Abstract
Purpose
The composition of thrombi retrieved during endovascular thrombectomy (EVT) in acute ischemic stroke (AIS) due to large vessel occlusion (LVO) may differ depending on their origin. In this study, we investigated the association between thrombus composition and stroke etiology in a large population of patients from the Dutch MR CLEAN Registry treated with EVT in daily clinical practice.
Methods
The thrombi of 332 patients with AIS were histologically analyzed for red blood cells (RBC), fibrin/platelets (F/P), and white blood cells (leukocytes) using a machine learning algorithm. Stroke etiology was assessed using the Trial of Org 10,172 in acute stroke treatment (TOAST) classification.
Results
The thrombi of cardioembolic origin contained less RBC and more F/P than those of non-cardioembolic origin (25.8% vs 41.2% RBC [p = 0.003] and 67.1% vs 54.5% F/P [p = 0.004]). The likelihood of a non-cardioembolic source of stroke increased with increasing thrombus RBC content (OR 1.02; [95% CI 1.00–1.06] for each percent increase) and decreased with a higher F/P content (OR 1.02; [95% CI 1.00–1.06]). Thrombus composition in patients with a cardioembolic origin and undetermined origin was similar.
Conclusion
Thrombus composition is significantly associated with stroke etiology, with an increase in RBC and a decrease in F/P raising the odds for a non-cardioembolic cause. No difference between composition of cardioembolic thrombi and of undetermined origin was seen. This emphasizes the need for more extensive monitoring for arrhythmias and/or extended cardiac analysis in case of an undetermined origin.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00234-023-03115-y.
Keywords: Ischemic stroke, Mechanical thrombectomy, Stent-retriever, Endovascular treatment, Thrombus, Microscopy
Introduction
With the emergence of endovascular thrombectomy (EVT) as part of standard treatment for acute ischemic stroke (AIS) caused by a large vessel occlusion (LVO), occluding thrombo-emboli have become available for histopathologic analysis. Insight in the relationship between thrombus composition and stroke etiology could be of value for secondary stroke prevention, as the appropriate preventive treatment depends on stroke etiology. Several causes of AIS have been identified, and the most prevalent being large artery atherosclerosis and cardiac embolism; both of which may lead to thrombo-embolic occlusions in the intracranial circulation. Stroke etiology currently remains unknown in approximately 30–50% of patients despite extensive clinical workup [1]. If a clear association exists between thrombus composition and stroke etiology, examination of extracted thrombi could be useful to guide therapeutic choices for secondary prevention of recurrent stroke [2].
Various previous studies have studied the relationship between thrombus composition and stroke etiology [3–21]. Most studies so far, however, did not find an association, possibly due to small sample sizes [3, 5, 6, 8, 11–15, 17]. While several studies did find an association between thrombus composition and etiology, they yielded contradictory results: some reported a higher amount of red blood cells (RBC) in thrombi associated with large artery atherosclerosis than in thrombi of cardiac or unknown origin [9, 10, 16, 18, 20, 21], while others reported the opposite with a higher amount of RBC in thrombi related to a cardiac source of embolization [7, 19, 22].
The aim of this study was to assess the relationship between thrombus composition and stroke etiology in a large population of AIS patients, treated with EVT for a large vessel occlusion of the anterior circulation, in daily clinical practice. In contrast to previous studies, we were able to compare clinical baseline characteristics of patients with and without thrombi available for analysis.
Patients and methods
Study population and sample selection
The MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) Registry was a prospective observational study of all patients who underwent EVT for AIS in the Netherlands (Appendix) [23]. Enrolment started March 2014, directly after the final inclusion in the MR CLEAN trial [24]. All patients undergoing EVT for AIS in the anterior or posterior circulation (defined as at least entry into the angiography suite and receiving arterial puncture), in one of the sixteen centers performing EVT in the Netherlands, were registered. The central medical ethics committee evaluated the study protocol and granted permission to carry out the study as a registry. For this histopathologic substudy, patients who met the following criteria were included: age 18 years and older, a proximal intracranial vessel occlusion in the anterior circulation as shown on CT angiography (CTA), availability of clinical and imaging data for the assessment of stroke etiology, and available thrombus for histological assessment. Data of patients treated until 15 June 2016 were collected and analyzed for this study.
Thrombus analysis
After EVT, the thrombi were immediately stored in 4% buffered formaldehyde before embedding in paraffin. The thrombi were mostly retrieved by mechanical thrombectomy using a stent retriever, with only 14.8% using aspiration thrombectomy. For each paraffin block, 5-µm sections were cut at two depths, generally at a depth of 170 and 230 µm (Microm HM335 S, Microm International GmbH, Waldorf, Germany), as it has been shown that partial sectioning of the thrombus provides a good estimate of thrombus composition [25]. The two sections at these depths were collected on a single slide, stained by hematoxylin–eosin (HE), digitized at 20 × magnification (228 nm/pixel, Hamamatsu Nano-Zoomer, Hamamatsu Photonics K.K., Hamamatsu City, Japan), and images were stored as raw Hamamatsu.ndpi datafiles.
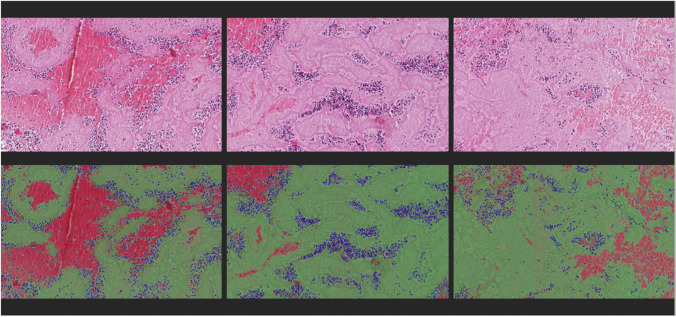
An analysis of stained sections was performed using Orbit Image Analysis software (Orbit Image Analysis, Idorsia Ltd.) [26]. Orbit enables the analysis of native Hamamatsu.ndpi files and utilizes machine learning algorithms for image segmentation, classification, and quantification. First, a unified foreground/background segmentation model was trained to exclude background from further analysis. Using one unified classification model for all thrombi yielded less classification accuracy due to slight differences in staining between samples; therefore, separate classification models were created for each slide containing two sections. All models were trained to quantify percentages of red blood cells (RBC), fibrin and platelets (F/P), and white blood cells (leukocytes), as the main components of extracted EVT thrombi [3, 5–19, 22]. The weighted average of the two sections was considered representative for the whole thrombus [25]. Orbit was then used in conjunction with a custom script to enable batch analysis of all sections with individual classification models applied for each slide, generating both a Jason data file containing the quantification results per sample and a classification overlay image per sample (Fig. 1) to allow for visual inspection of the classification accuracy. Thrombus sections with large central defects in RBC rich sample regions, generally difficult to cut, were corrected by manually outlining and measuring these defects and adding the missing RBC surface area after comparison with the other section, and the original paraffin blocks (biomedical scientist (HH), validated by a pathobiologist (HB)).
