INTRODUCTION
Rhythms in the Brain
Much of human biology is organized around the 24-hour day. Exercise, eating, digestion, and sleep occur at particular times, requiring highly ordered physiology. To achieve this, “clocks” throughout the body synchronize with 24-hour cycles in light, activity, and other salient cues to enable appropriately aligned and adaptive biological functions.
The central clock in the suprachiasmatic nucleus (SCN) of the hypothalamus receives light input from the retina and transmits time-of-day cues to cellular clocks throughout the rest of the brain and body.1 In this way, the SCN organizes sleep-wake timing and also many aspects of peripheral physiology. At the cellular level, the clock is a feedback loop involving transcriptional activators (BMAL1, CLOCK, NPAS2) and repressors (CRYs and PERs) that generates a 24-hour molecular oscillation and drives waves of expression in thousands of target genes. Half of mammalian protein-coding genes are rhythmically expressed somewhere in the body,2,3 driving dozens of physiologic functions.4
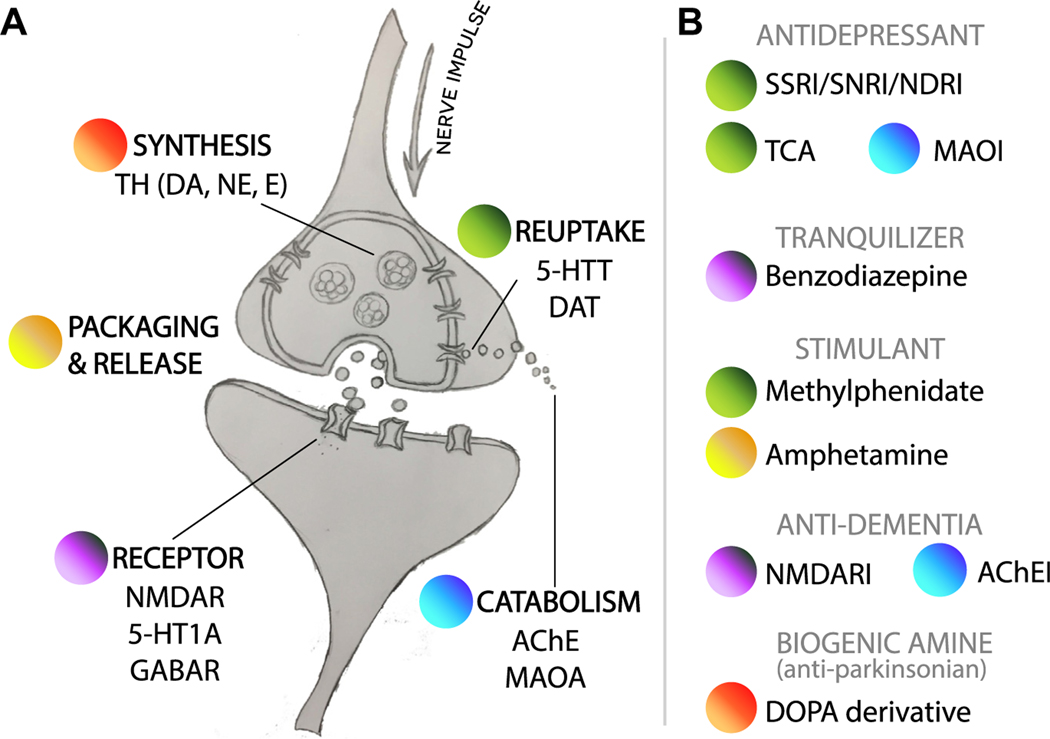
In the brain, key steps in intercellular signaling are time-of-day regulated, from transmitter synthesis5,6 to degradation7,8 (Fig. 1A). Other fundamental neural processes, including endocytosis/exocytosis,9 waste removal,10,11 regulation of synaptic strength,12–14 and permeability of the brain15 and cerebrospinal fluid16 to blood, also show daily variation. Therefore, it is not surprising that circadian disruption and neurologic disease often go hand in hand. Most neurodegenerative (eg, Alzheimer, Parkinson, Huntington) and psychiatric diseases (eg, schizophrenia, bipolar disorder, and depression) are accompanied by sleep fragmentation and circadian rhythm disorders (SFCRDs) (as reviewed in Refs.17–19). Many of these diseases are without effective therapies, increasing in prevalence, and represent enormous unmet medical needs.
Fig. 1.
Daily variation in mammalian neurotransmission. (A) Protein-level 24-hour variation at points in neurotransmitter life cycle: synthesis, packaging/release, reception, reuptake, and catabolism. (B) Standard medical therapies in neuropsychiatric or neurodegenerative disease. Those listed have primary targets whose abundance and/or function were shown to be time dependent somewhere in the mammalian brain (see Table 1). DOPA, dihydroxyphenylalanine; 5-HT, serotonin; 5-HTT, serotonin transporter; AChE, acetylcholinesterase; AChEI, acetylcholinesterase inhibitor; E, epinephrine; MAOA, monoamine oxidase A; MAOI, monoamine oxidase inhibitor; NDRI, norepinephrine and dopamine reuptake inhibitor; NE, norepinephrine; NMDAR, N-methyl-D-aspartate receptor; SNRI, selective serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TH, tyrosine hydroxylase.
Circadian Disruption: Chicken or Egg?
Although the association between neurologic disease and SFCRD is well established,20–22 the direction of causation remains uncertain. This association was a topic in prior reviews17,23–25 and continues to pose a challenge in research and patient care.
