Abstract
Purpose of the Review
Bone adapts structure and material properties in response to its mechanical environment, a process called mechanoadpatation. For the past 50 years, finite element modeling has been used to investigate the relationships between bone geometry, material properties, and mechanical loading conditions. This review examines how we use finite element modeling in the context of bone mechanoadpatation.
Recent Findings
Finite element models estimate complex mechanical stimuli at the tissue and cellular levels, help explain experimental results, and inform the design of loading protocols and prosthetics.
Summary
FE modeling is a powerful tool to study bone adaptation as it complements experimental approaches. Before using FE models, researchers should determine whether simulation results will provide complementary information to experimental or clinical observations and should establish the level of complexity required. As imaging technics and computational capacity continue increasing, we expect FE models to help in designing treatments of bone pathologies that take advantage of mechanoadaptation of bone.
Keywords: Bone, Finite element model, Mechanoadpatation
Introduction
Finite element (FE) modeling is a mathematical representation of a structure that incorporates geometry, material properties, and loading conditions to understand the mechanical environment. Because bone adapts geometry and material properties to its mechanical environment, FE models have been widely used to explore the effects of loading on bone growth and fracture healing, predict fracture risk, and explore bone mechanoadaptation to loading conditions. This review presents a brief historical summary of the use of FE modeling in the context of bone mechanoadaptation, bone adapting to mechanical loading. (We do not cover models of endochondral ossification during growth or fracture healing nor the use of FE to predict fracture risk.) We explore why we use FE models, how we validate our models, the required complexity of the models, current limitations, and potential benefits for future applications.
Historical Context
In 1972, Brekelmans et al. [1] presented a 2D model of a femur with less than 1000 triangular elements and showed stress distribution under different loading conditions. Loading conditions were determined by taking into account hip anatomy and center of gravity [2]. These 2D models explored heterogeneous material properties and the influence of trabecular bone heterogeneity on the stress distribution in the femoral head [3]. Additionally, a 2D model of the patella was used to investigate the relationship between trabecular bone architecture and stress distribution [4].
The first 3D bone finite element models examined stress distributions during slow walking in human femurs [5, 6]. Computational results were compared to ex vivo measurements using an extensiometer [5, 7] and strain gauges [6]. Numerous studies during this time used FE models for stress analysis and implant design. Huiskes and Chao detailed the first decade of applications of FE models between 1972 and 1982 [8]. At this time (early 1980s), models were limited by computational power, thereby limiting the complexity of bone geometry and material properties (heterogeneous, nonlinear, anisotropic).
Between the late 1980s and early 1990s, improvements in computational power and advancement in imaging capabilities allowed more complex FE model geometry and investigation of mechanical stimulus predicting changes in bone density, material properties, and architecture due to loading history [9–12]. Carter et al. implemented a time-dependent approach to simulate bone remodeling and predict changes in bone density under various loading conditions applied on a 2D femoral head model [13, 14]. Expanding this work in 3D, Fyhrie et al. [11] compared cancellous bone morphology following different effective stresses (Von Mises, strain energy density, spherical stresses) and showed that Von Mises stress cannot accurately predict bone apparent density. The development of computed tomography (CT) led to image-based finite element modeling that accounts for three-dimensional architecture [15]. Image voxels were directly converted to brick elements to create the model mesh [15, 16]. The development of imaging techniques was critical to the first subject specific FE-model in which both geometry and material properties were estimated based on CT scan data [17]. In their review, Huiskes and Hollister detailed progress in structure optimization, bone remodeling, and fracture studies between 1983 and 1993 [10].
In addition to imaged-based geometric meshes, automatic meshing algorithms also improved, in the late 1990s,to allow the meshing of complex structures [18]. Tetrahedral meshes are easily constructed automatically from complex 3D geometry; hexahedral meshes require more manual manipulation of the mesh and computational power, but offers a more mathematically stable element compared to tetrahedral meshes [19]. More complex material properties were also integrated into the models, specifically heterogeneity (i.e. correlation between CT attenuation coefficient and elastic modulus) [20–22] and non-linearity (i.e. poroelasticity) [23–25]. Imaging with micro-computed tomography (microCT) provided a method for obtaining the micro-structure of the trabecular architecture [15, 16, 26, 27]. Micro-finite element models improved the quality of trabecular bone architecture modeling and allowed computational assessment of bone material properties [21, 28]. MicroCT-based 3D finite element models became commonly used in the context of animal models of bone adaptation [26, 29–31] to study bone mechanical environment induced by the experimental setup [26, 32–36]. Development of in vivo microCT allowed researchers to acquire multiple scans of the same bone over time and identify regions of bone formation and resorption between scans [31, 32, 37] and link regions of adaptation to the mechanical environment [31, 38].
FE models enable studies of the distribution of complex mechanical stimuli at different scales of the bone structure: from the tissue [23, 25, 33, 36, 39–42], to the cellular level [43–47]. Multi-scale FE models have been used to better understand the mechanical environment experienced at the cellular level based on the estimated stimulus in the whole bone [48, 49]. Specifically, modeling of an idealized lacuna canaliculi network (LCN) provided the first insights into the mechanical environment around osteocytes [47]. Image-based FE modeling enabled a more representative LCN geometry of a single or multiple lacunae, with or without osteocytes [44–46, 50]
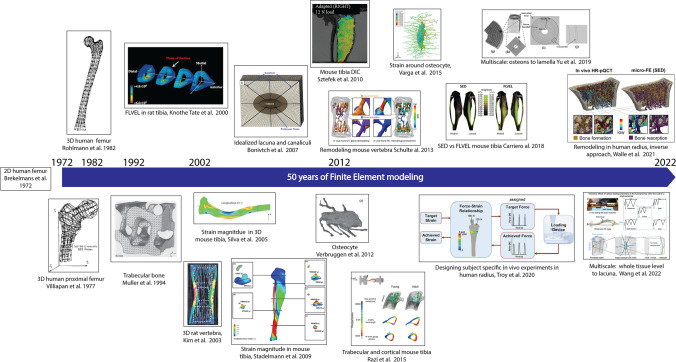
To summarize, since 1972, increasing image resolution, computational resources, and advanced mechanical testing methods enabled more representative and complex bone FE models. FE models greatly contributed to the understanding of bone mechanoadpatation. Figure 1 illustrates the evolution of FE models used in bone adaptation studies over the past 50 years. The following section will detail recent uses of FE models in the context of bone adaptation.
Fig. 1.
Evolution of FE models for bone adaptation within the last 50 years: from 2D human femur to 3D multi-scale models
Why Do We Use FE Models in Bone Adaptation Studies?
FE models are used to estimate the bone mechanical environment under specific loading conditions. Simulation results can then be used to explain experimental measures of bone formation and resorption and to inform experimental design.
To Explain Experimental Observations
In vivo animal models of adaptation explore how imposed mechanical loading or unloading results in bone formation and resorption. Typically, the applied mechanical loading is well-defined and controlled. FE models allow estimation of the mechanical environment within a region of interest [33, 42] that can be correlated to areas of bone adaptation [31, 38, 41]. Recently, mechanical environments have been compared between males and females [51], after ovariectomy [52, 53], after pharmaceutical treatment [54], and with genetic modifications [55, 56•].