Fig. 1.
Examples of generated classification overlay images into RBC (red), F/P (green), and leukocytes (blue) to allow for visual inspection of the classification accuracy using Orbit. The top three images are original HE stained images; the bottom three images are the semi-automatically segmented images
Assessment of stroke etiology
All patients underwent CTA or magnetic resonance angiography (MRA) of the cervical arteries before EVT. Furthermore, 12-lead ECG followed by ECG-monitoring for at least 24 h was performed. Additional etiologic workup was performed in accordance with local protocols. Stroke etiology was determined based on data provided in the discharge letters and imaging by two trained observers who were blinded for histological thrombus composition. The presumed cause of stroke was determined for each patient using the TOAST criteria as a guideline: large artery atherosclerosis (TOAST 1), cardioembolism (TOAST 2), stroke of other determined cause (TOAST 4), and stroke of undetermined cause (TOAST 5). In our cohort of patients who underwent EVT for AIS, there were no patients with small vessel disease as cause of stroke (TOAST 3). A patient was considered to have large artery atherosclerotic stroke, if there was > 50% atherosclerotic stenosis or atherosclerotic occlusion at the bifurcation of the carotid artery on the symptomatic side. Patients were considered to have undetermined stroke etiology, if more than one possible cause was identified; if no cause was identified despite complete workup, as described above; or if diagnostic workup was incomplete.
For statistical analysis, each patient was allocated to one of three predefined etiologic categories: (a) “non-cardioembolic” for both large artery atherosclerotic disease and other determined causes (TOAST 1 and 4), (b) “cardioembolic” (TOAST 2), or (c) “undetermined” (TOAST 5). For graphical representation of thrombus composition within groups, we further subdivided cardioembolic stroke into medium-risk or high-risk based on the evidence of the relative propensity for embolization, according to the original TOAST classification [27]. We also subdivided non-cardioembolic into large artery atherosclerosis, carotid artery dissection, and other determined cause. Stroke of undetermined etiology was subdivided in “ > 1 cause” (more than one possible cause was identified) and “cryptogenic” (no cause was identified despite complete workup, as described).
Statistical analysis
Clinical characteristics and histological thrombus composition were described using standard statistics. Since thrombus composition did not follow a normal distribution, a Kruskal–Wallis test was performed first to assess differences in thrombus composition between all three etiologic groups (cardioembolic, non-cardioembolic, and undetermined). The association of histological components (percentages of RBC, F/P, and leukocytes) with stroke etiology (non-cardioembolic stroke, cardioembolic stroke, and stroke with undetermined etiology) was estimated with univariable and multivariable multinomial logistic regression and presented as (adjusted) odds ratios (aOR) with 95% confidence intervals (CI), with cardioembolic stroke as the reference category. We adjusted for potentially relevant differences in baseline characteristics by performing Kruskal–Wallis, χ2 test and Fisher-Freeman-Halton tests and including characteristics with a p-value of < 0.20 in the multivariable models (supplemental Table S1). For the regression analyses, single imputation was performed for missing values (supplemental Table S2). To assess the representativeness of the patients with an available thrombus to all patients who underwent EVT in clinical practice, we compared patient, clinical, and imaging characteristics of the included patients from the MRCLEAN Registry to those who were not included using ANOVA and Mann–Whitney U tests. Statistical analyses were performed using Stata (StataCorp. 2017. Stata Statistical Software: Release 15.1 SE. College Station, TX: StataCorp LLC) and SPSS (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp).
Results
Patient population
Thrombus samples of 332 patients from the MR CLEAN Registry were included for histological analysis in this study (Fig. S1). Baseline characteristics of these patients did not differ with those not included from the registry. Importantly, there were also no statistically significant differences in stroke etiology. For included patients, stroke etiology was categorized as cardioembolic in 114 patients (34.3%), non-cardioembolic in 58 patients (17.5%), and undetermined in 160 patients (48.2%), compared to 364 (30.5%), 199 (16.7%), and 631 (52.8%), respectively, in patients from the registry who were not included for histological analysis (Table 1).
Table 1.