There is genetic evidence that disruptions to the circadian system may be causal factors in the development of mental illness. Single nucleotide polymorphisms (SNPs) in several human circadian clock genes (CLOCK, BMAL1, NPAS2, PER2, CRY1, TEF, RORB) were associated with incidence and severity of attention-deficit/hyperactivity disorder (ADHD),26,27 schizophrenia,28,29 major depressive disorder (MDD),30 bipolar disorder (BP),31,32 posttraumatic stress disorder,33 and seasonal-affective disorder.34 All these diseases are complex, multigenic, and distinct from one another. It is noteworthy that so-called circadian entrainment was the top enriched pathway from the small set of 23 clinically relevant genes common to bipolar disorder (BP), schizophrenia, and autism.35
Studies of shift workers provide additional support for a causal link between circadian disruption and mental illnesses. Rotating schedules can lead to the loss of proper temporal coordination between organ systems (ie, circadian misalignment). These people have an increased risk of major depression and other mood disorders,36,37 likely a consequence of chronic circadian misalignment.
Shift work is also associated with the risk of dementia,38,39 suggesting that SFCRD may be a primary contributor in neurodegenerative disease as well. In Parkinson and Lewy body disease, rapid eye movement (REM) sleep disorders can manifest decades before the hallmark parkinsonian symptoms.40 Early (presymptomatic) SFCRD is also seen in Alzheimer disease (AD) and other neurodegenerative disorders.41,42 However, experiments in animals showed that early does not necessarily imply causal.43,44 For example, in a mouse model of AD, the deposition of β-amyloid preceded the onset of sleep abnormalities, and its removal restored normal circadian rhythms.44
So, is SFCRD a primary contributor to neurodegenerative disease? It is difficult to say. Two studies linked SNPs in BMAL1 and CLOCK with incidence of AD,45–47 but the associations were only significant in the subpopulation without the AD-risk allele apolipoprotein E (APOE e4). Although small, these studies suggest that the impact of genetic disruptions to the clock on dementia depends on genetic makeup, as expected in a multifactorial disease. Regardless of underlying cause, abnormal sleep-wake patterns can occur very early in neurodegenerative disease, predict risk of progression (in both animal48–50 and clinical studies51,52), and are a major primary complaint from patients seen in neurology clinics.
Where Do Sleep and Circadian Medicine Fit in?
Sleep medicine is an interdisciplinary field based on the diagnosis and treatment of sleep disorders. Therapeutic modalities are broad and may or may not directly address circadian disruption (eg, cognitive behavior therapy for insomnia, medical therapy for symptoms of narcolepsy, or surgical therapy for resistant obstructive sleep apnea). In contrast, circadian medicine is intended to specifically incorporate knowledge of 24-hour biological rhythms to administer more effective treatment.
There are 2 broad approaches for circadian medicine. In the first, the therapeutic target is directly or indirectly the molecular oscillator (discussed later). Light therapy for phase disorders is the hallmark example and is effective at consolidating sleep in dementia.53 In addition to light, drugs (eg, melatonin, zolpidem, tasimelteon) and diet54 can also modulate the clock’s amplitude or phase to potentially influence neurologic health.
A second approach in circadian medicine is intended to harmonize treatment with the clock’s rhythmic outputs (discussed later). Timing the administration of a drug to coincide with peak levels of its physiologic target showed clinical benefits in hypertension, hypercholesterolemia, cancer, and several other areas.55 Some of the key pathways targeted by standard-of-care drugs in neurology (monoamine and gamma-aminobutyric acid [GABA] neurotransmission, and others) are circadian regulated in the mammalian brain (Fig. 1). Can time of day be leveraged to improve existing treatments?
This article highlights how advances in circadian biology might translate to neurologic patient care. It focuses on neuropsychiatric and neurodegenerative conditions because they (1) share the common feature of SFCRD, and (2) are areas of high unmet medical need.
TREATING THE BRAIN’S CLOCK
Multiple factors lead to SFCRD in patients with dementia, including normal age-related changes, disease pathophysiology, medical comorbidities, side effects from medications, environmental and behavioral factors (eg, inadequate sleep hygiene), or some combination thereof.56 Regardless of cause, sleep disorders impair cognition57 and are a common cause of institutionalization in patients with dementia.58 Consolidating sleep can markedly improve quality of life for the patients and caregivers.59 However, clinicians face major challenges in achieving this.
Which Therapy in Which Patients, and When?
Options for treating sleep disruption include drug or light therapy, cognitive-behavioral intervention, and sleep hygiene recommendations. Although each of these affects the circadian clock, the mechanisms (and outcomes) are varied. Problematically, it is not clear which patients are the best candidates for a particular treatment. For example, sedative-hypnotics (ie, benzodiazepines) are widely used to treat sleep onset insomnia.60 The Canadian Institute for Health reported 15% benzodiazepine use in the community, increasing to 30% in long-term care facilities61 where half of the residents aged 80 years or older have dementia.62 However, the American Academy of Sleep Medicine (AASM) strongly recommends against sleep-promoting medications to treat sleep-wake rhythm disorder in elderly demented patients63 because of fallrelated injuries64 and impaired cognition.65 This type of discordance between guidelines and practice is common in sleep medicine, and likely caused by reliance on therapies that are not well supported by large clinical trials and treatment practices that are driven by anecdotal experience.
The use of nonpharmacologic therapies for SFCRD in neurodegenerative disease is also equivocal. The AASM recommends bright light therapy (BLT) for patients with sleep and circadian rhythm disorders,66,67 which can improve total sleep time in patients with dementia.68 However, BLT was not effective for all SFCRD-related symptoms (eg, daytime behavior) in this patient population, and in some cases even exacerbated the symptoms.69 How can the inconsistent results be explained?