Historically, stain magnitude was considered the mechanical stimulus triggering bone adaptation [57–59]. However, studies showed that other mechanical parameters such as strain rate, strain energy density, and fluid flow Could drive the adaptation [31, 59–63]. FE models have also been used to examine less commonly-considered stimuli such as the piezoelectric capacity of the bone [64•, 65]. FE models combined with remodeling algorithms can simulate changes in bone geometry and properties due to changes in their mechanical environment (i.e., application of external loads, insertion of implants). This approach involves hypotheses regarding the mechanical stimulus responsible for the adaptation (i.e., strain energy density, strain magnitude, fluid flow) and the adaptive response (i.e. change in structure or modulus). Typically remodeling algorithms use a mechanoadaptive theory [57, 66, 67]: when the mechanical signal of interest is above/below a certain threshold, bone formation/resorption is implemented according to the proposed algorithm [53, 68••, 69]. Studies have also implemented a lazy zone in which no formation or resorption occurs [63, 70•, 71, 72•]. In addition, response to heavy mechanical loading can be simulated to consider woven bone formation [73•] or damage [72•]. The adaptation that occurs in response to the stimuli is often modeled as a change in density or modulus [52, 70•, 74], or a change in bone geometry [68••, 73•]. Remodeling algorithms enable the prediction of bone adaptation under different loading conditions. Simulation results can then be compared to experimental results to assess the accuracy of the prediction [68••, 75••, 76••]. Because algorithms can be tuned to adjust the amount of bone formation (or increase in density or modulus), comparisons between in vivo experiments and computer models more often compare location of adaptive changes, which is much more dependent on the distribution of the stimulus. Thus, comparing computational and experimental results help to determine the mechanical stimuli that best predict adaptation. Using this approach, fluid flow was a better predictor of regions of adaptation than strain magnitude [63, 68••]. Using FE modeling and circuit theory, van Tol et al. [68••] estimated strain magnitude and load-induced fluid flow by taking into account the lacunar canalicular network and predicted the bone mechanoresponse. Computational bone formation and resorption predictions were compared to in vivo microCT measurements. Authors showed that fluid flow within the LCN predicted bone remodeling better than strain. Other models have used multi-scale stimuli. Goyal et al. used Strain Energy Density (SED) and diffusion of calcium ions as the stimuli driving adaptation under heavy mechanical loading to predict the formation of woven bone.
Table 1 details some studies in the past four years that have used remodeling algorithms in FE mechanoadpatation studies.
Table 1.
Remodeling algorithm used in recent FE studies in the context of bone adaptation
| Mechanical signal | Response simulated | References |
|---|---|---|
| Strain energy density | Change in bone density/modulus |
Gonzalez et al., 2020 [70•] Anijs et al., 2022 [71] Park et al., 2022 [78] Poovarodom et al., 2022 [79] |
| Change in bone density and geometry | Cheong et al., 2020 [52] | |
| Strain gradient | Change in bone density and geometry | Du et al., 2021 [72•] |
| Stress/strain | Change in bone density/modulus | Levadnyi et al., 2021 [74] |
| Tissue differentiation and changes in material properties | Mathai et al., 2022 [80] | |
| Strain and ions flow | Woven or lamellar bone formation (geometry) |
Goyal et al., 2022 [73•] Prasad et al., 2019 [69] |
| Fluid flow | Bone formation and resorption (geometry only) | van Tol et al., 2020 |
| Electric charges (piezoelectric strain) | Change in bone density/modulus | Bansod et al., 2021 [64•] |
Remodeling algorithms can be applied to cortical and trabecular bone. A recent review [81] detailed and compared computational models for trabecular bone remodeling.
In investigating bone remodeling, a typical approach is to apply a load on the model and simulate the bone formation and resorption. However, inverse approaches have also been developed to back-calculate the applied load based on the trabecular bone architecture and measured remodeling from animal experiments [69] and clinical data [82••].
Under the assumption of a specific driving mechanical stimulus, FE models can help identify regions of interest to further investigate the mechanism driving bone adaptation using dynamic histomorphometry, microCT analysis, and protein and gene expression analysis. Recently, Chlebek [83•] used FE simulation results to investigate gene regulation in bone regions experiencing different strain magnitudes and showed that, under loading, the transcriptomic response of cortical bone varied along the bone length, correlating with strain magnitude.
In all approaches discussed so far, a hypothesis regarding the stimulus driving adaptation is required. Thus, FE models provide only a potential explanation for in vivo measurement. One advantage of using models is the ability to explore multiple stimuli to see which match regions of bone formation most closely. For example, in a FE model of murine tibial loading model, strain energy density and fluid flow were examined and correlated to regions of bone formation [63, 77]. While strain energy density predicted formation on the periosteal surface, fluid flow predicted bone formation on both the endosteal and periosteal surfaces, as seen in the experiments. Because the mechanical stimuli are often related, contributions from different stimuli are challenging to tease apart. Indeed, increasing strain magnitude with the same loading frequency will result in an increased load rate, which also affects the fluid flow velocity. Thus, attributing bone formation only to changes in strain magnitude may provide an incomplete explanation of the experimental measurements [29, 35, 84].
To Design and Inform
FE models are commonly used to inform the design of orthopedic implants. Appropriate bone adaptation around implants is critical to guarantee their integration. The mechanical behavior of prosthetics was one of the first applications of FE models in bone [10]. In their review, Taylor et al. [85] detailed the different models used for orthopedic applications until 2015. Today, FE models are still used in implant design. Levadnyi et al. used FE modeling, strain gages, and digital image correlation (DIC) and showed that the hip implant collar affects bone density over time [74]. Implant design and position can alter the bone adaptation around the prosthesis under loading. Thus, bone remodeling algorithms are also important to consider in the evaluation of prosthetics [74, 78, 79]. Investigations of dental implant positioning [79] showed that implant depth affects cancellous bone remodeling. FE models can also be used to predict bone ingrowth within the implant coating [80]. FE models of bone adaptation in prosthesis applications are often compared to clinical data [71].
In addition to implant design FE models can help in designing in vivo experiments, such that simulation results inform the loading protocol. Troy et al. used subject specific FE models of the human radius to inform their clinical experiments [86••]. They investigated the impact of strain magnitudes and strain rates on human bone adaptation, using a voluntary upper limb compressive task in healthy adult women. Subjects were assigned target forces based on FE simulation results to achieve low or high strain magnitudes and rates. This model was validated via multiple strain gauges measurement using ex vivo forelimb [87, 88]. They demonstrated that strain magnitude, rate, and number of applied loading bouts contribute to bone adaptation in healthy adult women.
In animal studies, FE models enable the design of loading conditions to help determine the loading parameters to apply in vivo. Eller et al. [89•] used a strain-matched approach to ensure equivalent strain magnitude in tibiae from control and high fat diet mice. High fat diet mice have significantly larger cortical bone cross sectional areas, requiring a larger load to obtain the same strain. Meslier et al. [90••] adapted this strain-matched approach to fluid flow. They used a mouse tibia model to estimate strain magnitude and fluid velocity in the cortical bone in response to various loading profiles. Simulation results were used to design their in vivo experiment, which aimed to dissociate the contribution of fluid flow compared to strain on bone adaptation.
FE models can be a powerful comparative tool to inform experimental design but is currently underutilized. We expect an increase in the use of FE models for protocol development in human and animal experiments in which the mechanical stimulus is challenging to measure in vivo non-invasively.
How Complex Does the Model Need to Be?
With the development of imaging techniques and computational power, there is an increase in our ability to build more complex models. Model intricacy can be related to the geometry, materials properties, loading conditions, and the multi-scale aspect. However, the model complexity should be adapted to the research question.
Geometry
One of the most common applications of FE modeling is to evaluate the distribution of mechanical stimuli in the model. The geometric complexity of the FE model influences the accuracy of the solution. Pickering et al. [91] compared beam theory and FE model of a mouse tibia to investigate load-induced strain distribution. They reported that by correcting the beam theory model with a loading correction factor, the estimation of strain distribution was comparable to the FE model. The correction factor must be adapted to the age, sex, and strain of the mice (i.e., calibrated). This work introduces the idea that simplified models that do not require advanced modeling skills can be used for simple estimation of the mechanical environment. However, beam theory can only be applied to the bone diaphysis and at the macroscopic level. Due to the curvature of most of the bones and specific features, having an accurate representation of the tissue geometry is important to obtain a representative distribution of the mechanical stimulus. For example, the fibula significantly impacts the strain distribution in the murine tibial bone [92], but adding a growth plate does not affect diaphyseal strains [93•].