Baseline characteristics for patients from the MR CLEAN cohort with histological analysis as compared to those without. ANOVA was performed; p-values > 0.05 are printed bold
| Histology n = 332, (%) | No histology n = 1194, (%) | p-value | |
|---|---|---|---|
| Age (median) | 70 | 71 | 0.690a |
| Sex (male) | 177 (53.3%) | 632 (52.9%) | 0.902 |
| NIHSS baseline (median) | 17 | 15 | 0.00a |
| IVT | 251 (75.6%) | 919 (77.2%) | 0.551 |
| Atrial fibrillation | 92 (27.9%) | 243 (20.7%) | 0.006 |
| Peripheral arterial disease | 43 (13.3%) | 95 (8.1%) | 0.004 |
| Previous stroke | 66 (19.9%) | 187 (15.8%) | 0.072 |
| Myocardial infarction | 55 (16.8%) | 178 (15.2%) | 0.486 |
| Antiplatelet use | 105 (32.2%) | 401 (34%) | 0.555 |
| Coumarin use | 54 (16.5%) | 140 (11.8%) | 0.025 |
| NOAC use | 11 (3.4%) | 26 (2.2%) | 0.228 |
| Occlusion segment based on CTA | |||
| Intracranial ICA | 12 (3.8%) | 73 (6.5%) | 0.072 |
| ICA-T | 90 (28.3%) | 232 (20.5%) | 0.003 |
| M1 | 190 (59.7%) | 652 (57.6%) | 0.502 |
| M2 | 25 (7.9%) | 156 (13.8%) | 0.005 |
| Other: M3/anterior | 1 (0.3%) | 18 (1.6%) | 0.077 |
| Etiology (TOAST) | |||
| Cardioembolic | 114 (34.3%) | 364 (30.5%) | 0.181 |
| Non-cardioembolic | 58 (17.5%) | 199 (16.7%) | 0.729 |
| Undetermined | 160 (48.2%) | 631 (52.8%) | 0.133 |
aMann-Whitney U test
Differences in thrombus histology between etiologic groups
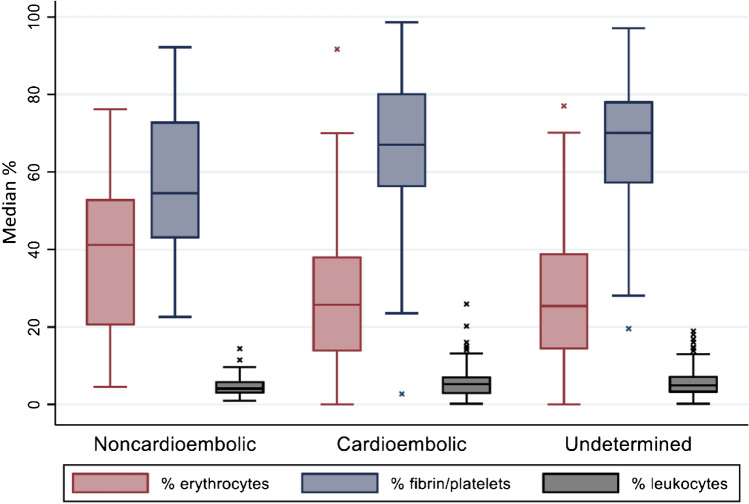
Histopathologic evaluation showed the thrombi typically contained areas being predominantly RBC, F/P, or leukocyte rich (Fig. 2). A large heterogeneity in overall composition was seen in our cohort (supplemental Fig. S2). For all thrombi, median RBC content was 27.1% (IQR 15.9–42.4), median F/P content was 67.0% (IQR 53.1–78.1) and median leukocyte content was 4.8% (IQR 3.0–7.1). RBC content in thrombi from patients with non-cardioembolic etiology (median 41.2%, IQR 20.5–53.0), was higher than in thrombi from patients with a cardiac etiology (median 25.8%, IQR 13.8–38.2) and thrombi from patients with an undetermined origin (median 25.4%, IQR 14.3–39.0). Inversely, the F/P content in thrombi from patients with non-cardioembolic etiology (median 54.5%, IQR 55.1–80.3) was lower than in thrombi from patients with cardioembolic etiology (median 67.1%, IQR 55.1–80.3) and thrombi with an undetermined origin (median 70.0%, IQR 57.1–78.2). Using a Kruskal–Wallis test for group differences, a significant difference was seen between the three etiologic groups for RBC content (p < 0.001) and F/P content (p = 0.002), but not for leukocyte content (p = 0.24) (Table 2 and Fig. 3). These differences were more pronounced for high-risk cardioembolic etiologies (supplemental Fig. S3). After correction for baseline differences in the regression analysis, RBC and F/P significantly differed between non-cardioembolic and cardioembolic strokes. Increased RBC (OR, 1.02 [95% CI 1.01–1.05]) and decreased F/P (OR, 0.98 [95% CI 0.95–0.99]) were associated with non-cardioembolic stroke, as opposed to cardioembolic stroke (Table 3). In other words, for every 1% increase in RBC content, the OR for a non-cardioembolic stroke (as opposed to cardioembolic stroke) was 1.02 (Fig. 4). Leukocyte content did not differ between non-cardioembolic and cardioembolic stroke. Furthermore, we did not observe any differences in thrombus composition between strokes with undetermined etiology and cardioembolic strokes in the univariable regression analyses (OR 1.00 [95% CI 0.99–1.01] for RBC, OR 1.00 [95% CI 0.99–1.01] for F/P and OR 1.00 [95% CI 0.94–1.07] for leukocytes) and multivariable regression analyses (Table 3).
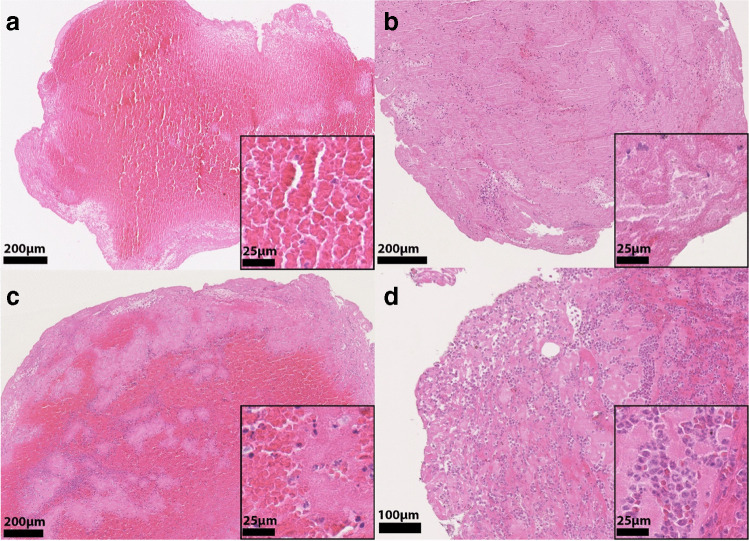
Fig. 2.
Examples of RBC rich (A), F/P rich (B), mixed (C), and leukocyte rich (D) thrombus areas. The inserted boxes represent magnifications of the underlying area of each example. HE-stain, bar = 200 µm (A–C), 100 µm (D), and 25 µm (inserts). RBC are red, F/P purple, and nuclei of leukocytes blue
Table 2.
Thrombus composition stratified by stroke etiology
| Non-cardioembolic (n = 58) | Cardioembolic (n = 114) | Undetermined (n = 160) | |
|---|---|---|---|
| Median (IQR) RBC % | 41.2 (20.5–53.0) | 25.8 (13.8–38.2) | 25.4 (14.3–39.0) |
| Median (IQR) F/P % | 54.5 (42.9–73.0) | 67.1 (56.1–80.3) | 70.0 (57.1–78.2) |
| Median (IQR) leukocytes % | 4.1 (2.9–6.0) | 5.2 (2.7–7.2) | 5.0 (3.1–7.4) |
Fig. 3.
Bar-whisker plot of thrombus composition for the three etiologic groups. P-values are given based on Kruskal Wallis test. x = outliers > 1.5 times box height. Significant differences were found for both erythrocyte content (p < 0.001) and fibrin/platelet content (p = 0.002), but not for leukocytes (p = 0.240)
Table 3.