One possibility is that clinicians have not fully delineated the appropriate timing of treatment. Guidelines for BLT recommend exposure before bedtime (to delay sleep phase) or on awakening (to advance sleep phase).67 Critically, in order for BLT to impose strong and properly phased sleep-wake rhythms, it must be administered in narrow windows of circadian clock time, which presents a challenge. For example, in elderly patients with irregular sleep-wake patterns, it is difficult to measure circadian phase using current clinical tools, and therefore difficult to know when BLT is likely to be most effective. This difficulty is further complicated by the SCN becoming less responsive to light stimuli as people age,70 emphasizing the importance of proper timing of administration in this population.
Are Some Therapies Worsening Sleep Fragmentation and Circadian Rhythm Disorders?
All of the major psychotherapeutic drug classes can affect sleep architecture (Table 1). For example, treatment-induced insomnia occurred in close to a quarter of patients taking venlafaxine for generalized anxiety disorders.71 Although the mechanisms are broadly understood (eg, GABA agonists produce sedative effects), the specific effects on the central clock and sleep homeostat are not clear. This uncertainty is important when clinicians are trying to determine the primary and secondary causes of sleep disruption: will this patient require (1) therapy for a primary sleep disorder or (2) changes to medications prescribed for the neurologic disease? It is even more complex in patients that require polydrug therapy.
Table 1.
Effects of common psychotherapeutic drug classes on sleep
| Drug Class | Time to Sleep Onset | Sleep Continuity | Slow Wave Sleep | REM Sleep |
|---|---|---|---|---|
|
| ||||
| SSRI | Increased | Decreased | Decreased | Decreased |
|
| ||||
| SNRI | Unchanged | Decreased | Decreased | Decreased |
|
| ||||
| MAOI | Increased | Decreased | Unchanged | Decreased |
|
| ||||
| TCA | Decreased | Increased | Unchanged | Decreased |
|
| ||||
| Trazodone | Decreased | Increased | Increased | Decreased |
|
| ||||
| Antipsychotic | Decreased | Increased | Increased | Decreased |
|
| ||||
| Anxiolytic | Decreased | Increased | Decreased | Decreased |
These are general class effects on sleep. Each drug has its own profile.
Abbreviations: MAOI, monoamine oxidase inhibitor; SNRI, serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Adapted from Barkoukis TJ, Avidan AY, service) S (online. Review of Sleep Medicine; with permission.
There is an even broader challenge here: because of the inextricable link between sleep, circadian rhythms, and neural function, it is not always clear what is being treated. Indeed, recent studies suggest that the benefits from some psychotherapeutics (eg, lithium, selective serotonin reuptake inhibitors [SSRIs]) may derive from their effects on the circadian system.72 The SSRI citalopram, prescribed for depression and anxiety, acutely increased the sensitivity of the human circadian system to light,73 which may realign the circadian clock and contribute to the drug’s antidepressant effect.
Moving Sleep and Circadian Medicine Forward
Advancements in sleep medicine will depend on improved approaches to (1) measure internal circadian phase, (2) reliably shift circadian phase, (3) strengthen rhythms (ie, amplitude), and (4) understand how SFCRD fits in the context of other diseases (eg, neurodegeneration).
Current methods for determining internalcircadian phase are limited and not used clinically. The gold-standard dim-light melatonin onset (DLMO) assay is based on the circadian clock–regulated evening increase in melatonin levels74 and requires the serial sampling of saliva over a 24-hour period. DLMO is time consuming, costly, difficult to standardize, and therefore rarely used to diagnose circadian rhythm disorders. However, lastyear3separatestudiesshowedthefeasibilityofgeneexpressionsignaturesfrom1or 2minimallyinvasive(skinorblood)samplestodetermineaperson’scircadianphase.75–77 As mentioned, the efficacy of BLT, and potentially all therapies aimed at circadian realignment, depends on the time at which they are administered. A single-sample point-of-use biomarker could therefore have broad diagnostic and therapeutic value.
In addition to light, exercise and feeding can influence the circadian clock. Although not routinely used in practice, exercise can help consolidate sleep in the elderly.78,79 The type and timing of nutrient intake can also influence circadian clocks in health.49,80 In order to determine the use for these newer methods to treat SFCRD, larger clinical trials must be completed. New methods for determining circadian time should lead to guidelines for how and when these therapies should be given.
Circadian disruption occurs presymptomatically in AD,41 as mentioned. Several genes implicated in neurodegenerative (and neuropsychiatric) disease are circadian regulated in mice,2 including AD-risk genes β-secretase enzyme 1 (Bace1), Bace2, and APOE in the hippocampus.81 There is evidence that genetically disrupted clock function can predispose to neurodegeneration.45,47,82 Can treatment of SFCRD, if early and effective, attenuate or delay AD?
LEVERAGING THE CLOCK’S RHYTHMIC OUTPUT
Timing Treatment to Target Dynamics
Timing treatment to coincide with daily rhythms in clock output is not new. The short-acting statin, simvastatin, is more effective at reducing lipid levels if taken at night, when its target, the rate-limiting enzyme in cholesterol biosynthesis, is at its peak levels.83 However, apart from simvastatin, which has US Food and Drug Administration–labeled time-of-day dosing instructions, circadian time has had very little role in drug development or clinical practice. One reason for this is a lack of understanding about how the circadian clock regulates human physiology and pathophysiology.
However, a series of advances have renewed translational interest. First, circadian regulation is far more pervasive than previously appreciated.2,4 In a multitissue time-series study in mice, nearly half of the protein-coding genome was circadian regulated somewhere in the body. In the nervous system alone, many basic neural functions have been shown to vary by time of day, as mentioned earlier. Second, genome-wide and systems-wide circadian profiling, historically limited to time-series studies in animal models, is now feasible in humans. A recent study by our group applied a bioinformatic approach called cyclic ordering by periodic structure (CYCLOPS)84 to assemble a population-scale atlas of circadian gene expression across 13 different human tissue types.3 The study found ~1000 genes that were rhythmically expressed somewhere in the human body that encode known drug targets.
