Abstract
Aims
Patients who undergo permanent pacemaker (PPM) implantation after transcatheter aortic valve replacement (TAVR) have a worse outcome. The aim of this study was to identify risk factors of worse outcomes in patients with post-TAVR PPM implantation.
Methods and results
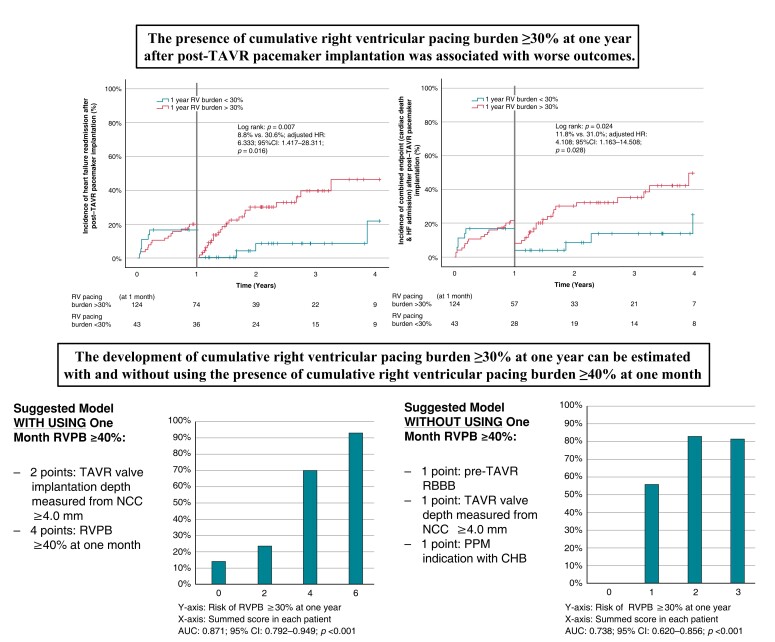
This is a single-centre, retrospective study of consecutive patients who underwent post-TAVR PPM implantation from 11 March 2011 to 9 November 2019. Clinical outcomes were evaluated by landmark analysis with cut-off at 1 year after the PPM implantation. Of the 1389 patients underwent TAVR during the study duration and a total of 110 patients were included in the final analysis. Right ventricular pacing burden (RVPB) ≥ 30% at 1 year was associated with a higher likelihood of heart failure (HF) readmission [adjusted hazard ratio (aHR): 6.333; 95% confidence interval [CI]: 1.417–28.311; P = 0.016] and composite endpoint of overall death and/or HF (aHR: 2.453; 95% CI: 1.040–5.786; P = 0.040). The RVPB ≥30% at 1 year was associated with higher atrial fibrillation burden (24.1 ± 40.6% vs. 1.2 ± 5.3%; P = 0.013) and a decrease in left ventricular ejection fraction (−5.0 ± 9.8% vs. + 1.1 ± 7.9%; P = 0.005). The predicting factors of the RVPB ≥30% at 1 year were the presence of RVPB ≥40% at 1 month and the valve implantation depth measured from non-coronary cusp ≥4.0 mm (aHR: 57.808; 95% CI: 12.489–267.584; P < 0.001 and aHR: 6.817; 95% CI: 1.829–25.402; P = 0.004).
Conclusions
The RVPB ≥30% at 1 year was associated with worse outcomes. Clinical benefit of minimal RV pacing algorithms and biventricular pacing needs to be investigated.
Keywords: Aortic stenosis, Dyssynchrony, Heart failure, Left ventricular ejection fraction, Pacemaker, Transcatheter aortic valve replacement
Graphical Abstract
Graphical Abstract.
What’s new?
Contrary to the cumulative right ventricular pacing burden (RVPB) ≥ 40% which is a well-known predictor of worse outcomes after permanent pacemaker implantation, this present study demonstrated that a lower RVPB ≥30% at 1 year was predictive of worse outcomes, atrial tachyarrhythmia, and left ventricular systolic dysfunction in post-transcatheter aortic valve replacement (TAVR) patients.
The presence of RVPB ≥40% at 1 month was the strongest predictor of the cumulative RVPB ≥30% at 1 year, in addition to the deep TAVR valve implantation and other pre-TAVR factors.
The observation of RVPB ≥40% at 1 month can be used to identify patients likely to develop RVPB ≥30% at 1 year and the prompt identification of high-risk population may improve their outcomes.
If such high-risk patients require continuous pacing, in addition to providing optimal heart failure medications, clinical benefits of left bundle branch or biventricular pacing can be considered for well-selected post-TAVR patients.
Introduction
Transcatheter aortic valve replacement (TAVR) is an effective alternative to surgical treatment of severe symptomatic aortic stenosis (AS), and TAVR is now widely utilized for patients at low to high risk for cardiac surgery. However, acute high-grade atrioventricular block requiring new permanent pacemaker (PPM) implantation has remained one of the most common complications following the conventional TAVR procedure.1 Indeed, the rate of post-TAVR PPM implantation with new generation valves is reported to be between 2.3% and 36.1%.1 Overall, large observational studies have demonstrated that patients treated with post-TAVR PPM have an increase in all-cause mortality and heart failure (HF) readmission.2,3
Historically, a cumulative right ventricular pacing burden (RVPB) more than 40% has been associated with higher long-term mortality and HF readmission.4–6 We previously reported that the presence of RVPB ≥40% in patients receiving post-TAVR PPM implantation was associated with a higher mortality and HF readmission at 1 year follow-up after TAVR.7 However, given that TAVR-indicated patients are generally older, have underlying structural heart disease as well as other medical comorbidities, we hypothesized that other factors may contribute to these worse outcomes. The goal of this present study is to identify the threshold at which RVPB contributes to the worse outcomes and the predicting factors of the high-risk RVPB after the post-TAVR PPM implantation.
