if their score was two or greater (social isolation = 1).
2.2.2. Assessment of cognitive functioning
The Digit Symbol Substitution Test (DSST), a performance module from the Wechsler Adult Intelligence Scale III, was the only cognitive test in NHANES 1999–2002. DSST assesses the processing speed, sustained attention, and working memory (Wechsler & Psychological Corporation, 1997). As a screening tool, the DSST has been used extensive to measure cognitive function in screenings, epidemiological, and clinical studies (Jaeger, 2018) and was administered during the in-home interviews to adults aged 60 and older. The DSST is highly sensitive to various types of brain dysfunction and discriminates well between mild cognitive impairment and ADRD (Tsatali et al., 2021). The test is conducted using a paper form with a top key containing nine numbers paired with symbols. Participants have 120 seconds to copy the corresponding symbols in the 133 boxes that adjoin the numbers. The DSST score is the total number of correct matches. A higher score represents better cognitive functioning, with a maximum score of 133.
2.2.3. Markers of chronic inflammation
We measured chronic inflammation using three inflammatory markers available in the NHANES 1999–2002: CRP, plasma fibrinogen, and serum albumin (National Center for Health Statistics (U.S.), 2015). CRP was examined using latex-enhanced nephelometry, an analysis of light scattering of antigen-antibody complexes with latex particles. Fibrinogen was measured through thrombin clotting time, in which thrombin was used to convert fibrinogen into fibrin enzymatically. Albumin was measured by dye binding using bromocresol green reagents and an Astra 8 analyzer (Beckman Instruments, Brea, CA). These markers’ detailed laboratory measurements and assay procedures have been described elsewhere (National Center for Health Statistics (U.S.), 2015). We used natural continuous log-transformed CRP, fibrinogen, and albumin to account for the skewness in the distribution of continuous inflammatory markers.
2.2.4. Covariates
Covariates were selected based on studies that examined the association between social isolation and cognitive functioning (Joyce et al., 2022; Shen et al., 2022; Yu et al., 2020). These covariates included: 1) sociodemographic variables: age, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanics, or others), education (less than high school, high school graduate, more than high school), and annual household income (<$20,000, $20,000-$75,000, or >$75,000); 2) behavioral characteristics: smoking status (nonsmokers [<100 cigarettes during one’s lifetime], former smoker [100 or more cigarettes during one’s lifetime, but not actively smoking during recent time frame], or current smoker [ongoing smoking habit]), alcohol intake (≥12 drinks/year or <12 drinks/year), and did moderate/vigorous leisure time physical activities over the past month (yes/no); 3) health-related characteristics: obesity defined as body mass index of at least 30 kg/m2, hypertension defined as systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg, and diabetes mellitus defined as self-reported diabetes diagnosis or glycated hemoglobin level of ≥ 5.7%.
2.3. Statistical analyses
Continuous variables were described as mean (standard deviation, SD) for normal distributions and median (interquartile range, IQR) for skewed distributions. Categorical variables were described as numbers (percentage, %). Comparisons among socially isolated/not isolated participants were performed using t-tests for continuous variables and the chi-square tests for categorical variables. Step-wise linear regression models were conducted to investigate the association of social isolation with the three log-transformed inflammatory markers and cognitive functioning. Model 1 was unadjusted, and the relevant sociodemographic, behavioral, and health factors, as described above, were incorporated into the regression model in Model 2–4. The standardized regression coefficients (β) and their standard errors (SEs) were presented.
We conducted formal mediation analyses to investigate whether each inflammatory marker mediates the associations between social isolation and cognitive functioning. As shown in Supplementary Figure 1, we set three pathways to establish mediation: 1) exposure to mediator (path a), 2) mediator to outcome (path b), and 3) exposure to outcome (path c). The total effect reflected the sum of a direct effect (path c’) and an indirect (mediated, a × b) effect. The percentage of variance in the outcome explained by the mediated effect was calculated using the following formula: (βtotal effect – βdirect effect) / βtotal effect × 100% (Ditlevsen et al., 2005). All the mediation analyses standardized estimates with SEs and p-values were based on nonparametric bootstrapping with 1,000 resamplings.
To demonstrate the robustness of the results, we performed a sensitivity analysis using dichotomous inflammatory markers to assess possible threshold effects. We identified cut points to dichotomize continuous inflammation marker measurements into variables that distinguished high risk for chronic inflammation. We dichotomized CRP at ≥3.0 mg/dL, albumin at 4.0 μg/mL, and fibrinogen at the top quartile. The cut-points for high risk for chronic inflammation were defined by clinical practice and previous studies (Myers et al., 2004; Osimo et al., 2019; Pearson et al., 2004). Step-wise logistic regression was used for the analogous models with outcomes of dichotomous inflammatory markers. Based on the conceptual model depicted in Appendix 2, we evaluated the two sets of models for each inflammation marker: 1) social isolation alone, 2) social isolation and dichotomized inflammatory markers. All models were adjusted to all covariates. Additionally, since our focus is on chronic inflammation, we did another sensitivity analysis by excluding six participants whose CRP values were greater than 10 mg/dL, which indicates acute infection or active inflammatory disorders (Pearson et al., 2004). Our results and conclusions were unchanged after excluding individuals with CRP ≥10.0 mg/dL.
Estimates for means, SD, proportions, effect sizes and their SEs or 95% confidence intervals (CIs) were adjusted for the complex survey design, survey nonresponse, and post-stratification of NHANES. Considering the low percentage of missing data for all the covariates, complete-case multivariable regression was conducted. The comparison of individuals with complete data and those with missing data showed no significant difference in social isolation and cognitive functioning. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using STATA 15.1 (StataCorp, College Station, TX, USA).
3. Results
3.1. Participants’ characteristics
Table 1 presents the characteristics of 1,267 men and 1,268 women by social isolation, with weighted descriptive statistics. The mean age was 71.05 (SD = 7.77) years old. A total of 190 (15.0%) older men and 270 (21.3%) older women were socially isolated. Bivariate analyses showed that socially isolated men had a significantly elevated inflammation measured by CRP and fibrinogen (p < 0.05). Social isolated older adults had lower cognitive functioning scores (p < 0.001) than those not isolated in both sexes.