Are there neurologic therapies whose efficacy or safety might depend on time of dosing?
Time for Targeting Neurotransmission
Monoamine neurotransmission is a major therapeutic target in neurology. Six different serotonin-specific and norepinephrine-specific reuptake inhibitor drugs(indicated for depression, anxiety, and other mood disorders) are among the top 50 most prescribed drugs in the United States.85 The first SSRI (fluoxetine/Prozac) was developed in the 1970s86 based on evidence of low serotonin and/or norepinephrine levels in depression.87 However, although widely prescribed, many patients (most, by some accounts) do not benefit from SSRI therapy.88
Accumulating evidence has shown that monoamine transmitters are circadian regulated in brain loci implicated in arousal and mood, as reviewed by Albrecht89 (Fig. 1B; Table 2). However, knowledge of these 24-hour dynamics has not factored into clinical practice or drug development. Most SSRI/serotonin norepinephrine reuptake inhibitor formulations are long acting (half-lives >12 hours). Could a short-acting SSRI be used to restore normal 24-hour rhythms in synaptic serotonin? In a mouse model of moodlike behavior, effects of the short-acting SSRI milnacipran were circadian-time dependent.90 Future studies should test whether these findings are generalizable. Several other drug classes routinely prescribed in neuropsychiatric and neurodegenerative disease also target time-dependent physiology (see Fig. 1B, Table 2).
Table 2.
Drug classes with time-dependent targets in mammalian brain tissues
| Drug Class | Approved Uses | Time-dependent Target (Protein Levels or Function) | Study |
|---|---|---|---|
|
| |||
| SSRI/SNRI | MDD, GAD, SAD, PD, OCD, PMDD, PTSD, bulimia nervosa | 5-HTT | Cortex (human)103 |
|
| |||
| NDRI | MDD | DAT | Striatum (mouse)104 |
|
| |||
| Methylphenidate | ADD, narcolepsy | DAT | Striatum (mouse)104 |
|
| |||
| Amphetamine | ADD, narcolepsy | Vesicle recycling machinery | SCN (mouse)9 |
|
| |||
| MAOI | MDD, atypical depression, BP, AD, PD | MAOA | VTA, striatum (mouse)7 |
|
| |||
| AChEI | AD, LBD, PD, MG, SCZ | AChE | Cerebrum, cerebellum, medulla, optic lobe (rat)8 |
|
| |||
| NMDAR antagonist |
AD, PD, drug-induced extrapyramidal reactions | NMDARs | SCN, motor cortex (rat)105–106 |
|
| |||
| Benzodiazepine | Insomnia, GAD, PD | GABAA receptors | Cerebral cortex, SCN (hamster)107,108 |
|
| |||
| Biogenic amine | PD | TH | VTA, nucleus accumbens, pineal gland (rat)109,110 |
References are not exhaustive, and many other known targets of these drugs in neuropsychiatric and neurodegenerative disease were shown to be circadian regulated at the transcriptional level but are not shown here.
Abbreviations: AChE, acetylcholinesterase; AChEI, acetylcholinesterase inhibitor; ADD, attention-deficit disorders; BP, bipolar disorder; DAT, dopamine transporter; GAD, generalized anxiety disorder; 5-HTT, serotonin transporter; LBD, Lewy body dementias; MAOA, monoamine oxidase A; MG, myasthenia gravis; NDRI, norepinephrine and dopamine reuptake inhibitor; NMDAR, N-methyl-D-aspartate receptor; OCD, obsessive-compulsive disorder; PD, panic disorder; PMDD, premenstrual dysphoric disorder; PTSD, posttraumatic stress disorder; SAD, social anxiety disorder; SCZ, schizophrenia; TH, tyrosine hydroxylase; VTA, ventral tegmental area.
Timing Off Targets and Drug Disposition
Alternatively, drug delivery can be timed to coincide with trough levels of an undesired target. The idea here is that adverse drug-related events can be minimized if drug exposure is out of phase with the mechanisms responsible for the adverse events. A study of the influence of dosing time on the effects of the antipsychotic aripiprazole (ARPZ) provides (retrospective) clinical support for this strategy.91 ARPZ can cause metabolic abnormalities because of off-target antagonism of D2 dopamine receptors (D2Rs) in the pancreas. This antagonism can lead to weight gain, a primary reason for drug discontinuation. The study found that patients who took ARPZ at bedtime had worse metabolic outcomes (poor lipid profiles) compared with morning dosing.91 The precise mechanism needs to be verified, but it was proposed that pancreatic D2R levels are clock regulated, highest at bedtime, and thus antagonism at this time leads to abnormally increased insulin levels during sleep and consequent metabolic dysregulation.
Another approach is to harmonize the administration of a drug with rhythms in its disposition. Passage through the blood-brain barrier (BBB) is a challenge in drug development; increased doses can deliver more drug to the central nervous system (CNS), but toxicity in the periphery can be dose limiting. Recent work in Drosophila showed a clinically relevant time dependency in BBB permeability,92 and evidence suggests a similar mechanism may exist in mammals.15 Time-dependent BBB permeability could be leveraged to improve therapeutic windows for both CNS-targeting and peripheral nervous system (PNS)–targeting drugs. As an example of the latter, there is a need in peripheral neuropathic pain for drugs with better-tolerated CNS-side-effect profiles.93,94 Future models will define the scope of potential benefit from harmonizing treatment with BBB permeability.
Very few clinical trials have tested the influence of time of day on CNS-targeting and PNS-targeting agents.95–98 As the understanding of circadian-expressed targets and physiology in neurologic disease expands, so does the potential for time-stipulated therapy.