Methods
Study design
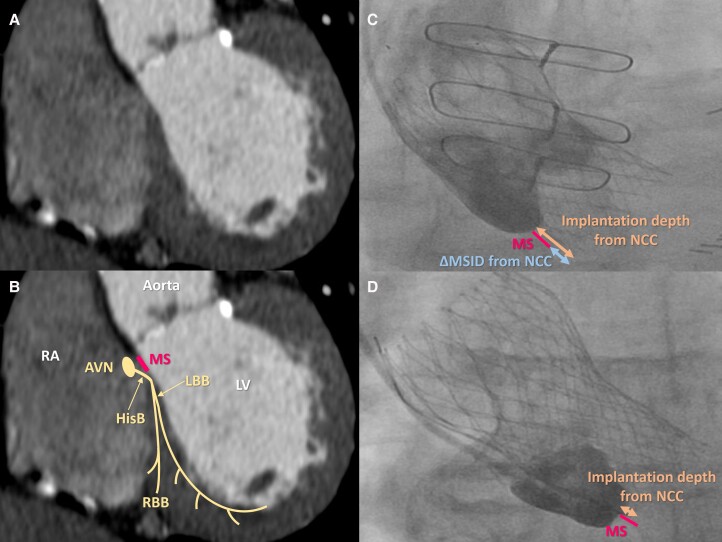
This is a single-centre retrospective cohort study of consecutive patients who underwent TAVR from 10 March 2011 to 9 November 2019 at University Hospitals Cleveland Medical Center. Patients with a pre-existing cardiac implantable electronic device or those who received new cardiac resynchronization therapy after TAVR were excluded from this study. A variety of clinical outcomes were evaluated by landmark analysis with cut-off at 1 year after the device implantation, respectively: all-cause mortality, cardiac mortality, HF readmission, the composite endpoint of all-cause mortality and/or HF readmission, and the composite endpoint of cardiac mortality and/or HF readmission. We collected patient characteristics, pre- and post-TAVR electrocardiogram (ECG) and echocardiography, pre-TAVR computed tomography (CT) imaging, intra-procedural data, and PPM information. The cumulative RVPB was calculated and reported based on the interrogation algorithm provided by each manufacture. The membranous septum (MS) length was measured in a dedicated coronal view as the perpendicular distance from the annular plane to the beginning of the muscular septum with using the pre-TAVR CT imaging.8 The TAVR valve implantation depth was measured at the final aortic angiogram.8 The difference between the MS length and the implantation depth was measured to define the distance between the two structures (ΔMSID)8 (Figure 1). All measurements were performed by three experienced structural interventional cardiologists (L.A.P.D, A.F, and SH.Y) who were unaware of clinical outcomes. Medtronic CoreValve (Medtronic, Minneapolis, MN, USA), Edwards SAPIEN Valve (Edwards Lifesciences, Irvine, CA, USA), and Edwards SAPIEN XT were considered as the early generation devices. Edwards SAPIEN 3, Medtronic Evolut R, and Medtronic Evolut PRO were considered as new generation prostheses. Patients receiving other types of TAVR valves were excluded. This study utilized data extracted from our institutional TAVR research registry which was approved by the institutional review board at University Hospitals Cleveland Medical Center. All patients provided signed informed consent for the data collection. The research reported in this paper adhered to Helsinki Declaration as revised in 2013.
Figure 1.
Schematic illustration of cardiac conduction system on cardiac computed tomography and aortic angiogram. Reconstructed coronal view of cardiac computed tomography (A), schematic illustration of cardiac conduction system on cardiac computed tomography (B), aortic aortogram in patient with deep valve implantation (C), and aortic aortogram in patient with shallow valve implantation (D). AVN, atrioventricular node; HisB, His bundle; LBB, left bundle branch; LV, left ventricle; MS, membranous septum; NCC, non-coronary cusp; RA, right atrium; RBB, right bundle branch; ΔMSID, the difference between the MS length and the implantation depth.
Transcatheter aortic valve replacement procedure and post-transcatheter aortic valve replacement permanent pacemaker implantation
Patient selection and the required procedural technique for TAVR were decided by a team which included an interventional cardiologist and a cardiac surgeon, per Centers for Medicare & Medicaid Services policy.9 The indication for post-TAVR PPM implantation was determined after consultation with a cardiac electrophysiologist. In patients with preserved sinus rhythm, the dual-chamber PPM was indicated and all ventricular leads were placed at the right ventricular septal apex. When available, the algorithms to reduce the RVPB were utilized appropriately.10
Statistical analysis
Qualitative variables were reported as number (percentage) and quantitative variables as mean ± standard deviation or median [interquartile range (IQR)] based on each variable distribution. Categorical variables were compared with Pearson’s χ2 test or Fisher’s exact test. Mann–Whitney test and Wilcoxon signed rank test were used for quantitative variables with non-normal distribution. Two-sided Student’s t-test was used for quantitative variables with normal distribution. All P-values were two-sided, and P-values less than 5% were considered as significant. Survival rates were reported by Kaplan–Meier analysis, and log-rank test was used to compare the outcome between groups. Cox proportional hazard models (cumulative outcomes) were utilized to evaluate clinical outcomes, and all multivariable models were adjusted based on baseline differences with P-value less than 5%. The area under the receiving curve in receiver-operator curve with Youden’s index was used to assess the predictive accuracy. Multivariable logistic regression analysis with forward stepwise algorithm (likelihood ratio) using the significance level 5% was used to extract the predictive covariates. The number of points assigned to each variable equalled its regression coefficient rounded to the nearest whole number and all points were summed for obtaining the risk score in each patient. The linearity assumption of continuous variables were evaluated using Cox regression with spline transformed continuous variables and the hazard ratio (HR) of 1 (y-axis = 0) was chosen as the transitional point to convert the analyzed quantitative variables to the categorical ones. The spline regression analyses were performed with R statistical software package (version 3.5.1 for Mac, R Foundation for Statistical Computing, Vienna, Austria). All of other analysis was performed with IBM® SPSS® Statistics Version 27 software (SPSS, Chicago, IL).