Univariable and multivariable multinomial logistic regression for the relationship of thrombus composition with stroke etiology
| Univariable model | Multivariable model* | |
|---|---|---|
| Base outcome: cardioembolic | OR (95% CI) | aOR (95% CI) |
| Non-cardioembolic | ||
| RBC | 1.03 (1.01; 1.05) | 1.02 (1.00; 1.04) |
| F/P | 0.97 (0.95; 0.99) | 0.98 (0.96; 1.00) |
| Leukocytes | 0.91 (0.83; 1.01) | 0.90 (0.81; 1.01) |
| Undetermined | ||
| RBC | 1.00 (0.99; 1.01) | 1.00 (0.98; 1.01) |
| F/P | 1.00 (0.99; 1.01) | 1.00 (0.99; 1.02) |
| Leukocytes | 1.00 (0.94; 1.07) | 1.00 (0.92; 1.06) |
All analyses were done with thrombus components as a continuous variable, expressed as % of the thrombus. Odds ratios for stroke etiology are shown per percentage increase of thrombus components, with 95% confidence intervals. Interpretation: for every 1% increase in RBCs, OR for a non-cardioembolic stroke (compared with cardioembolic stroke) is 1.03 (95% CI 1.01–1.05). Non-cardioembolic indicates TOAST 1 (large artery sclerosis) + TOAST 4 (other determined cause)
RBC red blood cells, F/P fibrin/platelets, and leukocytes white blood cells
*Adjusted for: age, sex, IV thrombolysis, coumarin/DOAC use, and thrombus location
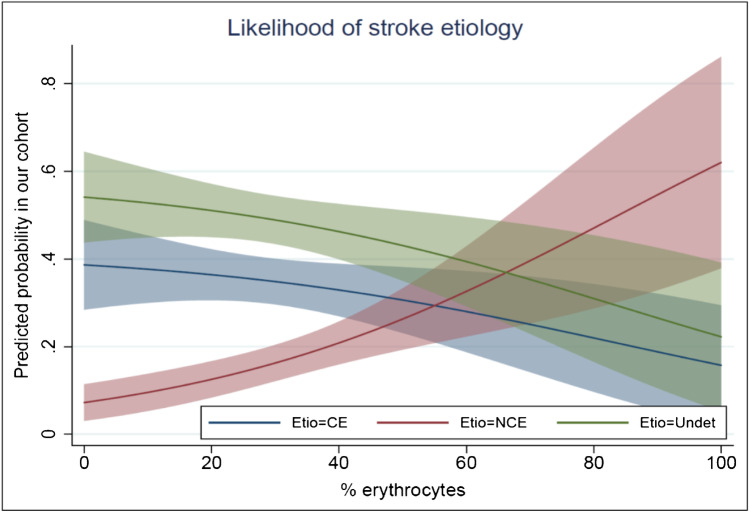
Fig. 4.
Likelihood of cardioembolic (CE), non-cardioembolic (NCE) and undetermined (Undet) etiology (Etio) based on erythrocyte content after univariable multinomial regression. For every 1% increase in RBC content, the OR for a non-cardioembolic stroke (as opposed to cardioembolic stroke) was 1.02
Discussion
We studied the association between histopathologic composition of mechanically extracted thrombi and stroke etiology in patients with AIS included in the Dutch MR CLEAN Registry. RBC and F/P contents of thrombi retrieved from patients with AIS differed significantly between strokes of cardioembolic and non-cardioembolic origin. The RBC content in thrombi from patients with non-cardioembolic etiology was higher than in thrombi from patients with a cardiac etiology and thrombi from patients with an undetermined origin. Inversely, the F/P content in thrombi from patients with non-cardioembolic etiology was lower than in thrombi from patients with cardioembolic etiology and thrombi with an undetermined origin. In addition, cardioembolic thrombi had a similar histopathologic composition to thrombi from strokes of undetermined origin.
Our findings of a significant correlation between stroke etiology and thrombus composition are in line with several previous studies [10, 16, 20, 21]. In our cohort, the wide confidence intervals and overlap in composition hamper reliable prediction of stroke etiology based on thrombus composition alone in individual cases. However, an increasingly high percentage of RBC in thrombi is associated with a higher likelihood of a non-cardioembolic etiology.
Like some previous studies [9, 10, 16, 21, 28], a similarity in thrombus composition was found for thrombi of cardioembolic origin and those of undetermined origin. Our results suggest that patients with an undetermined stroke origin and F/P rich thrombi have a higher likelihood of a cardiac source and may benefit from more extensive monitoring for arrhythmias and/or extended cardiac analysis.
Contradictory results in previous studies regarding the relationship between thrombus histology and etiology may in part be caused by random variation in relatively small samples, and a lack of consensus on histopathological processing and analysis [29]. Indeed, studies with larger sample sizes did find significantly higher fractions of RBC in ischemic stroke caused by large artery disease [10, 16, 20, 21], while smaller studies often failed to show such a correlation. Interestingly, all previous studies that found a higher RBC percentage in cardioembolic stroke were performed in an Asian population [9, 30, 31]. Lastly, many previous studies included thrombi from patients with posterior circulation stroke [3, 6, 8–10, 12, 13, 15, 18], while the determination of large artery atherosclerosis as a cause of AIS is based on stenosis grading of the anterior circulation.
Recently, studies have started to make use of machine learning software for image analysis [28, 30, 32], which implies training and validating a segmentation model first. Differences in accuracy of these segmentation models will affect quantification of thrombus components. In contrast to previous studies, we used a custom script in conjunction with Orbit image analysis software which enabled visual quality control in batch mode, enabling the possibility of verification of the segmented components RBC, F/P, and leukocytes by an experienced pathobiologist (HB). This diminished uncertainties normally inherent to the use of machine learning algorithms for segmentation purposes, ensuring high quality thrombus classification.