Commonly, FE models are used to estimate the mechanical environment in the bone at a specific time point, therefore they do not take into account changes in geometry that could be induced by loading protocols [76••] or changes in material properties [94]. Using image-based models representative of different time points could address this limitation but such models are more labor intensive [76••, 95, 96•, 97]. When investigating the mechanical environment of the whole bone, considering only the cortical shell can help simplify the model. Nevertheless, depending on the research question, the trabecular bone can also be included [41, 98•].
In all FE models, the accuracy of the solution depends on the element size and a critical first step of modeling is to check for mesh convergence. Mesh density is of particular importance at material interfaces; gradients in material properties help to avoid stress concentrations.
Material Properties
In most models, homogeneous material properties are assumed to simplify the model. However, bone is a heterogenous material, which can be taken into account in the model [68••, 76••, 99]. Heterogenous bone properties can lead to variation in the strain distribution [41, 55, 99]. To account for heterogeneity, Young moduli are based on attenuation coefficients from computed tomography images, which indicate bone density. Heterogeneous models can lead to small differences in strain distribution compared to homogeneous models, for example, in the mid-diaphysis of the mouse tibia [55]. However, in their micro FE model of the mouse tibia, Oliveiro et al. [100•] reported that homogenous models were the best compromise between accuracy and computational time to predict structural mechanical properties.
When FE models are used to study strain distribution in the bone, material properties are typically elastic. Characterization of fluid flow in the model requires consideration of the bone poroelastic properties. Viscoelastic properties are usually not taken into account but can be important in determining matrix deformation at different strain rates. Considering the viscoelastic properties of the pericellular matrix in the LCN could help distinguish the direct impact of fluid flow compared to the pericellular matrix deformation on osteocytes activation.
Current desktop computers have the computational power to model complex heterogeneous material properties and dynamic loading conditions. The limitation in modeling non-linear viscoporoelastic materials is in determining the bone viscoelastic and poroelastic properties from experiments.
Loading Conditions
Loading conditions applied on the FE model often aim to emulate the load experienced within the experimental setup or during a typical physical activity such as walking. To do so, defined magnitudes and educated guesses on placement and direction are necessary. Loading conditions can be informed by imaging the sample during loading. Poulet et la [101] imaged the mouse tibia under uniaxial compression and showed the extreme flexed position made the femoral condyles contact with the posterior part of the tibia. Additional methods such as gait analysis, instrumented implants equipped with load sensors [102, 103], and musculoskeletal simulation software [104] help in defining physiological loading conditions [105, 106•]. Muscle forces can be included in the FE model as point loads or loads distributed over an attachment area. In some instances, muscle forces may not affect mechanical stimuli in the region of interest and can be neglected. For example, we found femoral muscles contribute significantly to the hip joint contact force, but do not affect stresses in the growth plate [126].
FE models have been recently used to estimate the influence of loading location in the mouse tibia on the strain distribution in the bone [93•]. Results suggested that in order to obtain a similar strain distribution, load location must be adjusted between specimens. Boundary conditions are assumed to be similar between computational models and in vivo setup and are expected to be reproducible between samples. To address the potential variability of load applied between samples, subject-specific FE models can be used for human studies [86••, 107] or in vivo imaging during loading for animal studies.
Multi-scale
Osteocytes are mechanosensitive cells that play a crucial role in the bone adaptation. Characterizing the pericellular mechanical environment of osteocytes is critical for determining the mechanical stimuli necessary for activation. The mechanical environment around the osteocytes is challenging to assess experimentally and depends on the stimulus distribution and magnitude at the tissue level. Multi-scale models of bone adaptation have related the organ and tissue length scale to the cellular length scale during loading [48, 49, 108••, 109•, 110•]. FE models of bone lacunae demonstrated that the size and orientation of the lacuna affect the strain magnitude around osteocytes [111•]. A parametric study varying lacunar morphology (i.e., density, volume, equancy) and perilacunar bone properties indicated that increasing lacunar density resulted in increased deformation around the lacunae [112••]. Results from FE models that include lacunae suggest that alteration of lacunae morphology and properties due to aging or pathological processes might affect osteocyte mechanosensitive due to a change in their mechanical environment. Other parametric studies were conducted to examine the effect of permeability and porosity on the fluid flow [113] and showed that microstructural changes related to osteoporotic conditions could alter fluid flow around osteocytes. An osteocyte FE model has been used to investigate the effect of TGF-β deficiency and LCN degeneration on osteocytes’ mechanical environment [114••]. Results suggested that the expansion of the modeled pericellular space restored the mechanical environment around the aged osteocytes to young levels.
One of the challenging aspects of multi-scale modeling is linking the organ level to the lacuna canalicular system (LCS) and the osteocytes. These challenges were reviewed by Paul et al. [115]. In addition, FE modeling of strain and fluid flow at the cellular and LCS levels have been reviewed in detail [110•, 116••]. Recently, a multi-scale model was used to study the effect of multiple loading parameters on fluid flow in the lacuna canalicular network [108••]. Results from a continuum model were applied as boundary conditions to an idealized osteocyte lacuna. This multi-scale model demonstrated that strain magnitude and strain rate both affect the fluid velocity and shear stress around the osteocyte. However, these two stimuli do not seem to have an additive effect. Determining the mechanical environment at the cellular level will help to inform the remodeling algorithms.
The Importance of Model Validation
Validation of finite element models is often performed by comparing simulation results to measured strain magnitudes using strain gages [55, 93•, 99, 108••, 117•, 118•, 119]. This method validates the estimated strain at single-point locations. Strain distribution is heterogenous in bones due to their complex shape and material properties. Thus, assumptions are necessary to extrapolate the validated area to the rest of the bone.
To address this limitation, digital image correlation (DIC) was used to validate FE models along the bone [120]. DIC allows researchers to characterize surface strain within a definite region of interest [74, 77, 120]. Expanding on this method, digital volume correlation (DVC) combined mechanical testing and microCT scan of undeformed and deformed bones allowing direct measurement of the strain in the tissue [100•, 121].
The precise strain magnitude values will vary between species, genotype, sex, and location on the bone due to differences in bone geometry, density, and material properties [122•]. Accurate estimation of a mechanical stimulus is critical for tuning experiments to the same stimulus across groups. However, an accurate measurement of the mechanical stimulus is not always possible nor necessary. For many experiments comparison across groups requires only higher/lower designation. For example, validation of simulated fluid flow velocity is complex. In Meslier et al., the model was validated for strain stimuli patterns with DIC but was used to compare high versus low fluid velocity in different loading conditions. An unvalidated model is likely sufficient for this purpose. FE models can estimate regions of high fluid flow velocity, explore the fluid flow dependence on material properties, allow a relative comparison between loading conditions, and identify the changes in the flow due to geometry alterations.
Conclusion
FE is a powerful tool for investigating the distribution of complex mechanical signals in the bone under various experimental loading conditions and simulating the mechanoadaptive response with a change in material properties or geometry. FE models cannot supplant experiments. However, FE models can help to explain experimental results and to design experiments efficiently. Consideration of bone adaptation is critical to the design of implants and is facilitated by FE modeling. When embarking on modeling bone mechanoadaptation, it is essential to understand the research question clearly. Specifically, what will the model tell you that experiments cannot? Models should only be as complex as required by the research question; and unvalidated models may be sufficient for comparing across groups. As the model becomes more complex, more unknown variables are added, which may affect the model's ability to predict adaptive outcomes efficiently.