Results
Of the 1389 patients who underwent TAVR during the study duration, a total of 198 patients received post-TAVR PPM implantation. The device follow-up data at 1 year after the PPM implantation was available on 110 patients who were included in the final analysis. The median age was 83.0 (IQR: 77.0–88.0) years old, and the median of the observational period was 1.5 (IQR: 0.50–2.70) years after the device implantation.
Clinical impact of the cumulative right ventricular pacing burden on post-transcatheter aortic valve replacement outcomes
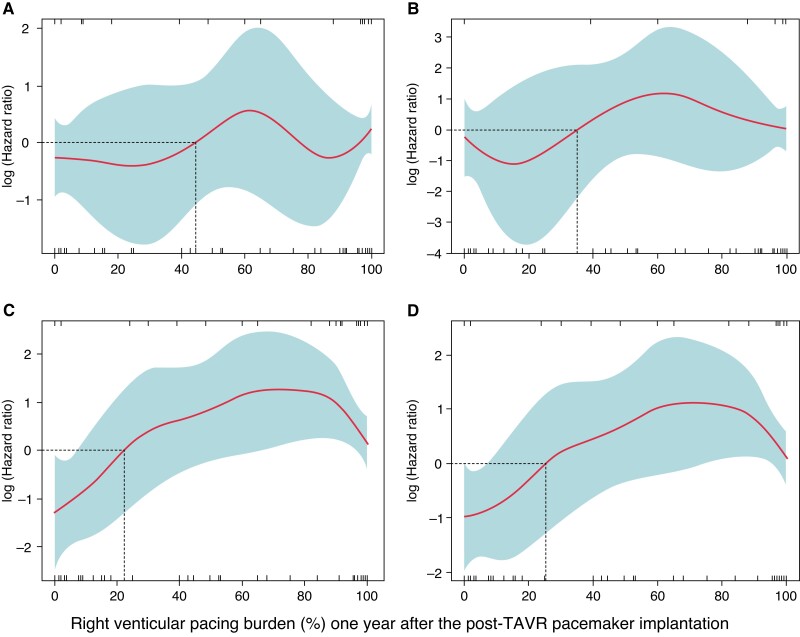
With linear spline regression analysis, a cumulative RVPB ≥30% 1 year after the post-TAVR PPM implantation was associated with higher cardiac mortality, HF readmission, and the composite endpoint of cardiac mortality and/or HF readmission (Figure 2). The baseline characteristics between patients with RVPB <30% and ≥30% at 1 year were summarized in Table 1.
Figure 2.
Linear-spline cox regression analysis of right ventricular pacing burden. All-cause mortality (A), cardiac mortality (B), heart failure readmission (C), and the composite endpoint of cardiac mortality and/or heart failure readmission (D). TAVR, transcatheter aortic valve replacement.
Table 1.
Baseline characteristics in patients who survived 1 year after post-TAVR pacemaker implantation
| 1 year RVPB <30% (n = 36) | 1 year RVPB ≥30% (n = 74) | P-value | |
|---|---|---|---|
| Baseline characteristics | |||
| ȃMale | 19 (52.8%) | 44 (59.5%) | 0.506 |
| ȃAge | 82.4 ± 8.8 | 81.5 ± 8.1 | 0.324 |
| ȃBMI | 29.3 ± 9.3 | 30.7 ± 7.0 | 0.080 |
| ȃHypertension | 35 (97.2%) | 70 (94.6%) | 1.000 |
| ȃDiabetes mellitus | 15 (41.7%) | 33 (44.6%) | 0.726 |
| ȃChronic kidney disease | 11 (30.6%) | 28 (37.8%) | 0.395 |
| ȃHyperlipidaemia | 26 (72.2%) | 55 (74.3%) | 0.726 |
| ȃPrior stroke | 4 (11.1%) | 11 (14.9%) | 0.769 |
| ȃPeripheral artery disease | 6 (16.7%) | 20 (27.0%) | 0.203 |
| ȃPre-existing CAD | 17 (47.2%) | 45 (60.8%) | 0.130 |
| ȃPrior PCI | 10 (27.8%) | 19 (25.7%) | 0.806 |
| ȃPrior CABG | 6 (16.7%) | 25 (33.8%) | 0.073 |
| ȃPrior surgical AVR | 1 (2.8%) | 1 (1.4%) | 0.542 |
| ȃHistory of AF | 4 (11.1%) | 20 (27.0%) | 0.067 |
| ȃNew-onset AF after the device implantation | 3 (8.3%) | 16 (21.6%) | 0.094 |
| ȃSTS score | 7.5 ± 5.8 | 6.7 ± 4.9 | 0.558 |
| Baseline electrocardiogram | |||
| ȃPre-existing RBBB | 11 (30.6%) | 48 (64.9%) | <0.001 |
| ȃPre-existing LBBB | 2 (5.6%) | 1 (1.4%) | 0.241 |
| ȃPre-existing AVBa | 9 (25.0%) | 17 (23.0%) | 0.758 |
| ȃNew-onset LBBB | 12 (33.3%) | 13 (17.6%) | 0.054 |
| ȃBaseline PR (milliseconds) | 192.5 ± 51.7 | 191.4 ± 43.9 | 0.735 |
| ȃBaseline QRS (milliseconds) | 110.8 ± 25.7 | 126.0 ± 28.9 | 0.018 |