CONCLUDING THOUGHTS
The next Decade: Implementing Circadian Medicine
There are unmet needs for more effective therapies in several areas of neurology. Drug development in neurodegenerative disease is marked with pivotal failures,99 and controversy surrounds the clinical value of standard-of-care therapies in mental illness.100 In 2018, soon after unsuccessful attempts to translate molecular targets into efficacious new therapies, Pfizer, the world’s third largest pharmaceutical company, announced plans to abandon all research and development into new neuroscience programs.101
Circadian medicine alone is not a panacea. However, recent advances in the understanding of 24-hour dynamics in physiology and disease create a path for near-term improvements in medicine. This article concludes with 3 specific clinical scenarios of how technologic advances in circadian biology might change patient care in the next decade.
By administering a single minimally invasive test for internal time, a clinician diagnoses circadian sleep-wake phase disorders in patients.Biomarkers of internal time based on gene expression signatures can replace DLMO, actigraphy, and unreliable sleep diaries.
The neurologist stipulates timing of light therapy, exercise, and feeding to strengthen clock function in a patient with low-amplitude or irregular sleep-wake rhythms, such as in neurodegenerative disorders.The circadian clock in the SCN is maximally sensitive to light-resetting during narrow windows of time (phases). With an accurate test for internal phase, treatment can be delivered at the most appropriate time.
Guided by a map of 24-hour dynamics in 5-HT neurotransmission in the brain, researchers initiate a clinical trial to test administration-time dependency of SSRIs in depression. Current maps of circadian dynamics in the human brain remain poorly resolved, but this is likely to change. Genomic repositories are ever growing, some with extensive coverage across brain regions (eg, GTEx102). A circadian human brain atlas is foreseeable, and should spark mechanism-based prospective trials in circadian medicine.
KEY POINTS.
Fundamental neural processes are normally time-of-day regulated, likely explaining the tight link between disrupted circadian rhythms and neurologic disease.
Time of day is rarely considered for central nervous system–targeting therapies. Circadian medicine incorporates knowledge of 24-hour biological rhythms to administer more effective treatment.
It is conceivable that (1) determining a patient’s internal circadian time, and (2) prescribing time-stipulated therapy will be common practice in neurology clinics in the coming decade.
Footnotes
Disclosures: None.
REFERENCES
- 1.Weaver DR. Introduction to circadian rhythms and mechanisms of circadian oscillations. In: Gumz ML, editor. Circadian clocks: role in health and disease. New York: Springer New York; 2016. p. 1–55. [Google Scholar]
- 2.Zhang R, Lahens NF, Ballance HI, et al. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 2014;111(45):16219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruben MD, Wu G, Smith DF, et al. A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci Transl Med 2018;10(458). 10.1126/scitranslmed.aat8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skarke C, Lahens NF, Rhoades SD, et al. A pilot characterization of the human chronobiome. Sci Rep 2017;7(1):17141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X, Zecharia A, Zhang Z, et al. Circadian factor BMAL1 in histaminergic neurons regulates sleep architecture. Curr Biol 2014;24(23):2838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung S, Lee EJ, Yun S, et al. Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell 2014;157(4): 858–68. [DOI] [PubMed] [Google Scholar]
- 7.Hampp G, Ripperger JA, Houben T, et al. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol 2008;18(9):678–83. [DOI] [PubMed] [Google Scholar]
- 8.Mohan C, Radha E. Circadian rhythm in acetylcholinesterase activity during aging of the central nervous system. Life Sci 1974;15(2):231–7. [DOI] [PubMed] [Google Scholar]
- 9.Deery MJ, Maywood ES, Chesham JE, et al. Proteomic analysis reveals the role of synaptic vesicle cycling in sustaining the suprachiasmatic circadian clock. Curr Biol 2009;19(23):2031–6. [DOI] [PubMed] [Google Scholar]
- 10.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342(6156):373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundgaard I, Lu ML, Yang E, et al. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab 2017;37(6):2112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014;81(1): 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, et al. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci 2008;11(2):200–8. [DOI] [PubMed] [Google Scholar]
- 14.Hinard V, Mikhail C, Pradervand S, et al. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J Neurosci 2012;32(36):12506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kervezee L, Hartman R, van den Berg D-J, et al. Diurnal variation in P-glycoprotein-mediated transport and cerebrospinal fluid turnover in the brain. AAPS J 2014;16(5):1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan W, Cornélissen G, Halberg F, et al. Selected contribution: circadian rhythm of tumor necrosis factor-alpha uptake into mouse spinal cord. J Appl Physiol 2002;92(3):1357–62 [discussion: 1356]. [DOI] [PubMed] [Google Scholar]
- 17.Vadnie CA, McClung CA. Circadian Rhythm Disturbances in Mood Disorders: Insights into the Role of the Suprachiasmatic Nucleus. Neural Plast 2017; 2017:1504507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Videnovic A, Lazar AS, Barker RA, et al. “The clocks that time us”–circadian rhythms in neurodegenerative disorders. Nat Rev Neurol 2014;10(12):683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iranzo A Sleep in neurodegenerative diseases. Sleep Med Clin 2016;11(1): 1–18. [DOI] [PubMed] [Google Scholar]