The FDA now considers finite element modeling a critical part of medical device design [123–125]. In the future, the FDA may accept simulation results as an important piece of evidence to indicate bone adaptation to implants or treatment. While the last 50 years of FE modeling have provided insight into mechanoadaptation of bone; the next 50 years will allow us to utilize this insight to design effective devices, therapies, and experiments to tap into the therapeutic potential of bone adaptation.
Funding
Open access funding provided by Northeastern University Library This work was funded by NSF #2010010.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Biomechanics
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Brekelmans WAM, Poort HW, Slooff TJJH. A new method to analyse the mechanical behaviour of skeletal parts. Acta Orthop Scand. 1972;43(5):301–317. doi: 10.3109/17453677208998949. [DOI] [PubMed] [Google Scholar]
- 2.McLeish RD, Charnley J. Abduction forces in the one-legged stance. J Biomech. 1970;3(2):191–209. doi: 10.1016/0021-9290(70)90006-0. [DOI] [PubMed] [Google Scholar]
- 3.Brown TD, Ferguson AB. The development of a computational stress analysis of the femoral head. J Bone Joint Surg. 1978;60(5):619–629. doi: 10.2106/00004623-197860050-00006. [DOI] [PubMed] [Google Scholar]
- 4.Hayes WC, Snyder B, Levine BM, Ramaswamy S. Stress-morphology relationships in trabecular bone of the patella. Finite Elem Biomech, John Wiley. 1982;12:223–268. [Google Scholar]
- 5.Valliappan S, Svensson NL, Wood RD. Three dimensional stress analysis of the human femur. Comput Biol Med. 1977;7(4):253–264. doi: 10.1016/0010-4825(77)90031-2. [DOI] [PubMed] [Google Scholar]
- 6.Rohlmann A, Mössner U, Bergmann G, Kölbel R. Finite-element-analysis and experimental investigation of stresses in a femur. J Biomed Eng. 1982;4(3):241–246. doi: 10.1016/0141-5425(82)90009-7. [DOI] [PubMed] [Google Scholar]
- 7.Brockhurst PJ, Svensson NL. Design of total hip prosthesis: the femoral stem. Med Prog Technol. 1977;5(2):73–102. [PubMed] [Google Scholar]
- 8.Huiskes R, Chao EYS. A survey of finite element analysis in orthopedic biomechanics: the first decade. J Biomech. 1983;16(6):385–409. doi: 10.1016/0021-9290(83)90072-6. [DOI] [PubMed] [Google Scholar]
- 9.Hollister SJ, Kikuchi N, Goldstein SA. Do bone ingrowth processes produce a globally optimized structure? J Biomech. 1993;26(4–5):391–407. doi: 10.1016/0021-9290(93)90003-W. [DOI] [PubMed] [Google Scholar]
- 10.Huiskes R, Hollister SJ. From structure to process, from organ to cell: recent developments of FE-analysis in orthopaedic biomechanics. J Biomech Eng. 1993;115(4B):520–527. doi: 10.1115/1.2895534. [DOI] [PubMed] [Google Scholar]
- 11.Fyhrie DP, Carter DR. Femoral head apparent density distribution predicted from bone stresses. J Biomech. 1990;23(1):1–10. doi: 10.1016/0021-9290(90)90363-8. [DOI] [PubMed] [Google Scholar]
- 12.Orr TE, Beaupré GS, Carter DR, Schurman DJ. Computer predictions of bone remodeling around porous-coated implants. J Arthroplasty. 1990;5(3):191–200. doi: 10.1016/S0883-5403(08)80074-5. [DOI] [PubMed] [Google Scholar]
- 13.Beaupré GS, Orr TE, Carter DR. An approach for time-dependent bone modeling and remodeling-theoretical development: time-dependent modeling and remodeling. J Orthop Res. 1990;8(5):651–661. doi: 10.1002/jor.1100080506. [DOI] [PubMed] [Google Scholar]
- 14.Beaupré GS, Orr TE, Carter DR. An approach for time-dependent bone modeling and remodeling-application: a preliminary remodeling simulation: time-dependent remodeling theory. J Orthop Res. 1990;8(5):662–670. doi: 10.1002/jor.1100080507. [DOI] [PubMed] [Google Scholar]
- 15.Feldkamp LA, Goldstein SA, Parfitt MA, Jesion G, Kleerekoper M. The direct examination of three-dimensional bone architecture in vitro by computed tomography. J Bone Miner Res. 1989;4(1):3–11. doi: 10.1002/jbmr.5650040103. [DOI] [PubMed] [Google Scholar]
- 16.Hollister SJ, Riemer BA. Digital-image-based finite element analysis for bone microstructure using conjugate gradient and Gaussian filter techniques. San Diego, CA. 1993;95–106. 10.1117/12.146616.
- 17.Keyak JH, Meagher JM, Skinner HB, Mote CD. Automated three-dimensional finite element modelling of bone: a new method. J Biomed Eng. 1990;12(5):389–397. doi: 10.1016/0141-5425(90)90022-F. [DOI] [PubMed] [Google Scholar]
- 18.Viceconti M, Bellingeri L, Cristofolini L, Toni A. A comparative study on different methods of automatic mesh generation of human femurs. Med Eng Phys. 1998;20(1):1–10. doi: 10.1016/S1350-4533(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 19.Tadepalli SC, Erdemir A, Cavanagh PR. Comparison of hexahedral and tetrahedral elements in finite element analysis of the foot and footwear. J Biomech. 2011;44(12):2337–2343. doi: 10.1016/j.jbiomech.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourne BC, van der Meulen MCH. Finite element models predict cancellous apparent modulus when tissue modulus is scaled from specimen CT-attenuation. J Biomech. 2004;37(5):613–621. doi: 10.1016/j.jbiomech.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 21.van Rietbergen B, Weinans H, Huiskes R, Odgaard A. A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models. J Biomech. 1995;28(1):69–81. doi: 10.1016/0021-9290(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 22.Taddei F, Schileo E, Helgason B, Cristofolini L, Viceconti M. The material mapping strategy influences the accuracy of CT-based finite element models of bones: an evaluation against experimental measurements. Med Eng Phys. 2007;29(9):973–979. doi: 10.1016/j.medengphy.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Smit TH, Huyghe JM, Cowin SC. Estimation of the poroelastic parameters of cortical bone. J Biomech. 2002;35(6):829–835. doi: 10.1016/S0021-9290(02)00021-0. [DOI] [PubMed] [Google Scholar]
- 24.Pereira AF, Shefelbine SJ. The influence of load repetition in bone mechanotransduction using poroelastic finite-element models: the impact of permeability. Biomech Model Mechanobiol. 2014;13(1):215–225. doi: 10.1007/s10237-013-0498-8. [DOI] [PubMed] [Google Scholar]
- 25.Steck R, Niederer P, Knothe Tate ML. A finite element analysis for the prediction of load-induced fluid flow and mechanochemical transduction in bone. J Theor Biol. 2003;220(2):249–259. doi: 10.1006/jtbi.2003.3163. [DOI] [PubMed] [Google Scholar]
- 26.Guo XE, Eichler MJ, Takai E, Kim CH. Quantification of a rat tail vertebra model for trabecular bone adaptation studies. J Biomech. 2002;35(3):363–368. doi: 10.1016/S0021-9290(01)00212-3. [DOI] [PubMed] [Google Scholar]
- 27.Ulrich D, van Rietbergen B, Weinans H, Rüegsegger P. Finite element analysis of trabecular bone structure: a comparison of image-based meshing techniques. J Biomech. 1998;31(12):1187–1192. doi: 10.1016/S0021-9290(98)00118-3. [DOI] [PubMed] [Google Scholar]
- 28.van Rietbergen B. Micro-FE analyses of bone: state of the art. In Noninvasive Assess Trabecular Bone Archit Competence Bone. 496, S. Majumdar and B. K. Bay, Eds. Boston, MA: Springer US. 2001;21–30. 10.1007/978-1-4615-0651-5_3. [DOI] [PubMed]