Table 1.
Selected characteristics among 2,535 older adults aged ≥60 and stratified by social isolation and sex: NHANES 1999–2002 a
| Characteristics | Overall (N = 2,535) | Men (n = 1,267) | Women (n = 1,268) | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Socially isolated (n = 190) | Not isolated (n = 1,077) | P - Value | Socially isolated (n = 270) | Not isolated (n = 998) | P - Value | ||
|
|
|||||||
| Age (years), mean (SD) | 71.05 (7.77) | 71.20 (7.26) | 70.87 (7.65) | 0.586 | 71.89 (8.21) | 71.01 (7.87) | 0.104 |
| Race/ethnicity (%) | |||||||
| Non-Hispanic white | 59.6 | 46.8 | 63.3 | <0.001 | 47.8 | 61.3 | <0.001 |
| Non-Hispanic black | 14.8 | 14.2 | 14.2 | 17.0 | 14.8 | ||
| Hispanics | 23.4 | 37.4 | 20.2 | 32.6 | 21.7 | ||
| Others | 2.2 | 1.6 | 2.2 | 2.6 | 2.1 | ||
| Educational attainment (%) | |||||||
| Less than high school | 40.1 | 60.0 | 36.7 | <0.001 | 48.0 | 37.8 | <0.01 |
| High school graduate | 24.0 | 13.7 | 22.7 | 23.4 | 27.6 | ||
| More than high school | 35.9 | 26.3 | 40.6 | 28.6 | 34.6 | ||
| Annual household income (%) | |||||||
| <$20,000 | 43.7 | 65.1 | 35.7 | <0.001 | 59.9 | 44.0 | <0.001 |
| $20,000-$75,000 | 43.8 | 29.1 | 49.2 | 35.2 | 43.0 | ||
| >$75,000 | 12.5 | 5.8 | 15.2 | 4.9 | 13.0 | ||
| Smoking status (%) | |||||||
| Nonsmoker | 46.3 | 31.6 | 31.3 | <0.01 | 57.8 | 62.2 | 0.296 |
| Former smoker | 41.7 | 47.4 | 55.7 | 30.4 | 28.6 | ||
| Current smoker | 12.0 | 21.1 | 13.0 | 11.9 | 9.2 | ||
| Alcohol intake >12 drinks/year (%) | 60.9 | 77.1 | 77.1 | 0.996 | 44.1 | 47.4 | 0.336 |
| Moderate/vigorous physical activities (%) | 47.0 | 39.0 | 55.0 | <0.001 | 32.7 | 43.8 | <0.001 |
| Obesity (%) | 31.4 | 26.5 | 27.6 | 0.767 | 36.0 | 35.3 | 0.829 |
| Hypertension (%) | 51.7 | 48.4 | 46.9 | 0.702 | 60.0 | 55.3 | 0.168 |
| Diabetes mellitus (%) | 19.6 | 23.6 | 20.3 | <0.05 | 23.7 | 17.1 | <0.01 |
| Chronic inflammatory markers | |||||||
| CRP (mg/dL) b, medium (IQR) | 0.29 (0.45) | 0.30 (0.45) | 0.23 (0.39) | <0.05 | 0.41 (0.57) | 0.32 (0.50) | <0.05 |
| <3.0 (normal/low risk) | 91.7 | 89.5 | 93.7 | <0.05 | 89.6 | 90.3 | 0.739 |
| ≥3.0 (high risk) | 8.3 | 10.5 | 6.3 | 10.4 | 9.7 | ||
| Plasma fibrinogen | 393.39 | 401.69 | 381.58 | <0.01 | 411.72 | 397.25 | <0.05 |
| (mg/dL), mean (SD) | (83.60) | (92.84) | (84.66) | (85.31) | (79.24) | ||
| Serum albumin (μg/mL) b, medium (IQR) | 4.60 (6.9) | 4.60 (10.55) | 4.70 (7.65) | 0.845 | 4.6 (6.95) | 4.50 (5.20) | 0.264 |
| ≥4.0 (normal) | 86.0 | 93.5 | 89.4 | 0.089 | 84.3 | 81.5 | 0.308 |
| <4.0 (high risk) | 14.0 | 6.5 | 10.6 | 15.7 | 18.5 | ||
| Cognitive functioning (DSST), mean (SD) | 41.69 (18.59) | 34.07 (16.99) | 41.28 (17.81) | <0.001 | 35.79 (17.45) | 45.17 (19.13) | <0.001 |
Note:
Descriptive statistics are weighted to adjust for complex survey design.
Non-normal distribution continuous variable, median (interquartile range).
Abbreviations: SD, standard deviation; IQR, interquartile range; CRP, C-reactive protein; DSST, digit symbol substitution test.
3.2. Association of social isolation with cognitive functioning
As shown in Model 1 (Table 2), socially isolated older adults were found to have a poorer cognitive functioning than those not isolated (β [SE] = −8.795 [1.772], p < 0.01 for men; β [SE] = −9.190 [1.492], p < 0.001 for women). The significant association persists after controlling for sociodemographic, behavioral, and health-related characteristics in Model 2–4. Compared with older adults not isolated, socially isolated older adults had poorer cognitive functioning (β [SE] = −2.445 [1.180], p < 0.01 for men; β [SE] = −5.478 [1.167], p < 0.001 for women).
Table 2.