| ȃDelta PR (post-pre) (milliseconds) | 22.6 ± 60.0 | 38.8 ± 89.2 | 0.405 |
| ȃDelta QRS (POST-PRE) (milliseconds) | 26.2 ± 25.3 | 31.1 ± 27.0 | 0.484 |
| Echocardiographic data | |||
| ȃBaseline LVEF (%) | 56.1 ± 10.8 | 58.0 ± 10.0 | 0.308 |
| ȃBaseline Vmax (m/sec) | 4.1 ± 0.8 | 4.1 ± 0.6 | 0.788 |
| ȃBaseline AVA (cm2) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.948 |
| ȃBaseline mean AVPG (mmHg) | 43.4 ± 15.3 | 41.6 ± 13.6 | 0.538 |
| ȃLVEF 1 year after TAVR (%) | 56.6 ± 10.1 | 53.3 ± 11.7 | 0.097 |
| ȃDelta LVEF (%) | 1.1 ± 7.9 | −5.0 ± 9.8 | 0.005 |
| Procedural Values | |||
| ȃSelf-expanding valve | 27 (75.0%) | 56 (75.7%) | 0.882 |
| ȃBAV before deployment | 23 (63.9%) | 53 (71.6%) | 0.536 |
| ȃBAV after deployment | 6 (16.7%) | 6 (8.1%) | 0.202 |
| ȃNew-generation valve | 26 (72.2%) | 49 (66.2%) | 0.416 |
| ȃTransfemoral approach | 36 (100.0%) | 70 (94.6%) | 0.174 |
| ȃImplantation depth from NCC (mm) | 5.4 ± 3.3 | 6.3 ± 2.9 | 0.072 |
| ȃImplantation depth from NCC more than 4.0 mm | 17 (48.6%) | 57 (78.1%) | 0.004 |
| ȃImplantation depth from LCC (mm) | 5.5 ± 3.2 | 6.0 ± 3.0 | 0.393 |
| ȃLength of membranous septum (mm) | 5.0 ± 2.7 | 5.1 ± 1.7 | 0.164 |
| ȃΔMSID from NCC (mm) | 0.8 ± 4.2 | 1.1 ± 3.5 | 0.700 |
| ȃΔMSID from LCC (mm) | 0.5 ± 4.6 | 1.0 ± 3.5 | 0.600 |
| PPM information | |||
| ȃPPM implantation without defibrillator | 34 (94.4%) | 74 (100.0%) | 0.593 |
| ȃCHB as the PPM indication | 17 (47.2%) | 62 (83.8%) | <0.001 |
| ȃTiming of PPM implantation after TAVR (days) | 4.5 ± 5.9 | 3.7 ± 10.7 | 0.007 |
| ȃPacing mode (DDD) | 32 (88.9%) | 66 (89.2%) | 0.363 |
| ȃ1 year RAPB (%) | 37.2 ± 28.4 | 28.9 ± 30.8 | 0.050 |
| ȃ1 month RVPB (%) | 29.8 ± 38.5 | 85.1 ± 27.4 | <0.001 |
| ȃ1 year RVPB (%) | 5.1 ± 7.8 | 87.6 ± 20.7 | <0.001 |
| ȃ1 year AF burden (%) | 1.2 ± 5.3 | 24.1 ± 40.6 | 0.013 |
Values are n (%) or mean ± SD.
AF, atrial fibrillation; AVA, aortic valvular area; AVB, atrioventricular block; AVPG, aortic valvular pressure gradient; AVR, aortic valve replacement; BAV, balloon aortic valvuloplasty; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHB, complete heart block; ΔMSID, difference between membranous septum and valve implantation depth; LBBB, left bundle branch block; LCC, left coronary cusp; LVEF, left ventricular ejection fraction; NCC, non-coronary cusp; PCI, percutaneous coronary intervention; PPM, permanent pacemaker; RAPB, right atrium pacing burden; RBBB, right bundle branch block; RVPB, right ventricular pacing burden; TAVR, transcatheter aortic valve replacement, and Vmax, maximum transvalvular aortic velocity.
The prior AVB includes any kinds of AVB before TAVR and all of them were either first or second-degree AVB.