- 29.De Souza RL, Matsuura M, Eckstein F, Rawlinson SCF, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37(6):810–818. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Fritton J, Myers E, Wright T, Vandermeulen M. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36(6):1030–1038. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Schulte FA, et al. Local mechanical stimuli regulate bone formation and resorption in mice at the tissue level. PLoS One. 2013;8(4):e62172. doi: 10.1371/journal.pone.0062172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birkhold AI, Razi H, Duda GN, Weinkamer R, Checa S, Willie BM. The influence of age on adaptive bone formation and bone resorption. Biomaterials. 2014;35(34):9290–9301. doi: 10.1016/j.biomaterials.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 33.Patel TK, Brodt MD, Silva MJ. Experimental and finite element analysis of strains induced by axial tibial compression in young-adult and old female C57Bl/6 mice. J Biomech. 2014;47(2):451–457. doi: 10.1016/j.jbiomech.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moustafa A, et al. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23(4):1225–1234. doi: 10.1007/s00198-011-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis KJ, et al. Osteocyte calcium signals encode strain magnitude and loading frequency in vivo. Proc Natl Acad Sci USA. 2017;114(44):11775–11780. doi: 10.1073/pnas.1707863114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva MJ, Brodt MD, Hucker WJ. Finite element analysis of the mouse tibia: estimating endocortical strain during three-point bending in SAMP6 osteoporotic mice. Anat Rec. 2005;283A(2):380–390. doi: 10.1002/ar.a.20171. [DOI] [PubMed] [Google Scholar]
- 37.Willie BM, et al. Diminished response to in vivo mechanical loading in trabecular and not cortical bone in adulthood of female C57Bl/6 mice coincides with a reduction in deformation to load. Bone. 2013;55(2):335–346. doi: 10.1016/j.bone.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Birkhold AI, Razi H, Duda GN, Weinkamer R, Checa S, Willie BM. The periosteal bone surface is less mechano-responsive than the endocortical. Sci Rep. 2016;6(1):23480. doi: 10.1038/srep23480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manfredini P, Cocchetti G, Maier G, Redaelli A, Montevecchi FM. Poroelastic finite element analysis of a bone specimen under cyclic loading. J Biomech. 1999;32(2):135–144. doi: 10.1016/S0021-9290(98)00162-6. [DOI] [PubMed] [Google Scholar]
- 40.Gross TS, Edwards JL, Mcleod KJ, Rubin CT. Strain gradients correlate with sites of periosteal bone formation. J Bone Miner Res. 1997;12(6):982–988. doi: 10.1359/jbmr.1997.12.6.982. [DOI] [PubMed] [Google Scholar]
- 41.Razi H, et al. Skeletal maturity leads to a reduction in the strain magnitudes induced within the bone: a murine tibia study. Acta Biomater. 2015;13:301–310. doi: 10.1016/j.actbio.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 42.Stadelmann VA, et al. 3D strain map of axially loaded mouse tibia: a numerical analysis validated by experimental measurements. Comput Methods Biomech Biomed Engin. 2009;12(1):95–100. doi: 10.1080/10255840802178053. [DOI] [PubMed] [Google Scholar]
- 43.Mccreadie BR, Hollister SJ. Strain concentrations surrounding an ellipsoid model of lacunae and osteocytes. Comput Methods Biomech Biomed Engin. 1997;1(1):61–68. doi: 10.1080/01495739708936695. [DOI] [PubMed] [Google Scholar]
- 44.McCreadie BR, Hollister SJ, Schaffler MB, Goldstein SA. Osteocyte lacuna size and shape in women with and without osteoporotic fracture. J Biomech. 2004;37(4):563–572. doi: 10.1016/S0021-9290(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 45.Verbruggen SW, Vaughan TJ, McNamara LM. Strain amplification in bone mechanobiology: a computational investigation of the in vivo mechanics of osteocytes. J R Soc Interface. 2012;9(75):2735–2744. doi: 10.1098/rsif.2012.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varga P, et al. Synchrotron X-ray phase nano-tomography-based analysis of the lacunar–canalicular network morphology and its relation to the strains experienced by osteocytes in situ as predicted by case-specific finite element analysis. Biomech Model Mechanobiol. 2015;14(2):267–282. doi: 10.1007/s10237-014-0601-9. [DOI] [PubMed] [Google Scholar]
- 47.RathBonivtch A, Bonewald LF, Nicolella DP. Tissue strain amplification at the osteocyte lacuna: a microstructural finite element analysis. J Biomech. 2007;40(10):2199–2206. doi: 10.1016/j.jbiomech.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deligianni DD, Apostolopoulos CA. Multilevel finite element modeling for the prediction of local cellular deformation in bone. Biomech Model Mechanobiol. 2008;7(2):151–159. doi: 10.1007/s10237-007-0082-1. [DOI] [PubMed] [Google Scholar]
- 49.Fan L, Pei S, Lucas LuX, Wang L. A multiscale 3D finite element analysis of fluid/solute transport in mechanically loaded bone. Bone Res. 2016;4(1):16032. doi: 10.1038/boneres.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider P, Ruffoni D, Larsson D, Chiapparini I, Müller R. Image-based finite element models for the investigation of osteocyte mechanotransduction. J Biomech. 2012;45:S436. doi: 10.1016/S0021-9290(12)70437-2. [DOI] [Google Scholar]
- 51.Javaheri B, et al. Sexually dimorphic tibia shape is linked to natural osteoarthritis in STR/Ort mice. Osteoarthr Cartil. 2018;26(6):807–817. doi: 10.1016/j.joca.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheong VS, Roberts BC, Kadirkamanathan V, Dall’Ara E. Bone remodelling in the mouse tibia is spatio-temporally modulated by oestrogen deficiency and external mechanical loading: A combined in vivo/in silico study. Acta Biomater. 2020;116:302–317. doi: 10.1016/j.actbio.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 53.Cheong VS, Kadirkamanathan V, Dall’Ara E. The role of the loading condition in predictions of bone adaptation in a mouse tibial loading model. Front Bioeng Biotechnol. 2021;9:676867. doi: 10.3389/fbioe.2021.676867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson ST, Shyu PT, Guo XE. Mechanical loading and parathyroid hormone effects and synergism in bone vary by site and modeling/remodeling regime. Bone. 2021;153:116171. doi: 10.1016/j.bone.2021.116171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albiol L, et al. Sost deficiency leads to reduced mechanical strains at the tibia midshaft in strain-matched in vivo loading experiments in mice. J R Soc Interface. 2018;15(141):20180012. doi: 10.1098/rsif.2018.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.• Yang H, et al. Cortical bone adaptation to a moderate level of mechanical loading in male Sost deficient mice. Sci Rep. 2020;10(1):22299. 10.1038/s41598-020-79098-0. Authors used FE modeling to compared mechanical strain environment between SOST deficient male mice of various age and control mice. Authors concluded that long-term inhibition of sclerostin in male mice does not significantly affect bone adaptation under moderate levels of loading. [DOI] [PMC free article] [PubMed]
- 57.Frost HM. Bone ‘mass’ and the ‘mechanostat’: a proposal. Anat Rec. 1987;219(1):1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 58.Turner CH, Forwood MR, Rho J-Y, Yoshikawa T. Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res. 2009;9(1):87–97. doi: 10.1002/jbmr.5650090113. [DOI] [PubMed] [Google Scholar]
- 59.Hsieh Y-F, Turner CH. Effects of loading frequency on mechanically induced bone formation. J Bone Miner Res. 2001;16(5):918–924. doi: 10.1359/jbmr.2001.16.5.918. [DOI] [PubMed] [Google Scholar]
- 60.You L, Cowin SC, Schaffler MB, Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34(11):1375–1386. doi: 10.1016/S0021-9290(01)00107-5. [DOI] [PubMed] [Google Scholar]
- 61.Knothe Tate ML, Steck R, Forwood MR, Niederer P. In vivo demonstration of load-induced fluid flow in the rat tibia and its potential implications for processes associated with functional adaptation. J Exp Biol. 2000;203(18):2737–2745. doi: 10.1242/jeb.203.18.2737. [DOI] [PubMed] [Google Scholar]