Associations of social isolation with the digit symbol substitution test score (n = 2,535)
| Weighted β coefficients (Standard Error) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Men (n = 1,267) | Women (n = 1,268) | |||||||
|
|
||||||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 1 | Model 2 | Model 3 | Model 4 | |
|
| ||||||||
| Variables | ||||||||
|
| ||||||||
| Social isolation (Ref. No) | −8.795 (1.772)*** | −3.542 (1.471)** | −2.828 (1.188)** | −2.445 (1.180)** | −9.190 (1.492)*** | −5.072 (1.213)*** | −5.761 (1.166)*** | −5.478 (1.167)*** |
| Age | −0.688 (0.061)*** | −0.710 (0.063)*** | −0.724 (0.067)*** | −0.870 (0.069)*** | −0.827 (0.074)*** | −0.816 (0.079)*** | ||
| Race/ethnicity (Ref. Non-Hispanic white) | ||||||||
| Non-Hispanic black | −7.392 (1.679)*** | −7.163 (1.620)*** | −7.258 (1.669)*** | −14.162 (1.649)*** | −13.330 (1.607)*** | −13.045 (1.648)*** | ||
| Hispanics | −3.489 (1.937) | −4.127 (1.920)* | −3.362 (1.977) | −0.160 (1.829) | −0.097 (1.821) | −0.222 (1.856) | ||
| Others | −11.573 (3.649)** | −12.075 (3.744)** | −12.230 (3.795)** | −6.758 (3.796) | −4.650 (3.545) | −4.584 (3.594) | ||
| Educational attainment (Ref. Less than high school) | ||||||||
| High school graduate | 8.635 (1.362)*** | 7.957 (1.387)*** | 8.108 (1.425)*** | 9.880 (1.393)*** | 9.862 (1.382)*** | 9.604 (1.420)*** | ||
| More than high school | 12.943 (1.263)*** | 11.952 (1.271)*** | 12.120 (1.305)*** | 13.774 (1.397)*** | 13.030 (1.362)*** | 12.039 (1.414)*** | ||
| Annual household income (Ref. <$20,000) | ||||||||
| $20,000-$75,000 | 6.860 (1.108)*** | 6.233 (1.141)*** | 5.978 (1.184)*** | 4.236 (1.228)*** | 4.046 (1.238)** | 4.249 (1.259)*** | ||
| >$75,000 | 11.912 (1.533)*** | 10.945 (1.515)*** | 10.518 (1.568)*** | 5.292 (1.757)** | 4.952 (1.755)** | 4.929 (1.745)** | ||
| Smoking status (Ref. Nonsmoker) | ||||||||
| Former smoker | −2.716 (1.038)** | −2.407 (1.087)* | 0.413 (1.261) | 0.902 (1.293) | ||||
| Current smoker | −3.295 (1.590)* | −3.565 (1.671)* | 0.795 (1.663) | 1.115 (1.703) | ||||
| Alcohol intake ≥12 drinks/year (Ref. No) | 2.143 (1.081)* | 1.750 (1.127) | 2.361 (1.185)* | 1.817 (1.223) | ||||
| Moderate/vigorous physical activities (Ref. No) | 2.310 (1.050)* | 1.981 (0.880)* | 2.530 (1.096)* | 2.360 (1.107)* | ||||
| Obesity (Ref. No) | −0.987 (1.062) | −0.601 (1.185) | ||||||
| Hypertension (Ref. No) | −1.479 (0.457)* | −0.671 (0.576) | ||||||
| Diabetes mellitus (Ref. No) | −1.818 (0.476)** | −2.869 (0.654)** | ||||||
|
| ||||||||
| Model Fit | ||||||||
|
| ||||||||
| F | 24.633 | 81.630 | 43.033 | 56.245 | 37.924 | 79.809 | 43.591 | 57.390 |
| Significance | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| R2 | 0.027 | 0.439 | 0.439 | 0.447 | 0.037 | 0.427 | 0.438 | 0.443 |
Note:
Model 1: unadjusted.
Model 2: adjusted for sociodemographic variables (age, race/ethnicity, education, and income).
Model 3: additionally adjusted for behavioral characteristics (smoking, alcohol intake, and physical exercise).
Model 4: additionally adjusted for health-related characteristics (obesity, hypertension, and diabetes).
p < 0.05,
p < 0.01,
p < 0.001
3.3. Association of social isolation with inflammatory markers
Social isolation was related to higher levels of inflammation measured by log-transformed CRP and fibrinogen in older men but not older women (Table 3). For older men, adjusting for all covariates, Model 2 shows elevated CRP (β [SE] = 0.226 [0.110], p < 0.05) and fibrinogen (β [SE] = 0.058 [0.026], p < 0.05) for those who were socially isolated. By contrast, for older women, Model 1 reports only slightly significant elevated fibrinogen for the socially isolated than are not (β [SE] = 0.060 [0.022], p < 0.01). After adjusting all covariates, social isolation did not significantly affect the inflammatory markers in older women.
Table 3.
Associations of social isolation with inflammatory markers
| Log C-reactive Protein | Log Plasma Fibrinogen | Log Serum Albumin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Weighted β coefficients (Standard Error) | ||||||||||||