- 20.Harper DG, Volicer L, Stopa EG, et al. Disturbance of endogenous circadian rhythm in aging and Alzheimer disease. Am J Geriatr Psychiatry 2005;13(5): 359–68. [DOI] [PubMed] [Google Scholar]
- 21.Hatfield CF, Herbert J, van Someren EJW, et al. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain 2004;127(Pt 5):1061–74. [DOI] [PubMed] [Google Scholar]
- 22.Bliwise DL, Mercaldo ND, Avidan AY, et al. Sleep disturbance in dementia with Lewy bodies and Alzheimer’s disease: a multicenter analysis. Dement Geriatr Cogn Disord 2011;31(3):239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hastings MH, Goedert M. Circadian clocks and neurodegenerative diseases: time to aggregate? Curr Opin Neurobiol 2013;23(5):880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan T, Malkani R. Sleep and circadian rhythm disruption and stress intersect in Alzheimer’s disease. Neurobiol Stress 2018. 10.1016/j.ynstr.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 2016;354(6315):1004–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kissling C, Retz W, Wiemann S, et al. A polymorphism at the 3’-untranslated region of the CLOCK gene is associated with adult attention-deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 2008;147(3):333–8. [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Breen G, Chen C-K, et al. Association study between a polymorphism at the 3’-untranslated region of CLOCK gene and attention deficit hyperactivity disorder. Behav Brain Funct 2010;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takao T, Tachikawa H, Kawanishi Y, et al. CLOCK gene T3111C polymorphism is associated with Japanese schizophrenics: a preliminary study. Eur Neuropsychopharmacol 2007;17(4):273–6. [DOI] [PubMed] [Google Scholar]
- 29.Kishi T, Kitajima T, Ikeda M, et al. Association study of clock gene (CLOCK) and schizophrenia and mood disorders in the Japanese population. Eur Arch Psychiatry Clin Neurosci 2009;259(5):293–7. [DOI] [PubMed] [Google Scholar]
- 30.Benedetti F, Radaelli D, Bernasconi A, et al. Clock genes beyond the clock: CLOCK genotype biases neural correlates of moral valence decision in depressed patients. Genes Brain Behav 2008;7(1):20–5. [DOI] [PubMed] [Google Scholar]
- 31.Serretti A, Benedetti F, Mandelli L, et al. Genetic dissection of psychopathological symptoms: insomnia in mood disorders and CLOCK gene polymorphism. Am J Med Genet B Neuropsychiatr Genet 2003;121B(1):35–8. [DOI] [PubMed] [Google Scholar]
- 32.Soria V, Martínez-Amorós E, Escaramís G, et al. Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacology 2010;35(6):1279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linnstaedt SD, Pan Y, Mauck MC, et al. Evaluation of the association between genetic variants in circadian rhythm genes and posttraumatic stress symptoms identifies a potential functional allele in the transcription factor TEF. Front Psychiatry 2018;9:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Partonen T, Treutlein J, Alpman A, et al. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann Med 2007;39(3):229–38. [DOI] [PubMed] [Google Scholar]
- 35.Khanzada NS, Butler MG, Manzardo AM. geneanalytics pathway analysis and genetic overlap among autism spectrum disorder, bipolar disorder and schizophrenia. Int J Mol Sci 2017;18(3). 10.3390/ijms18030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asaoka S, Aritake S, Komada Y, et al. Factors associated with shift work disorder in nurses working with rapid-rotation schedules in Japan: the nurses’ sleep health project. Chronobiol Int 2013;30(4):628–36. [DOI] [PubMed] [Google Scholar]
- 37.Scott AJ, Monk TH, Brink LL. Shiftwork as a risk factor for depression: a pilot study. Int J Occup Environ Health 1997;3(Supplement 2):S2–9. [PubMed] [Google Scholar]
- 38.Bokenberger K, Sjölander A, Dahl Aslan AK, et al. Shift work and risk of incident dementia: a study of two population-based cohorts. Eur J Epidemiol 2018; 33(10):977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jørgensen JT, Karlsen S, Stayner L, et al. Shift work and overall and cause-specific mortality in the Danish nurse cohort. Scand J Work Environ Health 2017;43(2):117–26. [DOI] [PubMed] [Google Scholar]
- 40.Boeve BF. Idiopathic REM sleep behaviour disorder in the development of Parkinson’s disease. Lancet Neurol 2013;12(5):469–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musiek ES, Bhimasani M, Zangrilli MA, et al. Circadian rest-activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol 2018; 75(5):582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abbott SM, Videnovic A. Chronic sleep disturbance and neural injury: links to neurodegenerative disease. Nat Sci Sleep 2016;8:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pallier PN, Maywood ES, Zheng Z, et al. Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington’s disease. J Neurosci 2007; 27(29):7869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roh JH, Huang Y, Bero AW, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med 2012;4(150):150ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Q, Peng XD, Huang CQ, et al. Association between ARNTL (BMAL1) rs2278749 polymorphism T >C and susceptibility to Alzheimer disease in a Chinese population. Genet Mol Res 2015;14(4):18515–22. [DOI] [PubMed] [Google Scholar]
- 46.Chen H-F, Huang C-Q, You C, et al. Polymorphism of CLOCK gene rs 4580704 C> G is associated with susceptibility of Alzheimer’s disease in a Chinese population. Arch Med Res 2013;44(3):203–7. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y-K, Peng X-D, Li Y-H, et al. The polymorphism of CLOCK gene 3111T/C C>T is associated with susceptibility of Alzheimer disease in Chinese population. J Investig Med 2013;61(7):1084–7. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Y, Zhan G, Fenik P, et al. Chronic sleep disruption advances the temporal progression of tauopathy in P301S mutant mice. J Neurosci 2018. 10.1523/JNEUROSCI.0275-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whittaker DS, Loh DH, Wang H-B, et al. Circadian-based treatment strategy effective in the BACHD mouse model of Huntington’s disease. J Biol Rhythms 2018;33(5):535–54. [DOI] [PubMed] [Google Scholar]