- 62.LaMothe JM, Hamilton NH, Zernicke RF. Strain rate influences periosteal adaptation in mature bone. Med Eng Phys. 2005;27(4):277–284. doi: 10.1016/j.medengphy.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 63.Pereira AF, Javaheri B, Pitsillides AA, Shefelbine SJ. Predicting cortical bone adaptation to axial loading in the mouse tibia. J R Soc Interface. 2015;12(110):20150590. doi: 10.1098/rsif.2015.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.• Bansod YD, Kebbach M, Kluess D, Bader R, van Rienen U. Finite element analysis of bone remodelling with piezoelectric effects using an open-source framework. Biomech Model Mechanobiol. 2021;20(3)1147–1166. 10.1007/s10237-021-01439-3. Authors simulated and predicted bone remodeling in human femur by considering piezoelectric effects. Result suggested that electrical stimulation could improve bone density. [DOI] [PMC free article] [PubMed]
- 65.Mohammadkhah M, Marinkovic D, Zehn M, Checa S. A review on computer modeling of bone piezoelectricity and its application to bone adaptation and regeneration. Bone. 2019;127:544–555. doi: 10.1016/j.bone.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 66.Huiskes R, Weinans H, Grootenboer HJ, Dalstra M, Fudala B, Slooff TJ. Adaptive bone-remodeling theory applied to prosthetic-design analysis. J Biomech. 1987;20(11–12):1135–1150. doi: 10.1016/0021-9290(87)90030-3. [DOI] [PubMed] [Google Scholar]
- 67.Cowin SC, Hegedus DH. Bone remodeling I: theory of adaptive elasticity. J Elasticity. 1976;6(3):313–326. doi: 10.1007/BF00041724. [DOI] [Google Scholar]
- 68.•• van Tol AF, et al. The mechanoresponse of bone is closely related to the osteocyte lacunocanalicular network architecture. Proc Natl Acad Sci USA. 2020;117(51):32251–32259. 10.1073/pnas.2011504117. Authors investigated strain magnitude and fluid flow distribution in the mouse tibia. Bone remodeling simulation showed that considering fluid flow and the lacunar canalicular network led to a better prediction of the bone remodeling compared to strain magnitude. [DOI] [PMC free article] [PubMed]
- 69.Prasad J, Goyal A. An invertible mathematical model of cortical bone’s adaptation to mechanical loading. Sci Rep. 2019;9(1):5890. doi: 10.1038/s41598-019-42378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.• Gonzalez J, Nacy S, Youssef G. Finite element analysis of human skull bone adaptation to mechanical loading. 13. A finite element model and bone remodeling algorithm were used to investigate changes in properties in a human skull following loading. This study provides an approach valuable to design implants and investigate their prolonged effect on the surrounding bone properties.
- 71.Anijs T, Eemers S, Minoda Y, Wolfson D, Verdonschot N, Janssen D. Computational tibial bone remodeling over a population after total knee arthroplasty: a comparative study. J Biomed Mater Res. 2022;110(4):776–786. doi: 10.1002/jbm.b.34957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.• Du J, Li S, Silberschmidt VV. Remodelling of trabecular bone in human distal tibia: a model based on an in-vivo HR-pQCT study. J Mech Behav Biomed Mater. 2021;119:104506. 10.1016/j.jmbbm.2021.104506. Using FE modeling, authors investigated the effect of strain and strain gradient on density and microstructural changes in human trabecular bone remodeling. This study participates in establishing the relationship between mechanical loading and bone response. [DOI] [PubMed]
- 73.• Goyal A, Prasad J. An in silico model for woven bone adaptation to heavy loading conditions in murine tibia. Biomech Model Mechanobiol. 2022, 10.1007/s10237-022-01599-w. Authors reported a new bone adaptation model to predict woven bone formation. Strain and diffusion of calcium ions were considered stimuli. The model contributes to a better understanding of the cellular mechanism of bone in forming woven bone. [DOI] [PubMed]
- 74.Levadnyi I, et al. Comparative analysis of the biomechanical behavior of collar and collarless stems: experimental testing and finite element modelling. J Med Biol Eng. 2021;41(6):844–855. doi: 10.1007/s40846-021-00652-w. [DOI] [Google Scholar]
- 75.•• Scheuren AC, et al. Mechano-regulation of trabecular bone adaptation is controlled by the local in vivo environment and logarithmically dependent on loading frequency. Front Bioeng Biotechnol. 2020;8:566346. 10.3389/fbioe.2020.566346. A micro-FE model of a mouse vertebra was used to correlated bone remodeling and local in vivo mechanical environment. Results suggested that bone resorption and formation occurred respectively at sites of lower and higher strain energy density and strain energy density gradient. Authors also reported a logarithmic dependency between bone remodeling and loading frequency. [DOI] [PMC free article] [PubMed]
- 76.•• Javaheri B, et al. Lasting organ-level bone mechanoadaptation is unrelated to local strain. Sci Adv. 2020;6(10):eaax8301. 10.1126/sciadv.aax8301. In this study, FE modeling was used to investigate strain magnitude and distribution in the mouse tibia under loading. Strain environment was correlated to load-related remodeling and bone shape alteration. Results suggested that external load induced significant lasting modifications in mouse tibia shape and mass, independent of strain magnitude. [DOI] [PMC free article] [PubMed]
- 77.Carriero A, et al. Spatial relationship between bone formation and mechanical stimulus within cortical bone: combining 3D fluorochrome mapping and poroelastic finite element modelling. Bone Reports. 2018;8:72–80. doi: 10.1016/j.bonr.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park S, Park J, Kang I, Lee H, Noh G. Effects of assessing the bone remodeling process in biomechanical finite element stability evaluations of dental implants. Comput Methods Prog Biomed. 2022;221:106852. doi: 10.1016/j.cmpb.2022.106852. [DOI] [PubMed] [Google Scholar]
- 79.Poovarodom P, et al. Effect of implant placement depth on bone remodeling on implant-supported single zirconia abutment crown: a 3D finite element study. J Prosthodont Res. JPR_D_22_00054. 10.2186/jpr.JPR_D_22_00054. [DOI] [PubMed]
- 80.Mathai B, Gupta S. Bone ingrowth around an uncemented femoral implant using mechanoregulatory algorithm: a multiscale finite element analysis. J Biomech Eng. 2022;144(2):021004. doi: 10.1115/1.4052227. [DOI] [PubMed] [Google Scholar]
- 81.Smotrova E, Li S, Silberschmidt VV. Mechanoregulated trabecular bone adaptation: progress report on in silico approaches. Biomater Biosyst. 2022;7:100058. doi: 10.1016/j.bbiosy.2022.100058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.•• Walle M, Marques FC, Ohs N, Blauth M, Müller R, Collins CJ. Bone mechanoregulation allows subject-specific load estimation based on time-lapsed micro-CT and HR-pQCT in vivo. Front Bioeng Biotechnol. 2021;9:677985. 10.3389/fbioe.2021.677985. Authors present an approach to back calculate in vivo loading based on bone remodeling measurements. This work could link changes in loading and bone strength and help in the development of personalized treatment approaches. [DOI] [PMC free article] [PubMed]
- 83.• Chlebek C, Moore JA, Ross FP, van der Meulen MCH. Molecular identification of spatially distinct anabolic responses to mechanical loading in murine cortical bone. J Bone Miner Res. 2022;p. jbmr.4686. 10.1002/jbmr.4686. Authors investigate gene regulation in bone regions experiencing different strain magnitudes. Under loading, the transcriptomic response of cortical bone varied along the bone length, correlating with strain magnitude. This study participates in a better understanding of bone mechanotransduciton. [DOI] [PubMed]