|
|
||||||||||||
| Men | Women | Men | Women | Men | Women | |||||||
|
|
||||||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
|
| ||||||||||||
| Variables | ||||||||||||
|
| ||||||||||||
| Social isolation (Ref. No) | 0.284 (0.109)** | 0.226 (0.110)* | 0.040 (0.099) | 0.011 (0.103) | 0.069 (0.025)** | 0.058 (0.026)* | 0.060 (0.022)** | 0.040 (0.022) | 0.375 (0.157)* | 0.280 (0.156) | 0.135 (0.102) | 0.035 (0.101) |
| Age | 0.017 (0.006)** | −0.015 (0.006)** | 0.005 (0.001)*** | 0.004 (0.001)*** | 0.012 (0.006)* | 0.019 (0.007)* | ||||||
| Race/ethnicit y (Ref. Non-Hispanic white) | ||||||||||||
| Non-Hispanic black | 0.062 (0.139) | 0.040 (0.122) | 0.050 (0.037) | 0.036 (0.023) | 0.119 (0.161) | 0.166 (0.152) | ||||||
| Hispanics | 0.429 (0.172)* | −0.073 (0.141) | 0.127 (0.040)** | 0.023 (0.025) | 0.261 (0.197) | 0.208 (0.180) | ||||||
| Others | −0.503 (0.411) | −0.572 (0.286)* | 0.004 (0.061) | −0.010 (0.048) | −0.160 (0.203) | 0.308 (0.341) | ||||||
| Educational attainment (Ref. Less than high school) | ||||||||||||
| High school graduate | 0.068 (0.108) | −0.161 (0.110) | 0.019 (0.022) | −0.015 (0.021) | −0.071 (0.131) | −0.123 (0.128) | ||||||
| More than high school | 0.087 (0.112) | −0.107 (0.107) | 0.013 (0.023) | 0.007 (0.020) | −0.119 (0.117) | −0.054 (0.123) | ||||||
| Annual household income (Ref. <$20,000) | ||||||||||||
| $20,000–$75,000 | −0.101 (0.098) | −0.026 (0.090) | 0.001 (0.020) | −0.026 (0.017) | −0.022 (0.106) | −0.053 (0.090) | ||||||
| >$75,000 | −0.218 (0.151) | −0.123 (0.146) | −0.007 (0.028) | −0.066 (0.023)** | 0.011 (0.133) | −0.212 (0.125) | ||||||
| Smoking status (Ref. Nonsmoker) | ||||||||||||
| Former smoker | 0.195 (0.095)* | 0.132 (0.097) | 0.015 (0.018) | 0.011 (0.017) | −0.075 (0.092) | 0.120 (0.095) | ||||||
| Current smoker | 0.794 (0.157)*** | 0.030 (0.142) | 0.157 (0.028)*** | 0.044 (0.025) | 0.020 (0.132) | 0.000 (0.116) | ||||||
| Alcohol intake ≥12 drinks/year (Ref. No) | −0.077 (0.111) | 0.052 (0.088) | −0.031 (0.021) | −0.006 (0.015) | 0.122 (0.109) | 0.031 (0.100) | ||||||
| Moderate/vigorous physical activities (Ref. No) | −0.167 (0.092) | −0.168 (0.084)* | −0.026 (0.018) | −0.043 (0.015)** | −0.092 (0.100) | −0.090 (0.085) | ||||||
| Obesity (Ref. No) | 0.417 (0.090)*** | 0.492 (0.091)*** | 0.032 (0.017) | 0.056 (0.016)*** | 0.129 (0.098) | 0.177 (0.115) | ||||||
| Hypertension (Ref. No) | 0.041 (0.085) | 0.164 (0.084) | 0.012 (0.016) | −0.016 (0.015) | 0.136 (0.078) | 0.109 (0.081) | ||||||
| Diabetes mellitus (Ref. No) | 0.022 (0.114) | 0.075 (0.113) | 0.057 (0.022)** | 0.044 (0.021)* | 0.425 (0.141)** | 0.142 (0.147) | ||||||
|
| ||||||||||||
| Model Fit | ||||||||||||
|
| ||||||||||||
| F | 5.668 | 4.162 | 4.940 | 6.759 | 3.845 | 4.749 | 6.380 | 7.246 | 5.669 | 6.200 | 6.320 | 7.736 |
| Significance | 0.017 | 0.000 | 0.000 | 0.000 | 0.050 | 0.005 | 0.000 | 0.000 | 0.017 | 0.004 | 0.000 | 0.188 |
| R2 | 0.007 | 0.098 | 0.106 | 0.112 | 0.006 | 0.099 | 0.108 | 0.128 | 0.012 | 0.059 | 0.062 | 0.092 |
Note:
Model 1: unadjusted.
Model 2: adjusted for sociodemographic variables (age, race/ethnicity, education, and income), behavioral characteristics (smoking, alcohol intake, and physical exercise), and health-related characteristics (obesity, hypertension, and diabetes).
p < 0.05
p < 0.01
p < 0.001
3.4. Mediating roles of CRP and fibrinogen
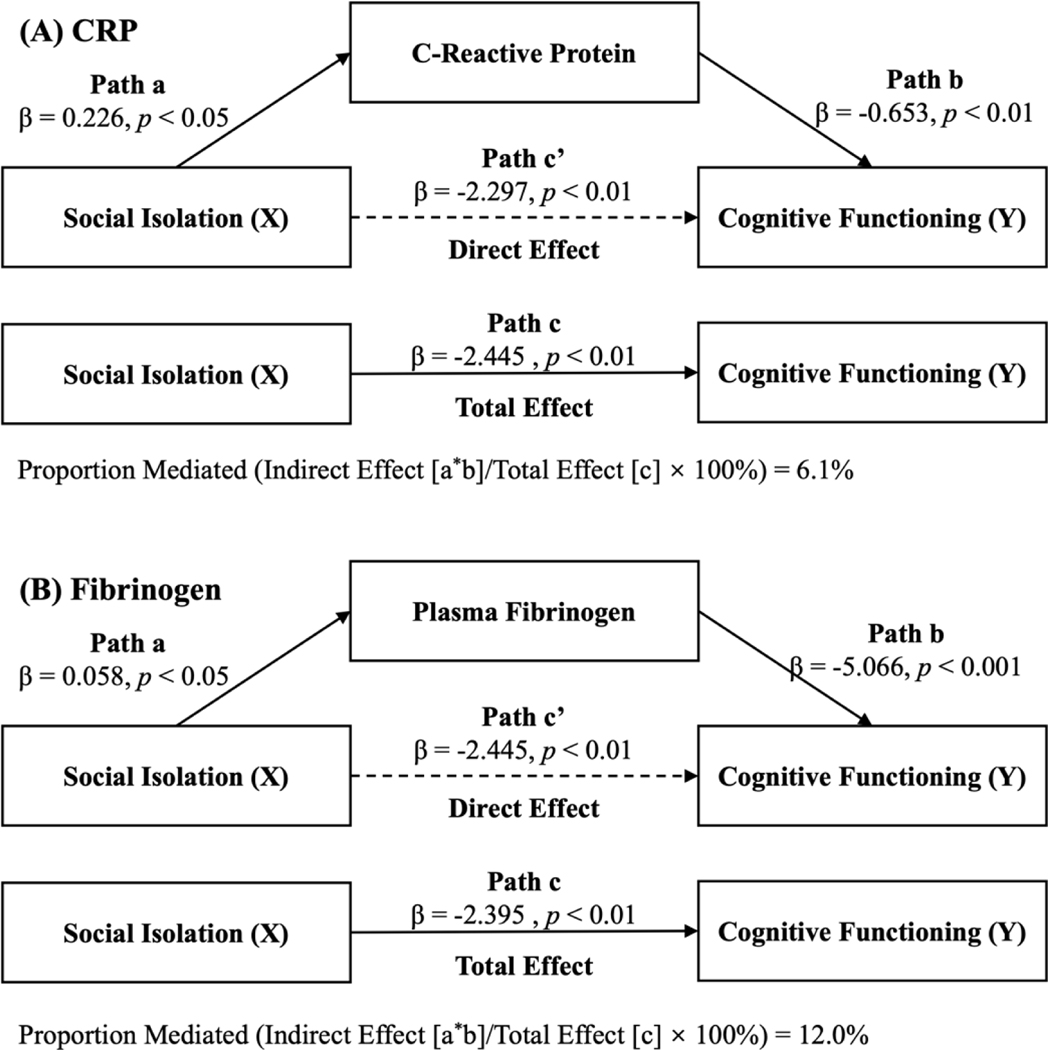
Because of the lack of significant associations between social isolation and albumin for older men and all three inflammatory markers for older women, we focused on the CRP and fibrinogen in the mediation analysis among older men. After adjustment for covariates, the CRP and fibrinogen level was inversely associated with cognitive functioning (β [SE] = −0.653 [0.215], p < 0.01 for CRP; β [SE] = −5.066 [1.713], p < 0.001 for fibrinogen). The association between social isolation and cognitive functioning mediated by CRP and fibrinogen was 6.1% and 12.0%, respectively (Figure 2). Supplementary Table S1 summarized all standardized path estimates.