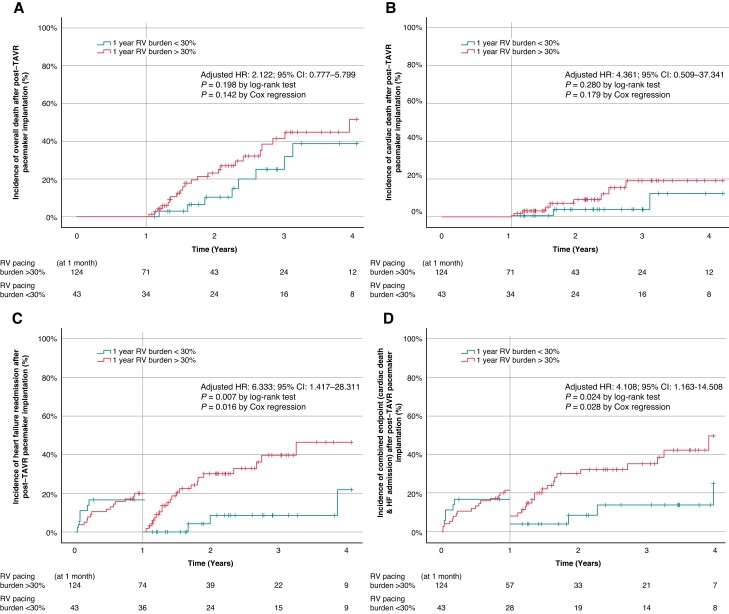
During the observed period, the presence of 1 year RVPB ≥30% was significantly associated with HF readmission and the composite endpoints (Table 2 and Figure 3). On the other hand, in comparison to the development of 1 year RVPB ≥30%, clinical outcomes in patients with 1 year RVPB <40% and ≥40% were insignificant in this present study (see Supplementary material online, Table S1). In addition, the development of 1 year RVPB ≥30% was significantly related to higher atrial fibrillation (AF) burden and the reduction in left ventricular systolic function at 1 year after the PPM implantation (Table 1). With multivariate Cox regression analysis, baseline (pre-TAVR) QRS ≥150 milliseconds and the presence of RVPB ≥30% at 1 year after the post-TAVR PPM implantation were significantly associated with the development of HF readmission and the composite endpoints (see Supplementary material online, Tables S2–S4).
Table 2.
Clinical outcomes of right ventricular pacing burden more than 30% 1 year after post-TAVR pacemaker implantation
| RVPB <30% (n = 36) | RVPB ≥30% (n = 74) | Unadjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| All-cause mortality | 8 (22.2%) | 23 (31.1%) | 1.687 (0.754–3.777) | 0.203 | 2.122 (0.777–5.799) | 0.142 |
| Cardiac mortality | 2 (5.6%) | 8 (10.8%) | 2.296 (0.487–10.827) | 0.293 | 4.361 (0.509–37.341) | 0.179 |
| HF readmission | 3 (8.3%) | 22 (29.7%) | 4.615 (1.375–15.494) | 0.013 | 6.333 (1.417–28.311) | 0.016 |
| Composite (all-cause mortality and/or HF) | 10 (27.8%) | 31 (41.9%) | 2.001 (0.978–4.094) | 0.058 | 2.453 (1.040–5.786) | 0.040 |
| Composite (cardiac mortality and/or HF) | 4 (11.1%) | 22 (29.7%) | 3.213 (1.101–9.373) | 0.033 | 4.108 (1.163–14.508) | 0.028 |
CI, confidence interval; HF, heart failure; HR, hazard ratio; RVPB, right ventricular pacing burden.
Figure 3.
Clinical outcomes of 1 year right ventricular pacing burden ≥30% cumulative incidence curves evaluated by landmark analysis with cut-off at 1 year for long-term outcomes: all-cause mortality (A), cardiac mortality (B), heart failure readmission (C), and the composite of cardiac mortality and/or heart failure readmission (D). CI, confidence interval, HR, hazard ratio; TAVR, transcatheter aortic valve replacement.
Risk factors associated with a higher right ventricular pacing burden
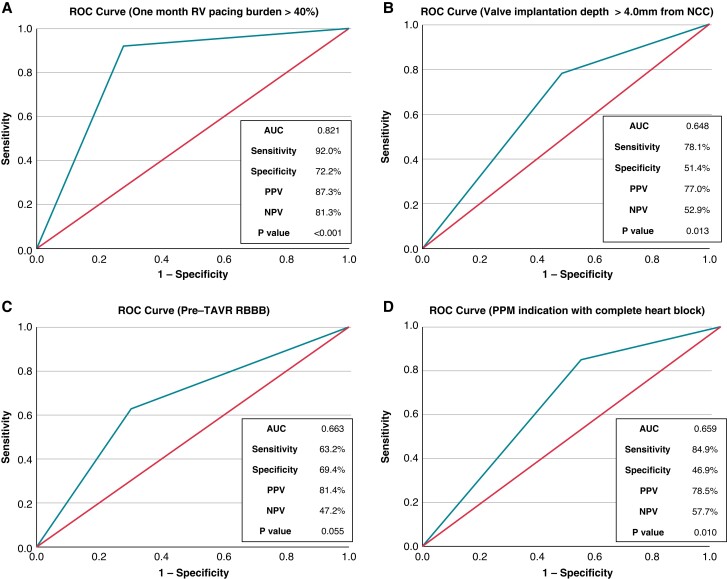
The development of RVPB ≥30% at 1 year after post-TAVR PPM implantation was significantly associated with the presence of RVPB ≥40% at 1 month after the post-TAVR PPM implantation and the TAVR valve implantation depth measured from non-coronary cusp (NCC) ≥ 4.0 mm (see Supplementary material online, Table S5). These values demonstrated good predictive accuracy (Figure 4). Given the RVPB ≥40% at 1 month was not available at the timing of the initial post-TAVR PPM implantation, we also performed the multivariable logistic regression analysis without including the RVPB ≥40% at 1 month (see Supplementary material online, Table S6). The identified predictors of 1 year RVPB ≥30% were pre-TAVR RBBB, TAVR valve implantation depth measured from NCC ≥4.0 mm, and PPM indication with complete heart block (CHB).