- 84.Sugiyama T, Meakin LB, Browne WJ, Galea GL, Price JS, Lanyon LE. Bones’ adaptive response to mechanical loading is essentially linear between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition. J Bone Miner Res. 2012;27(8):1784–1793. doi: 10.1002/jbmr.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor M, Prendergast PJ. Four decades of finite element analysis of orthopaedic devices: where are we now and what are the opportunities? J Biomech. 2015;48(5):767–778. doi: 10.1016/j.jbiomech.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 86.•• Troy KL, Mancuso ME, Johnson JE, Wu Z, Schnitzer TJ, Butler TA. Bone adaptation in adult women is related to loading dose: a 12‐month randomized controlled trial. J Bone Miner Res. 2020;35(7):1300–1312. 10.1002/jbmr.3999. Authors used FE modeling to design their in vivo loading experiments and investigate the influence of strain magnitude and strain rate on bone adaptation in healthy adult women. Results showed that strain magnitude, strain rate, and number of loading bouts all participate to bone adaptation in healthy women. This study could be use in designing future clinical studies investigating bone adaptation. [DOI] [PMC free article] [PubMed]
- 87.Troy KL, Edwards WB, Bhatia VA, Bareither ML. In vivo loading model to examine bone adaptation in humans: a pilot study: prospective bone adaptation in women. J Orthop Res. 2013;31(9):1406–1413. doi: 10.1002/jor.22388. [DOI] [PubMed] [Google Scholar]
- 88.Bhatia VA, Edwards WB, Troy KL. Predicting surface strains at the human distal radius during an in vivo loading task — finite element model validation and application. J Biomech. 2014;47(11):2759–2765. doi: 10.1016/j.jbiomech.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.• Eller K, et al. Mechanoadaptation of the bones of mice with high fat diet induced obesity in response to cyclical loading. J Biomech. 2021;124:110569. 10.1016/j.jbiomech.2021.110569. Authors modeled the tibia of control and high fat diet mice to match the strain environment between experimental conditions. This study provides new insights regarding the effect of adolescent obesity on bone mechanoresponse. [DOI] [PubMed]
- 90.•• Meslier QA, DiMauro N, Somanchi P, Nano S, Shefelbine SJ. Manipulating load-induced fluid flow in vivo to promote bone adaptation. Bone. 2022;165:116547. 10.1016/j.bone.2022.116547. Authors estimated strain magnitude and fluid flow velocity distribution in a mouse tibia using FE modeling. Simulations results were used to design in vivo loading experiments and investigate the contribution of strain vs fluid flow on bone adaptation. The study participates in better understanding of bone mechanoadpatation the driving mechanical stimulus. [DOI] [PubMed]
- 91.Pickering E, Trichilo S, Delisser P, Pivonka P. Beam theory for rapid strain estimation in the mouse tibia compression model. Biomech Model Mechanobiol. 2022;21(2):513–525. doi: 10.1007/s10237-021-01546-1. [DOI] [PubMed] [Google Scholar]
- 92.Yang H, Butz KD, Duffy D, Niebur GL, Nauman EA, Main RP. Characterization of cancellous and cortical bone strain in the in vivo mouse tibial loading model using microCT-based finite element analysis. Bone. 2014;66:131–139. doi: 10.1016/j.bone.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 93.• Pickering E, Silva MJ, Delisser P, Brodt MD, Gu Y, Pivonka P. Estimation of load conditions and strain distribution for in vivo murine tibia compression loading using experimentally informed finite element models. J Biomech. 2021;115:110140. 10.1016/j.jbiomech.2020.110140. Authors presented an experimentally informed approach to identify load application region on the mouse tibial plateau. Results showed that loading conditions commonly used in mouse tibia FE models can lead to different strain distribution through the bone. This work highlights the importance of load calibration. [DOI] [PMC free article] [PubMed]
- 94.Main RP, Lynch ME, van der Meulen MCH. Load-induced changes in bone stiffness and cancellous and cortical bone mass following tibial compression diminish with age in female mice. J Exp Biol. 2014;p. jeb.085522. 10.1242/jeb.085522. [DOI] [PMC free article] [PubMed]
- 95.Malhotra A, Walle M, Paul GR, Kuhn GA, Müller R. Application of subject-specific adaptive mechanical loading for bone healing in a mouse tail vertebral defect. Sci Rep. 2021;11(1):1861. doi: 10.1038/s41598-021-81132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.• Paul GR, Wehrle E, Tourolle DC, Kuhn GA, Müller R. Real-time finite element analysis allows homogenization of tissue scale strains and reduces variance in a mouse defect healing model. Sci Rep. 2021;11(1)13511. 10.1038/s41598-021-92961-y. This work emphasizes the benefits of designing subject-specific loading condition to reduce the variance in tissue mechanical environment across specimen. [DOI] [PMC free article] [PubMed]
- 97.Willie BM, et al. Diminished response to in vivo mechanical loading in trabecular and not cortical bone in adulthood of female C57Bl/6 mice coincides with a reduction in deformation to load. Bone. 2013;55(2):335–346. doi: 10.1016/j.bone.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 98.• Yang H, Bullock WA, Myhal A, DeShield P, Duffy D, Main RP. Cancellous bone may have a greater adaptive strain threshold than cortical bone. JBMR Plus. 2021;5(5). 10.1002/jbm4.10489. Authors used a FE model to determine the strain environment in cortical and trabecular mouse bone and correlate this mechanical environment to mechanosensitive genes expression and bone formation. Results suggest that trabecular bone might have a greater adaptative strain threshold compared to cortical bone. This study participates in a better understanding of bone mechanoadpatation. [DOI] [PMC free article] [PubMed]
- 99.Yang H, et al. Examining tissue composition, whole-bone morphology and mechanical behavior of GorabPrx1 mice tibiae: a mouse model of premature aging. J Biomech. 2017;65:145–153. doi: 10.1016/j.jbiomech.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.• Oliviero S, Roberts M, Owen R, Reilly GC, Bellantuono I. Dall’Ara E. Non-invasive prediction of the mouse tibia mechanical properties from microCT images: comparison between different finite element models. Biomech Model Mechanobiol. 2021;20(3):941–955. 10.1007/s10237-021-01422-y. In this study, authors investigate the ability of different microCFE models to predict mouse tibia mechanical properties under loading. The results provide a useful comparison of the models complexity and their potential benefits for modeling bone adaptation. [DOI] [PMC free article] [PubMed]
- 101.Poulet B, Hamilton RW, Shefelbine S, Pitsillides AA. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 2011;63(1):137–147. doi: 10.1002/art.27765. [DOI] [PubMed] [Google Scholar]
- 102.Rohlmann A, Gabel U, Graichen F, Bender A, Bergmann G. An instrumented implant for vertebral body replacement that measures loads in the anterior spinal column. Med Eng Phys. 2007;29(5):580–585. doi: 10.1016/j.medengphy.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 103.Navacchia A, Rullkoetter PJ, Schütz P, List RB, Fitzpatrick CK, Shelburne KB. Subject-specific modeling of muscle force and knee contact in total knee arthroplasty: modeling of knee contact in total knee arthroplasty. J Orthop Res. 2016;34(9):1576–1587. doi: 10.1002/jor.23171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Delp SL, et al. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng. 2007;54(11):1940–1950. doi: 10.1109/TBME.2007.901024. [DOI] [PubMed] [Google Scholar]