Figure 2. Mediating role of chronic inflammation on the association between social isolation and cognitive functioning in older men.
Note: All models are adjusted for sociodemographic variables (age, race/ethnicity, education, and income), behavioral characteristics (smoking, alcohol intake, and physical exercise), and health-related characteristics (obesity, hypertension, and diabetes). The mediated proportion is calculated based on the formula: (a × b)/((a × b) + c), where a × b is the indirect effect of social isolation on cognitive functioning mediated by each inflammatory marker, c is the direct effect of social isolation on cognitive functioning, and (a × b) + c is the total effect of social isolation on cognitive functioning. (A) mediating effect of C-reactive protein. (B) mediating effect of plasma fibrinogen.
3.5. Sensitivity analyses
Results using dichotomized inflammatory markers were qualitatively similar to those for log-transformed markers. For older men, adjusting for all covariates, social isolation was associated with higher risks of elevated CRP (odds ratios [OR] = 1.83, 95% CI = 1.03–3.26) and being at the highest quartile of fibrinogen (OR = 1.66, 95% CI = 1.05–2.62), and the ORs were not significant in the albumin model. For older women, adjusting for all covariates, there was no significant difference in any of these inflammatory markers for the socially isolated than those not socially isolated (Supplementary Table S2). Supplementary Table S3 presents the results of testing the mediating effects of CRP and fibrinogen for older men. Overall, the inclusion of CRP and fibrinogen significantly reduced the sizes of coefficients of social isolation.
4. Discussion
Using data from NHANES 1999–2002, our hypothesis was supported: social isolation is associated with poorer cognitive functioning through physiological upregulation of chronic inflammation in older men. Further analyses in the older male sample indicated that 6.1% and 12.0% proportion of the association of social isolation on cognitive functioning were mediated by CRP and fibrinogen, respectively. The better cognitive health conveyed by social connection, widely documented in animal and human studies (Biddle et al., 2019; Joyce et al., 2022; Shen et al., 2022; Yu et al., 2020) can be attributed partly to ameliorating subclinical chronic inflammation in older men.
Our findings on the association between social isolation and cognitive functioning in both sexes are in line with studies that indicate social isolation increases the risks of dementia (Joyce et al., 2022; Shen et al., 2022). According to the stress process framework (Pearlin, 2010), social disconnection is a potent factor that can induce stress (Pearlin, 2010) and is thought to cause cognitive decline and dementia by elevating higher amyloid-β levels (Biddle et al., 2019) and lowing gray matter volumes (Shen et al., 2022). Chronic stress exposure and reduced coping strategies associated with social disconnection can induce a cascade of immune, neuroendocrine, and cardiovascular changes (Cacioppo et al., 2015). From a cognitive reserve perspective, social engagement and social participation is cognitively beneficial and contributes to building cognitive reserve and enhancing cognitive function (Stern, 2002). This was potently demonstrated in the Nun Study that an active religious life with numerous opportunities for social connection might stimulate cognition and hence expand cognitive reserve (Bennett et al., 2014).
In this study, the association of social isolation with cognitive functioning is comparable with other well-recognized risk factors to cognitive decline, which include lower education, less household income, smoking, physical inactivity, hypertension, and diabetes mellitus. This finding is in accordance with the Lancet Commission report that estimated social isolation is associated with a 4% reduction in dementia prevalence if this risk factor is addressed. Notably, this reduction is greater than combatting physical inactivity in later life (2%) or hypertension in midlife (2%) alone (Livingston et al., 2020). Given the high prevalence (24%) of social isolation among older adults in the US (Cudjoe et al., 2020), more interventions should be designed in the future to counter the effects of social isolation on the diminished cognitive functioning.
We find that social isolation is strongly associated with a high fibrinogen level in both sexes. The commonly used CRP also appears to be related to social isolation, but this relationship is less consistent across sex. Albumin does not seem to be associated with social isolation, because it is less specific and is affected by many physiological processes besides inflammation (Yang et al., 2013). The associations between social isolation and markers of chronic inflammation suggested different physiological pathways important to consider in further analysis of the interplay between social and biological factors in dementia etiology. Studies have also demonstrated the association between social isolation and inflammation. Laboratory research has long documented that stress directly affects multiple regulatory systems through activation of the HPA axis and sympathetic nervous system (SNS), which modulate immune function and inflammatory processes (Seeman et al., 2001). And it has been suggested that social isolation may operate to reduce such effects by dampening physiological arousal or reactivity (Shen et al., 2022), whereas social isolation is itself a stressor that produces negative reactivity and affect (e.g., anxiety, depression), promotes chronic elevations in HPA and SNS activation (Cacioppo et al., 2015), and increases inflammation (Cudjoe et al., 2021; Das, 2013; Glei et al., 2012; Shankar et al., 2011). Furthermore, the stress-buffering functions of social support and interpersonal psychological resources can be linked with inflammatory processes in various ways (Ditzen & Heinrichs, 2014). It has been suggested that socially isolated individuals are deprived of opportunities for emotional support and coping resources, which can decrease their sense of control and psychological well-being (Qi et al., 2022).