Our study has several limitations. Due to the ex vivo nature of thrombus analysis, all studies investigating the association between thrombus composition and stroke etiology theoretically suffer from a selection bias, since data from patients with thrombi resistant to thrombectomy as well as thrombi completely decomposed by IVT are not available for analysis. This study is the first to also have clinical data of all patients in the cohort without thrombus material available for analysis, enabling us to compare characteristics of included patients to those who were not included in the study. Indeed, this comparison was not possible in prior studies, including STRIP [20], through lack of sufficient clinical data in the LVO patient group as a whole. It is a feature that is available in, and a unique characteristic of, the MR CLEAN studies. We can therefore validate that no significant differences were found in stroke etiology between the two groups, and only minor differences in patient baseline characteristics were found. Therefore, we believe our results are valid for the general stroke population eligible for EVT. Using only HE staining makes a reliable differentiation between fibrin and platelets impossible; therefore, these components were combined into one category, F/P. Differentiation of platelets as a separate component and even adding more in-depth immunohistochemical analysis or even 3-D characterization of thrombi could certainly be of interest, as it has been recently shown that platelet content also differs between etiologic groups [32]. Also, since most patients received IVT prior to mechanical thrombectomy, it is possible that (partial) thrombolysis altered thrombus composition; we did, however, adjust for IVT in our regression analysis. Lastly, using the TOAST classification possibly underestimates the number of patients with large artery atherosclerosis as the cause for stroke, since it only values the degree of stenose at the carotid bifurcation. However, we did adhere to this classification, as it is the most used classification system in practice, enabling comparison of our results with previous studies.
Conclusion
Thrombus composition is significantly associated with stroke etiology, with an increase in RBC and a decrease in F/P, raising the odds for a non-cardioembolic cause. Secondly, thrombus composition of cardioembolic and undetermined etiology are similar. These results suggest that patients with an undetermined origin and F/P-rich thrombi are more likely to have a cardiac cause and may benefit from more extensive monitoring for arrhythmias and/or extended cardiac analysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the MR CLEAN investigators; a list of all investigators is provided in the Appendix. Also, we want to thank Mr. Manuel Stritt, founder of Orbit image analysis software, for his help with the implementation of his software in our analysis of extracted LVO stroke thrombi during and after the 2018 OME Annual Users Meeting, where we first pitched our idea for this manuscript.
Appendix
MR CLEAN Registry Investigators — group authors
Executive committee
Diederik W. J. Dippel1, Aad van der Lugt2, Charles B. L. M. Majoie3, Yvo B. W. E. M. Roos4, Robert J. van Oostenbrugge5, Wim H. van Zwam6, Jelis Boiten14, Jan Albert Vos8
Study coordinators
Ivo G. H. Jansen3, Maxim J. H. L. Mulder1,2, Robert- Jan B. Goldhoorn5,6, Kars C. J. Compagne2, Manon Kappelhof3, Josje Brouwer4, Sanne J. den Hartog1,2,40, Wouter H. Hinsenveld5,6
Local principal investigators
Diederik W. J. Dippel1, Bob Roozenbeek1, Aad van der Lugt2, Adriaan C. G. M. van Es2, Charles B. L M. Majoie3, Yvo B. W. E. M. Roos4, Bart J. Emmer3, Jonathan M. Coutinho4, Wouter J. Schonewille7, Jan Albert Vos8, Marieke J. H. Wermer9, Marianne A. A. van Walderveen10, Julie Staals5, Robert J. van Oostenbrugge5, Wim H. van Zwam6, Jeannette Hofmeijer11,41, Jasper M. Martens12, Geert J. Lycklama à Nijeholt13, Jelis Boiten14, Sebastiaan F. de Bruijn15, Lukas C. van Dijk16, H. Bart van der Worp17, Rob H. Lo18, Ewoud J. van Dijk19, Hieronymus D. Boogaarts20, J. de Vries22, Paul L. M. de Kort21, Julia van Tuijl21, Jo P. Peluso26, Puck Fransen22, Jan S. P. van den Berg22, Boudewijn A. A. M. van Hasselt23, Leo A. M. Aerden24, René J. Dallinga25, Maarten Uyttenboogaart28, Omid Eschgi29, Reinoud P. H. Bokkers29, Tobien H. C. M. L. Schreuder30, Roel J. J. Heijboer31, Koos Keizer32, Lonneke S. F. Yo33, Heleen M. den Hertog22, Tomas Bulut35, Paul J. A. M. Brouwers34
Imaging assessment committee
Charles B. L. M. Majoie3 (chair), Wim H. van Zwam6, Aad van der Lugt2, Geert J. Lycklama à Nijeholt13, Marianne A. A. van Walderveen10, Marieke E. S. Sprengers3, Sjoerd F. M. Jenniskens27, René van den Berg3, Albert J. Yoo38, Ludo F. M. Beenen3, Alida A. Postma6, Stefan D. Roosendaal3, Bas F. W. van der Kallen13, Ido R. van den Wijngaard13, Adriaan C. G. M. van Es2, Bart J. Emmer3, Jasper M. Martens12, Lonneke S. F. Yo33, Jan Albert Vos8, Joost Bot36, Pieter-Jan van Doormaal2, Anton Meijer27, Elyas Ghariq13, Reinoud P. H. Bokkers29, Marc P. van Proosdij37, G. Menno Krietemeijer33, Jo P. Peluso26, Hieronymus D. Boogaarts20, Rob Lo18, Wouter Dinkelaar2, Auke P. A. Appelman29, Bas Hammer16, Sjoert Pegge27, Anouk van der Hoorn29, Saman Vinke20
Writing committee
Diederik W. J. Dippel1 (chair), Aad van der Lugt2, Charles B. L. M. Majoie3, Yvo B. W. E. M. Roos4, Robert J. van Oostenbrugge5, Wim H. van Zwam6, Geert J. Lycklama à Nijeholt13, Jelis Boiten14, Jan Albert Vos8, Wouter J. Schonewille7, Jeannette Hofmeijer11,41, Jasper M. Martens12, H. Bart van der Worp17, Rob H. Lo18
Adverse event committee
Robert J. van Oostenbrugge5 (chair), Jeannette Hofmeijer11,41, H. Zwenneke Flach23
Trial methodologist
Hester F. Lingsma40
Research nurses/local trial coordinators
Naziha el Ghannouti1, Martin Sterrenberg1, Wilma Pellikaan7, Rita Sprengers4, Marjan Elfrink11, Michelle Simons11, Marjolein Vossers12, Joke de Meris14, Tamara Vermeulen14, Annet Geerlings19, Gina van Vemde22, Tiny Simons30, Gert Messchendorp28, Nynke Nicolaij28, Hester Bongenaar32, Karin Bodde24, Sandra Kleijn34, Jasmijn Lodico34, Hanneke Droste34, Maureen Wollaert5, Sabrina Verheesen5, D. Jeurrissen5, Erna Bos9, Yvonne Drabbe15, Michelle Sandiman15, Nicoline Aaldering11, Berber Zweedijk17, Jocova Vervoort21, Eva Ponjee22, Sharon Romviel19, Karin Kanselaar19, Denn Barning10