Figure 4.
Risk factors of 1 year right ventricular pacing burden ≥30% the area under the receiving curve in receiver-operator curve demonstrated predictive accuracy of each parameter: the cumulative right ventricular pacing burden ≥40% 1 month after the post-TAVR PPM implantation (A), the TAVR valve implantation depth measured from non-coronary cusp (NCC) ≥ 4.0 mm (B), Pre-TAVR RBBB (C), and PPM indication with CHB (D). AUC, area under the receiving curve in receiver-operator curve; CHB, complete heart block; CI, confidence interval; NCC, non-coronary cusp; NPV, negative predictive value; PPM, permanent pacemaker; PPV, positive predictive value; RBBB, right bundle branch block; ROC, receiver-operator curve; RV, right ventricular; TAVR, transcatheter aortic valve replacement.
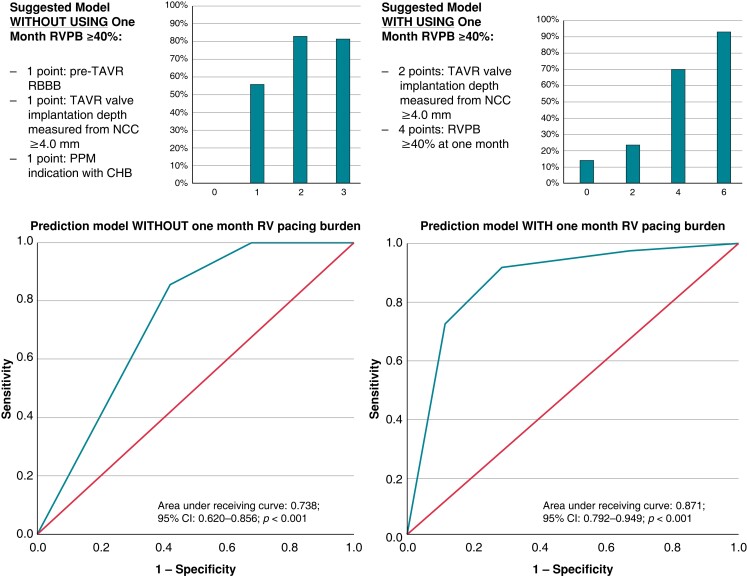
The extraction of risk prediction models for the development of RVPB ≥30% at 1 year was attempted in this present study. Without including the RVPB ≥40% at 1 month, the regression coefficient estimates were pre-TAVR RBBB (1.000), TAVR valve implantation depth measured from NCC ≥4.0 mm (1.071), and PPM indication with CHB (1.293). Therefore, the suggested covariates were 1 point, respectively. With including the RVPB ≥40% at 1 month, the regression coefficient estimates were TAVR valve implantation depth measured from NCC ≥4.0 mm (1.919) and the RVPB ≥40% at 1 month (4.057). The suggested covariates were 2 and 4, respectively. The performance of these risk prediction models was summarized in Figure 5.
Figure 5.
Risk-prediction models on 1 year right ventricular pacing burden ≥30%. Regarding the figures about the suggested models in figure 5, the Y-axis means the estimated risk (%) of development of RVPB >30% at one year after the post-TAVR PPM implantation. The X-axis means the summed score in each patient. CHB, complete heart block; CI, confidence interval; NCC, non-coronary cusp; PPM, permanent pacemaker; RBBB, right bundle branch block, RV, right ventricular; RVPB, right ventricular pacing burden; TAVR, transcatheter aortic valve replacement.
Discussion
The main findings of the present study are highlighted as follows: (i) the presence of RVPB ≥30% at 1 year after the post-TAVR PPM implantation portended worse outcomes, (ii) the presence of RVPB ≥30% at 1 year was associated with higher AF burden and left ventricular (LV) systolic dysfunction after the PPM implantation, (iii) the presence of RVPB ≥40% at 1 month after the post-TAVR PPM implantation was the strongest predictor of the development of RVPB ≥30% at 1 year, and (iv) some of pre-TAVR factors and the deep valve implantation depth measured from non-coronary cusp ≥4.0 mm were also related to the development of RVPB ≥30% at 1 year.
The clinical impact of the post-TAVR PPM implantation has varied among different studies, and the inconsistency can be related to limitations in each study design and follow-up duration. The largest systematic review and meta-analysis (n = 42 927) showed that post-TAVR PPM implantation was associated with both all-cause mortality and HF readmission.3 These findings were consistent with other large-sample studies.1–3
Right ventricular (RV) pacing has been associated in some patients with LV systolic dysfunction and subsequent HF readmission.4–6 RV pacing can provoke LV electro-mechanical dyssynchrony, functional mitral regurgitation, and subsequent LV systolic dysfunction after the PPM implantation.5–6,11 Post hoc analysis of both MOST and DAVID trials demonstrated the cumulative RVPB ≥40% was an independent predictor of HF readmission.4–6 This present study demonstrated that a lower RVPB ≥30% was predictive of worse outcomes in post-TAVR population. In comparison with the baseline characteristics of MOST and DAVID trials, our present cohort was characterized by advanced age and high prevalence of multiple medical comorbidities.4,5 These clinical features can be related to underlying myocardial fibrosis which is newly recognized as contributing to pathophysiology of post-TAVR outcomes.11 In patients who underwent TAVR, the baseline myocardial fibrosis was significantly associated with LV systolic dysfunction, higher extent of pathological LV remodelling, and the development of clinical HF and cardiac death after TAVR.11 Furthermore, the initiation of RV apical pacing in patients with pre-existing myocardial fibrosis demonstrated both immediate and medium-term deterioration in LVEF with a significant increase in the mechanical dyssynchrony index.12 In that study, 20% of patients were upgraded to cardiac resynchronization therapy (CRT). Patients undergoing CRT upgrades were characterized by significant pre-existing myocardial fibrosis.12 Based on these findings, it is possible that post-TAVR patients with significant baseline myocardial fibrosis, who subsequently undergo RV apical pacing, will develop acute dyssynchrony with resultant decrease in LVEF and worse outcomes.