- 105.Kersh ME, Martelli S, Zebaze R, Seeman E, Pandy MG. Mechanical loading of the femoral neck in human locomotion. J Bone Miner Res. 2018;33(11):1999–2006. doi: 10.1002/jbmr.3529. [DOI] [PubMed] [Google Scholar]
- 106.• Martelli S, Beck B, Saxby D, Lloyd D, Pivonka P, Taylor M. Modelling human locomotion to inform exercise prescription for osteoporosis. Curr Osteoporos Rep. 2020;18(3):301–311. 10.1007/s11914-020-00592-5. This review provides useful information regarding hip strain during various exercise type and exercise recommendation for osteoporosis. [DOI] [PMC free article] [PubMed]
- 107.Warden SJ, Wright CS, Fuchs RK. Bone microarchitecture and strength adaptation to physical activity: a within-subject controlled HRpQCT study. Med Sci Sports Exerc. 2021;53(6):1179–1187. doi: 10.1249/MSS.0000000000002571. [DOI] [PubMed] [Google Scholar]
- 108.•• Wang H, Du T, Li R, Main RP, Yang H. Interactive effects of various loading parameters on the fluid dynamics within the lacunar-canalicular system for a single osteocyte. Bone. 2022;158:116367. 10.1016/j.bone.2022.116367. Authors reported a multiscale model to investigate the combined effect of various loading parameters on fluid flow in the lacunar-canalicular system. Results suggested a combination of loading parameter to promote fluid flow in the LCS. This study participates in a better understanding of bone mechanical environment under loading and could help in designing future loading experiments. [DOI] [PubMed]
- 109.• Yu W, et al. Study on the biomechanical responses of the loaded bone in macroscale and mesoscale by multiscale poroelastic FE analysis. BioMed Eng OnLine. 2019;18(1):122. 10.1186/s12938-019-0741-3. Authors used a multi-scale poroelastic FE model to investigate strain distribution and fluid flow behavior in loaded bone. This work provides a better understanding of fluid flow and mechanotransduction in bone remodeling. [DOI] [PMC free article] [PubMed]
- 110.• Ganesh T, Laughrey LE, Niroobakhsh M, Lara-Castillo N. Multiscale finite element modeling of mechanical strains and fluid flow in osteocyte lacunocanalicular system. Bone. 2020;137:115328. 10.1016/j.bone.2020.115328. This review presents some studies that contributed to a better understanding of the lacunae mechanical environment and associated osteocytes activation. [DOI] [PMC free article] [PubMed]
- 111.• Kola SK, et al. Osteocyte lacunar strain determination using multiscale finite element analysis. Bone Rep. 2020;12:100277. 10.1016/j.bonr.2020.100277. Using a multiscale approach, authors showed that osteocyte lacunae size, orientation and geometry affect osteocytes strain environment. This study participates in better understanding the process of osteocytes activation in response to mechanical loading. [DOI] [PMC free article] [PubMed]
- 112.•• Sang W, Ural A. Quantifying how altered lacunar morphology and perilacunar tissue properties influence local mechanical environment of osteocyte lacunae using finite element modeling. J Mech Behav Biomed Mater. 2022;135:105433. 10.1016/j.jmbbm.2022.105433. Authors modeled osteocytes lacunae and conducted a parametric study to investigate the influence of lacunar morphology (density, volume, equancy) and perilacunar bone properties on osteocytes mechanical environment. Results suggest that alteration of lacunae morphology and properties alter the induced mechanical environment, which might affect osteocytes mechanosensitivity. [DOI] [PubMed]
- 113.Gatti V, Azoulay EM, Fritton SP. Microstructural changes associated with osteoporosis negatively affect loading-induced fluid flow around osteocytes in cortical bone. J Biomech. 2018;66:127–136. doi: 10.1016/j.jbiomech.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.•• Schurman CA, Verbruggen SW, Alliston T. Disrupted osteocyte connectivity and pericellular fluid flow in bone with aging and defective TGF-β signaling. Proc Natl Acad Sci USA. 2021;118(25):e2023999118. 10.1073/pnas.2023999118. An osteocyte FE model was used to investigate the change in mechanical environment around the cell with age and LCN disruption. Simulation suggested a strategy to restore restore physical stimulation to osteocytes.
- 115.Paul GR, Malhotra A, Müller R. Mechanical stimuli in the local in vivo environment in bone: computational approaches linking organ-scale loads to cellular signals. Curr Osteoporos Rep. 2018;16(4):395–403. doi: 10.1007/s11914-018-0448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.•• Smit TH. Finite element models of osteocytes and their load-induced activation. Curr Osteoporos Rep. 2022;20(2):127–140. 10.1007/s11914-022-00728-9. This recent review covers finite elements models used to investigate osteocytes mechanical environment and associated limitations. [DOI] [PMC free article] [PubMed]
- 117.• Srinivasan S, et al. Static preload inhibits loading-induced bone formation: static preload inhibits bone adaptation. JBMR Plus. 2019;3(5):e10087. 10.1002/jbm4.10087. Authors designed a new loading device and used finite element model combined with strain gauges to characterize the mechanical environment induced the mouse tibia. [DOI] [PMC free article] [PubMed]
- 118.• Gao J, Liu B, Zhang M, Gong H, Gao B. Strain distribution evaluation of rat tibia under axial compressive load by combining strain gauge measurement and finite element analysis. Appl Bionics Biomech. 2019;2019:1–14. 10.1155/2019/1736763. Using strain gauge and FEM, the strain environment in a whole rat tibia were characterized under axial compressive load. Positions of high and low strain magnitude could be used in future mechanobiology study in the rat tibia. [DOI] [PMC free article] [PubMed]
- 119.Webster DJ, Morley PL, van Lenthe GH, Müller R. A novel in vivo mouse model for mechanically stimulated bone adaptation – a combined experimental and computational validation study. Comput Methods Biomech Biomed Engin. 2008;11(5):435–441. doi: 10.1080/10255840802078014. [DOI] [PubMed] [Google Scholar]
- 120.Sztefek P, Vanleene M, Olsson R, Collinson R, Pitsillides AA, Shefelbine S. Using digital image correlation to determine bone surface strains during loading and after adaptation of the mouse tibia. J Biomech. 2010;43(4):599–605. doi: 10.1016/j.jbiomech.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 121.Oliviero S, Giorgi M, Dall’Ara E. Validation of finite element models of the mouse tibia using digital volume correlation. J Mech Behav Biomed Mater. 2018;86:172–184. doi: 10.1016/j.jmbbm.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 122.• Carriero A, Javaheri B, Bassir Kazeruni N, Pitsillides AA, Shefelbine SJ. Age and sex differences in load‐induced tibial cortical bone surface strain maps. JBMR Plus. 2021;5(3). 10.1002/jbm4.10467. This study showed that load-induced strain environment in the mouse tibia vary with age and sex. Results will help to more precisely match strain environment between experimental conditions. They also participate in understanding hoe bone mechanoresponsive is influenced by aging and sex. [DOI] [PMC free article] [PubMed]
- 123.Liu C. FDA recognizes simulation essential to evaluate medical devices. Adv Mater Process. 2013.
- 124.Reporting of computational modeling studies in medical device submissions - guidance for industry and food and drug administration staff. U.S Food Drug Adm. 2016.
- 125.Assessing the credibility of computational modeling and simulation in medical device submissions. U.S Food Drug Adm. 2021.
- 126.Carriero A, Jonkers I, Shefelbine SJ. Mechanobiological prediction of proximal femoral deformities in children with cerebral palsy. Comput Methods Biomech Biomed Engin. 2011;14(3):253–262. doi: 10.1080/10255841003682505. [DOI] [PubMed] [Google Scholar]