The mediating analysis provided direct evidence that chronic inflammation can act as important physiological link between social isolation and cognitive functioning in older men. Consistent with findings from previous research that indicate health-risk behaviors and psychological stress as the main explanatory mechanisms (Donovan et al., 2017; Shankar et al., 2011), we found that the independent effects of social isolation on cognitive functioning largely remain after adjusting for a wide array of sociodemographic, behavioral, and health factors. This suggests that inflammation should be included as one physiological mechanism contributing to the detrimental effects of social isolation on dementia independent of conventional factors in future studies.
We also found the sex differences in the extent to which the inflammatory process mediates the effect of social isolation on cognitive functioning. The associations between social isolation and inflammatory markers are significant in older men but not women. Some psychosocial mechanisms have been proposed to explain this difference. For example, when a loss of social support (e.g., loss of a spouse) is expected, it can improve women’s capacity to foresee such a loss, encourage them to replace the lost ties with other close ties, or help them cope with the demands and rewards of social support. This may improve women’s resilience, which is essential to their health and survival after such loss (Berkman & Syme, 1979). Presumably, socially isolated men are more likely to suffer from lower life satisfaction and less resilient than socially isolated women (Zebhauser et al., 2014). Women have a greater capacity to buffer social isolation than men because they have more multifaceted networks that include close friendships and neighbors, resulting in greater social support (Zebhauser et al., 2014). Physiological mechanisms may also play important roles in sex differences. Previous studies indicate sexual dimorphism in behavioral stress responses (Lu et al., 2015). Men would display the classic “fight-or-flight” response to stressors. In contrast, women react with “tend-and-befriend” responses, characterized by nurturing behaviors that downregulate stress reactivity and by affiliating with social groups to reduce risk (Taylor et al., 2000). The physiological response typical of women has likely evolved as an adaptation to their maternal and caregiving roles (i.e., tend-and-befriend pattern) and acts to downregulate innate immune responses such as inflammation. Prior studies also found that social isolation and a lack of social support have been related to higher inflammatory responses (Yang et al., 2013) and allostatic load (Seeman et al., 2001), with deleterious effects being more pronounced in men than women.
5. Strengths and limitations
This study has several noteworthy strengths. First, this is a large nationally representative sample of older adults in the U.S., with equal distribution of sexes. Second, we performed sensitivity analyses and arrived at the same results and conclusions, lending robustness to our conclusions. Third, this study is one of the few studies to date that examined social isolation, inflammatory biomarkers, and cognitive functioning in a single study using mediation model.
Several limitations need to be acknowledged. First, the measures of social isolation available in the NHANES 1999–2002 are restricted to social support and social networks that represent structural aspects of social connection. Qualitative and functional aspects of social connection are important to consider (National Academies of Sciences, Engineering, and Medicine, 2020) as psychosocial mechanisms mediating the link between social isolation and cognitive functioning and sex differences therein. Second, we only tested the inflammation mechanism due to the limited biomarkers available in the NHANES 1999–2002. Additional neuroendocrinological mechanisms, such as decreased HPA and sympathoadrenal activities and stress-related neurohormones such as cortisol and norepinephrine, could be explored in future studies to illustrate how social factors impact cognitive health (Cacioppo et al., 2015). Third, it is important to note that a reciprocal relationship between social isolation and cognitive functioning cannot be ruled out due to the cross-sectional nature of this study. Early symptoms of diminished cognitive functioning may also lead to social withdrawal and isolation. Causal direction should be investigated in further longitudinal or interventional studies. Fourth, although Lancet Commission identified 12 modifiable risk factors for dementia (Livingston et al., 2020), we did not test for all of them as measures on head injury, hearing loss, air pollution, and depression were unavailable among older adults in NHANES 1999–2002. Finally, the biobehavioral model of sex differences further suggests that health advantages for women may be conferred through neurobiological mechanisms, such as decreased cholesterol and HPA response to stress, instead of via inflammation (Grant et al., 2009). Future studies that include additional biological measures are needed to elucidate the neuroendocrine processes that mediate the effects of social isolation on cognitive functioning in sex-specific contexts.
6. Conclusions
Using a nationally representative and population-based sample, we found that social isolation was partially associated with poorer cognitive functioning via elevated inflammatory responses. There are sex differences in that they are greater for men and can be attributed partly to their heightened inflammatory responses. While biological mechanisms may account for the associations between social isolation and poorer cognitive functioning, longitudinal and interventional research are needed to further examine the changes in cognitive functioning induced by social isolation that may predispose older adults to cognitive impairment.
Supplementary Material
Highlights.
Social isolation was associated with poorer cognitive functioning in both older men and women in the United States.
Social isolation is associated with elevated levels of C-reactive protein and plasma fibrinogen in older men.
The associations between social isolation and cognition can be attributed in part to heightened inflammatory responses.
Funding Source
This study is partially supported by the National Institutes of Health (P30AG059304, P50MD017356).