It is well known that long-term asynchronous ventricular pacing can lead to AF secondary to the left atrial overload and the subsequent remodelling of the atrium.5,13 However, even patients with ‘physiologic’ dual-chamber pacing with atrioventricular synchronization remain at high risk of developing new AF after PPM implantation.14 Pre-existing hypertension, left atrial enlargement, advanced age, and high cumulative RVPB were independent predictors of new AF development after dual-chamber PPM implantation.15,16 Patients with sinus node dysfunction exposed to the cumulative RVPB >50% were highly likely to develop new AF after dual-chamber PPM implantation.14 Given the conventional TAVR-indicated patients had age-relevant risk factors for atrial fibrosis and sinus node dysfunction in spite of their predominant presentation of normal sinus rhythm prior to TAVR, hypothetically patients with 1 year RVPB ≥30% might develop high AF burden.17
Several studies attempted to predict RV pacing dependency following post-TAVR PPM implantation.8,18,19 In these studies, PPM dependency was generally defined as the presence of complete atrioventricular dissociation in patients with normal sinus rhythm or the presence of a lower escape rhythm <50 bpm in AF over 30–60 s.8,18,19 However, there are no prior studies to predict high RVPB following post-TAVR PPM implantation. Our present study reported that the presence of RVPB ≥40% at 1 month after post-TAVR PPM implantation was the strongest predictor of RVPB ≥30% at 1 year. In clinical practice, patients are routinely seen within 4–6 weeks of device implantation. Therefore, the observation of RVPB ≥40% at 1 month can be used to identify patients likely to have RVPB ≥30% at 1 year and the prompt identification of high-risk population may improve their outcomes. If such high-risk patients presented with some recovery of the infra-nodal conduction, there may be an opportunity to utilize algorithms that minimize RV pacing. If pacing is necessary, then the timing and indication for an upgrade to biventricular pacing can be considered with providing optimal HF management to such post-TAVR patients.20 Alternatively, the development of RVPB ≥30% at 1 year can be estimated at the end of TAVR procedure without waiting for the development of RVPB ≥40% at 1 month. The present study showed three suggestive risk factors. If the patient was considered as high-risk of RVPB ≥30% at 1 year, clinical benefits of left bundle branch pacing or biventricular PPM can be considered for well-selected post-TAVR patients at the timing of initial PPM implantation.
Study limitations
This is a single-centre retrospective observational study, and the sampling bias cannot be excluded. There was no centralized core laboratory to evaluate the utilized cardiovascular imaging and no central committee to verify the reported clinical events in our present study. Because some patients were mainly followed by outside medical healthcare systems after TAVR, there is a possibility of missing some longitudinal follow-up results. Related to the relatively small study sample size, there is a substantial risk of overfitting a limited number of predictors in the present multivariable models. Finally, the suggestive risk prediction models were not internally validated in this present study.
Conclusions
In patients with post-TAVR PPM implantation, the presence of RVPB ≥30% at 1 year was significantly associated with atrial tachyarrhythmia, LV systolic dysfunction, and worse clinical outcomes. In addition to other predictors, the presence of RVPB ≥40% at 1 month was highly predictive of the future progression to RVPB ≥30% at 1 year. Whether pacing strategies to provide cardiac resynchronization or to minimize RV pacing can improve patient outcomes in this population will need to be evaluated. Additional prospective and multi-centre randomized trials should be undertaken.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Contributor Information
Takahiro Tsushima, Department of Medicine, Division of Cardiology, University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University School of Medicine, 11100 Euclid Avenue, Cleveland, OH 44106, USA.
Sadeer Al-Kindi, Department of Medicine, Division of Cardiology, University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University School of Medicine, 11100 Euclid Avenue, Cleveland, OH 44106, USA.
Luis Augusto Palma Dallan, Department of Medicine, Division of Cardiology, University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University School of Medicine, 11100 Euclid Avenue, Cleveland, OH 44106, USA.
Anas Fares, Department of Medicine, University Hospitals Cleveland Medical Center, Case Western Reserve University School of Medicine, Cleveland, OH, USA.
Sung-Han Yoon, Department of Medicine, Division of Cardiology, University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University School of Medicine, 11100 Euclid Avenue, Cleveland, OH 44106, USA.
Heather L Wheat, Department of Medicine, University Hospitals Cleveland Medical Center, Case Western Reserve University School of Medicine, Cleveland, OH, USA.
Guilherme F Attizzani, Department of Medicine, Division of Cardiology, University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University School of Medicine, 11100 Euclid Avenue, Cleveland, OH 44106, USA.
Cristian R Baeza, Department of Surgery, Division of Cardiac Surgery, University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University School of Medicine, Cleveland, OH, USA.
Marc P Pelletier, Department of Surgery, Division of Cardiac Surgery, University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University School of Medicine, Cleveland, OH, USA.