PhD/Medical students
Esmee Venema40, Vicky Chalos140, Ralph R. Geuskens3, Tim van Straaten19, Saliha Ergezen1, Roger R. M. Harmsma1, Daan Muijres1, Anouk de Jong1, Olvert A. Berkhemer1,3,6, Anna M. M. Boers3,39, J. Huguet3, P. F. C. Groot3, Marieke A. Mens3, Katinka R. van Kranendonk3, Kilian M. Treurniet3, Manon L. Tolhuisen3,39, Heitor Alves3, Annick J. Weterings3, Eleonora L.F. Kirkels3, Eva J. H. F. Voogd11, Lieve M. Schupp3, Sabine L. Collette28,29, Adrien E. D. Groot4, Natalie E. LeCouffe4, Praneeta R. Konduri39, Haryadi Prasetya39, Nerea Arrarte-Terreros39, Lucas A. Ramos39
1Department of Neurology, Erasmus MC University Medical Center, Rotterdam, The Netherlands
2Department of Radiology and Nuclear Medicine, Erasmus MC University Medical Center, Rotterdam, The Netherlands
3Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
4Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
5Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM), Maastricht, The Netherlands
6Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM), Maastricht, The Netherlands
7Department of Neurology, Sint Antonius Hospital, Nieuwegein, The Netherlands
8Department of Radiology, Sint Antonius Hospital, Nieuwegein, The Netherlands
9Department of Neurology, Leiden University Medical Center, Leiden, The Netherlands
10Department of Radiology, Leiden University Medical Center, Leiden, The Netherlands
11Department of Neurology, Rijnstate Hospital, Arnhem, The Netherlands
12Department of Radiology, Rijnstate Hospital, Arnhem, The Netherlands
13Department of Radiology, Haaglanden MC, The Hague, The Netherlands
14Department of Neurology, Haaglanden MC, The Hague, The Netherlands
15Department of Neurology, HAGA Hospital, The Hague, The Netherlands
16Department of Radiology, HAGA Hospital, The Hague, The Netherlands
17Department of Neurology, University Medical Center Utrecht, Utrecht, The Netherlands
18Department of Radiology, University Medical Center Utrecht, Utrecht, The Netherlands
19Department of Neurology, Radboud University Medical Center, Nijmegen, The Netherlands
20Department of Neurosurgery, Radboud University Medical Center, Nijmegen, The Netherlands
21Department of Neurology, Elisabeth-TweeSteden Ziekenhuis, Tilburg, The Netherlands
22Department of Neurology, Isala Klinieken, Zwolle, The Netherlands
23Department of Radiology, Isala Klinieken, Zwolle, The Netherlands
24Department of Neurology, Reinier de Graaf Gasthuis, Delft, The Netherlands
25Department of Radiology, Reinier de Graaf Gasthuis, Delft, The Netherlands
26Department of Radiology, Elisabeth-TweeSteden Ziekenhuis, Tilburg, The Netherlands
27Department of Radiology, Radboud University Medical Center, Nijmegen, The Netherlands
28Department of Neurology, University Medical Center Groningen, Groningen, The Netherlands
29Department of Radiology, University Medical Center Groningen, Groningen, The Netherlands
30Department of Neurology, Atrium Medical Center, Heerlen, The Netherlands
31Department of Radiology, Atrium Medical Center, Heerlen, The Netherlands
32Department of Neurology, Catharina Hospital, Eindhoven, The Netherlands
33Department of Radiology, Catharina Hospital, Eindhoven, The Netherlands
34Department of Neurology, Medisch Spectrum Twente, Enschede, The Netherlands
35Department of Radiology, Medisch Spectrum Twente, Enschede, The Netherlands
36Department of Radiology, Amsterdam UMC, Vrije Universiteit van Amsterdam, Amsterdam, The Netherlands
37Department of Radiology, Noordwest Ziekenhuisgroep, Alkmaar, The Netherlands
38Department of Radiology, Texas Stroke Institute, TX, USA
39Department of Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
40Department of Public Health, Erasmus MC University Medical Center, Rotterdam, The Netherlands
41Department of Clinical Neurophysiology, University of Twente, Enschede, The Netherlands
Author contribution
H. M. Hund: conceptualization, methodology, software, formal analysis, and writing — original draft; N. Boodt: formal analysis, resources, data curation, writing — review and editing; D. Hansen: investigation and data curation; W.A. Haffmans: methodology and software; G. J. Lycklama à Nijeholt: conceptualization, methodology, and writing — review and editing; J. Hofmeijer: writing — review and editing; D.W.J. Dippel: conceptualization, methodology, and writing — review and editing; A. van der Lugt: conceptualization, methodology, and writing — review and editing; A. C. G. M. van Es: conceptualization, methodology, and writing —review and editing; H. M. M. van Beusekom: conceptualization, resources, methodology, and writing — review and editing.
Guarantor
The scientific guarantor of this publication is Dr. H. M. M. van Beusekom, Department of Cardiology, Erasmus University Medical Center, Rotterdam, the Netherlands.
Statistics and biometry
More than one of the authors have significant statistical expertise.
Study subjects or cohorts overlap
Some study subjects have been previously reported in MR CLEAN Registry studies; however, there is no overlap with regard to the research question addressed in the current paper.
Methodology
This study is a retrospective analysis of data from the MR CLEAN Registry, which was a prospective, observational, multicenter study of all patients who underwent EVT in the Netherlands.
Funding
This study was funded and carried out by the Erasmus University Medical Center, the Academic Medical Center Amsterdam, and the Maastricht University Medical Center. The study was additionally funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 777072 (IN-SIlico trials for treatment of acute Ischemic STroke, INSIST), which played no role in the trial design and patient enrolment or in data the collection, analysis, or writing of the manuscript.
Data Availability
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research, supporting data is not available.
Declarations
Conflict of interest
We declare that we have no conflict of interest.
Ethical approval
The central medical ethics committee of the Erasmus Medical Center Rotterdam, the Netherlands, approved the study protocol and granted permission to carry out the study as a registry (MEC-2014–235).
Informed consent
Written informed consent was waived by the Institutional Review Board.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
HMH and NB contributed equally to this work.