Footnotes
Disclosure Statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval
The National Health and Nutrition Examination Survey (NHANES) protocol was approved by the Institutional Review Board (IRB) of the Centers for Disease Control and Prevention, and all patients provided written informed consent. This project is the result of a secondary analysis of NHANES and is exempt from local IRB approval.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- Alzheimer’s Association. (2021). 2021 Alzheimer’s Disease Facts and Figures. https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf [DOI] [PubMed]
- Ayoub SS (2010). Fundamentals of Inflammation (Serhan CN, Ward PA, & Gilroy DW, Eds.; 1st ed.). Cambridge University Press. 10.1017/CBO9781139195737 [DOI] [Google Scholar]
- Bennett DA, Arnold SE, Valenzuela MJ, Brayne C, & Schneider JA (2014). Cognitive and social lifestyle: Links with neuropathology and cognition in late life. Acta Neuropathologica, 127(1), 137–150. 10.1007/s00401-013-1226-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF, & Syme SL (1979). SOCIAL NETWORKS, HOST RESISTANCE, AND MORTALITY: A NINE-YEAR FOLLOW-UP STUDY OF ALAMEDA COUNTY RESIDENTS. American Journal of Epidemiology, 109(2), 186–204. 10.1093/oxfordjournals.aje.a112674 [DOI] [PubMed] [Google Scholar]
- Biddle KD, d’Oleire Uquillas F, Jacobs HIL, Zide B, Kirn DR, Rentz DM, Johnson KA, Sperling RA, & Donovan NJ (2019). Social Engagement and Amyloid-β-Related Cognitive Decline in Cognitively Normal Older Adults. The American Journal of Geriatric Psychiatry, 27(11), 1247–1256. 10.1016/j.jagp.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, & Boomsma DI (2014). Evolutionary mechanisms for loneliness. Cognition and Emotion, 28(1), 3–21. 10.1080/02699931.2013.837379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, Capitanio JP, & Cole SW (2015). The Neuroendocrinology of Social Isolation. Annual Review of Psychology, 66(1), 733–767. 10.1146/annurev-psych-010814-015240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle CE, Steinman BA, & Chen J (2017). Visual Acuity and Self-Reported Vision Status: Their Associations With Social Isolation in Older Adults. Journal of Aging and Health, 29(1), 128–148. 10.1177/0898264315624909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudjoe TKM, Roth DL, Szanton SL, Wolff JL, Boyd CM, & Thorpe RJ (2020). The Epidemiology of Social Isolation: National Health and Aging Trends Study. The Journals of Gerontology: Series B, 75(1), 107–113. 10.1093/geronb/gby037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudjoe TKM, Selvakumar S, Chung S, Latkin CA, Roth DL, Thorpe RJ, & Boyd CM (2021). Getting under the skin: Social isolation and biological markers in the National Health and Aging Trends Study. Journal of the American Geriatrics Society, jgs.17518. 10.1111/jgs.17518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin LR, Mohadjer LK, Dohrmann SM, Montaquila JM, Kruszan-Moran D, Mirel LB, Carroll MD, Hirsch R, Schober S, & Johnson CL (2012). The National Health and Nutrition Examination Survey: Sample Design, 1999–2006. Vital and Health Statistics. Series 2, Data Evaluation and Methods Research, 155, 1–39. [PubMed] [Google Scholar]
- Das A (2013). How does race get “under the skin”?: Inflammation, weathering, and metabolic problems in late life. Social Science & Medicine, 77, 75–83. 10.1016/j.socscimed.2012.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditlevsen S, Christensen U, Lynch J, Damsgaard MT, & Keiding N (2005). The Mediation Proportion: A Structural Equation Approach for Estimating the Proportion of Exposure Effect on Outcome Explained by an Intermediate Variable. Epidemiology, 16(1), 114–120. 10.1097/01.ede.0000147107.76079.07 [DOI] [PubMed] [Google Scholar]
- Ditzen B, & Heinrichs M (2014). Psychobiology of social support: The social dimension of stress buffering. Restorative Neurology and Neuroscience, 32(1), 149–162. 10.3233/RNN-139008 [DOI] [PubMed] [Google Scholar]
- Donovan NJ, Wu Q, Rentz DM, Sperling RA, Marshall GA, & Glymour MM (2017). Loneliness, depression and cognitive function in older U.S. adults: Loneliness, depression and cognition. International Journal of Geriatric Psychiatry, 32(5), 564–573. 10.1002/gps.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Moieni M, Inagaki TK, Muscatell KA, & Irwin MR (2017). In Sickness and in Health: The Co-Regulation of Inflammation and Social Behavior. Neuropsychopharmacology, 42(1), 242–253. 10.1038/npp.2016.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Ryff CD, Lin Y-H, & Weinstein M (2012). Social relationships and inflammatory markers: An analysis of Taiwan and the U.S. Social Science & Medicine, 74(12), 1891–1899. 10.1016/j.socscimed.2012.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB (2010). Role of inflammation in cognitive impairment: Results of observational epidemiological studies and clinical trials: Gorelick. Annals of the New York Academy of Sciences, 1207(1), 155–162. 10.1111/j.1749-6632.2010.05726.x [DOI] [PubMed] [Google Scholar]
- Grant N, Hamer M, & Steptoe A (2009). Social Isolation and Stress-related Cardiovascular, Lipid, and Cortisol Responses. Annals of Behavioral Medicine, 37(1), 29–37. 10.1007/s12160-009-9081-z [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, & Dixon RA (1999). Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging, 14(2), 245–263. 10.1037/0882-7974.14.2.245 [DOI] [PubMed] [Google Scholar]
- Jaeger J (2018). Digit Symbol Substitution Test: The Case for Sensitivity Over Specificity in Neuropsychological Testing. Journal of Clinical Psychopharmacology, 38(5), 513–519. 10.1097/JCP.0000000000000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce J, Ryan J, Owen A, Hu J, McHugh Power J, Shah R, Woods R, Storey E, Britt C, Freak‐Poli R, & ASPREE Investigator Group. (2022). Social isolation, social support, and loneliness and their relationship with cognitive health and dementia. International Journal of Geriatric Psychiatry, 37(1), gps.5644. 10.1002/gps.5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipinoinen T, Toppala S, Rinne JO, Viitanen MH, Jula AM, & Ekblad LL (2022). Association of Midlife Inflammatory Markers With Cognitive Performance at 10-Year Follow-up. Neurology, 10.1212/WNL.0000000000201116. 10.1212/WNL.0000000000201116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis NA, & Knight JE (2021). Longitudinal associations between C-reactive protein and cognitive performance in normative cognitive ageing and dementia. Age and Ageing, 50(6), 2199–2205. 10.1093/ageing/afab152 [DOI] [PubMed] [Google Scholar]