- 50.Kang J-E, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009;326(5955):1005–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim ASP, Kowgier M, Yu L, et al. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep 2013;36(7): 1027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benedict C, Byberg L, Cedernaes J, et al. Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men. Alzheimers Dement 2015; 11(9):1090–7. [DOI] [PubMed] [Google Scholar]
- 53.Hanford N, Figueiro M. Light therapy and Alzheimer’s disease and related dementia: past, present, and future. J Alzheimers Dis 2013;33(4):913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wehrens SMT, Christou S, Isherwood C, et al. Meal timing regulates the human circadian system. Curr Biol 2017;27(12):1768–75.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaur G, Phillips CL, Wong K, et al. Timing of administration: for commonly-prescribed medicines in Australia. Pharmaceutics 2016;8(2). 10.3390/pharmaceutics8020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCurry SM, Reynolds CF, Ancoli-Israel S, et al. Treatment of sleep disturbance in Alzheimer’s disease. Sleep Med Rev 2000;4(6):603–28. [DOI] [PubMed] [Google Scholar]
- 57.Vitiello MV, Borson S. Sleep disturbances in patients with Alzheimer’s disease. CNS Drugs 2001;15(10):777–96. [DOI] [PubMed] [Google Scholar]
- 58.Hope T, Keene J, Gedling K, et al. Predictors of institutionalization for people with dementia living at home with a carer. Int J Geriatr Psychiatry 1998;13(10): 682–90. [DOI] [PubMed] [Google Scholar]
- 59.Deschenes CL, McCurry SM. Current treatments for sleep disturbances in individuals with dementia. Curr Psychiatry Rep 2009;11(1):20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCall WV. Diagnosis and management of insomnia in older people. J Am Geriatr Soc 2005;53(7 Suppl):S272–7. [DOI] [PubMed] [Google Scholar]
- 61.Proulx J, Hunt J. Drug use among seniors on public drug programs in Canada, 2012. Healthc Q 2015;18(1):11–3. [DOI] [PubMed] [Google Scholar]
- 62.Wong SL, Gilmour H, Ramage-Morin PL. Alzheimer’s disease and other dementias in Canada. Health Rep 2016;27(5):11–6. [PubMed] [Google Scholar]
- 63.Auger RR, Burgess HJ, Emens JS, et al. Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015. J Clin Sleep Med 2015;11(10): 1199–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamblyn R, Abrahamowicz M, du Berger R, et al. A 5-year prospective assessment of the risk associated with individual benzodiazepines and doses in new elderly users. J Am Geriatr Soc 2005;53(2):233–41. [DOI] [PubMed] [Google Scholar]
- 65.By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63(11):2227–46. [DOI] [PubMed] [Google Scholar]
- 66.Chesson AL, Littner M, Davila D, et al. Practice parameters for the use of light therapy in the treatment of sleep disorders. Sleep 1999;22(5):641–60. [DOI] [PubMed] [Google Scholar]
- 67.Morgenthaler TI, Lee-Chiong T, Alessi C, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. Sleep 2007; 30(11):1445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lyketsos CG, Lindell Veiel L, Baker A, et al. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry 1999;14(7):520–5. [PubMed] [Google Scholar]
- 69.Barrick AL, Sloane PD, Williams CS, et al. Impact of ambient bright light on agitation in dementia. Int J Geriatr Psychiatry 2010;25(10):1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skene DJ, Swaab DF. Melatonin rhythmicity: effect of age and Alzheimer’s disease. Exp Gerontol 2003;38(1–2):199–206. [DOI] [PubMed] [Google Scholar]
- 71.Wichniak A, Wierzbicka A, Walęcka M, et al. Effects of Antidepressants on Sleep. Curr Psychiatry Rep 2017;19(9):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rybakowski JK, Dmitrzak-Weglar M, Kliwicki S, et al. Polymorphism of circadian clock genes and prophylactic lithium response. Bipolar Disord 2014;16(2): 151–8. [DOI] [PubMed] [Google Scholar]
- 73.McGlashan EM, Nandam LS, Vidafar P, et al. The SSRI citalopram increases thesensitivity of the human circadian system to light in an acute dose. Psychopharmacology 2018. 10.1007/s00213-018-5019-0. [DOI] [PubMed] [Google Scholar]
- 74.Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms 1999;14(3):227–36. [DOI] [PubMed] [Google Scholar]
- 75.Wu G, Ruben MD, Schmidt RE, et al. Population level rhythms in human skin: implications for circadian medicine. bioRxiv 2018. 10.1101/301820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wittenbrink N, Ananthasubramaniam B, Münch M, et al. High-accuracy determination of internal circadian time from a single blood sample. J Clin Invest 2018. 10.1172/JCI120874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Braun R, Kath WL, Iwanaszko M, et al. Universal method for robust detection ofcircadian state from gene expression. Proc Natl Acad Sci U S A 2018. 10.1073/pnas.1800314115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baehr EK, Eastman CI, Revelle W, et al. Circadian phase-shifting effects of nocturnal exercise in older compared with young adults. Am J Physiol Regul Integr Comp Physiol 2003;284(6):R1542–50. [DOI] [PubMed] [Google Scholar]
- 79.King AC, Oman RF, Brassington GS, et al. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA 1997; 277(1):32–7. [PubMed] [Google Scholar]
- 80.Oike H Modulation of circadian clocks by nutrients and food factors. Biosci Biotechnol Biochem 2017;81(5):863–70. [DOI] [PubMed] [Google Scholar]