Mauricio S Arruda, Department of Medicine, Division of Cardiology, University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University School of Medicine, 11100 Euclid Avenue, Cleveland, OH 44106, USA.
Judith A Mackall, Department of Medicine, Division of Cardiology, University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University School of Medicine, 11100 Euclid Avenue, Cleveland, OH 44106, USA.
Sergio G Thal, Department of Medicine, Division of Cardiology, University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University School of Medicine, 11100 Euclid Avenue, Cleveland, OH 44106, USA.
Data availability
Data will be made available based on reasonable requests.
References
- 1. Sammour Y, Krishnaswamy A, Kumar A, Puri R, Tarakji KG, Bazarbashi Net al. Incidence, predictors, and implications of permanent pacemaker requirement after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2021;14:115–34. [DOI] [PubMed] [Google Scholar]
- 2. Fadahunsi OO, Olowoyeye A, Ukaigwe A, Li Z, Vora AN, Vemulapalli Set al. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: analysis from the U. S. Society of Thoracic Surgeons/American College of Cardiology TVT registry. JACC Cardiovasc Interv 2016;9:2189–99. [DOI] [PubMed] [Google Scholar]
- 3. Faroux L, Chen S, Muntané-Carol G, Regueiro A, Philippon F, Sondergaard Let al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: a systematic review and meta-analysis. Eur Heart J 2020;41:2771–81. [DOI] [PubMed] [Google Scholar]
- 4. Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia Het al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the dual chamber and VVI implantable defibrillator (DAVID) trial. JAMA 2002;288:3115–23. [DOI] [PubMed] [Google Scholar]
- 5. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KLet al. MOde selection trial investigators. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932–7. [DOI] [PubMed] [Google Scholar]
- 6. Sharma AD, Rizo-Patron C, Hallstrom AP, O’Neill GP, Rothbart S, Martins JBet al. Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm 2005;2:830–4. [DOI] [PubMed] [Google Scholar]
- 7. Nadeem F, Tsushima T, Ladas TP, Thomas RB, Patel SM, Saric Pet al. Impact of right ventricular pacing in patients who underwent implantation of permanent pacemaker after transcatheter aortic valve implantation. Am J Cardiol 2018;122:1712–7. [DOI] [PubMed] [Google Scholar]
- 8. Fovino L N, Cipriani A, Fabris T, Massussi M, Scotti A, Lorenzoni Get al. Anatomical predictors of pacemaker dependency after transcatheter aortic valve replacement. Circ Arrhythm Electrophysiol 2021;14:e009028. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Medicare & Medicaid Services . Decision Memo for Transcatheter Aortic Valve Replacement (TAVR) (CAG-00430N). Available at: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx? NCAId=257.
- 10. Jankelson L, Bordachar P, Strik M, Ploux S, Chinitz L. Reducing right ventricular pacing burden: algorithms, benefits, and risks. Europace 2019;21:539–47. [DOI] [PubMed] [Google Scholar]
- 11. Puls M, Beuthner BE, Topci R, Vogelgesang A, Bleckmann A, Sitte Met al. Impact of myocardial fibrosis on left ventricular remodelling, recovery, and outcome after transcatheter aortic valve implantation in different haemodynamic subtypes of severe aortic stenosis. Eur Heart J 2020;41:1903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saunderson CED, Paton MF, Brown LAE, Gierula J, Chew PG, Das Aet al. Detrimental immediate- and medium-term clinical effects of right ventricular pacing in patients with myocardial fibrosis. Circ Cardiovasc Imaging 2021;14:e012256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sparks PB, Mond HG, Vohra JK, Jayaprakash S, Kalman JM. Electrical remodeling of the atria following loss of atrioventricular synchrony: a long-term study in humans. Circulation 1999;100:1894–900. [DOI] [PubMed] [Google Scholar]
- 14. Cheung JW, Keating RJ, Stein KM, Markowitz SM, Iwai S, Shah BKet al. Newly detected atrial fibrillation following dual chamber pacemaker implantation. J Cardiovasc Electrophysiol 2006;17:1323–8. [DOI] [PubMed] [Google Scholar]
- 15. Chen XL, Ren XJ, Liang Z, Han ZH, Zhang T, Luo Z. Analyses of risk factors and prognosis for new-onset atrial fibrillation in elderly patients after dual-chamber pacemaker implantation. J Geriatr Cardiol 2018;15:628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Z, Chen X, Ge J, Su Y. The risk factors of new-onset atrial fibrillation after pacemaker implantation. Herz 2021;46:61–8. [DOI] [PubMed] [Google Scholar]
- 17. Nattel S. Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin Electrophysiol 2017;3:425–35. [DOI] [PubMed] [Google Scholar]
- 18. Naveh S, Perlman GY, Elitsur Y, Planer D, Gilon D, Leibowitz Det al. Electrocardiographic predictors of long-term cardiac pacing dependency following transcatheter aortic valve implantation. J Cardiovasc Electrophysiol 2017;28:216–23. [DOI] [PubMed] [Google Scholar]
- 19. Lader JM, Barbhaiya CR, Subnani K, Park D, Aizer A, Holmes Det al. Factors predicting persistence of AV nodal block in post-TAVR patients following permanent pacemaker implantation. Pacing Clin Electrophysiol 2019;42:1347–54. [DOI] [PubMed] [Google Scholar]
- 20. Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee Let al. Biventricular versus right ventricular pacing in heart failure patients with atrioventricular block (BLOCK HF) trial investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 2013;368:1585–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available based on reasonable requests.