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, … Mukadam N (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet, 396(10248), 413–446. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks EB, Berkman LF, Gruenewald TL, & Seeman TE (2006). Relation of Social Integration to Inflammatory Marker Concentrations in Men and Women 70 to 79 Years. The American Journal of Cardiology, 97(7), 1010–1016. 10.1016/j.amjcard.2005.10.043 [DOI] [PubMed] [Google Scholar]
- Lu J, Wu X-Y, Zhu Q-B, Li J, Shi L-G, Wu J-L, Zhang Q-J, Huang M-L, & Bao A-M (2015). Sex differences in the stress response in SD rats. Behavioural Brain Research, 284, 231–237. 10.1016/j.bbr.2015.02.009 [DOI] [PubMed] [Google Scholar]
- Mick P, Kawachi I, & Lin FR (2014). The Association between Hearing Loss and Social Isolation in Older Adults. Otolaryngology–Head and Neck Surgery, 150(3), 378–384. 10.1177/0194599813518021 [DOI] [PubMed] [Google Scholar]
- Myers GL, Rifai N, Tracy RP, Roberts WL, Alexander RW, Biasucci LM, Catravas JD, Cole TG, Cooper GR, Khan BV, Kimberly MM, Stein EA, Taubert KA, Warnick GR, & Waymack PP (2004). CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: Report From the Laboratory Science Discussion Group. Circulation, 110(25). 10.1161/01.CIR.0000148980.87579.5E [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (Ed.). (2020). Social isolation and loneliness in older adults: Opportunitiies for the health care system. the National Academies Press. [PubMed] [Google Scholar]
- National Center for Health Statistics (U.S.) (Ed.). (2015). National Health and Nutrition Examination Survey (NHANES) biospecimen program: NHANES III (1988–1994) and NHANES 1999–2014. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. [Google Scholar]
- Osimo EF, Baxter LJ, Lewis G, Jones PB, & Khandaker GM (2019). Prevalence of low-grade inflammation in depression: A systematic review and meta-analysis of CRP levels. Psychological Medicine, 49(12), 1958–1970. 10.1017/S0033291719001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI (2010). The Life Course and the Stress Process: Some Conceptual Comparisons. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 65B(2), 207–215. 10.1093/geronb/gbp106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Hong Y, & Smith SC (2004). CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: Overview. Circulation, 110(25). 10.1161/01.CIR.0000148979.11121.6B [DOI] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, McCammon RJ, Fisher GG, Potter GG, Burke JR, Steffens DC, Foster NL, Giordani B, Unverzagt FW, Welsh-Bohmer KA, Heeringa SG, Weir DR, & Wallace RB (2011). Incidence of dementia and cognitive impairment, not dementia in the united states. Annals of Neurology, 70(3), 418–426. 10.1002/ana.22362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Zhang W, Wang K, Pei Y, & Wu B (2022). Social isolation and psychological well-being among older Chinese Americans: Does resilience mediate the association? International Journal of Geriatric Psychiatry, 37(8), gps.5791. 10.1002/gps.5791 [DOI] [PubMed] [Google Scholar]
- Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, & Tzourio C (2012). Circulating IL-6 and CRP are associated with MRI findings in the elderly: The 3C-Dijon Study. Neurology, 78(10), 720–727. 10.1212/WNL.0b013e318248e50f [DOI] [PubMed] [Google Scholar]
- Seeman TE, & Berkman LF (1988). Structural characteristics of social networks and their relationship with social support in the elderly: Who provides support. Social Science & Medicine, 26(7), 737–749. 10.1016/0277-9536(88)90065-2 [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, & Singer BH (2001). Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences, 98(8), 4770–4775. 10.1073/pnas.081072698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, McMunn A, Banks J, & Steptoe A (2011). Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychology, 30(4), 377–385. 10.1037/a0022826 [DOI] [PubMed] [Google Scholar]
- Shen C, Rolls E, Cheng W, Kang J, Dong G, Xie C, Zhao X-M, Sahakian B, & Feng J (2022). Associations of Social Isolation and Loneliness With Later Dementia. Neurology, 10.1212/WNL.0000000000200583. 10.1212/WNL.0000000000200583 [DOI] [PubMed] [Google Scholar]
- Stern Y (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8(3), 448–460. 10.1017/S1355617702813248 [DOI] [PubMed] [Google Scholar]
- Tavakol M, & Dennick R (2011). Making sense of Cronbach’s alpha. International Journal of Medical Education, 2, 53–55. 10.5116/ijme.4dfb.8dfd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, & Updegraff JA (2000). Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review, 107(3), 411–429. 10.1037/0033-295X.107.3.411 [DOI] [PubMed] [Google Scholar]
- Tsatali M, Poptsi E, Moraitou D, Agogiatou C, Bakoglidou E, Gialaouzidis M, Papasozomenou C, Soumpourou A, & Tsolaki M (2021). Discriminant Validity of the WAIS-R Digit Symbol Substitution Test in Subjective Cognitive Decline, Mild Cognitive Impairment (Amnestic Subtype) and Alzheimer’s Disease Dementia (ADD) in Greece. Brain Sciences, 11(7), 881. 10.3390/brainsci11070881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Hoogeveen RC, Folsom AR, Ballantyne CM, Knopman DS, Windham BG, Jack CR, & Gottesman RF (2017). Midlife systemic inflammatory markers are associated with late-life brain volume: The ARIC study. Neurology, 89(22), 2262–2270. 10.1212/WNL.0000000000004688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D & Psychological Corporation. (1997). WAIS-III: Wechsler adult intelligence scale. Psychological Corp. [Google Scholar]
- Yang YC, McClintock MK, Kozloski M, & Li T (2013). Social Isolation and Adult Mortality: The Role of Chronic Inflammation and Sex Differences. Journal of Health and Social Behavior, 54(2), 183–203. 10.1177/0022146513485244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Steptoe A, Chen Y, & Jia X (2020). Social isolation, rather than loneliness, is associated with cognitive decline in older adults: The China Health and Retirement Longitudinal Study. Psychological Medicine, 1–8. 10.1017/S0033291720001014 [DOI] [PubMed] [Google Scholar]
- Zebhauser A, Hofmann-Xu L, Baumert J, Häfner S, Lacruz ME, Emeny RT, Döring A, Grill E, Huber D, Peters A, & Ladwig KH (2014). How much does it hurt to be lonely? Mental and physical differences between older men and women in the KORA-Age Study: Gender differences of loneliness in older people. International Journal of Geriatric Psychiatry, 29(3), 245–252. 10.1002/gps.3998 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Wang P, & Fang Y (2018). Loneliness and the risk of dementia among older Chinese adults: Gender differences. Aging & Mental Health, 22(4), 519–525. 10.1080/13607863.2016.1277976 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.