- 81.Ma Z, Jiang W, Zhang EE. Orexin signaling regulates both the hippocampal clock and the circadian oscillation of Alzheimer’s disease-risk genes. Sci Rep 2016;6:36035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gu Z, Wang B, Zhang Y-B, et al. Association of ARNTL and PER1 genes with Parkinson’s disease: a case-control study of Han Chinese. Sci Rep 2015;5: 15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saito Y, Yoshida S, Nakaya N, et al. Comparison between morning and evening doses of simvastatin in hyperlipidemic subjects. A double-blind comparative study. Arterioscler Thromb 1991;11(4):816–26. [DOI] [PubMed] [Google Scholar]
- 84.Anafi RC, Francey LJ, Hogenesch JB, et al. CYCLOPS reveals human transcriptional rhythms in health and disease. Proc Natl Acad Sci U S A 2017;114(20): 5312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Top 200 Prescribed Medicines of 2018. ClinCalc. 2018. Available at: http://clincalc.com/DrugStats/Top200Drugs.aspx. Accessed September 2018. [Google Scholar]
- 86.Wong DT, Horng JS, Bymaster FP, et al. A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine. Life Sci 1974;15(3):471–9. [DOI] [PubMed] [Google Scholar]
- 87.Schildkraut JJ. Neuropharmacology of the affective disorders. Annu Rev Pharmacol 1973;13:427–54. [DOI] [PubMed] [Google Scholar]
- 88.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163(11):1905–17. [DOI] [PubMed] [Google Scholar]
- 89.Albrecht U Molecular Mechanisms in Mood Regulation Involving the Circadian Clock. Front Neurol 2017;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kawai H, Machida M, Ishibashi T, et al. Chronopharmacological Analysis of Antidepressant Activity of a Dual-Action Serotonin Noradrenaline Reuptake Inhibitor (SNRI), Milnacipran, in Rats. Biol Pharm Bull 2018;41(2):213–9. [DOI] [PubMed] [Google Scholar]
- 91.Chipchura DA, Freyberg Z, Edwards C, et al. Does the time of drug administration alter the metabolic risk of aripiprazole? Front Psychiatry 2018;9:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang SL, Yue Z, Arnold DM, et al. A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell 2018;173(1):130–9.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murphy KL, Bethea JR, Fischer R. Neuropathic pain in multiple sclerosis—current therapeutic intervention and future treatment perspectives. In: Zagon IS, McLaughlin PJ, editors. Multiple sclerosis: perspectives in treatment and pathogenesis. Brisbane (AU): Codon Publications; 2017. [PubMed] [Google Scholar]
- 94.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14(2):162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Badiyan SN, Ferraro DJ, Yaddanapudi S, et al. Impact of time of day on outcomes after stereotactic radiosurgery for non-small cell lung cancer brain metastases. Cancer 2013;119(19):3563–9. [DOI] [PubMed] [Google Scholar]
- 96.Rahn DA 3rd, Ray DK, Schlesinger DJ, et al. Gamma knife radiosurgery for brain metastasis of nonsmall cell lung cancer: is there a difference in outcome between morning and afternoon treatment? Cancer 2011;117(2):414–20. [DOI] [PubMed] [Google Scholar]
- 97.Chan S, Rowbottom L, McDonald R, et al. Could time of whole brain radiotherapy delivery impact overall survival in patients with multiple brain metastases? Ann Palliat Med 2016;5(4):267–79. [DOI] [PubMed] [Google Scholar]
- 98.Shappell SA, Kearns GL, Valentine JL, et al. Chronopharmacokinetics and chronopharmacodynamics of dextromethamphetamine in man. J Clin Pharmacol 1996;36(11):1051–63. [DOI] [PubMed] [Google Scholar]
- 99.Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 2014;370(4):322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 2008;5(2):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Terry M Pfizer to cut 300 jobs as it ends Alzheimer’s, Parkinson’s quest j BioSpace. BioSpace; 2018. Available at: https://www.biospace.com/article/unique-pfizer-to-cut-300-jobs-as-it-ends-alzheimer-s-parkinson-s-quest/. Accessed October 23, 2018.
- 102.Consortium GTEx. The Genotype-Tissue Expression (GTEx) project. Nat Genet 2013;45(6):580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matheson GJ, Schain M, Almeida R, et al. Diurnal and seasonal variation of the brain serotonin system in healthy male subjects. Neuroimage 2015;112:225–31. [DOI] [PubMed] [Google Scholar]
- 104.Ferris MJ, Espanã RA, Locke JL, et al. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc Natl Acad Sci U S A 2014; 111(26):E2751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bendová Z, Sumová A, Mikkelsen JD. Circadian and developmental regulation of N-methyl-d-aspartate-receptor 1 mRNA splice variants and N-methyl-daspartate-receptor 3 subunit expression within the rat suprachiasmatic nucleus. Neuroscience 2009;159(2):599–609. [DOI] [PubMed] [Google Scholar]
- 106.Estrada-Rojo F, Morales-Gomez J, Coballase-Urrutia E, et al. Diurnal variation of NMDA receptor expression in the rat cerebral cortex is associated with traumatic brain injury damage. BMC Res Notes 2018;11(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kanterewicz BI, Rosenstein RE, Golombek DA, et al. Daily variations in GABA receptor function in Syrian hamster cerebral cortex. Neurosci Lett 1995; 200(3):211–3. [DOI] [PubMed] [Google Scholar]
- 108.Walton JC, McNeill JK 4th, Oliver KA, et al. Temporal regulation of GABAA receptor subunit expression: role in synaptic and extrasynaptic communication in the suprachiasmatic nucleus. eNeuro 2017;4(2). 10.1523/ENEURO.0352-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McGeer EG, McGeer PL. Circadian rhythm in pineal tyrosine hydroxylase. Science 1966;153(3731):73–4. [DOI] [PubMed] [Google Scholar]
- 110.Webb IC, Baltazar RM, Wang X, et al. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J Biol Rhythms 2009;24(6):465–76. [DOI] [PubMed] [Google Scholar]