Contributor Information
Heleen M. M. van Beusekom, Email: h.vanbeusekom@erasmusmc.nl
on behalf of the MR CLEAN Registry Investigators:
Charles B. L. M. Majoie, Yvo B. W. E. M. Roos, Robert J. van Oostenbrugge, Wim H. van Zwam, Jelis Boiten, Jan Albert Vos, Ivo G. H. Jansen, Maxim J. H. L. Mulder, Robert- Jan B. Goldhoorn, Kars C. J. Compagne, Manon Kappelhof, Josje Brouwer, Sanne J. den Hartog, Wouter H. Hinsenveld, Bob Roozenbeek, Bart J. Emmer, Jonathan M. Coutinho, Wouter J. Schonewille, Marieke J. H. Wermer, Marianne A. A. van Walderveen, Julie Staals, Jasper M. Martens, Sebastiaan F. de Bruijn, Lukas C. van Dijk, H. Bart van der Worp, Rob H. Lo, Ewoud J. van Dijk, Hieronymus D. Boogaarts, J. de Vries, Paul L. M. de Kort, Julia van Tuijl, Jo P. Peluso, Puck Fransen, Jan S. P. van den Berg, Boudewijn A. A. M. van Hasselt, Leo A. M. Aerden, René J. Dallinga, Maarten Uyttenboogaart, Omid Eschgi, Reinoud P. H. Bokkers, Tobien H. C. M. L. Schreuder, Roel J. J. Heijboer, Koos Keizer, Lonneke S. F. Yo, Heleen M. den Hertog, Tomas Bulut, Paul J. A. M. Brouwers, Marieke E. S. Sprengers, Sjoerd F. M. Jenniskens, René van den Berg, Albert J. Yoo, Ludo F. M. Beenen, Alida A. Postma, Stefan D. Roosendaal, Bas F. W. van der Kallen, Ido R. van den Wijngaard, Joost Bot, Pieter-Jan van Doormaal, Anton Meijer, Elyas Ghariq, Marc P. van Proosdij, G. Menno Krietemeijer, Wouter Dinkelaar, Auke P. A. Appelman, Bas Hammer, Sjoert Pegge, Anouk van der Hoorn, Saman Vinke, H. Zwenneke Flach, Hester F. Lingsma, Naziha el Ghannouti, Martin Sterrenberg, Wilma Pellikaan, Rita Sprengers, Marjan Elfrink, Michelle Simons, Marjolein Vossers, Joke de Meris, Tamara Vermeulen, Annet Geerlings, Gina van Vemde, Tiny Simons, Gert Messchendorp, Nynke Nicolaij, Hester Bongenaar, Karin Bodde, Sandra Kleijn, Jasmijn Lodico, Hanneke Droste, Maureen Wollaert, Sabrina Verheesen, D. Jeurrissen, Erna Bos, Yvonne Drabbe, Michelle Sandiman, Nicoline Aaldering, Berber Zweedijk, Jocova Vervoort, Eva Ponjee, Sharon Romviel, Karin Kanselaar, Denn Barning, Esmee Venema, Vicky Chalos, Ralph R. Geuskens, Tim van Straaten, Saliha Ergezen, Roger R. M. Harmsma, Daan Muijres, Anouk de Jong, Olvert A. Berkhemer, Anna M. M. Boers, J. Huguet, P. F. C. Groot, Marieke A. Mens, Katinka R. van Kranendonk, Kilian M. Treurniet, Manon L. Tolhuisen, Heitor Alves, Annick J. Weterings, Eleonora L.F. Kirkels, Eva J. H. F. Voogd, Lieve M. Schupp, Sabine L. Collette, Adrien E. D. Groot, Natalie E. LeCouffe, Praneeta R. Konduri, Haryadi Prasetya, Nerea Arrarte-Terreros, and Lucas A. Ramos
References
- 1.Arsava EM, Helenius J, Avery R, Sorgun MH, Kim GM, Pontes-Neto OM, et al. Assessment of the predictive validity of etiologic stroke classification. JAMA Neurol. 2017;74(4):419–426. doi: 10.1001/jamaneurol.2016.5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gounis MJ, Chapot R. Histological composition and the origin of the thrombus: a new diagnostic assay for secondary stroke prevention? Stroke. 2017;48(8):2040–2041. doi: 10.1161/STROKEAHA.117.017630. [DOI] [PubMed] [Google Scholar]
- 3.Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37(8):2086–2093. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- 4.Almekhlafi MA, Hu WY, Hill MD, Auer RN. Calcification and endothelialization of thrombi in acute stroke. Ann Neurol. 2008;64(3):344–348. doi: 10.1002/ana.21404. [DOI] [PubMed] [Google Scholar]
- 5.Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42(5):1237–1243. doi: 10.1161/strokeaha.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niesten JM, van der Schaaf IC, van Dam L, Vink A, Vos JA, Schonewille WJ, et al. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One. 2014;9(2):e88882. doi: 10.1371/journal.pone.0088882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SK, Yoon W, Kim TS, Kim HS, Heo TW, Park MS. Histologic analysis of retrieved clots in acute ischemic stroke: correlation with stroke etiology and gradient-echo MRI. AJNR Am J Neuroradiol. 2015;36(9):1756–1762. doi: 10.3174/ajnr.A4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simons N, Mitchell P, Dowling R, Gonzales M, Yan B. Thrombus composition in acute ischemic stroke: a histopathological study of thrombus extracted by endovascular retrieval. J Neuroradiol. 2015;42(2):86–92. doi: 10.1016/j.neurad.2014.01.124. [DOI] [PubMed] [Google Scholar]
- 9.Ahn SH, Hong R, Choo IS, Heo JH, Nam HS, Kang HG, et al. Histologic features of acute thrombi retrieved from stroke patients during mechanical reperfusion therapy. Int J Stroke. 2016;11(9):1036–1044. doi: 10.1177/1747493016641965. [DOI] [PubMed] [Google Scholar]
- 10.Boeckh-Behrens T, Kleine JF, Zimmer C, Neff F, Scheipl F, Pelisek J, et al. Thrombus histology suggests cardioembolic cause in cryptogenic stroke. Stroke. 2016;47(7):1864–1871. doi: 10.1161/STROKEAHA.116.013105. [DOI] [PubMed] [Google Scholar]
- 11.Boeckh-Behrens T, Schubert M, Forschler A, Prothmann S, Kreiser K, Zimmer C, et al. The impact of histological clot composition in embolic stroke. Clin Neuroradiol. 2016;26(2):189–197. doi: 10.1007/s00062-014-0347-x. [DOI] [PubMed] [Google Scholar]
- 12.Dargazanli C, Rigau V, Eker O, RiquelmeBareiro C, Machi P, Gascou G, et al. High CD3+ cells in intracranial thrombi represent a biomarker of atherothrombotic stroke. PLoS One. 2016;11(5):e0154945. doi: 10.1371/journal.pone.0154945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto T, Hayakawa M, Funatsu N, Yamagami H, Satow T, Takahashi JC, et al. Histopathologic analysis of retrieved thrombi associated with successful reperfusion after acute stroke thrombectomy. Stroke. 2016;47(12):3035–3037. doi: 10.1161/strokeaha.116.015228. [DOI] [PubMed] [Google Scholar]
- 14.Sallustio F, Arnó N, Legge SD, Koch G, Martorana A, Rossi C, et al. Histological features of intracranial thrombo-emboli predict response to endovascular therapy for acute ischemic stroke. J Neurol Disord Stroke. 2016;3:1105. [Google Scholar]
- 15.Schuhmann MK, Gunreben I, Kleinschnitz C, Kraft P. Immunohistochemical analysis of cerebral thrombi retrieved by mechanical thrombectomy from patients with acute ischemic stroke. Int J Mol Sci. 2016;17(3):298. doi: 10.3390/ijms17030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sporns PB, Hanning U, Schwindt W, Velasco A, Minnerup J, Zoubi T, et al. Ischemic stroke: what does the histological composition tell us about the origin of the thrombus? Stroke. 2017;48(8):2206–2210. doi: 10.1161/strokeaha.117.016590. [DOI] [PubMed] [Google Scholar]
- 17.Choi MH, Park GH, Lee JS, Lee SE, Lee SJ, Kim JH, et al. Erythrocyte fraction within retrieved thrombi contributes to thrombolytic response in acute ischemic stroke. Stroke. 2018;49(3):652–659. doi: 10.1161/strokeaha.117.019138. [DOI] [PubMed] [Google Scholar]
- 18.Maekawa K, Shibata M, Nakajima H, Mizutani A, Kitano Y, Seguchi M, et al. Erythrocyte-rich thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc Dis Extra. 2018;8(1):39–49. doi: 10.1159/000486042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin JW, Jeong HS, Kwon HJ, Song KS, Kim J. High red blood cell composition in clots is associated with successful recanalization during intra-arterial thrombectomy. PLoS One. 2018;13(5):e0197492. doi: 10.1371/journal.pone.0197492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brinjikji W, Nogueira RG, Kvamme P, Layton KF, Delgado Almandoz JE, Hanel RA, et al. Association between clot composition and stroke origin in mechanical thrombectomy patients: analysis of the Stroke Thromboembolism Registry of Imaging and Pathology. J Neurointerv Surg. 2021;13(7):594–598. doi: 10.1136/neurintsurg-2020-017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzgerald S, Rossi R, Mereuta OM, Jabrah D, Okolo A, Douglas A, et al. Per-pass analysis of acute ischemic stroke clots: impact of stroke etiology on extracted clot area and histological composition. J Neurointerv Surg. 2020 doi: 10.1136/neurintsurg-2020-016966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong L, Zheng X, Feng L, Zhang X, Dong Q, Zhou X et al (2019) Bridging therapy versus direct mechanical thrombectomy in patients with acute ischemic stroke due to middle cerebral artery occlusion: a clinical-histological analysis of retrieved thrombi. 10.1177/963689718823206 [DOI] [PMC free article] [PubMed]
- 23.Jansen IGH, Mulder M, Goldhoorn RB, investigators MCR (2018) Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN Registry). BMJ 360:k949. 10.1136/bmj.k949 [DOI] [PMC free article] [PubMed]
- 24.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 25.Staessens S, Fitzgerald S, Andersson T, Clarencon F, Denorme F, Gounis MJ et al (2019) Histological stroke clot analysis after thrombectomy: technical aspects and recommendations. Int J Stroke 1747493019884527. 10.1177/1747493019884527 [DOI] [PubMed]
- 26.Fitzgerald S, Wang S, Dai D, Murphree DH, Jr, Pandit A, Douglas A, et al. Orbit image analysis machine learning software can be used for the histological quantification of acute ischemic stroke blood clots. PLoS One. 2019;14(12):e0225841. doi: 10.1371/journal.pone.0225841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams HP, Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL et al (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24(1):35–41. 10.1161/01.str.24.1.35 [DOI] [PubMed]
- 28.Duffy S, McCarthy R, Farrell M, Thomas S, Brennan P, Power S, et al. Per-pass analysis of thrombus composition in patients with acute ischemic stroke undergoing mechanical thrombectomy. Stroke. 2019;50(5):1156–1163. doi: 10.1161/STROKEAHA.118.023419. [DOI] [PubMed] [Google Scholar]
- 29.De Meyer SF, Andersson T, Baxter B, Bendszus M, Brouwer P, Brinjikji W, et al. Analyses of thrombi in acute ischemic stroke: a consensus statement on current knowledge and future directions. Int J Stroke. 2017;12(6):606–614. doi: 10.1177/1747493017709671. [DOI] [PubMed] [Google Scholar]
- 30.Gong L, Zheng X, Feng L, Zhang X, Dong Q, Zhou X, et al. Bridging Therapy Versus Direct Mechanical Thrombectomy in Patients with Acute Ischemic Stroke due to Middle Cerebral Artery Occlusion: A Clinical- Histological Analysis of Retrieved thrombi. Cell Transplant. 2019;28(6):684–690. doi: 10.1177/0963689718823206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin JW, Jeong HS, Kwon H-J, Song KS, Kim J. High red blood cell composition in clots is associated with successful recanalization during intra-arterial thrombectomy. PLoS One 13(5):e0197492. 10.1371/journal.pone.0197492 [DOI] [PMC free article] [PubMed]
- 32.Fitzgerald S, Dai D, Wang S, Douglas A, Kadirvel R, Layton KF, et al. Platelet-rich emboli in cerebral large vessel occlusion are associated with a large artery atherosclerosis source. Stroke. 2019;50(7):1907–1910. doi: 10.1161/STROKEAHA.118.024543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research, supporting data is not available.